Abstract
Background
Postoperative ileus is common after gastrointestinal surgery. This network meta-analysis aimed to compare the effectiveness of gum chewing and coffee and caffeine intake on ileus-related outcomes.
Methods
A systematic literature review was performed to identify randomized controlled trials (RCTs) comparing noninvasive treatments for ileus after gastrointestinal surgery. The main analyses included random effects network meta-analyses using frequentist methods with simultaneous direct and indirect comparisons of time to first flatus, time to first defecation, and length of stay. Bayesian network meta-analysis using Markov chains was also used.
Results
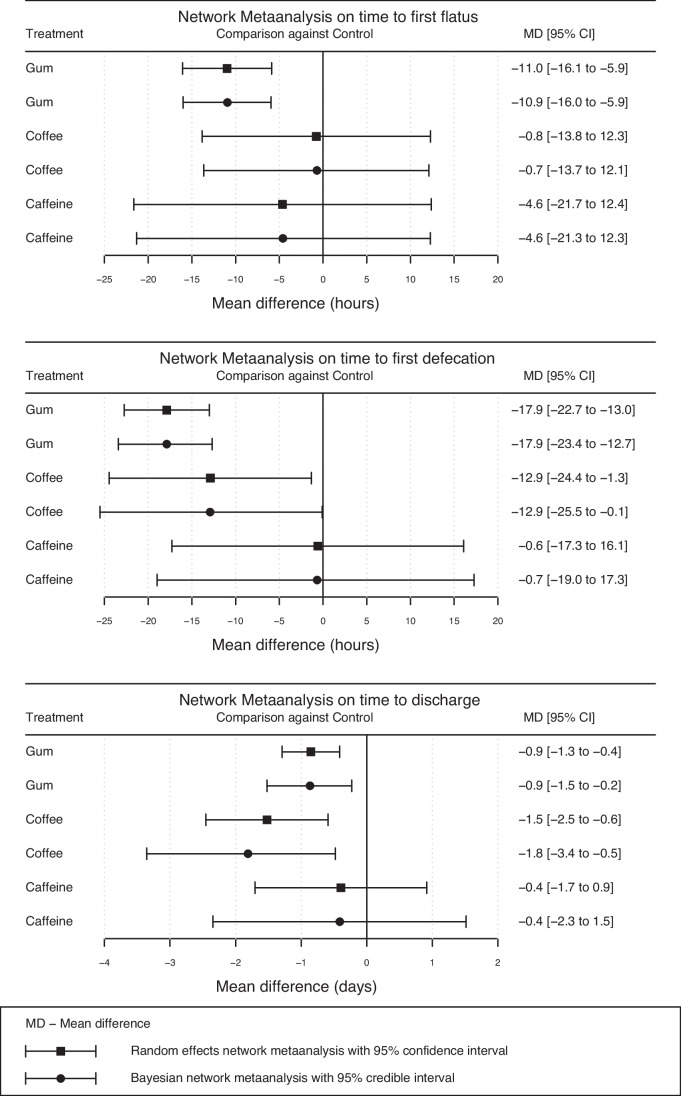
A total of 32 RCTs comparing 4999 patients were included in this network meta-analysis. Time to flatus was reduced by gum chewing (mean difference compared to control (MD): -11 h, 95% confidence interval (95% CI) − 16 to − 5 h, P < 0.001). Time to defecation was reduced by gum chewing and coffee, with MDs of -18 h (95% CI − 23 to − 13 h, P < 0.001) and -13 h (95% CI − 24 to − 1 h, P < 0.001), respectively. Length of stay was reduced by coffee and gum chewing with MDs of − 1.5 days (95% CI: − 2.5 to − 0.6 days, P < 0.001) and − 0.9 days (95% CI: − 1.3 to − 0.4 days, P < 0.001), respectively.
Conclusion
Coffee and gum chewing were proven to be effective noninvasive approaches for shortening the postoperative length of hospital stay and time to first defecation, especially in open gastrointestinal surgery; thus these actions should be recommended after gastrointestinal surgery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11605-023-05702-z.
Keywords: Coffee, Caffeine, Gum chewing, Bowel function, Postoperative ileus, Network meta-analysis, Systematic review, Gastrointestinal surgery, Bowel surgery, Abdominal surgery
Introduction
Postoperative ileus (POI) is a common and significant complication of gastrointestinal surgery. The overall incidence of postoperative ileus varies according to its definition and occurs in approximately 10–30% of patients after abdominal surgery. It is characterized by delayed passage of flatus and defecation, abdominal distension, nausea, vomiting, and the inability to tolerate oral food. Commonly, nasogastric tubes must be inserted, the patient suffers nutritional deficits, and the risk of associated postoperative morbidity increases. 1–3
The annual costs of treating POI in the USA are estimated to be at least 750 million dollars. 4,5 This is mainly due to the significantly increased duration of hospital stay (approximately 4–9 days) in patients with POI compared to patients with normal recovery of gut function but also due to medication costs, required imaging and personnel costs. 6
However, financial burden and POI can increase the patient’s risk of postoperative morbidities such as aspiration pneumonia (0.44–1.4%), anastomotic leakage (up to 5%), or even death (0.9%). 7–9
As many factors influence postoperative bowel function, such as anesthesia, postoperative medication, or the surgical procedure per se, numerous different and partially invasive management solutions are discussed.
Gum chewing, caffeine intake, or coffee consumption in the early postoperative period to reduce the occurrence and severity of POI is a promising noninvasive approach that does not contain major side effects. Similar to chewing gum, caffeine and coffee are inexpensive and widely available products that most patients are familiar with and have good experience with, they are well-tolerated interventions in postoperative stages.
Many studies have been conducted to investigate these different approaches with variable outcomes. It has been suggested that gum chewing (GC) and coffee/caffeine intake after surgery may help to recover gastrointestinal function by early stimulation. 10,11 The effects of coffee, caffeine, or gum chewing on postoperative bowel movement are debated. Earlier studies reported ambiguous results regarding whether coffee or caffeine had a larger effect; however, there was no difference between coffee and caffeine in terms of an improvement in postoperative bowel movement and a decrease in the length of hospital stay. 12
This systematic literature review and network meta-analysis aimed to assess the treatment effect of coffee, caffeine, and gum chewing on postoperative bowel movement in terms of time to first flatus, time to first defecation, and length of stay by simultaneous direct and indirect comparisons. The primary analyses included random effects network meta-analyses using frequentist methods. 13,14 Bayesian network meta-analyses served as sensitivity analyses.
Methods
This systematic review and network meta-analysis was registered in research registry unique identifying number (UIN) of “reviewregistry1541.”
Data Collection
This review complies with the recommendations of the Cochrane Handbook for Systematic Reviews and Interventions and is reported in line with the PRISMA guidelines and their extension statement for network meta-analyses. 15–17 A systematic literature search was performed using PubMed, the Cochrane Library, and Google Scholar. The search string was configured using Boolean operators and medical subject headings (MeSH).
The inclusion criteria were solely randomized controlled studies (RCTs) showing a comparison of at least two treatments measuring at least one of the following outcome parameters: time to first flatus, time to first bowel movement, and length of hospital stay after gastrointestinal surgery. No language restrictions were applied. We excluded reviews, meta-analyses, case reports, letters, comments, and non-RCTs.
A literature search was conducted in the PubMed, Google Scholar, and Cochrane Databases until August 8th, 2022. The search algorithm in PubMed was ((caffeine*) OR (coffein*) OR (coffee) OR (chewing gum) OR (gum chewing) OR (Chew*)) AND ((systematic review) OR (meta-analysis) OR (randomized controlled trial) OR (RCT) OR (randomized)) AND ((bowel function) OR (bowel movement) OR (ileus) OR (postoperative ileus) OR (gastrointestinal motility) OR (recovery)) AND ((colorectal surgery) OR (bowel surgery) OR (colon* surgery) OR (Gastrointestinal surgery) OR (Abdominal surgery) OR (colectomy)).
In the Cochrane database, the search items “chewing gum bowel function surgery,” “coffee bowel function surgery,” and “caffeine bowel function surgery” were used. The Google Scholar search was performed for review articles: ((caffeine*) OR (coffein*) OR (coffee) OR (chewing gum) OR (gum chewing) OR (chew*)) AND ((systematic review) OR (meta-analysis) OR (randomized controlled trial) OR (RCT) OR (randomized)) AND ((bowel function) OR (bowel movement) OR (ileus) OR (postoperative ileus)). Cross-referencing and manual searches of the bibliographies of eligible publications were actively performed to identify further relevant studies for the review. The selection of relevant articles was performed in two stages. First, the titles and abstracts of all retrieved references were screened to determine whether they met the inclusion criteria. Studies considered irrelevant were discarded. Second, we analyzed the full-text articles of each selected abstract. For data extraction, a dedicated predefined spreadsheet was used. Study selection was performed by two researchers with discrepancies resolved through discussion with the involvement of a third researcher. The selection process is illustrated in a PRISMA flow chart. 18
Data Extraction
An Excel sheet was created extracting the type of surgery, measured outcomes, and type of treatment (gum, coffee, or caffeine) from the included studies.
The number of patients in the intervention and control groups and the mean and standard deviation (SD) of the outcome parameters were recorded from text, tables, or figures. Some of the studies reported nonparametric measures instead of the mean and SD for the treatment effects. Hence, the mean and SD for treatment effects were estimated from the mean and SD in the treatment groups. In studies showing only quantiles, the mean and SD were estimated using the Box‒Cox power transformation into the sample mean estimators by Luo et al. and the sample SD estimators by Wan et al. 19–21 If possible, missing data were retrieved manually from the figures.
Publication Bias
Publication bias was separately assessed in pairwise comparisons of the interventions for each outcome parameter because, to the best of the authors’ knowledge, there was no method readily available to examine it in the framework of network meta-analysis. Assessment of publication bias was performed using contour-enhanced funnel plots and significance funnel plots as appropriate. 22,23 The latter distinguishes between affirmative studies (i.e., those with a statistically significant and positive estimate) and nonaffirmative studies (i.e., those with a nonsignificant or negative estimate). The ratio eta expresses the likelihood of an affirmative study to be published compared to a nonaffirmative study. 22
The risk of bias was assessed by two authors using the ROB-2 tool. 24 Consensus was performed with a third author, if necessary.
Statistical Analysis
Statistical analyses were performed using the R environment version 4.2.1 (http://www.r-project.org) using the recent R libraries “meta,” “netmeta” 25, and “gemtc.” Random effects network meta-analyses (NMA) using frequentist methods were the main analyses. They are based on a graph-theoretical method and random effects models. 14
Bayesian NMA was performed as a sensitivity analysis. The summary measure for the outcomes was the mean difference (MD) compared to the control.
First, pooled point estimates for each of the outcomes were estimated based on random effects models. Second, pairwise random effects meta-analyses comparing each intervention against the control were performed for all outcomes because none of the included studies compared more than one treatment against the control, hindering such pairwise analyses. These pairwise meta-analyses primarily served to assess statistical heterogeneity to overcome a lack of proven statistical methods to assess statistical heterogeneity in NMA. Statistical heterogeneity was assessed by visual examination of the forest plots, quantified using I2 and formally tested with Cochran’s Q statistic.
Third, the main analysis, NMA with random effects models based on the frequentist approach, was performed. The network geometry was assessed by network plots. Treatments were ranked using the P scores. P scores measure the extent of certainty that a treatment is better than another treatment, averaged over all competing treatments. 26 This interpretation is comparable to that of the surface under the cumulative ranking curve (SUCRA), which is the rank of a certain treatment within the range of treatments, measured on a scale from 0 (worst) to 1 (best). 27 The MD was estimated compared to the control. Due to the spider-like network, net heat plots 28 to elaborate inconsistency in the network were not feasible.
Finally, Bayesian NMA was performed as random effects models with Markov chain Monte Carlo (MCMC) simulations based on “Just another Gibbs sampler” (JAGS) 29,30 with 25,000 burn-ins, 50,000 inference iterations, and a thinning factor of 10. Point estimates and 95% credible intervals (95% CrI) for the comparison against the control were calculated, and the results were considered statistically significant if zero was not included in the credible intervals. Heterogeneity was assessed by the residual deviance and the deviance information criteria (DIC). Time series, density plots of the samples, and Gelman and Rubin’s plots were used to ensure convergence.
Results
Study Selection
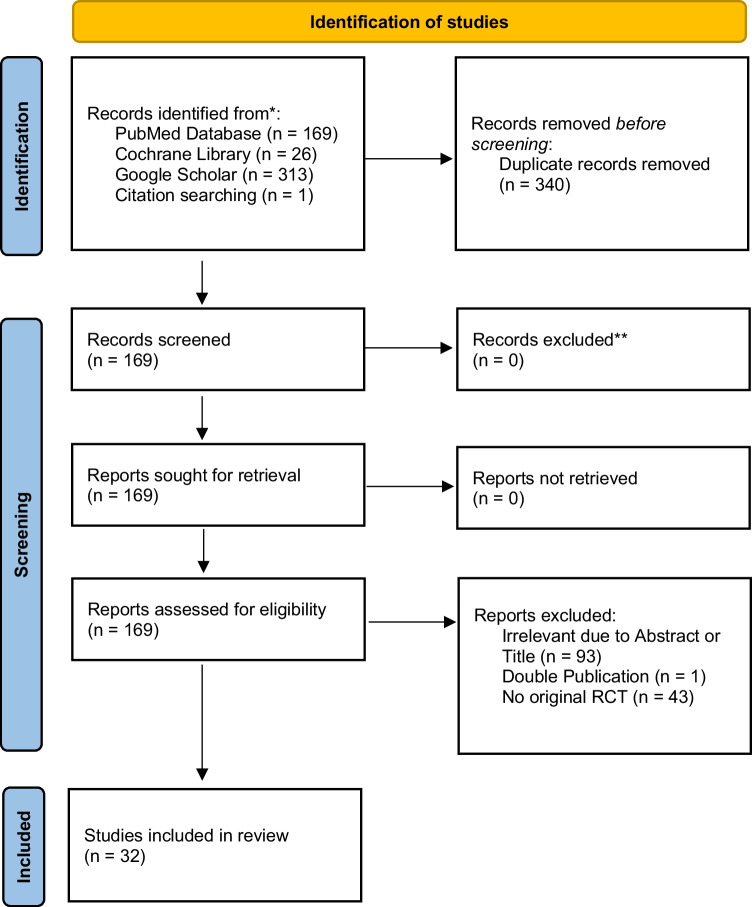
A total of 509 studies were identified. After removing 340 duplicates, 169 studies were screened. After excluding noneligible studies (one study was excluded due to double publication, 43 studies were non-RCTs, and 93 studies did not report the required outcome parameters or were not related to gastrointestinal surgery), 32 studies comparing at least one of the treatments against the control and assessing at least one of the outcomes were included in this network meta-analysis (Fig. 1).
Fig. 1.
PRISMA flow chart showing the selection of articles for review
Study Characteristics
The 32 studies included 4999 patients in total. Twenty-six studies reported on gum chewing, and the remaining 6 studies reported on coffee consumption or caffeine intake. Furthermore, 5 out of 32 studies additionally reported on other therapy methods (one studied candy usage, one studied an attention control adhesive patch, one studied the intake of olive oil, one studied the application of a bracelet, and another studied acupuncture). These third arms were not considered in quantitative analyses because they were only assessed once. None of the studies compared more than one treatment against the control (Table 1).
Table 1.
Systematic review of the studies with descriptions of the type of surgery, measured outcomes, and type of treatment
| First author (year) | Total number of included patients | Specification of surgery | Type of surgery | Outcome measured | Treatment |
|---|---|---|---|---|---|
| Abbassi (2022) | 60 | Elective colectomy (RC, LC, PC, SR, RR) | L | FF, FD, LOS | Caffeine |
| Asao (2022) | 19 | Elective colectomy not specified | L | FF, FD, LOS | Gum |
| Atkinson (2016) | 402 | Elective colorectal resection (TC, RC, LC, RR) | M | FF, FD, LOS | Gum |
| Bahena-Aponte (2010) | 32 | Elective LC | O | FF, FD, LOS | Gum |
| Bhatti (2021) | 100 | Elective ISC | O | FF, FD, LOS | Gum |
| Bonventre (2014) | 50 | Colorectal surgery (RC, LC, RR, HP, TG, GR) | O | FF, FD, LOS | Gum |
| 25 | Colorectal surgery (RC, LC, RR, HP, TG, GR) | O | FF, FD, LOS | Olive oil | |
| Byrne (2018) | 158 | Elective bowel surgery (RC, LC, TC, SR, RR, Ileostomy closure, SBR) | M | FF, FD, LOS | Gum |
| Crainic (2009) | 42 | Elective colectomy (RC, LC, SR, RR) | M | FF, FD | Gum |
| 19 | Elective colectomy (RC, LC, SR, RR) | M | FF, FD | Hard candy | |
| de Leede (2018) | 1941 | Elective Abdominal surgery (large and small intestine, esophagus/stomach) | M | FF, FD, LOS | Gum |
| Dulskas (2015) | 90 | Elective LC | L | FF, FD, LOS | Coffee |
| Duluklu (2020) | 34 | Elective LC and/or RR | O | FF, FD, LOS | Gum |
| Forrester (2014) | 31 | Elective SR/LC | M | FF, FD, LOS | Gum |
| 17 | Elective SR/LC | M | FF, FD, LOS | Attention control | |
| Ge (2017) | 75 | Elective TG, GR | L | FF, FD, LOS | Gum |
| Hasler-Gehrer (2019) | 115 | Elective RC, LC, SR, RR | L | FF, FD, LOS | Coffee |
| Hirayama (2006) | 24 | Elective colon resection (RC, LC, SR, RR) | O | FF, FD | Gum |
| Kobayashi (2015) | 43 | Elective LC | O | FF, FD, LOS | Gum |
| Lim (2013) | 157 | Elective colorectal surgery (RC, LC, SC, RR) | M | FF, FD, LOS | Gum |
| Marwah (2012) | 100 | Elective ISC | O | FF, FD, LOS | Gum |
| Matros (2006) | 43 | Elective colectomy (APR, colostomy reversal, RR, RC, LC, PC | O | FF, FD, LOS | Gum |
| 22 | Elective colectomy (APR, colostomy reversal, RR, RC, LC, PC | O | FF, FD, LOS | Bracelet | |
| Müller (2012) | 79 | Elective colectomy (RC, LC, RR) | M | FF, FD, LOS | Coffee |
| Ngowe (2010) | 46 | Emergency appendectomy | O | FF, FD, LOS | Gum |
| Parnasa (2021) | 58 | Elective colectomy (RC, LC, SR, PC, RR) | L | FF, FD, LOS | Caffeine |
| Piric (2015) | 59 | Elective colon resection (RC, LC, SR) | O | FD, LOS | Coffee |
| Quah (2006) | 38 | Elective colorectal cancer surgery (LC, RR, SR, HP, APR) | O | FF, FD, LOS | Gum |
| Schuster (2006) | 34 | Elective SR | O | FF, FD, LOS | Gum |
| Shum (2016) | 82 | Colorectal resection (RC, LC, SC, TC, RR, APR) | L | FF, FD, LOS | Gum |
| Topcu (2016) | 60 | Colorectal surgery (RC, LC, RR) | O | FF, FD, LOS | Gum |
| van den Heijkant (2015) | 112 | Elective colorectal surgery (RC, LC, SR, RR) | O | FF, FD, LOS | Gum |
| Vergara-Fernandez (2016) | 64 | Elective colon or rectal resection (RC, SR, LC, TC) | M | LOS | Gum |
| Yang (2017) | 379 | Elective colorectal cancer resection (TC, LC, RC, RR) | M | FF, FD, LOS | Gum |
| 186 | Elective colorectal cancer resection (TC, LC, RC, RR) | M | FF, FD, LOS | Acupuncture | |
| Yang (2018) | 89 | Elective proctectomy for rectal cancer | O | FF, FD | Gum |
| Zaghiyan (2013) | 114 | Colorectal surgery (RC, LC, RR, SR, APR, IPAA, SC, SBR, TPC, IPAA, ileostomy closure, APR, creation of ileostomy) | M | FF, FD, LOS | Gum |
RC right hemicolectomy, LC left hemicolectomy, SR sigmoid resection, SC subtotal colectomy, TC total colectomy, PC partial/segmental colonic resection, RR rectum resection, HP Hartmann procedure, TG total gastrectomy, GR gastric resection, SBR small bowel resection, APR abdominoperineal resection, TPC total proctocolectomy, IPAA ileal pouch-anal anastomosis, ISC intestinal stoma closure, FF time to first flatus, FD time to first defecation, LOS length of stay, L laparoscopic, O open, M mixed laparoscopic and open
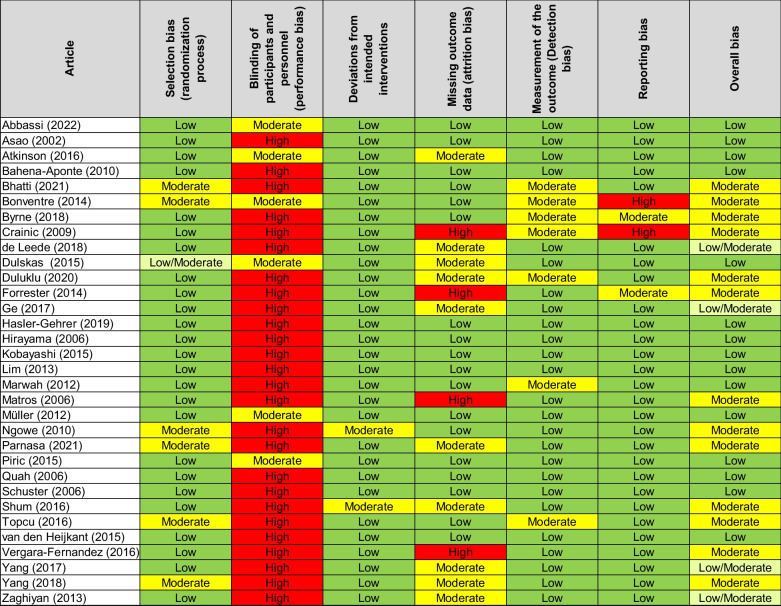
Risk of Bias
Of the included studies, 15 had a low risk of bias, 4 had a low to moderate risk of bias, and 13 had a moderate overall risk of bias. None of the studies showed a high risk of bias. As nearly no intervention was able to blind the patients, nearly all studies therefore had a high risk of performance bias (n = 26). Only three studies 31–33 were able to blind their patients using either capsules with and without caffeine or by administering coffee with and without caffeine. The remaining coffee consumption groups 34–36 were not able to blind their patients, as they compared coffee to placebo (mostly tea or water).
Additionally, missing outcome data (attribution bias) were causative for overall bias (n = 14). Reporting bias was only suspected in 4 studies, and 2 studies had high risk (Table 2).
Table 2.
Risk of bias
Assessment of Publication Bias
The contour-enhanced funnel plots for gum chewing, coffee consumption, and caffeine intake in first flatus, first defecation, and length of hospital stay were fairly robust to publication bias and did not indicate the presence of a strong publication bias (Supplementary Figure S1). Network graphs for the presence of publication bias including time to first flatus, time to first defecation, and discharge were compared to controls. Each node represents a treatment. The thickness of each line indicates the number of studies comparing the two treatments. The control group constituted the center of the spider-like network as there was no study with a head-to-head comparison between the treatments (Supplementary Figure S2).
Meta-analysis
Pooled point estimates for time to first flatus were 50.8 h (95% CI: 46.0 to 55.6 h), for time to first defecation 69.4 h (95% CI: 62.0 to 76.7 h), and for length of hospital stay 6.4 days (95% CI: 5.7 to 7.1 days) independent of the treatment.
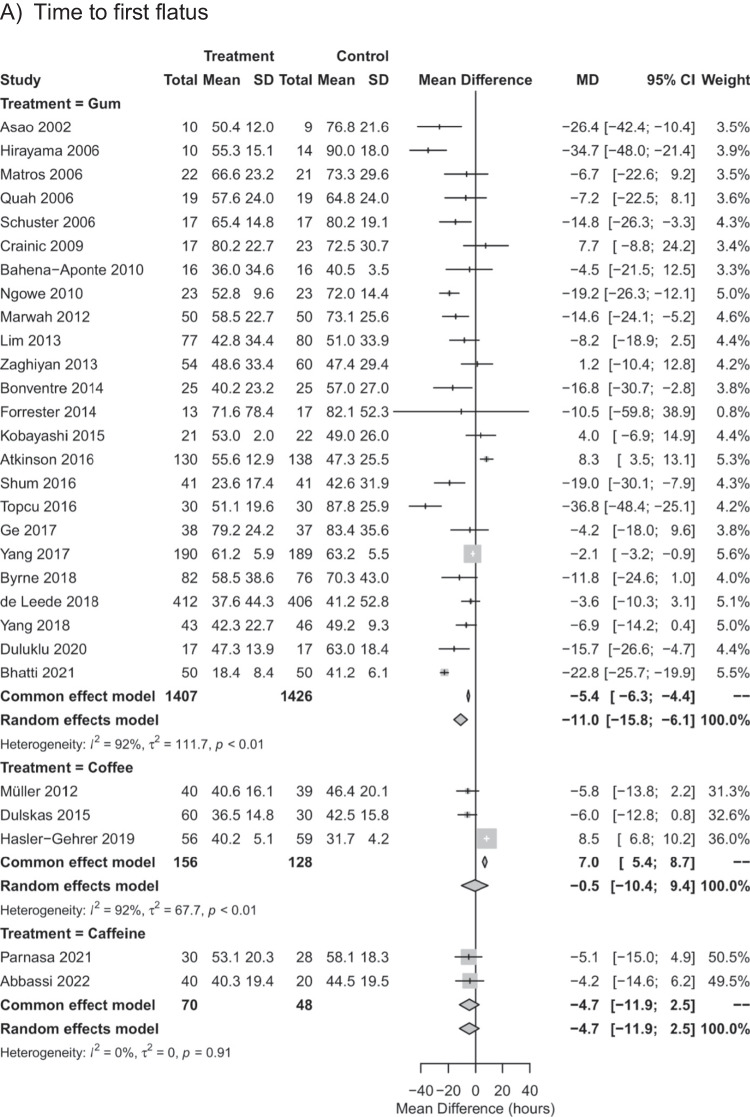
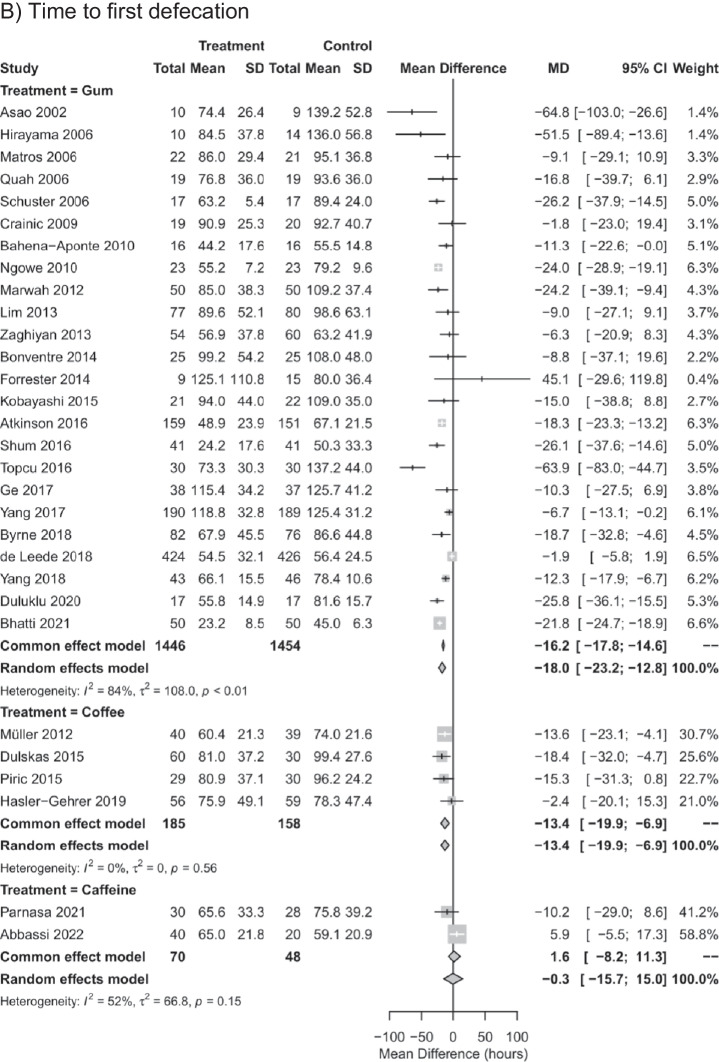
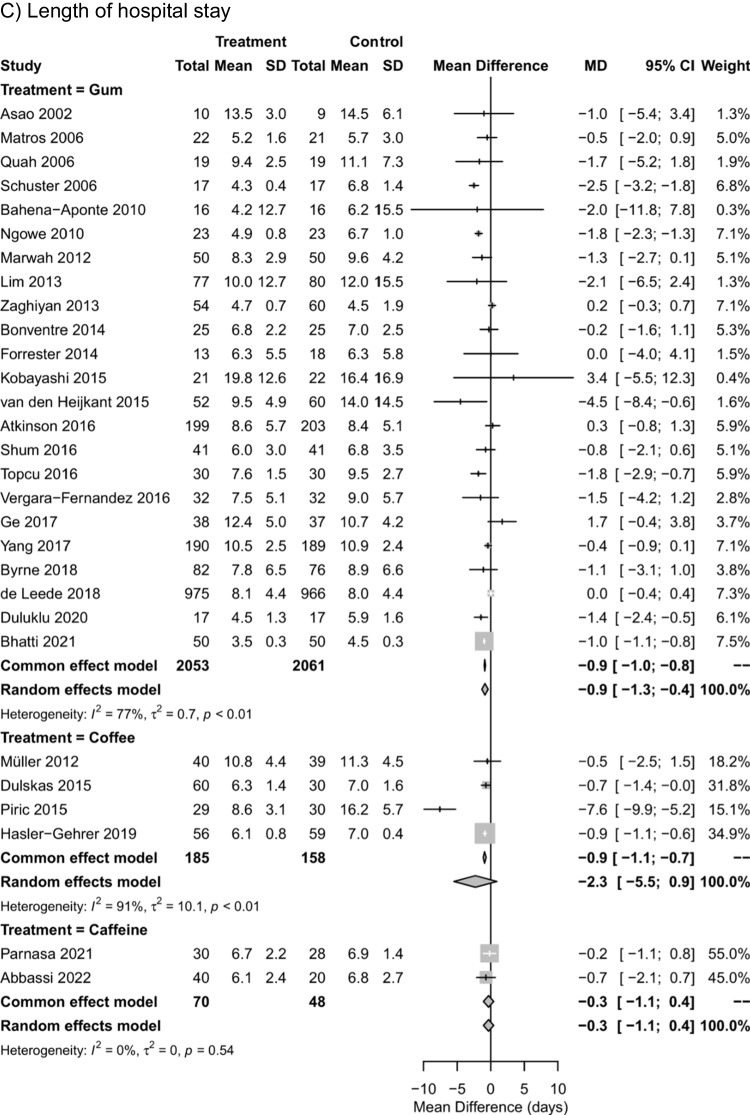
Pairwise meta-analyses comparing each intervention against control for time to first flatus, time to first defecation, and length of hospital stay (Fig. 2) indicated substantial to considerable statistical heterogeneity for gum chewing by visual inspection of the forest plot and by I2 ranging from 77 to 92%. For coffee intake, considerable statistical heterogeneity was observed for time to first flatus and for length of hospital stay (I2 = 91% and I2 = 92%) but not for time to first defecation (I2 = 0%). For caffeine, moderate statistical heterogeneity was observed for time to first defecation (I2 = 52%). In pairwise meta-analyses, gum chewing reduced the time to first flatus, time to first defecation, and length of hospital stay by MD of -11.0 h (95% CI: -15.8 to -6.1 h), -18.0 h (95% CI: -23.2 to -12.8 h), and -0.9 days (95% CI: 1.3 to -0.4 days), respectively, compared to the control. Coffee intake reduced the time to first defecation by a MD of -13.4 h (95% CI: -19.9 to -6.9 h), but not the time to first flatus (MD of -0.5 h with 95% CI: -10.4 to 9.4 h) or length of hospital stay (MD of -2.3 days with 95% CI: -5.5 to 0.9 days). Caffeine intake had no effect on time to first flatus (MD of -4.7 h with 95% CI: -11.9 to 2.5 h), time to first defecation (MD of -0.3 h with 95% CI: -15.7 to 15.0 h) or length of hospital stay (MD of -0.3 days with 95% CI: -1.1 to 0.4 days).
Fig. 2.
Forest plot for univariable random-effects meta-analyses of the mean difference in pairwise comparisons against control for time to first flatus (A), time to first defecation (B), and length of hospital stay (C)
Network Meta-analysis
The network graphs revealed spider-like nets and no closed loops due to the lack of studies with a head-to-head comparison between the treatments. For time to first defecation, 25, 4, and 2 studies compared gum chewing, coffee, and caffeine intake, respectively, with control (Table 1).
Substantial statistical heterogeneity was confirmed for all three outcomes (P < 0.001), especially for gum chewing. Gum chewing was ranked best for time to first flatus and first defecation whereas coffee consumption was ranked best for length of hospital stay. The P values for gum chewing, coffee consumption, and caffeine intake for time to first flatus were 0.89, 0.33, and 0.53, respectively; the P values for time to first defecation were 0.92, 0.69, and 0.22, respectively; and the P values for length of hospital stay were 0.61, 0.94, and 0.35, respectively. The time to first flatus was reduced by gum chewing with a MD of -11 h, (95% CI − 16 to − 5 h, P < 0.001) (Fig. 3). Time to first defecation was reduced by gum chewing with a MD of -18 h (95% CI − 23 to − 13 h, P < 0.001) and by coffee with a MD -13 h (95% CI − 24 to − 1 h, P < 0.001). Length of stay was reduced by coffee and gum chewing with MDs of − 1.5 days (95% Cl: − 2.5 to − 0.6 days, P < 0.001) and − 0.9 days (95% CI: − 1.3 to − 0.4 days, P < 0.001). Coffee was not superior to gum chewing in terms of length of hospital stay, with a MD of − 0.7 days (95% CI: − 1.7 to 0.4 days, P = 0.203). No significant effect was observed for caffeine. These results were confirmed by sensitivity analysis with Bayesian NMA with quite similar point estimates and 95% CI (Fig. 3).
Fig. 3.
Summary plot for point estimates and their corresponding 95% confidence and credible intervals in the frequentist and Bayesian network meta-analyses
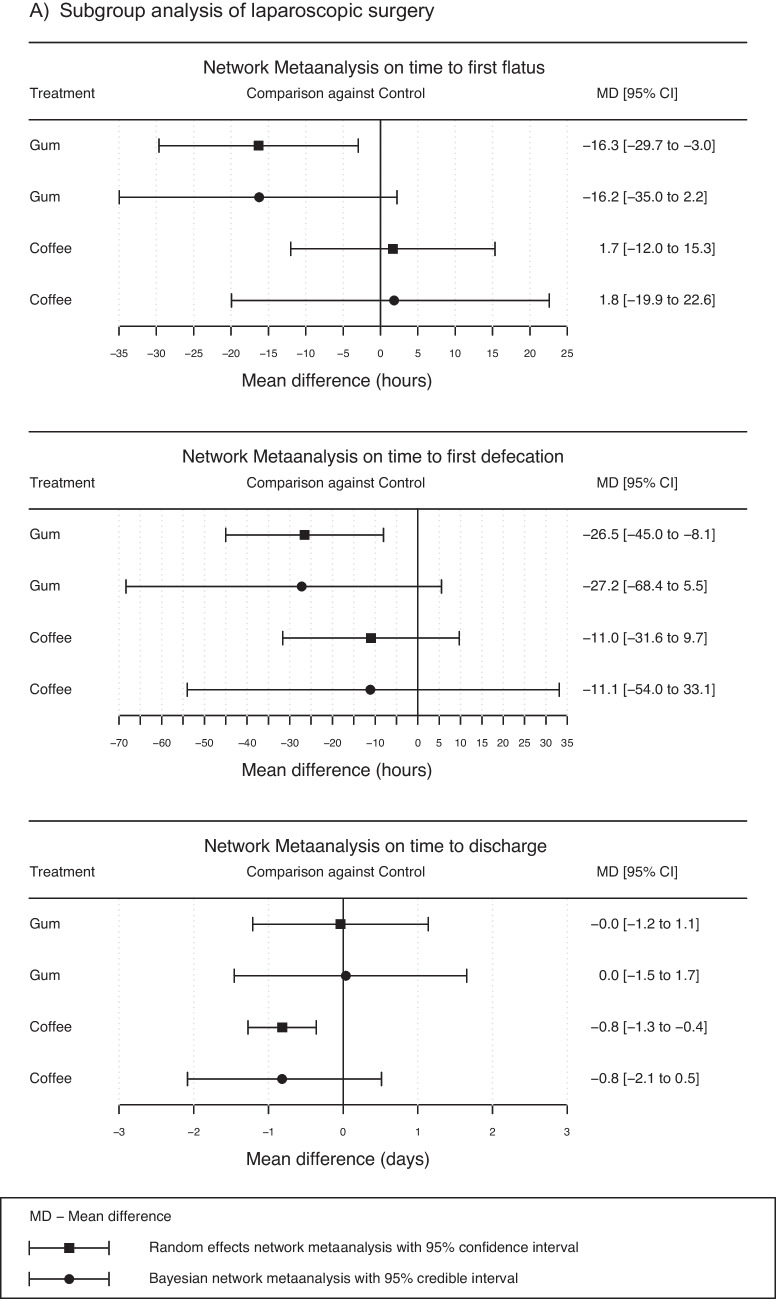
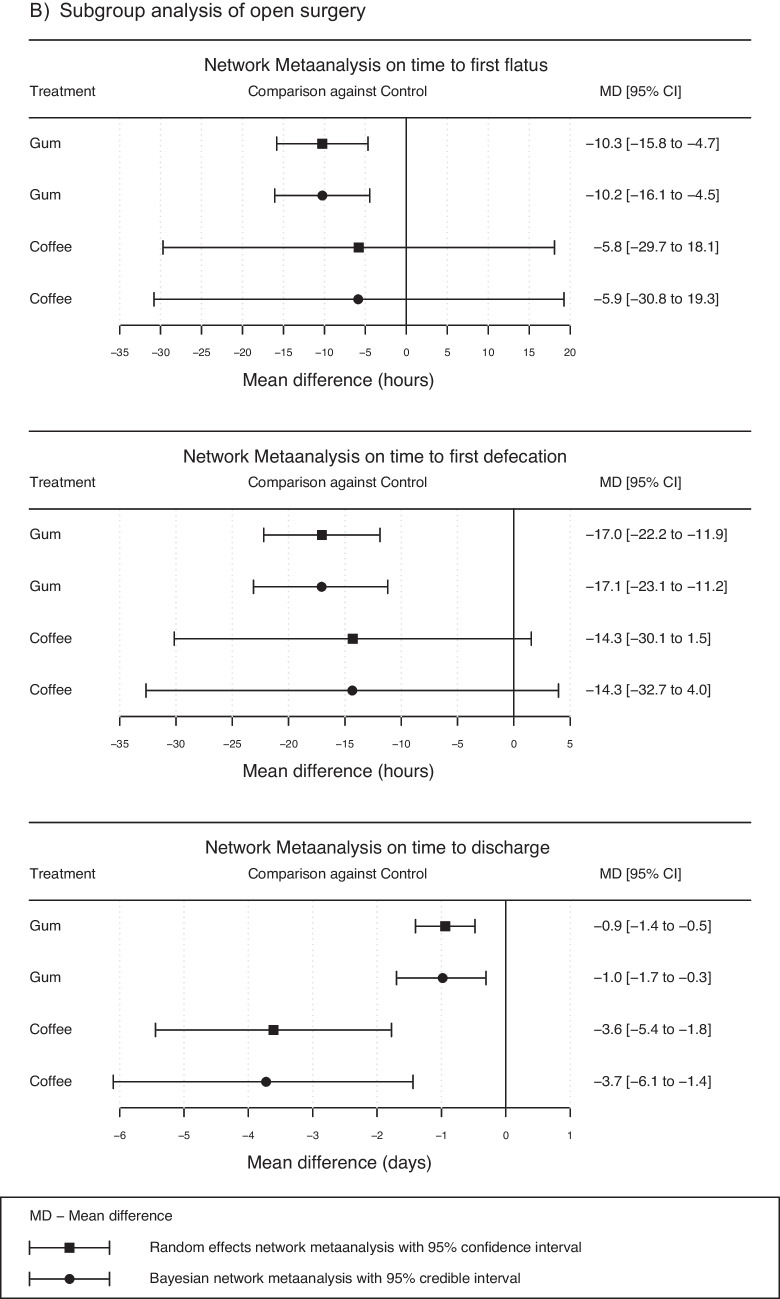
The results of two subgroup analyses after laparoscopic and open gastrointestinal surgery are summarized in Figs. 4a and b to elaborate possible differences in gum chewing, coffee consumption, and caffeine intake. Studies with mixed laparoscopic and open surgeries were added to the open group, as there were either many conversions in those studies or missing specifications in the percentage of conversion rates. The subgroup analyses of gum chewing and coffee consumption showed a significant reduction in time to discharge in the open surgery group (95% CI (gum): -1.7 to -0.3 days, respectively 95% CI (coffee): -6.1 to -1.4 days). In the laparoscopic group, there was no significant effect on time to discharge (95% CI (gum): -1.2 to 1.1, 95% CI (coffee): -2.1 to 0.5).
Fig. 4.
Subgroup analysis for point estimates and their corresponding 95% confidence and credible intervals in random effects and Bayesian network meta-analysis in laparoscopic (A) and open (B) gastrointestinal surgery
The time to first defecation in the gum-chewing open group (95% CI: -23.1 to 11.2 h) showed a significant reduction but did not carry weight in the time to discharge in this group.
Attempts to further elucidate the within-group heterogeneity by mixed effects meta-regression analyses using year of publication and laparoscopic versus open surgery as moderator variables did not succeed in relevantly decreasing the within-group heterogeneity. The I2 in the meta-regressions remained high and ranged from 51 to 84% compared to 77 to 92% in pairwise meta-analyses with laparoscopic versus open surgery as a significant moderator variable for all outcomes comparing gum chewing with control.
Discussion
This systematic review found clinically relevant and statistically significant effects on postoperative ileus after gastrointestinal surgery for gum chewing and coffee consumption but not for caffeine intake. These findings were confirmed by pairwise meta-analysis, random effects NMA using frequentist methods, and Bayesian network meta-analyses. Gum chewing was associated with an improvement in all assessed outcomes. Coffee consumption shortened the time to first defecation and length of hospital stay but not the time to first flatus. A numerically stronger effect for coffee consumption compared to gum chewing on the length of hospital stay could not be proven. The grade of evidence of this review was relevantly impaired by statistical heterogeneity, more pronounced for gum chewing than for coffee consumption.
Subgroup analysis in laparoscopic and open surgery confirmed a significant reduction in time to discharge in open surgery. In the subgroup analysis with laparoscopic surgery, the effect of gum could not be confirmed. One probable explanation might be the introduction of enhanced recovery programs in laparoscopic surgery. As the laparoscopic approach reduces abdominal wall trauma and the resultant milieu of inflammatory, neurohumoral, and pain responses, the recovery process might be accelerated. 37,38
For the research question at hand, length of hospital stay could be seen as the most objective measure and the easiest one to record. For coffee consumption and gum chewing, clinically relevant shortening of 1.5 days and 0.9 days was observed. In contrast, no effect was observed in the caffeine group, thus confirming recent research reporting no effect of caffeine on POI in a homogeneous RCT assessing exclusively laparoscopic colectomy. 31
Coffee consumption and gum chewing reduce the costs associated with the length of hospital stay. As they are inexpensive products that many people are familiar with, their use is widely accepted by patients. Watanabe et al. and Eamudomkarn et al. described similar findings, which were a shortening of LOS with postoperative coffee consumption, especially with the increasing complexity of the surgical procedure. 39,40 In addition, a shortening of the average length of hospital stay leads to a reduction in health care costs. 41
Physiologically the gastrointestinal system is stimulated by meals with high calories, acidity, or osmolarity or due to its volume. In the case of chewing gum, coffee consumption, and caffeine intake, their ingredients must exert biochemical effects, as they are almost free of calories and have low osmolality. 33
Coffee and its bioactive compounds are suggested to influence the gastrointestinal mucosa (permeability, secretion, and proliferation), the gut wall (and its neural and nonneural components), and the brain–gut axis. 42 Coffee consumption also induces cholecystokinin release, gallbladder contraction 43, and a gastrocolic response with increased colonic motility. 44,45 As different ingredients in coffee and caffeine exist, the different outcomes in the length of stay, time to first flatus, and time to the first defecation are explained by the different ingredients, such as melanoidins or chlorogenic acid. 46 These different compositions are due to the different coffee bean species as well as the roasting process, which leads to a strong variation in composition. 46,47
Chlorogenic acid is supposed to have an anti-inflammatory effect by inhibiting the production of interleukin-6 and tumor necrosis factor alpha and therefore reduces inflammation and pain with the improvement of gastrointestinal recovery, whereas melanoidins partially behave as dietary fiber, as shown in in vivo experiments, and have the ability to influence the contractility of gastric smooth muscles by activating cholinergic receptors. 46,48
Considering the physiological mechanism by which gum chewing improves bowel function after surgery, it is assumed that gum chewing activates the cephalic-vagal pathway via the parasympathetic nervous system. This stimulates intestinal myoelectric activity and bowel motility by counteracting the activation of the gastrointestinal μ-opioid receptor. It also seems to release gastrointestinal hormones and increase the secretion of saliva and pancreatic juice and therefore stimulates bowel movement. 4,49,50 Short et al. found some evidence that gum chewing may influence the digestive system to recover, especially bowel sounds and decrease the length of stay, but the involved studies were less reliable due to poor quality and a lack of description of methodology and allocation. 51
In contrast, de Leede et al. described no evidence of gum chewing in the postoperative care pathway to reduce the time to bowel recovery or length of stay in their RCT, which might be due to the heterogeneity of the frequency and duration of gum chewing. 52
There are several limitations of the present study, particularly with respect to the blinding of the participants. First, the participants in the gum-chewing group were not blinded due to its impracticability, which can lead to a high risk of performance bias. In the caffeine group, only two studies were able to blind their patients by using capsules with and without caffeine. The coffee consumption group contained only one study that blinded the participants by administering coffee with and without caffeine. As coffee compounds vary by region, bean type, and roasting method, the effect of coffee consumption may not be generalizable to all populations, which limits the validity of the RCT analysis. The characteristics of regular and nonregular coffee drinkers were not reported in the studies. Additionally, the dose‒response relationship between coffee and caffeine consumption is unknown, as it was not evaluated. Furthermore, the relevant in-group heterogeneity could not be explained, further limiting the generalizability of the present study, particularly for treatment with gum chewing, for which a significant treatment effect could only be proven after open surgery but not after laparoscopic surgery. Finally, the small number of included studies has also to be mentioned. Only four, respectively two studies reported on coffee consumption and caffeine intake. Given the small number of studies with a quite small included number of participants, the power to differentiate between these two treatments has to be considered strongly limited. Further evidence is needed to discriminate between these two treatments with certainty.
The main strength of the present investigation is the fact that only RCTs were included in the study. This is the first network meta-analysis comparing chewing gum, coffee consumption, and caffeine intake. Despite relevant heterogeneity in the univariable meta-analyses, the random effects NMA using frequentist methods and the Bayesian analyses gave quite similar results. The present study demonstrated that time to first flatus and defecation should be considered surrogate parameters in the gum chewing and coffee consumption groups for the length of hospital stay, as there were significant reductions in the univariate analysis but not in the network analysis.
Conclusion
Coffee and gum chewing were proven to be effective and noninvasive approaches for shortening the postoperative length of hospital stay and time to first defecation, especially after open gastrointestinal surgery; thus, these actions should be recommended after gastrointestinal surgery.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by University of St.Gallen
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest
The authors report no conflicts of interest. TS holds a grant from the “Stiftung Chirurgie,” St. Gallen, Switzerland, to financially support scientific publications in surgery.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Su'a BU, Pollock TT, Lemanu DP, MacCormick AD, Connolly AB, Hill AG. Chewing gum and postoperative ileus in adults: a systematic literature review and meta-analysis. International journal of surgery (London, England) 2015;14:49–55. doi: 10.1016/j.ijsu.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Wolthuis AM, Bislenghi G, Fieuws S, van Buck Overstraeten deBoeckxstaens AG, D'Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Disease. 2016;18(1):O1–9. doi: 10.1111/codi.13210. [DOI] [PubMed] [Google Scholar]
- 3.Flores-Funes D, Campillo-Soto Á, Pellicer-Franco E, Aguayo-Albasini JL. Uso de café, chicle y gastrografín en el manejo del íleo postoperatorio: revisión de la evidencia actual. Cirugia espanola. 2016;94(9):495–501. doi: 10.1016/j.ciresp.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Stakenborg N, Gomez-Pinilla PJ, Boeckxstaens GE. Postoperative Ileus: Pathophysiology, Current Therapeutic Approaches. Handbook of experimental pharmacology. 2017;239:39–57. doi: 10.1007/164_2016_108. [DOI] [PubMed] [Google Scholar]
- 5.Senagore AJ. Pathogenesis and clinical and economic consequences of postoperative ileus. American journal of health-system pharmacy AJHP official journal of the American Society of Health-System Pharmacists. 2007;64(20 Suppl 13):S3–7. doi: 10.2146/ajhp070428. [DOI] [PubMed] [Google Scholar]
- 6.Mao H, Milne TGE, O'Grady G, Vather R, Edlin R, Bissett I. Prolonged Postoperative Ileus Significantly Increases the Cost of Inpatient Stay for Patients Undergoing Elective Colorectal Surgery: Results of a Multivariate Analysis of Prospective Data at a Single Institution. Diseases of the colon and rectum. 2019;62(5):631–637. doi: 10.1097/DCR.0000000000001301. [DOI] [PubMed] [Google Scholar]
- 7.Venara A, Alfonsi P, Cotte E, Loriau J, Hamel J-F, Slim K. Postoperative ileus concealing intra-abdominal complications in enhanced recovery programs-a retrospective analysis of the GRACE database. International journal of colorectal disease. 2019;34(1):71–83. doi: 10.1007/s00384-018-3165-9. [DOI] [PubMed] [Google Scholar]
- 8.Scarborough JE, Schumacher J, Kent KC, Heise CP, Greenberg CC. Associations of Specific Postoperative Complications With Outcomes After Elective Colon Resection: A Procedure-Targeted Approach Toward Surgical Quality Improvement. JAMA surgery. 2017;152(2):e164681. doi: 10.1001/jamasurg.2016.4681. [DOI] [PubMed] [Google Scholar]
- 9.Sparn MB, Widmann B, Pietsch U, Weitzendorfer M, Warschkow R, Steffen T. Risk factors and outcomes of postoperative aspiration pneumonia in abdominal surgery patients: An exact matching and weighting analysis. Surgery. 2021;170(5):1432–1441. doi: 10.1016/j.surg.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Asao T, Kuwano H, Nakamura J, Morinaga N, Hirayama I, Ide M. Gum chewing enhances early recovery from postoperative ileus after laparoscopic colectomy1 1No competing interests declared. Journal of the American College of Surgeons. 2002;195(1):30–32. doi: 10.1016/S1072-7515(02)01179-1. [DOI] [PubMed] [Google Scholar]
- 11.Lim P, Morris OJ, Nolan G, Moore S, Draganic B, Smith SR. Sham feeding with chewing gum after elective colorectal resectional surgery: a randomized clinical trial. Annals of surgery. 2013;257(6):1016–1024. doi: 10.1097/SLA.0b013e318286504a. [DOI] [PubMed] [Google Scholar]
- 12.Yang T-W, Wang C-C, Sung W-W, Ting W-C, Lin C-C, Tsai M-C. The effect of coffee/caffeine on postoperative ileus following elective colorectal surgery: a meta-analysis of randomized controlled trials. International journal of colorectal disease. 2022;37(3):623–630. doi: 10.1007/s00384-021-04086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in medicine. 2014;33(25):4353–4369. doi: 10.1002/sim.6236. [DOI] [PubMed] [Google Scholar]
- 14.Rücker G. Network meta-analysis, electrical networks and graph theory. Research synthesis methods. 2012;3(4):312–324. doi: 10.1002/jrsm.1058. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). (ed.). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition.: Chichester (UK): John Wiley & Sons, 2019.
- 17.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of internal medicine. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 18.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed.) 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 19.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Statistical methods in medical research 2020:962280219889080. [DOI] [PMC free article] [PubMed]
- 20.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statistical methods in medical research. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC medical research methodology. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathur MB, VanderWeele TJ. Sensitivity analysis for publication bias in meta‐analyses. Journal of the Royal Statistical Society. Series C, Applied Statistics 2020;69(5):1091–119. [DOI] [PMC free article] [PubMed]
- 23.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of clinical epidemiology. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. Cham: Springer International Publishing; 2015. [Google Scholar]
- 26.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC medical research methodology. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of clinical epidemiology. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC medical research methodology. 2013;13:35. doi: 10.1186/1471-2288-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Research synthesis methods. 2012;3(4):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 30.Plummer M. JAGS: A Program for Analysis of Bayesian Graphical Models using Gibbs Sampling. 3rd International Workshop on Distributed Statistical Computing (DSC 2003); Vienna, Austria 2003;124.
- 31.Abbassi F, Müller SA, Steffen T, Schmied BM, Warschkow R, Beutner U et al. Caffeine for intestinal transit after laparoscopic colectomy: randomized clinical trial (CaCo trial). The British journal of surgery 2022. [DOI] [PubMed]
- 32.Parnasa SY, Marom G, Bdolah-Abram T, Gefen R, Luques L, Michael S, et al. Does caffeine enhance bowel recovery after elective colorectal resection? A prospective double-blinded randomized clinical trial. Techniques in coloproctology. 2021;25(7):831–839. doi: 10.1007/s10151-021-02450-7. [DOI] [PubMed] [Google Scholar]
- 33.Dulskas A, Klimovskij M, Vitkauskiene M, Samalavicius NE. Effect of Coffee on the Length of Postoperative Ileus After Elective Laparoscopic Left-Sided Colectomy: A Randomized, Prospective Single-Center Study. Diseases of the colon and rectum. 2015;58(11):1064–1069. doi: 10.1097/DCR.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 34.Hasler-Gehrer S, Linecker M, Keerl A, Slieker J, Descloux A, Rosenberg R, et al. Does Coffee Intake Reduce Postoperative Ileus After Laparoscopic Elective Colorectal Surgery? A Prospective, Randomized Controlled Study: The Coffee Study. Diseases of the colon and rectum. 2019;62(8):997–1004. doi: 10.1097/DCR.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 35.Müller SA, Rahbari NN, Schneider F, Warschkow R, Simon T, von Frankenberg M, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. The British journal of surgery. 2012;99(11):1530–1538. doi: 10.1002/bjs.8885. [DOI] [PubMed] [Google Scholar]
- 36.Piric M, Pasic F, Rifatbegovic Z, Konjic F. The Effects of Drinking Coffee While Recovering from Colon and Rectal Resection Surgery. Medical archives (Sarajevo, Bosnia and Herzegovina) 2015;69(6):357–361. doi: 10.5455/medarh.2015.69.357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leissner KB, Shanahan JL, Bekker PL, Amirfarzan H. Enhanced Recovery After Surgery in Laparoscopic Surgery. Journal of laparoendoscopic & advanced surgical techniques. Part A. 2017;27(9):883–891. doi: 10.1089/lap.2017.0239. [DOI] [PubMed] [Google Scholar]
- 38.Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World journal of surgery. 2019;43(3):659–695. doi: 10.1007/s00268-018-4844-y. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe J, Miki A, Koizumi M, Kotani K, Sata N. Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients 2021;13(12). [DOI] [PMC free article] [PubMed]
- 40.Eamudomkarn N, Kietpeerakool C, Kaewrudee S, Jampathong N, Ngamjarus C, Lumbiganon P. Effect of postoperative coffee consumption on gastrointestinal function after abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Scientific reports. 2018;8(1):17349. doi: 10.1038/s41598-018-35752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. Journal of managed care pharmacy JMCP. 2009;15(6):485–494. doi: 10.18553/jmcp.2009.15.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wald A, Back C, Bayless TM. Effect of caffeine on the human small intestine. Gastroenterology. 1976;71(5):738–742. doi: 10.1016/S0016-5085(76)80353-8. [DOI] [PubMed] [Google Scholar]
- 43.Douglas BR, Jansen JB, Tham RT, Lamers CB. Coffee stimulation of cholecystokinin release and gallbladder contraction in humans. The American journal of clinical nutrition. 1990;52(3):553–556. doi: 10.1093/ajcn/52.3.553. [DOI] [PubMed] [Google Scholar]
- 44.Brown SR, Cann PA, Read NW. Effect of coffee on distal colon function. Gut. 1990;31(4):450–453. doi: 10.1136/gut.31.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boekema PJ, Samsom M, van Berge Henegouwen GP, Smout AJ. Coffee and gastrointestinal function: facts and fiction. A review. Scandinavian journal of gastroenterology. Supplement 1999;230:35–9. [DOI] [PubMed]
- 46.Ludwig IA, Clifford MN, Lean MEJ, Ashihara H, Crozier A. Coffee: biochemistry and potential impact on health. Food & function. 2014;5(8):1695–1717. doi: 10.1039/C4FO00042K. [DOI] [PubMed] [Google Scholar]
- 47.Gloess AN, Schönbächler B, Klopprogge B, D`Ambrosio L, Chatelain K, Bongartz A et al. Comparison of nine common coffee extraction methods: instrumental and sensory analysis. Eur Food Res Technol 2013;236(4):607–27.
- 48.Argirova MD, Stefanova ID, Krustev AD, Turiiski VI. Testing biological activity of model Maillard reaction products: studies on gastric smooth muscle tissues. Amino Acids. 2010;38(3):797–803. doi: 10.1007/s00726-009-0286-z. [DOI] [PubMed] [Google Scholar]
- 49.Arosio M, Ronchi CL, Beck-Peccoz P, Gebbia C, Giavoli C, Cappiello V, et al. Effects of modified sham feeding on ghrelin levels in healthy human subjects. The Journal of clinical endocrinology and metabolism. 2004;89(10):5101–5104. doi: 10.1210/jc.2003-032222. [DOI] [PubMed] [Google Scholar]
- 50.Stern RM, Crawford HE, Stewart WR, Vasey MW, Koch KL. Sham feeding. Cephalic-vagal influences on gastric myoelectric activity. Digestive diseases and sciences 1989;34(4):521–7. [DOI] [PubMed]
- 51.Short V, Herbert G, Perry R, Atkinson C, Ness AR, Penfold C et al. Chewing gum for postoperative recovery of gastrointestinal function. The Cochrane database of systematic reviews 2015(2):CD006506. [DOI] [PMC free article] [PubMed]
- 52.de Leede EM, van Leersum NJ, Kroon HM, van Weel V, van der Sijp JRM, Bonsing BA. Multicentre randomized clinical trial of the effect of chewing gum after abdominal surgery. The British journal of surgery. 2018;105(7):820–828. doi: 10.1002/bjs.10828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.