Abstract
Low and very-low-birth-weight (V/LBW) neonates are highly susceptible to bacterial sepsis and meningitis. Bacterial infections caused by Staphylococcus aureus can be particularly dangerous for neonates and can result in high mortality and long-term disabilities. Antibody-based strategies have been attempted to protect V/LBW neonates against staphylococcal disease. However, these efforts have so far been unsuccessful. Failures were attributed to the immaturity of the neonatal immune system but did not account for the anti-opsonic activity of Staphylococcal protein A (SpA). Here we show that monoclonal antibody 3F6, which blocks SpA activity, promotes complement-dependent cell-mediated phagocytosis of S. aureus in human umbilical cord blood. A substitution in the crystallizable fragment (Fc) region of 3F6 that enhances recruitment of complement component C1q further increases the phagocytic activity of cord blood. Our data demonstrate that the neonatal immune system possesses bactericidal activity that can be harnessed by antibodies that circumvent a key innate immune strategy of S. aureus.
Keywords: Staphylococcus aureus, Staphylococcal protein A, Monoclonal antibody, Neonatal bacteremia/meningitis, Antibody-mediated opsonophagocytosis, Umbilical cord blood
1. Introduction
Neonatal infections are a leading cause of morbidity and mortality worldwide, particularly in low and very-low-birth-weight (V/LBW) and preterm babies [1]. These newborns are at increased risk for infections due to their immature immune systems, as well as their exposure to a variety of microorganisms rapidly after birth. Staphylococcus aureus is the second most common cause of late-onset sepsis and meningitis in V/LBW babies admitted to neonatal intensive care units [2] and even with appropriate antibiotic therapy, neonatal infections can lead to long-term neurological and developmental problems [3]. The development of effective strategies to prevent S. aureus infections in newborns remain a challenge, as the bacterium is highly adaptive and can develop resistance to antibiotics.
An FDA approved antibody that prevents the incidence of S. aureus sepsis/meningitis in newborns and infants is not available. Early studies by C.J. Baker and colleagues suggested that administration of pooled human immunoglobulin (hIg) to V/LBW neonates may have a protective effect against bacterial infection [4]. This intervention however, did not reduce disease incidence or mortality [5]. Further efforts examined the clinical efficacy of human antibodies against staphylococcal capsule (Altastaph®), clumping factor A (ClfA) and serine-aspartate repeat protein G (SdrG) (INH-A21) or monoclonal antibody against polyglycerol-phosphate lipoteichoic acid (Pagibaximab®) [6]. None of these clinical trials met their study endpoints toward the protection of V/LBW neonates against S. aureus infections [6].
We reported earlier that the failure of antibodies to provide protection against S. aureus is based on the immune evasive attributes of staphylococcal protein A (SpA). SpA captures the Fcγ domain of IgG molecules as well as Fab domains of VH3-type IgG, IgM and IgE molecules [7]. Fcγ binding effectively neutralizes opsonophagocytic IgG antibodies directed against the pathogen. Fab binding and crosslinking of VH3-type IgM B cell receptors (BCR) triggers non-specific B cell expansion while crosslinking of IgG/IgE on surface of mast cells and basophils triggers histamine release [7]. Earlier we isolated 3F6-hIgG1, a humanized IgG1 antibody that binds SpA in an immune-dependent manner, promotes killing of S. aureus in adult murine and human blood, and protects neonatal and adult mice from S. aureus disease [8], [9], [10], [11], [12]. The binding of 3F6-hIgG1 to the surface of S. aureus was shown to enhance interactions with Fcγ receptors (FcγR) on innate immune cells and with the six-headed globular domains of complement molecule C1q, promote antibody-dependent cell-mediated phagocytosis (ADCP) and complement-dependent cell-mediated phagocytosis (CDCP), respectively [11], [12].
Passive transfer of SpA-neutralizing antibodies could be a useful addition to conventional antibiotic treatments in newborns at risk of S. aureus infection. Human umbilical cord blood cells have been used to compare the capacity of neonatal and adult immune cells to produce immune responses [13]. Here, we use human cord blood as a model to evaluate antibodies that target SpA for their ability to promote phagocytic uptake of S. aureus in newborns.
2. Materials and methods
2.1. Ethics statement
Adult and umbilical cord blood samples were collected from informed, consenting volunteers under approved University of Chicago Institutional Review Board (IRB) protocols. S. aureus experiments were conducted under the University of Chicago Institutional Biosafety Committee (IBC) supervision.
2.2. Collection of blood
Healthy adult blood was collected in sodium heparin-coated tubes (BDScience), used within 30 min at room temperature. Cord blood, collected post-delivery, was stored in solubilized sodium heparin (30 USP/mL; Sigma-Aldrich) on ice and removed 30 min before bacterial incubation.
2.3. Complete blood count (CBC) analysis of blood
Blood was immediately transported to the University of Chicago Hematology Lab for processing. Clotted samples were excluded from analysis. Complete blood counts were obtained using a Sysmex XN-10 analyzer (XN-9100 system).
2.4. Bacterial strain
S. aureus USA300 LAC, a methicillin-resistant clinical isolate (MRSA), was grown in tryptic soy broth or agar (TSB/TSA) at 37 °C. Overnight cultures were diluted (1:100) into fresh TSB and grown for 3 h at 37 °C. Staphylococci were centrifuged, washed twice and diluted in PBS to A600 0.4 (2 × 108 CFU ml−1).
2.5. Phagocytic activity in blood
Blood was pre-incubated with cytochalasin D (CD, 0.04 mM) and antibodies (up to 10 μg ml−1) for 10 min where indicated. Isotype hIgG1 was from Jackson ImmunoResearch, and 3F6-hIgG1 and 3F6-hIgG1AESP were produced as described [11], [12]. Staphylococcal survival was assessed by mixing a 50 μl S. aureus suspension (5 × 106 CFU / PBS) in 0.45 ml anticoagulated blood, incubating at 37 °C for 0 and 60 min, and adding 0.5 ml SK lysis buffer (phosphate-buffered saline [PBS] containing 0.5 % saponin, 100 U streptokinase [SK], 50 μg trypsin, 1 μg DNase, and 5 μg RNase) for 10 min at 37 °C before plating on agar for CFU enumeration.
2.6. Staphylococcal antigen matrix (SAM)
Staphylococcal antigens (2 μg each of the following proteins: SpAKKAA, a variant unable to bind to Fcγ- and Fab-domains of Ig [14]; ClfA and ClfB; iron-regulated surface determinant A, IsdA; iron-regulated surface determinant B, IsdB; coagulase, Coa; von-Willebrand factor binding protein, vWbp; alpha-hemolysin, Hla; fibronectin binding protein A, FnbpA and FnbpB) were blotted onto nitrocellulose, blocked with 5 % milk and incubated with human plasma. Signal intensities (A700) were quantified using IRDye 680-conjugated anti-human IgG (Rockland) and the Odyssey™ infrared imaging system (Li-cor) as described [14].
2.7. Blood smear, Giemsa stain and microscopy
Blood mixed with bacteria (1 µl) was smeared on a glass slide, fixed with methanol, and stained with Wright-Giemsa. Stained slides were visualized using a Zeiss Axioscope upright histology microscope.
3. Results
3.1. Comparing the phagocytic activity of adult and cord blood toward S. aureus.
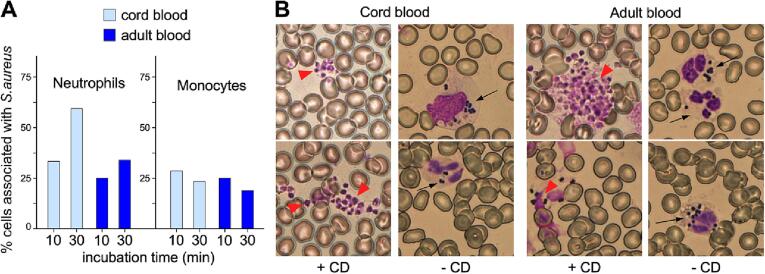
Umbilical cord blood provides a window into the in-utero environment, containing all components of the innate immune system newborns utilize to fend off infections. We incubated anticoagulated cord blood from full-term newborn deliveries, or peripheral blood from healthy adult donors, with S. aureus strain USA300, and then analyzed the association of immune cells with the pathogen. The cellular composition of the cord blood samples did not significantly deviate from reported values (Table 1). S. aureus strain USA300 represents the multi-locus sequencing (MLST) sequence type (ST) 8-MRSA-IV, first identified in the United States, but now globally prevalent [15]. After incubation with S. aureus, blood smears were stained with Giemsa and visualized by microscopy. Leukocytes were identified based on cellular morphology, and the pattern of immune cells associated with staphylococci was determined. These observations revealed that staphylococci did not associate with lymphocytes or basophils. In comparison to adult blood, cord blood exhibited an increase in the number of staphylococci-associated neutrophils within 10 to 30 min of incubation (Fig. 1A). The association of bacteria with monocytes was similar in both cord blood and adult blood, with no apparent increase after 10- and 30-minute incubation periods (Fig. 1A).
Table 1.
Differential cell analysis and complement levels of blood specimens.
| Cell Type1,# | Specimen ID1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M10 (A) | P5 (A) | P4 (A) | M9 (C) | M11 (C) | M3 (C) | P6 (C) | P1 (C) | P2 (C) | P3 (C) | |
|
Neutrophils (A) 40–70 %, (C) 41–81 % |
54.2 % | 61.0 % | – | – | – | 49.0 % | 52.0 % | – | – | – |
|
Lymphocytes (A) 20–40 %, (C) 19–36 % |
37.4 % | 31.0 % | – | – | – | 37.0 % | 35.0 % | – | – | – |
|
Monocytes (A) 2–8 %, (C) 0.4–7 % |
5.2 % | 6.0 % | – | – | – | 7.0 % | 5.0 % | – | – | – |
|
Eosinophils (A) 1–4 %, (C) 0–2 % |
2.2 % | 1.0 % | – | – | – | 2.0 % | 1.5 % | – | – | – |
|
Basophils (A) 0.5–1 %, (C) 0–1 % |
1.0 % | 1.0 % | – | – | – | 0.0 % | 0.5 % | – | – | – |
| Metamyelocyte | – | – | – | – | – | 2.0 % | 2.0 % | – | – | – |
| Myelocyte | – | – | – | – | – | 3.0 % | 4.0 % | |||
|
Absolute Neutrophils (range in cells × 103/μL) (A) 1.56–6.45, (C) 3–28 |
2.40 | 4.53 | – | – | – | 8.56 | 7.21 | – | – | – |
|
Absolute Lymphocytes (range in cells × 103/μL) (A) 0.95–3.07, (C) 2–11 |
1.70 | 2.26 | – | – | – | 6.25 | 5.13 | – | – | – |
|
Absolute Monocytes (range in cells × 103/μL) (A) 0.1–1.3, (C) 0.1–4.4 |
0.20 | 0.43 | – | – | – | 1.20 | 1.30 | – | – | – |
|
Absolute Eosinophils (range in cells × 103/μL) (A) 0.03–0.3, (C) 0.01–1.5 |
0.10 | 0.05 | – | – | 0.34 | 0.20 | – | – | – | |
|
Absolute Basophils (range in cells × 103/μL) (A) 0.0–0.2, (C) 0.0–0.7 |
0.00 | 0.04 | – | – | – | 0.00 | 0.00 | – | – | – |
| Absolute Metamyelocyte | – | – | – | – | – | 0.26 | 0.28 | – | – | – |
| Absolute Myelocyte | – | – | – | – | – | 0.51 | 0.48 | – | – | – |
|
WBC (range in cells × 103/μL) (A) 3.5–11, (C) 9.0–35.0 |
5.9 | 7.3 | – | 9.0 | 11.1 | 17.1 | 10.5 | – | – | – |
|
Hemoglobin (A) 11.5–15.5 g/dL, (C) 14.0–18.8 g/dL |
13.0 | 13.6 | – | 14.6 | 11.6 | 14.7 | 13.6 | – | – | – |
|
MCV (A) 81–99 fL, (C) 95–125 |
91.3 | 90.9 | – | 93.0 | 98.0 | 111.6 | 103.2 | – | – | – |
|
RBCDIST WIDTH (A) < 15 %, (C) 13.0–18.0 % |
13.0 | 12.9 | – | 13.0 | 16.9 | 15.9 | 12.9 | – | – | – |
|
Platelets count (A) and (C) 150–450 x103/μL |
232.0 | 254.0 | – | 170.0 | 177.0 | 179.0 | 189.0 | – | – | – |
| Complement | ||||||||||
| C3 (mg/dL) | – | 99 | 248 | – | – | – | 97 | 70 | 99 | 94 |
| C4 (mg/dL) | – | 17 | 19 | – | – | – | 50 | – | 15 | 13 |
Denotes (A) = adult blood, (C) = cord blood.
Normal reported values indicated for adult (A) and (C) cord blood.
Fig. 1.
Phagocytosis of S. aureus by immune cells in cord and adult blood. (A) Association of staphylococci with neutrophils and monocytes in cord or adult blood after incubation for 10 and 30 min. Ten images were collected per time point and approximately 300 immune cells were counted to establish the distribution of cells associated with bacteria. (B) Giemsa stains of whole blood samples incubated with bacteria for 30 min in presence (+CD) and absence (−CD) of cytochalasin D. Red arrows point to extracellular agglutinated staphylococci. Black arrows point to neutrophils filled with bacteria. Data displayed is from 1 of 2 experiments, conducted independently.
Most staphylococci were associated with neutrophils and monocytes following the 30-minute incubation; isolated bacteria diffusing freely were seldom observed. We surmised that the majority of bacteria adhere to the cell surface of immune cells or are internalized. The inhibition of phagocytosis by Cytochalasin D (CD), which blocks actin polymerization [16], resulted in the formation of large agglutinated masses of extracellular S. aureus (Fig. 1B). This result suggests that phagocytosis must be the primary mechanism underlying the observed association between staphylococci and immune cells.
3.2. Phagocytosis of S. aureus in cord blood is enhanced with 3F6-hIgG1.
We examined the replication of S. aureus in cord and adult blood samples after a 60-minute incubation, in the presence of either 3F6-hIgG1 or control hIgG1. It is noteworthy that S. aureus is the only known pathogen capable of coagulating plasma and agglutinating within fibrin cables, making accurate enumeration of bacteria in blood samples challenging [17]. To liberate bacteria from blood agglutinates, extracellular DNA traps, and host cells, blood samples were treated with streptokinase, nucleases and saponin, respectively prior to agar plating. Staphylococcal replication after 60-min incubation in blood was reported as the average of at least two measurements and calculated as the % of the initial bacterial inoculum by counting colony-forming units (CFUs).
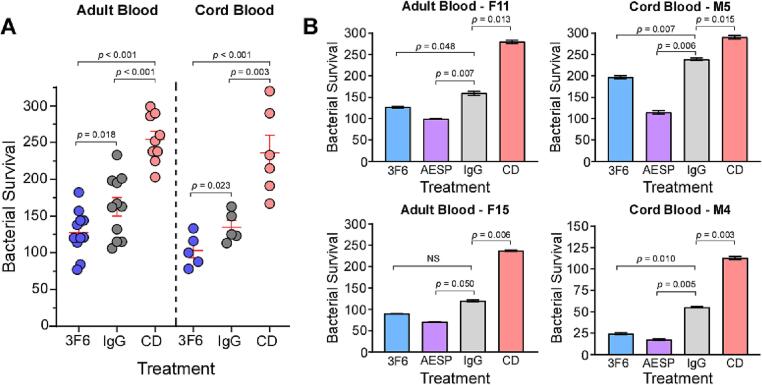
Consistent with the notion that CD treatment blocks phagocytic uptake (Fig. 1B), a significant increase in CFUs was observed in cord blood treated with CD compared to control hIgG1 (134.8 % ± 9.3 % with hIgG1 vs 236.0 % ± 24.0 % with CD) (Fig. 2A). Adult blood samples treated with CD showed a similar trend, with higher CFU of staphylococci recovered after incubation compared to control hIgG1 (162.5 % ± 12.6 % with IgG1 vs 254.6 % ± 10.8 % with CD) (Fig. 2A). Bacterial counts were reduced by 24 % upon addition of 10 µg ml−1 of 3F6-hIgG1 to cord blood (103.0 % ± 9.9 % with 3F6-IgG1 vs 134.8 % ± 9.3 % with hIgG1) (Fig. 2). Similarly, a 22 % reduction was observed in adult blood with 10 µg ml−1 of 3F6-hIgG1 (127.4 % ± 9.2 % with 3F6-IgG1 vs 162.5 % ± 12.6 % with IgG1) (Fig. 2A). These findings suggest that cord blood effectively inhibits S. aureus growth through phagocytosis, comparable to adult blood. Furthermore, the addition of 3F6-IgG1 enhances this phagocytic uptake mechanism.
Fig. 2.
Antibody enhanced phagocytic clearance of S. aureus in cord and adult blood. (A) Bacterial survival in cord or adult blood pre-treated with 10 µg ml−1 3F6-hIgG1 (3F6), 10 µg ml−1 control hIgG1 (IgG) or 0.04 mM Cytochalasin D (CD) after 60 min. The data represent the average ± SEM of colony forming units (CFU) after 60 min of incubation in blood, relative to the CFU of the initial inoculum (set as 100 %). Each condition was performed in duplicate and statistical significance was determined using unpaired independent Student’s t test. The data displayed represents experiments from 11 adult blood samples and 5 cord blood samples, conducted independently. (B) Bacterial survival in individual cord or adult blood samples after pre-treatment with 5 µg ml−1 3F6-hIgG1 (3F6), 5 µg ml−1 3F6-hIgG1AESP (AESP), 5 µg ml−1 control hIgG1 (IgG) or 0.04 mM CD after 60 min. Incubations with each blood sample were performed in duplicate and statistical significance was determined using Dunnett's multiple comparisons test. Data displayed is representative of experiments from 5 adult blood samples and 3 cord blood samples, conducted independently.
SpA molecules displayed on the surface of S. aureus bind to the Fcγ domains of IgG, including 3F6-hIgG1, which competes with Fcγ-Complement 1q (C1q) interaction, thereby diminishing complement activation and optimal opsonization of S. aureus [18]. Previously, we engineered 3F6-hIgG1 with four amino acid substitutions (AESP: S254A, Q311E, L432S, and N434P) abolishing the SpA-Fcγ domain interaction, thereby enhancing C1q, C3 and C4 recruitment on the surface of S. aureus [11]. Addition of 5 μg ml−1 3F6-hIgG1AESP further enhanced staphylococcal uptake compared to unmodified 3F6-hIgG1 in both cord blood and adult blood (Fig. 2B). Although cord blood exhibited slightly reduced levels of complement proteins C3/C4 compared to adult blood (Table 1), neonatal complement and immune cells retained the ability to effectively phagocytose and inhibit S. aureus growth in cord blood, similar to adult blood, and this capability was further enhanced with 3F6-hIgG1AESP.
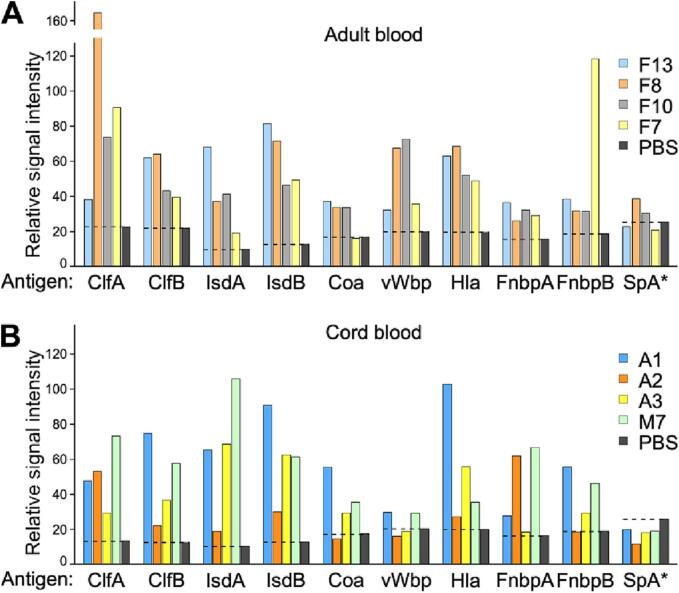
Using Enzyme-Linked Immunosorbent Assay (ELISA), we detected comparable levels of pre-existing S. aureus antibodies in both cord and adult blood plasma, suggesting successful maternal antibody transmission to cord blood donors (Fig. 3). However, the abundance of S. aureus-specific antibodies varied among donors and did not correlate with the extent of staphylococcal uptake in either cord blood or adult blood (Fig. 2). As anticipated, we measured negligible levels of antibodies to SpA (detected with SpAKKAA, a variant of SpA that does not bind to Fcγ and Fab) in all of the donors.
Fig. 3.
Anti-S. aureus antibodies for representative donors. Plasma samples collected from (A) adult blood and (B) umbilical cord blood were analyzed for antibodies against ClfA, ClfB, IsdA, IsdB, Coa, vWbp, Hla, FnbpA, FnbpB, and SpAKKAA. The dashed line represents the background signal obtained for PBS (in grey).
4. Discussion
Susceptibility of newborns to invasive bacterial infections has been attributed to their immature immune system, impaired function of phagocytes, and hyperactive innate immune responses at birth [19]. Newborns exhibit lower levels of Ig and are heavily reliant on maternal IgG passively transferred across the placenta during the final trimester of pregnancy [20]. Neonatal T cells are as potent as adult T cells, but complement activity is reduced in newborns as compared to adults.
The failure of antibodies to protect against staphylococcal sepsis in clinical trials might be due to the inability of neonatal immune cells to eliminate the pathogen. Our study found a stronger association of S. aureus with neutrophils in anticoagulated cord blood than in adult blood. However, this association did not correspond to improved pathogen clearance. The increased association of S. aureus with neonatal neutrophils, without an accompanying improvement in bacterial clearance, may reflect an impairment in bactericidal activities. Yet, as with adult blood, the presence of 3F6-hIgG1 enhanced the uptake of S. aureus in cord blood; such uptake was further improved with 3F6-hIgG1AESP even with an underdeveloped neonatal complement system, characterized by reduced levels of C1q, C4, C3, properdin, and factor B in newborns ([19] and Table 1).
While we detected substantial levels of pre-existing antibodies against S. aureus antigens, we hypothesize that these antibodies do not contribute to opsonophagocytic uptake as surface-bound SpA likely diverts them from their physiological targets. Consequently, a strategy neutralizing SpA, may be additive to pre-existing opsonizing antibodies and ultimately stimulate and enhance the neonatal immune system to combat staphylococcal infections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Padmini Srikantiah, Jacqueline Kirchner, and Keith Klugman for their support and suggestions. This project was supported by funds from the National Institute of Allergy and Infectious Diseases [AI148543] and the Bill & Melinda Gates Foundation, Seattle, WA [INV-003909]. Microscopy was performed in the Integrated Light Microscopy Core at University of Chicago, which receives financial support from the Cancer Center Support Grant (P30CA014599) RRID: SCR_019197.
Data availability
Data will be made available on request.
References
- 1.Lake J.G., et al. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the national healthcare safety network, 2011–2014. Infect Control Hosp Epidemiol. 2018;39(1):1–11. doi: 10.1017/ice.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cailes B., et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. 2018;103(6):F547–F553. doi: 10.1136/archdischild-2017-313203. [DOI] [PubMed] [Google Scholar]
- 3.Stoll B.J., et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 4.Baker C.J., Kasper D.L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 5.Sandberg K., et al. Preterm infants with low immunoglobulin G levels have increased risk of neonatal sepsis but do not benefit from prophylactic immunoglobulin G. J Pediatr. 2000;137:623–628. doi: 10.1067/mpd.2000.109791. [DOI] [PubMed] [Google Scholar]
- 6.Sause W.E., et al. Antibody-based biologics and their promise to combat staphylococcus aureus infections. Trends Pharmacol Sci. 2016;37(3):231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thammavongsa V., et al. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13(9):529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H.K., et al. Protein A-specific monoclonal antibodies and the prevention of Staphylococcus aureus disease in mice. Infect Immun. 2012;80:3460–3470. doi: 10.1128/IAI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thammavongsa V., et al. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine. 2015;33(4):523–526. doi: 10.1016/j.vaccine.2014.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., et al. Staphylococcus aureus decolonization of mice with monoclonal antibody neutralizing protein A. J Infect Dis. 2019;219(6):884–888. doi: 10.1093/infdis/jiy597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Schneewind O., Missiakas D. Engineered human antibodies for the opsonization and killing of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2022;119(4) doi: 10.1073/pnas.2114478119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., et al. Glycosylation-dependent opsonophagocytic activity of staphylococcal protein A antibodies. Proc Natl Acad Sci USA. 2020;117(37):22992–23000. doi: 10.1073/pnas.2003621117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basha S., Surendran N., Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10(9):1171–1184. doi: 10.1586/1744666X.2014.942288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.K., et al. Non-toxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections. J Exp Med. 2010;207:1863–1870. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss L., et al. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc Natl Acad Sci USA. 2017;114(49):E10596–E10604. doi: 10.1073/pnas.1702472114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casella J.F., Flanagan M.D., Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293(5830):302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- 17.Cheng A.G., et al. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsgren A., Quie P.G. Effects of staphylococcal protein A on heat labile opsonins. J Immunol. 1974;112:1177–1180. [PubMed] [Google Scholar]
- 19.Cuenca A.G., et al. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30(2):105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.