Abstract
The natural ligands for the CCR5 chemokine receptor, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES (regulated on T-cell activation, normal T-cell expressed and secreted), are known to inhibit human immunodeficiency virus (HIV) entry, and N-terminally modified RANTES analogues are more potent than native RANTES in blocking infection. However, potent CCR5 blocking agents may select for HIV-1 variants that use alternative coreceptors at less than fully inhibitory concentrations. In this study, two N-terminal chemical modifications of RANTES produced by total synthesis, aminooxypentane (AOP)-RANTES[2-68] and N-nonanoyl (NNY)-RANTES[2-68], were tested for their ability to prevent HIV-1 infection and to select for coreceptor switch variants in the human peripheral blood lymphocyte-SCID mouse model. Mice were infected with a CCR5-using HIV-1 isolate that requires only one or two amino acid substitutions to use CXCR4 as a coreceptor. Even though it achieved lower circulating concentrations than AOP-RANTES (75 to 96 pM as opposed to 460 pM under our experimental conditions), NNY-RANTES was more effective in preventing HIV-1 infection. However, in a subset of treated mice, these levels of NNY-RANTES rapidly selected viruses with mutations in the V3 loop of envelope that altered coreceptor usage. These results reinforce the case for using agents that block all significant HIV-1 coreceptors for effective therapy.
Primate lentiviruses initiate infection by binding to two cell membrane receptors, CD4 (24) and one of several chemokine receptors (1, 4, 6, 7, 10, 17, 18, 20). CCR5 is the coreceptor used by primary, macrophage-tropic human immunodeficiency virus type 1 (HIV-1) isolates which are most frequently transmitted between humans (13, 14). The CXCR4 chemokine receptor is utilized by T-cell-line-adapted HIV-1 isolates (6, 20), and viruses using this coreceptor are isolated from about one-half of infected individuals late in the course of disease (37). Viruses using CCR5 or CXCR4 coreceptors are now termed R5 and X4, respectively (5). Several other chemokine receptors, including CCR2b, CCR3, STRL33, and gpr15 and gpr1 can mediate virus entry (2, 9, 19), but CCR5 appears to be the most widely expressed and utilized (40). The natural ligands for CCR5 are the chemokines macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES (regulated on T-cell activation, normal T-cell expressed and secreted) (12). N-terminal modifications of RANTES result in antagonists that can block HIV-1 infection without signaling calcium flux (23, 32, 35). These modifications include N-terminal truncation (RANTES[9-68]) (3) and the addition of methionine (32) or the substitution of Ser-1 of RANTES by the n-pentane oxime of glyoxylic acid (AOP) at the N terminus of RANTES (35). AOP-RANTES was particularly effective at blocking infection with R5 HIV-1 isolates in vitro, perhaps due to its inhibition of receptor recycling (23).
These observations led us to investigate the activity of AOP-RANTES and a novel N-terminal modification, Nα-nonanoyl-RANTES[2-68] (hereafter referred to as NNY-RANTES), as antagonists of HIV-1 infection in a small animal model. SCID mice repopulated with human peripheral blood mononuclear cells (hu-PBL-SCID mice) are highly susceptible to HIV-1 infection by a variety of isolates, including R5, R5X4, and X4 viruses with minimal sequence differences (26–28, 31). A major concern about antagonists for a single chemokine coreceptor is their potential to select for viruses that use alternative coreceptors. Although the evolution from R5 to X4 viruses is very slow in patients, the selective pressure of a potent CCR5 blocking agent might rapidly select for X4 variants. To address this concern experimentally, we used virus derived from the 242 molecular clone to infect hu-PBL-SCID mice, since this R5 isolate needs only a single amino acid substitution to become R5X4, and it needs only three changes to become X4 (8, 36).
MATERIALS AND METHODS
Synthesis of AOP- and NNY-RANTES.
N-terminal-modified chemokines were prepared by total chemical protein synthesis as previously described (38). The N-terminal modifications were incorporated by an on-resin reaction of RANTES[2-33] with the preformed oxime n-pentyl-O-N⩵CHCOOH as the last step in the chain assembly to give AOP-RANTES[2-33] thioester or with nonanoic acid to give Nα-nonanoyl-RANTES[2-33] thioester. Native chemical ligation (16) of the purified unprotected peptide segments of AOP-RANTES[2-33] thioester with RANTES[34-68] in aqueous buffer gave the full-length polypeptide produced in reduced form, which was folded with disulfide formation in aqueous buffer and purified by reversed-phase high-pressure liquid chromatography (HPLC). The folded AOP-RANTES was homogeneous on HPLC and gave a molecular mass of 7,901.02 ± 0.8 Da on electrospray ionization mass spectroscopy (calculated average isotope composition, 7,901.2 Da). NNY-RANTES was similarly prepared by chemical ligation of Nα-nonanoyl-RANTES[2-33] thioester with RANTES[34-68]. The folded NNY-RANTES was homogeneous on HPLC and gave a molecular mass of 7,899.96 ± 0.01 Da on electrospray ionization mass spectroscopy (calculated average isotope composition, 7,900.21 Da). Large amounts (>50 mg) of purified folded proteins were obtained from a single research scale synthesis of each analogue. Multidimensional nuclear magnetic resonance measurements showed that both N-terminal analogue proteins were conformationally homogeneous (data not shown).
Generation of hu-PBL-SCID mice.
C.B-17 SCID mice were bred under specific-pathogen-free conditions at The Scripps Institute and tested for mouse immunoglobulin M (IgM) production at 8 weeks of age. Mice with <5 μg of IgM per ml were engrafted with peripheral blood mononuclear cells (PBMC) prepared from Epstein-Barr virus (EBV)-seronegative donors from the Scripps General Clinical Research Center pool. SCID mice were injected with 20 × 106 PBMC intraperitoneally and checked for plasma levels of human IgG after 12 to 13 days. Mice with >100 μg of human IgG per ml were used for HIV-1 infection. Each experiment used mice generated from a single, different EBV-negative donor. All three donors were genotyped for the CCR5 Δ32 mutation and were homozygous wild type.
HIV-1 virus pools.
Infectious stocks of the 242 molecular clone were made by transfecting 293 cells with a full-length molecular clone provided by Bruce Chesebro. Virus recovered from the culture after 48 h was used to infect PBMC cultured for 4 days with phytohemagglutinin (PHA; 2 μg/ml) and for 2 days with interleukin-2 (IL-2; 20 U/ml). Infectious virus was recovered after 7 to 10 days of culture, and the tissue culture infectious dose (TCID) of the virus was determined by endpoint titration. Mice were infected with 1,000 TCIDs of virus. Sequencing results showed that our original 242 infectious stock differed from the published sequence by having an H rather than R in position 21 of the V3 loop (see Table 2). This 242H variant was used for all of the experiments depicted in Fig. 2. A second lot of the 242 clone was prepared subsequently and shown to retain the original sequence. The original sequence was also recovered from one animal (NNY-2 R3 in Fig. 2B), which could have resulted from either mutation or selection of the original R sequence from a virus pool dominated by the 242H variant.
TABLE 2.
V3 envelope sequences of HIV-1 242 recovered from hu-PBL-SCID mice treated with AOP- or NNY-RANTES
| Virus clonea | Sequence |
|---|---|
| Expt A | 15 10 15 20 25 30 35 |
| Stock | CTRPNNNTRRSISIGPGRAFHTT-EIIGDIRQAHC |
| All clones | ----------------------------------- |
| Expt B | CTRPNNNTRRSISIGPGRAFRTT-EIIGDIRQAHC |
| NNY 2 R3-1 | --------------------R-------------- |
| NNY 2 R3-2 | --------------------R-------------- |
| NNY 2 R3-3 | --------------------R-------------- |
| NNY 2 R3-4 | --------------------R-------------- |
| NNY 2 R3-5 | --------------------R-------------- |
| CTRPNNNTRRSISIGPGRAFHTT-EIIGDIRQAHC | |
| NNY 2 R5-9 | ----------------------------------- |
| NNY 2 R5-8 | ----------------------------------- |
| NNY 2 R5-4 | ----------------------------------- |
| NNY 2 R5-2 | ----------------------------------- |
| NNY 2 R5-10 | -----------------------------T----- |
| Expt C | CTRPNNNTRRSISIGPGRAFHTT-EIIGDIRQAHC |
| NNY 3 R4-5 | ----------R---------R---K---------- |
| NNY 3 R4-7 | ----------R---------R---K---------- |
| CTRPNNNTRRSISIGPGRAFHTT-EIIGDIRQAHC | |
| NNY 3 R5-1 | ----------R---------R---K---------- |
| NNY 3 R5-3 | ----------R---------R---K---------- |
| NNY 3 R5-4 | ----------R---------R---K---------- |
| NNY 3 R5-10 | ----------R---------R---K---------- |
| NNY 3 R5-2 | ----------R---R-----R---K---------- |
FIG. 2.
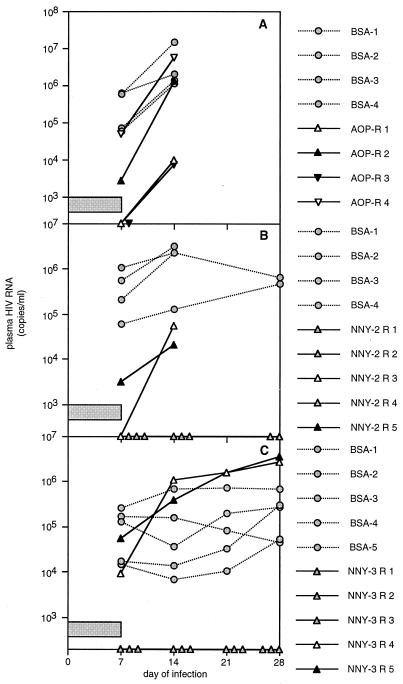
Inhibition in hu-PBL-SCID mice of HIV-1 infection by AOP- or NNY-RANTES. CCR5 antagonists were delivered by subcutaneously implanted osmotic pumps at the rate of 316 nM (2.5 μg)/h beginning 1 day before infection with the 242H isolate (see the text). A single dose of 126.6 μM (1 mg) of AOP-RANTES (A) or NNY-RANTES (B and C) was administered just prior to HIV-1 challenge. All animals thus received a bolus injection of compounds just prior to infection and continuous infusion of compounds from day −1 to at least day 7 after infection, as indicated by the horizontal bar in each panel. Data presented are plasma HIV RNA copies per milliliter at 1 to 4 weeks after infection, and each point represents the value for a single animal at each time point. Data collection was halted after 2 weeks in the first experiment (panel A), since all mice were positive. Two mice from each treatment group were sampled for human cell survival after 2 weeks of infection in the second experiment (panel B), so fewer values are recorded at later time points.
In vitro assays.
PBMC were collected from normal blood samples by density centrifugation. CD4+ T cells were separated by depletion of other cell types by antibody treatment and immunomagnetic bead separation. Whole PBMC or separated CD4+ T cells were cultured at 5 × 104 cells per well in 96-well microtiter plates. Cells were activated with PHA and IL-2 for 3 to 4 days, the medium was replaced with concentrations of AOP- or NNY-RANTES ranging from 12,660 to 1.27 pM (100 ng/ml to 1 pg/ml), and the cells were incubated for 30 min at 37°C and then infected with 100 TCIDs of HIV-1 in the continued presence of modified RANTES. After overnight incubation, free virus was removed, and fresh medium containing the original concentration of modified RANTES was added. The wells were sampled on days 4, 7, and 10 after infection, and p24 HIV capsid antigen was measured by enzyme-linked immunosorbent assay (ELISA) (NEN Life Sciences, Boston, Mass.).
Administration of CCR5 antagonists to mice.
AOP- or NNY-RANTES were dissolved in 0.9% saline at 2.5 mg/ml (316 μM). Alzet 2001 mini-osmotic pumps (ALZA Pharmaceuticals, Palo Alto, Calif.) were loaded with 200 to 225 μl of compounds or bovine serum albumin (BSA) as a control. Pumps were surgically implanted subcutaneously under halothane anesthesia between the scapulae, and the incision was closed with a single wound clip. Pumps were observed for proper placement during the course of the experiment. A single intraperitoneal (i.p.) injection of 1 mg in 0.4 ml of either RANTES compounds (126.6 μM) or BSA was administered just prior to virus infection. These concentrations were based on the solubility and the availability of the compounds and not on prior pharmacokinetic studies.
Virus infection in mice.
Infection of hu-PBL-SCID mice with HIV-1 was determined by plasma HIV-1 RNA levels measured by the quantitative Roche PCR assay (Amplicor HIV Monitor; Roche Molecular Systems, Somerville, N.J.). The limit of detection was 200 to 400 copies/ml, depending on the plasma volume available. Depletion of CD4+ T cells was measured by flow cytometry. Cells recovered from the peritoneal cavity or regional lymph nodes of hu-PBL-SCID mice were stained with fluorescein- or phycoerythrin-labeled antibodies to human CD3, CD4, CD8, or CD45 and mouse H-2Kd (Pharmingen, San Diego, Calif.) and analyzed with a FACScan (Becton Dickinson, Mountain View, Calif.) flow cytometer. CD4+ T cells are expressed as a percentage of total CD3+ cells recovered.
RANTES levels in mice.
Plasma from hu-PBL-SCID mice was analyzed for RANTES antagonists by ELISA (R & D Systems, Minneapolis, Minn.) by using standard curves for either AOP- or NNY-RANTES. Plasma was diluted either 1:10 or 1:100 to bring the RANTES concentration into the optimal sensitivity range of the assay.
V3 envelope sequences.
RNA was extracted from mouse plasma by using the Qiagen viral RNA kit (Qiagen, Valencia, Calif.). RNA was converted to cDNA by reverse transcriptase PCR. cDNA was amplified by nested PCR with the following primers: outer V3 sense, CCAATTCCCATACATTATTG; outer V3 antisense, ATTACAGTAGAAAAATTCCCC; inner V3 sense, CAGTACAATGTACACATGGAATT; and inner V3 antisense, AATTTCTGGGTCCCCTCCTGA. The final 356-bp product was cloned by using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, Calif.), and the resulting product was subjected to automated sequencing (ABI; Perkin-Elmer, Foster City, Calif.). The final sequence encodes 54 amino acids 5′ of V3 and 50 amino acids 3′ of V3. Although only the translated V3 sequence is reported in Table 2, the entire sequence was examined, and there were no mutations outside of V3. Note that HIV-1 242 has a V3 region of only 34 amino acids compared to the clade B consensus length of 35. The consensus G at position 24 is deleted in the 242 clone (8), so substitutions 3′ to this deletion are aligned to the consensus sequence (i.e., no position 24 residue exists in these sequences).
Use of coreceptors.
The coreceptor usage of viruses recovered in these experiments was tested by two independent methods. First, the viruses were used to infect PHA- and IL-2-activated PBMC cultures derived from a donor who is homozygous for the CCR5 Δ32 mutation (22) and thus fails to express CCR5. Second, virus isolates were grown on GHOST cells transfected with either CXCR4 or CCR5 and a reporter construct encoding enhanced green fluorescent protein. These cell lines were obtained through the NIAID/NIH AIDS Research and Reference Reagent Program and were contributed by Vineet N. KewalRamani and Dan R. Littman. Infection of GHOST cell lines was detected by flow cytometry at 5 days after the addition of virus.
RESULTS
Inhibitory activity of AOP- and NNY-RANTES in vitro.
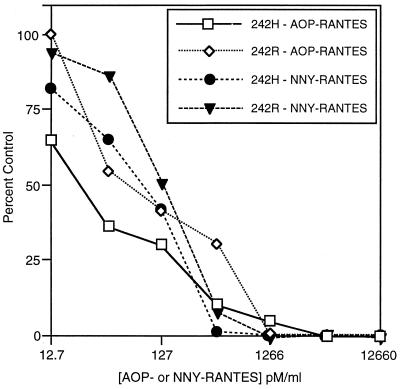
The ability of AOP-RANTES and NNY-RANTES to inhibit R5 virus infection, including the R5 242 isolate of Chesebro et al. (8), was confirmed by in vitro experiments. The results show that both AOP-RANTES and NNY-RANTES were effective at inhibiting the infection of activated PBMC with the R5 SF162 isolate (data not shown) as well as with the two variants of the R5 242 HIV-1 isolate (Fig. 1, also see Table 2). Both AOP-RANTES and NNY-RANTES failed to inhibit infection with X4 isolates (data not shown). In contrast to the more potent inhibition of SF162 (26a), ADA, and JR-CSF (30a), R5 HIV-1 isolates, NNY-RANTES was not more potent than AOP-RANTES at inhibiting the replication of either the 242H or 242R variants in vitro. HIV-1 242R (with an R rather than an H at position 21 of V3) was more resistant to inhibition than was 242H with either of the two CCR5 antagonists. This suggests that minor sequence changes in V3 may impact the affinity of the envelope-CCR5 interaction. AOP-RANTES thus demonstrates the previously observed specificity for CCR5-using HIV-1 isolates (35), and NNY-RANTES has a similar specificity and equal or higher potency (30a). The 242H isolate was used for all subsequent experiments.
FIG. 1.
Inhibition of HIV-1 infection by AOP- and NNY-RANTES in cultured primary human PBMC. Virus replication was measured by p24 capsid antigen production after 5 to 7 days of infection. Infection was with two R5 variants of HIV-1 242: the original 242 isolate with an R at position 21 of V3 and a spontaneous mutant with H at position 21. These viruses are referred to as 242R and 242H, respectively.
Activity of AOP- and NNY-RANTES in hu-PBL-SCID mice.
We performed three replicate experiments in hu-PBL-SCID mice to evaluate the in vivo efficacy of AOP- or NNY-RANTES. Because we anticipated rapid clearance from plasma and wished to maintain stable levels of the CCR5 antagonists, they were administered at the rate of 316.5 nM (2.5 μg)/h by continuous infusion by using subcutaneously implanted osmotic pumps. In addition, a single dose of 126.6 μM (1 mg; ∼50 mg/kg) of each antagonist was injected i.p. just prior to virus infection. Serial plasma HIV RNA determinations were performed on the treated and control hu-PBL-SCID mice after infection with HIV-1 242. In the experiment shown in Fig. 2A, mice were infused with AOP-RANTES or BSA as a control. Plasma concentrations of AOP-RANTES ranged from 157 to 604 pM on day 7 of infusion (Table 1, experiment A). Two of the four mice treated with AOP-RANTES had undetectable viral RNA levels at the end of the 7-day infusion period, but virus levels increased in all mice once AOP-RANTES administration was halted. Thus, as used here, AOP-RANTES was capable of reducing viral load, but it could not prevent HIV-1 infection despite plasma levels that were fully inhibitory in vitro (Fig. 1).
TABLE 1.
Plasma levels of AOP- or NNY-RANTES after 7 days of constant infusion, recovery of CD4+ human T cells, and plasma levels of HIV RNA in hu-PBL-SCID mice treated with CCR5 antagonists
| Expt | Mousea | Day 7 plasma AOP- or NNY-RANTES concn (pM/ml) | Day 14 % CD4+ T cellsb in peritoneal cavity | Day 14 plasma level of HIV RNA (log10 copies/ml) |
|---|---|---|---|---|
| A | AOP-R 1 | 571 | 17.1 | 3.98 |
| AOP-R 2 | 604 | 29.2 | 6.07 | |
| AOP-R 3 | 510 | 27.7 | 3.86 | |
| AOP-R 4 | 157 | 14.3 | 6.72 | |
| Mean ± SE | 460 ± 51 | 22.1 ± 3.7 | 5.16 ± 0.73 | |
| BSA-1 | <0.01 | 3.5 | 7.16 | |
| BSA-2 | <0.01 | 11.9 | 6.06 | |
| BSA-3 | <0.01 | 6.2 | 6.31 | |
| BSA-4 | <0.01 | 4.4 | 6.12 | |
| Mean ± SE | n6.5 ± 1.9 | 6.41 ± 0.25 | ||
| B | NNY 2 R1 | 104 | 86.1 | <2.30c |
| NNY-2 R2 | 86 | <2.30 | ||
| NNY-2 R3 | 110 | 32.7 | 4.72 | |
| NNY-2 R4 | 87 | <2.30 | ||
| NNY-2 R5 | 90 | 47.9 | 4.32 | |
| Mean ± SE | 96 ± 5 | 55.6 ± 15.9 | ||
| BSA-1 | <0.01 | 11.7 | 6.49 | |
| BSA-2 | <0.01 | 12.3 | 6.36 | |
| BSA-3 | <0.01 | 5.12 | ||
| BSA-4 | <0.01 | 6.34 | ||
| Mean ± SE | 12.0 ± 0.3 | 6.08 ± 0.32 | ||
| C | NNY-3 R1 | 62 | ND | <2.30 |
| NNY-3 R2 | 52 | ND | <2.30 | |
| NNY-3 R3 | 156 | ND | <2.30 | |
| NNY-3 R4 | 48 | ND | 6.02 | |
| NNY-3 R5 | 54 | ND | 5.59 | |
| Mean ± SE | 75 ± 20 | |||
| BSA-1 | <0.01 | ND | 4.16 | |
| BSA-2 | <0.01 | ND | 3.83 | |
| BSA-3 | <0.01 | ND | 5.85 | |
| BSA-4 | <0.01 | ND | 4.56 | |
| BSA-5 | <0.01 | ND | 5.21 | |
| Mean ± SE | 4.72 ± 0.36 |
Each mouse is individually designated as in Fig. 2. AOP-R or NNY-R means the animal was treated with either AOP- or NNY-RANTES.
CD4+ T cells are reported as a percentage of total CD3 T cells recovered from the peritoneal cavity of each animal. ND, not determined.
Below the limit of detection of 200 copies/ml.
We therefore tested the inhibitory capacity of NNY-RANTES in the next two experiments. Infusion of NNY-RANTES followed the same dose and schedule as AOP-RANTES (126.6 μM or a 1-mg bolus injection given i.p. followed by 316.5 nM or 2.5 μg/h delivered with an Alzet pump) but led to a mean plasma concentration of 96 pM (Table 1, experiment B) on day 7 of infusion, a level with less than complete inhibitory activity in vitro (Fig. 1). Nonetheless, four of five hu-PBL-SCID mice infused with NNY-RANTES had undetectable viral RNA levels on day 7 of the infusion period, and only one additional animal subsequently developed viremia (NNY-2 R3 in Fig. 2B). NNY-RANTES treatment was thus successful in preventing R5 HIV-1 infection in three of five mice, despite achieving five- to sixfold lower plasma concentrations than AOP-RANTES. This experiment was repeated with a different human donor to generate hu-PBL-SCID mice to confirm inhibition of infection at such low concentrations of NNY-RANTES. This experiment also used the same dose and schedule of NNY-RANTES administration and resulted in even lower mean plasma concentrations of NNY-RANTES (75 pM in Table 1, experiment C). However, the inhibition of virus infection was similar to the previous experiment, with NNY-RANTES preventing infection in three of five mice (Fig. 2C). Virus and viral sequences from the two mice that became infected were further characterized (see Table 2, below). NNY-RANTES thus was able to prevent HIV-1 infection in 6 of 10 hu-PBL-SCID mice (Fig. 2B and C) at plasma concentrations lower than the 50% inhibitory dose for HIV-1 242H (∼150 pM; Fig. 1) in vitro.
We also measured the relative survival of human CD4+ T lymphocytes in hu-PBL-SCID mice treated with each CCR5 antagonist. Table 1 compares the recovery of CD4+ T cells (as a percentage of the total CD3+ T cells) in the peritoneal cavity of individual mice treated either with BSA or with AOP- or NNY-RANTES at 2 weeks after infection. Both AOP- and NNY-RANTES were able to slow the depletion of CD4+ T cells, even in mice where HIV-1 infection was not prevented.
NNY-RANTES but not AOP-RANTES selected for coreceptor switch variants under these experimental conditions.
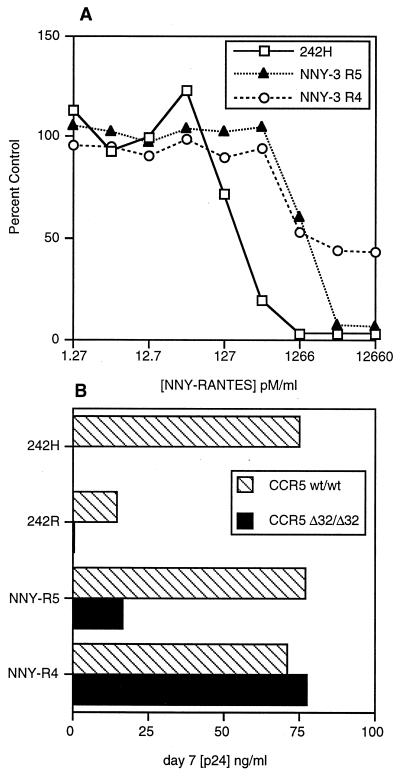
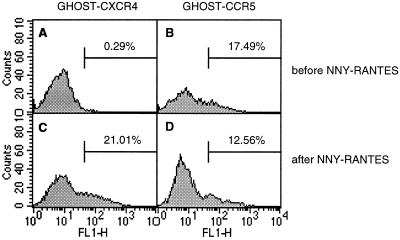
To determine whether virus from hu-PBL-SCID mice that became infected despite treatment with AOP- or NNY-RANTES was evading the antagonists by mutating from CCR5 to CXCR4 coreceptor utilization, we amplified proviral DNA envelope genes directly from hu-PBL-SCID mouse tissue and sequenced the region surrounding the V3 loop, a critical determinant of coreceptor usage (11). V3 sequences observed in the mice are shown in Table 2. In the first experiment (Fig. 2A), sequences obtained after 2 weeks of infection from all mice treated with AOP-RANTES were identical to the starting 242 virus isolate (which was found to contain an H in place of the published R at position 21, a change that had occurred prior to the initiation of these experiments). In the second experiment (Fig. 2B), HIV-1 sequences recovered after 4 weeks of infection from the two mice that became infected despite treatment with NNY-RANTES differed. One mouse had the sequence of the starting 242 isolate (except for one clone with a replacement mutation at position 30), while the other mouse showed a reversion of the H at position 21 to the R present in the original molecular clone. The presence of H or R at position 21 in these isolates did not impact CCR5 usage but may have impacted susceptibility to NNY-RANTES (Fig. 1) and the rate of replication in vitro (Fig. 3B). These results show that although sequence variation was occurring in this experiment and there may have been selection for sequence variants (either preexisting or generated by mutation) that were less sensitive to NNY-RANTES inhibition, there was not rapid selection for HIV-1 variants that used alternative coreceptors for viral entry. However, viral sequences amplified after 4 weeks of infection directly from two hu-PBL-SCID mice that became infected despite NNY-RANTES treatment (mice NNY-3 R4 and NNY-3 R5) in the third experiment (Fig. 2C) revealed the same three replacement mutations in V3 (Table 2, part C), although there were no other replacement or silent mutations in the remainder of the 356-bp PCR product (data not shown). The changes of S to R at position 11, H to R at position 21, and E to K at position 25 conferred upon these viruses reduced susceptibility to NNY-RANTES and the ability to use CXCR4 for infection. These two viral variants showed reduced sensitivity to inhibition by NNY-RANTES in primary PBMC cultures (Fig. 3A). The ability to use CXCR4 for virus entry was demonstrated by infection of CD4-transfected HeLa cells (MAGI cells [34], which are not permissive for R5 viruses [data not shown]) and by infection of PBMC cultures from an individual homozygous for the CCR5 Δ32 mutation (Fig. 3B). The coreceptor usage of the isolate from mouse NNY-3 R4 was further confirmed by the infection of GHOST cells transfected with either CXCR4 or CCR5. The results are shown in Fig. 4. The 242H isolate used for infection could infect only cells expressing CCR5 (Fig. 4A and B), but the NNY-3 R4 isolate with mutations in the V3 region was capable of infecting both CXCR4- and CCR5-expressing target cells (Fig. 4C and D). The V3 sequences present in the NNY-RANTES-treated animals showed rapid reversion to the 242H parental sequence during in vitro culture (a mixture of variant, parental, and partial revertant sequences were obtained after 7 days of culture [data not shown]), so the properties of the viruses recovered by in vitro propagation reflects a mixture of viral genotypes and phenotypes. This may explain the intermediate levels of sensitivity to NNY-RANTES demonstrated in Fig. 3A. Viruses recovered from tissue cultures containing 12,660 pM (100 ng/ml) NNY-RANTES retained the predominant sequence shown in Table 2, experiment C, as did viruses propagated on CCR5-negative cells (as in Fig. 3B), so continued selective pressure was required to maintain this genotype, and such viruses were highly resistant to NNY-RANTES. These results demonstrate that the viral sequences recovered directly from infected cells from mice NNY-3 R4 and NNY-3 R5 represent NNY-RANTES-resistant mutations.
FIG. 3.
(A) Inhibition of HIV-1 replication by NNY-RANTES in cultured PBMC. The HIV-1 isolates were the starting 242H virus and the HIV recovered from animals NNY-3 R4 and NNY-3 R5 from the experiment shown in Fig. 2C. These viruses were expanded in vitro for 7 days prior to the addition of NNY-RANTES. Envelope sequences were obtained from the virus at this time point and after 5 days of culture in the presence of 12.7 nM (100 mg/ml) NNY-RANTES. Sequences from the isolate NNY-3 R4 obtained after culture with NNY-RANTES matched the sequence shown in Table 2, part C, obtained directly from the infected hu-PBL-SCID mouse, but sequences obtained before the addition of NNY-RANTES showed several clones with a reversion to a 242 sequence. The NNY-RANTES inhibition data therefore reflect a population of virus sequences, with only a fractional representation of the NNY-RANTES resistant variants. (B) Replication of HIV-1 in cultured PBMC from a normal donor (CCR5 wt/wt) or a donor without CCR5 expression (CCR5 Δ32/Δ32). Both of the starting 242 virus isolates (H or R at position 21 of V3) were unable to replicate in the CCR5-negative cells, but the viruses recovered from mice NNY-3 R4 and NNY-3 R5 were able to replicate in these cells. As noted above, this experiment was done after 7 days of culture in normal PBMC, and there was no longer sequence homogeneity in the NNY-R4 and NNY-R5 isolates at this time.
FIG. 4.
Coreceptor usage after NNY-RANTES treatment in vivo. The starting 242H isolate and the NNY-3 R4 isolate recovered from an NNY-RANTES treated mouse (Fig. 2C and Fig. 3) were tested for infection of GHOST cells transfected either with CXCR4 (A and C) or CCR5 (B and D). The 242H isolate could only infect GHOST cells expressing CCR5 (B), but the NNY-3 R4 isolate could infect both CXCR4 (C)- and CCR5 (D)-expressing cells.
DISCUSSION
These results show that it is possible to block HIV-1 infection with N-terminally modified RANTES compounds in vivo but that the more effective inhibitor was able to select for coreceptor switch variants during the 1-week treatment period (Fig. 2 and 3). Inhibition of virus infection occurred with plasma levels of 50 to 113 pM NNY-RANTES and 500 to 630 pM AOP-RANTES during continuous administration of the antagonists, levels that are lower than the average concentration (∼2.5 nM) of native RANTES in human plasma (39) and, for NNY-RANTES, levels that were lower than the in vitro 50% inhibitory concentration (Fig. 1) for the 242H virus isolate. There has been one previous report of a chemokine receptor antagonist (AMD3100) that displayed efficacy against X4 HIV-1 infection in SCID-hu mice, albeit at concentrations of greater than 100 nM (15), but ours is the first report of antiviral activity of a CCR5 antagonist in vivo. The finding that AOP-RANTES was poorly effective at preventing infection and that even NNY-RANTES was not completely effective suggests that the pharmacokinetics of these molecules will need to be manipulated to ensure higher circulating concentrations and that further improvements may have to be made in receptor affinity. It should be noted that even the low doses of NNY-RANTES achieved were similar in activity to a potent neutralizing antibody for the prevention of HIV-1 infection of hu-PBL-SCID mice (21, 30). Mice that were not protected from infection had lower viral RNA levels and higher CD4+ T-cell counts than the controls, suggesting that CCR5 antagonists may be useful in treating established infection.
The duration of the cellular response to CCR5 antagonists is currently unknown. If compounds such as AOP- and NNY-RANTES inhibit virus entry by sequestering CCR5 in the cytoplasm (23), then CCR5 antagonists may have an extended period of activity despite their short half-life in plasma. Alternatively, if antagonists only interfere with gp120 binding by receptor occupancy, then they may need to be constantly present at effective inhibitory concentrations. We chose to administer AOP- and NNY-RANTES by both bolus injection and continuous infusion. It is not clear whether both are required for the observed inhibition of virus infection, but preliminary results suggest that neither a single bolus injection nor continuous infusion alone were sufficient to prevent HIV-1 infection. The steady-state concentrations of NNY-RANTES were lower than those of AOP-RANTES under identical administration conditions. This implies a more rapid turnover of NNY-RANTES, but differential rates of receptor recycling might also have the same effect. The low levels achieved make it even more surprising that 60% of the challenged hu-PBL-SCID mice were protected from HIV-1 infection by NNY-RANTES.
The emergence of viruses capable of using CXCR4 under the selective pressure of the concentrations of NNY-RANTES used in these experiments demonstrates that the inappropriate use of CCR5 antagonists could generate more pathogenic variants, since there is general agreement that the course of disease progression is accelerated with the switch from R5 to R5X4 viruses (14, 33). The experiments described here were conceived to test this possibility, and the choice of the HIV-1 242 isolate as the challenge virus should have increased the probability of coreceptor switch variants, since it is known that only a single amino change will generate the 241 sequence which is an R5X4 virus (36). However, both of the hu-PBL-SCID mice that developed NNY-RANTES resistant viruses independently generated the same three replacement mutations rather than the E-to-Q substitution at position 24 that distinguishes HIV-1 241 from HIV-1 242 (8). If primary patient R5 isolates require more mutations to generate altered coreceptor usage they may take longer to emerge, but our results suggest that this is very likely to happen. The selective amino acid replacements at positions 11 and 25 of V3, positions known to influence coreceptor usage (8, 25, 29), and the absence of other mutations (either silent or replacement) argue for highly selective pressure exerted by NNY-RANTES treatment under the conditions of these experiments. The H-to-R change at position 21 also occurred in these viruses as well as in the viral variant recovered from one additional mouse (NNY-R3 in experiment 2 [Fig. 2]) and was shown to decrease susceptibility to NNY-RANTES (Fig. 1) as well as the in vitro replication rate (Fig. 3). The rapid reversion of these viruses to the starting sequence in vitro in the absence but not in the presence of NNY-RANTES suggests that the viral variants are less fit for in vitro replication, but they persisted for 3 weeks after cessation of NNY-RANTES treatment in hu-PBL-SCID mice. It is thus not clear that these coreceptor switch variants would be more highly pathogenic than the starting virus in infected humans, although that possibility clearly exists. The rapid selection of CCR5 antagonist-resistant virus mutations observed in hu-PBL-SCID mice suggests that similar experiments could rapidly map the amino acid substitutions required for resistance to these and other antagonists in patient isolates.
These results strongly reinforce the view that clinical use of blocking agents for CCR5 alone would be unwise and that cocktails of antagonists directed toward known coreceptors or antagonists with broader specificity will be required for safe and effective therapy of HIV-1 infection in humans. Nonetheless, the potent activity of NNY-RANTES in preventing infection of 60% of challenged animals at very low concentrations suggests that HIV coreceptors are important targets for current and novel inhibitory agents.
ACKNOWLEDGMENTS
We appreciate the skilled technical assistance of Andrew Beernink and Michael Neal. We thank Kathy Wehrly and Bruce Chesebro for providing the HIV-1 242 isolate, and we acknowledge the dedicated animal care staff at The Scripps Research Institute. We appreciate the helpful comments of Stephen Kent. The choice of NNY-RANTES for in vivo studies was based on in vitro studies performed by L.P.
This study was supported by NIH grant AI29182 to D.E.M., NIH grant MO1 RR00833 to the Scripps General Clinical Research Center, and a grant from the Swiss National Science Foundation to R.E.O.
Footnotes
Publication no. 11734-IMM from The Scripps Research Institute.
REFERENCES
- 1.Alkhatib G, Broder C C, Berger E A. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos F, Virelizier J L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 4.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:3–16. [PubMed] [Google Scholar]
- 5.Berger E A, Doms R W, Fenyo E M, Korber B T, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 6.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K, Nishio J, Perryman S. Mapping of independent V3 envelope determinants of human immunodeficiency virus type 1 macrophage tropism and syncytium formation in lymphocytes. J Virol. 1996;70:9055–9059. doi: 10.1128/jvi.70.12.9055-9059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard C, Sodroski J. The orphan seven-transmembrane receptor apj supports the entry of primary T-cell-line-tropic and dual-tropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 12.Combadiere C, Ahuja S K, Tiffany H L, Murphy P M. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1α, MIP-1β, and RANTES. J Leukoc Biol. 1996;60:147–152. doi: 10.1002/jlb.60.1.147. [DOI] [PubMed] [Google Scholar]
- 13.Connor R I, Mohri H, Cao Y, Ho D D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datema R, Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune J M. Antiviral efficacy in vivo of the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM 3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–754. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson P E, Muir T W, Clark-Lewis I, Kent S B. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Gauduin M C, Parren P W, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 23.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N, Schlondorff D, Proudfoot A E. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 25.Milich L, Margolin B H, Swanstrom R. Patterns of amino acid variability in NSI-like and SI-like V3 sequences and a linked change in the CD4-binding domain of the HIV-1 Env protein. Virology. 1997;239:108–118. doi: 10.1006/viro.1997.8821. [DOI] [PubMed] [Google Scholar]
- 26.Mosier D E. Human immunodeficiency virus infection of human cells transplanted to severe combined immunodeficient mice. Adv Immunol. 1996;63:79–125. doi: 10.1016/s0065-2776(08)60855-x. [DOI] [PubMed] [Google Scholar]
- 26a.Mosier, D. E. Unpublished data.
- 27.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 28.Mosier D E, Gulizia R J, MacIsaac P D, Torbett B E, Levy J A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 29.Nelson J A, Fiscus S A, Swanstrom R. Evolutionary variants of the human immunodeficiency virus type 1 V3 region characterized by using a heteroduplex tracking assay. J Virol. 1997;71:8750–8758. doi: 10.1128/jvi.71.11.8750-8758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parren P W, Ditzel H J, Gulizia R J, Binley J M, Barbas III C F, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:1–6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 30a.Picard, L. Unpublished data.
- 31.Picchio G R, Gulizia R J, Wehrly K, Chesebro B, Mosier D E. The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes. J Virol. 1998;72:2002–2009. doi: 10.1128/jvi.72.3.2002-2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proudfoot A E, Power C A, Hoogewerf A J, Montjovent M O, Borlat F, Offord R E, Wells T N. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 33.Richman D D, Bozzette S A. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 34.Rocancourt D, Bonnerot C, Jouin H, Emerman M, Nicolas J F. Activation of a beta-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990;64:2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 36.Speck R F, Wehrly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tersmette M, Lange J M, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellekens P T, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;1:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 38.Ueda H, Siani M A, Gong W, Thompson D A, Brown G G, Wang J M. Chemically synthesized SDF-1alpha analogue, N33A, is a potent chemotactic agent for CXCR4/Fusin/LESTR-expressing human leukocytes. J Biol Chem. 1997;272:24966–24970. doi: 10.1074/jbc.272.40.24966. [DOI] [PubMed] [Google Scholar]
- 39.Weiss L, Si-Mohamed A, Giral P, Castiel P, Ledur A, Blondin C, Kazatchkine M D, Haeffner-Cavaillon N. Plasma levels of monocyte chemoattractant protein-1 but not those of macrophage inhibitory protein-1alpha and RANTES correlate with virus load in human immunodeficiency virus infection. J Infect Dis. 1997;176:1621–1624. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]