Abstract
Organotypic cultures support the stratification and differentiation of keratinocytes and the human papillomavirus (HPV) life cycle. We report transcription from four novel promoters in the HPV31b upstream regulatory region during the viral life cycle in organotypic cultures. Promoter initiation was not differentiation dependent; two promoters were down-regulated upon epithelial differentiation.
Human papillomaviruses (HPVs) have a tropism for squamous epithelium (25). HPV types with an increased risk of cervical malignancy include HPV types 16, 18, 31, and 33 (12). Genomic organization is highly conserved among HPVs, and the genomes can be divided into three segments (reviewed in reference 8). The upstream regulatory region (URR) contains promoter and enhancer elements involved in the control of gene expression, as well as sequences important for replication. The early region encodes proteins involved in genome replication and transcription. The late region codes for the viral structural proteins.
The life cycles of HPVs are tightly linked to the differentiation state of infected cells (5, 14, 23, 25). The dependence of the viral life cycle on epithelial differentiation has encumbered study of the virus in vitro. However, organotypic (raft) tissue culture systems have permitted the growth of differentiated keratinocytes in vitro and have provided a permissive environment for the complete HPV life cycle (14, 15, 17, 18). The addition of protein kinase C (PKC) pathway activators to the culture medium of raft tissues containing HPV sequences induces a more complete differentiation program (14, 16, 17). Specifically, we have shown that the enhanced epithelial differentiation of tissues containing episomal HPV genomes is concurrent with a strong induction of HPV late gene expression and efficient assembly of virions by days 10 to 12 in the raft system (14, 15, 17). The life cycle activities of HPV31b are the best characterized of those of the high-risk viruses due to the growth of the clonal CIN-612 9E cell line in the raft tissue culture system (3, 9, 10, 13, 17–19). CIN-612 9E cells maintain an average of 50 episomal copies of HPV31b per cell (3, 9). Seven spliced, polycistronic early RNAs and 19 late RNAs have been identified for HPV31b (9, 10, 17, 18). Four HPV31b promoters have been reported. P99, the major early promoter, P77, and P3320 are constitutively expressed during the viral life cycle (9, 19). P742 is induced upon epithelial differentiation and initiates a number of late gene transcripts (9, 17, 19).
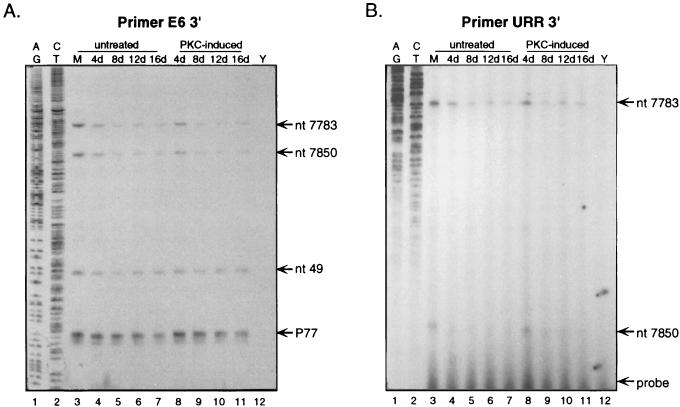
Cottontail rabbit papillomavirus, bovine papillomavirus type 1, HPV1, and HPV8 utilize promoters in the URR of their genomes (2, 20, 24, 26). To investigate the possibility that HPV31b initiates transcripts from promoters in this region, we performed nuclease protection assays using a probe which contained the URR just 3′ to the late polyadenylation signal and extended into the E6 open reading frame (ORF) (Fig. 1B). CIN-612 9E cells were grown as described previously and were harvested as subconfluent monolayers and as 12-day raft tissues PKC induced by treatment with 10 μM 1,2-dioctanoyl-sn-glycerol (C8:0; Sigma Chemical Co., St. Louis, Mo.) (14, 17, 19). Total RNAs were extracted, DNase I treated, and quantitated as previously reported (17, 19). RNA concentrations were based on optical densities; RNA concentrations and qualities were verified by electrophoresis through agarose gels containing ethidium bromide. A 5′-end-labeled, single-stranded probe was prepared by PCR amplification from a cloned segment of HPV31b DNA (17) and included HPV31b nucleotides (nt) 7239 to 173 (Fig. 1B). The primer E6 3′ (Table 1), complementary to the sense DNA strand, was 5′ end labeled and paired for PCR with an unlabeled M13(−40) primer complementary to the antisense strand and upstream of the cloned HPV31b URR sequences (Fig. 1B). The resulting 934-nt antisense probe was gel purified and used in nuclease S1 (S1) and exonuclease VII (exo VII) protection analyses with total RNAs as previously described (Fig. 1A) (17). The results from nuclease S1 and exo VII digestion assays were identical, indicating 5′ RNA ends rather than splice sites (Fig. 1A, lanes 1, 2, 5, and 6). The exo VII-digested samples migrated slightly slower than the nuclease S1-digested samples, as expected when analyzing large amounts of RNA (17, 19). In addition to the previously characterized 5′ RNA ends at HPV31b nt 99 and 77, four novel start sites were observed in the regions of nt 53, 7850, 7790, and 7375 (Fig. 1A and B). These start sites were observed in samples of RNAs from both the monolayer and raft tissue cultures (Fig. 1A, lanes 1, 2, 5, and 6). Yeast RNA controls yielded no specific protections (Fig. 1A, lanes 3 and 7). As we previously reported, similar levels of viral RNAs were found initiating from P99 in both monolayers and raft tissues (Fig. 1A, lanes 1, 2, 5, and 6) (19). Densitometric analyses demonstrated this was also true for RNAs initiating from P77, ≈nt 53, and ≈nt 7375, showing less than 2.5-fold differences between undifferentiated monolayer cells and differentiated, 12-day raft tissues (Fig. 1C). However, the levels of transcripts initiating from ≈nt 7850 and ≈nt 7790 were reduced an average of 6.6- and 7.8-fold, respectively, in differentiated raft tissues compared to those in undifferentiated monolayer cells (Fig. 1A, lanes 1, 2, 5, and 6, and C). These data were representative of several analyses and suggest the RNA start sites at ≈nt 7850 and ≈nt 7790 are negatively regulated by epithelial differentiation, whereas the start sites at P99, P77, ≈nt 53, and ≈nt 7375 are more constitutively active during the viral life cycle.
FIG. 1.
Nuclease S1 and exoVII nuclease protection analyses of HPV31b transcripts. (A) CIN-612 9E cells were cultured as monolayers (M) or as PKC-induced rafts harvested at day 12 (R). Forty micrograms of total RNAs or yeast RNAs was hybridized with the 5′-end-labeled probe and then digested with nuclease S1 or exo VII as indicated. Two yeast RNA samples were included as controls (Y): one yeast RNA sample was nuclease digested to show probe specificity (lanes 3 and 7), and the other was not nuclease digested to indicate the size of the input probe (lanes 4 and 8). The reactions were analyzed by electrophoresis through a 5% polyacrylamide–7 M urea sequencing gel. RNA Century Plus Markers (Ambion, Inc., Austin, Tex.) were used as size standards. The protected fragments are indicated to the right of the panel. MW, molecular weight; b, bases. (B) The position of the probe (thin and thick black lines) relative to the HPV31b genome is indicated. The HPV31b sequences of the URR and E6 and E7 ORFs (open boxes) are shown from the AccI site at nt 7239 to 1000 based on the sequence of HPV31 (7). Promoters P77, P99, and P742 are illustrated by bent arrows (9, 17, 19). The probe was PCR amplified from plasmid p31A-L1 by using primers M13(−10) and E6 3′ (17) and includes at the 3′ end 87 nt of plasmid sequences (thin line) plus 847 nt specific to the URR and E6 sequences from HPV31b nt 7239 to 173 (thick black line). The lengths and positions of viral RNAs protected by the probe are shown by shaded lines below the probe illustration. (C) Densitometric data from panel A; the autoradiogram was scanned in the regions containing the full-length protected fragments. Absolute readings for each RNA species were plotted and represent the average relative differences between the RNA levels in undifferentiated monolayer cells (shaded boxes) and differentiated raft epithelial tissues (hatched boxes).
TABLE 1.
Oligonucleotide primers used in analysis of HPV31b gene expression in CIN-612 9E monolayer cells and raft tissues
| Primer | Sequencea | Sense or antisense | ORFb | HPV31 position (nt)a |
|---|---|---|---|---|
| URR 5′ | 5′-TTA GGT GTC ACG CCA TAG-3′ | Sense | URR | 7381–7398 |
| URR-2 5′ | 5′-CTG CCA AGG TTG TGT CAT GC-3′ | Sense | URR | 7796–7815 |
| URR 3′ | 5′-GAA CAA TTG CTT GTA AAA CTG TAA CCT AAA AGG GTG-3′ | Antisense | URR | 7867–7902 |
| E6 3′ | 5′-GGG TAT TTC CAA TGC CGA GC-3′ | Antisense | E6 | 154–173 |
| E7E1 3′ | 5′-CTG GAT CAG CCA TTG TAG-3′ | Antisense | E7E1 | 852–874 |
| E1 3′ | 5′-TGT CCT CTT CCT CGT GC-3′ | Antisense | E1 | 2667–2683 |
| E4 3′ | 5′-CTT CAC TGG TGC CCA AGG-3′ | Antisense | E4 | 3378–3395 |
| L1-2 3′ | 5′-TAG CAC TGC CTG CGT G-3′ | Antisense | L1 | 5657–5672 |
Corresponding to the sequence and numbering of HPV31 (7).
ORF or region of HPV31.
To verify the data from the nuclease protections and more accurately map the two novel HPV31b start sites at ≈nt 7850 and ≈nt 7790, we performed primer extension analyses as previously described (19). The temporal usage of viral promoters during the HPV31b life cycle was investigated by harvesting the raft tissues for total RNAs at 4, 8, 12, and 16 days after lifting to the air-liquid interface (17). Antisense primers E6 3′ and URR 3′ (Table 1) were 5′ end labeled as previously reported (19). Reverse transcriptase-mediated extension of primer E6 3′ on the RNA samples resulted in the detection of a specific product corresponding to P77 as expected (Fig. 2A, lanes 3 to 11). Inclusion of sequencing ladder markers permitted accurate mapping of RNA start sites detected by nuclease protection analyses (≈nt 53, ≈nt 7850, and ≈nt 7790) to nt 49, 7850, and 7783, respectively (Fig. 2). As we previously reported, levels of transcripts initiating at P77 were generally similar whether the RNA samples were obtained from CIN-612 9E untreated monolayer cultures, untreated rafts, or PKC-induced raft tissues (19). Consistent with the results of the nuclease protection assays, the RNA start site at nt 49 (P49) also showed relatively unchanging initiation, albeit it was lower than that from P77, during the viral life cycle (Fig. 2A, lanes 3 to 11). Also in agreement with nuclease protection results, the primer extension reactions demonstrated the initiations from nt 7850 (P7850) and 7783 (P7783) were relatively strong in undifferentiated monolayer cells and 4-day raft tissues, but were considerably reduced upon increased epithelial differentiation in 8- to 16-day raft tissues (Fig. 2A and B, lanes 3 to 11). Given that the 12-day raft tissue embodies cells at various stages of differentiation, there could be a significant change in P99, P77, ≈nt 53, and ≈nt 7375 activity in a subpopulation(s) of the cells that might preclude detection. However, we do not believe this to be likely, because the levels of initiation from these promoters were similar between the fully differentiated 12-day rafts and the 4-day raft tissues, which consist of undifferentiated basal-like cells. Primer URR 3′ was used in extension assays to further verify the results obtained with primer E6 3′. Although hybridizations were weaker with primer URR 3′, likely due to a higher A+T/G+C ratio, this experiment substantiated that initiations from P7850 and P7783 were significantly down-regulated upon epithelial differentiation (Fig. 2B, lanes 3 to 11). It is also possible that the RNAs that initiated from P7850 and P7783 in differentiated tissues are less stable than the RNAs initiating from the other URR promoters. RNA samples derived from yeast, human foreskin keratinocyte monolayer cultures, and SCC-13 raft tissues were used as negative controls (19, 21) (Fig. 2A and B, lanes 12, and data not shown). The primer extension experiments were representative of several analyses using three separate RNA preparations.
FIG. 2.
Temporal analyses of HPV31b URR promoters by primer extension assays. Total RNAs were extracted from CIN-612 9E untreated monolayers (M); untreated rafts harvested at 4 days, 8, 12, and 16 days after lifting to the air-liquid interface; and rafts treated with C8:0 every second day (PKC induced) and harvested at 4, 8, 12, and 16 days after lifting to the air-liquid interface. Yeast RNAs (Y) are shown as controls. Primers were 5′-end labeled, gel purified, hybridized with RNAs, and extended with avian myeloblastosis virus reverse transcriptase, and the RNAs were digested with RNase A (19). The reactions were analyzed by electrophoresis through a 7% polyacrylamide–7 M urea sequencing gel. (A) Primer E6 3′ was hybridized to 25 μg of RNA. Sequencing ladders (AG/CT) were generated by using the E6 3′ primer on cloned HPV31b DNA template p31U*742L1 (17). (B) Primer URR 3′ was hybridized to 30 μg of RNA. Sequencing ladders (AG/CT) were generated by using the URR 3′ primer on cloned HPV31b DNA template p31bURRE1 (17).
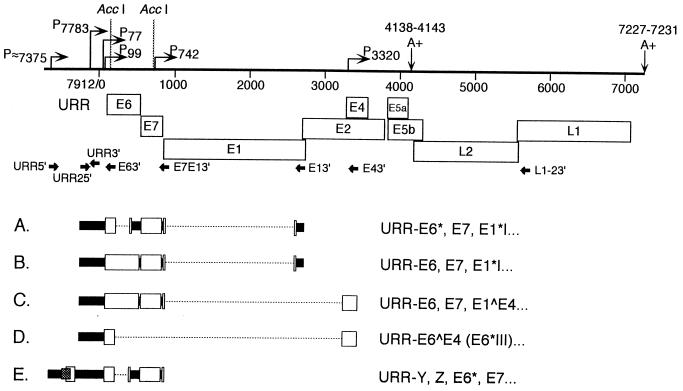
Reverse transcription (RT)-PCR was performed to identify the structures of transcripts initiating from the promoters in the URR (17, 19). Primer URR2 5′ was specific to the region just downstream of P7783, and primer URR 5′ was created to detect transcripts initiating from P≈7375 (Table 1 and Fig. 3 and 4). Total RNAs from CIN-612 9E monolayers and/or 12-day raft tissues were subjected to RT, and then PCR was performed with the 5′ URR primers paired with various 3′ primers (Fig. 3). The PCR products were cloned and sequences were determined with representative clones from each reaction as described previously (17, 19). The structures of the cloned cDNAs corresponding to each RNA are shown in Fig. 3; all contain splice combinations that have been reported previously (9, 18, 19). Transcripts A to D (Fig. 3) initiate at P7783 or upstream; no new ORFs were defined downstream of P7783. Transcript A contains the ORFs E6*, E7, E1*I, and potentially, E2 and E5a, whereas transcript B contains the ORFs E6, E7, E1*I, and, potentially, E2 and E5a. Transcript C contains the ORFs E6, E7, E1∧E4, and potentially E5a. Transcript D contains the E6∧E4 (E6*III) and, potentially, E5a ORFs. The cDNA corresponding to transcript E was cloned with primers URR 5′ and E7E1 3′, indicating initiation at ≈nt 7375 or upstream. Two novel overlapping ORFs are contained in the URR downstream of nt 7375 in addition to the E6* and E7 ORFs. The novel ORFs, designated Y and Z, potentially code for proteins of 29 and 27 amino acids, respectively. These RT-PCR results confirm that transcripts initiate in the HPV31b URR. Cottontail rabbit papillomavirus, bovine papillomavirus type 1, HPV1, and HPV8 cause cutaneous papillomas, and all initiate late gene transcripts in their URRs (2, 20, 24, 26). However, we previously demonstrated that none of the currently identified late gene transcripts initiated upstream of P77 in HPV31b, a mucosal-tropic virus (17, 19). Furthermore, we were unable using either of the 5′ URR primers to amplify cDNAs with primer L1-2 3′ as the downstream primer (Fig. 3), suggesting that no late gene transcripts are initiated in the upstream URR. Thus, we predict that each of the transcripts shown in Fig. 3 uses the early polyadenylation site. Bovine papillomavirus type 1 uses a promoter in the URR to initiate transcripts that use the early polyadenylation site in addition to those that use the late polyadenylation site (2); therefore, it is possible that some as yet unidentified HPV31b late gene RNAs might initiate in the URR upstream of P77. The low levels of RNAs initiating from these URR promoters and the potential length of RNAs may constrain RT and/or PCR amplification of such RNAs. Furthermore, because the ≈P7375 promoter was not finely mapped, it is possible that the URR 5′ primer was not appropriately positioned to detect a spliced transcript(s) initiating upstream of a weak 5′ splice site (AGGT) at nt 7384.
FIG. 3.
cDNA-derived HPV31b sequences from untreated CIN-612 9E monolayer cells and PKC-induced, 12-day raft tissues to verify the initiation of HPV31b transcripts in the URR. Total RNAs (1 to 2 μg) were reverse transcribed, and the cDNAs were PCR amplified; the products from the PCRs were cloned, and representative cDNAs were sequenced. The top schematic illustrates the HPV31b genome arbitrarily linearized following the late polyadenylation signal (A+). AccI restriction enzyme sites at HPV31 nt 198 and 745 were important for screening the clones. Bent arrows indicate the putative promoters P≈7375, P7783, and P7850; the constitutively expressed promoters P77, P99, and P3320; and the differentiation-induced promoter P742 (9, 19). The early polyadenylation site at nt 4138 to 4143 and the late polyadenylation site at nt 7226 to 7232 are designated by A+ (7). The URR is indicated and the major ORFs are shown by open boxes. PCR primer placement and orientation are shown by solid arrows. cDNAs characterized in this study are illustrated (A to E) with areas removed by splicing marked by broken lines. The amino acid coding potential for each cDNA is illustrated to the right. (A) RT-PCR with primers URR2 5′ (5′ end at nt 7796) and E1 3′ gave an 831-bp partial cDNA containing the URR, E6* ORF (E6* splice; nt 211∧413), E7 ORF, and the E1*I splice (877∧2646). (B) RT-PCR with primers URR2 5′ and E1 3′ yielded a 1,032-bp partial cDNA containing the URR, E6 ORF, E7 ORF, and the E1*I splice. (C) RT-PCR with primers URR2 5′ and E4 3′ produced a 1,095-bp partial cDNA containing the URR, E6 ORF, E7 ORF, and the E1∧E4 ORF (splice 877∧3295). (D) RT-PCR with primers URR2 5′ and E4 3′ gave a 429-bp partial cDNA containing the URR and E6∧E4 ORF (E6*III; splice 211∧3295). (E) RT-PCR with primers URR 5′ (5′ end at nt 7381) and E7E1 3′ gave a 1,205-bp partial cDNA containing the URR leader, Y ORF (stippled box), Z ORF (open box), E6* ORF, E7 ORF, and E1∧ region. The HPV31b nucleotide numbering is based upon the sequence of HPV31 (7).
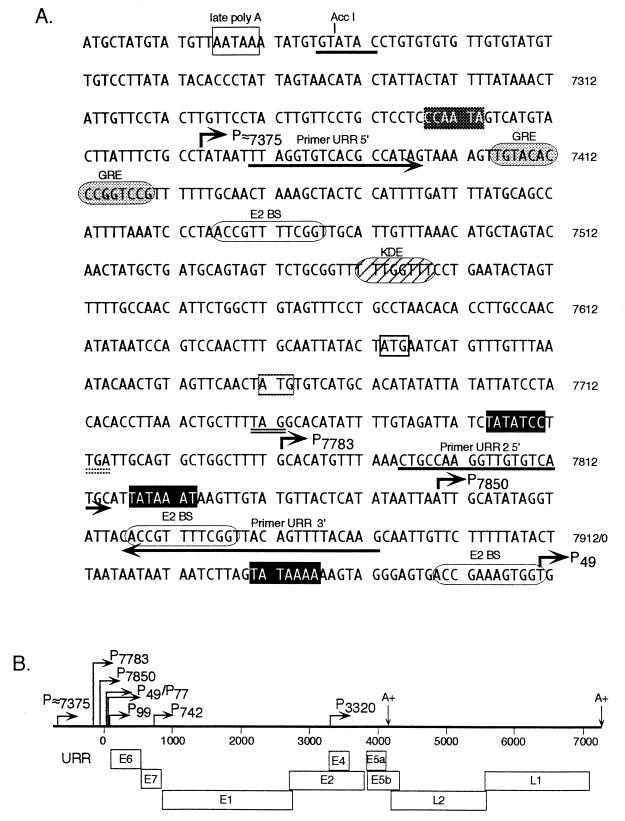
FIG. 4.
Analysis of the nucleotide sequences surrounding HPV31b URR promoters based upon the sequence for HPV31 (7). (A) The late polyadenylation site (nt 7227 to 7231) is shown by an open box, the AccI site at nt 7239 is underlined, and HPV31b genome nucleotide numbering is shown to the right of the sequence. Consensus nucleic acid sequence recognition sites are shown for the TATA binding protein (solid boxes indicate good consensus, and shaded boxes show weak consensus) (22), E2BS (ACCGN4CGGT) (1), a glucocorticoid responsive element (GRE) (6), and keratinocyte-dependent enhancer (KDE) (4). The promoters are shown by bent arrows with corresponding nucleotide numbers. The URR ORFs are marked by an open box on the AUG and a double-underlined stop codon (Y, thin lines; Z, stippled lines). PCR primers used in cloning viral cDNAs (URR 5′ and URR2 5′) and the primer used in primer extension assays (URR 3′) are indicated by underlining; their orientation is shown by arrows. (B) Summary of HPV31b genome indicating the URR, the major ORFs (open boxes), the four previously defined promoters (P77, P99, P742, and P3320), the four novel promoters characterized in this study (P≈7375, P7783, P7850, and P49), and the poly(A) sites (A+).
We analyzed the sequences adjoining the HPV31b URR transcriptional start sites for regulatory elements (Fig. 4). P49, P7850, and P7783 have consensus TATA boxes upstream of their initiation sites; the start site near nt 7375 has a weak consensus TATA box upstream. These minimal promoter elements may account for the relatively low levels of expression compared to that of the P99 major early promoter (Fig. 1A, lanes 1 and 2) which also contains initiator and Sp1 sequences (19). E2 binding sites (E2BS) are found near the start sites; the constitutively active P49 promoter has an E2BS abutting the initiation site, whereas each of the promoters negatively regulated by differentiation, P7850 and P7783, has an E2BS 85 and 18 nt downstream, respectively. However, it is unclear as to whether the E2BSs affect the temporal changes in expression from the URR promoters.
We have shown biochemical evidence for four novel HPV31b promoters in the URR and their usage throughout the viral life cycle. No uniquely spliced RNA structures were identified as initiating from the novel URR promoters, but two new ORFs were identified downstream of P≈7375. Neither novel ORF contains an AUG in a context that would suggest efficient translation; the long leader sequences upstream of the first ORF in each of the five identified transcripts also imply inefficient translation of the ORFs (11). Because P7850 and P7783 are negatively regulated by differentiation, they are likely to be preferentially expressed in undifferentiated basal cells. However, these experiments did not address the aspects of spatial tissue expression or the tissue-specific nature of these elements. The down-regulation of the P7850 and P7783 promoters during differentiation prompts us to speculate that they may be important in the earliest phases of infection in basal cells. A keratinocyte-dependent enhancer and a glucocorticoid responsive element are present in the URR, implying that these promoters may be important in regulation of this genital-specific virus during hormonal changes in its native cervical environment. We are currently performing experiments to address the latter two possibilities. Furthermore, our ability to produce infectious HPV stocks following the transfection of HPV genomic DNA into keratinocytes will allow us to construct mutant viruses whereby we can further explore the functions of these promoters in the differentiation-dependent life cycle of HPVs (15).
Acknowledgments
This work was supported by National Cancer Institute grants CA-64624 (C.M.) and CA-66316 (M.A.O.) and Howard Hughes Medical Institute Research Resources Program funds through the University of New Mexico School of Medicine (M.A.O.).
REFERENCES
- 1.Androphy E J, Lowy D R, Schiller J T. Bovine papillomavirus E2 trans-activating gene product binds to specific sites in papillomavirus DNA. Nature (London) 1987;325:70–73. doi: 10.1038/325070a0. [DOI] [PubMed] [Google Scholar]
- 2.Baker C C, Howley P M. Differential promoter utilization by the bovine papillomavirus in transformed cells and productively infected wart tissues. EMBO J. 1987;6:1027–1035. doi: 10.1002/j.1460-2075.1987.tb04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedell M A, Hudson J B, Golub T R, Turyk M E, Hosken M, Wilbanks G D, Laimins L A. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J Virol. 1991;65:2254–2260. doi: 10.1128/jvi.65.5.2254-2260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cripe T P, Haugen T H, Turk J P, Tabatabai F, Schmid III P G, Dürst M, Gissmann L, Roman A, Turek L P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987;6:3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crum C P, Nuovo G, Friedman D, Silverstein S J. Accumulation of RNA homologous to human papillomavirus type 16 open reading frames in genital precancers. J Virol. 1988;62:84–90. doi: 10.1128/jvi.62.1.84-90.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gloss B, Bernard H U, Seedorf K, Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6:3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldsborough M D, DiSilvestre D, Temple G F, Lorincz A T. Nucleotide sequence of human papillomavirus type 31: a cervical neoplasia-associated virus. Virology. 1989;171:306–311. doi: 10.1016/0042-6822(89)90545-x. [DOI] [PubMed] [Google Scholar]
- 8.Howley P M. Papillomavirinae: the viruses and their replication. In: Fields B N, Knipe D M, editors. Fields virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2045–2076. [Google Scholar]
- 9.Hummel M, Hudson J B, Laimins L A. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J Virol. 1992;66:6070–6080. doi: 10.1128/jvi.66.10.6070-6080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummel M, Lim H B, Laimins L A. Human papillomavirus type 31b late gene expression is regulated through protein kinase C-mediated changes in RNA processing. J Virol. 1995;69:3381–3388. doi: 10.1128/jvi.69.6.3381-3388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 12.Lorincz A T, Reid R, Jenson A B, Greenburg M D, Lancaster W, Kurman R J. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992;79:328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Mayer T J, Meyers C. Temporal and spatial expression of the E5a protein during the differentiation-dependent life cycle of human papillomavirus type 31b. Virology. 1998;248:208–218. doi: 10.1006/viro.1998.9262. [DOI] [PubMed] [Google Scholar]
- 14.Meyers C, Frattini M G, Hudson J B, Laimins L A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992;257:971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 15.Meyers C, Mayer T J, Ozbun M A. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J Virol. 1997;71:7381–7386. doi: 10.1128/jvi.71.10.7381-7386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozbun M A, Meyers C. Transforming growth factor β1 induces differentiation in human papillomavirus-positive keratinocytes. J Virol. 1996;70:5437–5446. doi: 10.1128/jvi.70.8.5437-5446.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozbun M A, Meyers C. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. J Virol. 1997;71:5161–5172. doi: 10.1128/jvi.71.7.5161-5172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozbun M A, Meyers C. Human papillomavirus type 31b E1 and E2 transcript expression correlates with vegetative viral genome amplification. Virology. 1998;248:218–230. doi: 10.1006/viro.1998.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozbun M A, Meyers C. Temporal usage of multiple promoters during the life cycle of human papillomavirus type 31b. J Virol. 1998;72:2715–2722. doi: 10.1128/jvi.72.4.2715-2722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palermo-Dilts D A, Broker T R, Chow L T. Human papillomavirus type 1 produces redundant as well as polycistronic mRNAs in plantar warts. J Virol. 1990;64:3144–3149. doi: 10.1128/jvi.64.6.3144-3149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rheinwald J G, Beckett M A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 22.Smale S T. Core promoter architecture for eukaryotic protein-coding sequences. In: Conway R C, Conway J W, editors. Transcription mechanisms and regulation. New York, N.Y: Raven Press; 1994. pp. 63–81. [Google Scholar]
- 23.Stoler M H, Wolinsky S M, Whitbeck A, Broker T R, Chow L T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989;172:331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 24.Stubenrauch F, Malejczyk J, Fuchs P G, Pfister H. Late promoter of human papillomavirus type 8 and its regulation. J Virol. 1992;66:3485–3493. doi: 10.1128/jvi.66.6.3485-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taichman L B, LaPorta R F. The expression of papillomaviruses in epithelial cells. In: Salzman N P, Howley P M, editors. The Papovaviridae. 2. The papillomaviruses. New York, N.Y: Plenum Press; 1987. pp. 109–139. [Google Scholar]
- 26.Wettstein F O, Barbosa M S, Nasseri M. Identification of the major cottontail rabbit papillomavirus late RNA cap site and mapping and quantitation of an E2 and minor E6 coding mRNA in papillomas and carcinomas. Virology. 1987;159:321–328. doi: 10.1016/0042-6822(87)90470-3. [DOI] [PubMed] [Google Scholar]