Abstract
Recently, compelling evidence points to dysbiosis and disruption of the epithelial intestinal barrier as major players in the pathophysiology of metabolic disorders, such as obesity. Upon the intestinal barrier disruption, components from bacterial metabolism and bacteria itself can reach peripheral tissues through circulation. This has been associated with the low-grade inflammation that characterizes obesity and other metabolic diseases. While circulating bacterial DNA has been postulated as a common feature of obesity and even type 2 diabetes, almost no focus has been given to the existence and effects of bacteria in peripheral tissues, namely the adipose tissue. As a symbiont population, it is expected that gut microbiota modulate the immunometabolism of the host, thus influencing energy balance mechanisms and inflammation. Gut inflammatory signals cause direct deleterious inflammatory responses in adipose tissue and may also affect key gut neuroendocrine mechanisms governing nutrient sensing and energy balance, like incretins and ghrelin, which play a role in the gut-brain-adipose tissue axis. Thus, it is of major importance to disclose how gut microbiota and derived signals modulate neuroendocrine and inflammatory pathways, which contribute to the dysfunction of adipose tissue and to the metabolic sequelae of obesity and related disorders. This review summarizes the current knowledge regarding these topics and identifies new perspectives in this field of research, highlighting new pathways toward the reduction of the inflammatory burden of metabolic diseases.
Keywords: Gut dysbiosis, Intestinal permeability, Endotoxemia, Adipose tissue microbiota, Metabolic disease
Introduction
The gastrointestinal (GI) tract is classically perceived as the main responsible for digestion, allowing nutrient absorption and fluids/electrolytes balance. Nonetheless, the gut orchestrates a plethora of other relevant functions, such as regulation of energy homeostasis, immune sensing, and protection from external harmful factors [1].
Energy balance is a delicate process relying on the rhythmic alignment between the central nervous system (CNS) and peripheral informants that sense nutrient arrival and scarcity, i.e., the gut, the pancreas, and the adipose tissue (AT) [2]. The energetic status, communicated to brain centers through neuronal and peripheral humoral effectors, will determine an appropriate response to adapt food intake, nutrient partitioning, and energy expenditure (catabolism of stored lipids and thermogenesis) accordingly [2, 3].
The unique anatomy of the GI tract, especially in the intestines, allows it to work as an important physical, but selective, wall of defense against external pathogens, while maintaining neutrality to gut commensals. The intestinal mucosal barrier is composed of different layers: the lumen, mucus layer, intestinal epithelium, and the lamina propria [reviewed by Farré et al. [1] and Vancamelbeke et al. [4]]. The outer mucus layer allocates a huge diversity of commensal microorganisms, known as the gut microbiota, and respective metabolites, as well as antimicrobial peptides. The mucus layer is mainly composed of water and mucins (glycosylated proteins secreted by goblet cells) and confers a semi-permeable environment, allowing the passage of factors from the lumen to the epithelial cells, while preventing the extravasation of microorganisms. An intricate relationship occurs between gut microbiota and the mucus gel since the antimicrobial peptides repel microorganisms that, in turn, can influence the gel composition [1, 4, 5]. In fact, the mucus phenotype is transmissible through fecal microbiota transplantation into germ-free mice, which acquire mucosal properties dependent on the microbiota of the donor [5]. Different types of epithelial cells constitute the intestinal epithelium in a single-layer structure that divides the lumen from the lamina propria. The enterocytes are the cells responsible for maintaining structural integrity and regulating absorption; the goblet cells secrete mucus; Paneth cells produce the antimicrobial peptides; M cells are involved in immunity responses, and the enteroendocrine cells produce hormones and neurotransmitters, allowing communication between the gut and several organs [1, 4, 6]. A rupture in the mucosal or epithelial barriers can compromise the gut-vascular interface (endothelium, pericytes, and enteric glial cells), allowing pathogens and bacterial products to disseminate mainly through the portal vein circulation to peripheral sites, such as the liver or visceral fat [7]. Thus, the gut barrier exists as a dynamic structure that regulates metabolic homeostasis, whose function is compromised in metabolic diseases such as obesity, metabolic-associated fatty liver disease (MAFLD), and type 2 diabetes (T2DM) [7–10].
In this review, we will highlight the contribution of gut inflammatory signals (endotoxemia) and bacteria itself on AT dysfunction in obesity, a main factor for the development of other metabolic disorders. We will briefly review the process of diet-driven dysbiosis, intestinal barrier disruption and metabolic endotoxemia (1), and will deeply cover two main points: (2) the consequences of dysbiosis and intestinal inflammation on gut neuroendocrine function and (3) the deleterious effects of impaired gut function on AT.
Diet-driven dysbiosis, gut inflammation, and intestinal barrier disruption
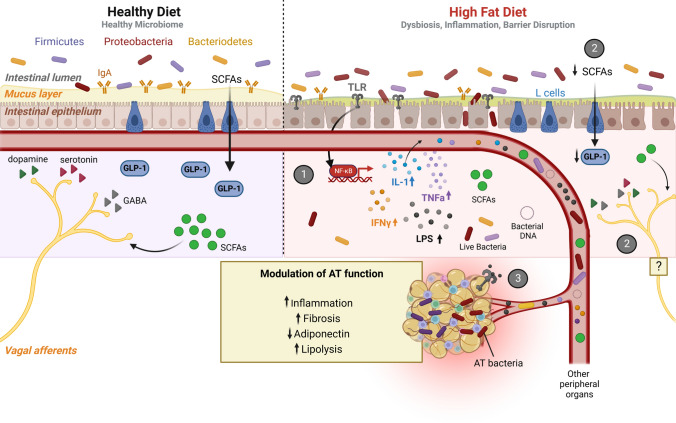
Trillions of microorganisms, most of them bacteria, inhabit the human gut in a symbiotic relationship with the host and this colonization is a dynamic and ongoing process throughout life. The gut microbiota plays an active part in the regulation of (1) host defenses, by training the immune system through pathogen-associated molecular patterns (PAMPs) and antigens exposure [11]; (2) digestion, as dietary indigestible products are degraded by bacteria [12]. Indeed, gnotobiology studies in germ-free mice show a deficient immune response, originating from morphological and functional malformations, insufficient mesenteric lymph nodes, and incapacity to produce antibodies [11]. During digestion, gut microbiota produce short-chain fatty acids (SCFAs) from the metabolism of dietary fibers, which are important not only to maintain the epithelial barrier but also to keep immune homeostasis [13]. SCFAs are correlated with better glycemic and lipid profiles and their contribution to improved metabolism arises from direct action on peripheral sites, such as AT (decrease lipolysis), liver (increase fatty acid oxidation), and pancreas (potentiate glucose-stimulated insulin secretion) [14, 15]. Other common bacterial metabolites are trimethylamine (derived from choline and carnitine), lipopolysaccharide (LPS) (derived from the Western diet), indole (derived from tryptophan), and p-cresol (derived from tyrosine) [7].
An adequate balance between the main bacterial phyla (Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, Verrucomicrobia) and the overall diversity of bacterial groups, as well as a healthy ratio of symbionts/pathobionts, should be maintained to avoid dysbiosis, which facilitates a disease-promoting environment [15]. Well-known contributors to dysbiosis are, to name a few, antibiotics, drugs, alcohol, stress, genetic susceptibility, and diet, in particular, the westernized high-fat diets (HFD) (reviewed by Weiss and Hennet) [15–17]. The first studies emerging on the link between diet-induced obesity and a shift in gut microbiota were performed by Gordon and co-workers [18, 19] (Table 1). Further, gut microbiota was shown to be an active part of fat storage regulation [20]. In fact, in C57BL/6 J mice, HFD consumption for 4 weeks, was sufficient to induce marked alterations in gut flora composition [21–24], and fecal transplantation to germ-free mice induced a massive increase in adiposity [19]. Similar effects were observed when diets were extended to 8, 12, or 16 weeks [25–27] (Table 1), meaning that a short period is enough to induce such marked alteration in gut flora and structure. Diet-induced obesity promotes dysbiosis mainly by increasing Firmicutes/Bacteroidetes ratio, in mice and rats, with an overall loss in genus diversity [15, 17, 21–32] (Table 1), which has been shown to promote the increased ability for energy accretion from food and generation of an inflammatory environment [33]. In an HFD-driven dysbiosis environment, with a high prevalence of LPS-producing Gram-negative bacteria, Toll-like receptors (TLRs) may be continuously activated, namely TLR4. This will promote downstream activation of nuclear factor kappa β (NF-ĸB)-dependent transcription programs for several pro-inflammatory cytokines -, interleukin (IL)-1β, IL-18, IL-6, IL-33, tumor necrosis factor α (TNFα) and interferon-gamma (IFNy), eventually contributing to colonic inflammation [25, 26, 28, 31, 32, 34, 35] (Fig. 1(1)). Yet, decreased amounts of TH17 cells and eosinophils have been reported in the small intestine of HFD-fed mice, during 1 to 4 weeks of diet, which are features of a reduced intestinal inflammatory process [23, 36] (Table 1). Moreover, no histological hallmarks of intestinal inflammation (e.g., mononuclear cell infiltrate, epithelial hyperplasia, or goblet cell depletion) were observed in most studies even when molecular markers of inflammation were present and under chronic regimens of HFD feeding [28, 36]. Albeit still controversial, sustained activation of inflammatory pathways, which appear to be mainly triggered by TLR4 activation [25], may perpetuate a chronic inflammation state, causing damage to the epithelial barrier (Fig. 1(1)). In fact, HFD-induced gut dysbiosis and inflammation compromise barrier integrity by decreasing tight-junction proteins, such as zonulin, occludin, and claudin-1/5 [25, 26, 29, 41] (Table 1). The influence of dysbiosis is clearly proven by antibiotic treatment, which indeed prevented diet-induced barrier breakage [22].
Table 1.
The putative relationship between diet-driven dysbiosis, gut inflammation, and intestinal barrier disruption with adiposity, in animal models and human studies
| Model/protocol | Gut microbiota (phylum/class, order, family or genus) | Gut inflammation | Intestinal permeability & endotoxemia | Body adiposity | References |
|---|---|---|---|---|---|
| C57BL/6 J mice/HF diet feeding (1 week) | N/A |
↓ TNF-α, MCP-1, = CD11b, CD11c, IL-1β mRNA ↓ eosinophils No histological features of inflammation |
↑ FITC-dextran | N/A | [36] |
| C57BL/6 J mice/HF diet feeding (4 weeks) |
↓ Bacteroidetes ↓ Actinobacteria/Bifidobacterium |
N/A |
↓ colonic zonulin and occludin ↑ FITC-dextran ↑ LPS (plasma) |
↑ | [21, 22] |
|
↓Bacteroidetes/Porphyromonadaceae ↑ Firmicutes/Peptostreptococcaceae |
↓ IL-22, IL-17, IL-10 mRNA and ↓Th17 cells | N/A | ↑ | [23] | |
|
↓ Bacteroidetes ↑ Firmicutes/Mollicutes |
N/A | N/A | ↑ | [24] | |
| C57BL/6 J mice/ HF diet feeding (8 weeks) |
↓ Bacteroidetes ↑ Firmicutes/Ruminococcaceae ↓ Proteobacteria |
↑ TNFa, IL-1β, IL-6, TLR4 and NF-kB mRNA |
↑ LPS (plasma) ↓ colonic claudin-1 and occludin |
↑ | [25] |
| C57BL/6 J mice/HF diet feeding (12 weeks) |
↑ Firmicutes/Oscillibacter ↓ Firmicutes/Lactobacillus ↓ Bacteroidetes |
↑ TNF-α, = IL-6 mRNA |
↓ transepithelial resistance ↓ colonic zonulin |
↑ | [26] |
| N/A | N/A |
↑ FITC-dextran ↓ colonic occludin and claudin-5 recruitment of immune cells into the intestinal epithelial layer (H&E staining) |
↑ | [41] | |
| C57BL/6 J mice/HF diet feeding (16 weeks) | N/A | ↑ ileum NF-kB, TNF-α | N/A | ↑ | [34] |
|
↓ Bacteroidetes ↑ Firmicutes |
N/A | N/A | ↑ | [27] | |
| C57BL/6 J mice/HF diet feeding (80 weeks) |
↓ Bacteroidetes ↑ Firmicutes ↓ Tenericutes/Anaeroplasma ↑ Actinobacteria/Adercreutzia |
↑ IL-6, = MCP-1, IL-10, IL-17 mRNA No histological features of inflammation |
N/A | ↑ | [28] |
| C57BL/6 J mice/HS or HFr diet feeding (12 weeks) |
↑ Firmicutes ↑ Proteobacteria/Desulfovibrio ↓ Bacteroidetes/Muribaculum ↑ Verrucomicrobia/Akkermansia |
↑ IL-1β, TNF-α mRNA |
↑ LPS (plasma) ↑ FITC-dextran ↓ colonic zonulin and occludin |
↑ | [29] |
| GF C57BL/6 J mice/fecal transplantation from HF/HS-fed donors |
↑ Firmicutes/Erysipelotrichi ↑ Firmicutes/Bacilli ↓ Bacteroidetes |
N/A | N/A | ↑ | [19] |
| Sprague–Dawley rats/ HF diet feeding (12 weeks) |
↓ Bacteroidetes ↑ Firmicutes/Clostridiales ↓ Firmicutes/Lactobacillus ↓Tenericutes |
N/A | N/A | N/A | [30] |
|
↑ Proteobacteria/Enterobacteriales ↑ Firmicutes/Clostridiales = Bacteroidetes/Bacteroidales |
↑ ileum TLR4/MD2 |
↑ LPS (plasma) ↑ FITC-dextran |
↑ | [31] | |
| Sprague–Dawley rats/HF-HS diet feeding (4 weeks) |
↓ Bacteroidetes ↑ Firmicutes ↑ Actinobacteria/Micrococcaceae ↑ Tenericutes/Anaeroplasmatales |
↑ IL-6, IL-1β, TNF-α mRNA |
↑ LPS (plasma) ↓ cecum occludin |
↑ | [32] |
| Adults with obesity | N/A | N/A | ↑ LPS (plasma) | N/A | [44] |
|
↑ Firmicutes ↓ Bacteroidetes |
N/A | N/A | N/A | [45] | |
| ↓ Actinobacteria/Bifidobacterium | = fecal calprotectin |
= lactulose/mannitol = lactulose/sucralose |
N/A | [48] | |
| Women with overweight or obesity | N/A | N/A | ↑ lactulose and sucralose/mannitol excretion | N/A | [46, 47] |
| Healthy adults on HF diet (5 days) |
↑ Firmicutes/Clostridiales ↑ Proteobacteria/Bilophila |
N/A | N/A | N/A | [49] |
| Women with obesity on a low-calorie diet (14 days) |
↓ Firmicutes/Agathobacter ↑ Actinobacteria/Bifidobacterium |
N/A |
↓ lactulose and sucralose excretion = mannitol excretion ↓ plasma zonulin and LBP |
↓ | [51] |
N/A Not applicable or not measured, HF high-fat, HS high-sugar, HFr high-fructose, LPS lipopolysaccharide, GF germ-free, MCP-1 monocyte chemoattractant protein-1, NF-kB nuclear factor kappa β, TLR4/MD2 Toll-like receptor 4/myeloid differentiation protein-2, FITC fluorescein isothiocyanate, IL interleukin, TNFα tumor necrosis factor alpha, LBP lipopolysaccharide-binding protein
Fig. 1.
The putative mechanisms linking HF diet-driven dysbiosis to impaired AT function. HFD initiates a loop of gut dysbiosis and inflammation, dampening epithelial barrier integrity, and thus increasing intestinal permeability. Because of dysbiosis, LPS levels rise while SCFAs production is impaired. 1—LPS-meditated TLR activation triggers NF-kB-dependent transcription programs for pro-inflammatory cytokines: IL-1, TNFα, and IFNy, contributing to colonic inflammation, that may spread into the circulation. 2—A reduction in the SCFAs production hinders GLP-1 secretion by L cells. Additionally, since SCFAs, mainly butyrate, directly stimulate vagal afferents in healthy conditions (left side), a decrease in the SCFAs production will likely result in decreased vagal tone, which might compromise the gut-brain axis and energy balance regulation (right side). 3—Increased intestinal permeability facilitates LPS, cytokines, and bacteria translocation and migration to distant peripheral targets, via the circulation. Colonization of AT by gut-derived bacteria and LPS-mediated TLR activation will culminate in an inflammatory response and overall impairment of AT function. Abbreviations: SCFAs short-chain fatty acids, GLP-1 glucagon-like peptide 1, GABA y-aminobutyric acid, TLR toll-like receptor, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, IL-1 interleukin 1, IFNy interferon-gamma, LPS lipopolysaccharide, TNFa Tumor necrosis factor-alpha, AT adipose tissue. Image created with Biorender.com
Another mechanism possibly involved in the regulation of barrier integrity is the endocannabinoid system, which is highly upregulated by HFD consumption [37]. Cannabinoid receptor 1 has been shown to modulate barrier permeability. Rimonabant administration to ob/ob mice reduces intestinal permeability (measured by circulating LPS levels), whereas agonism displayed differential effects, leading to increased (with anandamide) or decreased (with 2-arachidonoylglycerol) barrier integrity (reviewed by Cuddihey et al.) [38].
HFD is also associated with increased hepatic bile acids (BA) secretion, which are converted to secondary BA by the microbiota within the colon. Secondary BA play a role in fat digestion and can in turn control the gut microbiome (increasing Firmicutes/Bacteroidetes ratio) while reducing the risk of intestinal inflammation [7]. However, at chronically high levels, secondary BA are associated with colonic inflammation and intestinal barrier dysfunction [7, 39, 40]. A 2-week HFD regimen, in mice, potentiated secondary BA production by increasing Lachnospiraceae and Rumincoccaceae (Firmicutes/Clostridiales), which deconjugate and transform the liver-derived primary BA into secondary intestinal BA [39] while inducing gut epithelium proliferation, which may arise as an initial protective mechanism.
Furthermore, a 4-week HFD, in C57BL6/J mice, was already able to increase LPS levels in circulation, inducing LPS-dependent systemic inflammation, the so-called metabolic endotoxemia, which might initiate/contribute to the metabolic sequelae of obesity [21]. The relevance of impaired barrier integrity as a pathological mechanism for obesity (reviewed by Portincasa et al.) [7] was recently further validated, since extracellular vesicles from Akkermansia muciniphila (Verrucomicrobia) were able to reduce weight gain of HFD-fed mice, by improving tight-junctions, and thus decreasing intestinal permeability, through an AMP-activated protein kinase (AMPK)-dependent mechanism [41].
Glucose and fructose-enriched diets were also shown to induce loss in tight-junction proteins and increased gut barrier permeability assayed by plasma LPS levels and fluorescein isothiocyanate (FITC)-dextran administration, although to a lower extent than after the HFD feeding [29] (Table 1). Refined sugars, such as saccharides, are a typical component of Western diets and were linked to TLR4-mediated colonic inflammation and increased permeability [42]. Hyperglycemia enhanced glucose transporter 2 (GLUT2)-mediated retrograde glucose uptake into epithelial cells, leading to increased N-glycan biosynthesis, also dampening barrier integrity [43].
Although westernized diets (high sugar and HFD) are associated with a pattern of dysbiosis (increased Firmicutes/Bacteroidetes ratio), putative intestinal inflammatory response, and barrier disruption in rodents (Table 1), this link is less evident in humans, in part because it is harder to study. However, some studies in humans have already shown a relationship between HFD-feeding and/or obesity scenarios with alterations in gut colonization and intestinal barrier disruption (Table 1). The Firmicutes/Bacteroidetes ratio and circulating LPS levels (7.8 EU/mL) are increased in patients with obesity when compared to normal-weighted ones [44, 45] (Table 1). However, such measurements are merely indirect indicators of barrier integrity, since the definition of the normal range of values is uncertain. The lactulose/mannitol or sucralose/mannitol excretion ratios are more useful to assess intestinal permeability, allowing to discriminate between small intestinal permeability (lactulose/mannitol) or colonic permeability (sucralose/mannitol) [4]. In women with obesity, the permeability of the small intestine was increased, as measured by the lactulose/mannitol excretion, and correlated positively with waist/abdominal perimeter and with the homeostatic model assessment of insulin resistance (HOMA-IR) [46]. Moreover, gut permeability was also positively associated with visceral adiposity in healthy overweight women, but in advanced age, as measured by the sucralose/mannitol excretion ratio [47] (Table 1). Altogether these studies point to a relationship between whole gut permeability and visceral adiposity. A study by Brignardello et al. challenged this hypothesis, as no alterations in small intestine barrier permeability (through lactulose/mannitol excretion) in obese versus lean individuals were reported [48]. This discrepancy may arise from the inclusion of males in this study, as age and body mass index (BMI) were comparable to the study conducted by Teixeira et al. [46]. As aforementioned in pre-clinical studies, HFD seems to have a preponderant role in barrier impairment (Table 1) and, in the covered human studies, no data regarding dietary habits was evaluated, nor provided. Nonetheless, in healthy subjects, a five-day HFD regimen induced a marked increase in Firmicutes and Proteobacteria [49]. On the other hand, higher fiber intake was related to lower amounts of Proteobacteria, increased SCFA, and weight loss in overweight and obese subjects [50]. A very low-calorie diet (800 kcal/day) induced a marked amelioration of glycemic profile while decreasing Proteobacteria and improving gut barrier integrity, in obese women, as measured by lactulose/mannitol and polyethylene glycol excretion and by plasma zonulin levels [51] (Table 1). To the best of our knowledge, no studies in patients with obesity were conducted with more precise techniques that would allow visual detection of GI abnormalities, such as confocal endomicroscopy, a technique allowing in vivo imaging of GI lesions and structures, using a contrast agent during endoscopy, nor using in vitro assessment of intestinal biopsies. In animal models, GI integrity assessment by confocal endomicroscopy has been used in rats and mice to evaluate the gastric and colonic walls [52, 53], but no studies in obesity models were reported to date.
Altogether, these data show the powerful role of the diet itself in the fast modulation of gut microbiota and barrier permeability. Some dietary factors have been linked to a balanced microbiome and restored gut function, such as fiber and polyunsaturated fatty acids. Dietary interventions can be designed with greater precision now that the contribution of specific foods on gut microbiota and modulation of enterotypes and inflammation is starting to be unraveled [54]. Both bamboo fiber and omega-3 supplementation in HFD-fed mice restored the Firmicutes/Bacteroidetes ratio, and increased SCFAs production (by increasing Prevotella and Bacteroides), thus being associated with better metabolic outcomes [55, 56]. Cereals, vegetables, fish, and nuts are highly associated with microbiota balance, reduced inflammation, and increased SCFAs production in humans [57], highlighting the potential of healthy dieting in the management of intestinal health. On the other hand, environmental factors such as air pollutants, heavy metals and pesticides, can also shape the gut microbiota, by entering the food chain, thus contaminating aliments [58]. Indeed, microplastics, derived from the degradation of plastic litter, which accumulate in the soil, water and air, have been shown to promote gut dysbiosis in C57BL/6 J mice and alter the fecal BA profile and liver function [59]. Further, traffic-related air pollution is emerging as a possible inductor of gut dysbiosis in rodents [60] and humans [61], and correlates positively with the development of obesity from a young age [62], raising serious concerns about their negative impacts on metabolic health.
Arising as beneficial gut microbiota modulators, probiotic supplementation with Lactobacillus spp. has been shown as a promising strategy to counteract intestinal inflammation and barrier disruption, while reverting obesity in mice [8, 63]. Exercise training is also a potent modulator of the gut microbiota, by decreasing the Firmicutes/Bacteroidetes ratio and improving intestinal inflammation in sedentary adults with obesity [64]. By reshaping gut microbiota and alleviating inflammation and barrier disruption, these strategies will end up improving overall gut function, metabolic endotoxemia and peripheral tissue’s function, such as adipose tissue and the liver, constituting valuable weapons against metabolic diseases and related gut malfunction.
-
(2)
Consequences of dysbiosis and inflammation on gut function
The role of gut microbiota on regulating gut endocrine and nervous functions: the gut communicates with other organs either through nervous connections or endocrine signaling, in an intricate manner, to regulate energy balance [2]. Vagus nerve projections onto the nucleus tractus solitarius regulate food intake, inducing a positive response upon activation of epinephrine neurons co-expressing neuropeptide Y (NPY), or instead, inhibiting it, through activation of norepinephrine ones [65]. Moreover, the vagus-mediated gut-brain-liver connection controls glucose homeostasis by suppressing hepatic glucose production upon nutrient sensing in the duodenum-jejunum [66]. Additionally, the GI tract secretes a myriad of hormones, including the glucagon-like peptide 1 (GLP-1), peptide YY (PYY), glucose-dependent insulinotropic polypeptide (GIP), cholecystokinin (CCK) and ghrelin [67]. While nutrient digestion and absorption stimulate the secretion of anorexigenic signal—GLP-1, CCK and PYY–, nutrient shortage triggers ghrelin secretion from the stomach to promote feeding instead [67]. The synchronicity between neuronal and humoral signals traveling across the crossroads of the gut-brain axis will govern overall energy homeostasis.
A healthy microbiota regulates gut signals secretion by the production of SCFAs from dietary fiber digestion [14]. Within the gut, SCFAs, but mainly butyrate, stimulate GLP-1 and PYY secretion from L-cells, through a G protein-coupled receptor 43 (GPR43)-dependent mechanism [68, 69] (Fig. 1(2)), This favors an anorexigenic and incretin-stimulatory environment that might account for the beneficial metabolic effects. Secondary BA were also shown to stimulate GLP-1 secretion in vitro in enteroendocrine cell lines [70]. Other metabolites derived from bacterial metabolism, such as indole, are stimulants of gut hormones secretion. Indole is derived from bacteria-mediated tryptophan metabolism [71]. Acute indole action (5 min) stimulated GLP-1 secretion from GLUTag cells, by inhibiting voltage-gated K+ channels [71]. However, longer exposure to indole (30–240 min) induced a reduction of GLP-1 secretion rate, through a reduction of adenosine triphosphate production in GLUTag cells [71], showing that indole production by bacteria can have a major impact on host metabolism. Whether SCFAs, indole, or other bacterial metabolites can modulate the secretion of other gut hormones, such as CCK or ghrelin, remains yet to discover.
Despite not being able to contact directly with vagal afferent fibers, the gut microbiota produce several neurotransmitters—acetylcholine, dopamine, serotonin, γ-aminobutyric acid —, that can act either in the enteric nervous system or in brain centers through the vagal afferents or via circulation [72] (Fig. 1(2)). Additionally, butyrate was shown to induce a direct stimulatory effect on the vagus nerve in rats, independently of enteric neurotransmitters release/activation, but the mechanisms were not further investigated [73] (Fig. 1(2)).
Gut dysbiosis, inflammation and impaired gut endocrine and nervous functions: gut hormones secretion is highly dependent on diet composition (reviewed by Martin et al.) [74]. Diet-induced obesity, accelerated by HFDs, is a major deregulator of the gut-brain axis and gut inflammation has deleterious effects on the overall GI function [74]. Obesity is characterized by alterations in the gut hormones secretion profile, as shown by the decrease in GLP-1 and PYY post-prandial excursions, as well as impaired total and acylated ghrelin levels, which levels remain higher in the post-prandial state when compared to healthy lean controls [67]. Furthermore, obesity is linked to the desensitization of satiety signals in vagal afferent neurons, favoring constitutive orexigenic activity [75].
SCFAs production is also hampered in the context of obesity, as dysbiosis induces the loss of butyrate-producing bacteria [76]. Consequently, the SCFAs-mediated gut hormones secretion, such as GLP-1, may be reduced (Fig. 1(2)). Lactobacillus reduction in HFD-fed mice was associated with decreased GLP-1 responsiveness [77]. Moreover, circadian alterations of ileal microbiota were shown to regulate GLP-1 production in L cells, through ileum clock genes regulation [78].
Loss in SCFAs production might also impair the vagal tone since butyrate contributes to vagal afferents stimulation [73] (Fig. 1(2)). Indeed, germ-free mice conventionalized with microbiota from HFD-fed mice displayed a reduction in vagal innervation to the brain [79]. In rats, inducing dysbiosis by an HFD/high-sugar regimen, induces vagal innervation withdrawal at the gut and nucleus solitary tract, dampening the gut-brain axis [32]. Intestinal dysbiosis was also associated with alterations in serotonin availability, through serotonin transporter modulation [80]. Excessive reuptake of serotonin will induce precocious termination of its physiologic effects, impairing normal gut-brain connectivity.
The relationship between the compromise on gut endocrine function and vagal activation in scenarios of intestinal inflammation is yet poorly understood. Nonetheless, colonic GLP-1 and circulating GLP-1 and GLP-2 levels were decreased under inflammatory conditions, in rodent models and patients with inflammatory bowel disease (IBD) [81, 82]. Interestingly, promising data from pre-clinical studies support a protective role of GLPs in the amelioration of IBD, as agonism of their receptors was shown to alleviate colitis, by reducing the expression of inflammatory cytokines [83]. Clinical pilot studies using the GLP-2 analog teduglutide have demonstrated higher and quicker remission rates in Chron’s disease patients, than placebo-treated ones [84]. In a recent study, in patients with IBD, GLP-1-based therapies showed a decreased risk of hospitalization or need for a TNFα inhibitor treatment, compared to patients on other medications [85]. Similarly, plasma PYY levels, colonic PYY and oxyntomodulin-positive cells are decreased in IBD patients [86]. In turn, ghrelin levels were reported to be increased in patients with Crohn’s disease and ulcerative colitis [87]. Altogether, although the crosslink between gut inflammation and altered gut hormones secretion patterns is still not clear, the available data supports the notion that gut inflammatory milieu potentiates the impairment of neuroendocrine mechanisms involved in nutrient-sensing and energy balance. Future studies should address the mechanisms involved in gut hormone dysregulation by inflammation.
The vagus nerve is a major regulator of gut inflammation. In animal models of colitis, vagal nerve stimulation prevents disease worsening, through acetylcholine-mediated anti-inflammatory effects on macrophages [88]. Individuals who underwent vagotomy have an increased risk of developing IBD, highlighting the immunomodulatory effect of the vagus nerve activity [89]. Further, intestinal inflammation is characterized by an increase in serotonin and NPY circulating levels, neurotransmitters with relevant action within the enteric nervous system and in the gut-brain axis [90, 91]. NPY is abundantly expressed in the enteric nerves and plays a role in colonic relaxation. Npy knock-out mice were shown to be more protected from the development of dextran sodium sulfate-induced colitis [92]. Colitis induces a major upregulation of enteric neuronal NPY, which triggers an oxidative and inflammatory response through neuronal nitric oxide synthase activation [93]. The NPY system seems particularly relevant as a modulator of inflammatory activity, since NPY receptor 1 antagonism reduced inflammatory cytokines in the gut of an IBD rodent model [93]. Colonic serotonin is increased in IBD patients and colitis rodent models, and inhibition of serotonin synthesis is a sufficient factor to reduce experimental colitis severity [94].
Prebiotics are indigestible fermented foods, frequently used to restore balance in the gut flora, by increasing the amount of beneficial Bifidobacterium and Lactobacillus spp., and thus of SCFAs [95]. Modulation of the gut microbiota has shown beneficial effects on intestinal function, thus restoring normal gut hormones secretion. Prebiotic supplementation, with oligofructose, ameliorated gut inflammation and intestinal barrier integrity, while restoring GLP-1 and GLP-2 levels, through increased L cells density [95, 96]. Further, pre and probiotics supplementation has marked effects in AT metabolism [95], highlighting a relevant link between the gut and the AT, possibly mediated by both gut hormones and microbiota.
-
(3)
The involvement of gut dysbiosis and endotoxemia in AT dysfunction
Dysbiosis/inflammation-mediated changes of gut-AT crosstalk: Under physiological conditions, the gut hormones act on and modulate AT function, to regulate lipid metabolism (GLP-1 stimulates lipolysis, CCK and ghrelin stimulate lipogenesis), insulin sensitivity and glucose uptake (GLP-1), and tissue plasticity (GLP-1 and ghrelin) [67]. This intricate communication is a central piece in the whole-body energy balance regulation, allowing the AT reservoirs to behave accordingly to the energetic status, i.e., post-absorptive or post-prandial. Given that the secretion pattern of gut hormones is altered in obesity, this may be an important contributing factor to AT dysfunction, a major hallmark of both obesity and the metabolic syndrome [67]. Also, as covered above, gut dysbiosis and possible inflammation have deleterious effects not only on endocrine, but also nervous-mediated gut communication, which inevitably ends up by compromising other organs’ function. However, the real contribution of gut dysbiosis/inflammation for compromising gut endocrine and nervous functions is not yet established, neither is the role of the dietary signals for such relationship. One may note that many of the mechanisms and signals governing gut hormone secretion are not known, and only recently their direct action in AT has been disclosed in more detail (reviewed by Rosendo-Silva) [67]. It may be expectable that impaired GLP-1 secretion following loss of SCFA-producing bacteria could have a negative impact on AT plasticity and glucose metabolism, given the known GLP-1 actions in adipose fat [67, 97]. However, the impact of other gut signals is yet to be disclosed.
The direct contribution of gut signals to AT dysfunction in obesity: A reduction in the SCFA-producing bacteria, in the context of HFD-driven dysbiosis, is also associated with an increased prevalence of bacteria able to convert other food molecules (such as aromatic amino acids and histidine, among others) in metabolites (namely indoles, cresols, and imidazole propionate) that can affect metabolic function. For instance, this has been observed regarding glucose tolerance and insulin resistance, contributing also to an increase in overall pro-inflammatory effects [98–100]. In fact, while the symbiotic SCFA-producing bacteria contribute to metabolic homeostasis and gut barrier health, the prevalence of pathogenic bacteria contribute to metabolic deregulation and gut barrier impairment, on one side by losing the panoply of protective effects of SCFAs (including the secretion of mucin by goblet cells), and on the other side by presenting a pro-inflammatory profile in opposition to the anti-inflammatory effects of symbiotic bacteria [15, 16]. Gut-derived metabolites can directly impair the behavior of several extra-intestinal sites, such as the AT, especially the mesenteric one due to the close location, the liver, the pancreas, and the skeletal muscle [101]. Upon intestinal barrier leakiness, the whole organism is exposed to the metabolic by-products of the local microbiota (Fig. 1(3)). Some of these metabolites are important metabolic mediators, including SCFAs, H2S, p-cresol, and trimethylamine [100]. Namely, they display relevant effects in adipocytes. While SCFAs and H2S induce antilipolytic effects and favor lipid storage [102, 103], 4-cresol and secondary bile acids induce lipolysis [104]. Nonetheless, other microbiota-derived factors, can trigger harmful events in extra-intestinal organs. A well-known example is LPS, responsible for metabolic endotoxemia, giving rise to chronic low-grade inflammation, characteristic of obesity and metabolic syndrome [21]. By activating the TLR4/MD-2 complex in target organs, and after cluster of differentiation 14 (CD14)-dependent internalization, LPS-mediated signaling triggers the production of several pro-inflammatory cytokines, similar to its role in mediating gut inflammation [105]. The primary site of action of LPS is suspected to be the liver, through the portal vein circulation [106]. In fact, hepatocytes and Kupffer cells highly express CD14 [107]. LPS migration through portal circulation is mainly mediated by high-density lipoprotein (HDL), specifically HDL3, which paradoxically plays a protective role against LPS-mediated liver injury [106]. Still to uncover is how HDL3 suppresses LPS bioactivity, possibly by inducing its clearance, and if and how this mechanism is impaired in obesity, which is usually associated with alterations in HDL cholesterol levels and properties. Indeed, a recent study reported increased HDL3 levels in patients with obesity, but LPS levels were not simultaneously assayed [108]. Circulating LPS has been suggested as a biomarker of MAFLD [109]. Indeed, TLR4 activation is required for fructose-induced experimental MAFLD in mice, highlighting the role of diet-driven dysbiosis as a fuel for metabolic diseases [110]. Similarly, the secondary BA deoxycholic acid can cause liver injury, by activation of the inflammasome [111].
Circulating LPS eventually reaches other peripheral organs besides the liver, such as AT. The relevance of LPS signaling on AT emerged from the early studies revealing increasing adiposity as one of the main outcomes of metabolic endotoxemia [23]. In fact, human AT macrophages highly express the TLR4/CD14 machinery, and so do adipocytes, although to a lower extent [112]. LPS can modulate several AT events—inflammation, matrix remodeling/plasticity, metabolic activity—possibly being involved in tissue dysfunction (Fig. 1(3)). Subcutaneous AT from patients with obesity displayed a marked increase in the LPS-signaling machinery and LPS-treated adipocytes initiated an immunogenic response, relying on NF-ĸB and the inflammasome [113]. Accordingly, NLRP3 silencing prevented LPS-induced inflammation in human visceral adipocytes [114]. Vatier et al. reported higher sensitivity of subcutaneous, rather than visceral adipocytes, to the deleterious effects of LPS, with lower amount of LPS triggering a more powerful immune response in abdominal subcutaneous AT explants [115]. Metabolic endotoxemia impairs the AT matrix remodeling by inducing fibrosis both in vivo and ex vivo, through a transforming growth factor beta 1 (TGFβ1)-dependent mechanism [101]. LPS is also able to directly stimulate the lipolytic activity of AT, inducing hormone-sensitive lipase phosphorylation (S650), thus increasing serum fatty acids levels [115, 116]. It has also been shown that adiponectin gene expression was downregulated in 3T3-L1 cells after LPS treatment, and so was the adiponectin receptor in muscle cells [117]. Moreover, adiponectin suppressed LPS-induced NF-ĸB activation, and consequent inflammation, in pig subcutaneous adipocytes and 3T3-L1 cells [118]. Given that circulating adiponectin is decreased in subjects with obesity, as young as 5 years of age, and in individuals with the metabolic syndrome [118, 119], this may increase the susceptibility of AT to LPS-mediated deleterious effects. Low-dose of LPS infusion (600 µg kg/day) during 4 weeks in C57BL/6N mice induced insulin resistance, as shown by an aberrant increased insulin secretion without proportional clearance of plasma glucose levels [120]. Co-administration of the anti-inflammatory resveratrol, restored normal insulinogenic index, suggesting inflammation of insulin-dependent organs to be the cause of LPS-mediated impaired glucose homeostasis [120]. However, Stevens et al. demonstrated that an acute low-dose LPS (0.1 µg/kg) had a positive impact in glucose tolerance in C57BL/6N mice, but without increasing glucose uptake [121]. Thus, for further accuracy, it is important to reflect on the definition of high and low LPS dosages. LPS levels in circulation fluctuate along the day (from 1 to 50 pg/mL) [122], increasing after a single high-fat meal, even in lean healthy subjects [123].
Although controversial at the beginning, the number of reports on bacterial DNA and bacteria itself in the blood has been drastically increasing, leaving no doubt as to the existence of the blood microbiome. In healthy individuals, the blood-borne bacteria resemble that of the skin and mouth, substantially different from intestinal microbiota [124]. Remarkably, in patients with obesity and T2DM, the blood microbiota might be more closely related to that of the intestines [125, 126]. In fact, Escherichia–Shigella is increased in plasma and stool samples from patients with T2DM [127], highlighting once again the involvement of gut bacteria and respective metabolites on the pathophysiology of obesity and related metabolic disorders. The idea of blood-resident bacteria, with no association with an ill state or infection, needs further reflection, as it could lead to the discernment between healthy versus unhealthy blood microbiome, and thus, be a useful biomarker aiding the prediction or diagnosis of a specific disease.
More recently, and with the advances in sequencing techniques, the easiness to dig deeper into human physiology from a microbial perspective allowed ground-breaking discoveries. Today, we acknowledge our “microbial-selves”, as referred by Castillo et al., not only because our intestines house more bacteria than our body houses cells, but also, since apparently several organs have a resident microbiota [124, 128]. Major players in the pathophysiology of obesity and metabolic syndrome—the liver, AT depots (mesenteric, omental and subcutaneous), skeletal muscle and the pancreas -, have been described to harbor their own microbiota (reviewed by Massier et al.) [128, 129]. The “tissue microbiota hypothesis”, as first raised by Burcelin et al. postulates that gut-derived bacteria colonize extra-intestinal tissues, modulating their function and causing the onset of metabolic diseases [130]. The first evidence arrived from pre-clinical studies of Rémy Burcelin and co-workers. HFD-induced obese mice showed translocation of gut-derived live bacteria to blood and mesenteric AT [131]. However, studies with human AT samples, were very controverse at the beginning. In 2016, the first study testing the hypothesis of the tissue microbiota in humans, failed to identify endogenous bacterial DNA in subcutaneous and visceral AT [132]. Nonetheless, in the following year, two studies reported successful characterization of bacterial DNA in human AT [133, 134]. Ralstonia spp. (Gram-) was enriched in the mesenteric AT from subjects with obesity. Despite lacking appropriate control conditions, the occurrence of contamination may be discarded, as subcutaneous and omental AT did not yield 16S rRNA amplicons determined by pyrosequencing [134]. Pedicino et al. also showed the presence of bacterial DNA in epicardial AT from patients with acute coronary syndrome, but not in control subjects, which was positively associated with inflammasome activation [133]. More recently, in 2020, studies employing the Illumina sequencing technology have confirmed the hypothesis of AT being colonized with microbiota [127, 135]. Being aware of possible sample contamination as a confounding factor, Anhê et al. designed an elegant study, considering a set of controls, from the operating room to sample manipulation in the laboratory [127]. Successful 16S rRNA quantification and sequencing, in different AT depots, revealed higher bacterial DNA deposition in the omental AT. However, the mesenteric AT bacterial signature presented the closest resemblance to the gut microbiota, suggesting the gut to be the main source of bacterial DNA to the surrounding AT [127]. Interestingly, the mesenteric AT microbiota, was substantially different between individuals with obesity, with or without T2DM [127], suggesting a close relationship between extra-intestinal bacterial colonization and dysglicemia. More compelling evidence arose from the work of Massier et al. who employed a protocol, enabling to detect live bacteria in human subcutaneous AT of patients with obesity [135]. Findings regarding bacteria compartmentalization and quantity in various AT depots were according to those aforementioned [127]. The bacterial load in omental AT was highly correlated with macrophage infiltration and inflammatory genes, in obese subjects with T2DM. Despite being merely associative, these data advert a possible involvement of the tissue microbiota in the development of local inflammation. Further, HOMA-IR provided good explanatory power on Bray–Curtis dissimilarity analysis, in all fat depots (subcutaneous, omental, and mesenteric), suggesting an interplay between dysglycemia and shifted bacteria signatures in obesity [135].
Nonetheless, some important questions remain to be answered: (1) how to disclose the gut as the main source of extra-intestinal bacterial colonization? As discussed above, in vivo evaluation of intestinal barrier integrity is challenging, especially in humans. Nonetheless, the link between metabolic endotoxemia, obesity and intestinal permeability must be further investigated by employing more reliable techniques and finding new biomarkers of the leaky gut to correlate with in vivo tests, especially since increased intestinal permeability is also a hallmark of other obesity-related metabolic diseases, such as MAFLD and T2DM. (2) When does AT depots colonization takes place? The difficulty to include normal-weighted subjects in these studies has led to uncertainty regarding this matter, since the doubt persists on whether AT colonization exclusively happens during chronic inflammatory diseases, such as obesity and related T2DM. Further, the aforementioned studies only focused on the obesity scenario with or without T2DM. However, one must interpret the metabolic sequelae of obesity as a spectrum, ranging from metabolically healthy obesity (normoglycemia and insulin sensitivity) to the metabolically unhealthy phenotype, comprising the consequent ill-stages of insulin resistance, progressive dysglycemia and established T2DM. Hence, future studies should address the chronological timepoint of AT bacterial colonization and how it responds to the consequent metabolic dysregulation that may follow obesity; (3) what are the implications of AT colonization in tissue function? Doubt remains on what could be the influence of a given bacteria signature on AT function. Existing data focused only on AT inflammation and relied on associative links, derived from correlation analyses, that do not necessarily reflect causality. More studies are needed to unravel the contribution of specific bacteria to the modulation of AT (dys)function. In particular, the reshaping of resident and infiltrating AT immune cells population may arise as a determinant factor for the development of a pro-inflammatory environment that may trigger insulin resistance and dysmetabolism.
Conclusion
Many of the components of the diet (fibers, macro, and micronutrients) are digested by the microbiota and most of the physiological functions of the gut are regulated by the metabolites resulting from such modifications. Westernized diets with a lipid and sugar overload, and potentially also contaminated with residual levels of chemicals and pollutants, induce rapid and marked dysbiosis (alterations in gut microbiota composition and its metabolites), that may be accompanied by gut inflammation and barrier disruption. While the role of Westernized diets in inducing dysbiosis is overall consistent (increasing the Firmicutes/Bacteroidetes ratio) (Table 1), in rodents and humans, the development of gut inflammation is a more complex process to decipher, as there is conflicting evidence regarding a pro-inflammatory response (Table 1). Despite some studies showing increased colonic inflammatory markers upon HF or high-sugar diet consumption in rodents, there is a lack of observation of histological features of inflammation, even when diets were taken to an extreme duration (Table 1) [28]. However, this does not exclude that a chronic low-grade inflammation might be taking place in the colon, resulting from increased levels of pro-inflammatory factors. Nevertheless, studies with germ-free mice have shown the central role of microbiota in Westernized diet-induced gut inflammation [34]. Slight alterations in the microbiota (at family or genus level), possibly attributed to differences in diet composition, might be the explanation for the discrepancy in the inflammatory response.
The gut microbiota exerts a modulatory effect on vagal tone and the production of GLP-1, PYY, serotonin, mainly through SCFAs, having a major impact in gut-brain communication (Fig. 1(2)). Diet-induced dysbiosis was shown to hamper gut hormones production and to cause vagal withdrawal in rodents, although their consequences in the secretion of gut neuroendocrine factors are still unknown [31, 73, 75]. However, it is necessary to depict the involved triggering agents, from specific bacteria to inflammatory markers, as this investigation is still in its first steps. Thus, there is still a long way to understand the relation between gut microbiota and impaired energy balance, at central and peripheral levels.
Westernized diets consumption and metabolic diseases, such as obesity, MAFLD and T2DM, are associated with the development of chronic low-grade inflammation [17, 136]. Intriguingly, this feature is also present in patients with T2DM without obesity and some patients with obesity do not have increased markers of systemic inflammation, raising questions about the mechanisms linking metabolically (un)healthy obesity with gut dysbiosis/endotoxemia. The diet-induced modulation on gut microbiota is associated with increased intestinal permeability and endotoxemia (Table 1), which can be the initial trigger for low-grade inflammation by activating TLR4 in the liver and adipose tissue (Fig. 1(3)). Moreover, the extravasation of bacteria itself to the blood and tissues is arising as a putative modulator of inflammatory pathways. Despite their role in the establishment of low-grade inflammation is also still to unravel, this highlights the importance of diet manipulation as an important factor not only to ameliorate gut dysbiosis, but also in regulating pathogenic bacteria and metabolites translocation to the adipose tissue.
Acknowledgements
The image was created with Biorender.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by the Portuguese Foundation of Science and Technology (Strategic Projects UID/NEU/04539/2013, UID/NEU/04539/2019, UIDB/04539/2020, UIDP/04539/2020 and LA/P/0058/2020) D.R.S was supported by Ph.D. Grant from the Portuguese Foundation for Science and Technology (2021.08160.BD).
Data availability
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Human and animal rights statement and Informed consent
The article is a review analysis. Human Participants and/or Animals have not been involved in the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farré R, Fiorani M, Abdu Rahiman S, Matteoli G. Intestinal permeability, inflammation and the role of nutrients. Nutrients. 2020;12:1185. doi: 10.3390/nu12041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 3.Yi C-X, Tschöp MH. Brain–gut–adipose-tissue communication pathways at a glance. Dis Model Mech. 2012;5(5):583–587. doi: 10.1242/dmm.009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC, Johansson ME. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164–177. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portincasa P, Bonfrate L, Khalil M, Angelis MD, Calabrese FM, D’Amato M, Wang DQ-H, Di Ciaula A. Intestinal barrier and permeability in health obesity NAFLD. Biomedicines. 2021;10:83. doi: 10.3390/biomedicines10010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, Kang X, Yang H, Liu H, Yang X, Liu Q, Tian H, Xue Y, Ren P, Kuang X, Cai Y, Tong M, Li L, Fan W. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol Res. 2022;175:106020. doi: 10.1016/j.phrs.2021.106020. [DOI] [PubMed] [Google Scholar]
- 9.Rehman AU, Siddiqui NZ, Farooqui NA, Alam G, Gul A, Ahmad B, Asim M, Khan AI, Xin Y, Zexu W, Song Ju H, Xin W, Lei S, Wang L. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front Nutr. 2022;9:984695. doi: 10.3389/fnut.2022.984695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie F, Xu H-F, Zhang J, Liu X-N, Kou B-X, Cai M-Y, Wu J, Dong J-L, Meng Q-H, Wang Y, Chen D, Zhang Y. Dysregulated hepatic lipid metabolism and gut microbiota associated with early-stage NAFLD in ASPP2-deficiency mice. Front Immunol. 2022;13:974872. doi: 10.3389/fimmu.2022.974872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Round JL, Mazmanian SK. The gut microbiome shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Y, Ahmed S, Yao W, You L, Zheng J, Hileuskaya K. Regulation effects of indigestible dietary polysaccharides on intestinal microflora: an overview. J Food Biochem. 2021;45:e13564. doi: 10.1111/jfbc.13564. [DOI] [PubMed] [Google Scholar]
- 13.Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal barrier in human health and disease. Int J Environ Res Public Health. 2021;18:12836. doi: 10.3390/ijerph182312836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller M, Hernández MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, van Eijk H, Canfora EE, Blaak EE. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. 2019;9:12515. doi: 10.1038/s41598-019-48775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74:2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buttó LF, Haller D. Dysbiosis in intestinal inflammation: cause or consequence. Int J Med Microbiol. 2016;306:302–309. doi: 10.1016/j.ijmm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-fat, western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10:3164. doi: 10.3390/cells10113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009 doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 22.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 23.Garidou L, Pomié C, Klopp P, Waget A, Charpentier J, Aloulou M, Giry A, Serino M, Stenman L, Lahtinen S, Dray C, Iacovoni JS, Courtney M, Collet X, Amar J, Servant F, Lelouvier B, Valet P, Eberl G, Fazilleau N, Douin-Echinard V, Heymes C, Burcelin R. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 2015;22:100–112. doi: 10.1016/j.cmet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K-A, Gu W, Lee I-A, Joh E-H, Kim D-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7(10):e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam YY, Ha CWY, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heisel T, Montassier E, Johnson A, Al-Ghalith G, Lin Y-W, Wei L-N, Knights D, Gale CA. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. Msphere. 2017;2:e00351–e417. doi: 10.1128/mSphere.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velázquez KT, Enos RT, Bader JE, Sougiannis AT, Carson MS, Chatzistamou I, Carson JA, Nagarkatti PS, Nagarkatti M, Murphy EA. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J Hepatol. 2019;11:619–637. doi: 10.4254/wjh.v11.i8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do MH, Lee E, Oh M-J, Kim Y, Park H-Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. 2018;10:761. doi: 10.3390/nu10060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H, An Y, Hao F, Wang Y, Tang H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci Rep. 2016;6:21618. doi: 10.1038/srep21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen T, Cawthon CR, Ihde BT, Hajnal A, DiLorenzo PM, de La Serre CB, Czaja K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol Behav. 2017;173:305–317. doi: 10.1016/j.physbeh.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 34.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbara G, Barbaro MR, Fuschi D, Palombo M, Falangone F, Cremon C, Marasco G, Stanghellini V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. 2021;8:718356. doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AMF, Costanzo A, Gareau MG, Armando AM, Quehenberger O, Jameson JM, Olefsky JM. High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS ONE. 2015;10:e0122195. doi: 10.1371/journal.pone.0122195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuipers EN, Kantae V, Maarse BCE, van den Berg SM, van Eenige R, Nahon KJ, Reifel-Miller A, Coskun T, de Winther MPJ, Lutgens E, Kooijman S, Harms AC, Hankemeier T, van der Stelt M, Rensen PCN, Boon MR. High fat diet increases circulating endocannabinoids accompanied by increased synthesis enzymes in adipose tissue. Front Physiol. 2018;9:1913. doi: 10.3389/fphys.2018.01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuddihey H, MacNaughton WK, Sharkey KA. Role of the endocannabinoid system in the regulation of intestinal homeostasis. Cell Mol Gastroenterol Hepatol. 2022 doi: 10.1016/j.jcmgh.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J-Y, Gillilland M, Lee AA, Wu X, Zhou S-Y, Owyang C. Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation. JCI Insight. 2022;7:e148309. doi: 10.1172/jci.insight.148309. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Zeng H, Safratowich BD, Cheng W-H, Larson KJ, Briske-Anderson M. Deoxycholic acid modulates cell-junction gene expression and increases intestinal barrier dysfunction. Molecules. 2022;27:723. doi: 10.3390/molecules27030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, Jeon J, Kim M-S, Jee Y-K, Gho YS, Park H-S, Kim Y-K, Ryu SH. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fajstova A, Galanova N, Coufal S, Malkova J, Kostovcik M, Cermakova M, Pelantova H, Kuzma M, Sediva B, Hudcovic T, Hrncir T, Tlaskalova-Hogenova H, Kverka M, Kostovcikova K. Diet rich in simple sugars promotes pro-inflammatory response via gut microbiota alteration and TLR4 signaling. Cells. 2020;9:2701. doi: 10.3390/cells9122701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 44.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, Sabico S, O’Hare JP, Ceriello A, Saravanan P, Kumar S, McTernan PG. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira TFS, Souza NCS, Chiarello PG, Franceschini SCC, Bressan J, Ferreira CL, Maria CGD. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–740. doi: 10.1016/j.clnu.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Gummesson A, Carlsson LMS, Storlien LH, Bäckhed F, Lundin P, Löfgren L, Stenlöf K, Lam YY, Fagerberg B, Carlsson B. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity. 2011;19:2280–2282. doi: 10.1038/oby.2011.251. [DOI] [PubMed] [Google Scholar]
- 48.Brignardello J, Morales P, Diaz E, Romero J, Brunser O, Gotteland M. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010;32:1307–1314. doi: 10.1111/j.1365-2036.2010.04475.x. [DOI] [PubMed] [Google Scholar]
- 49.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medawar E, Haange S-B, Rolle-Kampczyk U, Engelmann B, Dietrich A, Thieleking R, Wiegank C, Fries C, Horstmann A, Villringer A, von Bergen M, Fenske W, Veronica Witte A. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl Psychiatry. 2021;11:500. doi: 10.1038/s41398-021-01620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ott B, Skurk T, Hastreiter L, Lagkouvardos I, Fischer S, Büttner J, Kellerer T, Clavel T, Rychlik M, Haller D, Hauner H. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep. 2017;7:11955. doi: 10.1038/s41598-017-12109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li T, Hui H, Hu C, Ma H, Yang X, Tian J. Multiscale imaging of colitis in mice using confocal laser endomicroscopy, light-sheet fluorescence microscopy, and magnetic resonance imaging. J Biomed Opt. 2019;24:1–8. doi: 10.1117/1.JBO.24.1.016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quero G, Saccomandi P, Kwak J-M, Dallemagne B, Costamagna G, Marescaux J, Mutter D, Diana M. Modular laser-based endoluminal ablation of the gastrointestinal tract: in vivo dose-effect evaluation and predictive numerical model. Surg Endosc. 2019;33:3200–3208. doi: 10.1007/s00464-018-6603-4. [DOI] [PubMed] [Google Scholar]
- 54.Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in prevotella- versus bacteroides-dominated gut microbiota. Sci Rep. 2017;7:2594. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Y, Wang Y, Gao H, Li D, Jiang R, Ge L, Tong C, Xu K. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators Inflamm. 2021 doi: 10.1155/2021/8879227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Guo J, Ji K, Zhang P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci Rep. 2016;6:32953. doi: 10.1038/srep32953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolte LA, Vich Vila A, Imhann F, Collij V, Gacesa R, Peters V, Wijmenga C, Kurilshikov A, Campmans-Kuijpers MJE, Fu J, Dijkstra G, Zhernakova A, Weersma RK. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70:1287–1298. doi: 10.1136/gutjnl-2020-322670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mousavi SE, Delgado-Saborit JM, Adivi A, Pauwels S, Godderis L. Air pollution and endocrine disruptors induce human microbiome imbalances: A systematic review of recent evidence and possible biological mechanisms. Sci Total Environ. 2022;816:151654. doi: 10.1016/j.scitotenv.2021.151654. [DOI] [PubMed] [Google Scholar]
- 59.Jiang P, Yuan G, Jiang B, Zhang J, Wang Y, Lv H, Zhang Z, Wu J, Wu Q, Li L. Effects of microplastics (MPs) and tributyltin (TBT) alone and in combination on bile acids and gut microbiota crosstalk in mice. Ecotoxicol Environ Saf. 2021;220:112345. doi: 10.1016/j.ecoenv.2021.112345. [DOI] [PubMed] [Google Scholar]
- 60.van den Brule S, Rappe M, Ambroise J, Bouzin C, Dessy C, Paquot A, Muccioli GG, Lison D. Diesel exhaust particles alter the profile and function of the gut microbiota upon subchronic oral administration in mice. Part Fibre Toxicol. 2021;18(1):7. doi: 10.1186/s12989-021-00400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurmann F, Gilliland FD, Goran MI. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ Res. 2018;161:472–478. doi: 10.1016/j.envres.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jerrett M, McConnell R, Wolch J, Chang R, Lam C, Dunton G, Gilliland F, Lurmann F, Islam T, Berhane K. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014;13(1):49. doi: 10.1186/1476-069X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monteros MJM, Galdeano CM, Balcells MF, Weill R, De Paula JA, Perdigón G, Cazorla SI. Probiotic lactobacilli as a promising strategy to ameliorate disorders associated with intestinal inflammation induced by a non-steroidal anti-inflammatory drug. Sci Rep. 2021;11:571. doi: 10.1038/s41598-020-80482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motiani KK, Collado MC, Eskelinen J-J, Virtanen KA, Löyttyniemi E, Salminen S, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise training modulates gut microbiota profile and improves endotoxemia. Med Sci Sports Exerc. 2020;52:94–104. doi: 10.1249/MSS.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Cheng M, Wang L, Zhang L, Xu D, Cao P, Wang F, Herzog H, Song S, Zhan C. A vagal-NTS neural pathway that stimulates feeding. Curr Biol. 2020;30:3986–3998.e5. doi: 10.1016/j.cub.2020.07.084. [DOI] [PubMed] [Google Scholar]
- 66.Wang PYT, Caspi L, Lam CKL, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TKT. Upper intestinal lipids trigger a gut–brain–liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 67.Rosendo-Silva D, Matafome P. Gut–adipose tissue crosstalk: A bridge to novel therapeutic targets in metabolic syndrome? Obes Rev. 2021;22:e13130. doi: 10.1111/obr.13130. [DOI] [PubMed] [Google Scholar]
- 68.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, Blottiere HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei M, Huang F, Zhao L, Zhang Y, Yang W, Wang S, Li M, Han X, Ge K, Qu C, Rajani C, Xie G, Zheng X, Zhao A, Bian Z, Jia W. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine. 2020;55:102766. doi: 10.1016/j.ebiom.2020.102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9:1202. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 74.Martin AM, Sun EW, Keating DJ. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J Endocrinol. 2020;244:R1–R15. doi: 10.1530/JOE-19-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loper H, Leinen M, Bassoff L, Sample J, Romero-Ortega M, Gustafson KJ, Taylor DM, Schiefer MA. Both high fat and high carbohydrate diets impair vagus nerve signaling of satiety. Sci Rep. 2021;11:10394. doi: 10.1038/s41598-021-89465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Tercé F, Burcelin R. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric no-dependent and gut-brain axis mechanism. Cell Metab. 2017;25:1075–1090.e5. doi: 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Martchenko SE, Martchenko A, Cox BJ, Naismith K, Waller A, Gurges P, Sweeney ME, Philpott DJ, Brubaker PL. Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes. 2020;69:2589–2602. doi: 10.2337/db20-0262. [DOI] [PubMed] [Google Scholar]
- 79.Kim JS, Kirkland RA, Lee SH, Cawthon CR, Rzepka KW, Minaya DM, de Lartigue G, Czaja K, de La Serre CB. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiol Behav. 2020;225:113082. doi: 10.1016/j.physbeh.2020.113082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M, Dong W, Wang S, Yan F, Jiang K, Wang B. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. 2017;7:10322. doi: 10.1038/s41598-017-10835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunt JE, Holst JJ, Jeppesen PB, Kissow H. GLP-1 and intestinal diseases. Biomedicines. 2021;9:383. doi: 10.3390/biomedicines9040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zatorski H, Sałaga M, Fichna J. Role of glucagon-like peptides in inflammatory bowel diseases—current knowledge and future perspectives. Naunyn-Schmiedeberg’s Arch Pharmacol. 2019;392:1321–1330. doi: 10.1007/s00210-019-01698-z. [DOI] [PubMed] [Google Scholar]
- 83.Moore BA, Peffer N, Pirone A, Bassiri A, Sague S, Palmer JM, Johnson DL, Nesspor T, Kliwinski C, Hornby PJ. GLP-2 receptor agonism ameliorates inflammation and gastrointestinal stasis in murine postoperative ileus. J Pharmacol Exp Ther. 2010;333:574–583. doi: 10.1124/jpet.109.161497. [DOI] [PubMed] [Google Scholar]
- 84.Teduglutide Study Group. Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2010;16:962–973. doi: 10.1002/ibd.21117. [DOI] [PubMed] [Google Scholar]
- 85.Villumsen M, Schelde AB, Jimenez-Solem E, Jess T, Allin KH. GLP-1 based therapies and disease course of inflammatory bowel disease. eClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Salhy M, Hatlebakk JG, Hausken T. Possible role of peptide YY (PYY) in the pathophysiology of irritable bowel syndrome (IBS) Neuropeptides. 2020;79:101973. doi: 10.1016/j.npep.2019.101973. [DOI] [PubMed] [Google Scholar]
- 87.El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications (review) Int J Mol Med. 2009;24:727–732. doi: 10.3892/ijmm_00000285. [DOI] [PubMed] [Google Scholar]
- 88.Mikami Y, Tsunoda J, Kiyohara H, Taniki N, Teratani T, Kanai T. Vagus nerve-mediated intestinal immune regulation: therapeutic implications of inflammatory bowel diseases. Int Immunol. 2022;34:97–106. doi: 10.1093/intimm/dxab039. [DOI] [PubMed] [Google Scholar]
- 89.Liu B, Wanders A, Wirdefeldt K, Sjölander A, Sachs MC, Eberhardson M, Ye W, Ekbom A, Olén O, Ludvigsson JF. Vagotomy and subsequent risk of inflammatory bowel disease: a nationwide register-based matched cohort study. Aliment Pharmacol Ther. 2020;51:1022–1030. doi: 10.1111/apt.15715. [DOI] [PubMed] [Google Scholar]
- 90.Chandrasekharan B, Boyer D, Owens JA, Wolfarth AA, Saeedi BJ, Dhere T, Iskandar H, Neish AS. Intracolonic neuropeptide Y Y1 receptor inhibition attenuates intestinal inflammation in murine colitis and cytokine release in IBD biopsies. Inflamm Bowel Dis. 2022;28:502–513. doi: 10.1093/ibd/izab243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 92.Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS ONE. 2008;3:e3304. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruiz HH, Becker S, Bai Y, Cortes-Burgos LA, Eckersdorff MM, Macdonald LE, Croll SD. Pharmacological inhibition of NPY receptors illustrates dissociable features of experimental colitis in the mouse DSS model: implications for preclinical evaluation of efficacy in an inflammatory bowel disease model. PLoS ONE. 2019;14:e0220156. doi: 10.1371/journal.pone.0220156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghia J-E, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Côté F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 95.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32–37. doi: 10.1017/S0007114507691648. [DOI] [PubMed] [Google Scholar]
- 97.Rodrigues T, Borges P, Mar L, Marques D, Albano M, Eickhoff H, Carrêlo C, Almeida B, Pires S, Abrantes M, Martins B, Uriarte C, Botelho F, Gomes P, Silva S, Seiça R, Matafome P. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharmacol Res. 2020;161:105198. doi: 10.1016/j.phrs.2020.105198. [DOI] [PubMed] [Google Scholar]
- 98.Croci S, D’Apolito LI, Gasperi V, Catani MV, Savini I. Dietary strategies for management of metabolic syndrome: role of gut microbiota metabolites. Nutrients. 2021;13:1389. doi: 10.3390/nu13051389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molinaro A, Bel Lassen P, Henricsson M, Wu H, Adriouch S, Belda E, Chakaroun R, Nielsen T, Bergh P-O, Rouault C, André S, Marquet F, Andreelli F, Salem J-E, Assmann K, Bastard J-P, Forslund S, Le Chatelier E, Falony G, Pons N, Prifti E, Quinquis B, Roume H, Vieira-Silva S, Hansen TH, Pedersen HK, Lewinter C, Sønderskov NB, Køber L, Vestergaard H, Hansen T, Zucker J-D, Galan P, Dumas M-E, Raes J, Oppert J-M, Letunic I, Nielsen J, Bork P, Ehrlich SD, Stumvoll M, Pedersen O, Aron-Wisneswky J, Clément K, Bäckhed F. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. 2020;11:5881. doi: 10.1038/s41467-020-19589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomasova L, Grman M, Ondrias K, Ufnal M. The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health. Nutr Metab. 2021;18:72. doi: 10.1186/s12986-021-00598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vila IK, Badin P-M, Marques M-A, Monbrun L, Lefort C, Mir L, Louche K, Bourlier V, Roussel B, Gui P, Grober J, Štich V, Rossmeislová L, Zakaroff-Girard A, Bouloumié A, Viguerie N, Moro C, Tavernier G, Langin D. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7:1116–1129. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 102.Cai J, Shi X, Wang H, Fan J, Feng Y, Lin X, Yang J, Cui Q, Tang C, Xu G, Geng B. Cystathionine γ lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim Biophys Acta. 2016;1861:419–429. doi: 10.1016/j.bbalip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Jocken JWE, González Hernández MA, Hoebers NTH, van der Beek CM, Essers YPG, Blaak EE, Canfora EE. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front Endocrinol (Lausanne) 2017;8:372. doi: 10.3389/fendo.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brial F, Alzaid F, Sonomura K, Kamatani Y, Meneyrol K, Le Lay A, Péan N, Hedjazi L, Sato T-A, Venteclef N, Magnan C, Lathrop M, Dumas M-E, Matsuda F, Zalloua P, Gauguier D. The natural metabolite 4-cresol improves glucose homeostasis and enhances β-cell function. Cell Rep. 2020;30:2306–2320.e5. doi: 10.1016/j.celrep.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 105.Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78:1233–1261. doi: 10.1007/s00018-020-03656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han Y-H, Onufer EJ, Huang L-H, Sprung RW, Davidson WS, Czepielewski RS, Wohltmann M, Sorci-Thomas MG, Warner BW, Randolph GJ. Enterically derived high-density lipoprotein restrains liver injury via the portal vein. Science. 2021;373:6729. doi: 10.1126/science.abe6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su GL, Dorko K, Strom SC, Nüssler AK, Wang SC. CD14 expression and production by human hepatocytes. J Hepatol. 1999;31:435–442. doi: 10.1016/S0168-8278(99)80034-8. [DOI] [PubMed] [Google Scholar]
- 108.Stadler JT, Lackner S, Mörkl S, Trakaki A, Scharnagl H, Borenich A, Wonisch W, Mangge H, Zelzer S, Meier-Allard N, Holasek SJ, Marsche G. Obesity affects HDL metabolism composition and subclass distribution. Biomedicines. 2021;9:242. doi: 10.3390/biomedicines9030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soppert J, Brandt EF, Heussen NM, Barzakova E, Blank LM, Kuepfer L, Hornef MW, Trebicka J, Jankowski J, Berres M-L, Noels H. Blood endotoxin levels as biomarker of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;S1542–3565(22):01110–1117. doi: 10.1016/j.cgh.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 110.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 111.Holtmann TM, Inzaugarat ME, Knorr J, Geisler L, Schulz M, Bieghs V, Frissen M, Feldstein AE, Tacke F, Trautwein C, Wree A. Bile acids activate NLRP3 inflammasome, promoting murine liver inflammation or fibrosis in a cell type-specific manner. Cells. 2021;10:2618. doi: 10.3390/cells10102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Single cell type CD14–The human protein atlas. https://www.proteinatlas.org/ENSG00000170458-CD14/single+cell+type, Accessed in 28th Aug 2022
- 113.Creely SJ, McTernan PG, Kusminski CM, Fisher FM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol-Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 114.Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, Frühbeck G, Catalán V. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol Immunol. 2021;18:1045–1057. doi: 10.1038/s41423-019-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vatier C, Kadiri S, Muscat A, Chapron C, Capeau J, Antoine B. Visceral and subcutaneous adipose tissue from lean women respond differently to lipopolysaccharide-induced alteration of inflammation and glyceroneogenesis. Nutr Diabetes. 2012;2:e51–e51. doi: 10.1038/nutd.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rittig N, Bach E, Thomsen HH, Pedersen SB, Nielsen TS, Jørgensen JO, Jessen N, Møller N. Regulation of lipolysis and adipose tissue signaling during acute endotoxin-induced inflammation: a human randomized crossover trial. PLoS ONE. 2016;11:0162167. doi: 10.1371/journal.pone.0162167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hall A, Leuwer M, Trayhurn P, Welters ID. Lipopolysaccharide induces a downregulation of adiponectin receptors in-vitro and in-vivo. PeerJ. 2015;3:e1428. doi: 10.7717/peerj.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol-Regul Integr Comp Physiol. 2005;288:R1220–R1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 119.Barbosa P, Melnyk S, Bennuri SC, Delhey L, Reis A, Moura GR, Børsheim E, Rose S, Carvalho E. Redox imbalance and methylation disturbances in early childhood obesity. Oxid Med Cell Longev. 2021 doi: 10.1155/2021/2207125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nøhr MK, Dudele A, Poulsen MM, Ebbesen LH, Radko Y, Christensen LP, Jessen N, Richelsen B, Lund S, Pedersen SB. LPS-enhanced glucose-stimulated insulin secretion is normalized by resveratrol. PLoS ONE. 2016;11:e0146840. doi: 10.1371/journal.pone.0146840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stevens JR, McMillan RP, Resendes JT, Lloyd SK, Ali MM, Frisard MI, Hargett S, Keller SR, Hulver MW. Acute low-dose endotoxin treatment results in improved whole-body glucose homeostasis in mice. Metabolism. 2017;68:150–162. doi: 10.1016/j.metabol.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation. 2019;16:180. doi: 10.1186/s12974-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 124.Castillo DJ, Rifkin RF, Cowan DA, Potgieter M. The healthy human blood microbiome: fact or fiction? Front Cell Infect Microbiol. 2019;9:148. doi: 10.3389/fcimb.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, Lepage P, Klopp C, Mariette J, Bouchez O, Perez L, Courtney M, Marre M, Klopp P, Lantieri O, Doré J, Charles MA, Balkau B, Burcelin R. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54:3055–3061. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- 126.Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, Komiya K, Kawaguchi M, Shimizu T, Ogihara T, Tamura Y, Sakurai Y, Yamamoto R, Mita T, Fujitani Y, Fukuda H, Nomoto K, Takahashi T, Asahara T, Hirose T, Nagata S, Yamashiro Y, Watada H. Gut Dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37:2343–2350. doi: 10.2337/dc13-2817. [DOI] [PubMed] [Google Scholar]
- 127.Anhê FF, Jensen BAH, Varin TV, Servant F, Van Blerk S, Richard D, Marceau S, Surette M, Biertho L, Lelouvier B, Schertzer JD, Tchernof A, Marette A. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat Metab. 2020;2:233–242. doi: 10.1038/s42255-020-0178-9. [DOI] [PubMed] [Google Scholar]
- 128.Massier L, Blüher M, Kovacs P, Chakaroun RM. Impaired intestinal barrier and tissue bacteria: pathomechanisms for metabolic diseases. Front Endocrinol. 2021 doi: 10.3389/fendo.2021.616506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17:53–64. doi: 10.1038/s41575-019-0242-7. [DOI] [PubMed] [Google Scholar]
- 130.Burcelin R, Serino M, Chabo C, Garidou L, Pomié C, Courtney M, Amar J, Bouloumié A. Metagenome and metabolism: the tissue microbiota hypothesis. Diabetes Obes Metab. 2013;15:61–70. doi: 10.1111/dom.12157. [DOI] [PubMed] [Google Scholar]
- 131.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zulian A, Cancello R, Cesana E, Rizzi E, Consolandi C, Severgnini M, Panizzo V, Di Blasio AM, Micheletto G, Invitti C. Adipose tissue microbiota in humans: an open issue. Int J Obes (Lond) 2016;40:1643–1648. doi: 10.1038/ijo.2016.111. [DOI] [PubMed] [Google Scholar]
- 133.Pedicino D, Severino A, Ucci S, Bugli F, Flego D, Giglio AF, Trotta F, Ruggio A, Lucci C, Iaconelli A, Paroni Sterbini F, Biasucci LM, Sanguinetti M, Glieca F, Luciani N, Massetti M, Crea F, Liuzzo G. Epicardial adipose tissue microbial colonization and inflammasome activation in acute coronary syndrome. Int J Cardiol. 2017;236:95–99. doi: 10.1016/j.ijcard.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 134.Udayappan SD, Kovatcheva-Datchary P, Bakker GJ, Havik SR, Herrema H, Cani PD, Bouter KE, Belzer C, Witjes JJ, Vrieze A, de Sonnaville N, Chaplin A, van Raalte DH, Aalvink S, Dallinga-Thie GM, Heilig HGHJ, Bergström G, van der Meij S, van Wagensveld BA, Hoekstra JBL, Holleman F, Stroes ESG, Groen AK, Bäckhed F, de Vos WM, Nieuwdorp M. Intestinal Ralstonia pickettii augments glucose intolerance in obesity. PLoS ONE. 2017;12:e0181693. doi: 10.1371/journal.pone.0181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Massier L, Chakaroun R, Tabei S, Crane A, Didt KD, Fallmann J, von Bergen M, Haange S-B, Heyne H, Stumvoll M, Gericke M, Dietrich A, Blüher M, Musat N, Kovacs P. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut. 2020;69:1796–1806. doi: 10.1136/gutjnl-2019-320118. [DOI] [PubMed] [Google Scholar]
- 136.Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, George J. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.