Highlights
-
•
Identified metabolite abnormalities in multiple brain areas in EPM1 patients.
-
•
Metabolic abnormalities associated with cognitive performance of EPM1 patients.
-
•
Widespread metabolic changes suggest the presence of neurodegeneration.
Keywords: EPM1, MRS, Spectroscopy, Brain, Neuropsychology, Cognition
Abstract
Purpose
Progressive myoclonic epilepsy, type 1A (EPM1, Unverricht-Lundborg disease), is a rare neurodegenerative autosomal recessive disorder characterized by stimulus-sensitive and action myoclonus and tonic-clonic epileptic seizures. Patients develop neurological symptoms, including ataxia, intention tremor, and dysarthria, over time, with relatively limited and nonspecific MRI atrophy findings. The effects of the disease on brain metabolism are largely unknown.
Method
Eighteen EPM1 patients (9 M, 9F) underwent clinical evaluation and neuropsychological testing, which included the assessment of intellectual ability, verbal memory, and psychomotor and executive functions. Magnetic resonance spectroscopy (MRS) and imaging (MRI) were performed on a 1.5 T MRI system. 2D MRS chemical shift imaging (CSI) maps (TE = 270) were obtained from the following regions of the brain: basal ganglia, thalamus, insula, splenium, and occipital white and gray matter, and N-acetyl-aspartate (NAA)-, choline (Cho)-, and lactate (Lac)-to-creatine (Cr) ratios were analyzed. Ten healthy age-and sex-matched subjects (5M, 5F) were used as controls for MRS.
Results
We found significant brain metabolic changes involving lactate, NAA, and choline, which are widespread in the basal ganglia, thalamic nuclei, insula, and occipital areas of EPM1 patients. Changes, especially in the right insula, basal ganglia, and thalamus, were associated with intellectual abilities and impairment of the psychomotor and executive functions of EPM1 patients.
Conclusion
Multiple brain metabolic alterations suggest the presence of neurodegeneration associated with EPM1 progression. The changes in metabolite ratios are associated with the neurocognitive dysfunction caused by the disease. However, the role of MRS findings in understanding pathophysiology of EPM1 warrants further studies.
1. Introduction
Progressive myoclonic epilepsy, type 1A (EPM1, Unverrich-Lundborg disease, OMIM #254800) is a rare neurodegenerative autosomal recessive disorder characterized by stimulus-sensitive and action myoclonus and tonic-clonic epileptic seizures. Disease onset occurs in late childhood or adolescence (6–18 years) (Kälviäinen et al., 2008). As EPM1 progresses, patients develop neurological symptoms, including ataxia, intention tremor and dysarthria, while their verbal memory remains largely intact (Kälviäinen et al., 2008, Canafoglia et al., 2017, Äikiä et al., 2021, Ferlazzo et al., 2009).
EPM1 is caused by a pathogenic variant in the gene encoding cystatin B (CSTB), a cysteine protease inhibitor. The 12-nucleotide dodecamer repeat expansion pathogenic variant in the CSTB accounts for approximately 90% of cases worldwide and 99% of EPM1 cases in Finland (Joensuu et al., 2008). Based on previous mouse model studies, cystatin B is involved in biological processes such as neurogenesis and apoptosis, oxidative stress response and inflammation, cell cycle regulation, and synapse physiology. However, the precise mechanism of cystatin deficiency leading to clinical disease is still uncertain in mouse models and humans. A full cystatin B deficiency causes neonatal-onset encephalopathy that manifests with severe developmental delay, progressive microcephaly, and hypomyelination in MRI (Mancini et al., 2016).
In contrast, in dodecamer expansion homozygous EPM1 patients’ imaging findings rarely show abnormalities at the time of EPM1 diagnosis. However, as the disease progresses, cerebral atrophy may evolve. Moreover, compound heterozygous patients reported to have a more severe clinical course of the disease were shown to have mild to moderate cerebellar and cerebral atrophy by visual MRI assessment (Koskenkorva et al., 2011). Recent studies have examined EPM1 patients with voxel-based morphometry and show gray matter volume loss in the motor cortex (including premotor and primary motor areas and supplementary motor cortex), precuneus, and thalami, which are affected bilaterally. The changes, especially those found in motor areas, parallel the motor symptoms characteristic of EPM1 (Koskenkorva et al., 2009). Cortical thickness analysis has demonstrated widespread cortical thinning in EPM1 patients, for instance, in the sensorimotor cortex, visual cortex, Broca's area, and primary auditory cortex, as well as in premotor and supplementary motor cortices (Koskenkorva et al., 2012). Cortical thinning correlated significantly with the severity of the myoclonus.
Magnetic resonance spectroscopy (MRS) imaging can provide in vivo information on the N-acetyl-aspartate (NAA), choline (Cho), creatine (Cr), lactate (Lac), glutamine (Glu), and gamma-aminobutyric acid (GABA) levels in different areas of the brain. Previously, in EPM1 patients, metabolite peak ratios have been measured only in the pons and dentate nucleus (Mascalchi et al., 2002). However, more extensive cerebral metabolic changes in EPM1 remain unknown. This study aimed to examine possible metabolic changes in the supratentorial brain regions of EPM1 patients via MRS, evaluate analogies between this and earlier radiologic studies of EPM1, and provide additional information on causative and co-associated factors in the clinical picture of EPM1.
2. Subjects and methods
2.1. Subjects
Eighteen genetically verified adult EPM1 patients (9 males, 9 females) homozygous for the dodecamer expansion pathogenic variant in the CSTB gene participated in the study. The patients are a part of the large EPM1 cohort reported previously (Hyppönen et al., 2015, Äikiä et al., 2021, Danner et al., 2013, Koskenkorva et al., 2012). Ten age- and sex-matched healthy volunteers (5 males, 5 females) were selected as controls for MRS. The main demographic characteristics of the EPM1 patients and controls are presented in Table 1. All patients and control subjects were right-handed. The Ethical Committee of Kuopio University Hospital approved the study protocol, and informed consent was obtained from all participants.
Table 1.
Demographic data, including the ambulatory and functional status of the EPM1 patients. The numbers indicate the mean (SD) [range] and the number of subjects where applicable.
| EPM1 | Control | |
|---|---|---|
| Age (years) | 32 (10) [19–49] | 31 (11) [16–48] |
| Sex (F/M) | 9F; 9 M | 5F; 5 M |
| Age at onset (years) | 10 (2) [6–16] | |
| Disease duration (years) | 22 (10) [8–41] | |
| UMRS SS: total score | 2 (2) [0–8] | |
| UMRS Myoclonus with action: total score | 46 (26) [12–90] | |
| UMRS FT: total score | 10 (7) [1–22] | |
| VIQ | 88 (12) [69–109] | |
| PIQ | 78 (13) [55–103] |
Mean (Std. Dev) [Minimum-Maximum]; F, female, M, male; UMRS, unified myoclonus rating scale; SS, stimulus sensitivity; FT, functional tests; VIQ, Verbal Intelligence Quotients; PIQ, Performance Intelligence Quotients.
2.2. Clinical and neuropsychological assessment
Detailed clinical and neuropsychological characteristics of the cohort have been published earlier (Hyppönen et al., 2015, Äikiä et al., 2021). Previously collected clinical histories, including age at disease onset and antiseizure medication (ASM) history, were used in this study. The results of the video-recorded unified myoclonus rating scale (UMRS) test panel (Frucht et al., 2002), including stimulus sensitivity (SS), myoclonus with action (AM), and functional tests (FT), were used for the analyses. The patients were classified based on their myoclonus and action scores (max. score = 160) into three myoclonus severity groups: mild myoclonus (score = 1–30), moderate myoclonus (score = 31–59), and severe myoclonus (score > 60). Neuropsychological assessments were performed by the same experienced neuropsychologist (M.Ä.). The battery of tests included intellectual ability, verbal learning and memory, and psychomotor and executive functions. Six subtests of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (David, 1981)—information, similarities, digit span, digit symbol, picture completion, and block design—were used to calculate the patients’ verbal intelligence quotients (VIQ) and performance intelligence quotients (PIQ). A detailed protocol for evaluating verbal memory, executive function and psychomotor function has been described previously (Äikiä et al., 2021). WAIS-R digit symbol, picture completion, and block design tests were used to evaluate patients’ perceptional reasoning and processing speed. List learning, story recall, and delayed recall were used to evaluate patients' verbal learning and memory.
2.3. Imaging and spectroscopy
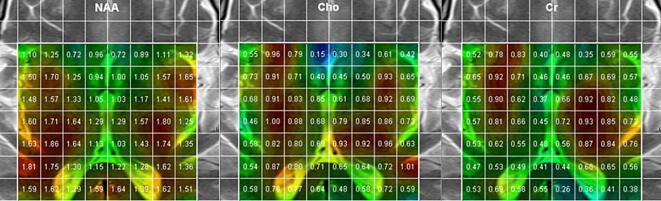
The MRI and MR spectroscopy were conducted at Kuopio University Hospital with a Siemens Avanto 1.5 T MRI system (Siemens AG, Erlangen, Germany). 2D MR chemical shift imaging (CSI) spectral maps were obtained, as indicated by Fig. 1 (based on an MR spectroscopy protocol with TE = 270 ms, TR = 1500, NA = 4). The area was chosen to encompass as much of the basal ganglia as possible, together with the insular cortices and occipital white matter, including the dorsal splenium and even occipital gray matter. Spectroscopy analysis was performed using vendor workstation software in research mode, with baseline correction and frequency domain fitting using Lorentzian line shapes. The 270 ms echo time was chosen for reliable fitting and spectral clarity. The identified and analyzed metabolite groups were NAA, creatine (Cr), choline (Cho), and lactate (Lac). Metabolite concentrations are expressed according to standards in relation to the Cr signal calculated from the 2D CSI images and analyzed in the following regions of the brain: caudate nucleus, putamen, globus pallidus, anterior thalamus, dorsomedial thalamus, lateral thalamus, splenium, insula, occipital white matter, and occipital gray matter (visual cortex).
Fig. 1.
Typical TE = 270 ms MR spectroscopic imaging data from a patient, indicating the covered anatomical area, voxels, and intrinsic NAA, Cho, and Cr metabolite signal areas (AU). NAA, N-acetyl-aspartate; Cho, Choline; Cr, Creatine.
2.4. Statistical analysis
Statistical analyses were performed with the IBM Statistical Package for the Social Sciences (SPSS) version 26 (SPSS Inc., Chicago IL, USA). All variables were tested for normality of distribution using the Shapiro–Wilk test. Independent samples t-tests or non-parametric Kruskal-Wallis tests were used in comparisons between metabolite concentrations of patients and control subjects where appropriate. Pearson or Spearman correlation tests were carried out to investigate the relationship between metabolite levels and clinical variables. To ensure the clinical utility of the findings, only correlations of moderate effect size (r2 of 0.30) or large effect size (r2 of 0.50) (Akoglu, 2018) were interpreted as evidence of a significant association between variables. The interhemispheric deviation was evaluated with a non-parametric test (Wilcoxon signed-rank test).
3. Results
The main demographic characteristics of the EPM1 patients are presented in Table 1. According to the severity of myoclonus, 6 patients had mild myoclonus, 6 – moderate, and 6 – severe. All patients received treatment with ASMs with individually adjusted combinations and doses. None of the patients were on ASM monotherapy. Valproate was included in the ASM regime in all patients.
3.1. MRS results of EPM1 patients and healthy controls
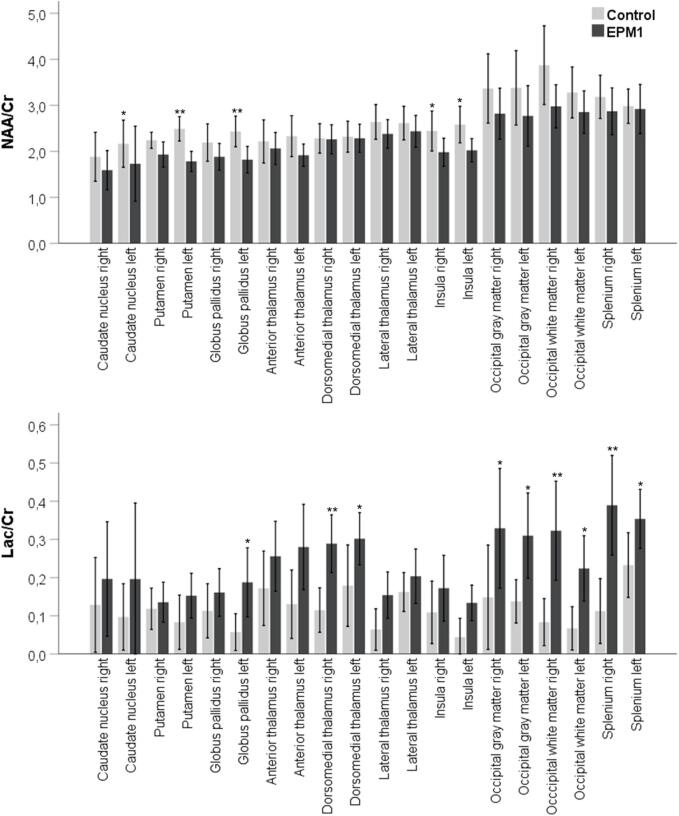
The main finding was the Lac/Cr ratio increase in EPM1 patients compared to healthy controls bilaterally in the dorsomedial area of the thalamus, splenium, and occipital white and gray matter (Fig. 2). On the left, the Lac/Cr ratio also increased in the insula and globus pallidus. NAA/Cr levels were significantly lower in EPM1 patients than in controls in the insula bilaterally and left caudate nucleus, globus pallidus, and anterior thalamus. Cho/Cr levels showed no statistically significant difference between the EPM1 patients and the controls. Metabolite ratios compared between EPM1 patients and healthy control subjects for each region are presented separately for both hemispheres in Supplemental Table 1 and Fig. 2.
Fig. 2.
Metabolite levels in different brain regions of EPM1 patients and healthy controls. Significant differences are marked with an asterisk. The mean average of both hemispheres is presented for each brain region. NAA/Cr, N-acetyl-aspartate-to-Creatine ratio; Lac/Cr, Lactate-to-Creatine ratio; * p < 0.05; ** p < 0.01.
3.2. MRS and clinical phenotypes of EPM1 patients
We found no global association of metabolic profiles with the age of EPM1 onset or disease duration, or systematic global association with the severity of myoclonus (measured by the AM or FT scores of the UMRS test panel). There were, however, some regional changes, such as in the caudate nucleus associated with the age at onset, as EPM1 patients with the later disease onset had higher Cho/Cr ratio in the right caudate nucleus (r2 0.615, p = 0.019), higher NAA/Cr in the left caudate nucleus (r2 0.632, p = 0.020), and bilateral increases in Lac/Cr ratios (right r2 0.591, p = 0.026, left r2 0.753, p = 0.003). The bilateral Cho/Cr ratio of occipital white matter was moderately associated with AM (right r2 0.623, p = 0.006, left r2 0.557, p = 0.016) and FT (right r2 0.661, p = 0.003, left r2 0.481, p = 0.043). All correlation results of Lac/Cr, NAA/Cr, and Cho/Cr ratios for each brain region with clinical parameters are presented in Supplemental Table 2.
3.3. MRS and neuropsychological findings in EPM1 patients
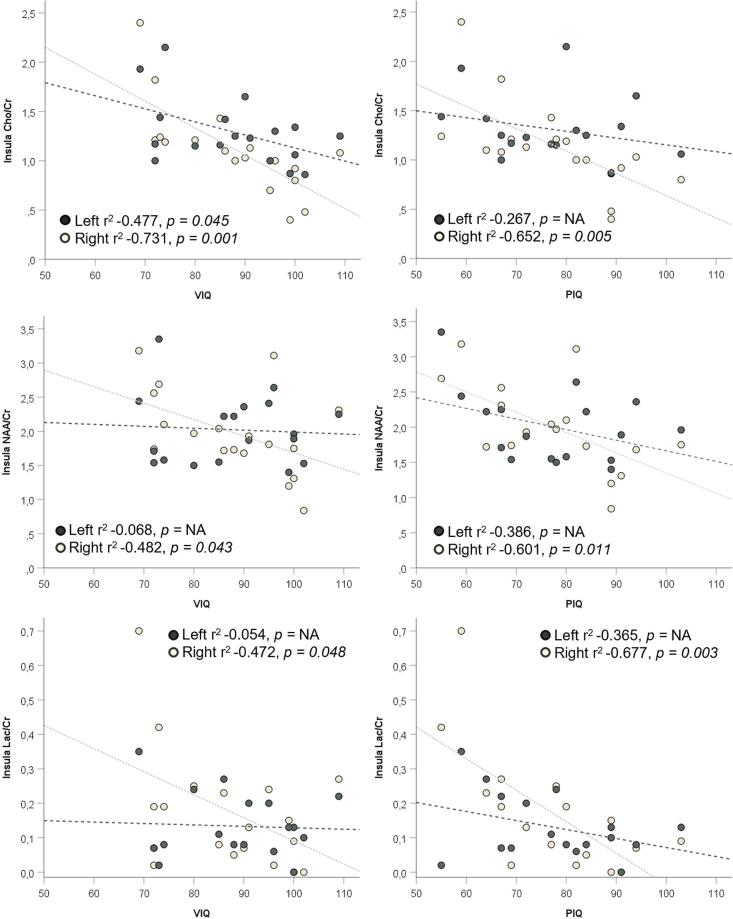
Higher VIQ and PIQ were correlated with significantly lower NAA/Cr (r2 −0.482, p = 0.043 for VIQ and r2 −0.601, p = 0.011 for PIQ) and Lac/Cr ratios (r2 −0.472, p = 0.048 for VIQ and r2 −0.677, p = 0.003 for PIQ) in the right insula and significantly lower Cho/Cr ratios bilaterally in the insula (left r2 −0.477, p = 0.045 and right r2 −0.731, p = 0.001 for VIQ and left r2 −0.267, p = NA, right r2 −0.652, p = 0.005 for PIQ)(Fig. 3). In addition, the Cho/Cr ratio of the right anterior thalamus presented a moderate, negative association with both VIQ and PIQ (r2 −0.535, p = 0.027 for VIQ and r2 −0.544, p = 0.029 for PIQ).
Fig. 3.
Association of insular Cho/Cr, NAA/Cr, and Lac/Cr levels with verbal and performance IQs. VIQ, verbal IQ; PIQ performance IQ; Cho/Cr, Choline-to-Creatine ratio; NAA/Cr, N-acetyl-aspartate-to-Creatine ratio; Lac/Cr, Lactate-to-Creatine ratio.
A lower Cho/Cr in the left caudate nucleus was associated with better verbal learning (r2 −0.543, p = 0.045 for list learning and r2 −0.634, p = 0.015 for delayed recall). In addition, lower Lac/Cr in the right globus pallidus was associated with better list learning (r2 −0.585, p = 0.011). In addition, lower Lac/Cr in the caudate nucleus (r2 −0.611, p = 0.020) and nucleus pallidus (r2 −0.529, p = 0.024) were associated with delayed recall of words (Supplemental Table 3). Additionally, significantly higher levels of Lac/Cr in the left dorsomedial thalamus and significantly lower levels of NAA/Cr in the right anterior and lateral thalamic nuclei were associated moderately with better immediate and delayed recall of the story (Supplemental Table 3).
Simple psychomotor speed in the tapping task was positively associated with the NAA/Cr ratio bilaterally in the dorsomedial thalamus area (r2 0.522, p = 0.038 for right-hand tapping, and r2 0.579, p = 0.019 for left-hand tapping). Additionally, non-dominant hand tapping was negatively associated with Cho/Cr and NAA/Cr in the right occipital white matter (r2 −0.508, p = 0.037 for Cho/Cr and r2 −0.483, p = 0.050 for NAA/Cr).
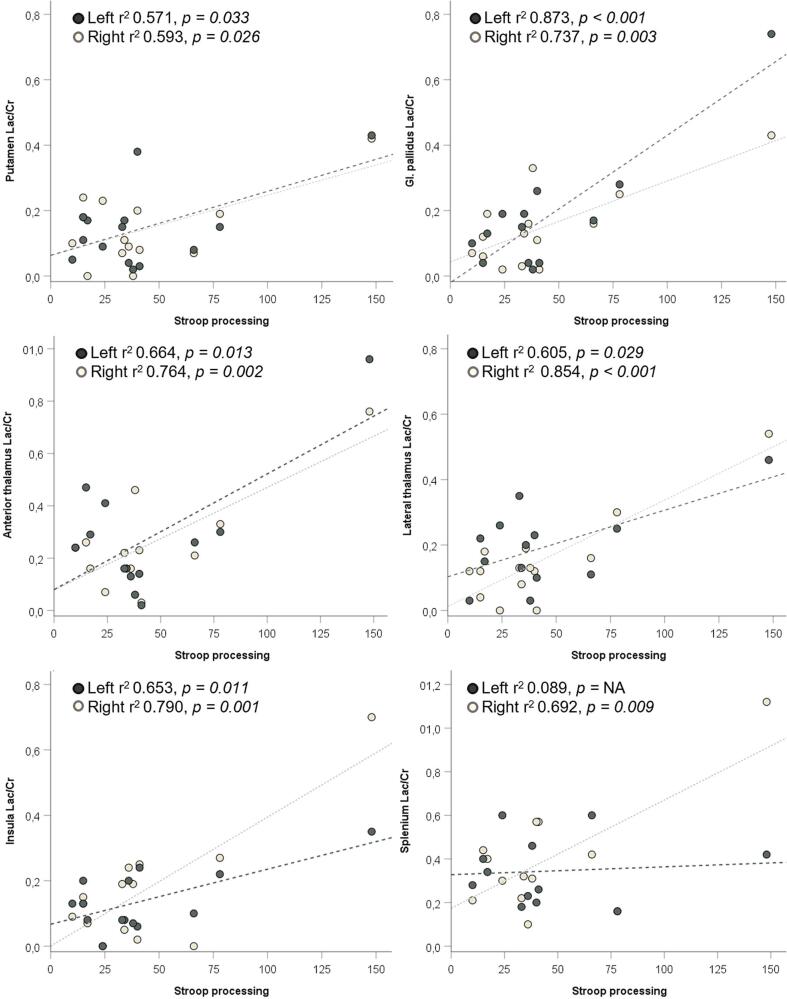
Lower Lac/Cr ratios bilaterally in the globus pallidus, the right anterior and lateral thalamus, and the insula were associated with better performance in the WAIS-R perceptional reasoning and processing speed tests. There was also a significant, moderate association between the lower Cho/Cr of the left occipital white matter with these tests (Table 2 and Suppl Table 3). Lower Lac/Cr levels bilaterally in the putamen, globus pallidus, anterior and lateral thalamus, and insula were associated with better performance in the Stroop test (Fig. 4). One patient was a strong outlier regarding Stroop test results and high lactate levels; however, this individual also had a significantly lower PIQ than the group average. The associations between metabolite ratios and different cognitive domains are summarized in Table 2 (all detailed correlation results are presented in Supplemental Table 3).
Table 2.
Overview summary of the metabolite/Cr ratio association with cognitive test outcomes.
| Cho/Cr | VIQ | PIQ | List learning | List delayed recall | Story recall | Story delayed recall | WAIS-R: Digit symbol | WAIS-R: Block design | WAIS-R: Picture completion | Tapping | TMT processing | Stroop processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal ganglia* | – | – | ↓ | ↓ | – | – | – | ↓ | – | – | – | ↓ |
| Thalamus** | ↓ | ↓ | – | – | ↓ | – | – | – | ↓ | – | – | – |
| Insula | ↓ | ↓ | – | – | – | – | – | – | ↓ | – | – | ↓ |
| Occipital gray matter | – | – | – | – | – | – | – | – | – | – | ↓ | – |
| Occipital white matter/splenium | – | ↓ | – | – | – | – | ↓ | ↓ | ↓ | ↓ | – | – |
| NAA/Cr | ||||||||||||
| Basal ganglia* | – | – | – | – | ↓ | – | – | ↓ | – | – | ↓ | |
| Thalamus** | – | ↓ | – | – | ↓ | ↓ | – | – | – | ↑ | – | – |
| Insula | ↓ | ↓ | – | – | – | – | – | – | ↓ | – | ↓ | – |
| Occipital gray matter | – | – | – | – | – | – | – | – | – | – | ↓ | – |
| Occipital white matter/splenium | – | – | – | – | – | – | – | – | – | ↓ | – | – |
| Lac/Cr | ||||||||||||
| Basal ganglia* | – | – | ↓ | ↓ | – | – | ↓ | ↓ | ↓ | – | – | ↓ |
| Thalamus** | – | – | – | – | ↑ | ↑ | ↓ | – | ↓ | – | – | ↓ |
| Insula | ↓ | ↓ | – | – | – | – | – | ↓ | ↓ | – | ↓ | ↓ |
| Occipital gray matter | – | ↑ | – | – | ↑ | – | ↑ | – | ↑ | – | – | – |
| Occipital white matter /splenium | – | – | – | – | – | – | – | – | – | – | – | ↓ |
↑ High metabolite/Cr ratio is associated with better cognitive test results.
↓ Low metabolite/Cr ratio is associated with better cognitive test results.
Cho/Cr, Choline-to-Creatine ratio; NAA/Cr, N-acetyl-aspartate-to-Creatine ratio; Lac/Cr, Lactate-to-Creatine ratio; * Basal ganglia includes any significant association observed for either putamen or globus pallidus or caudate nucleus; ** Thalamus includes any significant association observed for either anterior or lateral or dorsomedial thalamic nuclei.
Fig. 4.
Association of Lac/Cr in basal ganglia, anterior and lateral thalamus, insula, and splenium with Stroop processing. Lac/Cr, Lactate-to-Creatine ratio.
4. Discussion
This is the first clinical MR spectroscopy study of the supratentorial brain in EPM1 patients. We show that EPM1 patients have significantly higher Lac/Cr concentrations and decreased NAA/Cr ratios than healthy controls in multiple brain regions. However, we did not find significant changes in Cho/Cr compared to the healthy controls. Moreover, widespread changes in Lac/Cr, NAA/Cr, and Cho/Cr were associated with cognitive, verbal, and performance abilities in EPM1 patients.
In MRI studies of EPM1 patients, volume losses have been observed in motor cortices and thalamic areas, along with cortical thinning in sensorimotor, visual, and auditory cortices. However, they are not discernible to the naked eye (Koskenkorva et al., 2009, Koskenkorva et al., 2012). Brain metabolite peak ratios have previously only been measured in the pons and dentate nucleus, demonstrating a lower than normal NAA/Cr ratio in the pons of EPM1 patients (Mascalchi et al., 2002). In our study, the most pronounced NAA/Cr decreases were observed in the insular cortex and basal ganglia, especially of the left dominant hemisphere, and Lac/Cr increases in the thalamus (particularly the dorsomedial part), the insula, white matter of occipital lobe and splenium, and occipital gray matter.
NAA is a free amino acid found in relatively high concentrations in the central and peripheral nervous systems. The NAA concentration in the central nervous system is considered an indicator of neural density. Previous studies have shown changes in NAA/Cr in mesial temporal sclerosis patients in the mesial temporal region (Fernández-Vega et al., 2021). However, it is known that the NAA concentration can vary between different types of neurons, and changes in NAA levels may also reflect neuronal dysfunction instead of actual neuronal loss (Simmons et al., 1991). Decreased NAA concentrations have been found in cortical dysplasia when the cell density has been normal or even elevated (Kuzniecky et al., 1997, Li et al., 1998). In patients with genetic generalized epilepsy (GGE), decreased NAA/Cr levels in the thalamic areas have been reported (Yıldırım et al., 2022). Moreover, in patients with Lafora disease (EPM2), the NAA/Cho ratio decreased in the cerebellum and correlated significantly with myoclonus and ataxia severity scores (Altindag et al., 2009). NAA is metabolized via aminohydrolase, which is released in cell dysfunction. Elevated aminohydrolase activity, such as that caused by mitochondrial dysfunction and detected as neurodegeneration, can lead to decreased NAA concentration (Goldstein, 1976; Savic et al., 2000). Therefore, it seems plausible that the metabolic changes involving NAA in this study could suggest the presence of neurodegeneration and actual neuronal loss. To support this possibility, a decrease in NAA/Cr ratios may reflect neuronal degeneration, which can eventually be seen in previously mentioned morphometry as a volume loss of the thalamus (Koskenkorva et al., 2009). Previous EPM1 studies on animal models (cstb -/- mice) have also found proof of thalamic atrophy by diffusion tensor imaging (Manninen et al., 2013).
Interestingly, decreased NAA/Cr ratio in the motor cortices together with decreased frontal lobe and thalamic NAA concentrations and NAA/Cr ratios were observed in JME patients (Lin et al., 2009, Haki et al., 2007, Savic et al., 2000, Savic et al., 2004). EPM1 and JME share similarities early in the course of the disease. While JME does not progress and patients do not have neurological symptoms other than epilepsy, EPM1 patients show a large variability in their disease progression. Although EPM1 and JME share some similarities in the early clinical picture, the metabolic changes in subcortical regions show differences in distribution. In contrast to previous JME studies, we found decreased NAA/Cr in the basal ganglia and insular cortex. It is plausible that the involvement of the thalamus and basal ganglia is associated with the presence of myoclonus in both diseases. The basal ganglia and thalamus are known to play essential roles in the integration of motor behavior and motor learning. They also integrate information from multiple brain areas and connect it to the primary motor cortex (McHaffie et al., 2005, Nagano-Saito et al., 2014, Fama and Sullivan, 2015). Metabolic changes in the thalamic area and basal ganglia may account for action-activated myoclonus, which is the most disabling progressive symptom in EPM1 patients. We did not, however, find any global association of myoclonus severity (UMRS score) with the measured metabolites, which implies a more general metabolic imbalance associated with the disease.
Choline is the primary substrate for phospholipid synthesis, and changes in its concentrations may be related to the turnover of the cell membrane or changes in cell density. The role of choline as a biomarker of demyelination has also been suggested (Rae, 2014). We did not find significant changes in Cho/Cr in EPM1 patients compared to controls. However, there was some association of Cho/Cr in the white matter with action myoclonus severity and functional test scores. We also showed a significant increase in Lac/Cr in occipital white matter and splenium, as well as in the occipital gray matter. Our research has previously identified cortical thinning in the visual cortex (Koskenkorva et al., 2012). White matter degeneration is observed in both cstb-/- mice and EPM1 patients (Manninen et al., 2013). Photosensitivity is a significant early feature of EPM1, but no association between measured metabolites and stimulus sensitivity was observed in our study population. The results, however, may reflect disturbances in lactate metabolism in occipital gray and white matter and further support the notion that these brain areas are involved in EPM1 pathophysiology.
Lactate has long been considered a marker of hypoxic pathology. However, under physiological conditions, lactate is also a predominant glycolytic substrate in the brain formed in astrocytes and further trafficked to glutamatergic neurons through the astrocyte–neuron lactate shuttle pathway (Magistretti and Allaman, 2018, Patel et al., 2019). Lactate may be involved in neuronal excitability homeostasis. Studies have mainly indicated that lactate influences the excitability of glutamatergic and GABAergic neurons (Bozzo et al., 2013, Patel et al., 2019, Sada et al., 2015). Animal experiments have shown that lactate is a necessary mediator for learning and memory consolidation (Suzuki et al., 2011). We show an association of multiple metabolite changes, including an increase of Lac/Cr in the insula, with patients’ VIQ and PIQ. Moreover, an increase in Cho/Cr and Lac/Cr widely in the basal ganglia, thalamus, and insula was associated with the performance in the neuropsychological tests assessing perceptional reasoning and psychomotor speed. Decreased NAA/Cr in the thalamus and insula were associated with better cognitive performance. In comparison, increased Lac/Cr levels were associated with poorer results in cognitive tests, though verbal learning and memory were inconsistently associated with both an increase of Lac/Cr in the thalamus and a decrease in the basal ganglia.
Our previous study showed that the cognitive function of EPM1 patients is associated with disease severity (Äikiä et al., 2021). In this study population, the cognitive test results also correlated with the severity of myoclonus. However, since we did not find a significant correlation between the measured metabolites and myoclonus severity, the association between the metabolite changes and cognitive performance is likely independent of the patients’ motor system symptoms. The basal ganglia, thalamus, and insula are complex structures in various integrative motor and cognitive functions. Each structure consists of multiple substructures and different nuclei with separate connection pathways, structure-specific cytoarchitectonic organization, and neurotransmitter profiles. Therefore, the more precise role of measured metabolites in the cognitive functioning of EPM1 patients remains to be elucidated.
Overall, we observed that Lac/Cr was increased in EPM1 patients and that increased Lac/Cr was associated with poorer performance in multiple cognitive tests. One could speculate that increased Lac/Cr in EPM1 might reflect abnormal glial activation and cell death, complementing the findings previously reported in cstb-/- mice (Okuneva et al., 2015). This could also reflect the previously suggested possibility of mitochondrial dysfunction (Kopitar-Jerala, 2015a, Kopitar-Jerala, 2015b).
A limitation of our study is the lack of MRS data for other cortical regions, such as the primary and supplementary motor and sensory areas. Although we use creatine as an internal reference with adherence to common practice in clinical MRS, theoretical fluctuations in creatine concentrations could occur (Rae, 2014). However, Cr levels have consistently been stable in epilepsies and movement disorders (Govindaraju et al., 2000, Saunders et al., 1999). Our finding that Cho/Cr ratios remain stable compared to control subjects and between the different brain areas further corroborates this assumption. We are also acutely aware of the possibility of drug-induced metabolic effects: the patients were treated with multiple ASMs, and all our patients received sodium valproate, which could theoretically affect brain metabolite levels. There are, however, indications in the literature that, when used at therapeutic concentrations, valproate does not affect the MRS-measured NAA signal in the brain (Seyfert et al., 2003, Silverstone et al., 2003). There is also no evidence that other ASMs would lead to abnormal brain metabolite levels of Cho, Cr, and NAA on 1H MRS (van Veenendaal et al., 2015) and that low NAA/Cr ratios may actually be an indicator of suboptimal medication status or treatment response (Campos et al., 2010). Also, since our study is cross-sectional, we have included patients of different disease severities equally. Although we did not observe any significant changes related to disease duration or myoclonus severity, a larger study size might be needed to show more progressive changes in brain metabolic profiles.
5. Conclusions
To summarize, the findings of this study point to a broad range increase of Lac/Cr and reduction of NAA/Cr in basal ganglia, thalamic nuclei, insula, and occipital white and gray matter, which were previously unknown. These findings are corroborated by previous morphometry, cortical thickness analyses, and similar findings in other myoclonic epilepsies. However, understanding these metabolic changes and assuming neurodegeneration is still inadequate, and further research is needed, particularly regarding the role of energy metabolism. Overall, our findings bring additional evidence that EPM1, like other progressive myoclonic epilepsies, show structural degeneration and metabolic disturbances in the course of the disease progression.
CRediT authorship contribution statement
Jelena Hyppönen: Formal analysis, Visualization, Writing – original draft. Vili Paanila: Investigation, Data curation, Visualization. Marja Äikiä: Data curation, Methodology, Writing – review & editing. Päivi Koskenkorva: Investigation, Resources. Mervi Könönen: Methodology, Data curation. Ritva Vanninen: Resources, Project administration. Esa Mervaala: Resources, Writing – review & editing. Reetta Kälviäinen: Project administration, Resources, Writing – review & editing. Juhana Hakumäki: Conceptualization, Supervision, Methodology, Data curation, Formal analysis, Visualization, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are very grateful to all the patients and their family members for participating and making this research possible. We also thank our study coordinator, R.N. Pirjo Lavi, for the excellent organization of the study.
Funding
The Academy of Finland [grant number 331867], Kuopio University Hospital State Research Funding, and Saastamoinen and Vaajasalo Foundations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103459.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Äikiä M., Hyppönen J., Mervaala E., Kälviäinen R. Cognitive Functioning in Progressive Myoclonus Epilepsy Type 1 (Unverricht-Lundborg Disease, EPM1) Epilepsy Behav.: E&B. 2021;122(September) doi: 10.1016/j.yebeh.2021.108157. 108157. [DOI] [PubMed] [Google Scholar]
- Akoglu H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altindag E., Kara B., Baykan B., Terzibasioglu E., Sencer S., Onat L., Sirvanci M. MR Spectroscopy Findings in Lafora Disease. J. Neuroimag.: Off. J. Am. Soc. Neuroimag. 2009;19(4):359–365. doi: 10.1111/j.1552-6569.2008.00325.x. [DOI] [PubMed] [Google Scholar]
- Bozzo L., Puyal J., Chatton J.-Y., Amédée T. Lactate Modulates the Activity of Primary Cortical Neurons through a Receptor-Mediated Pathway. PLoS One. 2013;8(8):e71721. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos B.A.G., Yasuda C.L., Castellano G., Bilevicius E., Li L.M., Cendes F. Proton MRS May Predict AED Response in Patients with TLE. Epilepsia. 2010;51(5):783–878. doi: 10.1111/j.1528-1167.2009.02379.x. [DOI] [PubMed] [Google Scholar]
- Canafoglia L., Ferlazzo E., Michelucci R., Striano P., Magaudda A., Gambardella A., Pasini E., Belcastro V., Riguzzi P., Fanella M., Granata T., Beccaria F., Trentini C., Bianchi A., Aguglia U., Panzica F., Franceschetti S. Variable Course of Unverricht-Lundborg Disease: Early Prognostic Factors. Neurology. 2017;89(16):1691–1697. doi: 10.1212/WNL.0000000000004518. [DOI] [PubMed] [Google Scholar]
- Danner N., Julkunen P., Hyppönen J., Niskanen E., Säisänen L., Könönen M., Koskenkorva P., Vanninen R., Kälviäinen R., Mervaala E. Alterations of Motor Cortical Excitability and Anatomy in Unverricht-Lundborg Disease. Movement Disord.: Off. J. Movement Disord. Soc. 2013;28(13):1860–2187. doi: 10.1002/mds.25615. [DOI] [PubMed] [Google Scholar]
- David W. Psychological Corporation; San Antonio, TX: 1981. Wechsler Adult Intelligence Scale-Revised. Book, Whole. [Google Scholar]
- Fama R., Sullivan E.V. Thalamic Structures and Associated Cognitive Functions: Relations with Age and Aging. Neurosci. Biobehav. Rev. 2015;54(July):29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo E., Gagliano A., Calarese T., Magaudda A., Striano P., Cortese L., Cedro C., Laguitton V., Bramanti P., Carbonaro M., Albachiara A., Fragassi N., Italiano D., Sessa E., Coppola A., Genton P. Neuropsychological Findings in Patients with Unverricht-Lundborg Disease. Epilepsy Behav.: E&B. 2009;14(3):545–549. doi: 10.1016/j.yebeh.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Fernández-Vega N., Ramos-Rodriguez J.R., Alfaro F., Barbancho M.Á., García-Casares N. Usefulness of Magnetic Resonance Spectroscopy in Mesial Temporal Sclerosis: A Systematic Review. Neuroradiology. 2021;63(9):1395–1405. doi: 10.1007/s00234-021-02704-z. [DOI] [PubMed] [Google Scholar]
- Frucht S.J., Leurgans S.E., Hallett M., Fahn S. The Unified Myoclonus Rating Scale. Adv. Neurol. 2002;89 Journal Article): 361. [PubMed] [Google Scholar]
- Govindaraju V., Young K., Maudsley A.A. Proton NMR Chemical Shifts and Coupling Constants for Brain Metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Haki C., Gümüştaş O.G., Bora I., Gümüştaş A.U., Parlak M. Proton Magnetic Resonance Spectroscopy Study of Bilateral Thalamus in Juvenile Myoclonic Epilepsy. Seizure. 2007;16(4):287–295. doi: 10.1016/j.seizure.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Hyppönen J., Äikiä M., Joensuu T., Julkunen P., Danner N., Koskenkorva P., Vanninen R., Lehesjoki A.-E., Mervaala E., Kälviäinen R. Refining the Phenotype of Unverricht-Lundborg Disease (EPM1): A Population-Wide Finnish Study. Neurology. 2015;84(15):1529–1536. doi: 10.1212/WNL.0000000000001466. [DOI] [PubMed] [Google Scholar]
- Joensuu T., Lehesjoki A.-E., Kopra O. Molecular Background of EPM1-Unverricht-Lundborg Disease. Epilepsia. 2008;49(4):557–563. doi: 10.1111/j.1528-1167.2007.01422.x. [DOI] [PubMed] [Google Scholar]
- Kälviäinen R., Khyuppenen J., Koskenkorva P., Eriksson K., Vanninen R., Mervaala E. Clinical Picture of EPM1-Unverricht-Lundborg Disease. Epilepsia. 2008;49(4):549–556. doi: 10.1111/j.1528-1167.2008.01546.x. [DOI] [PubMed] [Google Scholar]
- Kopitar-Jerala N. Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front. Mol. Neurosci. 2015;8:73. doi: 10.3389/fnmol.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala N. The Role of Stefin B in Neuro-Inflammation. Front. Cell. Neurosci. 2015;9:458. doi: 10.3389/fncel.2015.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskenkorva P., Hyppönen J., Aikiä M., Mervaala E., Kiviranta T., Eriksson K., Lehesjoki A.E., Vanninen R., Kälviäinen R. Severer Phenotype in Unverricht-Lundborg Disease (EPM1) Patients Compound Heterozygous for the Dodecamer Repeat Expansion and the c.202C>T Mutation in the CSTB Gene. Neurodegener. Dis. 2011;8(6):515–522. doi: 10.1159/000323470. [DOI] [PubMed] [Google Scholar]
- Koskenkorva P., Khyuppenen J., Niskanen E., Könönen M., Bendel P., Mervaala E., Lehesjoki A.E., Kälviäinen R., Vanninen R. Motor Cortex and Thalamic Atrophy in Unverricht-Lundborg Disease: Voxel-Based Morphometric Study. Neurology. 2009;73(8):606–611. doi: 10.1212/WNL.0b013e3181b3888b. [DOI] [PubMed] [Google Scholar]
- Koskenkorva P., Niskanen E., Hyppönen J., Könönen M., Mervaala E., Soininen H., Kälviäinen R., Vanninen R. Sensorimotor, Visual, and Auditory Cortical Atrophy in Unverricht-Lundborg Disease Mapped with Cortical Thickness Analysis. AJNR Am. J. Neuroradiol. 2012;33(5):878–883. doi: 10.3174/ajnr.A2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniecky R., Hetherington H., Pan J., Hugg J., Palmer C., Gilliam F., Faught E., Morawetz R. Proton Spectroscopic Imaging at 4.1 Tesla in Patients with Malformations of Cortical Development and Epilepsy. Neurology. 1997;48(4):1018–1024. doi: 10.1212/wnl.48.4.1018. [DOI] [PubMed] [Google Scholar]
- Li L.M., Cendes F., Bastos A.C., Andermann F., Dubeau F., Arnold D.L. Neuronal Metabolic Dysfunction in Patients with Cortical Developmental Malformations: A Proton Magnetic Resonance Spectroscopic Imaging Study. Neurology. 1998;50(3):755–779. doi: 10.1212/wnl.50.3.755. [DOI] [PubMed] [Google Scholar]
- Lin K., Carrete Jr H., Lin J., Peruchi M.M., de Araújo Filho G.M., Guaranha M.S.B., Guilhoto L.M.F.F., Sakamoto A.C., Yacubian E.M.T. Magnetic Resonance Spectroscopy Reveals an Epileptic Network in Juvenile Myoclonic Epilepsy. Epilepsia. 2009;50(5):1191–1200. doi: 10.1111/j.1528-1167.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- Magistretti P.J., Allaman I. Lactate in the Brain: From Metabolic End-Product to Signalling Molecule. Nat. Rev. Neurosci. 2018;19(4):235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- Mancini G.M.S., Schot R., Marie C.Y., de Wit R.F., de Coo R., Oostenbrink Heus K.-d., Berger L.P.V., et al. CSTB Null Mutation Associated with Microcephaly, Early Developmental Delay, and Severe Dyskinesia. Neurology. 2016;86(9):877–888. doi: 10.1212/WNL.0000000000002422. [DOI] [PubMed] [Google Scholar]
- Manninen O., Koskenkorva P., Lehtimäki K.K., Hyppönen J., Könönen M., Laitinen T., Kalimo H., Kopra O., Kälviäinen R., Gröhn O., Lehesjoki A.-E., Vanninen R. White Matter Degeneration with Unverricht-Lundborg Progressive Myoclonus Epilepsy: A Translational Diffusion-Tensor Imaging Study in Patients and Cystatin B-Deficient Mice. Radiology. 2013;269(1):232–239. doi: 10.1148/radiol.13122458. [DOI] [PubMed] [Google Scholar]
- Mascalchi M., Brugnoli R., Guerrini L., Belli G., Nistri M., Politi L.S., Gavazzi C., Lolli F., Argenti G., Villari N. Single-Voxel Long TE 1H-MR Spectroscopy of the Normal Brainstem and Cerebellum. J. Magn. Reson. Imag.: JMRI. 2002;16(5):532–557. doi: 10.1002/jmri.10189. [DOI] [PubMed] [Google Scholar]
- McHaffie J.G., Stanford T.R., Stein B.E., Coizet V., Redgrave P. Subcortical Loops through the Basal Ganglia. Trends Neurosci. 2005;28(8):401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A., Martinu K., Monchi O. Function of Basal Ganglia in Bridging Cognitive and Motor Modules to Perform an Action. Front. Neurosci. 2014;8:187. doi: 10.3389/fnins.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuneva O., Körber I., Li Z., Tian L.i., Joensuu T., Kopra O., Lehesjoki A.-E. Abnormal Microglial Activation in the Cstb(-/-) Mouse, a Model for Progressive Myoclonus Epilepsy, EPM1. Glia. 2015;63(3):400–411. doi: 10.1002/glia.22760. [DOI] [PubMed] [Google Scholar]
- Patel D.C., Tewari B.P., Chaunsali L., Sontheimer H. Neuron-Glia Interactions in the Pathophysiology of Epilepsy. Nat. Rev. Neurosci. 2019;20(5):282–297. doi: 10.1038/s41583-019-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.D. A Guide to the Metabolic Pathways and Function of Metabolites Observed in Human Brain 1H Magnetic Resonance Spectra. Neurochem. Res. 2014;39(1):1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Sada Nagisa, Lee Suni, Katsu Takashi, Otsuki Takemi, Inoue Tsuyoshi. Epilepsy Treatment. Targeting LDH Enzymes with a Stiripentol Analog to Treat Epilepsy. Sci. (New York, N.Y.) 2015;347(6228):1362–1367. doi: 10.1126/science.aaa1299. [DOI] [PubMed] [Google Scholar]
- Saunders D.E., Howe F.A., van den Boogaart A., Griffiths J.R., Brown M.M. Aging of the Adult Human Brain. In Vivo Quantitation of Metabolite Content with Proton Magnetic Resonance Spectroscopy. J. Magn. Reson. Imag.: JMRI. 1999;9(5):711–776. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Savic I., Lekvall A., Greitz D., Helms G. MR Spectroscopy Shows Reduced Frontal Lobe Concentrations of N-Acetyl Aspartate in Patients with Juvenile Myoclonic Epilepsy. Epilepsia. 2000;41(3):290–326. doi: 10.1111/j.1528-1157.2000.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Savic I., Osterman Y., Helms G. MRS Shows Syndrome Differentiated Metabolite Changes in Human-Generalized Epilepsies. Neuroimage. 2004;21(1):163–172. doi: 10.1016/j.neuroimage.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Seyfert S., Bernarding J., Braun J. Volume-Selective 1H MR Spectroscopy for in Vivo Detection of Valproate in Patients with Epilepsy. Neuroradiology. 2003;45(5):295–329. doi: 10.1007/s00234-003-0973-5. [DOI] [PubMed] [Google Scholar]
- Silverstone P.H., Wu R.H., O’Donnell T., Ulrich M., Asghar S.J., Hanstock C.C. Chronic Treatment with Lithium, but Not Sodium Valproate, Increases Cortical N-Acetyl-Aspartate Concentrations in Euthymic Bipolar Patients. Int. Clin. Psychopharmacol. 2003;18(2):73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Simmons M.L., Frondoza C.G., Coyle J.T. Immunocytochemical Localization of N-Acetyl-Aspartate with Monoclonal Antibodies. Neuroscience. 1991;45(1):37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Stern S.A., Bozdagi O., Huntley G.W., Walker R.H., Magistretti P.J., Alberini C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veenendaal T.M., IJff D.M., Aldenkamp A.P., Hofman P.A.M., Vlooswijk M.C.G., Rouhl R.P.W., de Louw A.J., Backes W.H., Jansen J.F.A. Metabolic and Functional MR Biomarkers of Antiepileptic Drug Effectiveness: A Review. Neurosci. Biobehav. Rev. 2015;59:92–99. doi: 10.1016/j.neubiorev.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Yıldırım F., Aydin Z., Sakcı Z., Destînâ Yalçın A.E. Investigation of Patients With Eye Closure Sensitive Epilepsy With Magnetic Resonance Spectroscopy. Clin. EEG Neurosci. 2022;53(1):45–53. doi: 10.1177/15500594211040953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.