Introduction
Persistent chemotherapy-induced alopecia (PCIA) is defined as the absence of hair regrowth after more than 6 months of treatment discontinuation [1]. Topical minoxidil has been considered as the drug of choice in prevention or treatment of CIA. In a case of PCIA the efficacy of topical minoxidil may be limited [2,3]. Moreover, it may cause scalp irritation with itching, dryness and scaling. The use of low-dose oral minoxidil (LDOM) for the treatment of various forms of hair loss has demonstrated great efficacy [4]. To date, data considering efficacy of LDOM in PCIA are limited [2,5].
Case Presentation
A retrospective analysis of case series of 15 patients treated with LDOM for PCIA (Table 1) was performed. All patients with biopsy-proven PCIA with no efficacy from topical minoxidil used for at least one year were included. Patients with diagnosis of other form of hair loss or using other, than oral minoxidil, drug for alopecia were excluded.
Table 1.
Characteristics of patients with persistent chemotherapy-induced alopecia treated with low dose oral minoxidil.
| Patients numbers | 15 |
| females | 13 |
| males | 2 |
|
| |
| Age (years), mean (range) | 49 (35–71) |
|
| |
| Disease | |
| breast adenocarcinoma | 12 |
| lung adenocarcinoma | 1 |
| myelodysplasia | 1 |
| Hodgkin lymphoma | 1 |
|
| |
| Taxane-based therapy | |
| docetaxel | 3 |
| paclitaxel | 7 |
|
| |
| Non-taxane based therapy | |
| carboplatin | 3 |
| cisplatin | 1 |
| cyclophosphamide | 10 |
| dacarbazine | 1 |
| doxorubicin | 2 |
| epirubicin | 3 |
| etoposide | 3 |
| 5-fluorouracil | 1 |
| vincristine | 1 |
| vinorelbine | 1 |
|
| |
| Targeted therapy | |
| pertuzumab | 3 |
| trastuzumab | 4 |
|
| |
| Alopecia grade | |
| grade 1 | 7 |
| grade 2 | 8 |
|
| |
| Trichoscopy | |
| circle hairs / yellow dots | 3 |
| hair thinning / empty follicules | 15 |
All patients presented with thinned and sparse hair, with more or less pronounced areas of bare scalp. Trichoscopy showed a reduction of follicular units with an increase in vellus hair formation in the absence of inflammation and scarring. LDOM was used at a daily dose of 1.5 mg in less severe cases (seven cases with grade 1 alopecia according to CTCAEv5.0) and 2.5 mg in more severe ones (eight cases with grade 2 alopecia). Efficacy of LDOM was assessed based on clinical and trichoscopic pictures. After six to 12 months, clinical improvement was observed in seven (100%) patients with grade 1 and in six (75%) patients with grade 2 alopecia. Trichoscopy revealed an increased hair thickness and growth of new hair (Figure 1). Two out of eight patients affected by grade 2 alopecia were not able to abandon the wig, but partial hair regrowth and increased thickness made them feel more confident. Dose reduction from 2.5 mg to 1.5 mg was necessary, at the three months follow up, in three females due to non-acceptable facial hypertrichosis. No other, including cardiological, side effects were reported. No cardiological tests were needed during and after the therapy. All patients refused a post-treatment scalp biopsy.
Figure 1.
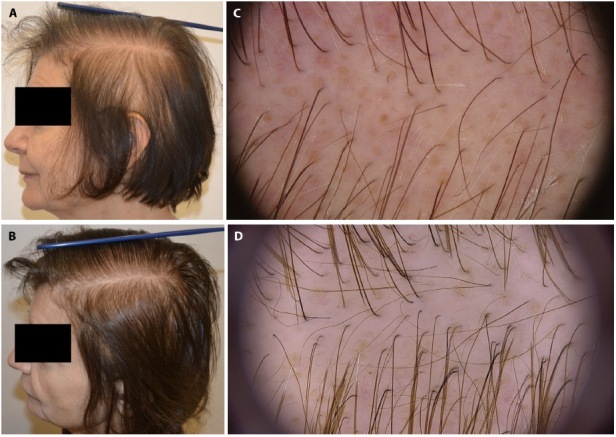
Clinical and trichoscopy images of a patient with persistent chemotherapy-induced alopecia (A,B) before treatment. (C,D) after 6 months of treatment with oral minoxidil 1.5 mg/day.
The results of the present study is consistent with the study conducted by Kang et al. (5) who observed efficacy of combination of LDOM and topical minoxidil in patients with PCIA. In our study, LDOM used in monotherapy was effective.
The limitation of the study is a small group of the patients included into analysis and the lack of post-treatment histology which would allow to define improvement on pathology grounds. Further studies are needed to better clarify the mechanism of action of LDOM in patients affected by PCIA and to draw conclusions concerning optimal drug dosages.
Conclusions
To conclude, we believe that LDOM is a promising therapeutic option for patients with PCIA who do not benefit from the topical solution or report disadvantages related to friction on a delicate scalp with thin hair.
Footnotes
Funding: None.
Competing interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80(5):1199–1213. doi: 10.1016/j.jaad.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57(4):e130–e132. doi: 10.1111/ajd.12350. [DOI] [PubMed] [Google Scholar]
- 3.Bhoyrul B, Asfour L, Lutz G, et al. Clinicopathologic Characteristics and Response to Treatment of Persistent Chemotherapy-Induced Alopecia in Breast Cancer Survivors. JAMA Dermatol. 2021;157(11):1335–1342. doi: 10.1001/jamadermatol.2021.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84(6):1644–1651. doi: 10.1016/j.jaad.2021.02.054. [DOI] [PubMed] [Google Scholar]
- 5.Kang J, Lee JW, Kwon O. Efficacy of low-dose oral minoxidil in the management of anticancer therapy-induced alopecia in patients with breast cancer: A retrospective cohort study. J Am Acad Dermatol. 2023;88(5):1170–1173. doi: 10.1016/j.jaad.2022.12.005. [DOI] [PubMed] [Google Scholar]


