Objectives:
Our objective was to evaluate the short- and long-term safety and efficacy of teduglutide treatment in infants and children with short bowel syndrome with intestinal failure (SBS-IF).
Methods:
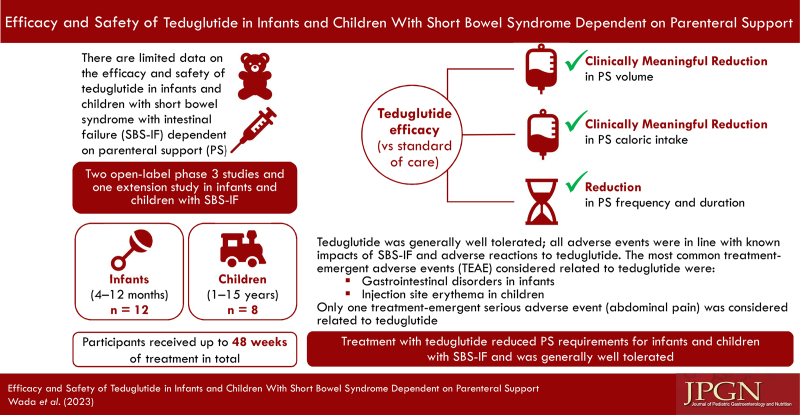
Two open-label phase 3 studies and 1 extension study investigated the short- and long-term safety and efficacy of teduglutide (0.05 mg/kg/day) in infants and children with SBS-IF: NCT03571516, 24-week study of infants who were randomized to receive teduglutide or standard of care (SoC); NCT02980666, 24-week study of infants and children who all received teduglutide; and NCT03268811, 24-week extension study of patients who completed NCT02980666 (patients could receive up to 48 weeks of total treatment).
Results:
Twelve infants and 8 children enrolled in the core studies, and 2 infants and 7 children in the extension study. After 24 weeks of treatment, parenteral support (PS) requirements reduced by ≥20% from baseline for 4 infants (57.1%) and 4 children (66.7%) receiving teduglutide and for 2 infants receiving SoC (50.0%). One infant (50.0%) and 4 children (80.0%) receiving teduglutide maintained the ≥20% reduction in PS at 48 weeks of treatment. Two children receiving teduglutide achieved enteral autonomy, after 12 weeks and 28 weeks of treatment, respectively. All adverse events (AEs) were in line with known impacts of SBS-IF and adverse reactions to teduglutide. Only one serious AE (abdominal pain) was considered related to teduglutide.
Conclusions:
Short- and long-term treatment with teduglutide resulted in clinically meaningful reductions in PS requirements for infants and children with SBS-IF. Teduglutide was well tolerated, and efficacy improved with longer-term treatment.
Keywords: GLP-2, intestinal adaptation, pediatric, SBS-IF
What Is Known
Teduglutide has proven efficacy and safety in adult patients with short bowel syndrome with intestinal failure (SBS-IF).
There are currently limited data on the efficacy and safety of teduglutide in pediatric patients with SBS-IF.
What is New
This analysis provides the first data on the efficacy and safety of teduglutide in infants under 1 year of age and additional data on children with SBS-IF.
Infants receiving teduglutide experienced similar reductions in parenteral support requirements to older children.
Infants may have comparable or fewer safety concerns than children when receiving teduglutide.
Short bowel syndrome (SBS) is a malabsorptive condition that occurs following extensive intestinal resection (1,2). SBS in infants or younger children is most commonly the result of congenital abnormalities or acquired through diseases that result in patients requiring massive bowel resection (3–7). New-onset SBS in older children is commonly caused by midgut volvulus or trauma (3).
Once gut function falls below the minimum required to absorb sufficient nutrients and fluids, patients require parenteral support (PS), at which point patients have SBS with intestinal failure (SBS-IF) (8,9). Long-term PS can lead to complications in pediatric patients (10–13). Promoting intestinal adaptation following intestinal resection allows pediatric patients to absorb more nutrients through enteral feeding and minimizes dependence on PS (10).
Teduglutide is an analog of glucagon-like peptide 2 that supports intestinal adaptation in SBS-IF by regulating the functional and structural integrity of cells lining the gastrointestinal tract (1,14,15). Teduglutide 0.05 mg/kg once daily is approved for treating adults and children (≥1 year of age) with SBS-IF in the United States, Europe, and Japan (16–19). Expert opinion from Italy provided treatment strategies due to limited data from pediatric trials (19). Since 2021, teduglutide has also been approved for treating patients with a corrected age of ≥4 months and a body weight of ≥10 kg in Japan (20), but not in Europe or the United States.
The objective of this current analysis is to evaluate the short- and long-term safety and efficacy of teduglutide treatment in pediatric patients (infants and children) with SBS dependent on PS.
METHODS
Clinical Studies
Data were collected from 2 core (NCT03571516, NCT02980666) and 1 extension (NCT03268811) open-label, phase 3 clinical studies in pediatric patients with SBS-IF. These studies were conducted at 7 study sites across Finland, France, and the United Kingdom (NCT03571516) and 6 sites in Japan (NCT02980666, NCT03268811). Teduglutide was not approved in Japan at the time these studies were conducted.
All studies were conducted in accordance with the World Medical Association Declaration of Helsinki and the International Council for Harmonisation and Good Clinical Practice guidelines for medical research in humans. Written informed consent was obtained from each patient and patient’s parent/guardian prior to study participation. Written informed assent was obtained from patients in elementary school or above. The protocols and associated materials were reviewed and approved by local institutional review boards.
Study Population
The studies recruited infants with a corrected gestational age of 4–12 months and children 1–15 years of age. The population sizes were based on the enrollment feasibility for this rare condition and young age of the patients. The inclusion criteria included a diagnosis of SBS with stable PS requirements, and patients had to have been receiving a minimum of 30% (NCT02980666) or 50% (NCT03571516) of fluids or calories parenterally (Table 1, Supplemental Digital Content 1, http://links.lww.com/MPG/D201).
Study Design
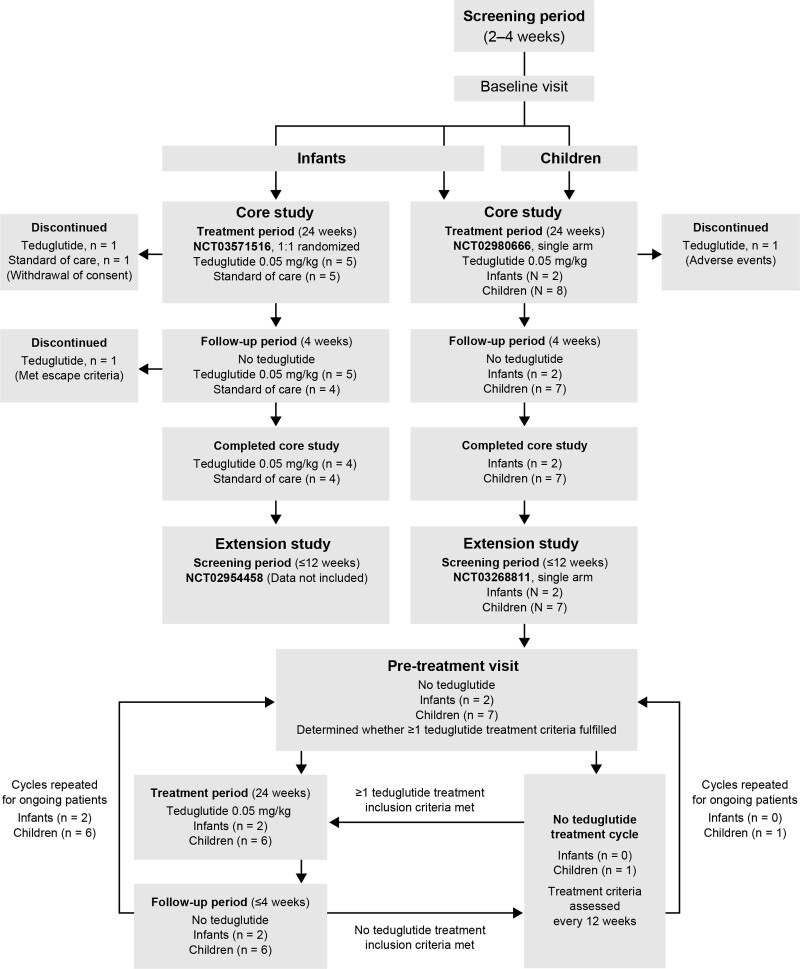
The designs of the core studies were based on the previously conducted 24-week, multicenter, multinational pediatric study (Fig. 1) (21). Patients received either teduglutide 0.05 mg/kg administered once daily by subcutaneous injection for a 24-week treatment period in addition to standard medical therapy for SBS-IF [standard of care (SoC)] or SoC only. In study NCT03571516, infants were randomized 1:1 to teduglutide or SoC groups using an interactive response system (stratified according to the presence of a small bowel ostomy). Study NCT02980666 had a single treatment arm in which all infants and children received teduglutide. At the end of the treatment period, patients who had not discontinued prematurely or enrolled into treatment extension studies entered a 4-week follow-up period, during which all patients received no teduglutide. Patients who were receiving teduglutide and achieved enteral autonomy at any time during the treatment period continued to receive teduglutide treatment throughout that study.
FIGURE 1.
Study design for the 2 core and 1 extension pediatric clinical trials.
Eligible infants and children who had completed and responded to treatment in the core study NCT02980666 could participate in the extension study (28-week treatment cycle; 24-week teduglutide treatment period and 4-week follow-up). If patients did not meet treatment inclusion criteria, they could join the no teduglutide treatment arm until at least one of the teduglutide treatment inclusion criteria was met. The current analysis is up to an interim cut-off of 24 weeks (totaling 48 weeks of treatment) so data can be used for a Japanese new drug application filing.
A protocol amendment was made in the core study NCT02980666 after a proportion of children had already enrolled. Changes included improved training for parents to administer doses of teduglutide and updated ancillary kits for preparing and administering teduglutide. The amended protocol continued to be used in the extension study.
Efficacy analyses were conducted on the intent-to-treat population of infants and children (all enrolled infants and only children who were enrolled after the protocol amendment). Safety analyses were performed on the safety population (core studies, all patients who had received at least 1 dose of teduglutide or SoC and had at least 1 post-baseline safety assessment; extension study, all enrolled patients).
Weekly PS volume and calorie intake were recorded using patient diary data and investigator-prescribed data. Efficacy analysis focused on patient diary data, unless otherwise specified. Treatment compliance was defined as patients receiving ≥80% of planned doses and was measured by using patient diaries, asking the patient’s parent/guardian if they had administered the study drug according to instructions, and by counting and examining used and unused vials.
Endpoints
Efficacy endpoints included the proportion of patients with a reduction in PS volume of ≥20% (patients who achieved ≥20% reduction in weight-normalized PS volume were considered responders), absolute and percentage change in PS volume and caloric intake, and changes in PS frequency and duration at week 24 or 48 compared with the baseline of the core studies. The changes in PS volumes were weight normalized for infants.
Safety endpoints included study drug exposure, adverse events (AEs), and changes in growth parameters between baseline and week 24 or 48. Treatment-emergent AEs (TEAEs) and treatment-emergent serious AEs (TESAEs) were coded using preferred terms from the Medical Dictionary for Regulatory Activities central coding dictionary, version 21.0/version 20.0 (22). Laboratory tests for clinical biochemistry, hematology, coagulation, and urinalysis were assessed at screening and follow-up visits.
Statistical Analysis
Data were pooled for infants who received teduglutide from studies NCT03571516 and NCT02980666. Descriptive statistics were used to summarize data from all studies, with data values reported as mean ± standard deviation unless otherwise stated. All statistical procedures were completed using SAS statistical software (SAS Institute, Cary, NC, USA) version 9.4 or later.
RESULTS
Patients
A total of 12 infants (n = 5, SoC; n = 7, teduglutide) and 8 children participated in the core studies; 2 infants and 7 children continued into the extension study. Baseline demographics and characteristics are summarized in Table 1. Most infants in the core studies were White (teduglutide, 57.1%; SoC, 80.0%), and all other patients in the core and extension studies were Asian. The most common primary underlying reasons for SBS diagnosis were gastroschisis and necrotizing enterocolitis (NEC) in infants and midgut volvulus in children. The residual small intestinal lengths for infants and children in the core and extension studies were 2.0–95.0 cm (teduglutide, 2.0–40.0 cm; SoC, 5.0–95.0 cm) and 1.5–48.0 cm, respectively.
TABLE 1.
Patient demographics and baseline values
| Parameter | Infants | Children* | |||
|---|---|---|---|---|---|
| Core study | Extension study (N = 2) | Core study (N = 8) | Extension studyठ(N = 7) | ||
| SoC (n = 5) | Teduglutide†(n = 7) | ||||
| Age (months‖ or years¶) | |||||
| Mean (SD) | 8.3 (2.7)‖ | 8.9 (2.7)‖ | 10.0 (1.2)‖ | 7.0 (3.6)¶ | 6.7 (3.8)¶ |
| Median (min, max) | 7.5 (5.0, 12.0)‖ | 9.7 (4.6, 11.7)‖ | 10.0 (9.1, 10.8)‖ | 5.9 (2.7, 12.0)¶ | 5.7 (2.7, 12.0)¶ |
| 4 to <6 months, n (%) | 1 (20.0)‖ | 2 (28.6)‖ | 0‖ | N/A | N/A |
| 6 to 12 months, n (%) | 4 (80.0)‖ | 5 (71.4)‖ | 2 (100.0)‖ | N/A | N/A |
| Gestational age, wk, mean (SD) [range] | 31.3 (5.2) [26.6–40.0] | 34.2 (2.3) [30.9–37.8] | N/A | N/A | N/A |
| Sex, n (%) | |||||
| Male | 3 (60.0) | 5 (71.4) | 1 (50.0) | 7 (87.5) | 6 (85.7) |
| Female | 2 (40.0) | 2 (28.6) | 1 (50.0) | 1 (12.5) | 1 (14.3) |
| Race, n (%) | |||||
| White | 4 (80.0) | 4 (57.1) | 0 | 0 | 0 |
| Asian | 1 (20.0) | 3 (42.9) | 2 (100.0) | 8 (100.0) | 7 (100.0) |
| Primary reason for SBS diagnosis, n (%) | |||||
| Necrotizing enterocolitis | 2 (40.0) | 1 (14.3) | 0 | 0 | 0 |
| Midgut volvulus | 0 | 1 (14.3) | 1 (50.0) | 6 (75.0) | 6 (85.7) |
| Intestinal atresia | 0 | 1 (14.3) | 0 | 1 (12.5) | 1 (12.5) |
| Gastroschisis | 2 (40.0) | 3 (42.9) | 0 | 1 (12.5) | 0 |
| Other | 1 (20.0)# | 1 (14.3)** | 1 (50.0)** | 0 | 0 |
| Patients with a stoma, n (%) | 1 (20.0)†† | 1 (14.3)‡‡ | 0 | 0 | 0 |
| Total estimated remaining small intestinal length, cm | |||||
| n | 5 | 7 | 2 | 7 | 6 |
| Mean (SD) | 35.0 (34.9) | 18.4 (12.4) | 6.0 (5.7) | 23.1 (15.9) | 22.0 (17.1) |
| Median (min, max) | 23.0 (5.0, 95.0) | 15.0 (2.0, 40.0) | 6.0 (2.0, 10.0) | 25.0 (1.5, 48.0) | 22.0 (1.5, 48.0) |
| Patients with remaining colon | |||||
| n (%) | 5 (100.0) | 7 (100.0) | 2 (100.0) | 8 (100.0) | 7 (100.0) |
| Estimated percent of colon remaining, mean (SD) | 51.7 (27.5) | 69.9 (28.7) | 75.0 (35.4) | 82.5 (32.4) | 90.0 (26.5) |
| Median (min, max) | 65.0 (20.0, 70.0) | 75.0 (19.0, 100.0) | 75.0 (50.0, 100.0) | 100.0 (30.0, 100.0) | 100.0 (30.0, 100.0) |
| Number of patients with colon in continuity, n (%) | 4 (80.0) | 7 (100.0) | 2 (100.0) | 8 (100.0) | 7 (100.0) |
| Number of patients with distal/terminal ileum, n (%) | 0 | 2 (28.6) | 1 (50.0) | 5 (62.5) | 5 (71.4) |
| Ileocecal valve present, n (%)§§ | 0 | 2 (100.0) | 1 (100) | 4 (80.0) | 4 (80.0) |
| Duration of prior PS dependence | 7.4 (4.5)‖‖ | 8.0 (4.8)‖‖ | 5.7 (8.9)‖‖ | 6.7 (3.8)¶¶ | 6.5 (4.1)¶¶ |
Values were based on the safety population unless otherwise specified.
N/A = not applicable; PS = parenteral support; SBS = short bowel syndrome; SD = standard deviation; SoC = standard of care; wk = week.
For study NCT02980666 and its extension NCT03268811, 6 of 8 children were enrolled and received teduglutide treatment after protocol amendment, which included improved training for parents to administer doses of teduglutide to patients.
Data pooled from studies NCT03571516 (n = 5; patients randomized to receive teduglutide treatment compared with SoC group) and NCT02980666 (N = 2; patients only received teduglutide).
Of the children enrolled in the extension study, 1 child did not receive teduglutide owing to ongoing enteral autonomy from the core study.
Data from core study (NCT02980666) baseline.
Corrected gestational age in months.
Age in years.
#Necrotic terminal ileum.
Congenital absence of the midgut.
Type of stoma was colostomy.
Type of stoma was “other.”
Percentages based on the number of patients with distal/terminal ileum present in each treatment group.
Duration in months.
Duration in years.
Almost all patients who received teduglutide achieved ≥80% treatment compliance; 1 infant in the core study achieved 79.2% compliance owing to a dosing error. Two infants (1 SoC and 1 teduglutide) discontinued during the core treatment period (withdrawal by parent/guardian) and 1 discontinued during the follow-up period (met several escape criteria). One child in the core study, who received treatment prior to the protocol amendment, discontinued during the treatment period (TEAE). No infants or children in the extension study discontinued during the 24-week treatment extension period.
Reduction in PS Volume of ≥20%
Reductions in PS volumes of ≥20% were observed in all patient groups (Table 2, Supplemental Digital Content 2, http://links.lww.com/MPG/D202). One infant who received SoC had data missing from their diary at week 24, so prescribed data were used (Table 3, Supplemental Digital Content 3, http://links.lww.com/MPG/D203). At 24 weeks, 4 infants (57.1%) and 4 children (66.7%) receiving teduglutide and 2 infants (50.0%) receiving SoC experienced a ≥20% reduction in PS volume. One infant (50.0%) achieved a ≥20% reduction in PS volume at day 1 of the extension study, which was maintained through week 24 (48 weeks of total treatment). Two children (40.0%) achieved a ≥20% reduction in PS volume at day 1 of the extension study; by week 48 of total treatment, this increased to 4 children (80.0%).
Changes in PS Volume
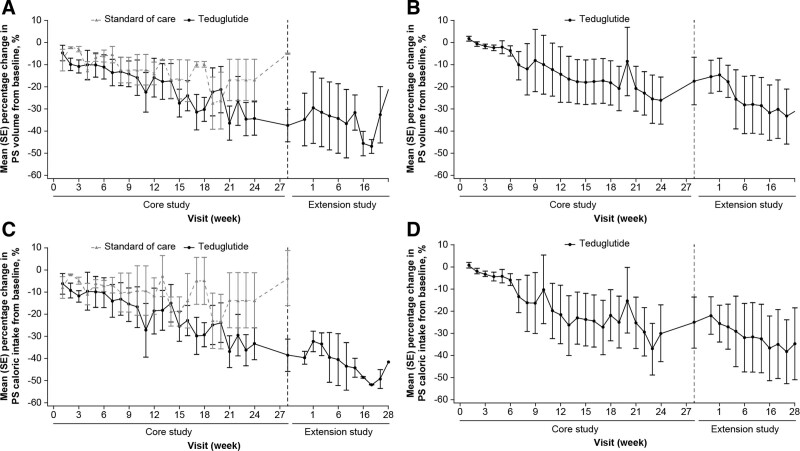
All infants and children who received teduglutide had reduced PS volume requirements by week 24 of treatment (Fig. 2; Table 2, Supplemental Digital Content 2, http://links.lww.com/MPG/D202). At week 24, infants who received teduglutide (n = 6) had a 34.2 ± 18.8% reduction in PS volume from baseline, whereas infants who received SoC (n = 3) had a 16.8 ± 16.4% reduction (Fig. 2A). PS volume requirements reduced by 35.7 ± 33.2% at week 24 for children who received teduglutide (N = 6) and increased slightly during the follow-up period (Fig. 2B). The infants who continued into the extension study (N = 2) had little subsequent change in PS volume requirements from baseline (of the core study) at 48 weeks of treatment (−32.5 ± 18.1%) compared with 24 weeks of treatment (Fig. 2A). For children (n = 5), PS volume reduced by 42.1 ± 38.4% at week 48, which was maintained during the follow-up period (Fig. 2B; Table 2, Supplemental Digital Content 2, http://links.lww.com/MPG/D202).
FIGURE 2.
Mean (SE) percentage change in PS volume from baseline for (A) infants receiving teduglutide or standard of care and (B) children receiving teduglutide, based on patient diary data. Mean (SE) percentage change in PS calories from baseline for (C) infants receiving teduglutide or standard of care and (D) children receiving teduglutide, based on patient diary data. PS = parenteral support; SE = standard error.
Changes in PS Caloric Intake
Overall, the changes in PS caloric intake between baseline and week 24 mirrored the changes observed in PS volume (Fig. 2C and D; Table 2, Supplemental Digital Content 2, http://links.lww.com/MPG/D202). At week 24, the PS caloric intake had reduced by 33.4 ± 17.8% for infants receiving teduglutide (n = 6), 13.7 ± 21.9% for infants receiving SoC (n = 3), and 35.2 ± 33.7% for the children (N = 6). Further reductions in PS caloric intake from baseline were observed at week 48, with reductions of 49.2 ± 6.1% and 42.0 ± 38.3% from baseline for infants (N = 2) and children (n = 5), respectively.
Changes in PS Frequency and Duration
At week 24, the mean frequency (days/week) and duration (hours/day) that infants who received teduglutide received PS reduced by approximately one-quarter, whereas infants who received SoC had no change in frequency and almost no change in duration (Table 2, Supplemental Digital Content 2, http://links.lww.com/MPG/D202). The frequency reduced by 16.7% and the duration of PS reduced by approximately one-quarter for children at week 24. At week 48, neither infant had a reduction in the frequency of PS from baseline, but there was a slight reduction in the duration of PS. At week 48, the frequency and duration of PS for children was similar to week 24.
Enteral Autonomy
No infants in the core and extension studies achieved enteral autonomy. Two children (25.0%) receiving teduglutide achieved enteral autonomy from week 12 and week 28 of treatment; both sustained this to week 48.
Teduglutide Exposure
Infants who received teduglutide had a mean of 305.3 ± 186.3 and 133.0 ± 50.9 days of exposure to teduglutide in the core and extension studies, respectively. Children had a mean of 164.4 ± 11.1 and 169.3 ± 0.8 days of exposure to teduglutide in the core and extension studies, respectively.
Adverse Events
AEs reported by all infants and children in both the core and the extension studies were predominantly mild-to-moderate in severity. No deaths or TEAEs of special interest were reported for any patients (Table 2). Overall, the most frequently reported TEAEs were related to infections and gastrointestinal disorders (Table 4, Supplemental Digital Content 4, http://links.lww.com/MPG/D204). Of the TEAEs considered related to teduglutide, gastrointestinal disorders were most frequently reported for infants, and administration site conditions were most frequently reported for children, in particular injection site erythema (Table 5, Supplemental Digital Content 5, http://links.lww.com/MPG/D205). The TESAE abdominal pain (Table 6, Supplemental Digital Content 6, http://links.lww.com/MPG/D206) was considered related to teduglutide; this was reported by 1 child after teduglutide was withdrawn during the core study follow-up period. No clinically significant changes considered to be related to teduglutide were reported in the laboratory tests.
TABLE 2.
Reported treatment-emergent adverse events and serious adverse events based on safety populations
| Infants | Children* | ||||
|---|---|---|---|---|---|
| n (m) | n (m) | ||||
| Core study | Extension study (N = 2) | Core study (N = 8) | Extension study‡ (N = 7) | ||
| SoC (n = 5) | Teduglutide† (n = 7) | ||||
| Any TEAE | 5 (29) | 7 (125) | 2 (12) | 8 (93) | 7 (101) |
| Severity | |||||
| Mild | 5 (23) | 6 (62) | 2 (9) | 8 (76) | 7 (86) |
| Moderate | 2 (4) | 5 (14) | 1 (2) | 4 (15) | 3 (15) |
| Severe | 2 (2) | 1 (1) | 1 (1) | 2 (2) | 0 |
| Related to teduglutide | N/A | 2 (9) | 0 | 6 (20) | 2 (2) |
| Led to treatment discontinuation | 0 | 1 (5) | 0 | 1 (1) | 0 |
| Led to death | 0 | 0 | 0 | 0 | 0 |
| Any TESAE | 3 (6) | 6 (14) | 2 (4) | 6 (15) | 6 (18) |
| Related to teduglutide | N/A | 0 | 0 | 1 (1) | 0 |
m = number of events; n = number of patients experiencing the event; N/A = not applicable; SoC = standard of care; TEAE = treatment-emergent adverse event; TESAE = treatment-emergent serious adverse event.
For study NCT02980666 and its extension NCT03268811, 6 of 8 children were enrolled and received teduglutide treatment after protocol amendment, which included improved training for parents to administer doses of teduglutide to patients.
Data pooled from studies NCT03571516 (n = 5; patients randomized to receive teduglutide treatment compared with SoC group) and NCT02980666 (N = 2; patients only received teduglutide).
Of the children enrolled in the extension study, 1 child did not receive teduglutide owing to ongoing enteral autonomy from the core study.
Growth Parameters
Both infants and children had low baseline growth parameters (Table 1). There were no clinically meaningful changes in growth parameters in the core studies for infants or in either the core or the extension studies for children (Table 7, Supplemental Digital Content 7, http://links.lww.com/MPG/D207). The 2 infants who continued into the extension study had increases in mean weight-for-age (3.3 ± 2.6), length (1.4 ± 0.3), weight-for-length (3.4 ± 4.3), and head circumference (2.4 ± 0.7) z scores after 48 weeks of treatment.
DISCUSSION
These studies provide valuable efficacy and safety data for teduglutide in infants and children, given the limited data currently available for children and the lack of published studies on infants <1 year of age with SBS-IF receiving teduglutide (21,23).
The infants in the teduglutide and SoC arms were similar in age and younger than participants in previous pediatric teduglutide clinical trials (21,23,24). Infants in the SoC arm had almost double the mean length of small intestine and a higher proportion had NEC as an underlying etiology than patients who received teduglutide—these features suggest the patients in the SoC arm are more likely to achieve enteral autonomy following intestinal adaptation within a shorter time frame than patients who received teduglutide (25,26). A similar proportion of infants receiving teduglutide experienced a ≥20% reduction in PS at week 24 as patients receiving SoC alone (57.1% vs 50.0%; prescribed data). However, infants who received teduglutide had clinically meaningful reductions in PS requirements and caloric intakes which were over twice that reported for infants who received SoC only (PS volume −34.2% teduglutide vs −16.8% SoC; PS caloric intake −33.4% teduglutide vs −13.7% SoC), suggesting teduglutide promoted intestinal adaptation in infants who may have greater difficulty achieving this based on the length of their small intestine and etiology of SBS. Of children who received teduglutide, the proportions who achieved a ≥20% reduction in PS at week 24 (66.7%) and reductions in PS volume (−35.7%) and PS caloric intake (−35.2%) were similar to those observed in infants, as well as in children in previous studies (21,23), indicating a similar efficacy of teduglutide and suggesting they experienced intestinal adaptation owing to treatment with teduglutide.
Most infants and children maintained a ≥20% reduction in PS volume to 48 weeks of total treatment. For infants, reductions in PS volumes after 48 weeks of treatment were similar to those recorded at 24 weeks; however, continued reductions were observed in PS caloric intake (−49.2% vs −33.4%). The low number of infants in the extension study (N = 2) and the lack of an SoC comparator arm mean further analyses are required to confirm that the changes were due to teduglutide and not the result of natural intestinal adaptation. In children, further reductions were observed at 48 weeks than at 24 weeks of treatment for PS volume (−42.1% vs −35.7%) and caloric intake (−42.0% vs −35.2%). This finding was in line with previous reports (21) and suggests a longer duration of treatment could provide further improvement in efficacy.
The proportion of children receiving teduglutide who achieved enteral autonomy during the core study (16.7%) is similar to that of previous studies, in which 11.5%–20.0% achieved enteral autonomy during short-term treatment (21,23). In this study, the absence of teduglutide treatment received during the extension study by the child who achieved enteral autonomy at week 12 of treatment suggests potential long-term efficacy of teduglutide; although the possibility they could have achieved enteral autonomy without treatment with teduglutide cannot be ruled out. The presence of a second child who achieved enteral autonomy during the extension study suggests some patients may require longer-term treatment with teduglutide to achieve enteral autonomy (24). Previous studies in adults have also suggested variability in how long it takes to achieve enteral autonomy with long-term teduglutide treatment (27–29). Teduglutide was well tolerated, and AEs reported were typically consistent with previous pediatric studies, with no new safety issues identified (21). Many of the frequently reported AEs are also known features of SBS (30). Although more TEAEs were reported for infants who received teduglutide than SoC (125 events vs 29 events), only 9 (12%) were reported as related to teduglutide, and no TEAEs related to teduglutide were reported for infants in the extension study. For children, TEAEs related to teduglutide accounted for 21.5% and 2.0% of total TEAEs for the core and extension studies, respectively. This led to the novel finding that infants may have comparable or even fewer safety concerns when receiving teduglutide than children.
The low mean baseline growth parameters for both infants and children suggest growth had been stunted owing to SBS-IF or nutritional deficiency. The lack of clinically meaningful changes in z scores in the core studies for infants and both core and extension studies for children could be related to the relatively short treatment period. Only infants showed increased z scores at 48 weeks of treatment, but the limited number of patients who continued into the extension study means it is difficult to draw conclusions.
A limitation of these studies was the small sample sizes, which are to be expected for pediatric studies in a rare disease. There is a possibility of imbalance in some factors between patients receiving teduglutide or SoC because of the small sample size in randomization. However, the pooled post hoc analysis of the infant studies did increase the data available. The patients had diverse etiologies for SBS-IF and a wide range of remaining small intestinal lengths; patients who received SoC had a mean small intestinal length that was longer than those selected to receive teduglutide, and a greater proportion had NEC as the primary reason for their SBS diagnosis. Although data recorded through patient diaries were preferred over prescribed data, some diary data were missing. Difficulties associated with caring for infants with SBS could have contributed to gaps in the patient’s diary by the parent/guardian. The open-label design of these studies may have biased the prescription of PS, with patients receiving teduglutide being weaned off more aggressively than those receiving SoC, as well as resulted in patients who received teduglutide being more inclined to report AEs.
CONCLUSIONS
This analysis has provided the first data on the efficacy and safety of teduglutide in infants younger than 1 year of age and additional data in children. Clinically meaningful reductions in PS volume were observed in infants and children after short- and long-term treatment with teduglutide. All AEs were in line with known impacts of SBS-IF and adverse reactions to teduglutide. Teduglutide was generally well tolerated, and no new safety issues were identified.
Supplementary Material
Footnotes
S.C. is an employee of Takeda Pharmaceutical Company Limited, Cambridge, MA, USA and is a stockholder of Takeda Pharmaceutical Company Limited. S.S. is an employee of Takeda Pharmaceutical Company Limited, Osaka, Japan and is a stockholder of Takeda Pharmaceutical Company Limited. E.U. was an employee of Takeda Pharmaceutical Company Limited, Tokyo, Japan. M.W. received research funding from Shire, a Takeda company. The remaining authors report no conflicts of interest.
Sources of Funding: This work was supported by Takeda Pharmaceuticals, Inc. The funder participated in the study design, data collection, data analysis, data interpretation, review and approval of the final clinical study reports, and provided the study drug and funded medical writing assistance. Medical writing support was provided by Elizabeth Coe, PhD, of Oxford PharmaGenesis, Oxford, UK, funded by Takeda.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
URL and trial identification number: https://clinicaltrials.gov/ct2/show/NCT03571516, NCT03571516; https://clinicaltrials.gov/ct2/show/NCT02980666, NCT02980666; https://clinicaltrials.gov/ct2/show/NCT03268811, NCT03268811.
REFERENCES
- 1.O’Keefe SJ, Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol 2013;11:815–23.e1–3. [DOI] [PubMed] [Google Scholar]
- 2.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012;143:1473–81.e3. [DOI] [PubMed] [Google Scholar]
- 3.Bruzoni M, Sudan DL, Cusick RA, Thompson JS. Comparison of short bowel syndrome acquired early in life and during adolescence. Transplantation 2008;86:63–6. [DOI] [PubMed] [Google Scholar]
- 4.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ; Eunice Kennedy Shriver NICHD Neonatal Research Network. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 2008;122:e573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr 2008;47:S33–6. [DOI] [PubMed] [Google Scholar]
- 6.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 2012;161:723–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin SC, Pappas C, Iyengar H, Maheshwari A. Short bowel syndrome in the NICU. Clin Perinatol 2013;40:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology 2006;130:S5–S15. [DOI] [PubMed] [Google Scholar]
- 9.Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124:1111–34. [DOI] [PubMed] [Google Scholar]
- 10.Chandra R, Kesavan A. Current treatment paradigms in pediatric short bowel syndrome. Clin J Gastroenterol 2018;11:103–12. [DOI] [PubMed] [Google Scholar]
- 11.Fullerton BS, Hong CR, Jaksic T. Long-term outcomes of pediatric intestinal failure. Semin Pediatr Surg 2017;26:328–35. [DOI] [PubMed] [Google Scholar]
- 12.Dibb M, Teubner A, Theis V, Shaffer J, Lal S. Review article: the management of long-term parenteral nutrition. Aliment Pharmacol Ther 2013;37:587–603. [DOI] [PubMed] [Google Scholar]
- 13.Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol 2016;30:173–85. [DOI] [PubMed] [Google Scholar]
- 14.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011;60:902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeppesen PB. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap Adv Gastroenterol 2012;5:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revestive®. teduglutide. Dublin, Ireland: Shire Pharmaceuticals Ireland Limited; 2019. [Google Scholar]
- 17.GATTEX®. teduglutide. Lexington, MA, USA: Shire-NPS Pharmaceuticals, Inc.; 2019. [Google Scholar]
- 18.Revestive®. teduglutide. Tokyo, Japan: Takeda Pharmaceutical Company Limited; 2021. [Google Scholar]
- 19.Diamanti A, Lezo A, D’Antiga L, et al. Teduglutide in pediatric intestinal failure: a position statement of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP). Dig Liver Dis 2022;54:1320–7. [DOI] [PubMed] [Google Scholar]
- 20.Revestive® [package insert]. Tokyo, Japan, Takeda Pharmaceutical Company Limited; 2021. [Google Scholar]
- 21.Kocoshis SA, Merritt RJ, Hill S, et al. Safety and efficacy of teduglutide in pediatric patients with intestinal failure due to short bowel syndrome: a 24-week, phase III study. JPEN J Parenter Enteral Nutr 2020;44:621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MedDRA. Medical dictionary for regulatory activities. https://www.meddra.org/. Accessed July 3, 2020.
- 23.Carter BA, Cohran VC, Cole CR, et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr 2017;181:102–111.e5. [DOI] [PubMed] [Google Scholar]
- 24.Ramos Boluda E, Redecillas Ferreiro S, Manrique Moral O, et al. Experience with teduglutide in pediatric short bowel syndrome: first real-life data. J Pediatr Gastroenterol Nutr 2020;71:734–9. [DOI] [PubMed] [Google Scholar]
- 25.Khan FA, Squires RH, Litman HJ, et al. Predictors of enteral autonomy in children with intestinal failure: a multicenter cohort study. J Pediatr 2015;167:29–34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks EA, Khan FA, Fisher JG, et al. Necrotizing enterocolitis is associated with earlier achievement of enteral autonomy in children with short bowel syndrome. J Pediatr Surg 2016;51:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeppesen PB, Gabe SM, Seidner DL, Lee H-M, Olivier C. Factors associated with response to teduglutide in patients with short-bowel syndrome and intestinal failure. Gastroenterology 2018;154:874–85. [DOI] [PubMed] [Google Scholar]
- 28.Seidner DL, Gabe SM, Lee HM, Olivier C, Jeppesen PB. Enteral autonomy and days off parenteral support with teduglutide treatment for short bowel syndrome in the STEPS trials. JPEN J Parenter Enteral Nutr 2020;44:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill S, Carter BA, Cohran V, et al. Safety findings in pediatric patients during long-term treatment with teduglutide for short-bowel syndrome-associated intestinal failure: pooled analysis of 4 clinical studies. JPEN J Parenter Enteral Nutr 2021;45:1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalaitzakis E, Carlsson E, Josefsson A, Bosaeus I. Quality of life in short-bowel syndrome: impact of fatigue and gastrointestinal symptoms. Scand J Gastroenterol 2008;43:1057–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.