Abstract
Rescue of negative-stranded RNA viruses from full-length genomic cDNA clones is an essential technology for genetic analysis of this class of viruses. Using this technology in our studies of measles virus (MV), we found that the efficiency of the measles virus rescue procedure (F. Radecke et al., EMBO J. 14:5773–5784, 1995) could be improved by modifying the procedure in two ways. First, we found that coculture of transfected 293-3-46 cells with a monolayer of Vero cells increased the number of virus-producing cultures about 20-fold. Second, we determined that heat shock treatment increased the average number of transfected cultures that produced virus another two- to threefold. In addition, heat shock increased the number of plaques produced by positive cultures. The effect of heat shock on rescue led us to test the effect on transient expression from an MV minireplicon. Heat shock increased the level of reporter gene expression when either minireplicon DNA or RNA was used regardless of whether complementation was provided by cotransfection with expression plasmids or infection with MV helper virus. In addition, we found that MV minireplicon gene expression could be stimulated by cotransfection with an Hsp72 expression plasmid, indicating that hsp72 likely plays a role in the effect of heat shock.
Measles virus (MV), like all other members of the Morbillivirus genus in the Paramyxoviridae family, is an enveloped virus that contains an nonsegmented, negative-sense RNA genome (24). Molecular genetic analysis of this family of viruses has proved difficult until recently because naked genomic RNA or RNA produced intracellularly from a transfected plasmid is not infectious (5). This technical problem has been overcome through development of clever cDNA rescue technology that permits isolation of recombinant negative-strand RNA viruses (38, 41, 47). The exact techniques used for rescue of different negative-strand viruses vary but follow a common sequence of steps (3, 8, 12, 18, 19, 23, 25, 41, 42, 46, 47, 51). After transfection of a genomic or antigenomic cDNA plasmid, an exact copy of genome (or antigenome) RNA is produced by the combined action of phage T7 RNA polymerase and a vector-encoded ribozyme sequence that cleaves the transcribed RNA to generate the 3′ terminus. This RNA is encapsidated and replicated by viral proteins initially supplied by cotransfected expression plasmids. In the case of the MV rescue system (42), a stable cell line (293-3-46) that expresses T7 RNA polymerase and the MV proteins N (nucleocapsid protein) and P (phosphoprotein) was prepared. Thus, MV rescue can be achieved by cotransfecting this helper cell line with a MV genomic cDNA clone and an expression plasmid that contains the MV polymerase gene (L).
Successful MV cDNA rescue apparently requires numerous molecular events to occur after transfection, including (i) accurate, full-length synthesis of genome RNA by T7 RNA polymerase and 3′ end processing by the ribozyme; (ii) synthesis of viral N, P, and L proteins at levels appropriate to initiate the de novo encapsidation of genomic RNA into transcriptionally active and replication-competent nucleocapsid structures; and (iii) expression of viral genes from newly formed nucleocapsids at levels sufficient for replication to progress. Exactly what steps may be rate limiting in successful rescue is unknown, but the efficiency of this relatively rare event may be improved by stimulating any one of the steps mentioned above.
We speculated that the efficiency of MV cDNA rescue could be improved if the host cell could be manipulated to stimulate MV gene expression. Subjecting cells to elevated temperature to induce heat shock has been shown to alter the course of morbillivirus infection. MV cell fusion activity was found to be increased at elevated temperatures, apparently because of increased levels of viral fusion protein expressed on the cell surface (36). In addition, the steady-state levels of canine distemper virus (CDV) mRNAs are increased when infected cells are exposed for a short period to elevated temperatures (34). Also, the RNA-synthesizing activity associated with purified CDV nucleocapsids is increased when they are isolated from cells that have been subjected to heat shock (34, 35). Thus, we chose to study the effect of heat shock on MV cDNA rescue efficiency and gene expression.
Heat shock induces the cellular stress response and the synthesis of a group of multifunctional proteins called the heat shock proteins (Hsps) (9, 17, 26). Many of the Hsps are encoded by highly inducible genes, and these proteins are synthesized at elevated levels to help the cell recover from stress. The inducible Hsps are also present in the cell at basal levels indicative of their various roles in normal cell function. Some of the Hsps are also called chaperones because they assist in proper protein folding (13, 28); other functions attributed to Hsps include roles in protein trafficking in the cell, modulation of enzyme and protein function, participation in DNA replication, and involvement in viral replication and pathogenesis (10, 11, 13, 14, 21, 27, 28, 39, 45).
The mammalian Hsp70 family consists of a group of related proteins of approximately 70 kDa. The major inducible form of Hsp70 (Hsp72; also referred to as HspA1 and Hsp70-1 [17]) has an apparent molecular mass of 72 kDa. The 73-kDa protein (hsp73) is expressed in the cell constitutively and has been termed a heat shock cognate protein (Hsc73 [17]). These proteins participate in some of the functions mentioned above and have been implicated as among the host cell factors that increases CDV gene expression in response to heat shock. The Hsp72 isoform copurifies with the fraction of CDV nucleocapsids that contain enhanced viral transcriptional activity (35), and this result implies that the effect of heat shock on CDV gene expression may be at least in part due to induction of Hsp70 family members.
In this study, we have tested the hypothesis that the effect of heat shock on morbillivirus gene expression may improve the efficiency of MV cDNA rescue. Our results indicate that heat shock does significantly improve recovery of recombinant virus. In addition, we demonstrate that MV minireplicon activity is increased by heat shock and that Hsp72 is a at least partially responsible for this effect.
MATERIALS AND METHODS
Cells, virus, and transfection.
293-3-46 cells (kindly provided by Martin Billeter and Frank Radecke) (42) constitutively express the MV-specific genes N and P and express the phage T7 RNA polymerase gene. The progenitor of 293-3-46 cells was 293 cells (15), a human embryonic kidney cell line transformed by adenovirus type 5 DNA. Both cell types were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (FBS). 293-3-46 cells were grown with selection in medium containing G418 (Geneticin; Life Technologies) at 1.5 mg per ml. Vero cells were grown in Dulbecco’s modified Eagle medium containing 5% FBS, and HeLa suspension cells were grown in minimal essential media supplemented with 10% FBS. MV (Edmonston B) was propagated in HeLa suspension cultures as described earlier (50).
Transfections were performed by the calcium phosphate precipitation method (2, 16). 293-3-46 or 293 cells used for transfection were seeded onto six-well plates and grown to about 50 to 75% confluence. Cells were fed 2 to 5 h before transfection with 4.5 ml of fresh medium lacking G418. DNA transfection mixtures were prepared by combining the appropriate DNAs in a final volume of 225 μl in water followed by addition of 25 μl of 2.5 M CaCl2. Minireplicon DNA was used at 1 μg per transfection, and 100 ng of L expression plasmid was determined to be optimal under our transfection conditions. Full-length genomic cDNA (plasmid p+MV) was used at 5 μg per transfection. DNA-calcium mixtures were vortexed gently while 250 μl of 2× HEPES-buffered saline (280 mM NaCl, 1.5 mM Na2HPO4, 50 mM HEPES [pH 7.05]) was added slowly. The precipitate was allowed to stand at room temperature for 20 min and then added to the cells. The cells were incubated overnight (14 to 16 h), then the transfection medium was removed, and the cells were rinsed and fed with fresh medium lacking G418. In some minireplicon experiments, MV-specific proteins were provided by infecting transfected cells with MV helper virus. Infection was performed by replacing the transfection medium and adding MV to the culture medium at a multiplicity of infection (MOI) of 5. Infected cells were incubated for 2 h before heat shock was performed. Dishes containing cells that were to be heat shocked were wrapped in Parafilm, transferred to a water bath at 43 to 44°C, and incubated for 3 h before being transferred to 37°C. Heat shock temperatures that exceeded 44°C produced high levels of cell death; temperatures below 43°C were less effective. Cells were harvested at 48 h after initiation of transfection for analysis of transient gene expression or harvested at 72 h for rescue experiments. Chloramphenicol acetyltransferase (CAT) assays were performed as described previously (37). 293-3-46 cells harvested for virus rescue were removed from the well by repeated pipetting of the medium over the monolayer to detach the cells and break the monolayer into small clumps. No cell-dissociating agents were used. The cells along with 5 ml of medium from the well were immediately distributed onto a near (about 75%)-confluent monolayer of Vero cells growing in 10 ml of medium on a 10-cm-diameter dish. In some experiments, 12 h after initiation of the coculture, the medium was replaced with medium containing 1% agarose. Four to five days later, plaques were visible and the monolayers were stained for plaque counting or harvested to prepare a recombinant virus stock.
RNA transfections were performed as described above for DNA, with some modification. RNA for transfection was prepared in vitro, using the T7 RNA polymerase reagents in the Megascript kit (Ambion, Inc.). Five micrograms of RNA was used to prepare RNA-calcium phosphate precipitates, which were incubated with 293 cells for 5 to 6 h. After this time, the transfection medium was removed and the cells were fed with fresh medium. Combined transfection-infection was carried out by addition of virus to the transfection medium. After replacement of the transfection-infection medium, appropriate cell samples were heat shocked. The cells were harvested at 24 to 28 h after the initiation of transfection-infection.
Recombinant DNA.
The full-length MV cDNA plasmid (p+MV) and the MV L-gene expression plasmid (pEMC-La) were generously provided by Martin Billeter and Frank Radecke (42). Preparation of the CAT minireplicon has been described elsewhere (48). The hsp72 cDNA (17, 22, 29) was cloned from RNA extracted from heat-shocked 293-3-46 cells. The cloned cDNA sequence predicts an amino acid sequence identical to those encoded by the hsp70-1 and hsp70-2 genes (17, 29). The cDNA was prepared by reverse transcription-PCR (RT-PCR) performed with the high-fidelity enzyme mixture containing Moloney murine leukemia virus reverse transcriptase, Taq DNA polymerase, and Pwo DNA polymerase provided with the Boehringer Mannheim Titan kit. The RT-PCR primer sequence CAAGCGGCCGCATGGCCAAAGCCGCGGCAGT was specific for the 5′ end of the cDNA, and the sequence GAAGGATCCGCAATCTTGGAAAGGCCCCTA was specific for the 3′ end. The hsp72 cDNA was cloned into expression plasmid pCGN (49) to generate an expression construct containing the influenza virus hemagglutinin (HA) epitope in place of the first five amino acids of Hsp72.
DNA sequencing.
The MV nucleotide sequence was determined by sequencing DNA amplified by RT-PCR. RNA from MV-infected cells was prepared by the guanidinium isothyocyanate-phenol-chloroform extraction method (7), and RT-PCR was performed with reagents in the Boehringer Mannheim Titan kit. Amplified DNA was gel purified in low-melting point agarose gels. The PCR fragment was sequenced by using dye terminator reactions (Applied Biosystems) and analyzed on an ABI Prism model 377 automated sequencer. Sequence confirmation of plasmid DNAs was performed also with the automated sequencer.
RESULTS
Heat shock improves rescue efficiency.
Molecular genetic analysis of MV requires effective cDNA rescue methods for isolation of recombinant viruses. The rescue method of Radecke et al. (42) was a very significant breakthrough that allowed isolation of recombinant MV. Recombinant MV can be isolated after cotransfection of 293-3-46 cells with only an MV genomic cDNA plasmid and a plasmid designed to express the MV L protein because this cell line constitutively produces MV N and P as well as phage T7 RNA polymerase. Radecke et al. (42) reported that about 30% of the transfected cultures produced recombinant virus detectable by syncytium formation. We also have used this rescue technique successfully but with lower efficiency. Thus, we felt it necessary to improve our rescue efficiency to enhance the likelihood of successful recovery of significantly impaired recombinant viruses. After testing various modifications of the technique, we have achieved significant improvement in recovery.
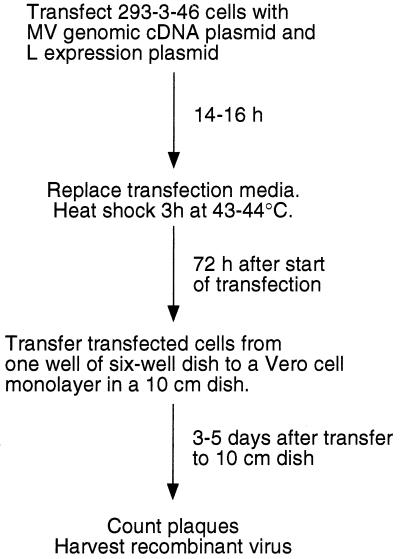
Figure 1 outlines the modified rescue technique that we now use, and Table 1 summarizes the results of a series of rescue experiments. The two modifications of the technique that we have found effective include a heat shock step and a plaque expansion step performed on Vero cells. These steps have improved our rescue efficiency in two ways: by increasing the percentage of transfected cultures that produce detectable virus, and by increasing the number of plaques generated from the virus-producing cultures. Before using these modifications, we estimate that approximately 1 to 2% of the transfected cultures produced syncytia. We now can recover recombinant virus from up to 90% of the transfected cultures.
FIG. 1.
Flow diagram of the modified cDNA rescue procedure. The use of a heat shock step and the coculture of transfected cells with Vero cells are the primary differences from the procedure described by Radecke et al. (42).
TABLE 1.
Effect of heat shocka
| Transfection | Expt 1 | Expt 2 | Expt 3 | Expt 4 | Expt 5 | Expt 6 | Expt 7 |
|---|---|---|---|---|---|---|---|
| −Heat shock | |||||||
| 1 | 0 | 0 | 0 | 22 | 14 | 0 | 50+ |
| 2 | 0 | 0 | 2 | 0 | 0 | 5 | 50+ |
| 3 | 0 | 3 | 0 | 3 | 37 | 15 | 0 |
| 4 | 0 | 1 | 0 | 6 | 0 | 49 | 1 |
| 5 | 0 | 50+ | 1 | 9 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 50+ | 0 | ||
| 8 | 0 | 7 | 2 | 1 | 0 | ||
| 9 | 0 | 50+ | 1 | 0 | 0 | ||
| 10 | 0 | 1 | 16 | 1 | 0 | ||
| 11 | 1 | 1 | 0 | 0 | 0 | ||
| 12 | 0 | 50+ | 0 | 0 | 0 | ||
| +Heat shock | |||||||
| 13 | + | 50+ | 1 | 50+ | 11 | 50+ | 50+ |
| 14 | + | 50+ | 50+ | 50+ | 50+ | 50+ | 4 |
| 15 | + | 50+ | 4 | 50+ | 50+ | 13 | 0 |
| 16 | + | 0 | 4 | 14 | 50+ | 1 | 1 |
| 17 | + | 50+ | 50+ | 29 | 50+ | 0 | 1 |
| 18 | + | 4 | 50+ | 50+ | 18 | 5 | 50+ |
| 19 | + | 1 | 32 | 50+ | 50+ | 50+ | |
| 20 | + | 50+ | 50+ | 50+ | 1 | 1 | |
| 21 | + | 3 | 50+ | 50+ | 41 | 0 | |
| 22 | + | 10 | 4 | 50+ | 2 | 50+ | |
| 23 | + | 3 | 50+ | 50+ | 50+ | 0 | |
| 24 | + | 8 | 50+ | 50+ | 15 | 0 |
Seven independent rescue experiments were performed to test the effect of heat shock. The MV cDNA used in all experiments contained Edmonston B sequences (42). Transfections were performed as described in Materials and Methods, and heat shock incubation was for 3 h at 44°C. Experiment 1 was scored for the presence (+) or absence (0) of plaques; in the remaining experiments, plaques were counted. In experiments 1, 2, and 6, several rescued viruses (indicated by boldface) were isolated and used to infect Vero cells for subsequent RNA preparation and sequence analysis to confirm the presence of recombinant virus markers (42). The coculture in experiment 7 was overlaid with medium containing agarose, whereas experiments 1 to 6 were maintained in medium without agarose.
The first modification involves replating the transfected 293-3-46 cells onto a monolayer of Vero cells. We tested this procedure because we were not successful in identifying plaques on transfected 293-3-46 cells. Expansion of the transfected 293-3-46 cells from one well of a six-well dish to a 10-cm plate did not improve plaque detection. We initially achieved a 2% success rate by harvesting transfected cells 72 h posttransfection, preparing a freeze-thaw lysate, and using this lysate to infect Vero cells. Although this procedure worked, we speculated that the freeze-thaw step may actually impair our recovery of a limited number of viable viruses, and so we modified the procedure by transferring intact transfected 293-3-46 cells to a near (about 75%)-confluent 10 cm-diameter dish of Vero cells. This was performed at 72 h posttransfection by removing the 293-3-46 cells from the well by repeated pipetting of the culture media over the cells. Clumps of transfected cells were then distributed over the Vero cell monolayer. Four to five days later, plaques were readily detectable on the Vero cell monolayer. This modification provided about a 20-fold increase in the number of transfected wells that eventually produced plaques (data not shown), and the results shown in Table 1 were obtained by using this modified coculture procedure.
Next, we tested the effect of heat shock on MV cDNA rescue efficiency (Table 1). Cells were transfected overnight (14 to 16 h) by the calcium phosphate procedure. After washing the cells and adding fresh medium, we wrapped cells in six-well plates in Parafilm and transferred them to a water bath at 43 to 44°C for 3 h. After heat shock, the cells were transferred to a 37°C incubator without further manipulation (except removal of the Parafilm). At 72 h after transfection, the 293-3-46 cells were transferred to a monolayer of Vero cells and the coculture was incubated for 4 to 5 days; then the cells were stained for plaque counting. The results revealed a significant improvement in rescue efficiency apparently due to heat shock. We routinely did not overlay the cocultured cells with agar because our primary goal was to develop a technique that maximized virus yield, but we did confirm in several later experiments that the effect of heat shock on rescue was detectable if the cocultured cells were cultured under agarose overlay (Table 1); one example is included in Table 1 (experiment 7). The results in Table 1 indicate that heat shock increased the average number of positive transfections in multiple experiments about two- to threefold, but in individual experiments the effect was more dramatic (experiments 1, 3, and 5). In addition, there was a large increase in the number of plaques found in each positive well. Without heat shock, the majority of positive cultures produced a few plaques, whereas many positive cultures that were heat treated generated more than 50 plaques. Also, temperatures between 43 and 44°C seemed to be optimal (data not shown). Temperatures above 44°C produced greater levels of cell death, and temperatures below 43°C seemed less effective. Sequence analysis of several isolates confirmed that we were indeed generating recombinant viruses during these experiments (Table 1). In combination, the coculture of transfected 293-3-46 cells with a monolayer of Vero cells and the heat shock step has enabled us to recover virus from up to 90% of the transfected cultures.
Heat shock increases expression from minireplicons.
To examine potential mechanisms for the improved rescue results after heat shock, we tested the effect of heat shock on gene expression from an MV minireplicon (Fig. 2). The plasmid minireplicon (pMV107CAT) is designed to direct T7 RNA polymerase-mediated synthesis of a negative-sense RNA copy of the CAT gene flanked by MV termini (48). This plasmid can be used to transfect 293-3-46 cells for intracellular synthesis of minireplicon RNA or can be used as the template for in vitro transcription to generate RNA for transfection. Replication and expression of minireplicon RNA can occur in 293-3-46 cells when complemented by MV proteins provided by infection or when complemented with an L expression plasmid, since the cells provide both MV-specific N and P proteins.
FIG. 2.
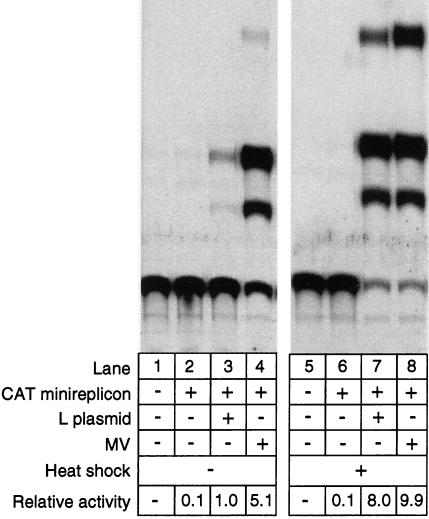
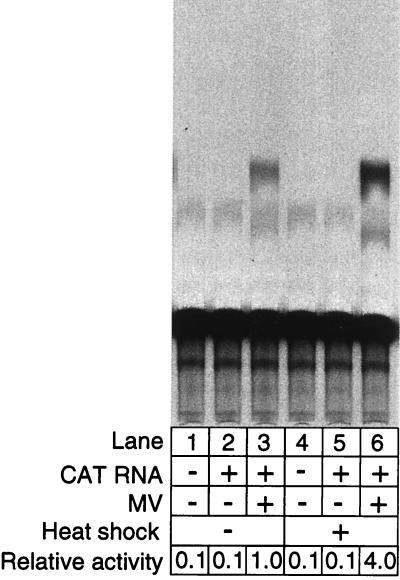
Effect of heat shock on minireplicon gene expression. 293-3-46 cells were transfected overnight with MV-CAT minireplicon plasmid DNA (1 μg). Some transfections (lanes 3 and 7) also received the MV L-gene expression plasmid (100 ng) to provide L complementation. About 14 h after transfection, the medium was replaced and the cells were infected with MV at an MOI of 5 per cell (lanes 4 and 8). After allowance of 2 h for infection, the indicated cell cultures (lanes 5 to 8) were heat shocked at 43 to 44°C for 3 h. Cells were harvested at 48 h after the start of transfection, and CAT assays were performed as described in Materials and Methods.
When we examined the effect of heat shock on expression of the minireplicon, we found that heat shock produced a strong increase in CAT gene activity (Fig. 2). The experiments shown in Fig. 2 were performed with minireplicon DNA and carried out similarly to rescue experiments except that cells were harvested 48 rather than at 72 h after transfection. Complementation by virus was performed by infecting transfected cells at an MOI of 5 after removal of the transfection medium. Complementation with the L expression plasmid was done simply by cotransfection with the minireplicon DNA. The results indicate that heat shock stimulated expression when either form of complementation was used. In multiple experiments, CAT expression generated by complementation with the L expression plasmid was increased from 2- to 10-fold by heat shock (Fig. 2; compare lanes 3 and 7). Similarly, CAT activity was also increased (averaging about fivefold) when viral complementation was used (lanes 4 and 8). As expected, negative control transfections that did not receive CAT plasmid (lanes 1 and 5) or a source of L complementation (lanes 2 and 6) produced very low levels of background CAT activity.
The possibility existed that the increased expression of the minireplicon was related to a higher level of T7 polymerase activity after heat shock. Higher T7 polymerase activity might result from increased expression of the gene in 293-3-46 cells after heat shock. The T7 polymerase gene is expressed from the cytomegalovirus (CMV) immediate-early promoter/enhancer in 293-3-46 cells (42), and the CMV promoter/enhancer has been shown to respond to heat shock (1). To rule out this possibility, we transfected cells with minireplicon RNA rather than DNA and performed a heat shock experiment (Fig. 3). In addition, to rule out the possibility that the effect of heat shock was related to increased expression of the MV genes present in the 293-3-46 cell line, we performed the RNA transfections with the progenitor cell line 293 (15), which does not constitutively express any MV genes. The transfection protocol used in this experiment was modified to accommodate RNA transfection (see Materials and Methods). Five micrograms of RNA was transfected by the calcium phosphate procedure. MV infection of the appropriate cells was performed by adding virus immediately after the transfection mixture was added to the medium. Six hours after transfection, the cells were washed and fed with fresh medium; cells that received heat shock treatment were incubated for 2 h at 44°C before being returned to 37°C. The cells were harvested at 24 to 28 h after the initiation of transfection.
FIG. 3.
Minireplicon RNA transfection. RNA prepared in vitro was transfected by the calcium phosphate procedure (Materials and Methods). After the precipitate was added to the cells, MV (MOI of 5; lanes 3 and 6) was added to the culture medium to initiate the infection immediately to lessen the chance of degradation of intracellular RNA before it could be packaged into nucleocapsids. After a 5- to 6-h transfection-infection incubation, the media was replaced and the indicated cell cultures were heat shocked 2 h at 43 to 44°C. Cell extracts were prepared 24 to 28 h after the start of transfection-infection for CAT assays.
The results from the RNA transfection were similar to the results of DNA transfection. Heat shock substantially increased the expression of CAT in cells that were infected (Fig. 3; compare lanes 3 and 6). As expected, no CAT activity was observed in cells that received no minireplicon RNA (lanes 1 and 4) or no viral complementation (lanes 2 and 5). The RNA transfection results also rule out the simple explanation that increased T7 RNA polymerase activity was responsible for the effect of heat shock in the DNA transfection experiment shown in Fig. 2A.
Stimulation of minireplicon expression by Hsp72.
We next examined the possibility that one of the Hsps may be involved in the stimulation of MV gene expression after heat shock. Studies of CDV have shown that the inducible Hsp70 isoform, Hsp72, copurifies with CDV nucleocapsids and that these nucleocapsids display enhanced in vitro transcriptional activity (32, 34, 35). Accordingly, we chose Hsp72 as a candidate to study further. To test the hypothesis that Hsp72 was involved in the stimulation of MV gene expression, we designed experiments that essentially substitute overexpression of the hsp72 gene for heat shock treatment (Fig. 4).
FIG. 4.
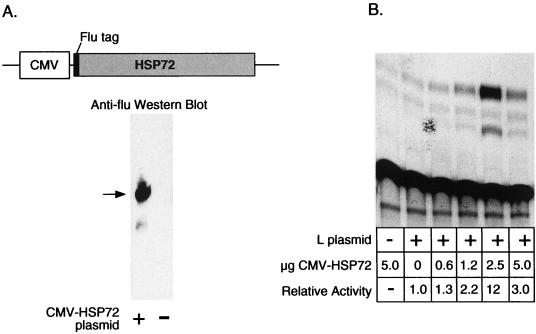
Stimulation of minireplicon gene expression by Hsp72. The hsp72 cDNA (17, 22, 29) was cloned into a CMV expression vector. The amino terminus coding region was fused to the influenza virus (flu) HA epitope tag (49). Whole-cell extracts prepared from transfected cells were analyzed by Western blotting using an antibody specific for the epitope tag (A). CAT assay results from cotransfection of 293-3-46 cells with the Hsp72 expression vector, minireplicon DNA, and L expression plasmid are also shown (B). Transfections were performed as described for Fig. 2.
We amplified the hsp72 cDNA (22, 29) from RNA prepared from heat-shocked 293-3-46 cells and cloned the cDNA into a CMV expression vector (49) that also encodes an influenza virus HA epitope tag. The hsp72 cDNA was cloned in frame to generate an Hsp72 protein with an amino terminus containing the HA epitope. Use of this plasmid allowed us to monitor expression of the hsp72 cDNA by using an antibody against the HA tag even in the presence of the background of endogenous Hsp70 isoforms. As expected, Western analysis of extracts from transfected cells showed that the expression plasmid (Fig. 4A) produced a tagged polypeptide slightly larger 70 kDa. Western analysis of transfected cells with an anti-Hsp72 antibody did not reveal a significant elevation of total Hsp72 in transfected cells (data not shown), but this is not unexpected since only a small percentage of cells received the expression plasmid during transfection and all cells produced basal levels of Hsp72.
Cotransfection of 293-3-46 cells with the Hsp72 expression vector along with the L expression plasmid and minireplicon DNA resulted in increased expression of CAT (Fig. 4B). In this transient assay system, the overexpression of Hsp72 increased the low level of L complementation as much as 20-fold. This increase in CAT expression induced by the Hsp72 expression vector was apparently specific because it required the presence of the L polymerase plasmid and did not increase the background CAT activity observed when L was absent or the CAT plasmid was omitted from the transfection. These results strongly suggest that Hsp72 was at least in part responsible for the effect of heat shock on minigenome expression.
DISCUSSION
We have modified the published MV rescue procedure and improved the recovery of recombinant virus. Using the published protocol (42), we successfully isolated recombinant virus but at a somewhat lower than expected frequency. The reason for our lower efficiency is not clear but may be related to slight differences in technical details, such as variables that affect transfection efficiency (e.g., transfection reagents, cell growth conditions, or cell passage number) or variables that affect detection and successful harvest of recombinant virus. Nevertheless, our modifications have improved efficiency and may prove useful for others working with MV as well as other negative-strand RNA viruses and related rescue systems.
Enhanced rescue efficiency and increased MV gene expression resulting from heat treatment of cells strongly implicate cellular proteins as mediators of this effect. Host cell factors have been suggested before as important components of the MV replication apparatus. Accurate and efficient in vitro transcription from purified MV requires cell extract proteins (4, 20), and results from in vitro transcription analysis using infected-cell extracts have suggested that two cellular matrix proteins, tubulin and actin, are involved in MV RNA synthesis (31). Additional cellular proteins that may play a role in MV transcription and replication have been found to interact with the cis-acting regulatory sequences in the MV genomic RNA (4). Finally, as mentioned earlier for CDV, cellular Hsp70 proteins have been found associated with nucleocapsids, and their presence correlates with increased transcriptional activity (35).
The mechanism of rescue enhancement by heat shock may be related to elevated levels Hsps. Our transient expression analysis showed that heat shock or overexpression of Hsp72 can increase the level of minireplicon gene expression. These findings correlate well with the results of Oglesbee et al. (34, 35), who found that (i) heat shock increases the expression of CDV RNA during infection and increases the transcriptional activity of purified nucleocapsids and (ii) Hsp72, an inducible Hsp70 protein (17), was associated with nucleocapsids containing the enhanced transcriptional activity. Consistent with the latter result, the hsp72 cDNA clone that we used in cotransfection experiments was prepared from an inducible, human hsp70 gene (hsp70-1 [17, 22, 29]) and not the constitutive heat shock cognate gene (hsp73 or hsc73 [17]). The implication that hsp72 increases MV gene expression can probably be extended to the rescue results; it appears reasonable to suggest that heat shock contributes to increased rescue efficiency by stimulating some step in the formation of or expression from MV nucleocapsids and that hsp72 is one participant in this process.
Preliminary attempts to stimulate full-length rescue by cotransfection with the Hsp72 expression vector have not enhanced rescue reproducibly. This may simply be indicative of the fact that we need to alter the transfection conditions (such as the amount of Hsp72 vector) from those used in the minireplicon system. We feel that a more likely explanation is that the stimulation of full-length rescue requires Hsp72 in combination with other components of the cellular stress response induced by heat shock. Some of these additional components may be one or more of the other Hsps such as Hsp40 or Hsp90 (21, 28). In addition, full-length rescue may benefit from the inhibition of cap-dependent mRNA translation induced by heat shock (6), providing a period of time when the translation of the L gene is favored because of the internal ribosome entry site encoded by plasmid vectors based on plasmid pTM1 (30). Cellular mRNA export from the nucleus also is inhibited by heat shock (44). Possibly this transient cessation of cellular mRNA accumulation in the cytoplasm provides another period of time that favors T7 RNA polymerase-mediated expression of the transfected plasmids in the cytoplasm. Finally, coexpression of Hsp72 may effectively stimulate minireplicon activity over the relatively brief period of a transient assay (24 to 48 h), but the more sustained period of Hsp72 synthesis driven by the expression plasmid in a transfected cell during the prolonged course of a full-length rescue experiment may prove inhibitory to viral replication and cell viability. Induction of the heat shock response generates a transient increase in the level of Hsps, and this may be what is tolerable for the cell and stimulatory for full-length rescue. In fact, it has been reported that attempts to generate stable cell lines that express high levels of Hsp72 from the β-actin promoter have not been successful, and neomycin-resistant clones established with this construct proved to have altered growth characteristics (52). Thus, it may be necessary to try stimulating full-length rescue using an inducible Hsp72 expression vector that could be effectively turned off after about 48 h.
The mechanism of Hsp72-mediated activation of MV gene expression is not understood. Our results and the results of Oglesbee et al. (34, 35) taken together indicate that MV mRNA synthesis is stimulated by the association of Hsp72 with viral nucleocapsids. This association may be indicative of numerous potential functions. Possibly, Hsp72 interacts with the L polymerase present in nucleocapsids, and this association may affect the activity of the polymerase. The capability of Hsps to modify protein function and conformation is well documented in the case of the steroid receptors (40), and the reverse transcriptase activity of the hepatitis B virus polymerase also appears to be modulated by interaction with several Hsps (21). In the case of MV, it is interesting to speculate that the association of Hsp72 with the L polymerase in the viral nucleocapsid may alter the conformation of L polymerase and stimulate mRNA synthesis. Furthermore, it is interesting to speculate that Hsp72 may participate in the molecular switch that affects the ratio of mRNA transcription to genome replication. It also is possible that the main effect of Hsp72 on viral transcription is exerted by association of Hsp72 with the N protein. Hsp72 may be a modifier of nucleocapsid structure, and in fact, several forms of MV and CDV nucleocapsids have been isolated (33, 43). Potentially, the association of Hsp72 with the N protein may promote assembly or stabilize a transcriptionally active form of nucleocapsid structure. Further analysis of the interactions between Hsp72 and the proteins found in the MV nucleocapsid should prove informative.
ACKNOWLEDGMENTS
We are grateful to Martin Billeter and Frank Radecke for helpful discussion and also thank them for providing plasmids and the 293-3-46 cell line.
REFERENCES
- 1.Andrews J M, Newbound G C, Lairmore M D. Transcriptional modulation of viral reporter gene constructs following induction of the cellular stress response. Nucleic Acids Res. 1997;25:1082–1084. doi: 10.1093/nar/25.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Siedman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1987. [Google Scholar]
- 3.Baron M D, Barrett T. Rescue of rinderpest virus from cloned cDNA. J Virol. 1997;71:1265–1271. doi: 10.1128/jvi.71.2.1265-1271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumberg M B, Chan J, Udem S A. Function of paramyxovirus 3′ and 5′ end sequences. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum; 1991. pp. 235–247. [Google Scholar]
- 5.Boyer J-C, Haenni A-L. Infectious transcripts and cDNA clones of RNA viruses. Virology. 1994;198:415–426. doi: 10.1006/viro.1994.1053. [DOI] [PubMed] [Google Scholar]
- 6.Brostrom C O, Brostrom M A. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acids Res. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory synctial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig E A. The heat shock response. Crit Rev Biochem. 1985;18:239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- 10.Franke E, Yaun H, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 11.Friedman D, Olson E, Georgopoulos C, Tilly K, Herskowitz I, Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984;48:299–335. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcin D, Pelet T, Calain P, Sakai Y, Shioda T, Roux L, Curran J, Kolakofsky D. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA; generation of a novel copyback nondefective interfering virus. EMBO J. 1995;14:6087–6094. doi: 10.1002/j.1460-2075.1995.tb00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gething M-J. Molecular chaperones: clasping the prize. Curr Biol. 1996;6:1573–1576. doi: 10.1016/s0960-9822(02)70775-6. [DOI] [PubMed] [Google Scholar]
- 14.Glick B S. Can Hsp70 proteins act as force-generating motors. Cell. 1995;80:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- 15.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 16.Graham F L, van der Eb A J. A technique for the assay of infectivity of human adenovirus DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 17.Gunther E, Walter L. Genetic aspects of the hsp70 multigene family in vertebrates. Experientia. 1994;50:987–1001. doi: 10.1007/BF01923453. [DOI] [PubMed] [Google Scholar]
- 18.He B, Paterson R G, Ward C D, Lamb R A. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman M A, Banerjee A K. An infectious clone of human parainfluenza virus type 3. J Virol. 1997;71:4272–4277. doi: 10.1128/jvi.71.6.4272-4277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horikami S M, Moyer S A. Synthesis of leader RNA and editing of the P mRNA during transcription by purified measles virus. J Virol. 1991;65:5342–5347. doi: 10.1128/jvi.65.10.5342-5347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Toft D O, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt C, Morimoto R I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato A, Sakai Y, Shioda T, Kondo T, Nakanishi M, Nagai Y. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells. 1996;1:569–579. doi: 10.1046/j.1365-2443.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]
- 24.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Monath T O, Melnick J L, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1177–1204. [Google Scholar]
- 25.Lawson N D, Stillman E A, Whitt M A, Rose J K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 27.Lund P A. The roles of molecular chaperones in vivo. Essays Biochem. 1995;29:113–123. [PubMed] [Google Scholar]
- 28.Martin J, Hartl F U. Chaperone-assisted protein folding. Curr Opin Struct Biol. 1997;7:41–45. doi: 10.1016/s0959-440x(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 29.Milner C M, Campbell R D. Structure and expression of three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- 30.Moss B, Elroy-Stein O, Mizukami T, Alexander W A, Fuerst T R. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 31.Moyer S A, Baker S C, Horikomi S M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990;71:775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- 32.Oglesbee M, Ringler S, Krakowka S. Interaction of canine distemper virus nucleocapsid variants with 70K heat-shock protein. J Gen Virol. 1990;71:1585–1590. doi: 10.1099/0022-1317-71-7-1585. [DOI] [PubMed] [Google Scholar]
- 33.Oglesbee M, Tatalick L, Rice J, Krakowka S. Isolation and characterization of canine distemper virus nucleocapsid variants. J Gen Virol. 1989;70:2409–2419. doi: 10.1099/0022-1317-70-9-2409. [DOI] [PubMed] [Google Scholar]
- 34.Oglesbee M J, Kenney H, Kenney T, Krakowka S. Enhanced production of Morbillivirus gene-specific RNAs following induction of the cellular stress response in stable persistent infection. Virology. 1993;192:556–567. doi: 10.1006/viro.1993.1072. [DOI] [PubMed] [Google Scholar]
- 35.Oglesbee M J, Zheng L, Kenney H, Brooks C L. The highly inducible member of the 70 kDa family of heat shock proteins increases canine distemper virus polymerase activity. J Gen Virol. 1996;77:2125–2135. doi: 10.1099/0022-1317-77-9-2125. [DOI] [PubMed] [Google Scholar]
- 36.Ogura H, Sato H, Kamiya S, Nakamura S. Temperature elevation enhances cell surface expression of measles fusion protein in infected cells. J Gen Virol. 1990;71:2475–2478. doi: 10.1099/0022-1317-71-10-2475. [DOI] [PubMed] [Google Scholar]
- 37.Parks C L, Shenk T. The serotonin 1a receptor gene contains a TATA-less promoter that responds to MAZ and Sp1. J Biol Chem. 1996;271:4417–4430. doi: 10.1074/jbc.271.8.4417. [DOI] [PubMed] [Google Scholar]
- 38.Pattnaik A K, Ball L A, LeGrone A W, Wertz G W. Use of T7 phage polymerase and hepatitis delta virus ribozyme sequences for cDNA synthesis and processing. Cell. 1992;69:1011–20. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- 39.Pratt W B. Control of steroid receptor function and cytoplasmic-nuclear transport by heat shock proteins. Bioessays. 1992;14:841–848. doi: 10.1002/bies.950141209. [DOI] [PubMed] [Google Scholar]
- 40.Pratt W B, Toft D O. Steroid receptor interactions with heat shock proteins and immunophilin chaperones. Endocrine Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 41.Radecke F, Billeter M A. Reverse genetics meets the nonsegmented negative-strand RNA viruses. Rev Med Virol. 1997;7:49–63. doi: 10.1002/(sici)1099-1654(199704)7:1<49::aid-rmv181>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 42.Radecke F, Spielhofer P, Schmeider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins S J, Bussell R H, Rapp F. Isolation and partial characterization of two forms of cytoplasmic nucleocapsids from measles virus-infected cells. J Gen Virol. 1980;47:301–310. doi: 10.1099/0022-1317-47-2-301. [DOI] [PubMed] [Google Scholar]
- 44.Saaverda C, Kuei-Shu T, Amberg D C, Hopper A K, Cole C N. Regulation of mRNA export in response to stress in Saccharomyces cerevisiae. Genes Dev. 1996;10:1608–1620. doi: 10.1101/gad.10.13.1608. [DOI] [PubMed] [Google Scholar]
- 45.Santoro M G. Viral infection. Experientia. 1996;77:337–357. doi: 10.1007/978-3-0348-9088-5_23. [DOI] [PubMed] [Google Scholar]
- 46.Schneider H, Spielhofer P, Kaelin K, Dotsch C, Radecke F, Sutter G, Billeter M A. Rescue of measles virus using a replication-deficient vaccinia-T7 vector. J Virol Methods. 1997;64:57–64. doi: 10.1016/s0166-0934(96)02137-4. [DOI] [PubMed] [Google Scholar]
- 47.Schnell M J, Mebatsion T, Conzelmann K-K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidhu M S, Chan J, Kaelin K, Spielhofer P, Radecke F, Schnieder H, Masurekar M, Dowling P C, Billeter M A, Udem S A. Rescue of synthetic measles virus minireplicons: measles genomic termini direct efficient expression and propagation of a reporter gene. Virology. 1995;208:800–807. doi: 10.1006/viro.1995.1215. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka M, Herr W. Differential transcriptional activation by oct-1 and oct-2: interdependent activation domains induce oct-2 phosphorylation. Cell. 1990;60:375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- 50.Udem S A. Measles virus: conditions for the propagation and purification of infectious virus in high yield. J Virol Methods. 1984;8:123–136. doi: 10.1016/0166-0934(84)90046-6. [DOI] [PubMed] [Google Scholar]
- 51.Whelan S P J, Ball L A, Barr J N, Wertz G T W. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams R S, Thomas J A, Fina M, German Z, Benjamin I J. Human heat shock protein 70 (hsp70) protects murine cells from injury during metabolic stress. J Clin Investig. 1993;92:503–508. doi: 10.1172/JCI116594. [DOI] [PMC free article] [PubMed] [Google Scholar]