Abstract
Background
Among MDR bacteria, carbapenem-resistant Acinetobacter baumannii (CRAB) is a major concern due to the limited therapeutic options. During the COVID-19 pandemic, a worrying increase in the spread of CRAB infections was reported.
Objectives
The study assessed the risk factors for CRAB bloodstream infection (BSI) in patients admitted to the ICU with CRAB colonization, and the related mortality risk factors.
Methods
We conducted a single-centre, observational, prospective study; all consecutive patients with CRAB colonization admitted to the ICU of a tertiary hospital in Rome from January 2021 to September 2022 were included in the study. Univariate and multivariate analyses were performed to investigate BSI and mortality risk factors.
Results
Overall, 129 patients were included in the study; 57 (44%) out of these developed BSI. In our study population, at the multivariable analysis the Charlson comorbidity index (CCI) (P = 0.026), COVID-19 (P < 0.001), multisite colonization (P = 0.016) and the need for mechanical ventilation (P = 0.024) were risk factors independently associated with BSI development. Furthermore, age (P = 0.026), CCI (P < 0.001), septic shock (P = 0.001) and Pitt score (P < 0.001) were independently associated with mortality in the BSI patients. Instead, early appropriate therapy (P = 0.002) and clinical improvement within 72 h (P = 0.011) were shown to be protective factors.
Conclusions
In critically ill patients colonized by CRAB, higher CCI, multisite colonization and the need for mechanical ventilation were identified as risk factors for BSI onset. These predictors could be useful to identify patients at highest risk of BSI.
Introduction
The spread of antimicrobial resistance (AMR) is a global emergency that threatens public health worldwide. Also referred to as the ‘hidden pandemic’, nowadays the AMR impact involves not only global health but is playing an increasingly important role in economic and social fields.1 In 2018, the WHO published a ranking of MDR bacteria with ‘critical priority’, on which carbapenem-resistant Acinetobacter baumannii (CRAB) is in the first place.2
CRAB infection has a mortality rate of up to 70%–80% with an increase in length of hospitalization and healthcare costs, especially in patients admitted to ICUs.3–6 Whether CRAB infection is an associated or attributable mortality risk factor in critically ill patients has still to be defined; however, when attributable mortality data are reported, the mortality rate in CRAB infections remains very high.1,7
Moreover, during the COVID-19 pandemic, there were several nosocomial outbreaks caused by CRAB in ICUs with a significant impact on in-hospital mortality.8–13 Among carbapenem-resistant Gram-negative bacilli bloodstream infections (BSIs), CRAB BSIs are the most serious infections with the highest mortality rates.7
Differently from carbapenem-resistant Enterobacterales (CRE), where there are well-established clinical risk scores for development of BSI in CRE-colonized patients, for CRAB, little is known about the relationship between colonization and BSI development.5,14–21
Appropriate early treatment is one of the most significant factors that may reduce the mortality rate of BSI, as reported for KPC-producing Klebsiella pneumoniae BSI.22 Therefore, it is plausible that knowing the specific risk factors for BSI development in CRAB-colonized critically ill patients might be crucial to prompt early appropriate therapy aimed to reduce associated mortality.
Based on these considerations, the primary objective of this study was to evaluate risk factors for CRAB BSI in ICU CRAB-colonized patients.
Materials and methods
Study design
We conducted a prospective, observational single-centre study in adult patients admitted to the ICUs of a tertiary academic hospital in Rome. From January 2021 to September 2022, all consecutive patients hospitalized in the ICUs with CRAB colonization were included in the study. Inclusion criteria were: (i) age ≥18 years; (ii) admission to ICU; and (iii) colonization by CRAB at any anatomical site. We excluded patients with prior CRAB colonization more than 30 days before the ICU admission, with CRAB BSI without prior colonization, and with expected survival less than 48 h from documented colonization.
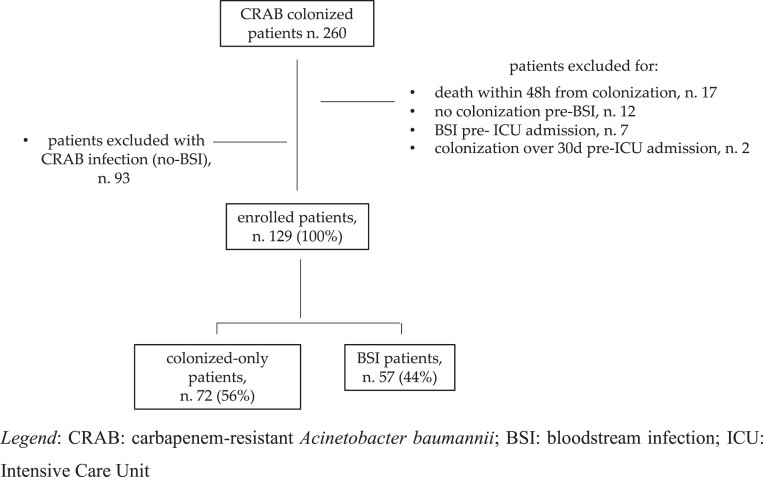
The study population was further divided into two groups: (i) BSI patients: patients who developed CRAB BSI throughout hospitalization with the same resistance profile of the colonizing pathogen; and (ii) colonized-only patients: patients with CRAB colonization who did not develop BSI or other CRAB infection during the entire hospitalization. Patients with CRAB infections other than BSI were excluded from the study population (Figure 1). The prospective nature of the study was based on the consecutive enrolment of patients. However, all complete data were retrospectively extracted afterwards.
Figure 1.
Flow chart of study population.
Medical charts were reviewed by trained doctors and the following information was anonymously recorded in an electronic database: demographics, comorbidities, hospitalization/antibiotic therapy/immunosuppression/surgery in the previous 90 days, previous SARS-CoV-2 infection, cause of hospitalization and ICU admission, laboratory and clinical data on the day of colonization and of BSI onset, site of colonization, timing of colonization and BSI onset, time from colonization to BSI onset, microbiological data and antibiotic regimens in the BSI group, duration of ICU and hospital stay, clinical cure, CRAB BSI recurrence, development of new CRAB infection, development of secondary infection, in-hospital mortality and 7 day, 14 day and 28 day mortality from colonization and BSI onset.
Endpoints
The primary outcomes were CRAB BSI onset and the risk factors associated with BSI development. The secondary outcomes were: (i) mortality from BSI onset at 7 days, 14 days and 28 days and its related predictors in the BSI group; and (ii) the impact of different treatment regimens on 28 day mortality.
ICU settings
The ICUs at the tertiary hospital in Rome, Policlinico Umberto I, are one general, one dedicated to the emergency department (ER-ICU), one dedicated to neurosurgery (NS-ICU), one dedicated to cardiothoracic surgery (CTS-ICU), one dedicated to transplantation and one dedicated to COVID-19. The beds are distributed as follows: 18 in the general ICU, 6 in the ER-ICU, 9 in the NS-ICU, 6 in the CTS-ICU and 6 in the transplantation ICU, while the number of COVID-19 ICU beds varied according to epidemiological needs during the study period, ranging from 15 to 54.
Microbiological analyses
The identification of CRAB strains was based accordingly on local laboratory techniques. Blood culture bottles were incubated in the automatic BacT/ALERT Virtuo system (bioMérieux, Inc., Marcy l’Étoile, France). Isolated colonies from blood cultures or other positive cultures were identified using a MALDI-TOF MS system (Bruker Daltonik GmbH, Bremen, Germany). Antimicrobial susceptibility was tested using the MicroScan WalkAway system (Beckman Coulter, Inc., Brea, CA, USA). The determination of cefiderocol susceptibility, when available for the clinicians, was performed with the disc diffusion method. A zone diameter of ≥17 mm for the cefiderocol 30 μg disc corresponded to MIC values below the pharmacokinetic/pharmacodynamic breakpoint of S ≤ 2 mg/L. The MICs of antibiotics were assessed by following EUCAST criteria.23
Definitions
Infections were defined according to the standard definitions of ECDC.24 CRAB BSI was defined when CRAB was isolated from blood cultures in the presence of clinical signs of infection and BSI onset was considered the date of the index blood culture collection. Primary BSI was defined as a BSI occurring in patients without a recognized source of infection.
MDR, XDR and pandrug-resistant (PDR) bacteria were defined according to the classification of Magiorakos et al.25
The burden of comorbidities was estimated by means of the Charlson comorbidity index (CCI), while patients’ severity at ICU admission was defined by the SAPS II score.26,27 The severity of BSI was defined according to the Pitt bacteraemia score at BSI onset.28 Septic shock was defined according to the international consensus.29
Immunosuppression was defined as either steroid therapy with prednisone (or its equivalent) at a dose of >0.5 mg/kg/day for at least 1 month or the receipt of chemotherapy, TNF-α inhibitors, cyclophosphamide, azathioprine, methotrexate or mycophenolate mofetil in the previous 90 days.
The source control was achieved when all those physical measures used to control the focus of BSI and to restore the optimal function of the affected area were performed.
According to the hospital’s guidelines for ICUs, rectal/stool swabs, respiratory and urine cultures were performed at ICU admission and routinely re-evaluated once a week for MDR organism (MDRO) strains; in the other hospital settings, regular MDRO screening has not been recommended unless suggested for clinical or epidemiological reasons. Screening for Gram-negative MDROs by throat and skin swabs is not performed in our hospital. Colonization was defined as a positive culture of a specimen from any anatomical site in the absence of clinical signs of infection. Multisite colonization was defined as a positive culture from more than one specimen from different anatomical sites in the same patient during the ICU hospitalization before BSI onset.
The differences between CRAB colonization and infection, in sites other than rectal swab isolation, were evaluated case by case according to patients’ clinical conditions, presence of signs/symptoms or laboratory parameters suggestive of infections and the eventual targeted treatment chosen by the dedicated Infectious Disease consultants. In case of doubt, a shared discussion was made case by case.
Appropriateness of therapy
Early appropriate therapy was defined as the use of at least one in vitro active drug within the first 24 h from BSI onset, definitive antibiotic therapy was defined as the specific antimicrobial treatment administered after the availability of susceptibility tests, and the time to definitive therapy was the number of days from BSI onset to the definitive therapy. Definitive antibiotic therapy was considered appropriate if the CRAB strain isolated was susceptible to the chosen drug regimen.
Outcomes
BSI onset was considered as a CRAB BSI occurring during hospitalization.
Clinical improvement at 48–72 h after initiation of antibiotic therapy was defined as at least one of the following: discontinuation of treatment with inotropic drugs if the patient was in septic shock, or disappearance of fever for at least 48 h, or reduction of serum procalcitonin values by at least 80% compared with initial value/achievement of a procalcitonin value of <0.5 ng/mL, or reduction of at least 75% of the maximum C-reactive protein (CRP) value achieved.30
Clinical cure was the resolution of symptoms after discontinuation of antibiotic therapy, whereas microbiological positive outcome was defined as negative follow-up blood cultures at 72 h, 7 days or 14 days after the start of antibiotic treatment.
CRAB BSI recurrence was defined as the onset of a second microbiologically documented CRAB BSI in the 30 days after the end of treatment in a patient who had previously achieved a clinical cure and microbiological positive outcome.
New CRAB infection was considered as isolation of CRAB causing infections other than BSI in the 30 days after the end of treatment and achieving a clinical cure.
Secondary infection was defined as an infection caused by an MDRO other than CRAB in the 30 days after the start of treatment for BSI.
All-cause mortality at 7, 14 and 28 days after documented colonization and BSI onset was recorded.
Statistical analyses
The data, unless otherwise stated, were given as medians with IQRs for continuous variables and as simple frequencies (n) and percentages (%) for categorical variables. Student’s t-test and Mann–Whitney test were used for unpaired samples, as appropriate. Dichotomous variables were compared using Fisher’s exact tests or chi-squared test statistics, as appropriate. Binary logistic regression analysis was used to identify the demographic characteristics and risk factors for the pre-specified outcome examined. Survival was analysed by Kaplan–Meier curves and the statistical significance of the differences between the two groups was assessed using the log-rank test.
Multivariable logistic regression model was performed to tease out the independent predictors for BSI onset and for 28 day mortality in patients with BSI. The multivariate model was constructed using a forward stepwise procedure, entering all variables shown to be significant at the univariable analysis and those deemed clinically significant for the chosen outcome. Interaction effects between variables were also taken into consideration in the final model. P value analyses were two-sided and a P value of <0.05 was considered statistically significant. All statistical analyses were performed with Statistical Program for the Social Sciences (SPSS, version 22, SPSS Inc., Chicago, IL, USA) software.
Ethics
The study was approved by the local Ethical Committee (no. 0341/2023), and informed consent was waived due to the observational nature of the research. The study was performed in line with the principles of the Declaration of Helsinki.
Results
General characteristics of study population
Overall, 129 patients were included in the study: 39 (30%) women and 90 (70%) men, with a median age of 64 (IQR 51–74) years. The general features of the study population are described in Table 1. Among the causes of ICU admission, the most common was respiratory failure, with 53 (41%) patients, followed by neurological disease (25; 19%) and polytrauma (20; 15.5%). All the enrolled COVID-19 patients presented with a critical illness with at least an acute respiratory distress syndrome condition and need for invasive or non-invasive ventilation. The median number of days from ICU admission to CRAB colonization was 12 (5–20) days, with the majority of patients (98%) receiving antibiotic therapy before colonization. Fifty-seven (44%) patients presented multisite CRAB colonization.
Table 1.
General characteristics of study population and comparison between colonized-only and BSI patients
| Characteristics | General population N = 129 | Colonized-only patients N = 72 | BSI patients N = 57 | P value |
|---|---|---|---|---|
| GENERAL, N (%) | ||||
| Age, years, median (IQR) | 64 (51–74) | 65.5 (48.5–73.7) | 61 (55–74) | 0.344 |
| Gender, female/male | 39(30)/90(70) | 24 (33)/48(77) | 15(26)/42(74) | 0.771 |
| Department of hospitalization, | ||||
| Medical | 35 (27) | 18 (25) | 17 (30) | 0.556 |
| Surgery | 20 (15) | 9 (12.5) | 11 (19) | 0.332 |
| ICU | 74 (58) | 45 (62.5) | 29 (51) | 0.212 |
| Cause of in-hospital admission, | ||||
| Lung diseases (including SARS-CoV-2 infection) | 47 (36) | 21 (29) | 26 (45.5) | 0.066 |
| Heart diseases | 6 (5) | 1 (1.5) | 5 (9) | 0.209 |
| Abdominal diseases | 12 (9) | 7 (9.5) | 5 (9) | 1.0 |
| Kidney diseases | 1 (1) | 1 (1.5) | 0 (0) | 1.0 |
| Neurological diseases | 35 (27) | 21 (29) | 14 (24.5) | 0.69 |
| Haematological diseases | 1 (1) | 1 (1.5) | 0 (0) | 1 |
| Sepsis or septic shock | 2 (1.5) | 2 (3) | 0 (0) | 0.502 |
| Trauma/polytrauma | 20 (15.5) | 15 (21) | 5 (9) | 0.086 |
| Other infection | 1 (1) | 0 (0) | 1 (1.5) | 0.442 |
| Other | 4 (3) | 3 (4) | 1 (1.5) | 0.689 |
| Cause of ICU admission | ||||
| Septic shock | 5 (4) | 4 (6) | 1 (1.5) | 0.382 |
| Respiratory failure | 53 (41) | 24 (33) | 29 (51) | 0.049 |
| Trauma/polytrauma | 20 (15.5) | 15 (21) | 5 (9) | 0.086 |
| Cardiogenic shock/cardiac arrest | 5 (4) | 4 (6) | 1 (1.5) | 0.382 |
| Neurological disease | 25 (19) | 16 (23) | 9 (16) | 0.381 |
| Post surgery | 18 (14) | 8 (11) | 10 (17.5) | 0.205 |
| Other | 3 (2.5) | 1 (1.5) | 2 (3.5) | 0.583 |
| Type of ICU setting | ||||
| Multidisciplinary | 34 (26) | 23 (32) | 11 (19) | 0.113 |
| COVID-19 | 50 (39) | 21 (29) | 29 (51) | 0.018 |
| Neurosurgery | 29 (22.5) | 18 (26) | 11 (19) | 0.526 |
| Transplantation | 2 (1.5) | 1 (1.5) | 0 (0) | 1.0 |
| Cardiothoracic surgery | 1 (1) | 1 (1.5) | 1 (1.5) | 1.0 |
| Emergency room | 13 (10) | 8 (11) | 5 (9) | 0.773 |
| IN PREVIOUS 90 DAYS FROM ADMISSION, N (%) | ||||
| Hospitalization | 26 (20) | 15 (21) | 11 (19) | 0.227 |
| ICU admission | 19 (15) | 9 (13) | 10 (18) | 0.939 |
| Antibiotic therapy | 37 (29) | 18 (25) | 19 (33) | 0.969 |
| MDRO infection | 4 (3) | 1 (1.5) | 3 (5) | 0.713 |
| Gram-negative MDR infection | 3 (2.5) | 2 (3) | 1 (1.5) | 0.797 |
| Immunosuppression | 7 (5.5) | 4 (6) | 3 (5) | 0.960 |
| Previous surgery | 50 (39) | 29 (40) | 21 (37) | 0.363 |
| COMORBIDITIES, N (%) | ||||
| Myocardial infarction | 12 (9) | 3 (4) | 9 (16) | 0.356 |
| Congestive heart failure | 17 (13) | 9 (13) | 8 (14) | 0.399 |
| Hypertension | 55 (43) | 28 (39) | 27 (47) | 0.034 |
| Obesity (BMI ≥ 30 kg/m2) | 15 (12) | 10 (14) | 5 (9) | 0.987 |
| Cerebrovascular disease | 16 (12) | 10 (14) | 6 (11) | 0.242 |
| COPD | 12 (9) | 6 (8) | 6 (11) | 0.851 |
| Diabetes mellitus | 22 (17) | 11 (15) | 11 (19) | 0.185 |
| Chronic kidney diseasea | 4 (3) | 0 (0) | 4 (7) | 0.025 |
| Solid tumour | 19 (15) | 8 (11) | 11 (19) | 0.952 |
| Leukaemia/lymphoma | 7 (5.5) | 3(4) | 4 (7) | 0.238 |
| CCI, median (IQR) | 3 (1–5) | 3 (1–4) | 3 (1–6) | 0.041 |
| SAPS II score, median (IQR) | 36 (27–49) | 35 (26–49.5) | 38 (29–46) | 0.172 |
| COVID-19 hospitalization | 54 (42) | 24 (33) | 30 (53) | 0.014 |
| Need for CPAP/HFNC/NIV | 45 (83) | 20 (28) | 25 (44) | 0.367 |
| Need for OTI | 23 (43) | 9 (13) | 14 (25) | 0.104 |
| CRAB COLONIZATION, N (%) | ||||
| First colonization in ICU | 123 (95) | 70 (97) | 53 (93) | 0.396 |
| Timing of colonization, median (IQR) | ||||
| From ER admission | 18 (10–27) | 17.5 (10–31.2) | 18 (10–24) | 0.011 |
| From ICU admission | 12 (5–20) | 13 (4–21.2) | 11 (5–18) | 0.016 |
| Site of colonization | ||||
| Respiratory tract | 35 (27) | 19 (26.5) | 16 (28) | 0.766 |
| Rectal swab | 93 (72) | 52 (72) | 41 (72) | 1.0 |
| Urine | 1 (1) | 1 (1.5) | 0 (0) | 0.987 |
| Multisite | 57 (44) | 25 (35) | 32 (56) | 0.003 |
| Number of sites | ||||
| 1 | 72 (56) | 47 (65) | 25 (44) | 0.082 |
| 2 | 49 (38) | 21 (29) | 28 (49) | 0.059 |
| 3 | 8 (6) | 4 (6) | 4 (7) | 0.679 |
| Mechanical ventilation | 81 (67) | 39 (54) | 42 (74) | 0.002 |
| ECMO | 5 (4) | 2 (3) | 3 (5) | 0.654 |
| CRRT | 9 (7) | 3 (4) | 6 (11) | 0.240 |
| Antibiotic therapy before colonization | 127 (98) | 71 (99) | 56 (98) | 0.321 |
| Days of antibiotic therapy, median (IQR) | 12 (4–21) | 13 (5–21.7) | 11 (3–20) | 0.044 |
| Steroids before colonization | 74 (57) | 42 (58) | 32 (56) | 0.427 |
| Days of steroids, median (IQR) | 11 (7–18) | 11 (6.2–15.7) | 12.5 (7–19) | 0.242 |
| CVC | 115 (89) | 63 (88) | 52 (91) | 0.897 |
| Vesical catheter | 127 (98) | 70 (97) | 57 (100) | 0.446 |
| Drainage | 17 (13) | 7 (10) | 10 (17.5) | 0.917 |
| OUTCOMES, MEDIAN (IQR) | ||||
| Hospitalization length | 45 (27–75) | 44.5 (26–70) | 51 (29.5–83) | 0.097 |
| ICU hospitalization length | 30 (18–54) | 24 (15.5–42) | 33.5 (20–69) | 0.024 |
| IN-HOSPITAL ALL-CAUSE MORTALITY, N(%) | 75 (58) | 36 (50) | 39 (68.5) | 0.002 |
| Mortality from colonization, N (%) | ||||
| 7 days | 26 (20) | 12 (17) | 14 (25) | 0.639 |
| 14 days | 33 (25) | 15 (21) | 18 (32) | 0.098 |
| 28 days | 50 (39) | 23 (32) | 27 (47) | 0.036 |
| Days from colonization to mortality | 19 (7–34) | 17 (5.5–33) | 21 (8.5–40.5) | 0.587 |
CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula; NIV, non-invasive ventilation; OTI, orotracheal intubation; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy. Bold type indicates statistical significance.
From moderate CKD (creatinine > 3 mg/dL) to dialysis or status post-kidney transplant.
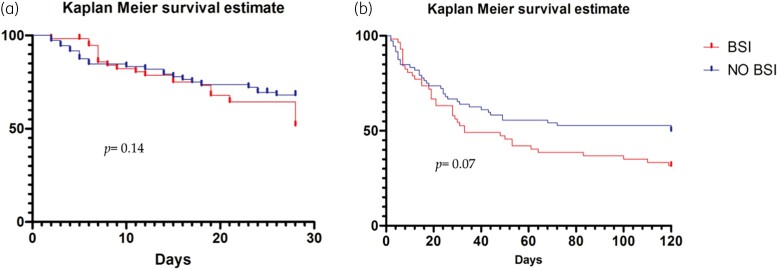
During hospitalization, 57 (44%) patients developed a CRAB BSI, whereas the remaining 72 (56%) did not. The overall in-hospital mortality was 58%, being 50% and 68.5% in the colonized-only and in BSI patients, respectively (P = 0.002). As for the 28 day mortality rate from CRAB colonization, we observed a statistically significant difference between colonized-only patients and BSI patients (32% versus 47%, respectively, P = 0.0036). However, no significant difference was observed for the cumulative survival analyses on 28 day and on overall in-hospital mortality. Of note in the overall mortality, there was a trend toward higher mortality in BSI patients than in colonized-only ones (Figure 2).
Figure 2.
Cumulative proportions of all-cause 28 day (a) and overall (b) in-hospital mortality. The cumulative proportions of in-hospital mortality were estimated from CRAB colonization between colonized-only patients (no BSI) and BSI patients (BSI).
Risk factors for BSI onset
Univariate analysis between colonized-only patients and BSI patients showed significant differences in terms of CCI (P = 0.041), hypertension (P = 0.034), COVID-19 (P = 0.014), colonization time from ICU admission (P = 0.016), need for mechanical ventilation at the time of colonization (P = 0.002), multisite colonization (P = 0.003) and days of antibiotic therapy prior to colonization (P = 0.044), whereas no significant difference was observed when considering the subgroups of single antibiotic classes used prior to colonization (data not shown). In multivariate analyses, an increased CCI (P = 0.026), COVID-19 (P < 0.001), mechanical ventilation (P = 0.012) and multisite colonization (P = 0.012) were independently associated with BSI onset (Table 2a).
Table 2.
Multivariable analysis
| Risk factors | OR (95% CI) | P value |
|---|---|---|
| Risk factors for BSI onset in patients with CRAB colonization | ||
| CCI | 1.34 (1.02–15.2) | 0.026 |
| COVID-19 | 2.32 (1.72–15.8) | <0.001 |
| Hypertension | 1.87 (0.91–3.87) | 0.089 |
| SAPS II | 2.5 (0.88–11.5) | 0.091 |
| Timing of ICU to colonization | 1.2 (0.84–9.9) | 0.122 |
| Multisite >1 | 2.4 (1.2–4.90) | 0.016 |
| Mechanical ventilation | 2.34 (1.1–5.02) | 0.024 |
| Mortality risk factors in the BSI patients | ||
| Age | 1.9 (1.72–15.2) | 0.026 |
| CCI | 2.41 (1.7–15.8) | <0.001 |
| COVID-19 | 1.2 (0.82–8.8) | 0.122 |
| SAPS II | 2.5 (0.87–11.5) | 0.091 |
| PMN/LYM | 1.2 (0.81–9.9) | 0.122 |
| Steroids | 0.9 (0.8–7.98) | 0.765 |
| BSI CVC-related | 0.7 (0.4–11.87) | 0.455 |
| Source control | 0.6 (0.1–16.6) | 0.217 |
| Early active therapy | 0.32 (0.02–0.68) | 0.002 |
| Septic shock | 1.5 (1.41–9.7) | 0.001 |
| Pitt score | 2.7 (1.6–14.4) | <0.001 |
| FUBCs | 1.14 (0.92–20.1) | 0.077 |
| Clinical improvement at 72 h | 0.4 (0.2–0.79) | 0.011 |
Multisite >1, patients with more than one colonization; PMN/LYM, neutrophil/lymphocyte ratio; early active therapy, use of at least one in vitro active drug within the first 24 h from the BSI onset; FUBCs, follow-up blood cultures performed. Bold type indicates statistical significance.
Patients with CRAB BSI
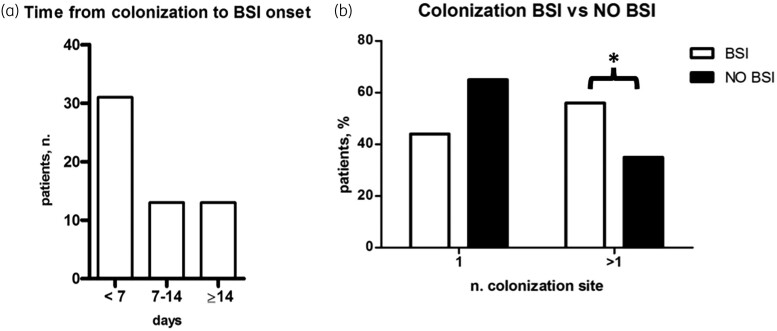
The characteristics of the BSI group are described in Table 1 and Table 3. The median time from colonization to BSI onset was 6 (0–10) days (Figure 3a); the majority of BSI patients (31; 54%) showed BSI onset within the first week from colonization. The patients were more likely to present rectal colonization (41; 72%), while 32 patients (56%) showed multisite colonization. The features of the number of colonization sites and the comparison between BSI and colonized-only patients are described in Figure 3b.
Table 3.
General characteristics of BSI population and comparison between survivors and non-survivors at 28 days from BSI onset
| Characteristics | BSI patients N = 57 | 28 day survivors N = 30 | 28 day non-survivors N = 27 | P value |
|---|---|---|---|---|
| GENERAL, MEDIAN (IQR) | ||||
| Age, years | 61 (55–74) | 59 (50.25–71.2) | 66 (59–76) | 0.038 |
| Gender, female/male, N (%) | 15(26)/42(74) | 10 (33)/20 (67) | 5 (19)/22(81) | 0.207 |
| Timing of colonization | ||||
| From ER admission | 24 (16–37) | 27 (16–41.5) | 21 (16.5–28) | 0.186 |
| From ICU admission | 19 (10–29) | 24.5 (12–39) | 15 (8.5–2-.5) | 0.053 |
| COMORBIDITIES, N (%) | ||||
| CCI, median (IQR) | 3 (1–6) | 2 (1–3) | 4 (2–7) | 0.024 |
| SAPS II score, median (IQR) | 38 (29–46) | 33.5 (26.2–41) | 44 (36.5–50.5) | 0.050 |
| COVID-19 hospitalization | 29 (53) | 10 (33) | 19 (70) | 0.010 |
| Clinical features at BSI onset, n (%) | ||||
| Inotropic support | 23 (40) | 7 (23) | 16 (59) | 0.006 |
| Mechanical ventilation | 40 (70) | 18 (60) | 22 (8) | 0.076 |
| ECMO | 3 (5) | 0 (0) | 3 (11) | 0.083 |
| CRRT | 7 (12) | 2 (7) | 5 (19) | 0.191 |
| Pitt score, median (IQR) | 5 (2–8) | 3 (1.25–6) | 8(4–8) | 0.001 |
| Septic shock | 17 (30) | 3 (10) | 14 (52) | 0.001 |
| Source of infection | ||||
| Lung (no VAP) | 6 (10.5) | 1 (3) | 5 (19) | 0.076 |
| VAP | 18 (31.5) | 9 (30) | 10 (37) | 0.583 |
| Urine tract | 1 (1.5) | 1 (3) | 0 (0) | 0.326 |
| Skin and soft tissue/wound | 1 (1.5) | 1 (3) | 0 (0) | 0.326 |
| Primary BSI | 20 (35) | 9 (30) | 11 (41 | 0.407 |
| CVC-related | 11 (19) | 9 (30) | 1 (4) | 0.007 |
| Source control | 12 (21) | 10 (33) | 2 (7) | 0.012 |
| Laboratory findings at BSI onset, median (IQR) | ||||
| PMN × 109 | 7.72 (5.2–14) | 7.1 (5.2–10.2) | 9.14 (6.4–15.6) | 0.103 |
| LYM × 109 | 0.76 (0.5–1.07) | 0.94 (0.68–1.16) | 0.6 (0.4–0.86) | 0.026 |
| PMN/LYM | 10.7 (5.3–25.6) | 9.1 (3.92–13.6) | 13.6 (8.3–30.3) | 0.030 |
| CRP, mg/dL | 14.1 (7.6–22.2) | 13.5 (4.65–20.5) | 14.5 (10.4–24.2) | 0.564 |
| PCT, ng/mL | 0.58 (0.19–3.4) | 0.37 (0.197–3.9) | 0.87 (0.19–3.43) | 0.817 |
| Albumin, g/dL | 2.6 (2.5–3) | 2.6 (2.5–3) | 2.7 (2.4- 3.1) | 0.808 |
| Microbiological data, n (%) | ||||
| MDR resistance profile | 9 (16) | 4 (13) | 5 (18) | 0.722 |
| XDR resistance profile | 48 (84) | 26 (87) | 22 (82) | 0.722 |
| Colistin resistant | 9 (16) | 2 (7.5) | 7 (26) | 0.070 |
| Availability of cefiderocol susceptibility | 46 (81) | 22 (73) | 24 (88) | 0.186 |
| Of which cefiderocol resistant | 2 (4.5) | 1 (7) | 1 (3) | 1.0 |
| Treatment data, n (%) | ||||
| Early active therapy | 38 (65.5) | 23 (77) | 15 (56) | 0.034 |
| Time to definitive therapy, median (IQR) | 1 (1–2) | 1 (1–1.5) | 1 (0.25- 3) | 0.838 |
| Definitive therapy within 48 h | 31 (54) | 19 (63) | 12 (44) | 0.189 |
| Definitive appropriate therapy | 45 (79) | 25 (83) | 20 (74) | 0.519 |
| Monotherapy | 11 (19) | 6 (20) | 5 (19) | 0.828 |
| Combination therapy | 40 (70) | 21 (70) | 19 (70) | 0.934 |
| Regimen based on | ||||
| Colistin | 41 (72) | 23 (77) | 18 (67) | 0.413 |
| Cefiderocol | 14 (24.5) | 7 (23) | 7 (26) | 0.825 |
| Fosfomycin | 29 (51) | 13 (43) | 15 (59) | 0.237 |
| Ampicillin/sulbactam | 18 (31.5) | 9 (30) | 8 (30) | 0.976 |
| Outcomes, n (%) | ||||
| Microbiological outcomes, FUBC performed | 39 (68.5) | 24 (80) | 15 (56) | 0.042 |
| FUBC negative at: | ||||
| 72 h | 7 (12.5) | 4 (13) | 3 (11) | 0.788 |
| 7 days | 26 (45.5) | 16 (53) | 10 (37) | 0.952 |
| 14 days | 36 (63) | 22 (73) | 14 (52) | 0.798 |
| Clinical improvement at 72 h | 17 (30) | 15 (50) | 2 (7) | 0.001 |
| Clinical cure | 28 (49) | 26 (87) | 2 (7) | 0.001 |
| BSI recurrence at 30 days | 5 (9) | 3 (10) | 2 (7) | 0.705 |
| New CRAB infection, no BSI (after BSI) | 14 (24.5) | 12 (40) | 2 (7) | 0.003 |
| Other MDRO infection at 30 days | 17 (30) | 15 (50) | 2 (7) | 0.002 |
| ICU hospitalization length, median (IQR) | 33.5 (20–69) | 64 (34–81) | 22 (18–32) | <0.001 |
| Hospitalization length, median (IQR) | 51 (29.5–83) | 73.5 (54.7–122.2) | 31 (20.5–43) | <0.001 |
| Mortality from BSI onset | ||||
| 7 days | 14 (25) | 0 (0) | 14 (52) | 0.001 |
| 14 days | 18 (32) | 0 (0) | 18 (67) | 0.001 |
| 28 days | 27 (47) | 0 (0) | 27 (100) | <0.001 |
| In-hospital all cause mortality | 39 (68.5) | 12 (43) | 27 (100) | 0.001 |
ER, Emergency Department; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; PMN, neutrophils; LYM, lymphocytes; PMN/LYM, neutrophil/lymphocyte ratio; CRP, C-reactive protein; PCT, procalcitonin; FUBC, follow-up blood culture. Bold type indicates statistical significance.
Figure 3.
CRAB colonization in BSI patients. (a) Timing from colonization to BSI onset in the BSI patients group. (b) Relationship between single versus multisite colonization in the no BSI patients and BSI patients. * P < 0.05.
Primary bacteraemia was the BSI source in 20 (35%) cases, followed by 18 (31.5%) cases with ventilator-associated pneumonia (VAP) and 11 (19%) cases of central venous catheter (CVC)-related BSI. As for microbiological data, 84% of the A. baumannii strains isolated showed an XDR resistance profile; resistance to colistin was demonstrated in 9 (16%) cases, while 2 (4.5%) out of 46 (81%) strains tested for cefiderocol showed in vitro resistance. As for treatment regimens, colistin was the only drug used in monotherapy (11 patients, 19%) and the most commonly used drug in combination regimes (30 patients, 75%), with the combination colistin + fosfomycin used in almost 25% of patients. Appropriate therapy in the first 24 h after the development of BSI was administered in 65.5% of patients, while definitive therapy was effective in 79%, with almost 55% of patients receiving it in the first 48 h.
Risk factors for 28 day mortality in patients with BSI
In the BSI population, we assessed risk factors for 28 day mortality. Characteristics of the surviving and non-surviving BSI patients are described in Table 3. On multivariate analysis, we observed that age (P = 0.021), an increased CCI (P < 0.001), septic shock (P = 0.001) and Pitt score (P < 0.001) were factors independently associated with mortality, while early appropriate therapy within 24 h (P = 0.002) and clinical improvement at 72 h (P = 0.011) demonstrated a protective prognostic effect on outcome (Table 2b).
Discussion
In this study, we observed that 44% of CRAB-colonized patients admitted to an ICU subsequently developed a CRAB BSI during hospitalization, more frequently in the first 7 days from colonization. Burden of comorbidities (expressed by the CCI), COVID-19, multisite colonization and the need for mechanical ventilation were risk factors for CRAB BSI. In addition, we confirmed that the patients’ comorbidities, as well as clinical severity at bacteraemia onset, were the most significant prognostic factors of poor outcome in BSI patients, whereas the prompt start of appropriate antibiotic therapy was a predictor of survival.
To the best of our knowledge, this is the first study in the COVID-19 era that explored risk factors for developing BSI in critically ill patients with previous CRAB colonization.
The role of CCI and pneumonia, particularly VAP, as factors associated with BSI onset has been already demonstrated.16,18,21 Although mechanical ventilation is a known risk factor for CRAB colonization and infection, often associated with pneumonia, in our study population we have observed how ventilation per se is associated with BSI development.31 A rather interesting aspect of our research is that we have identified the importance of COVID-19 and multisite colonization as risk factors for CRAB BSI. Indeed, our study confirms the negative impact of COVID-19 on patients admitted to the ICU, predisposing them to the development of invasive infections with high mortality rates.8,9 The relationship between SARS-CoV-2 infection and CRAB BSI onset could be explained by translocation phenomena both at the respiratory tract level, due to the severe pneumonia, and at the intestinal level, related to the combined effect of hypoxic and direct virus damage. Indeed, several authors have pointed out a relevant dysfunctional gut mucosal barrier in COVID-19 severe patients due to the expression of ACE-2 on enterocytes, which might act as an entrance site for SARS-CoV-2, and high IL-6 levels, which may promote and maintain systemic inflammation causing, at the end, the so-called cytokine storm typical of severe COVID-19 infection.32 Furthermore, recent reports showed that low-grade endotoxaemia is detectable in patients with COVID-19 and is associated with thrombotic events, probably due to activation of NOX-2, increased oxidative stress, low bioavailability of nitric oxide and endothelial dysfunction.33,34 A change in the microbiota and persistent microbial translocation has been observed during severe SARS-CoV-2 infections, a condition that could play a pathogenetic role in BSI development during COVID-19, a hypothesis supported by the high rate of primary bacteraemia that we observed.33,35,36 A recent study evaluated the possible role of a persistent high level of microbial translocation as the pathogenetic trigger for the development of primary BSI following Clostridioides difficile infection (CDI); the authors found that patients who developed primary BSI maintained high levels of LPS-binding protein (LBP) and low levels of EndoCAb IgM, both markers of microbial translocation, while patients who did not develop BSI showed a reduction of microbial translocation levels, similar to that of healthy donors.37
On the other hand, the impact of the pandemic on the healthcare systems, with its interference in infection control and antibiotic stewardship protocols, could have affected the diffusion of CRAB colonization and infection in ICU settings, as recently shown.10,11,38
However, one of the most important findings of the present research was the role of multisite colonization as an independent predictor of BSI. Indeed, while its importance had been previously demonstrated in Enterobacterales BSI, being incorporated in the Giannella score,14 no data regarding CRAB BSI exist so far. It could be speculated that the burden of colonization, expressed as the presence of CRAB at different sites, and the homeostasis alteration in particular organs, such as the gut and the lungs, may favour bacterial translocation and subsequent BSI onset.
Although the use of antibiotic therapy, particularly β-lactams, was a known risk factor for infection in previous studies, in our research, neither duration nor specific classes of antibiotics reached statistical significance as risk factors for BSI.19,20,39
Thus, following the results of our study, a patient colonized by CRAB is at highest risk of BSI development if colonization is localized at multiple sites and in the presence of a high burden of comorbidities at baseline, SARS-CoV-2 infection and mechanical ventilation. Furthermore, the first week after CRAB colonization seems to be the most vulnerable period. These factors could play a broader role in terms of infection control and antimicrobial stewardship programmes. As a matter of fact, on the one hand, highlighting which patients are at higher risk of BSI onset could help clinicians to start prompt appropriate therapy with an improvement in the clinical outcome; on the other hand, by understanding which patients are at low risk of BSI development, the use of broad-spectrum antibiotics active against CRAB could be avoided, with a reduction in consumption of WHO ‘reserve’ class drugs.
We confirmed that mortality in colonized-only or BSI patients remains very high.3,19,39,40 In our BSI patients, we found a 28 day mortality rate of 47%, similar to that published recently (42%) but lower than that observed previously in another study (73%).4,7
In BSI patients, we confirmed that the factors associated with mortality are age, the burden of comorbidities stratified by CCI, and clinical severity at BSI onset, defined in terms of Pitt score and septic shock. These risk factors have been demonstrated in numerous studies on A. baumannii and validated as prognostic factors for Enterobacterales BSIs in the INCREMENT score.4,15,19–21,40 However, no significant association was observed between mortality and clinical severity on ICU admission (SAPS II score), source of BSI, control of the source of infection, use of steroids, or prolonged hospitalization, as shown in other studies.4,16,41,42 A recent study reported risk factors for infection-related mortality in A. baumannii, dividing them into modifiable and non-modifiable factors. Improving the management of the modifiable risk factors (such as hospital stay, duration of antibiotic therapy, invasive procedures or ICU admission) should be key to improving clinical outcomes in these difficult-to-treat infections, as has been already described.42
COVID-19 did not have a direct negative impact on mortality either, despite the statistical significance observed in the univariate analysis between patients who survived and died by 28 days. Thus, COVID-19 does not appear to have had a direct impact on mortality in our study population, representing only a risk factor for the development of BSI. This is a noteworthy finding, which provides additional data about the relationship between COVID-19 and CRAB infection, a great challenge for clinicians in these pandemic years.10
Early appropriate therapy and related clinical improvement within 72 h are confirmed as important protective factors; however, in our study no specific drug regimen has been associated with survival.22,39,43 Indeed, neither colistin- nor cefiderocol-based regimens demonstrated higher survival rates and not even combination therapy compared with monotherapy achieved a significant difference between the two groups. Although these results are in line with the lack of strong scientific evidence in terms of optimal therapy for CRAB infections reported by the recent European guidelines, our study population is certainly too small to be able to analyse the efficacy of different treatment regimens, and further studies are needed to investigate this issue.44
Our study undoubtedly presents important limitations that should be acknowledged. Firstly, the observational nature of the study and the relatively small sample size of patients with BSI did not allow an exhaustive analysis in terms of BSI onset and risk factors for mortality as well as the effectiveness of the drug regimens used. Secondly, the retrospective data extraction afterwards to complete the data entry could have influenced the prospective nature of the study design, leading to some biases. Thirdly, having enrolled patients in the COVID-19 era makes it more complex to compare our findings with previous studies and to generalize our results to settings without SARS-CoV-2 infections. However, important relationships emerged between COVID-19 and CRAB, which will certainly have future clinical implications and require further studies.
We decided to exclude patients with expected survival less than 48 h from colonization in order to avoid bias unrelated to the CRAB colonization, but rather, related to the highly compromised clinical conditions of the patients. Indeed, by including colonized patients who died within 48 h, the associated risk factors for BSI development would have been probably biased by the patients’ conditions.
Finally, the single-centre nature of the study, reflecting the ecology and clinical management of intensivists and Infectious Disease consultants in our hospital, requires confirmation in further centres with similar colonization and CRAB infection epidemiology. Definitely, a larger and multicentre study will be essential to provide robust and generalizable results.
In conclusion, we showed that multisite colonization, burden of comorbidities, COVID-19 and need for mechanical ventilation are risk factors for CRAB BSI onset in critically ill patients with CRAB colonization. Of note, BSI occurred more frequently in the first 7 days from colonization.
These predictors for CRAB BSI could be useful to identify patients at highest risk of BSI, prompting clinicians to start the most appropriate empirical therapy early. Should our results be confirmed in future studies, these factors could become useful tools in antibiotic stewardship protocols.
Contributor Information
Francesco Cogliati Dezza, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Sara Covino, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Flavia Petrucci, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Federica Sacco, Microbiology and Virology Laboratory, Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.
Agnese Viscido, Microbiology and Virology Laboratory, Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.
Francesca Gavaruzzi, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Giancarlo Ceccarelli, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Gianmarco Raponi, Microbiology and Virology Laboratory, Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.
Cristian Borrazzo, Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Rome, Italy.
Francesco Alessandri, Department of General and Specialistic Surgery, Sapienza University of Rome, Rome, Italy.
Claudio Maria Mastroianni, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Mario Venditti, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Alessandra Oliva, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Funding
This study was carried out as a part of our routine work. This reserch did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Transparency declarations
The authors have no competing interests to declare that are relevant to the content of this article.
Author contributions
Conceptualization: Francesco Cogliati Dezza, Alessandra Oliva, Mario Venditti; methodology: Francesco Cogliati Dezza, Alessandra Oliva; formal analysis: Francesco Cogliati Dezza, Cristian Borrazzo; data curation: Francesco Cogliati Dezza, Flavia Petrucci, Sara Covino, Agnese Viscido, Federica Sacco, Giancarlo Ceccarelli, Francesco Alessandri, Francesca Gavaruzzi, Giammarco Ramponi; writing—original draft preparation: Francesco Cogliati Dezza; writing—review and editing: Alessandra Oliva, Mario Venditti; supervision: Alessandra Oliva, Mario Venditti, Claudio Maria Mastroianni. All authors have read and agreed to the published version of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Murray CJ, Ikuta KS, Sharara Fet al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tacconelli E, Carrara E, Savoldi Aet al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27. 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 3. Karakonstantis S, Gikas A, Astrinaki Eet al. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J Hosp Infect 2020; 106: 447–53. 10.1016/j.jhin.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 4. Russo A, Bassetti M, Ceccarelli Get al. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect 2019; 79: 130–8. 10.1016/j.jinf.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 5. Lee H, Lee H. Clinical and economic evaluation of multidrug-resistant Acinetobacter baumannii colonization in the intensive care unit. Infect Chemother 2016; 48: 174–80. 10.3947/ic.2016.48.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong D, Nielsen TB, Bonomo RAet al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30: 409–47. 10.1128/CMR.00058-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falcone M, Tiseo G, Carbonara Set al. Mortality attributable to bloodstream infections caused by different carbapenem-resistant Gram-negative bacilli: results from a nationwide study in Italy (ALARICO network). Clin Infect Dis 2023; 76: 2059–69. 10.1093/cid/ciad100 [DOI] [PubMed] [Google Scholar]
- 8. Russo A, Gavaruzzi F, Ceccarelli Get al. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection 2022; 50: 83–92. 10.1007/s15010-021-01643-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cogliati Dezza F, Arcari G, Alessi Fet al. Clinical impact of COVID-19 on multi-drug-resistant Gram-negative bacilli bloodstream infections in an intensive care unit setting: two pandemics compared. Antibiotics (Basel) 2022; 11: 926. 10.3390/antibiotics11070926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thoma R, Seneghini M, Seiffert SNet al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control 2022; 11: 12. 10.1186/s13756-022-01052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serapide F, Quirino A, Scaglione Vet al. Is the pendulum of antimicrobial drug resistance swinging back after COVID-19? Microorganisms 2022; 10: 957. 10.3390/microorganisms10050957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pascale R, Bussini L, Gaibani Pet al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: a multicenter before-and-after cross-sectional study. Infect Control Hosp Epidemiol 2022; 43: 461–6. 10.1017/ice.2021.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iacovelli A, Oliva A, Siccardi Get al. Risk factors and effect on mortality of superinfections in a newly established COVID-19 respiratory sub-intensive care unit at University Hospital in Rome. BMC Pulm Med 2023; 23: 30. 10.1186/s12890-023-02315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giannella M, Trecarichi EM, De Rosa FGet al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect 2014; 20: 1357–62. 10.1111/1469-0691.12747 [DOI] [PubMed] [Google Scholar]
- 15. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto Met al. A predictive model of mortality in patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae. Mayo Clin Proc 2016; 91: 1362–71. 10.1016/j.mayocp.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 16. Russo A, Giuliano S, Ceccarelli Get al. Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit patients. Antimicrob Agents Chemother 2018; 62: e02562-17. 10.1128/AAC.02562-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tacconelli E, Cataldo MA, De Pascale Get al. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J Antimicrob Chemother 2008; 62: 1130–7. 10.1093/jac/dkn289 [DOI] [PubMed] [Google Scholar]
- 18. Chopra T, Marchaim D, Johnson PCet al. Risk factors and outcomes for patients with bloodstream infection due to Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother 2014; 58: 4630–5. 10.1128/AAC.02441-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou H, Yao Y, Zhu Bet al. Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: a retrospective study from a Chinese hospital. Medicine (Baltimore) 2019; 98: e14937. 10.1097/MD.0000000000014937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gu Y, Jiang Y, Zhang Wet al. Risk factors and outcomes of bloodstream infections caused by Acinetobacter baumannii: a case-control study. Diagn Microbiol Infect Dis 2021; 99: 115229. 10.1016/j.diagmicrobio.2020.115229 [DOI] [PubMed] [Google Scholar]
- 21. Anggraini D, Santosaningsih D, Endraswari PDet al. Multicenter study of the risk factors and outcomes of bloodstream infections caused by carbapenem-non-susceptible Acinetobacter baumannii in Indonesia. Trop Med Infect Dis 2022; 7: 161. 10.3390/tropicalmed7080161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falcone M, Bassetti M, Tiseo Get al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care 2020; 24: 29. 10.1186/s13054-020-2742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. EUCAST . Clinical breakpoints (v 13.0). 2023. https://www.eucast.org/clinical_breakpoints.
- 24. ECDC . EU case definitions.2018. https://www.ecdc.europa.eu/en/all-topics/eu-case-definitions.
- 25. Magiorakos A-P, Srinivasan A, Carey RBet al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KLet al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 27. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270: 2957–63. 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 28. Al-Hasan MN, Lahr BD, Eckel-Passow JEet al. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect 2013; 19: 948–54. 10.1111/1469-0691.12085 [DOI] [PubMed] [Google Scholar]
- 29. Singer M, Deutschman CS, Seymour CWet al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oliva A, Volpicelli L, Di Bari Set al. Effect of ceftazidime/avibactam plus fosfomycin combination on 30 day mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae: results from a multicentre retrospective study. JAC Antimicrob Resist 2022; 4: dlac121. 10.1093/jacamr/dlac121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodríguez-Acelas AL, de Abreu Almeida M, Engelman Bet al. Risk factors for health care-associated infection in hospitalized adults: systematic review and meta-analysis. Am J Infect Control 2017; 45: e149–56. 10.1016/j.ajic.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 32. Cardinale V, Capurso G, Ianiro Get al. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: a working hypothesis. Dig Liver Dis 2020; 52: 1383–9. 10.1016/j.dld.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oliva A, Cammisotto V, Cangemi Ret al. Low-grade endotoxemia and thrombosis in COVID-19. Clin Transl Gastroenterol 2021; 12: e00348. 10.14309/ctg.0000000000000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ciacci P, Paraninfi A, Orlando Fet al. Endothelial dysfunction, oxidative stress and low-grade endotoxemia in COVID-19 patients hospitalised in medical wards. Microvasc Res 2023; 149: 104557. 10.1016/j.mvr.2023.104557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliva A, Miele MC, Di Timoteo Fet al. Persistent systemic microbial translocation and intestinal damage during coronavirus disease-19. Front Immunol 2021; 12: 708149. 10.3389/fimmu.2021.708149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spagnolello O, Pinacchio C, Santinelli Let al. Targeting microbiome: an alternative strategy for fighting SARS-CoV-2 infection. Chemotherapy 2021; 66: 24–32. 10.1159/000515344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oliva A, Aversano L, De Angelis Met al. Persistent systemic microbial translocation, inflammation, and intestinal damage during Clostridioides difficile infection. Open Forum Infect Dis 2020; 7: ofz507. 10.1093/ofid/ofz507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Segala FV, Bavaro DF, Di Gennaro Fet al. Impact of SARS-CoV-2 epidemic on antimicrobial resistance: a literature review. Viruses 2021; 13: 2110. 10.3390/v13112110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee H-Y, Chen C-L, Wu S-Ret al. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med 2014; 42: 1081–8. 10.1097/CCM.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 40. Lee CM, Kim C-J, Kim SEet al. Risk factors for early mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteraemia. J Glob Antimicrob Resist 2022; 31: 45–51. 10.1016/j.jgar.2022.08.010 [DOI] [PubMed] [Google Scholar]
- 41. Ballouz T, Aridi J, Afif Cet al. Risk factors, clinical presentation, and outcome of Acinetobacter baumannii bacteremia. Front Cell Infect Microbiol 2017; 7: 156. 10.3389/fcimb.2017.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alrahmany D, Omar AF, Alreesi Aet al. Acinetobacter baumannii infection-related mortality in hospitalized patients: risk factors and potential targets for clinical and antimicrobial stewardship interventions. Antibiotics (Basel) 2022; 11: 1086. 10.3390/antibiotics11081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Machuca I, Gutiérrez-Gutiérrez B, Gracia-Ahufinger Iet al. Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother 2017; 61: e00406-17. 10.1128/AAC.00406-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paul M, Carrara E, Retamar Pet al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect 2022; 28: 521–47. 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.