Abstract
Hepatocellular carcinoma (HCC) being a leading cause of cancer-related death, has high associated mortality and recurrence rates. It has been of great necessity and urgency to find effective HCC diagnosis and treatment measures. Studies have shown that microvascular invasion (MVI) is an independent risk factor for poor prognosis after hepatectomy. The abnormal expression of biomacromolecules such as circ-RNAs, lncRNAs, STIP1, and PD-L1 in HCC patients is strongly correlated with MVI. Deregulation of several markers mentioned in this review affects the proliferation, invasion, metastasis, EMT, and anti-apoptotic processes of HCC cells through multiple complex mechanisms. Therefore, these biomarkers may have an important clinical role and serve as promising interventional targets for HCC. In this review, we provide a comprehensive overview on the functions and regulatory mechanisms of MVI-related biomarkers in HCC.
Keywords: Hepatocellular carcinoma, Microvascular invasion, Biomarkers, Progress, Molecular mechanism
Graphical abstract
Introduction
Primary liver cancer is a common malignant tumor of the digestive system, and its morbidity and mortality rank sixth and third in the world. According to the classification of pathological types, primary liver cancer can be divided into hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and HCC-ICC mixed type, of which HCC accounts for 85–90%. In this article, liver cancer refers specifically to HCC.1 At present, radical resection of liver cancer (R0 resection) is the main treatment method for HCC. Advances in imaging procedures such as three-dimensional reconstruction, indocyanine green fluorescence fusion image guidance and other technologies led to better prognosis and treatment modalities. Nevertheless, high mortality and postoperative recurrence rate remain unsatisfactory.
Microvascular invasion (MVI) refers to the invasion of cancer cells into vascular spaces lined by endothelial cells and can only be observed by microscopy. MVI is graded based on the number and invasion sites to determine tumor stage, prognosis and provide basis for treatment selections: M0 is no MVI found; M1 (low-risk group) is ≤5 MVI and occurs in adjacent liver tissue that is <1 cm away; M2 (high-risk group) is >5 MVI, or MVI that occurs at a distance of >1 cm from the adjacent liver tissue.2 The presence of MVI is strongly associated with aggressive tumor behavior and distant metastasis.3,4 It is also a key prognostic factor for mortality and HCC recurrence. Studies have shown that recurrence rate in MVI-positive patients doubles that of MVI-negative patients.5 Moreover, it has been suggested that MVI is an important pathological hallmark in the process of intrahepatic metastasis of HCC, hence future studies should further explore the mechanisms of MVI-induced HCC development.6,7
To date, a large body of literature has suggested the upregulation of several biomarkers in the presence of MVI. Xu et al.8 found that the upregulation of circular RNA ciRS-7 (ciRS-7) in HCC tissue is an independent risk factor for liver MVI. Lu et al.9 found that by high-throughput sequencing, patients with significantly up-regulated expression of long noncoding RNA TSPAN12 (lncTSPAN12) showed a higher MVI positive rate (p=0.022). Ma et al.10 found that patients with high stress-induced phosphoprotein 1 (STIP1) expression had a poor prognosis, and that the expression of STIP1 was strongly correlated with MVI (p<0.001). Calderaro et al.11 found that high expression of PD-L1 in HCC was strongly associated with MVI (p<0.001). Nevertheless, previous studies have not been able to draw relationships between candidate biomarkers of HCC and MVI, and herein we will review the current understanding of these biomarkers and their association with MVI (Tables 1 and 29,12–46).
Table 1. Properties and expression of markers.
| Markers | Structure | Gene location | Detected position | Recipient cells | Expression level |
|---|---|---|---|---|---|
| CiRS-7 | An endogenous circular RNA with a closed loop structure. | Xq27.1 | HCC | HCC | High |
| CircAKT3 | An endogenous circular RNA with a closed loop structure. Full length: 524 nt. | 1q43-q44 | Serum exosomes | HCC | High |
| Lnc-TSPAN12 | A long noncoding RNA with a full length sequence of 1 577 bp. | 7q31.31 | HCC | HCC | High |
| LncRNA MVIH | A long noncoding RNA. | 10q22.3 | HCC | HCC | High |
| MicroRNA-188-5p | A noncoding RNA molecule of 21 nucleotides in length. | Xp11.23 | HCC | HCC | Low |
| MicroRNA-125b | A noncoding RNA molecule of 22 nucleotides in length. | 11q23-24 | Serum | HCC | Low |
| STIP1 | A protein with a length of 543 AA. Mass (Da)62,582. | 11q13.1 | HCC Serum | HCC | High |
| PD-L1 | A protein with a length of 290 AA. Mass (Da)33,275. | 9p24.1 | HCC | T cell | High |
| USP7 | A protein with a length of 1,129 AA. Mass (Da)130,446. | 16p13.2 | HCC | HCC | High |
| FN1 | A protein with a length of 2,477 AA. Mass (Da)272,320. | 2q35 | HCC | HCC | High |
| BOP1 | A protein with a length of 746 AA. Mass (Da)83,630. | 8q24.3 | HCC | HCC | High |
| MAGL | A protein with a length of 303 AA. Mass (Da)33,261. | 3q21.3 | HCC | HCC | High |
| MTDH | A protein with a length of 582 AA. Mass (Da)63,837. | 8q22.1 | HCC | HCC | High |
| STMN1 | A protein with a length of 149 AA. Mass (Da)17,303. | 1p36.11 | HCC | HCC | High |
| PIVKA-II | A protein with a molecular weight of 720 kDa. | – | Serum HCC | HCC | High |
| DDR1 | A protein with a length of 913 AA. Mass (Da)101,127. | 6p21.33 | Serum HCC | HCC | High |
| VEGF-A | A protein with a length of 395 AA. Mass (Da)43,597. | 6p21.1 | Serum | HCC | High |
| S100P | A protein with a length of 95 AA. Mass (Da)10,399. | 4p16.1 | Serum | HCC | High |
BOP1, block of proliferation 1; FN1, fibronectin 1; HCC, hepatocellular carcinoma; LncRNA MVIH, lncRNA associated with microvascular invasion; MAGL, monoacylglycerol lipase; MTDH, metadherin; PD-L1, programmed cell death-ligand 1; PIVKA-II, prothrombin induced by vitamin K absence II; STIP1, stress-induced phosphoprotein 1; STMN1, stathmin 1; USP7, ubiquitin-specific protease 7.
Table 2. Mechanisms and functions of markers.
| Markers | Mechanism | Functions or behaviors | References |
|---|---|---|---|
| CiRS-7 | Upregulate the expression of target genes CCNE1 and PIK3CD by sponge miR-7. | Promote the proliferation and invasion of HCC. | 12 |
| CircAKT3 | Circ-AKT3/microRNA-335 signaling pathway. | Promote the proliferation and migration of HCC. | 13 |
| Lnc-TSPAN12 | – | Promote the migration and invasion of HCC cells. | 9 |
| LncRNA MVIH | LncRNA MVIH/PGK1 signaling pathway; ARID1A gene regulate CDKN1A transcription by inhibiting lncRNA MVIH. | Increase tumor microvessel density; Promote the proliferation and migration of HCC. Inhibit HCC apoptosis. | 14,15 |
| MicroRNA-188-5p | MicroRNA-188-5p/FGF5/H-Ras/p-ERK signaling pathway; LncRNA PAPAS/MicroRNA-188-5p signaling pathway; LncRNA CASC11/MicroRNA-188-5p signaling pathway. | Inhibit HCC proliferation and metastasis. | 16–18 |
| MicroRNA-125b | MiR-125b inhibits the development of HCC by negatively regulating SUV39H1; Reduce the invasion ability of HCC by downregulating the expression of MMP-2 and -9; Inhibit the EMT process of HCC by targeting SMAD 2 and 4. | Inhibit HCC migration, Promote HCC apoptosis. | 19–23 |
| STIP1 | Promote the progression of EMT by activating Snail transcription; STIP1/PI3K/AKT signaling pathway; STIP1 activates β-catenin/TCF signaling by enhancing the interaction between axin and DVL2. | Promote HCC growth, colony formation and migration; Inhibit HCC apoptosis. | 24–26 |
| PD-L1 | Induce T cell inactivation, depletion, apoptosis, tolerance. | Promote the invasion and metastasis of HCC and accelerate the development of tumor. | 27–30 |
| USP7 | USP7 stabilizes TRIP12 by deubiquitylation, then inactivates p14ARF and promotes HCC progression. | Promote HCC proliferation and enhance tumor cell invasive ability. | 31 |
| FN1 | Oncogene MYC promotes HCC migration and invasion by upregulating FN1 transcription. | Promote HCC invasion and migration. | 32,33 |
| BOP1 | BOP1 enhances HCC invasion and metastasis by inducing EMT and promoting actin cytoskeleton remodeling. | Promote the invasion and metastasis of HCC. | 34 |
| MAGL | MAGL enhanced the activity of Snail by activating the NF-κB signaling pathway, leading to the downregulation of E-cadherin and triggering the EMT process. | Promote HCC proliferation, migration, and invasion. | 35 |
| MTDH | Promote HCC metastasis by inducing EMT process; MTDH/PI3K/AKT signaling pathway | Promote the proliferation, invasion, and migration of HCC. | 36,37 |
| STMN1 | Promote EMT of HCC cells through the signaling of “STMN1-Microtubule-EMT” axis. | Promote HCC cell metastasis. | 38 |
| PIVKA-II | HCC development may be promoted by inducing EMT process. | – | 39 |
| DDR1 | Promote HCC development by inducing EMT; DDR1/PSD4/ARF6 signaling axis. | Promote HCC migration, invasion, and proliferation. | 40,41 |
| VEGF-A | Promote the development of HCC by activating HSC; Promote HCC migration by promoting the phosphorylation of VEGFR2. | Promote HCC migration, invasion and growth. | 42–44 |
| S100P | Mediate HCC cell adhesion through CD44-dependent signal transduction; Promote the mitosis of HCC cell by upregulating the expression of cyclin D1 and CDK2 | Promote HCC proliferation, migration, and invasion. | 45,46 |
AKT, activating protein kinase B; BOP1, block of proliferation 1; EMT, epithelial-mesenchymal transition; FN1, fibronectin 1; FGF5, fibroblast growth factor 5; HCC, hepatocellular carcinoma; LncRNA, long noncoding RNA; LncRNA MVIH, lncRNA associated with microvascular invasion; MAGL, monoacylglycerol lipase; MMP, matrix metalloproteinase; MTDH, metadherin; PD-L1, programmed cell death-ligand 1; PIVKA-II, prothrombin induced by vitamin K absence II; STIP1, stress-induced phosphoprotein 1; STMN1, stathmin 1; USP7, ubiquitin-specific protease 7.
Biomarkers related to MVI
Circular RNAs
CiRS-7/CDr1as
CiRS-7/CDr1 as is an endogenous circular RNA (circ-RNA) with a closed loop structure. Since Hansen et al.47 first demonstrated that ciRS-7 can act as a competing endogenous RNA (ceRNA) in 2013, more studies have attempted to elucidate its function. Yu et al.12 were the first to demonstrate that the expression level of ciRS-7 in HCC cells is higher than that of adjacent nontumor tissues. Moreover, HCC proliferation and invasion were significantly diminished following ciRS-7 knockout. Xu et al.8 found that the expression of ciRS-7 was significantly associated with the clinicopathological including age <40 years, serum alpha-fetoprotein (AFP) ≥400 ng/µL, and the presence of MVI (p=0.03).In terms of prognosis, overexpression of ciRS-7 was strongly associated with poorer overall survival (OS), and to a lesser extent disease-free survival (DFS).48 It should be noted that this less significant correlation between ciRS-7 and DFS may be due to the small number of single-center samples that have been collected, whereas multicenter samples would have yielded a more significant result. In terms of molecular mechanisms, ciRS-7 acts as a ceRNA or the super spongemiR-7 that sequestered and subsequently inhibited the activity of miR-7. Target genes of miR-7 include several important oncogenes (CCNE1 and PIK3CD) in HCC, hence overexpression of ciRS-7 can promote HCC proliferation and invasion by increasing the expression of oncogenes while inhibiting tumor suppressor genes (Fig. 1). In conclusion, ciRS-7 has an oncogenic role and may represent an important biomarker in HCC with MVI.
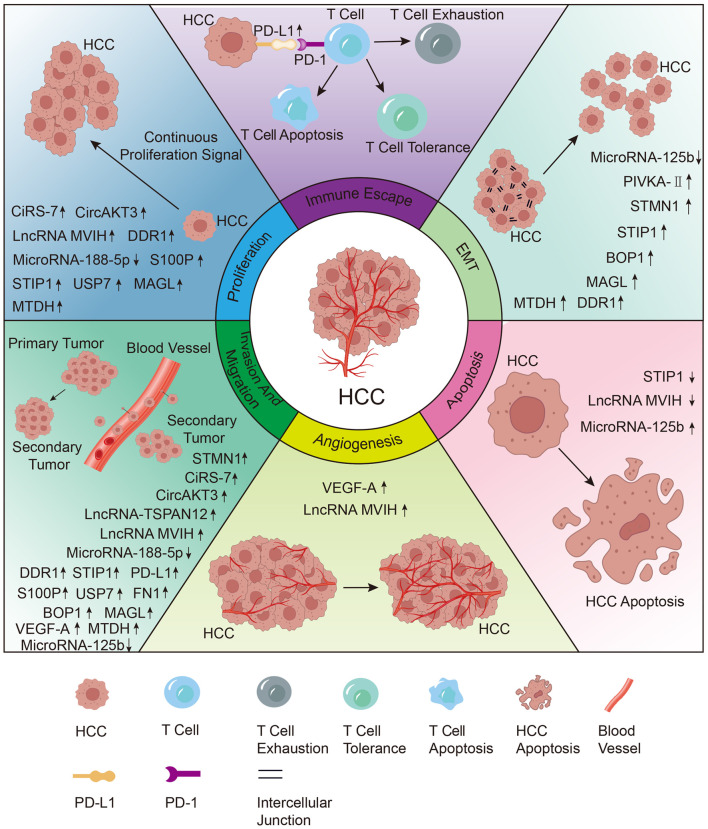
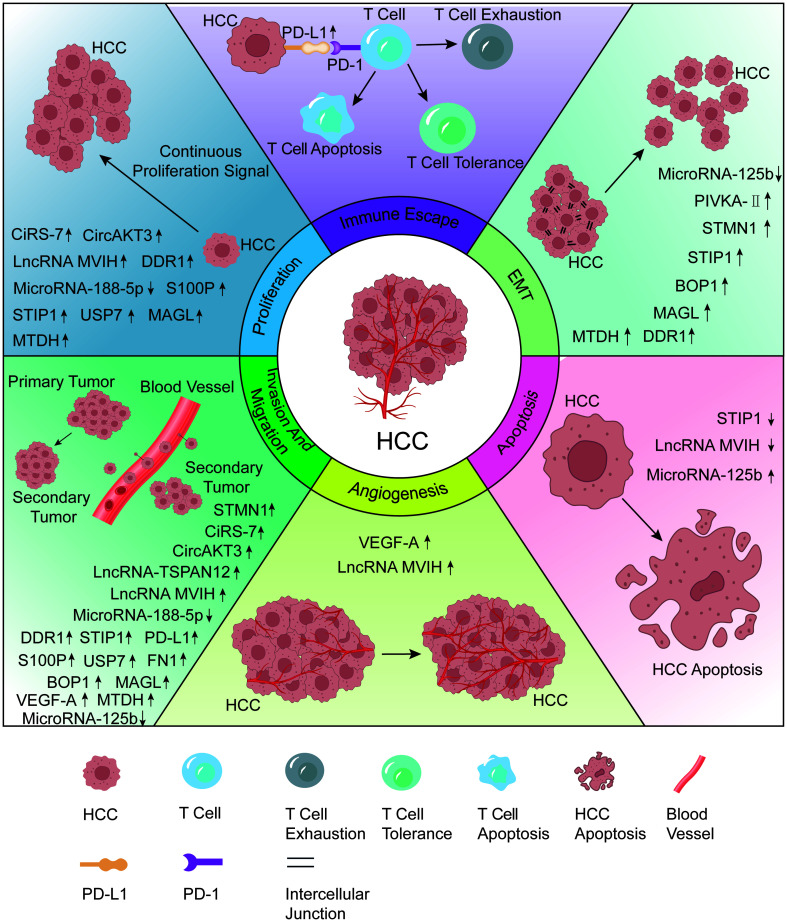
Fig. 1. Effect of biomarkers associated with MVI of HCC and their mechanisms.
Aberrantly expressed biomarkers in HCC with MVI can be involved in the process of cell proliferation and apoptosis, invasion and metastasis, angiogenesis, EMT, immune escape. BOP1, block of proliferation 1; EMT, epithelial-mesenchymal transition; FN1, fibronectin 1; FGF5, fibroblast growth factor 5; HCC, hepatocellular carcinoma; LncRNA MVIH, lncRNA associated with microvascular invasion; MAGL, monoacylglycerol lipase; MTDH, metadherin; MVI, microvascular invasion; PD-L1, programmed cell death-ligand 1; PIVKA-II, prothrombin induced by vitamin K absence II; STIP1, stress-induced phosphoprotein 1; STMN1, stathmin 1; USP7, ubiquitin-specific protease 7.
AKT3 (circAKT3)
CircAKT3 is a circular RNA generated from exons 3–7 of the AKT3 gene by back-splicing of mRNA. Xue et al.49 showed that circAKT3 functions as a tumor suppressor in clear cell renal cell carcinoma (ccRCC). Specifically, circAKT3 acts as a sponge and sequesters miR-296-3p, a microRNA that plays an important role in promoting metastasis of ccRCC cells. Huang et al.50 showed that in gastric cancer, circAKT3 acts as a sponge for the microRNA miR-198, which abolishes its suppressive effect on the target gene PIK3R1 and allows the upregulation of tumor suppressor gene BRCA1. The above studies suggested that the expression of circAKT3 is cancer-specific. Luo et al.51 found that compared with healthy subjects, circAKT3 expression was significantly increased in HCC patients, and was expressed in 65% of HCC patients. Pathologically, circAKT3 level was positively correlated with tumor sizes and MVI (p=0.0145). In terms of prognosis, overexpression of circAKT3 was associated with poorer OS and DFS in patients. In terms of molecular mechanisms, it was found that circAKT3 promotes HCC proliferation and invasion by downregulating the tumor suppressor miR-335. Future studies should continue to investigate the potential involvement of circAKT3 in MVI development, including downstream targets of miR-335 and other proteins involved in the signaling pathways.13 In conclusion, circAKT3 plays an oncogenic role and may represent an important biomarker in HCC with MVI.
Long noncoding RNAs (lncRNAs) that are related to MVI
Lnc-TSPAN12
Lnc-TSPAN12 is a long noncoding RNA that does not encode proteins. It has 1,577 bp and is located on chromosome 7q31.31. Lnc-TSPAN12 was first discovered in the abnormal expression profile of long noncoding RNA in HCC cells. The expression of lnc-TSPAN12 was significantly upregulated in HCC cells compared to adjacent nontumor tissues. Pathologically, lnc-TSPAN12 level was positively correlated with tumor sizes, advanced TNM stages and MVI (p<0.001). In terms of prognosis, overexpression of lnc-TSPAN12 in HCC patients was significantly associated with poorer OS and recurrence-free survival (RFS). Receiver operating characteristic curve analysis showed that lnc-TSPAN12 expression was highly distinguishable between HCC with MVI and adjacent nontumor liver tissues, suggesting that lnc-TSPAN12 could be a promising diagnostic biomarker in HCC with MVI. Moreover, lnc-TSPAN12 knockdown significantly suppressed migration and invasion of HCC cell lines (Huh7) in vitro, highlighting the ability of lnc-TSPAN12 to enhance invasiveness of HCC cells. Future studies should further elucidate the regulatory mechanisms of lnc-TSPAN12 in HCC with MVI. In conclusion, lnc-TSPAN12 plays an oncogenic role and may represent an important biomarker in HCC with MVI.9
LncRNA associated with MVI in HCC (LncRNA MVIH)
LncRNA MVIH is a long noncoding RNA that is highly expressed in HCC cells. It is generated from introns of the ribosomal protein S24 (RPS24) gene located on chromosome 10q22.3.52 Yuan et al.53 found that the expression of lncRNA MVIH was significantly upregulated in HCC cells compared to adjacent nontumor tissues. Pathologically, lncRNA MVIH level was positively correlated with advanced TNM stages and MVI (p=0.016). In terms of prognosis, patients with overexpressed lncRNA MVIH had poorer RFS and OS. In vitro and in vivo studies have shown that overexpression of lncRNA MVIH led to increased proliferation, invasion, and metastasis of HCC cells. The same group also found that tumor cells secrete the enzyme PGK1. PGK1 inhibits angiogenesis which is crucial for tumor growth and metastasis. Surprisingly, lncRNA MVIH modifies the PGK1 level by suppressing its secretion, hence allowing angiogenesis to be activated. Indeed, immunohistochemistry analysis has shown that lncRNA MVIH expression was positively correlated with microvessel density in HCC cells. This again supports the role of lncRNA MVIH in HCC with MVI. Shi et al.14 found that lncRNA MVIH promoted the growth of HCC and inhibited the apoptosis of HCC by inhibiting the expression of miR-199a. Other studies have shown that ARID1A gene acts as a tumor suppressor and prevents cancer development by inhibiting lncRNA MVIH. Although the other downstream targets of ARID1A remain unclear, lncRNA MVIH is nevertheless a potential interventional target in HCC with ARID1A mutation.15 In conclusion, lncRNA MVIH plays an oncogenic role by enhancing angiogenesis and may represent an important biomarker in HCC with MVI.
MicroRNAs (miRNAs) related to MVI
MiR-188-5p
miRNAs are a small noncoding RNA. Owing to high stability and detectability in serum, miRNAs, as noninvasive markers, have played an excellent diagnostic efficacy in the field of cancer.54 miR-188-5p contains 21 nucleotides. Xu et al.16 first found that the expression of miR-188-5p was significantly upregulated in UV-irradiated mouse skin in 2012. Fang et al.17 found that the expression of miR-188-5p in HCC cells was significantly lower than that of adjacent nontumor tissues. In >5 cm HCC, <5 cm HCC, nodular HCC, and venous tumor thrombus tissue, the expression of miR-188-5p decreased progressively, suggesting that it might be involved in the metastasis of HCC. Pathologically, miR-188-5p level was negatively correlated with nodular numbers and MVI (p=0.004), suggesting that miR-188-5p may be a tumor suppressor in HCC progression. In terms of prognosis, HCC patients with downregulated miR-188-5p had poorer OS and DFS. In vitro studies suggested that the downregulation of miR-188-5p led to faster HCC cell proliferation and increased colony numbers. In terms of regulatory mechanisms, it was found that miR-188-5p bound to a sequence within the 3′-UTR of fibroblast growth factor 5 (FGF5) mRNA directly and inhibited HCC cell proliferation and metastasis via FGF5-H-Ras-p-ERK signaling. Ma et al.18 found that lncRNA PAPAS was upregulated in HCC and it promotes cell proliferation by suppressing miR-188-5p. On the other hand, Cheng et al.55 found that lncRNA CASC11 was overexpressed in HCC and promoted cell proliferation by inhibiting miR-188-5p. In conclusion, miR-188-5p has an important tumor suppressive role and may be an important biomarker in HCC with MVI. Future studies should further elucidate the role of miR-188-5p in preventing MVI development.
MiR-125b
MiR-125b is a small noncoding multifunctional RNA molecule located on chromosome 11q23-24. It plays a key role in many cellular processes, such as regulating cell proliferation, differentiation, and apoptosis.56 Accumulating evidence indicates that miR-125b is abnormally expressed in a variety of tumors, and it is downregulated in HCC and plays a tumor suppressor role.57 Liu et al.58 analyzed miR-125b in the serum of 108 patients with HCC before surgery. The results showed that a level of miR-125b in the serum was significantly correlated with MVI. In terms of prognosis, previous studies have found that the expression of miR-125b in serum is significantly correlated with the RFS of patients.59 The molecular mechanisms of miR-125b promoting HCC are as follows. Fan et al.19 found that miR-125b inhibited the occurrence, development, and metastasis of HCC by negatively regulating suppressor of variegation 3-9 homolog 1 (SUV39H1). Alpini et al.20 found that epigenetic silencing of miR-125b can promote the degradation of extracellular matrix by upregulating the expression of matrix metalloproteinase (MMP)-2 and -9, thereby increasing the invasiveness of HCC. Zhou et al.21 found that miR-125b inhibited the EMT process of HCC by targeting small mothers against decapentaplegic (SMAD) 2 and 4, thereby inhibiting the development of HCC. Song et al.22 found that miR-125b induced HCC cell senescence and apoptosis by inhibiting SIRT6. In addition, some studies suggest that ganoderma lucidum polysaccharides inhibits the function and accumulation of Tregs by promoting the expression of miR-125b, and ultimately inhibit the development of HCC.23 In summary, miR-125b has a negative role in the development of HCC with MVI and may become a prognostic biomarker and a clinical therapeutic target in the future.
Proteins that are related to MVI
Stress-induced phosphoprotein 1 (STIP1)
Stress-induced phosphoprotein 1 (STIP1) functions as a co-chaperone of heat shock proteins (HSP) 70 and 90.60 Apart from its role in assisting with protein folding, STIP1 is also involved in various biological processes such as gene transcription, signal transduction, and cell division. Other studies also showed that STIP1 can be secreted by HCC cells and act as a cytokine to promote HCC progression.61,62 Recent studies have shown that STIP1 expression was significantly elevated in HCC cells compared with adjacent nontumor tissues. Pathologically, STIP1 level was positively correlated with tumor size, tumor capsule, and MVI (p<0.001).10,24 In terms of prognosis, overexpression of STIP1 was associated with poorer OS and shorter time to recurrence (TTR). The molecular mechanisms by which STIP1 promotes HCC progression are: (1) promoting metastasis by activating Snail transcription, followed by EMT in an HSP-dependent manner;25 (2) promoting metastasis via the PI3K/AKT signaling pathway, which upregulates HCC proliferation and inhibits HCC apoptosis;26 (3) enhancing the interaction between axin and DVL2, thereby activating β-catenin/TCF signaling, which is crucial for HCC growth, proliferation, and metastasis.24 In conclusion, STIP1 secreted by HCC cells has an oncogenic role and may be a biomarker of HCC with MVI. Future studies should further elucidate the involvement of STIP1 in MVI development.
Programmed cell death-ligand 1 (PD-L1)
PD-L1 is a member of the B7 gene family. It is a ligand for programmed death receptor-1 (PD-1) and is expressed on the surface of cancer cells, dendritic cells (DCs), monocytes, and macrophages. Studies have shown that binding of PD-1 to PD-L1 activates downstream signaling pathway and inhibits T cell activation.63–65 In HCC cells, the expression rate of PD-L1 is between 23.9% and 81.1%.66 Pathologically, PD-L1 level was positively associated with the number of nodules, AFP levels, CK19 levels, macrovascular infiltration and MVI (p<0.001). Less significant correlation was also found between PD-L1 expression on HCC cells and PD-1 expression on lymphocytes. In terms of prognosis, overexpression of PD-L1 on HCC cell surface was significantly associated with poorer OS and DFS.11,67 The molecular mechanisms through which PD-L1 promotes HCC progression are: (1) PD-L1 binding activates PD-1 and transmits inhibitory signals that attenuate TCR and CD28 activity, which results in inhibition of T-cell activation and proliferation, and eventually leads to cancer immune escape.27 (2) Macrophages have an essential role in the tumor immune microenvironment. It was found that interleukin (IL)4 induced macrophage activation that in turn upregulated tumor cell PD-L1 expression, thereby promoting T cell apoptosis.28 (3) Cancer cells hijack inflammatory mechanisms and induce immune cells to secrete interferon-gamma, which then acts on hepatic nonparenchyma cells and induces them to express PD-L1 on their surface.29,30 In conclusion, PD-L1 is a protumorigenic factor and may be an important biomarker in HCC with MVI. Future studies should further explore the involvement of PD-L1 in MVI development.
Ubiquitin-specific protease 7 (USP7)
The ubiquitin-proteasome pathway has a major role in protein degradation to ensure cell homeostasis. Correspondingly, Ubiquitin-specific protease 7 (USP7) belongs to members of the deubiquitinating enzymes families and is involved in disassembling ubiquitin conjugates. In other words, it rescues protein substrates from degradation.68 USP7 is composed of 1,102 amino acids and has a molecular weight of 135 kDa. It is located on chromosome 16p13.3 with a length of 4,013 bp.69 Studies have shown that USP7 mRNA and protein levels were significantly increased in HCC cells compared to adjacent nontumor tissues. Pathologically, USP7 expression was positively correlated with the number of tumor cells, tumor size, AFP level, and MVI (p=0.021). In terms of prognosis, overexpression of USP7 was significantly associated with poorer OS and DFS. In vitro studies also showed that downregulation of USP7 reduced proliferation of MHCC97-H cell lines and upregulation increased proliferation of Huh7 and Hep3B cell lines, suggesting that USP7 promoted HCC progression.31 Wang et al.70 performed wound healing assay and cell invasion assay to show that overexpression of USP7 can enhance invasion of HCC cells, while USP7-knockdown can suppress HCC invasion. In terms of molecular mechanisms, USP7 stabilizes TRIP12 by deubiquitination of the protein. USP7-TRIP12 complex in turn inactivates tumor suppressor gene p14(ARF) and subsequently promotes cell proliferation and invasion.31 In conclusion, USP7 plays an oncogenic role and may represent an important biomarker in HCC with MVI.
Fibronectin 1 (FN1)
FN1 is a 272kDa extracellular matrix glycoprotein that has an important role in cell adhesion and migration. It is involved in biological processes such as embryogenesis, host defense and cancer progression.71 Studies have shown that around 72% of HCC patients had increased expression of FN1. Compared with adjacent nontumor tissues, expression of FN1 was significantly increased in HCC cells. It is important to notice that overexpression of FN1 was also found in tumor cell emboli within microvessels. Pathologically, FN1 level was positively correlated with advanced TNM stages and MVI (p=0.001). In terms of prognosis, studies have shown that increased FN1 mRNA was associated with poorer RFS. Additionally, in vitro studies, basement membrane invasion test, and wound healing test showed that downregulating FN1 expression can significantly reduce the ability of cell invasion and migration, indicating that FN1 can promote HCC invasion and migration. In terms of molecular mechanisms, it was found that MYC oncogene has consistently been the upstream regulator of mRNA and proteins involved in MVI. Noticeably, FN1 contains multiple binding sites for MYC and was upregulated to promote cancer cell migration and invasion. Hence, FN1 may be a predictive biomarker of MVI in HCC.32,33 In conclusion, MYC oncogene is a potential interventional target of HCC with MVI, and FN1 overexpression is a promising predictor of those invasive tumors.
Block of proliferation 1 (BOP1)
BOP1 has a molecular mass of 83 kDa and belongs to the WD40 protein family. BOP1 is involved in rRNA processing and ribosome assembly.72 Its gene sequence is highly conserved and is located on chromosome 8q24.73 At present, studies have shown that BOP1 plays an important role in the progression of various cancers including gastric cancer, breast cancer, prostate cancer, and HCC. In HCC, BOP1 expression was significantly upregulated in HCC cells compared to adjacent nontumor tissues.34,74–76 Pathologically, BOP1 level was positively correlated with advanced TNM stages and MVI (p=0.0059). In terms of prognosis, overexpression of BOP1 was strongly associated with poorer OS and DFS. In vitro studies have found that downregulation of BOP1 in various HCC cell lines (HKCI-9 and Hep3B) led to profound inhibition on cell invasion. On the other hand, overexpression of BOP1 in normal liver cell line (L0-2) significantly enhanced cell invasion and migration. In terms of molecular mechanisms, BOP1 was proposed to be an upstream inducer of EMT, which mediates cancer progression including metastasis, invasion, and intravasation. BOP1-knockout led to upregulation of epithelial markers and downregulation of mesenchymal markers; the opposite is true when BOP-1 was overexpressed, suggesting that BOP1 enhances cell invasiveness through inducing EMT and promoting actin cytoskeleton remodeling that subsequently allows cells to acquire mobility.34 In conclusion, BOP1 has an oncogenic role and may represent an important biomarker in HCC with MVI.
Monoacylglycerol lipase (MAGL)
MAGL catalyzes the conversion of monoacylglycerol to free fatty acids and glycerol. Apart from its role in mediating pain and nociperception, MAGL is also involved in tumor progression.77,78 Previous studies have shown that MAGL expression was increased in various cancers including lung cancer, cervical cancer, colorectal cancer, and liver cancer.79–82 It was found that both mRNA and protein expression of MAGL were significantly higher in HCC cells compared with adjacent nontumor tissue. Pathologically, MAGL level was positively correlated with tumor sizes, advanced TNM stages and MVI (p=0.026). In terms of prognosis, overexpression of MAGL was associated with poorer OS and shorter TTR. In vivo studies have found that in HCC cell lines (HepG2) in which MAGL expression was upregulated, there were marked increase in cell migration and invasion. In terms of molecular mechanisms, MAGL was found to enhance the invasiveness of HCC cells by inducing EMT. This was supported by the findings of decreased epithelial markers and increased mesenchymal markers in HCC cell lines with higher MAGL levels. Additionally, it was found that MAGL induced EMT by upregulating the transcription factor Snail, which is dependent on the activation of nuclear factor kappa B (NF-κB) signaling. Snail in turn reduced the expression of the epithelial marker E-cadherin and mediated subsequent EMT processes.35 In conclusion, MAGL has an oncogenic role and may be an important biomarker of HCC with MVI.
Metadherin (MTDH)
MTDH is a single-channel transmembrane protein with its gene located on chromosome 8q22.83 MTDH was first discovered in fetal astrocytes of HIV-1 infected patients in 2002. Later studies showed that MTDH was abnormally expressed in various cancers, including esophageal, breast, gastric, liver cancer, osteosarcoma, and others.84–89 In HCC cells, MTDH expression was significantly higher than that in adjacent nontumor tissues. Pathologically, MTDH level was positively correlated with pathologic satellites, advanced TNM stages and MVI (p<0.001). In terms of prognosis, MTDH expression was strongly associated with poorer OS and shorter TTR. In vitro mobility assays showed that downregulation of MTDH expression in HCC cell lines greatly reduced cell motility. In vivo studies showed that overexpression of MTDH was associated with greater metastatic potential of HCC cells. Although the molecular mechanisms of MTDH regulation are still unclear, it has been reported that MTDH expression level was correlated with EMT markers. Specifically, upregulation of MTDH was associated with decreased E-cadherin and β-catenin, which are components of adherent junctions that mediate cell-cell adhesion. Overexpression of MTDH was also associated with increased Snail expression.36 Another study found that MTDH can promote the proliferation, invasion, and migration of HCC by activating PI3K/AKT signaling pathway.37 The findings support upregulation of MTDH leading to increased cell mobility and invasiveness of HCC cells. In conclusion, MTDH has an oncogenic role and may be an important biomarker in HCC with MVI.
Stathmin 1 (STMN1)
STMN1 has a molecular mass of 18 kDa and regulates microtubule dynamics by sequestering α/β-tubulin heterodimers and promoting microtubule destabilization.STMN1 is upregulated in many cancers such as HCC, lung cancer, breast cancer, and gastric cancer.38,90,91 In HCC, the expression of STMN1 was significantly upregulated in HCC cells compared to adjacent nontumor tissues. Pathologically, STMN1 level was positively correlated with portal vein tumor thrombus (PVTT) and MVI (p<0.001). In terms of prognosis, STMN1 expression was strongly associated with poorer OS and DFS. In vitro studies have found that after STMN1 knockdown, Snail2 and ZEB1 were significantly downregulated in Huh7 and MHCC97H cells. In vivo studies showed that lung metastasis by HCC cells the transplanted with STMN1 interfered cells was inhibited in terms of both tumor size and number. In terms of molecular mechanisms, STMN1 promoted EMT of HCC cells by regulating the dynamic balance of microtubules through the signaling by the STMN1-microtubule-EMTaxis.38 Chen et al.92 found that E2F1 was significantly correlated with STMN1 protein expression in HCC cells and in in vitro transactivation assays, suggesting that the STMN1 gene was transactivated by E2F1 protein. Zhang et al.93 found that STMN1 affected intricate crosstalk between HCC and hepatic stellate cells (HSC) by triggering the hepatocyte growth factor (HGF)/MET signaling pathway. In conclusion, STMN1 has an oncogenic role and may be an important biomarker of HCC with MVI.
Prothrombin induced by vitamin K absence II (PIVKA-II)
PIVKA-II, also known as des-γ-carboxy prothrombin, was first found to be significantly increased in the serum of HCC patients in 1984.94 Until now, elevated PIVKA-II levels have been rarely found in tumors other than HCC. The liver normally produces prothrombin in response to vitamin K, but PIVKA-II is produced in patients with vitamin K deficiency or HCC. That may be one a reason why it is highly expressed in HCC patients. Studies have shown that the serum PIVKA-II level in HCC patients was significantly higher than that in healthy people. Pathologically, serum PIVKA-II level was positively correlated with tumor sizes, differentiation and MVI (p<0.001). Interestingly, high PIVKA-II tissue expression was significantly associated with the presence of MVI (p=0.001).95 In terms of prognosis, overexpression of PIVKA-II was associated with poorer OS and DFS.96 Although the molecular mechanisms of PIVKA-II in HCC remain unclear, studies have demonstrated that the expression level of PIVKA-II is strongly correlated with EMT-related proteins such as MMP-9, Snail, vimentin, and E-cadherin. Therefore, PIVKA-II may promote HCC invasion and metastasis by promoting EMT.39 In conclusion, PIVKA-II plays an oncogenic role and may represent an important biomarker in HCC with MVI. Future studies should further explore the involvement of PIVKA-II in MVI development.
Discoidin domain receptor 1 (DDR1)
DDR1 has a molecular weight of 101 kDa and is located on 6p21.33. DDR1 is a member of the tyrosine kinase receptor family, and collagen is its specific ligand. DDR1 can interact with extracellular matrix (ECM) by binding to collagen.97 Existing studies have shown that DDR1 expression is increased in a variety of fibrotic diseases, including cirrhosis, idiopathic pulmonary fibrosis, skin hypertrophic scars, and renal fibrosis.98 Recent studies have found that DDR1 is associated with the development of many tumors, such as thyroid cancer, breast cancer, colorectal cancer, and others.99–101 Xu et al.102 found that the content of DDR1 in serum of HCC patients was significantly higher than that in chronic hepatitis patients and healthy people. Further studies showed that the level of DDR1 in tumors was higher than that in adjacent nontumor tissue, and had a strong correlation with the level of DDR1 in serum. In addition, overexpression of DDR1 in tumor tissues and serum are independent risk factors for MVI, and they are also significantly associated with the prognosis of HCC patients. In terms of mechanisms, the team found that DDR1 is closely related to EMT-related markers, so DDR1 may promote HCC development by inducing EMT. Pan et al.40 found that DDR1 stabilized SLC1A5 in a lysosomal-dependent manner to affect the mTORC1 signaling pathway, thereby controlling the proliferation of HCC cells. Another study found that DDR 1 promoted the migration and invasion of HCC cells through the DDR1/PSD4/ARF6 signaling axis.41 In summary, DDR1 is likely to become a clinical therapeutic target and biomarker for predicting MVI in the future.
Vascular endothelial growth factor A (VEGF-A)
VEGF-A has a molecular weight of 43 kDa and its gene is located at 6p21.1. VEGF-A is an important angiogenic factor secreted by tumor cells and stromal infiltrating cells. It might be involved in the regulation of angiogenesis and metastasis of many solid tumors.103,104 Recent studies have shown that serum VEGF-A concentrations ≥138.30 pg/ml is an independent risk factor for MVI.105 In terms of prognosis, studies have shown that high levels of VEGF-A in serum were strongly associated with worse OS.106 Current research describes mechanism of VEGF-A promoting HCC. Shen et al.42 found that hepatocyte-derived VEGF-A promoted the development of nonalcoholic fatty liver disease-related HCC by activating human HSCs. Vizio et al.43 found that thrombopoietin (THPO) and VEGF-A formed an interdependent autocrine system, and synergistically promoted the occurrence and development of HCC. In addition, it has been found that VEGF-A secreted by HCC cells promoted the formation of tubular structures of vascular endothelial cells, cell migration, and invasion by promoting the phosphorylation of VEGFR2.44 In summary, VEGF-A has an important role in the occurrence of HCC with MVI and is expected to become a prognostic marker and therapeutic target in the future.
S100P
The gene of S100P is located at 4p16.1, and its protein molecular weight is 104 kDa. S100P is a member of the S100 calcium-binding protein family, which was first identified in human placenta.107 As a signal molecule, S100P protein both extracellular and intracellular roles. In the extracellular space, S100P interacts with receptor for advanced glycation end products to activate signal transduction pathways.108 In cells, S100P interacts with cytoskeletal multidomain proteins through a Ca2+-dependent mechanism.109 More evidence shows that the expression of S100P is associated with tumors including HCC, pancreatic cancer, gallbladder cancer, etc.110 Qi et al.45 found that compared with healthy people, the serum S100P level of HCC patients was significantly increased, and the S100P mRNA level in HCC was significantly higher than that in adjacent nontumor tissues. In clinical features, high serum S100P level was significantly associated with the occurrence of PVTT and MVI. In addition, survival analysis showed that high S100P level in tumors was associated with poor prognosis. In vitro studies have shown that S100P could enhance the migration and invasion of HCC cells. In addition, in the study of molecular mechanism, the team found that S100P mediated HCC cell adhesion byCD44-dependent signal transduction and promote the formation of PVTT/MVI. Kim et al.46 found that S100P promoted mitosis of HCC cells by upregulating the expression of cyclin D1 and CDK2, thereby promoting the growth of HCC. In summary, serum S100P level can predict the occurrence of MVI, and S100P may become a new clinical therapeutic target in the future.
Potential serum biomarkers in HCC with MVI
Biomarkers indicators in blood, body fluids, and tissues that can be used to evaluate normal physiological processes, pathogenic processes, reactions to drugs, etc. HCC is characterized by a high postoperative recurrence rate. Compared with patients without MVI, HCC patients with MVI have a higher recurrence rate. However, a tough problem is that it is almost impossible to obtain a diagnosis of MVI before surgery. Therefore, if preoperative serum markers can be used to predict MVI in advance, and then a wider margin or anatomical hepatectomy is planned before surgery, the prognosis of HCC patients with MVI will be effectively improved.111 At present, there is no serum marker for predicting MVI in clinical practice, so this paper summarizes the serum markers significantly associated with MVI.
This review summarizes a total of 18 MVI-related markers, of which seven (circAKT3, microRNA-125b, STIP1, PIVKA-II, DDR1, VEGF-A, S100P) were found to be significantly abnormal in the serum of HCC patients. In the serum of HCC patients with MVI, the expression of microRNA-125b was significantly down-regulated, while the expression of circAKT3, STIP1, PIVKA-II, DDR1, VEGF-A, and S100P was significantly up-regulated. Among the serum markers mentioned in this paper, VEGF-A had the best predictive effect on MVI (AUC: 0.900; sensitivity: 0.805; specificity: 0.843; 95 % CI: (0.865–0.935), which may be related to its function of promoting angiogenesis. Although the prediction efficiency of other markers is relatively poor, the above markers can be combined to establish a prediction model in the future to improve the diagnostic ability of MVI. In addition, the expression of STIP1, PIVKA-II and DDR1 in serum and tumor tissues were significantly increased, and they were all related to EMT process. This shows that they have better characteristics and important value, and are potential biomarkers for predicting MVI. In the future, the above seven serum markers may become therapeutic targets for MVI and make outstanding contributions to the prediction of MVI.
Potential therapeutic values of biomarkers in HCC with MVI
With the in-depth study of HCC combined with MVI, the potential clinical application value of MVI-related biomarkers has been gradually explored. As discussed earlier in this article, 18 biomarkers have been reported to be dysregulated in HCC with MVI, and the amount is still increasing. The prognosis of HCC patients with MVI is poor, and the efficacy of postoperative drugs in HCC patients is not satisfactory. Therefore, the treatment of HCC is still a huge challenge. Both PD-L1 and VEGF-A mentioned in this review are related to MVI, and drugs developed based on these two substances (atezolizumab and bevacizumab) represent mature immunotherapy and targeted therapy, respectively. Moreover, the NCCN guidelines proposes that the combination of atezolizumab and bevacizumab can be used as the preferred treatment for advanced HCC.112 However, there is no team to study the therapeutic effect of atezolizumab and bevacizumab on HCC patients with MVI. In addition, this paper also mentioned some biomarkers that have not been applied to clinical treatment but may be used as therapeutic targets in the future. Shi et al.14 found that inhibiting the expression of lncRNA MVIH can promote the apoptosis of HCC cells and inhibit tumor growth. Fan et al.19 found that miR-125b inhibited the metastasis of HCC by inhibiting the expression of suppressor of variegation 3-9 homolog 1 (SUV39H1). Kim et al.46 have shown that inhibition of S100P can down-regulate the expression of cyclin D1 and CDK2 in HCC, thereby inhibiting the growth of HCC. Zhang et al.41 found that inhibition of DDR1 in HCC significantly reduced the migration and invasion of HCC cells. Fang et al.37 found that zingerone inactivated the PI3K/AKT signaling pathway by inhibiting the expression of MTDH, thereby inhibiting the proliferation, invasion, and migration of HCC cells. The abnormal expression of the above markers in HCC patients was significantly related to the malignant biological behavior of HCC. These findings might provide a new therapy for HCC patients.
Potential mechanisms in HCC with MVI
Currently, there is a large body of literature suggesting that EMT plays a pivotal role in promoting HCC progression by increasing cancer cell invasion and metastatic potential.113 EMT is a transient and reversible process whereby epithelial cells change in plasticity and switch back to mesenchymal phenotype. This process is particularly crucial during the early stages of cancer metastasis when cells lose tight cell-cell contacts because of downregulation of E-cadherin and β-catenin, which are key components of adherent junctions in the cell membrane. As a result, cells are detached from the basement membrane and acquire increased motility to disseminate into distant tissues.114 EMT can be divided into three types: type 1, which occurs during embryonic development; type 2, which occurs during wound healing and fibrosis; and type 3, which occurs mainly in the early stages of tumor development as cancer cells acquire the ability to break through basement membrane, intravasate and extravasate, gradually shifting the progression of malignancy toward metastasis.115 EMT can be reversed by mesenchymal-epithelial transition (MET), which allows the recovery of epithelial phenotype.116,117
In contrast to tumor cells that undergo EMT, passive shedding after a blood vessel is compromised also allows epithelial tumor cells to be released into circulation, but these circulating tumor cells (CTCs) can retain their original phenotype. In addition, epithelial CTC levels are significantly correlated with tumor sizes and BCLC stage, but not with recurrence rate and metastasis.118 Epithelial tumor cells that undergo EMT are detached from the extracellular matrix with the help of proteolytic enzymes. This allows tumor cells to invade beyond the basement membrane and intravasate into the circulation. Once in circulation, CTCs must overcome hemodynamic stresses and escape immune control and anoikis, an apoptotic mechanism that removes misplaced or detached cells. CTCs eventually extravasate and cause secondary or metastatic cancer. To date, a large body of literature have suggested that the presence of mesenchymal CTCs was associated with advanced TNM stages and MVI, as well as poorer prognosis and increased HCC recurrence rate.119 Moreover, it has been suggested that the hybrid epithelial/mesenchymal phenotypes are more adaptable to stressful environment for proceeding metastasis, and has increased stem-cell-like properties to enhance metastatic potential of cancer cells.120,121 The findings support that EMT enhanced the invasiveness of CTCs, and had a pivotal role in promoting HCC metastasis and the development of MVI.
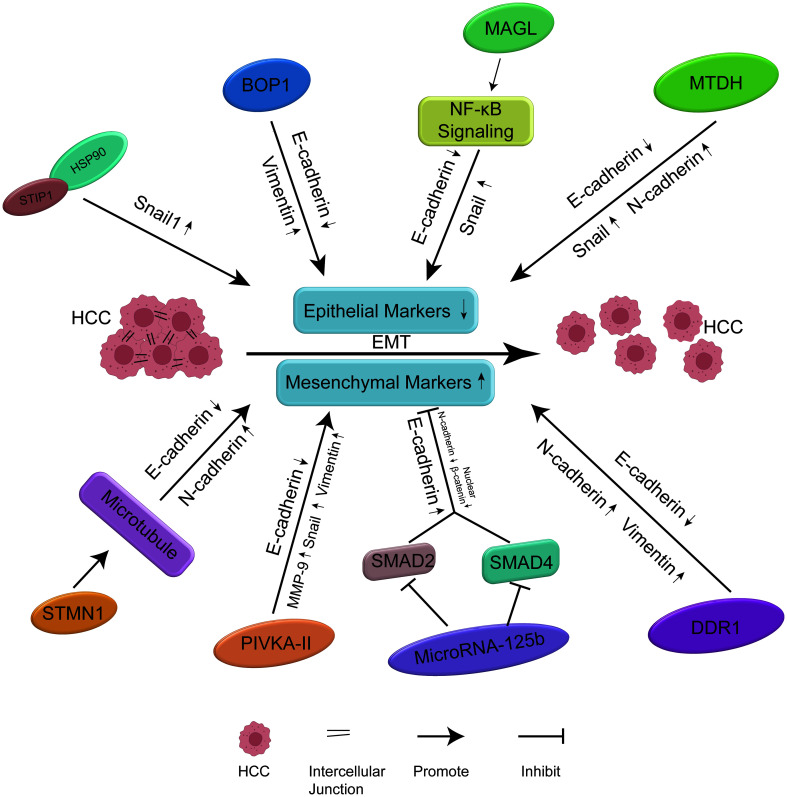
Wan et al.122 found that the expression of several EMT-related biomarkers including ZEB, Snail, Slug, and Twist1 was associated with poor prognostic factors, such as vascular invasion, intrahepatic metastasis, and poor OS in liver cancer. In this review, we have identified cancer biomarkers includingSTIP1, BOP1, MAGL, MTDH, STMN1, PIVKA-II, miR-125b and DDR1 that are strongly correlated with EMT markers such as E-cadherin and β-catenin (Fig. 2).STIP1-HSP90 protein complex upregulatedSnail1 and mediated heat-induced EMT in HCC cells.25 BOP1 deficiency caused an increase in E-cadherin expression and a reorganization of cytoplasmic keratin 18, while BOP1 overexpression significantly increased vimentin and fibronectin expression accompanied by an increase in the invasive phenotype of tumor cells, indicating that the increase of BOP1 induced EMT.34 The upregulation of MAGL enhances the activity of Snail and downregulates the expression of E-cadherin by activating NF-κB signaling, which then triggers the EMT process.35 After inhibiting the expression of MTDH, HCC cells showed down-regulated N-cadherin and Snail and up-regulated E-cadherin.36 After STMN1 was silenced, the expression of acetylated α-tubulin and E-cadherin increased, while the expression of pY397-FAK and intracellular N-cadherin decreased, indicating that STMN1 may promote EMT by regulating microtubule stability.38 PIVKA-II was significantly correlated with high levels of MMP-9, Snail, and vimentin, and low levels of E-cadherin, suggesting that PIVKA-II promoted HCC invasion and metastasis by promoting EMT.39 By targeting SMAD2, and SMAD4, miR-125b up-regulated the expression of E-cadherin and decreased the expression of N-cadherin and nuclear β-catenin, thereby weakening EMT-related characteristics.21 Overexpression of DDR1 increase expression of vimentin and N-cadherin, but reduced expression of E-cadherin, indicating that DDR1 activated the EMT pathway.102 Whether the biomarkers directly promoted HCC metastasis by upregulating EMT remain to be elucidated in future studies. Nevertheless, the eight biomarkers are potential targets in the underlying mechanisms of MVI and should be further explored.
Fig. 2. Biological role of the biomarkers in the development of EMT.
E-cadherin is an epithelial marker, and N-cadherin, vimentin, Snail, nuclear β-catenin and MMP-9 are mesenchymal markers in EMT-related proteins. In the pathological process of HCC with MVI, several markers can induce EMT by increasing the expression of mesenchymal markers and reducing the expression of epithelial markers, thereby promoting the migration, invasion, and metastasis of HCC cells. BOP1, block of proliferation 1; DDR1, discoidin domain receptor 1; EMT, epithelial-mesenchymal transition; HCC, hepatocellular carcinoma; MAGL, monoacylglycerol lipase; MTDH, metadherin; MVI, microvascular invasion; PIVKA-II, prothrombin induced by vitamin K absence II; STIP1, stress-induced phosphoprotein 1; STMN1, stathmin 1.
With regard to other biomarkers discussed previously here, the following regulatory mechanisms may contribute to the development of MVI. ciRS-7 promoted proliferation and invasion of HCC cells by sponging the tumor suppressor miR-7 to upregulate target genes including PIK3CD, CCNE1, and p70S6K. circAKT3 promotes the proliferation and invasion of HCC cells by downregulating the tumor suppressor miR-335; lncRNA MVIH inhibits PGK1 secretion to activate angiogenesis and promote HCC metastasis. miR-188-5p inhibits proliferation and invasion of HCC cells by targeting the FGF5-H-Ras-p-ERK signaling pathway. PD-L1 expression on cancer cells surface promotes HCC progression by inhibiting T cell activation and allowing immune escape. USP7-TRIP12 complex inactivates the tumor suppressor p14(ARF) to promote proliferation and invasion of HCC cells. MYC oncogene upregulates the transcription of FN1 to promote migration and invasion of HCC cells. TSC2, which was not mentioned previously, is correlated with MVI and inhibits proliferation and invasion of HCC cells by targeting the PI3K/AKT/mTOR signaling pathway.123,124
Biomarkers associated with MVI grade
Studies have shown that the prognosis of HCC patients is negatively correlated with the grade of MVI (M0, M1, M2). Therefore, exploring biomarkers significantly related to MVI grade has a strong guiding significance for future clinical work and scientific research.125 Existing studies have shown that VEGF-A and STMN1 are markers associated with MVI grade. Wang et al.105 found that the average serum VEGF-A concentrations in the M2 group, M1 group, and M0 group were 258.33 pg/ml, 167.60 pg/ml and 86.52 pg/ml, respectively (p<0.05). Cai et al.38 found that the content of STMN1 in HCC tissues increased with the increase of MVI grade (M0, M1, M2), and the results had statistical significance. No similar results were obtained for other markers mentioned in this paper, indicating that VEGF-A, and STMN1 may have more special significance for the prediction of MVI. In the future, we should carry out more studies to find more biomarkers related to MVI grade.
Conclusions and future perspectives
To sum up, MVI is an important prognostic factor related to survival of patients with HCC after surgery. Further understanding of the molecular mechanisms of MVI not only help to uncover the multistep process of HCC development, but also provide valuable insights into clinical applications such as early diagnosis, prognosis prediction, and treatment selections. Currently, the molecular mechanisms of the development of MVI are unclear. Previous reviews have not included the above-mentioned biomarkers and their relationship with MVI. Nevertheless, our review suggests that several biomarkers have important clinical application value in the early diagnosis of HCC patients with MVI, and also points to possible underlying mechanisms involved in MVI. More important, among the several markers associated with MVI described in this review, seven were abnormally expressed in serum, and eight markers simultaneously pointed out the possible molecular mechanism of MVI in EMT-related pathways. That means we could conduct additional research on those markers to reveal the molecular mechanisms of MVI in combination with the various signaling pathways discussed in this review. In addition, we can also attempt to combine the markers to construct a new model to predict the presence of MVI in advance, which is extremely important for perioperative preparation. Future studies should adopt multicenter and evaluation of large samples to further study the biological functions and molecular mechanisms of the potential biomarkers involved in MVI.
Abbreviations
- AKT
activating protein kinase B
- BOP1
block of proliferation 1
- ccRCC
clear cell renal cell carcinoma
- ceRNA
competing endogenous RNA
- circ-RNA
circular RNA
- CSC
cancer stem cell
- CTCs
circulating tumor cells
- DCs
dendritic cells
- DFS
disease-free survival
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- FN1
fibronectin 1
- FGF5
fibroblast growth factor 5
- HCC
hepatocellular carcinoma
- HSPs
heat shock proteins
- ICC
intrahepatic cholangiocarcinoma
- LncRNA
long noncoding RNA
- LncRNA MVIH
lncRNA associated with microvascular invasion
- MAGL
monoacylglycerol lipase
- MET
mesenchymal-epithelial transition
- MTDH
metadherin
- MVI
microvascular invasion
- OS
overall survival
- PD-L1
programmed cell death-ligand 1
- PIVKA-II
prothrombin induced by vitamin K absence II
- RFS
recurrence-free survival
- STIP1
stress-induced phosphoprotein 1
- STMN1
stathmin 1
- Treg
regulatory T cell
- TTR
time to recurrence
- USP7
ubiquitin-specific protease 7
References
- 1.Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Z, Guan R, Jianxi W, Zhao Z, Peng T, Liu C, et al. Microvascular Invasion in Hepatocellular Carcinoma: A Review of Its Definition, Clinical Significance, and Comprehensive Management. J Oncol. 2022;2022:9567041. doi: 10.1155/2022/9567041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Li J, Shen F, Lau W. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(2):347–354. doi: 10.1111/jgh.13843. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Perálvarez M, Luong T, Andreana L, Meyer T, Dhillon A, Burroughs A. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Zhang X, Wang H, Chai Z, Sun J, Guo W, et al. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB. 2019;21(8):935–944. doi: 10.1016/j.hpb.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Sheng X, Ji Y, Ren G, Lu C, Yun J, Chen L, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14(6):1034–1047. doi: 10.1007/s12072-020-10111-4. [DOI] [PubMed] [Google Scholar]
- 7.Isik B, Gonultas F, Sahin T, Yilmaz S. Microvascular Venous Invasion in Hepatocellular Carcinoma: Why Do Recurrences Occur? J Gastrointest Cancer. 2020;51(4):1133–1136. doi: 10.1007/s12029-020-00487-9. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(1):17–27. doi: 10.1007/s00432-016-2256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, Li B, Xiong X, Cheng N. RNA sequencing reveals the long noncoding RNA and mRNA profiles and identifies long noncoding RNA TSPAN12 as a potential microvascular invasion-related biomarker in hepatocellular carcinoma. Biomed Pharmacother. 2020;126:110111. doi: 10.1016/j.biopha.2020.110111. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Tang W, Yang M, Xie S, Wu M, Lin G, et al. Serum STIP1, a Novel Indicator for Microvascular Invasion, Predicts Outcomes and Treatment Response in Hepatocellular Carcinoma. Front Oncol. 2020;10:511. doi: 10.3389/fonc.2020.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship With clinical and pathological features. Hepatology. 2016;64(6):2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Gong X, Sun L, Zhou Q, Lu B, Zhu L. The Circular RNA Cdr1as Act as an Oncogene in Hepatocellular Carcinoma through Targeting miR-7 Expression. PLoS One. 2016;11(7):e0158347. doi: 10.1371/journal.pone.0158347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Dong B, Li H, Wang C, Qian X, Zhang R. Circ-AKT3 promotes the proliferation and migration of HCC cells via down-regulating microRNA-335 expression. Minerva Med. 2022;113(6):1040–1041. doi: 10.23736/s0026-4806.21.07715-6. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Song Q, Yu S, Hu D, Zhuang X. Microvascular invasion in hepatocellular carcinoma overexpression promotes cell proliferation and inhibits cell apoptosis of hepatocellular carcinoma via inhibiting miR-199a expression. Onco Targets Ther. 2015;8:2303–2310. doi: 10.2147/ott.S86807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, Wang L, Deng C, Du S, Han Z. ARID1A represses hepatocellular carcinoma cell proliferation and migration through lncRNA MVIH. Biochem Biophys Res Commun. 2017;491(1):178–182. doi: 10.1016/j.bbrc.2017.07.072. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Zhou B, Wu D, Yin Z, Luo D. Baicalin modulates microRNA expression in UVB irradiated mouse skin. J Biomed Res. 2012;26(2):125–134. doi: 10.1016/s1674-8301(12)60022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang F, Chang R, Yu L, Lei X, Xiao S, Yang H, et al. MicroRNA-188-5p suppresses tumor cell proliferation and metastasis by directly targeting FGF5 in hepatocellular carcinoma. J Hepatol. 2015;63(4):874–885. doi: 10.1016/j.jhep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Qin C, Yuan Z, Liu S. LncRNA PAPAS promotes hepatocellular carcinoma by interacting with miR-188-5p. J Cell Biochem. 2019;120(8):13494–13500. doi: 10.1002/jcb.28623. [DOI] [PubMed] [Google Scholar]
- 19.Fan DN, Tsang FH, Tam AH, Au SL, Wong CC, Wei L, et al. Histone lysine methyltransferase, suppressor of variegation 3-9 homolog 1, promotes hepatocellular carcinoma progression and is negatively regulated by microRNA-125b. Hepatology. 2013;57(2):637–647. doi: 10.1002/hep.26083. [DOI] [PubMed] [Google Scholar]
- 20.Alpini G, Glaser SS, Zhang JP, Francis H, Han Y, Gong J, et al. Regulation of placenta growth factor by microRNA-125b in hepatocellular cancer. J Hepatol. 2011;55(6):1339–1345. doi: 10.1016/j.jhep.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST, Nan X, et al. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology. 2015;62(3):801–815. doi: 10.1002/hep.27887. [DOI] [PubMed] [Google Scholar]
- 22.Song S, Yang Y, Liu M, Liu B, Yang X, Yu M, et al. MiR-125b attenuates human hepatocellular carcinoma malignancy through targeting SIRT6. Am J Cancer Res. 2018;8(6):993–1007. [PMC free article] [PubMed] [Google Scholar]
- 23.Li A, Shuai X, Jia Z, Li H, Liang X, Su D, et al. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J Transl Med. 2015;13:100. doi: 10.1186/s12967-015-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo X, Liu Y, Ma S, Liu L, Xie R, Li M, et al. STIP1 is over-expressed in hepatocellular carcinoma and promotes the growth and migration of cancer cells. Gene. 2018;662:110–117. doi: 10.1016/j.gene.2018.03.076. [DOI] [PubMed] [Google Scholar]
- 25.Su T, Liao J, Dai Z, Xu L, Chen S, Wang Y, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–3527. doi: 10.1038/s41388-018-0169-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Xu L, Su T, Ke Z, Peng Z, Zhang N, et al. Autocrine STIP1 signaling promotes tumor growth and is associated with disease outcome in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;493(1):365–372. doi: 10.1016/j.bbrc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Marconcini R, Spagnolo F, Stucci L, Ribero S, Marra E, Rosa F, et al. Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget. 2018;9(15):12452–12470. doi: 10.18632/oncotarget.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding T, Xu J, Wang F, Shi M, Zhang Y, Li S, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40(3):381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Chou H, Hsieh C, Charles R, Wang L, Wagner T, Fung J, et al. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93(3):272–282. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ang C, Klempner S, Ali S, Madison R, Ross J, Severson E, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget. 2019;10(40):4018–4025. doi: 10.18632/oncotarget.26998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai J, Shi G, Dong Z, Ke A, Ma H, Gao Q, et al. Ubiquitin-specific protease 7 accelerates p14(ARF) degradation by deubiquitinating thyroid hormone receptor-interacting protein 12 and promotes hepatocellular carcinoma progression. Hepatology. 2015;61(5):1603–1614. doi: 10.1002/hep.27682. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan M, Rajan Kd A, Park J, Arjunan V, Garcia Marques F, Bermudez A, et al. Genomic Analysis of Vascular Invasion in HCC Reveals Molecular Drivers and Predictive Biomarkers. Hepatology. 2021;73(6):2342–2360. doi: 10.1002/hep.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreos R, Ambrosini G, Groux R, Cavin Périer R, Bucher P. The eukaryotic promoter database in its 30th year: focus on non-vertebrate organisms. Nucleic Acids Res. 2017;45(D1):D51–D55. doi: 10.1093/nar/gkw1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung K, Cheng I, Ching A, Chu J, Lai P, Wong N. Block of proliferation 1 (BOP1) plays an oncogenic role in hepatocellular carcinoma by promoting epithelial-to-mesenchymal transition. Hepatology. 2011;54(1):307–318. doi: 10.1002/hep.24372. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W, Zhao Y, Zhou J, Wang X, Pan Q, Zhang N, et al. Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. J Hematol Oncol. 2016;9(1):127. doi: 10.1186/s13045-016-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu K, Dai Z, Pan Q, Wang Z, Yang G, Yu L, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–7302. doi: 10.1158/1078-0432.Ccr-11-1327. [DOI] [PubMed] [Google Scholar]
- 37.Fang J, Zhu H, Xu P, Jiang R. Zingerone suppresses proliferation, invasion, and migration of hepatocellular carcinoma cells by the inhibition of MTDH-mediated PI3K/Akt pathway. J Recept Signal Transduct Res. 2022;42(4):409–417. doi: 10.1080/10799893.2021.1988970. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, Fu Y, Liu C, Wang X, You P, Li X, et al. Stathmin 1 is a biomarker for diagnosis of microvascular invasion to predict prognosis of early hepatocellular carcinoma. Cell Death Dis. 2022;13(2):176. doi: 10.1038/s41419-022-04625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Liu X. PIVKA-II is an independent prognostic factor for overall survival of HCC patients and maybe associated with epithelial-mesenchymal transition. J Hepatol. 2015;63(4):1040–1041. doi: 10.1016/j.jhep.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Pan Y, Han M, Zhang X, He Y, Yuan C, Xiong Y, et al. Discoidin domain receptor 1 promotes hepatocellular carcinoma progression through modulation of SLC1A5 and the mTORC1 signaling pathway. Cell Oncol (Dordr) 2022;45(1):163–178. doi: 10.1007/s13402-022-00659-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Hu Y, Pan Y, Xiong Y, Zhang Y, Han M, et al. DDR1 promotes hepatocellular carcinoma metastasis through recruiting PSD4 to ARF6. Oncogene. 2022;41(12):1821–1834. doi: 10.1038/s41388-022-02212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen H, Yu H, Li QY, Wei YT, Fu J, Dong H, et al. Hepatocyte-derived VEGFA accelerates the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma via activating hepatic stellate cells. Acta Pharmacol Sin. 2022;43(11):2917–2928. doi: 10.1038/s41401-022-00907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vizio B, Bosco O, David E, Caviglia GP, Abate ML, Schiavello M, et al. Cooperative Role of Thrombopoietin and Vascular Endothelial Growth Factor-A in the Progression of Liver Cirrhosis to Hepatocellular Carcinoma. Int J Mol Sci. 2021;22(4):1818. doi: 10.3390/ijms22041818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang W, Li Z, Qin R, Wang X, An H, Wang Y, et al. YY1 Promotes Endothelial Cell-Dependent Tumor Angiogenesis in Hepatocellular Carcinoma by Transcriptionally Activating VEGFA. Front Oncol. 2019;9:1187. doi: 10.3389/fonc.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi LN, Ma L, Wu FX, Chen YY, Xing WT, Jiang ZJ, et al. S100P as a novel biomarker of microvascular invasion and portal vein tumor thrombus in hepatocellular carcinoma. Hepatol Int. 2021;15(1):114–126. doi: 10.1007/s12072-020-10130-1. [DOI] [PubMed] [Google Scholar]
- 46.Kim JK, Jung KH, Noh JH, Eun JW, Bae HJ, Xie HJ, et al. Targeted disruption of S100P suppresses tumor cell growth by down-regulation of cyclin D1 and CDK2 in human hepatocellular carcinoma. Int J Oncol. 2009;35(6):1257–1264. [PubMed] [Google Scholar]
- 47.Hansen T, Jensen T, Clausen B, Bramsen J, Finsen B, Damgaard C, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 48.Zou Y, Zheng S, Deng X, Yang A, Kong Y, Kohansal M, et al. Diagnostic and prognostic value of circular RNA CDR1as/ciRS-7 for solid tumours: A systematic review and meta-analysis. J Cell Mol Med. 2020;24(17):9507–9517. doi: 10.1111/jcmm.15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue D, Wang H, Chen Y, Shen D, Lu J, Wang M, et al. Circ-AKT3 inhibits clear cell renal cell carcinoma metastasis via altering miR-296-3p/E-cadherin signals. Mol Cancer. 2019;18(1):151. doi: 10.1186/s12943-019-1072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang X, Li Z, Zhang Q, Wang W, Li B, Wang L, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi: 10.1186/s12943-019-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y, Liu F, Gui R. High expression of circulating exosomal circAKT3 is associated with higher recurrence in HCC patients undergoing surgical treatment. Surg Oncol. 2020;33:276–281. doi: 10.1016/j.suronc.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Yang F, Zhang L, Huo X, Yuan J, Xu D, Yuan S, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54(5):1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 53.Yuan S, Yang F, Yang Y, Tao Q, Zhang J, Huang G, et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56(6):2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng N, Wu J, Yin M, Xu J, Wang Y, Chen X, et al. LncRNA CASC11 promotes cancer cell proliferation in hepatocellular carcinoma by inhibiting miRNA-188-5p. Biosci Rep. 2019;39(4):BSR20190251. doi: 10.1042/bsr20190251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Negrini M, Rasio D, Hampton GM, Sabbioni S, Rattan S, Carter SL, et al. Definition and refinement of chromosome 11 regions of loss of heterozygosity in breast cancer: identification of a new region at 11q23.3. Cancer Res. 1995;55(14):3003–3007. [PubMed] [Google Scholar]
- 57.Huang K, Dong S, Li W, Xie Z. The expression and regulation of microRNA-125b in cancers. Acta Biochim Biophys Sin. 2013;45(10):803–805. doi: 10.1093/abbs/gmt073. [DOI] [PubMed] [Google Scholar]
- 58.Liu M, Wang L, Zhu H, Rong W, Wu F, Liang S, et al. A Preoperative Measurement of Serum MicroRNA-125b May Predict the Presence of Microvascular Invasion in Hepatocellular Carcinomas Patients. Transl Oncol. 2016;9(3):167–172. doi: 10.1016/j.tranon.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Liu M, Zhu H, Rong W, Wu F, An S, et al. Identification of recurrence-related serum microRNAs in hepatocellular carcinoma following hepatectomy. Cancer Biol Ther. 2015;16(10):1445–1452. doi: 10.1080/15384047.2015.1071730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan J, Lee B, Lim J, Ma D, Goh J, Goh S, et al. Parkinson’s Disease-Specific Autoantibodies against the Neuroprotective Co-Chaperone STIP1. Cells. 2022;11(10):1649. doi: 10.3390/cells11101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krafft U, Tschirdewahn S, Hess J, Harke N, Hadaschik B, Nyirády P, et al. STIP1 Tissue Expression Is Associated with Survival in Chemotherapy-Treated Bladder Cancer Patients. Pathol Oncol Res. 2020;26(2):1243–1249. doi: 10.1007/s12253-019-00689-y. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Tsai C, Chang P, Chao A, Wu R, Chen S, et al. Positive associations between upregulated levels of stress-induced phosphoprotein 1 and matrix metalloproteinase-9 in endometriosis/adenomyosis. PLoS One. 2018;13(1):e0190573. doi: 10.1371/journal.pone.0190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman G, Long A, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latchman Y, Wood C, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 65.Carter L, Fouser L, Jussif J, Fitz L, Deng B, Wood C, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–643. doi: 10.1002/1521-4141(200203)32:3<634::Aid-immu634>3.0.Co;2-9. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Li J, Li H, Jiang T. Prognostic value of programmed cell death ligand 1 (PD-L1) for hepatocellular carcinoma: a meta-analysis. Biosci Rep. 2020;40(4):BSR20200459. doi: 10.1042/bsr20200459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung H, Jeong D, Ji S, Ahn T, Bae S, Chin S, et al. Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res Treat. 2017;49(1):246–254. doi: 10.4143/crt.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Everett R, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16(7):1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Zhang Q, Wang Y, Zhuang H, Chen B. Clinical Significance of Ubiquitin Specific Protease 7 (USP7) in Predicting Prognosis of Hepatocellular Carcinoma and its Functional Mechanisms. Med Sci Monit. 2018;24:1742–1750. doi: 10.12659/msm.909368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H, Zheng H. MicroRNA-200c represses migration and invasion of gastric cancer SGC-7901 cells by inhibiting expression of fibronectin 1. Eur Rev Med Pharmacol Sci. 2017;21(8):1753–1758. [PubMed] [Google Scholar]
- 72.Strezoska Z, Pestov D, Lau L. Functional inactivation of the mouse nucleolar protein Bop1 inhibits multiple steps in pre-rRNA processing and blocks cell cycle progression. J Biol Chem. 2002;277(33):29617–29625. doi: 10.1074/jbc.M204381200. [DOI] [PubMed] [Google Scholar]
- 73.Killian A, Sarafan-Vasseur N, Sesboüé R, Le Pessot F, Blanchard F, Lamy A, et al. Contribution of the BOP1 gene, located on 8q24, to colorectal tumorigenesis. Genes Chromosomes Cancer. 2006;45(9):874–881. doi: 10.1002/gcc.20351. [DOI] [PubMed] [Google Scholar]
- 74.Yang Y, Qin R, Zhao J, Qin X. BOP1 Silencing Suppresses Gastric Cancer Proliferation through p53 Modulation. Curr Med Sci. 2021;41(2):287–296. doi: 10.1007/s11596-021-2345-y. [DOI] [PubMed] [Google Scholar]
- 75.Vellky J, Ricke E, Huang W, Ricke W. Expression, Localization, and Function of the Nucleolar Protein BOP1 in Prostate Cancer Progression. Am J Pathol. 2021;191(1):168–179. doi: 10.1016/j.ajpath.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J, Horak C, Khanna C, Meng Z, Yu L, Veenstra T, et al. Alterations in Gemin5 expression contribute to alternative mRNA splicing patterns and tumor cell motility. Cancer Res. 2008;68(3):639–644. doi: 10.1158/0008-5472.Can-07-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlsson M, Contreras J, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272(43):27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 78.Bertrand T, Augé F, Houtmann J, Rak A, Vallée F, Mikol V, et al. Structural basis for human monoglyceride lipase inhibition. J Mol Biol. 2010;396(3):663–673. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, Li Z, Zhong L, Chen Y. Inhibition of monoacylglycerol lipase restrains proliferation, migration, invasion, tumor growth and induces apoptosis in cervical cancer. J Obstet Gynaecol Res. 2022;48(2):456–466. doi: 10.1111/jog.15110. [DOI] [PubMed] [Google Scholar]
- 80.Kienzl M, Hasenoehrl C, Maitz K, Sarsembayeva A, Taschler U, Valadez-Cosmes P, et al. Monoacylglycerol lipase deficiency in the tumor microenvironment slows tumor growth in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1965319. doi: 10.1080/2162402x.2021.1965319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye L, Zhang B, Seviour E, Tao K, Liu X, Ling Y, et al. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011;307(1):6–17. doi: 10.1016/j.canlet.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J, Liu Z, Lian Z, Liao R, Chen Y, Qin Y, et al. Monoacylglycerol Lipase: A Novel Potential Therapeutic Target and Prognostic Indicator for Hepatocellular Carcinoma. Sci Rep. 2016;6:35784. doi: 10.1038/srep35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang D, Su Z, Sarkar D, Emdad L, Volsky D, Fisher P. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353(1):8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Su Z, Kang D, Chen Y, Pekarskaya O, Chao W, Volsky D, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21(22):3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 85.Tokunaga E, Nakashima Y, Yamashita N, Hisamatsu Y, Okada S, Akiyoshi S, et al. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer. 2014;21(3):341–349. doi: 10.1007/s12282-012-0398-2. [DOI] [PubMed] [Google Scholar]
- 86.Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30(5):894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 87.Feng D, Yu X, Tian X, Meng H, Jiang Y, Song H, et al. Metadherin Promotes Malignant Phenotypes And Induces Beta-Catenin Nuclear Translocation And Epithelial-Mesenchymal Transition In Gastric Cancer. Cancer Manag Res. 2019;11:8911–8921. doi: 10.2147/cmar.S221422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014;47(5):427–434. doi: 10.1111/cpr.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ali D, Sabry N, Kabel A, Gaber R, Mokhtar H, Samy S, et al. The Expression of Circulating miR-497 and Metadherin in Hepatocellular Carcinoma: Relation to the Tumor Characteristics and Patients’ Survival. Medicina (Kaunas) 2021;57(9):866. doi: 10.3390/medicina57090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng P, Liu Y, Chen L, Liu X, Xiao Z, Zhao L, et al. Stathmin, a new target of PRL-3 identified by proteomic methods, plays a key role in progression and metastasis of colorectal cancer. J Proteome Res. 2010;9(10):4897–4905. doi: 10.1021/pr100712t. [DOI] [PubMed] [Google Scholar]
- 91.Hsieh S, Huang S, Yu M, Yeh T, Chen T, Lin Y, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49(5):476–487. doi: 10.1002/mc.20627. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y, Uen Y, Li C, Horng K, Chen L, Wu W, et al. The E2F transcription factor 1 transactives stathmin 1 in hepatocellular carcinoma. Ann Surg Oncol. 2013;20(12):4041–4054. doi: 10.1245/s10434-012-2519-8. [DOI] [PubMed] [Google Scholar]
- 93.Zhang R, Gao X, Zuo J, Hu B, Yang J, Zhao J, et al. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci. 2020;111(2):406–417. doi: 10.1111/cas.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liebman H, Furie B, Tong M, Blanchard R, Lo K, Lee S, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310(22):1427–1431. doi: 10.1056/nejm198405313102204. [DOI] [PubMed] [Google Scholar]
- 95.Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848–854. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Kim J, Hyuck C, Kwon D, Joh J, Lee J, Paik S, et al. Protein induced by vitamin K antagonist-II (PIVKA-II) is a reliable prognostic factor in small hepatocellular carcinoma. World J Surg. 2013;37(6):1371–1378. doi: 10.1007/s00268-013-1966-0. [DOI] [PubMed] [Google Scholar]
- 97.Agarwal G, Mihai C, Iscru DF. Interaction of discoidin domain receptor 1 with collagen type 1. J Mol Biol. 2007;367(2):443–455. doi: 10.1016/j.jmb.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 98.Moll S, Desmoulière A, Moeller MJ, Pache JC, Badi L, Arcadu F, et al. DDR1 role in fibrosis and its pharmacological targeting. Biochim Biophys Acta Mol Cell Res. 2019;1866(11):118474. doi: 10.1016/j.bbamcr.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Vella V, Nicolosi ML, Cantafio P, Massimino M, Lappano R, Vigneri P, et al. DDR1 regulates thyroid cancer cell differentiation via IGF-2/IR-A autocrine signaling loop. Endocr Relat Cancer. 2019;26(1):197–214. doi: 10.1530/erc-18-0310. [DOI] [PubMed] [Google Scholar]
- 100.Zhong X, Zhang W, Sun T. DDR1 promotes breast tumor growth by suppressing antitumor immunity. Oncol Rep. 2019;42(6):2844–2854. doi: 10.3892/or.2019.7338. [DOI] [PubMed] [Google Scholar]
- 101.Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V, et al. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. 2018;10(4):e7918. doi: 10.15252/emmm.201707918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu M, Cui C. Discoidin Domain Receptor Tyrosine Kinase 1 (DDR1): A Novel Predictor for Recurrence of Hepatocellular Carcinoma After Curative Resection. Med Sci Monit. 2021;27:e933109. doi: 10.12659/msm.933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Travaglino A, Raffone A, Saccone G, Migliorini S, Maruotti GM, Esposito G, et al. Placental morphology, apoptosis, angiogenesis and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2019;234:200–206. doi: 10.1016/j.ejogrb.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 104.Veenendaal LM, Jin H, Ran S, Cheung L, Navone N, Marks JW, et al. In vitro and in vivo studies of a VEGF121/rGelonin chimeric fusion toxin targeting the neovasculature of solid tumors. Proc Natl Acad Sci U S A. 2002;99(12):7866–7871. doi: 10.1073/pnas.122157899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang H, Lu Y, Liu R, Wang L, Liu Q, Han S. A Non-Invasive Nomogram for Preoperative Prediction of Microvascular Invasion Risk in Hepatocellular Carcinoma. Front Oncol. 2021;11:745085. doi: 10.3389/fonc.2021.745085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lacin S, Yalcin S. The Prognostic Value of Circulating VEGF-A Level in Patients With Hepatocellular Cancer. Technol Cancer Res Treat. 2020;19:1533033820971677. doi: 10.1177/1533033820971677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Becker T, Gerke V, Kube E, Weber K. S100P, a novel Ca(2+)-binding protein from human placenta. cDNA cloning, recombinant protein expression and Ca2+ binding properties. Eur J Biochem. 1992;207(2):541–547. doi: 10.1111/j.1432-1033.1992.tb17080.x. [DOI] [PubMed] [Google Scholar]
- 108.Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD. S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res. 2012;18(16):4356–4364. doi: 10.1158/1078-0432.Ccr-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Austermann J, Nazmi AR, Müller-Tidow C, Gerke V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J Biol Chem. 2008;283(43):29331–29340. doi: 10.1074/jbc.M806145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu BX, Tang CT, Dai XJ, Zeng L, Cheng F, Chen Y, et al. Prognostic Value of S100P Expression in Patients With Digestive System Cancers: A Meta-Analysis. Front Oncol. 2021;11:593728. doi: 10.3389/fonc.2021.593728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR, et al. Actual long-term survival in hepatocellular carcinoma patients with microvascular invasion: a multicenter study from China. Hepatol Int. 2021;15(3):642–650. doi: 10.1007/s12072-021-10174-x. [DOI] [PubMed] [Google Scholar]
- 112.Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 113.Erstad D, Tanabe K. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi: 10.1245/s10434-019-07227-9. [DOI] [PubMed] [Google Scholar]
- 114.Ortiz-Masiá D, Gisbert-Ferrándiz L, Bauset C, Coll S, Mamie C, Scharl M, et al. Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development. Cells. 2020;9(5):1104. doi: 10.3390/cells9051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thiery J, Acloque H, Huang R, Nieto M. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 116.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thiery J, Sleeman J. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 118.Celià-Terrassa T, Meca-Cortés O, Mateo F, Martínez de Paz A, Rubio N, Arnal-Estapé A, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122(5):1849–1868. doi: 10.1172/jci59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orrapin S, Udomruk S, Lapisatepun W, Moonmuang S, Phanphaisarn A, Phinyo P, et al. Clinical Implication of Circulating Tumor Cells Expressing Epithelial Mesenchymal Transition (EMT) and Cancer Stem Cell (CSC) Markers and Their Perspective in HCC: A Systematic Review. Cancers. 2022;14(14):3373. doi: 10.3390/cancers14143373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams E, Gao D, Redfern A, Thompson E. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. 2019;19(12):716–732. doi: 10.1038/s41568-019-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li C, Chen J, Ho Y, Hsu W, Wu L, Lan H, et al. Snail-induced claudin-11 prompts collective migration for tumour progression. Nat Cell Biol. 2019;21(2):251–262. doi: 10.1038/s41556-018-0268-z. [DOI] [PubMed] [Google Scholar]
- 122.Wan T, Zhang T, Si X, Zhou Y. Overexpression of EMT-inducing transcription factors as a potential poor prognostic factor for hepatocellular carcinoma in Asian populations: A meta-analysis. Oncotarget. 2017;8(35):59500–59508. doi: 10.18632/oncotarget.18352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ashworth R, Wu J. Mammalian target of rapamycin inhibition in hepatocellular carcinoma. World J Hepatol. 2014;6(11):776–782. doi: 10.4254/wjh.v6.i11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tee A, Fingar D, Manning B, Kwiatkowski D, Cantley L, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99(21):13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sheng X, Ji Y, Ren G, Lu C, Yun J, Chen L, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14(6):1034–1047. doi: 10.1007/s12072-020-10111-4. [DOI] [PubMed] [Google Scholar]