Abstract
DNA-encoded libraries have proven their tremendous value in the identification of new lead compounds for drug discovery. To access libraries in new chemical space, many methods have emerged to transpose traditional mol-scale reactivity to nmol-scale, on-DNA chemistry. However, procedures to access libraries with a greater fraction of C(sp3) content are still limited, and the need to “escape from flatland” more readily on-DNA remains. Herein, we report a Giese addition to install highly functionalized bicyclo[1.1.1]pentanes (BCPs) using tricyclo[1.1.1.01,3]pentane (TCP) as a radical linchpin, as well as other diverse alkyl groups, on-DNA from the corresponding organohalides as non-stabilized radical precursors. Telescoped procedures allow extension of the substrate pool by at least an order of magnitude to ubiquitous alcohols and carboxylic acids, allowing us to “upcycle” these abundant feedstocks to afford non-traditional libraries with different physicochemical properties for the small-molecule products (i.e., non-peptide libraries with acids). This approach is amenable to library production, as a DNA damage assessment revealed good PCR amplifiability and only 6% mutated sequences for a full-length DNA tag.
Graphical Abstract
INTRODUCTION
DNA-encoded library (DEL) platforms have emerged as powerful tools for drug discovery.1–10 They offer the advantage of requiring extremely small quantities of both the libraries and protein targets to reveal selective and potent small molecule binders, resulting in diminished costs for research and discovery efforts.5 In creating these libraries, standard organic reactions have been adapted to fit the constraints of this noncanonical and demanding discovery platform. Executing small molecule transformations in the presence of a DNA tag introduces several method limitations. Reaction requirements include chemoselectivity for the desired transformation, functional group compatibility with the encoding DNA, and being amenable to aqueous conditions at low concentrations.11 Furthermore, the DNA sequences must be conserved to be able to identify the attached small molecule binders following a DEL screen. The development of methods that operate within these allowances and also install the broadest diversity of scaffolds is of high interest to the DEL community.9
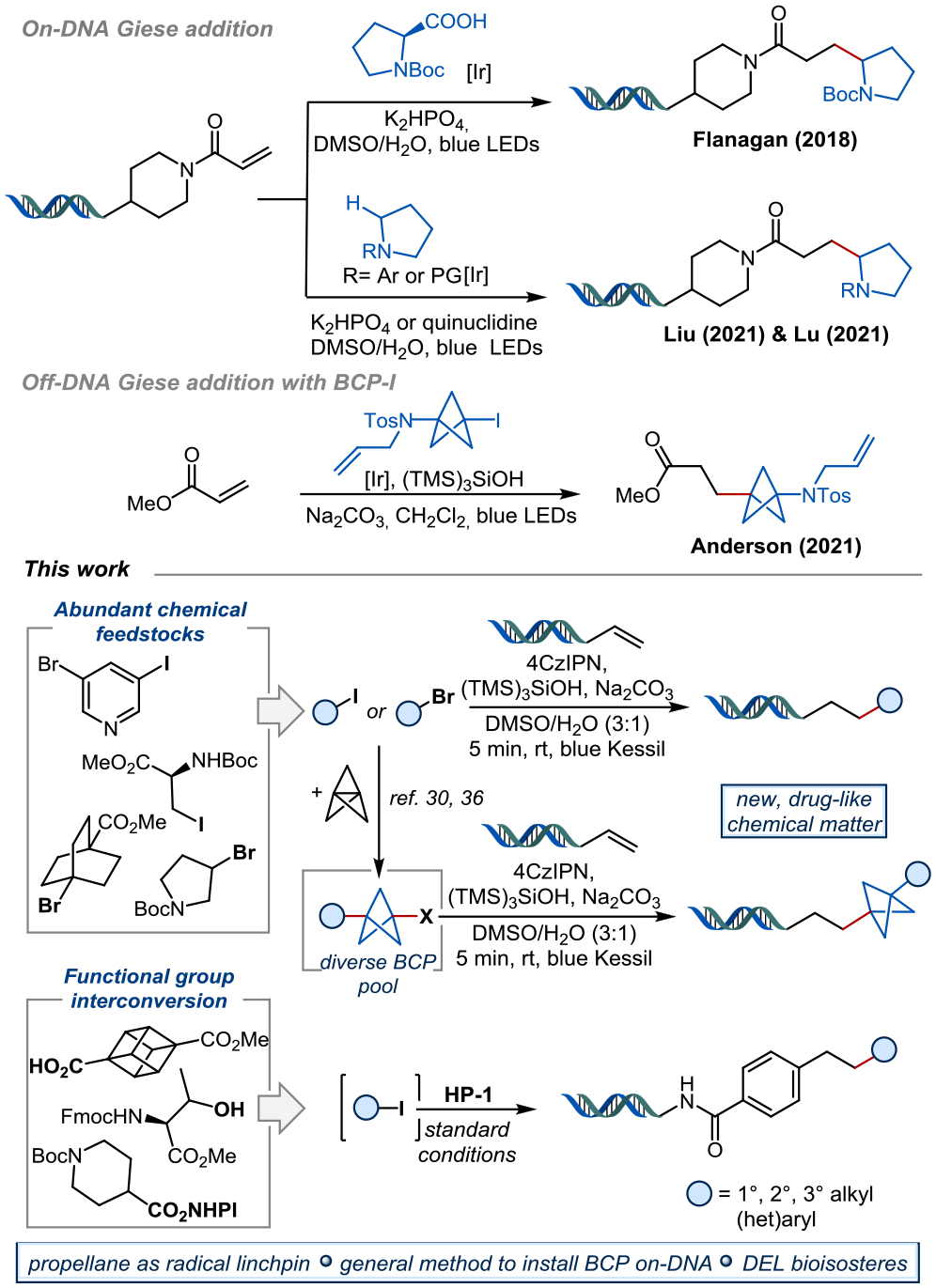
Within the DNA chemistry tool set, photoredox chemistry has demonstrated its usefulness to expand chemical space under extremely mild conditions,12–14 creating both carbon–carbon and carbon–heteroatom bonds. Among these, C(sp3)-C(sp3) bond formation has been of specific interest.7,14–19 Along these lines, Flanagan and coworkers developed a decarboxylative Giese-addition to introduce stabilized α-amino- or α-oxy radicals on-DNA (Figure 1).20,21
Figure 1.
State-of-the-art and this work
Following this report, Liu22 and Lu23 independently reported methods for α-amino radical addition to on-DNA alkenes, providing amino-alkylated products. Although accessing new, sp3-rich chemical space on-DNA, these methods and others24,25 are limited to stabilized radicals, while non-stabilized radical precursors react with low efficiency.
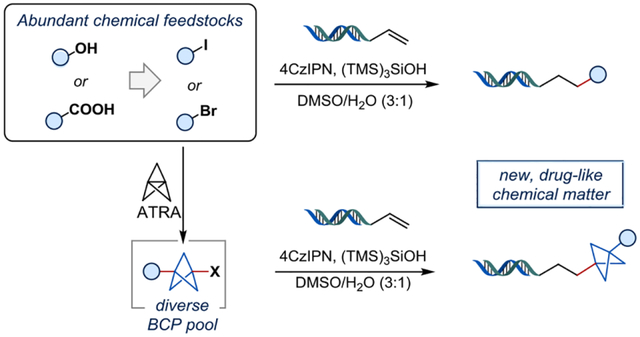
To improve the three-dimensionality and physicochemical properties of lead compounds, drug discovery chemists have long endeavored to replace the venerable arene ring in an effort to “escape from flatland”.26,27 Thus, arene bioisosteres such as bicyclo[1.1.1]pentanes (BCPs), bicyclo[2.2.2]octanes, or cubanes have been of interest to the synthesis community.28,29 Recent advances include efficient preparations of diversely substituted BCPs, or the Giese-type additions of non-stabilized BCP radical intermediates to alkene coupling partners, delivering sp3-rich products.30 Likewise, the ability to install arene bioisosteres on-DNA is of significant interest to the DEL field, but there exist only a few examples under DNA-compatible reaction conditions.31–33 In 2018, Baran and coworkers34 applied a bicyclo[2.2.2]octane N-hydroxyphthalimide (NHPI) redox active ester in an on-DNA Giese reaction with zinc nanopowder as a reductant, but BCP or cubane examples were not included. To the best of our knowledge, there has only been one example of a BCP radical addition on-DNA, likely because of the difficulty of generating the corresponding non-stabilized radical intermediate.30 Thus, there exists a gap of general methods to introduce diversely substituted arene bioisosteres on-DNA.
Inspired by the work of the Anderson group,30 we sought to develop radical couplings via halogen atom transfer (XAT) to access diverse on-DNA chemical matter (Figure 1). Furthermore, by using [1.1.1]propellane as an optional radical linchpin, two completely different products can be obtained from the same organic halide. We envisioned utilizing what has been historically viewed as limitations of DEL chemistry (low substrate concentration, excess of reagents, etc.) to our advantage to achieve desired reactivity and incorporate previously unprecedented substrates. As such, readily available and diverse alkyl- and (hetero)aryl halide feedstocks would be enabled for library preparation. Even further, we sought to upcycle carboxylic acid and alcohol feedstocks by using well-known methods for functional group interconversions (FG → I), which could then be telescoped into the on-DNA hydroalkylation chemistry. As an additional advantage, on-DNA photoredox methods have proven to be relatively less damaging to the DNA tag than typical DEL chemistry workhorses, such as Suzuki-Miyaura couplings or CuAAC.11,35 Toward this end, this approach offers access to more drug-like DEL libraries of higher fidelity with superior Fsp3 content.
RESULTS AND DISCUSSION
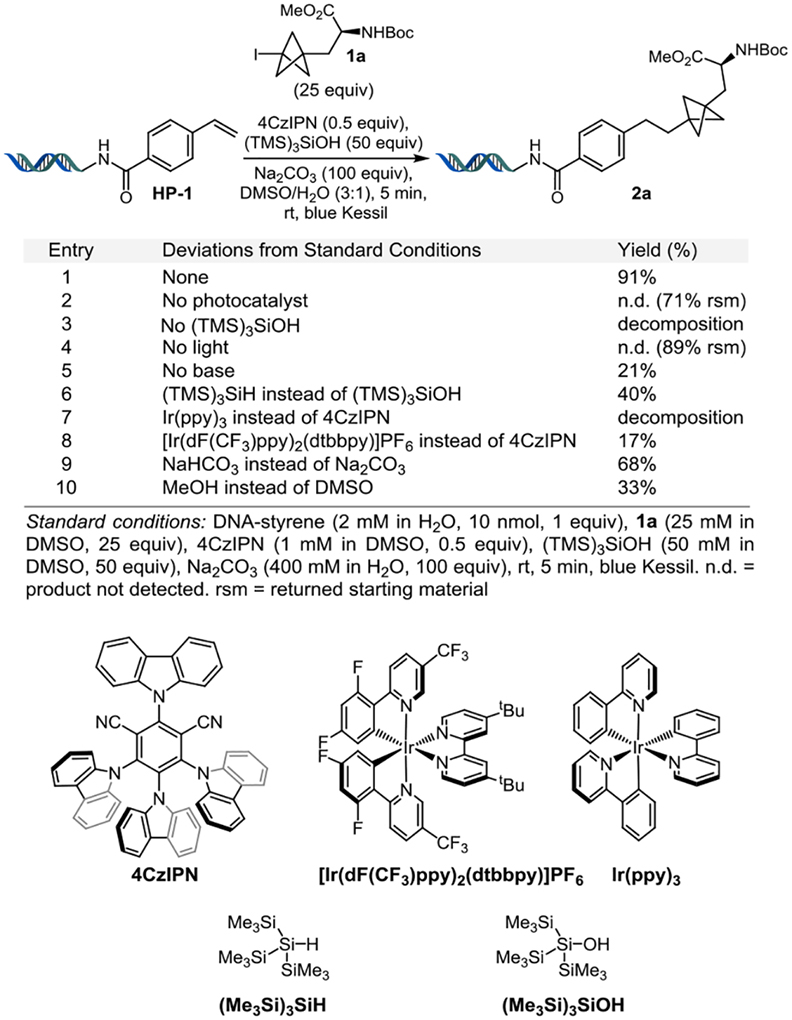
To initiate our investigation, p-styrene DNA headpiece HP-1 was used as a substrate because of its anticipated effectiveness as a radical acceptor (Table 1). The bicyclo[1.1.1]pentane derivative 1a was prepared from commercially available 3-iodoalanine in an excellent yield via atom transfer radical addition (ATRA).36
Table 1.
Optimization of on-DNA radical coupling.
|
The conditions were made amenable to on-DNA synthesis by using: 0.5 equivalent of 4CzIPN (1 mM in DMSO), 25 equivalents of BCP-I 1a (25 mM in DMSO), 50 equivalents of tris(trimethylsilyl)silanol [(TMS)3SiOH] (50 mM in DMSO), and 100 equivalents of Na2CO3 (400 mM in H2O), to afford the expected product after 5 min of irradiation using an H-150 blue Kessil lamp at room temperature, affording product 2a in a 91% yield (entry 1, Table 1). The developed on-DNA reaction requires a photosensitizer (entry 2), a radical mediator (entry 3), and light (entry 4) for reactivity. The use of a base led to increased reactivity (entry 5). In Anderson’s work, the use of a mediator was reported as being crucial to generate the BCP radical.30 Either tris(trimethylsilyl)silane [(TMS)3SiH] or (TMS)3SiOH led to the desired product, albeit the two mediators were proposed to function through two distinct mechanistic pathways. In the present case, when (TMS)3SiH was used instead of (TMS)3SiOH, the yield dropped to 40% (entry 6). In alignment with studies from the MacMillan group, we believe that the observed increased reactivity when (TMS)3SiOH is employed as the radical mediator is because of an efficient halogen atom transfer reaction (XAT) to generate the requisite BCP radical intermediate.37,38 The use of other photocatalysts such as Ir(ppy)3 or [Ir{dF(CF3)2ppy}2(dtbbpy)]PF6 led to DNA degradation and a decrease in yield, respectively (entries 7 and 8), in contrast to the off-DNA precedent, which proceeded most effectively with an iridium photocatalyst. Employing a weaker base gave a 68% yield (entry 9). Interestingly, although the off-DNA reaction occurred in a MeOH/H2O mixture,30 the on-DNA reaction gave a much lower yield (33%) (entry 10). Overall, this protocol is particularly noteworthy because of the ability to perform this reaction within minutes under air without degassing.
We then sought to evaluate the scope of this transformation with HP-1 using a set of BCP halides. Among them, a wide range of substrates served as competent partners, including those containing bifunctional handles (2a, 2j), a free alcohol (2d), N-Boc-protected amines (2f, 2h, 2i), a sugar (2g), and methyl esters (2e, 2o, 2l, 2r), all with moderate to excellent yield. The amino-substituted BCP30 (2n) performed well under the developed conditions.
BCP-iodides containing aryl substituents were also accessible under these conditions. Notably, arenes could be brought in through either functionalization at the benzylic position (2k, 2l, 2m) or through direct substitution of electron-deficient aryl iodides, generating C(sp2)-C(sp3) bonds prior to halo-BCP coupling (2p-v). In addition, by increasing the number of equivalents, BCP-bromides can also be used, affording products 2e and 2o in 63% and 46% yields, respectively. To the best of our knowledge, this represents the first time that BCP bromides could be leveraged for a Giese-type reaction on- or off-DNA. We believe that this result indicates a unique advantage that is available to on-DNA reactivity, as the small scale of reactions (nmol scale) and large excess of reagents (20–100 equivs), unlock challenging reactivity paradigms that would be prohibitive to investigate with canonical mol-scale reaction development conditions. Beyond BCP-containing substrates, we also demonstrated the generality of the developed on-DNA reactivity as applied to primary-, secondary-, and tertiary alkyl halides (Figure 2).
Figure 2.
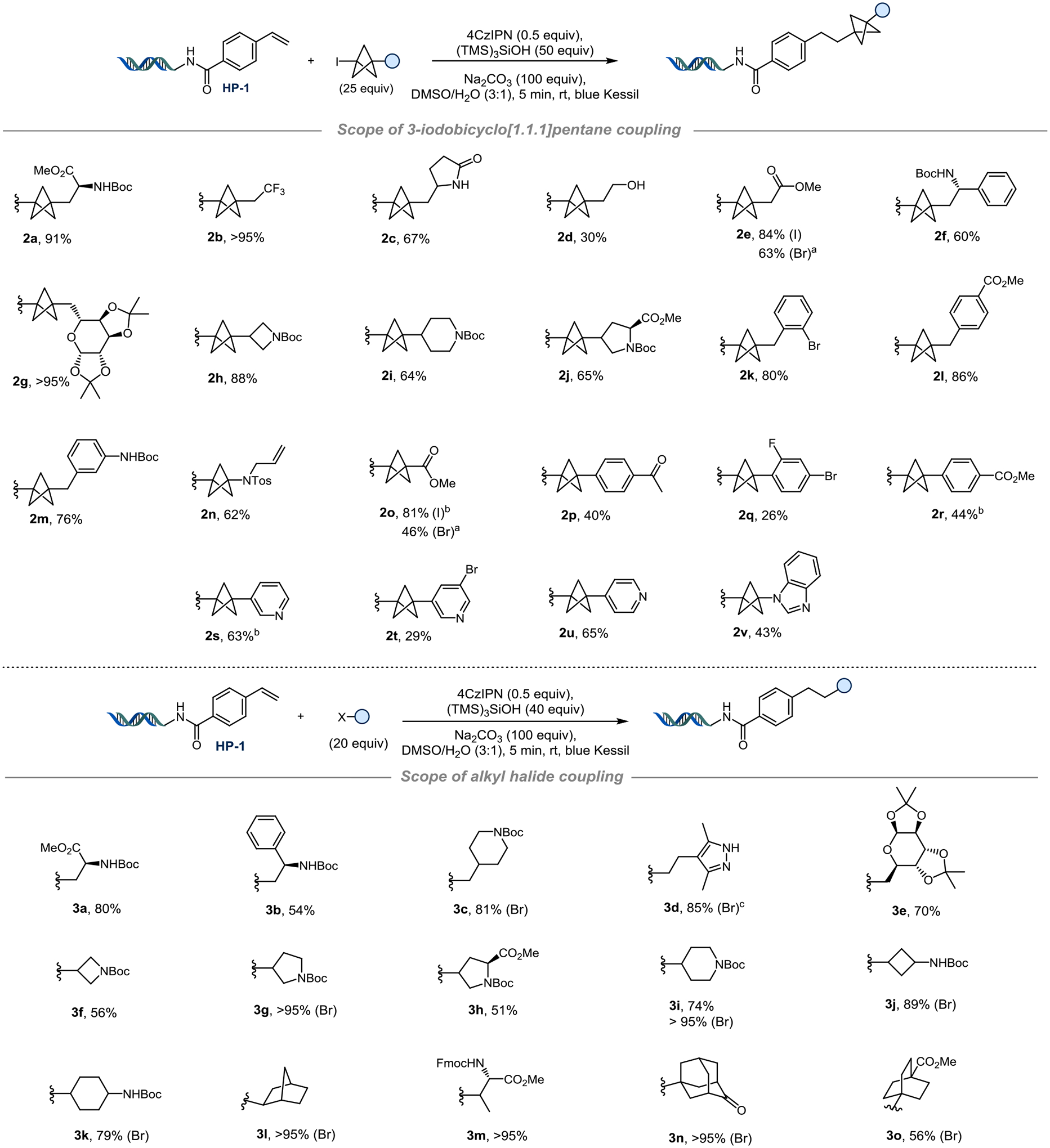
Top. Scope of radical coupling with 3-iodobicyclo[1.1.1]pentanes. The reaction was performed on 10 nmol scale for HP-1 (2 mM in H2O, 1.0 equiv), 4CzIPN (1 mM in DMSO, 0.5 equiv), BCP-I (25 mM in DMSO, 25 equiv), (TMS)3SiOH (50 mM in DMSO, 50 equiv), Na2CO3 (400 mM in H2O, 100 equiv), rt, 5 min, blue Kessil. a) 50 equiv of BCP-I (50 mM in DMSO) and 100 equiv of (TMS)3SiOH (100 mM in DMSO). b) 20 equiv of BCP-I (20 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO). Bottom. Evaluation of primary, secondary, and tertiary halides. Yields are indicated for alkyl iodides unless otherwise stated. HP-1 (2 mM in H2O, 10 nmol, 1 equiv), 4CzIPN (1 mM in DMSO, 0.5 equiv), alkyl halide (20 mM in DMSO, 20 equiv), (TMS)3SiOH (40 mM in DMSO, 40 equiv), Na2CO3 (400 mM in H2O, 100 equiv), rt, 5 min, blue Kessil. c) 40 equiv of alkyl-Br (40 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO)
The reaction accommodates both alkyl bromides and -iodides, albeit using a slightly decreased amount of alkyl halides and mediator than for the BCP halides (20 equivalents and 40 equivalents, respectively). This adjustment prevented the formation of byproducts corresponding to double addition by mass analysis of the alkyl radical (see SI, p S57 – S61). The unprotected pyrazole 3d afforded the desired product with HP-1 in 85% yield. Interestingly, the N-Fmoc-protected substrate 3m demonstrated excellent reactivity, with no observed deprotection despite the presence of sodium carbonate base. The tertiary adamantyl radical obtained from the brominated substrate gave product 3n in >95% yield. The [2.2.2]bicyclooctane 3o performed well in the reaction, with a 56% yield starting from the bromide derivative.
Exploration of the scope for on-DNA alkene acceptors (Figure 3) demonstrated that the meta-substituted HP-2 delivered the desired products in excellent yields with a secondary radical (4a) and a tertiary radical (4b). However, the addition of BCP-iodide (4c) with HP-2 failed to produce the product. ortho-Substituted styrene HP-3 provided moderate yields when reacted with substituted BCP-iodide substrates. As 3-alkyl-substituted BCP radicals are considered to be electron rich,39–41 they should react preferentially with electron-poor alkenes. This hypothesis is in line with our observation of the lack of reactivity of HP-2 with the BCP-iodide, as it does not benefit from the electron-withdrawing effect of the carboxamide. However, electron-deficient vinyl pyridines HP-4, HP-5 and HP-6 reacted smoothly with several BCP-iodides under the developed protocol. meta-Functionalization can be achieved with HP-5, and it is compatible with a variety of substrates: a deprotected piperidine 4j, quinoline- and pyrazole-containing BCPs (4o, 4p), and aryl BCPs possessing two useful handles for further post-functionalization (4m-n) were obtained in moderate to good yields. The substituted styrene HP-7 behaves as an excellent radical acceptor and offers the possibility to achieve further post-functionalization using the aryl halide. Methylacrylamide HP-8 required a more concentrated solution but was a very accommodating substrate. This activated alkene was of high interest because it allowed access to arene-free products (4y, 4z) and demonstrated the potency of the method to provide a further increase in Fsp3 in good yields. With the trifluoromethyl-substituted styrene (HP-9), the gem-difluoroalkene adduct was observed instead of the Giese-type addition product (4aa-ad) (Figure 4). This observed defluorinative alkylation mechanism provides access to interesting ketone isosteres on-DNA.42
Figure 3.
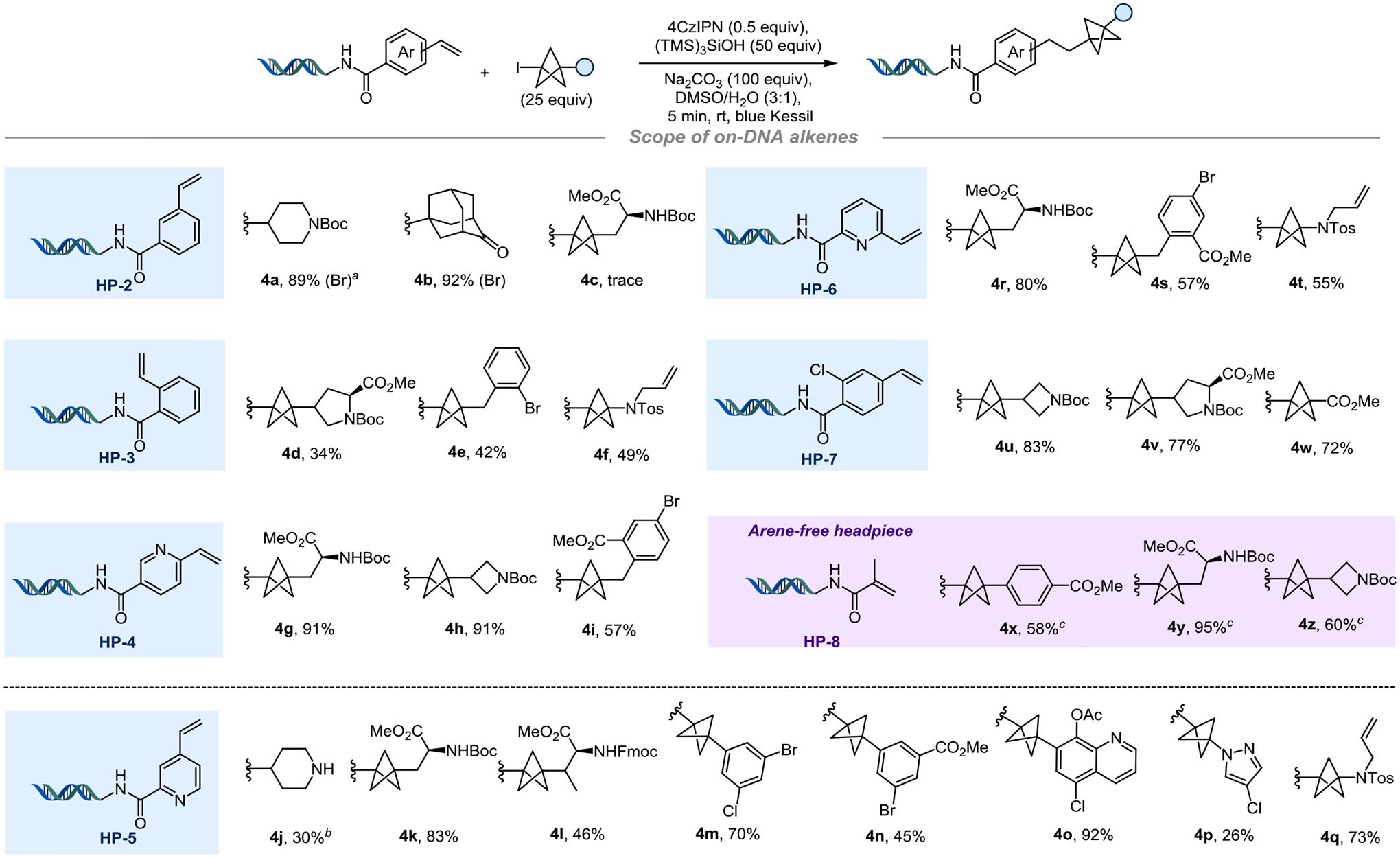
Scope of the olefin-functionalized DNA headpiece. a) alkyl bromide (20 mM in DMSO, 20 equiv) and (TMS)3SiOH (40 mM in DMSO, 40 equiv). b) 200 equiv of base, starting from the piperidine HBr salt. c) BCP-I (30 mM in DMSO, 15 equiv) and (TMS)3SiOH (60 mM in DMSO, 30 equiv).
Figure 4.
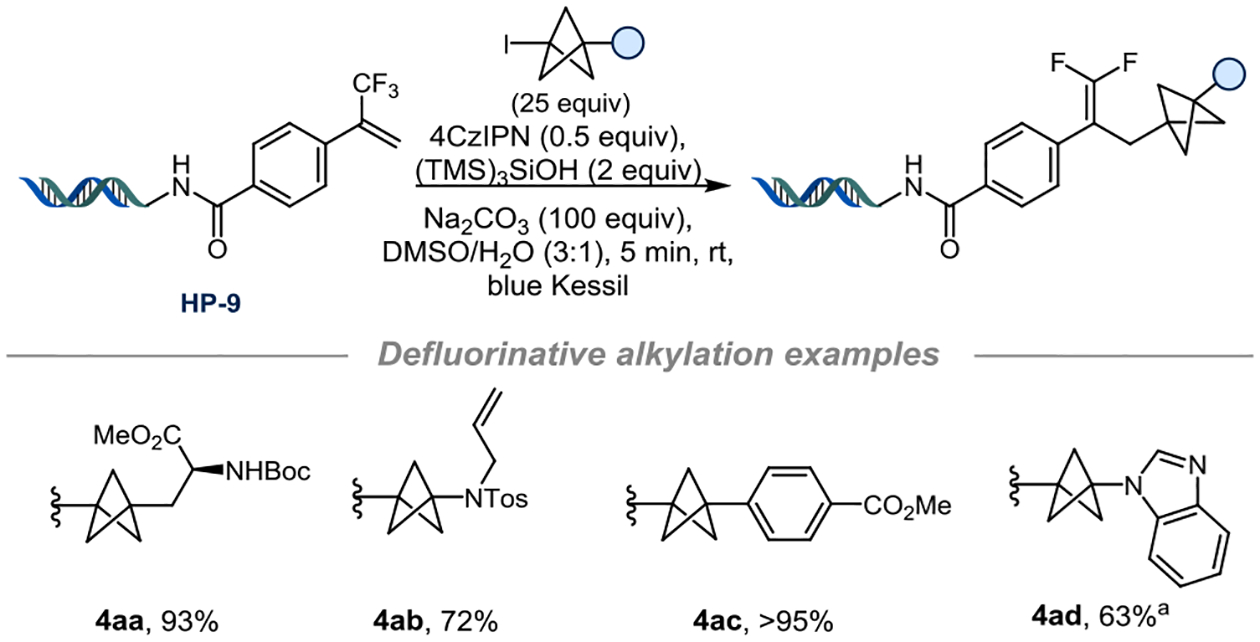
Defluorinative alkylation of CF3-alkene-functionalized DNA headpiece HP-9. a) BCP-I (20 mM in DMSO, 20 equiv), (TMS)3SiOH (20 mM in DMSO, 20 equiv)
We then sought to explore whether the scope could be expanded beyond alkyl- and (Het)Ar-halides to other abundant chemical feedstocks. Toward that end, “telescoped” processes for three substrate classes were pursued: activated esters, carboxylic acids, and alcohols. ‘Telescoped’ refers to performing two or more reactions without further purification in between (e.g., no chromatography, distillation, or crystallization), thereby streamlining the workflow and reducing resource waste. First, the redox active esters were used to form alkyl iodides,43 which were then reacted directly with the styrene headpiece. Secondary- (3i) and tertiary (3p) alkyl systems provided products in more than 90% yield, while the BCP substrate yielded the desired product 2o with only 28% yield under these conditions (Figure 5). A control experiment using redox active ester (S2a) directly as a radical precursor also provided product 3i in a low yield (< 25%), as it is known that a stoichiometric metal reducing agent is required for this purpose (see SI for details). The second strategy employed carboxylic acids. Previous photo-induced decarboxylative alkylations have been reported as shown in Figure 120, by our group44 in 2019. However, the present methods (from activated esters and carboxylic acids) allow non-stabilized secondary- and tertiary carboxylic acids to be used as precursors for the Giese addition to the less reactive DNA styrene, which has not been previously reported. Using this method,45 primary carboxylic acids performed smoothly and gave products 3q in 67% yield and 3r in 90% yield. A secondary carboxylic acid provided 3i in 76% yield. Iodocubane was generated from the corresponding carboxylic acid and reacted with HP-1 to give 3s in 84% yield, enabling the introduction of yet another interesting arene bioisostere (Figure 5).
Figure 5.
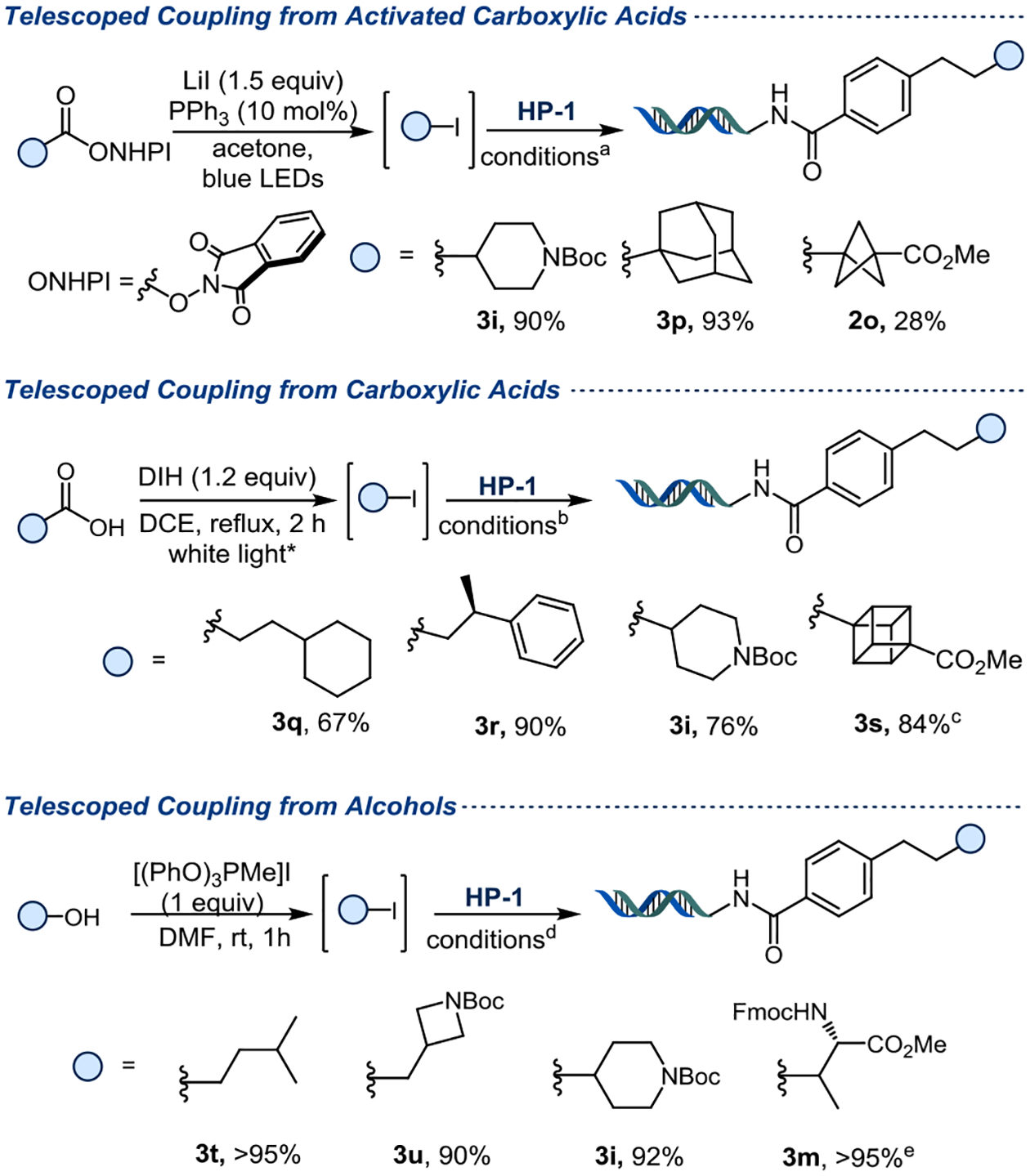
Telescoped reactions with additional chemical feedstocks. DIH = 1,3-diiodo-5,5’-dimethylhydantoin. The on-DNA reaction was performed on 10 nmol scale of HP-1 (2 mM in H2O, 1.0 equiv), 4CzIPN (1 mM in DMSO, 0.5 equiv), Na2CO3 (400 mM in H2O, 100 equiv), rt, 5 min, blue Kessil. a) 25 equiv of alkyl iodide (25 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO); b) 20 equiv of alkyl iodide (20 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO); c) 25 equiv of alkyl iodide (25 mM in DMSO) and 50 equiv of (TMS)3SiOH (50 mM in DMSO); d) 40 equiv of alkyl iodide (40 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO); e) off-DNA: 3 equiv of [(PhO)3PMe]I, overnight. On-DNA: 40 equiv of alkyl iodide (40 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO).
The last strategy employed alcohols in the telescoped reaction, another class of abundant and easily accessible building blocks. Using methyltriphenoxyphosphonium iodide to generate the iodide in situ46 allowed the introduction of primary- and secondary radicals with >90% yield (3t, 3u, 3i) (Figure 5). Of special note, the N-Fmoc-threonine-iodide derivative was successfully generated and added to HP-1 to give 3m in >95% yield.
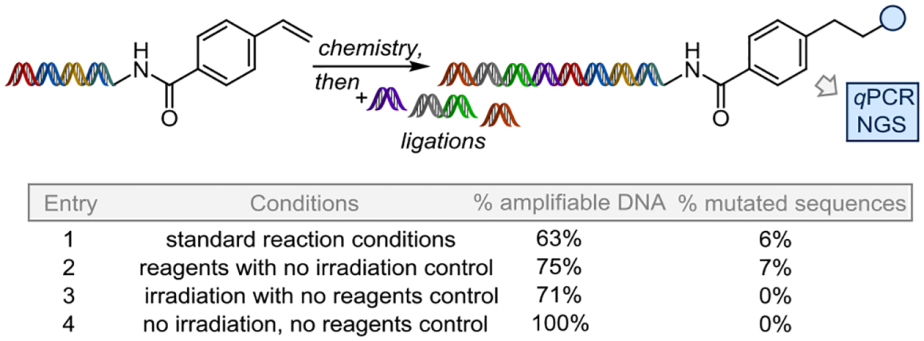
A critical metric for any new on-DNA chemistry is to ensure that the integrity of the encoding tag is maintained well enough to be employed in actual library production, an important aspect that is often not fully reported.11,35 It is essential that the encoding DNA is able to be amplified and sequenced following a DEL screen such that the corresponding small molecule and potential target binder can be revealed. If the dsDNA is significantly damaged, then the molecule cannot be identified, contributing to a false negative result.
Toward this end, the p-styrene substrate was prepared on an elongated DNA tag and then subjected to the reaction or other control conditions. Upon isolation of the single constructs by ethanol precipitation and filtration, a series of ligations were performed, mimicking the library production process, followed by quantitative polymerase chain reaction (qPCR) and next-generation sequencing (NGS) analysis of the full-length tag (see SI for details). Several controls were prepared in the same way. The ligations for all four samples proceeded with excellent efficiencies (97 –100%), as confirmed by LC-MS and/or gel. Amplification efficiency of the full-length sequence by qPCR was comparable for all four samples (90 – 92%) and the reaction maintained 63% amplifiable DNA, as compared to the no light, no reagents control (entry 1, Table 2). In contrast, some of the most often used, non-photonic on-DNA chemistries have only 30 – 50% amplifiable DNA remaining.35 Finally, NGS analysis revealed that the reaction sample had only 6% mutated sequences. Notably, the no light with reagents control had 7% mutated sequences, while the complementary control (light, but no reagents) had 0%. The observation that the reagents and not the irradiation were the cause of sequence mutations for the current method supports the hypothesis that photonic on-DNA chemistries offer a significant advantage over other protocols, particularly those methods that require high temperatures, longer reaction times, and metal-catalysis.
Table 2.
DNA damage assessment.
|
CONCLUSION
In conclusion, we have capitalized upon what have been considered limitations to on-DNA chemistry to develop a convenient, fast, general, and robust method to increase the Fsp3 content of DNA-encoded libraries with abundant chemical feedstocks. We successfully introduced a diverse set of arene bioisosteres, such as BCPs and cubanes, to radical acceptors on-DNA. Free alcohol, free amine, N-Fmoc-protected amine, ketone, bromide, and chloride functional groups are tolerated, introducing a possibility of further post-functionalization. A variety of DNA-conjugated olefin substrates are compatible with this transformation. Furthermore, the integrity of the DNA tag was preserved under these mild photonic reaction conditions, such that this method can be successfully employed to produce DNA-encoded libraries that cover previously inaccessible chemical space utilizing a larger subset of compatible and diverse building blocks.
Supplementary Material
ACKNOWLEDGMENT
The authors thank financial support provided by NIGMS (R35 GM 131680 to G.M.). The NSF Major Research Instrumentation Program (award NSF CHE-1827457), the NIH supplements awards 3R01GM118510-03S1 and 3R01GM087605-06S1, as well as the Vagelos Institute for Energy Science and Technology supported the purchase of the NMRs used in this study. We thank Dr. Charles W. Ross, III (UPenn) for mass spectral data and Gary O’Donovan (AbbVie) for his participation in the AbbVie-Molander collaboration. We thank Dr. Gaonan Wang and the WuXi AppTec HitS Unit for experimental execution of ligations, qPCR, NGS, and analysis for the DNA damage assessment. We thank Kessil for donations of LED lamps.
The authors declare the following competing financial interest(s): Some authors are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie and the agencies noted below. AbbVie participated in the interpretation of data, review, DNA damage assessment and approval of the publication.
Footnotes
Supporting Information. Preparation of on-DNA substrates, synthesis of alkyl halides and BCP-halides, general procedure for photoinduced transformations on-DNA, NMR spectra of small molecules prepared, UPLC/MS of on-DNA reactions, results of DNA damage assessment.
REFERENCES
- (1).Brenner S; Lerner RA Encoded combinatorial chemistry Proc. Natl. Acad. Sci. U. S. A 1992, 89, 5381–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Song M; Hwang GT DNA-Encoded Library Screening as Core Platform Technology in Drug Discovery: Its Synthetic Method Development and Applications in DEL Synthesis J. Med. Chem 2020, 63, 6578–6599. [DOI] [PubMed] [Google Scholar]
- (3).Neri D; Lerner RA DNA-Encoded Chemical Libraries: A Selection System Based on Endowing Organic Compounds with Amplifiable Information Annu. Rev. Biochem 2018, 87, 479–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Satz AL; Brunschweiger A; Flanagan ME; Gloger A; Hansen NJV; Kuai L; Kunig VBK; Lu X; Madsen D; Marcaurelle LA; Mulrooney C; O’Donovan G; Sakata S; Scheuermann J DNA-encoded chemical libraries Nat. Rev. Methods Prim 2022, 2, 1–17. [Google Scholar]
- (5).Goodnow RA; Dumelin CE; Keefe AD DNA-encoded chemistry: Enabling the deeper sampling of chemical space Nat. Rev. Drug Discov 2017, 16, 131–147. [DOI] [PubMed] [Google Scholar]
- (6).Gironda-Martínez A; Donckele EJ; Samain F; Neri D DNA-Encoded Chemical Libraries: A Comprehensive Review with Successful Stories and Future Challenges ACS Pharmacol. Transl. Sci 2021, 4, 1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Favalli N; Bassi G; Scheuermann J; Neri D DNA-encoded chemical libraries – achievements and remaining challenges FEBS Lett 2018, 592, 2168–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Melkko S; Scheuermann J; Dumelin CE; Neri D Encoded self-assembling chemical libraries Nat. Biotechnol 2004, 22, 568–574. [DOI] [PubMed] [Google Scholar]
- (9).Satz AL What Do You Get from DNA-Encoded Libraries? ACS Med. Chem. Lett 2018, 9, 408–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ottl J; Leder L; Schaefer JV; Dumelin CE Encoded library technologies as integrated lead finding platforms for drug discovery Molecules 2019, 24, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Malone ML; Paegel BM What is a “DNA-Compatible” Reaction? ACS Comb. Sci 2016, 18, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Patel S; Badir SO; Molander GA Developments in Photoredox-Mediated Alkylation for DNA-Encoded Libraries Trends Chem 2021, 3, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lechner VM; Nappi M; Deneny PJ; Folliet S; Chu JCK; Gaunt MJ Visible-Light-Mediated Modification and Manipulation of Biomacromolecules Chem. Rev 2022, 122, 1752–1829. [DOI] [PubMed] [Google Scholar]
- (14).Fair RJ; Walsh RT; Hupp CD The expanding reaction toolkit for DNA-encoded libraries Bioorganic Med. Chem. Lett 2021, 51, 128339. [DOI] [PubMed] [Google Scholar]
- (15).Xia B; Franklin GJ; Lu X; Bedard KL; Grady LC; Summerfield JD; Shi EX; King BW; Lind KE; Chiu C; Watts E; Bodmer V; Bai X; Marcaurelle LA DNA-Encoded Library Hit Confirmation: Bridging the Gap between On-DNA and Off-DNA Chemistry ACS Med. Chem. Lett 2021, 12, 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dickson P; Kodadek T Chemical composition of DNA-encoded libraries, past present and future Org. Biomol. Chem 2019, 17, 4676–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Madsen D; Azevedo C; Micco I; Petersen LK; Hansen NJV An overview of DNA-encoded libraries: A versatile tool for drug discovery, 1st ed.; Elsevier B.V., 2020; Vol. 59. [DOI] [PubMed] [Google Scholar]
- (18).Franzini RM; Randolph C Chemical space of DNA-encoded libraries: Miniperspective J. Med. Chem 2016, 59, 6629–6644. [DOI] [PubMed] [Google Scholar]
- (19).Clark MA; Acharya RA; Arico-Muendel CC; Belyanskaya SL; Benjamin DR; Carlson NR; Centrella PA; Chiu CH; Creaser SP; Cuozzo JW; Davie CP; Ding Y; Franklin GJ; Franzen KD; Gefter ML; Hale SP; Hansen NJV; Israel DI; Jiang J; Kavarana MJ; Kelley MS; Kollmann CS; Li F; Lind K; Mataruse S; Medeiros PF; Messer JA; Myers P; O’Keefe H; Oliff MC; Rise CE; Satz AL; Skinner SR; Svendsen JL; Tang L; Van Vloten K; Wagner RW; Yao G; Zhao B; Morgan BA Design, synthesis and selection of DNA-encoded small-molecule libraries Nat. Chem. Biol 2009, 5, 647–654. [DOI] [PubMed] [Google Scholar]
- (20).Kölmel DK; Loach RP; Knauber T; Flanagan ME Employing Photoredox Catalysis for DNA-Encoded Chemistry: Decarboxylative Alkylation of α-Amino Acids ChemMedChem 2018, 13, 2159–2165. [DOI] [PubMed] [Google Scholar]
- (21).For a recent review of photoinduced Giese reactions, see:; Gant Kanegusuku AL; Roizen JL Recent Advances in Photoredox-Mediated Radical Conjugate Addition Reactions: An Expanding Toolkit for the Giese Reaction Angew. Chemie - Int. Ed 2021, 60, 21116–21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wu R; Du T; Sun W; Shaginian A; Gao S; Li J; Wan J; Liu G Functionalization of DNA-tagged alkenes enabled by visible-light-induced C-H activation of N-aryl tertiary amines Org. Lett 2021, 23, 3486–3490. [DOI] [PubMed] [Google Scholar]
- (23).Shan J; Ling X; Liu JX; Wang X; Lu X DNA-encoded C–H functionality via photoredox-mediated hydrogen atom transformation catalysis Bioorganic Med. Chem 2021, 42, 116234. [DOI] [PubMed] [Google Scholar]
- (24).Chowdhury R; Yu Z; Tong ML; Kohlhepp SV; Yin X; Mendoza A Decarboxylative Alkyl Coupling Promoted by NADH and Blue Light J. Am. Chem. Soc 2020, 142, 20143–20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Badir SO; Sim J; Billings K; Csakai A; Zhang X; Dong W; Molander GA Multifunctional Building Blocks Compatible with Photoredox-Mediated Alkylation for DNA-Encoded Library Synthesis Org. Lett 2020, 22, 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lovering F; Bikker J; Humblet C Escape from flatland: Increasing saturation as an approach to improving clinical success J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]
- (27).Lovering F Escape from Flatland 2: Complexity and promiscuity Medchemcomm 2013, 4, 515–519. [Google Scholar]
- (28).Mykhailiuk PK Saturated bioisosteres of benzene: Where to go next? Org. Biomol. Chem 2019, 17, 2839–2849. [DOI] [PubMed] [Google Scholar]
- (29).Ma X; Nhat Pham L Selected Topics in the Syntheses of Bicyclo[1.1.1]Pentane (BCP) Analogues Asian J. Org. Chem 2020, 9, 8–22. [Google Scholar]
- (30).Pickford HD; Nugent J; Owen B; Mousseau JJ; Smith RC; Anderson EA Twofold Radical-Based Synthesis of N, C-Difunctionalized Bicyclo[1.1.1]pentanes J. Am. Chem. Soc 2021, 143, 9729–9736. [DOI] [PubMed] [Google Scholar]
- (31).Badir SO; Lipp A; Krumb M; Cabrera-Afonso MJ; Kammer LM; Wu VE; Huang M; Csakai A; Marcaurelle LA; Molander GA Photoredox-mediated hydroalkylation and hydroarylation of functionalized olefins for DNA-encoded library synthesis Chem. Sci 2021, 12, 12036–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ruff Y; Martinez R; Pellé X; Nimsgern P; Fille P; Ratnikov M; Berst F An Amphiphilic Polymer-Supported Strategy Enables Chemical Transformations under Anhydrous Conditions for DNA-Encoded Library Synthesis ACS Comb. Sci 2020, 22, 120–128. [DOI] [PubMed] [Google Scholar]
- (33).Liu W; Deng W; Sun S; Yu C; Su X; Wu A; Yuan Y; Ma Z; Li K; Yang H; Peng X; Dietrich J A Strategy for the Synthesis of Sulfonamides on DNA Org. Lett 2019, 21, 9909–9913. [DOI] [PubMed] [Google Scholar]
- (34).Wang J; Lundberg H; Asai S; Martín-Acosta P; Chen JS; Brown S; Farrell W; Dushin RG; O’Donnell CJ; Ratnayake AS; Richardson P; Liu Z; Qin T; Blackmond DG; Baran PS Kinetically guided radical-based synthesis of C(sp3)−C(sp3) linkages on DNA Proc. Natl. Acad. Sci. U. S. A 2018, 115, E6404–E6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sauter B; Schneider L; Stress C; Gillingham D An assessment of the mutational load caused by various reactions used in DNA encoded libraries Bioorganic Med. Chem 2021, 52, 116508. [DOI] [PubMed] [Google Scholar]
- (36).Nugent J; Arroniz C; Shire BR; Sterling AJ; Pickford HD; Wong MLJ; Mansfield SJ; Caputo DFJ; Owen B; Mousseau JJ; Duarte F; Anderson EA A General Route to Bicyclo[1.1.1]pentanes through Photoredox Catalysis ACS Catal 2019, 9, 9568–9574. [Google Scholar]
- (37).Zhang P; Le CC; MacMillan DWC Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling J. Am. Chem. Soc 2016, 138, 8084–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).For a recent example of XAT promoted Giese reaction, see:; Constantin T; Zanini M; Regni A; Sheikh NS; Juliá F; Leonori D Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides Science (80) 2020, 367, 1021–1026. [DOI] [PubMed] [Google Scholar]
- (39).Sterling AJ; Dürr AB; Smith RC; Anderson EA; Duarte F Rationalizing the diverse reactivity of [1.1.1]propellane through σ-π-delocalization Chem. Sci 2020, 11, 4895–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Walton JC; Whitehead L [1.1.1] Propellane and Alkyl Radicals : Verification of a Theoretical Prediction 1999, 1, 1399–1404. [Google Scholar]
- (41).Kim JH; Ruffoni A; Al-Faiyz YSS; Sheikh NS; Leonori D Divergent Strain-Release Amino-Functionalization of [1.1.1]Propellane with Electrophilic Nitrogen-Radicals Angew. Chemie - Int. Ed 2020, 59, 8225–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Leriche C; He X; Chang CWT; Liu HW Reversal of the apparent regiospecificity of NAD(P)H-dependent hydride transfer: The properties of the difluoromethylene group, a carbonyl mimic J. Am. Chem. Soc 2003, 125, 6348–6349. [DOI] [PubMed] [Google Scholar]
- (43).Fu MC; Wang JX; Shang R Triphenylphosphine-Catalyzed Alkylative Iododecarboxylation with Lithium Iodide under Visible Light Org. Lett 2020, 22, 8572–8577. [DOI] [PubMed] [Google Scholar]
- (44).Phelan JP; Lang SB; Sim J; Berritt S; Peat AJ; Billings K; Fan L; Molander GA Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis J. Am. Chem. Soc 2019, 141, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kulbitski K; Nisnevich G; Gandelman M Metal-free efficient, general and facile iododecarboxylation method with biodegradable co-products Adv. Synth. Catal 2011, 353, 1438–1442. [Google Scholar]
- (46).Kayser S; Temperini P; Poulie CBM; Staudt M; Nielsen B; Pickering DS; Bunch L A Diversity Oriented Synthesis Approach to New 2,3- trans-Substituted l -Proline Analogs as Potential Ligands for the Ionotropic Glutamate Receptors ACS Chem. Neurosci 2020, 11, 702–714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


