Abstract
Introduction
Preterm birth remains the most significant clinical and public health encounter. Preterm infant outcomes pose key evidence for clinicians and policymakers and are extensively used to set clinical and policy verdicts to improve services. It is necessary to conduct the outcomes of neonates frequently, as it varies from place to place and even from time to time in a similar place. There is limited literature in Ethiopia about preterm neonates’ outcomes and their predictors.
Objective
This study aimed to assess the neonatal outcomes of preterm neonates and their predictors in South Gondar zone public hospitals, Northwest Ethiopia, 2021.
Methods
A prospective observational study was employed on 462 preterm neonates in South Gondar Zone Public Hospitals. The data were entered into Epidata 4.6 and analyzed using STATA version 16/MP software. A parametric log-normal survival model was used to identify possible predictors for preterm neonate death. Statistical significance was declared at a P-value less than 0.05.
Result
The overall preterm survival rate was 71.1% (95% CI: 66.7, 75.1). Thirty-six percent of preterm neonates were diagnosed with sepsis. One-fourth of the neonates had respiratory distress syndrome. Gestational age greater than 34 weeks (β = 1.04; 95% CI: 0.53, 1.56), respiratory distress syndrome (β = 0.85; 95% CI: 0.49, 1.22), body mass index (β = −1.34; 95% CI: −1.87, −0.80), non-union marital status (β = −0.71; 95% CI: −1.34, −0.09), multiple pregnancies (β = −0.66; 95% CI: −0.99–0.32), multiparous (β = 0.35; 95% CI: 0.01, 0.69), hypothermia (β = −1.19; 95% CI: −1.76, −0.62), Kangaroo Mother Care (β = −1.9; 95% CI: −2.34, −1.41) and non-cephalic presentation (β = −1.23; 95% CI: −1.99,-0.46) were significant predictors.
Conclusion
In this study, the preterm survival rate was low. Gestational age greater than 34 weeks, no respiratory distress syndrome, and multiparous mothers were positively associated with the survival of preterm neonates. Though, high pre-pregnancy maternal body mass index, non-union marital status of mothers, multiple pregnancies, hypothermia, Kangaroo mother care is not given, and non-cephalic presentation were negatively associated. A significant focus should be given to implementing WHO recommendations on preventing and caring for preterm births.
Keywords: Neonatal outcome, Preterm neonate, Ethiopia, Preterm death, Preterm survival
1. Introduction
Preterm birth defines birth before 37 completed weeks of gestation or less than 259 days from the first day of a woman's last normal Menstrual Period (LMP). Preterm birth is often subdivided further based on birth gestational age. “Late preterm” infants are those born between 34 weeks and 37 weeks of gestation, “moderate preterm” is included as 32 weeks to less than 34 weeks of gestation, “very preterm” is defined as 28weeks of gestation to fewer than 32 weeks of gestation, and “extremely preterm,” is designated less than 28 weeks of gestational age [1].
Preterm infants are in varied groups with various possible outcomes [2]. Adverse neonatal outcomes are markedly higher in the developing world. Viability, a fifty percent probability of survival with or without medical care, is near 34 weeks gestational age in low and middle-income countries [3].
Preterm birth remains the most important clinical and public health challenge [4,5]. As a public health challenge, it was associated with high rates of occurrence of mortality, short-term perinatal complications, and lifelong difficulties such as learning infirmities from audiovisual, visual disabilities, and cognitive teething troubles [[6], [7], [8]].
In 2020, there were 2.4 million infant deaths worldwide and more than 6500 neonatal deaths per day. One-third of all newborn deaths occur on the first day after birth, and nearly 75% of all neonatal deaths happen within the first week of life. Half of (49%) all deaths among children under five worldwide occurred in just five nations, including Ethiopia [9,10]. Preterm birth, which accounts for 12.1% of under-five mortality in Sub-Saharan Africa, is the second most common cause of death [11]. Preterm birth and associated complications account for more than one-fifth of newborn death in Ethiopia [[11], [12], [13], [14], [15], [16]].
Common short-term morbidities of preterm neonates were sepsis, birth asphyxia, hyperbilirubinemia, and respiratory distress syndrome [17,18]. Late and moderate preterm neonates were significantly more likely to receive resuscitation at birth, respiratory and nutritional support, and were less likely to be fed breast milk than term infants [17]. Necrotizing enter colitis is a common morbidity among preterm neonates [19].
The risk of adverse outcomes of preterm neonates declines with increasing gestational age [17,20]. Birth weight, neonatal morbidities such as respiratory distress syndrome, sepsis, hypothermia, hypoglycemia, marital status, required resuscitation after delivery, no antenatal care, the plurality of pregnancy, sex of neonate, mode of delivery, non-cephalic presentation, fifth minute APGAR score <7, maternal complication and did not receive kangaroo mother care (KMC) were factors influencing preterm survival [[21], [22], [23], [24], [25], [26], [27]].
Despite WHO recommendations, preterm mortality is the primary cause of neonatal mortality nowadays. Implementation of the WHO recommendations of 2015 included corticosteroid injections for premature birth, antibiotics when her water breaks, and magnesium sulfate to prevent forthcoming neurological impairment of the infant and as well as thermal care, use of oxygen and surfactant to help infants breathe more effortlessly can decrease the chance of death and adverse health outcomes for preterm infants [28,29]. Even if Ethiopia has undertaken an initiative for measures to reduce the death of preterm babies at all stages of the healthcare system, the problem is still a public concern.
It is necessary to conduct the outcomes of neonates frequently as it varies from place to place and even from time to time in a similar place [30] because preterm infants' outcomes offer vital info to parents, clinicians, and policymakers for generally used to mark clinical and policy judgments, to design for the provision of medical care, to allocate resources and to advance the effectiveness of care. Preterm infants’ outcome data are also used for advocating and decision-making earlier and subsequently to birth, benchmarking regional outcomes, and identifying research questions and hypotheses.
Based on the health target of Sustainable Development Goals (SDGs) by 2030, all countries aimed to lessen neonatal mortality to as low as 12 per 1000 live births. Therefore, identifying preterm neonatal outcomes substantially affects the efforts to meet the SDG targets for windup-preventable child deaths by 2030 in Ethiopia.
2. Materials and methods
2.1. Study setting
The study was conducted in the South Gondar Zone public hospital in Northwest Ethiopia. The South Gondar zone is found in Amhara regional state. It is located in North West of Ethiopia. There are eight public hospitals in the South Gondar Zone. This study was done in Addis- Zemen primary Hospital, Wegeda primary hospital, Nefas Mewcha primary hospital, and Debretabor General Hospital.
2.2. Study design and period
A prospective institutional-based observational study design was employed on preterm neonates from December 1, 2020–May 30, 2021, delivered and admitted in neonatal intensive care units (NICU) in selected South Gondar zone public hospitals.
2.3. Sample size determination
The sample size was calculated using Open Epi Info 7 stat calc double population proportion formula with the assumptions of 95% CI, 80% power, and one to two exposed to unexposed ratio, by using gestational age as the exposure variable, the proportion of death among neonates delivered at a gestational age of 33 weeks is 22.6% and proportion of death among neonates delivered at a gestational age of 34 weeks is 9.6% in Addis Ababa teaching hospitals [31]. The final sample size with a 10% loss follow-up was 462 preterm neonates.
2.4. Sampling procedure
Preterm neonates were included from December 1, 2020, to May 30, 2021, when they were delivered and admitted to neonatal intensive care units (NICU) in selected South Gondar zone public hospitals. Four hospitals were selected based on the availability of organized NICU services and trained staff. Proportional allocation based on the monthly average number of preterm neonates delivered and admitted to the NICU was determined for each public hospital (Debre Tabor General Hospital, Nifas Mewch Primary Hospital, Adis Zemen Primary Hospital, and Wegeda Primary Hospital) for six months of the data collection period. A consecutive sampling technique was applied to those eligible participants. Incomplete charts and those women with unreliable LMP and who were unsure of their LMP and did not have first-trimester ultrasound records (up to and including 13 6/7 weeks of gestation) were excluded from the study.
2.5. Data collection instrument and variables
Data were collected using a pretested structured interviewer-administered questionnaire and checklists by reviewing medical records. The questionnaire was adapted by reviewing different literature pieces by critically evaluating the existing works focusing on socio-demographic characteristics, maternal medical, obstetric, Prenatal, and neonatal variables related to preterm neonatal outcomes.
Gestational age was estimated using reliable last normal menstrual period or the first-trimester ultrasound examination (up to and including 13 6/7 weeks of gestation). If there is an ultrasound measurement of the fetus in the first trimester, gestational age was determined based on ultrasound examination estimates because it is the most accurate method to confirm gestational age. Whereas for those women without an ultrasound examination in the first trimester but had reliable LNMP, gestational age was calculated based on their reliable LNMP.
Neonatal death is defined as a live-born neonate's death within 28 days after birth [32]. Immediate neonatal morbidity/complications include APGAR score at minutes 1 and 5, respiratory distress syndrome (RDS), hyperbilirubinemia, hypothermia, and sepsis of preterm infants within 42 PMAs after birth [25]. Neonatal sepsis is a severe infection that was diagnosed with the presence of at least two clinical symptoms and at least two laboratory abnormal findings in the presence of or as a result of a suspected or proven infection (positive culture) [33]. Respiratory distress Syndrome was diagnosed with one or more symptoms of tachypnea, intercostal muscle retraction, grunting, nasal flaring, and cyanosis [34].
Survival time is the quantity of follow-up starting from the date of birth to the occurrence of death [24]. Censored is a preterm neonate, referred, discharged, or alive at the end of the study period [35]. The survival time of a neonate beyond 42 postmenstrual ages, referred to and left with medical advice, was declared censored. Infant death between birth and 42 postmenstrual ages was declared as an event. The outcome variable coded 0 as censored and one as an event. Time-to-death is the death of a preterm neonate at a definite time during the follow-up period [24].
2.6. Data collection procedure and data quality control
The data were collected by twelve BSc neonatal nurses and four assistant lecturers in nursing supervisors. Trained data collectors interviewed mothers who were at 28 to less than 37 weeks (36 + 6 days) gestation at the labor ward and abstracted pregnancy, delivery, and infant data from the chart and followed during a 24-h period until discharge, transfer, death, or 42 postmenstrual ages after birth and their outcome was recorded.
Questionnaires were pretested with a 5% sample size at Mekane Eyesus general hospital. The training was given to data collectors and supervisors one day before and one day after the pretest regarding the study's objective, data collection tool, and ways of data collection and checking the completeness of the data collection tool.
2.7. Data analysis
Then data were entered using Epi-data version 4.6. Then exporting, cleaning, and analysis was done using STATA version 16/MP software. Kaplan Meier survival analysis was used to relate survival rates. Multicollinearity was assessed using the variance inflation factor (VIF). Multiple imputation techniques were used to deal with missing. The best-fit model was carefully chosen using Akakian Information Criteria (AIC). The lowest Akakian Information Criteria indicates the best-fit model. By a pure AIC-minimization criterion, a parametric log-normal survival model wins out. Parametric Log-normal survival model, the density of the “errors” is assumed to have a bell-shaped standard normal distribution. The function is Y = logT = α+σW, where W has a standard normal distribution. The hazard function of the log-normal distribution increases from 0 to reach a maximum and then decreases monotonically, approaching 0 as t → ∞. The hazards first rise and fall over time. This model has an accelerated life interpretation. This may be interpreted as by a factor exponent of the beta coefficient {β}. In the final model, a p-value <0.05 was considered significant.
2.8. Ethical approval
Ethical approval was obtained from Debre Tabor University, the College of Health Sciences, and the Institutional Review Board (IRB) with ethical number DTU/2751/2021.
2.9. Consent to participate
Study participants were informed about the purpose, risk, benefits, and confidentiality of the study, and an informed and voluntarily signed written consent (thumbprint for those unable to write) was obtained from all eligible mothers of the newborns before the study.
3. Results
3.1. Maternal socio-demographic characteristics
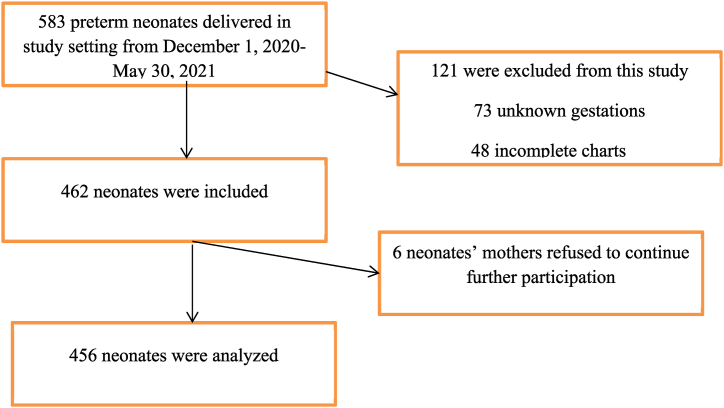
A total of 456 preterm neonates were involved in this study (Fig. 1).
Fig. 1.
Flow chart of participants' selection.
The median age of the respondents was 26 years. Most respondents (95.6%) were married, followed by single marital status (3.5%). Thirty-eight percent (177) of the mother's educational status was that she could not read or write. Regarding the occupation of the respondents, 364 (79.8%) of them were housewives, and 68 (14.9%) of them were employees. Three hundred (65.8%) respondents were rural residents (Table 1).
Table 1.
Socio-demographic characteristics among respondents’
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Marital status | Single | 16 | 3.5 |
| Married | 436 | 95.6 | |
| Divorced/windowed | 4 | 0.9 | |
| Educational status | Unable to read and write | 177 | 38.8 |
| Read and write | 17 | 3.7 | |
| Primary (1–8) | 166 | 36.4 | |
| Secondary (9–12) | 40 | 8.8 | |
| College and above | 56 | 12.3 | |
| Occupation | Housewife | 364 | 79.8 |
| Employed | 68 | 14.9 | |
| Merchant | 12 | 2.6 | |
| Student | 12 | 2.6 | |
| Residence | Urban | 156 | 34.2 |
| Rural | 300 | 65.8 | |
| Pre-pregnancy body mass index | Less than 18.5 kg/m2 | 88 | 19.3 |
| 18.5–24.99 kg/m2 | 220 | 48.2 | |
| Greater than 25 kg/m2 | 148 | 32.5 |
3.2. Maternal obstetric-related characteristics
Among the total respondents, one hundred seventy-two (37.2%) mothers were Para-one. Most mothers (97.4%) had antenatal care (ANC) follow-up during the current pregnancy. Among those mothers who have ANC follow-ups, 416 (93.7%) have four times and an above number of ANC follow-ups. Regarding the delivery route, the majority (91.2%) of mothers had vaginal route delivery. Seventy-six mothers (16.2%) encountered complications during the current pregnancy. Among those mothers who had a complication during the current pregnancy, antepartum hemorrhage (APH) 24 (31.6%) was the most common complication during the current pregnancy. Regarding the place of delivery, twenty mothers (4.4%) had home delivery. The median inter-birth interval of the mothers was 36 months (Table 2).
Table 2.
Obstetric-related characteristics among respondents’
| variables | Category | Frequency | Percent (%) |
|---|---|---|---|
| Parity | Null Para | 172 | 37.7 |
| Multiparous | 284 | 62.3 | |
| ANC | Yes | 444 | 97.4 |
| No | 12 | 2.6 | |
| No of ANC | Two | 4 | 0.9 |
| Three | 24 | 5.4 | |
| Four and above | 416 | 93.7 | |
| Route of Delivery | Vaginal route | 416 | 91.2 |
| Cesarean Section | 40 | 8.8 | |
| The plurality of the neonate | Singleton | 336 | 73.7 |
| Multiple | 120 | 26.3 | |
| Presentation | Cephalic | 440 | 96.5 |
| Non –Cephalic | 16 | 3.5 | |
| Complications during this Pregnancy | Yes | 76 | 16.7 |
| No | 380 | 83.3 | |
| Type of complication | Rhesus negative | 4 | 5.2 |
| Antepartum hemorrhage | 24 | 31.6 | |
| Pregnancy-induced hypertension | 16 | 21.1 | |
| Prolonged/obstructed labor | 8 | 10.4 | |
| Urinary tract infection | 4 | 5.2 | |
| Cord prolapse | 4 | 5.2 | |
| Premature rupture of membrane | 12 | 15.6 | |
| Maternal fever | 4 | 5.2 | |
| Antibiotics administration | Yes | 24 | 5.3 |
| No | 432 | 94.7 | |
| Pregnancy intention | Wanted | 452 | 99.1 |
| Unwanted | 4 | 0.9 | |
| Antenatal corticosteroid administered | Yes | 137 | 30.04 |
| No | 319 | 69.96 | |
| Magnesium sulfate administered | Yes | 0 | 0.0 |
| No | 456 | 100.0 |
3.3. Neonatal related characteristics
Of the total participants, 104 (40.4%) neonates were categorized between thirty-two and thirty-four weeks of gestational age. Two hundred twenty (52.6%) of the neonates were male (Table 3).
Table 3.
Neonatal-related characteristics among preterm neonates.
| variables | Category | Frequency | Percent (%) |
|---|---|---|---|
| Gestational age | Less than 32 weeks | 76 | 16.7 |
| 32–34 weeks | 196 | 43.0 | |
| Greater than 34 weeks | 184 | 40.4 | |
| Resuscitation at birth | Yes | 36 | 7.9 |
| No | 420 | 92.1 | |
| Sex | Male | 240 | 52.6 |
| Female | 216 | 47.4 | |
| Preterm | Spontaneous | 448 | 98.2 |
| Induced | 8 | 1.8 | |
| Type of Feeding | Breast milk | 252 | 55.3 |
| Express breast milk by nasogastric tube | 108 | 23.7 | |
| Formula milk | 4 | 0.9 | |
| Nothing per mouth | 92 | 20.2 | |
| Gestational Age for Weight | Appropriate for gestational age | 440 | 96.5 |
| Small for gestational age | 16 | 3.5 | |
| Birth weight | Greater than and equal to 2500 g | 34 | 7.5 |
| 1500–2499 g | 306 | 67.1 | |
| Less than 1500 g | 116 | 25.4 | |
| Kangaroo mother care (KMC) given | Yes | 110 | 24.1 |
| No | 346 | 75.9 | |
| 1st-minute APAGAR score | Less than 7 | 88 | 19.3 |
| ≥ 7 | 340 | 74.6 | |
| Not recorded | 28 | 6.1 | |
| 5 th minute APGAR score | Less than 7 | 69 | 15.1 |
| ≥ 7 | 387 | 84.9 |
3.4. Immediate morbidity of preterm neonates
Among the total preterm neonates, one-fourth (25.4%) of the neonates had respiratory distress syndrome (RDS). Most (89.9%) of preterm neonates had hypothermia (less than 36.5 °C). Thirty-six percent of preterm neonates were diagnosed with sepsis. Forty-four (9.6%) preterm neonates had perinatal asphyxia (PNA). Seven percent of preterm neonates had hypoglycemia (Table 4).
Table 4.
Frequency of immediate morbidity among preterm neonates.
| Morbidities | Category | Frequency | Percent (%) |
|---|---|---|---|
| RDS | Yes | 116 | 25.4 |
| No | 340 | 74.6 | |
| Hypothermia | Yes | 410 | 89.9 |
| No | 46 | 10.1 | |
| Sepsis | Yes | 164 | 36.0 |
| No | 292 | 64.0 | |
| Perinatal asphyxia | Yes | 44 | 9.6 |
| No | 412 | 90.4 | |
| Jaundice | Yes | 56 | 12.3 |
| No | 400 | 87.7 | |
| Congenital anomaly | Yes | 8 | 1.7 |
| No | 448 | 98.3 | |
| Hypoglycemia | Yes | 36 | 7.9 |
| No | 420 | 92.1 |
3.5. The survival rate of preterm neonates
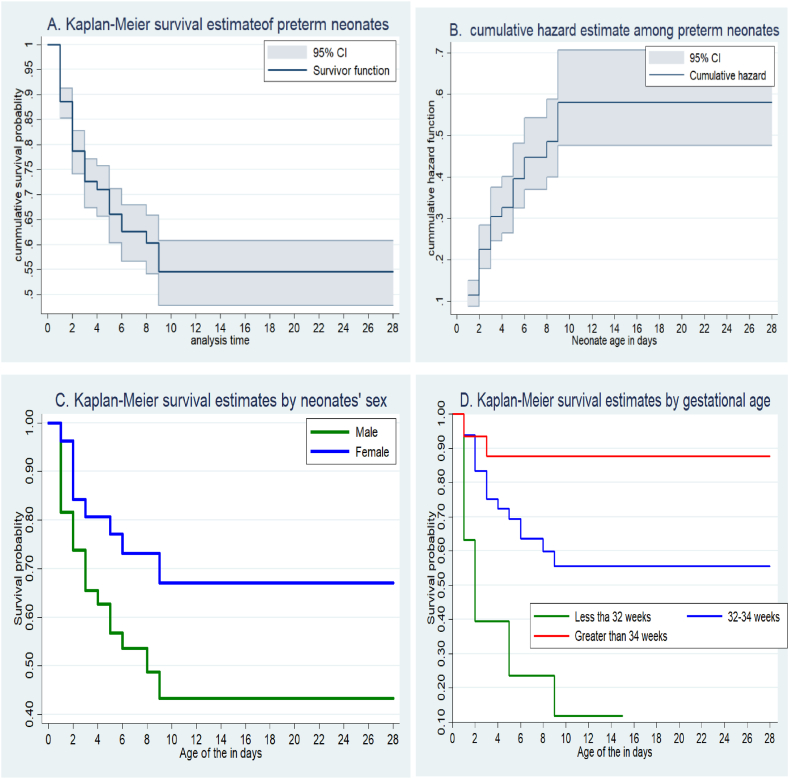
Four hundred fifty-six preterm neonates were followed from birth to 28 days, which gives 2159 days of risk time. The overall preterm survival rate was 71.1% (95% CI: 66.7, 75.1). The cumulative preterm neonates' survival probability of surviving at the end of the first 24 h was 86.3%. The Preterm neonates’ survival probability at the end of the first week was 56.4% (95% CI: 0.45, 0.58).
3.6. Kaplan-Meier preterm neonatal survival probability
The graph shows (Fig. 2A and B) that during the first 10 days of life, the graph went down progressively, indicating that a higher portion of preterm neonates was dying and there was a lesser probability of survival. While over the following days, the graph became straight, pointing out that the proportion of neonates who survived remained stable, signifying nearly no deaths. Male infants have a lower probability of survival related to female infants (Fig. 2C). The probability of death among preterm neonates whose gestational age was less than 32 weeks was higher (Fig. 2D).
Fig. 2.
Overall Kaplan Meier survival estimate of neonates (Fig A), cumulative hazard (Fig B), survival estimates by sex of neonates (Fig C), and survival estimates by gestational age (Fig D).
3.7. Predictors of preterm survival
Those variables with a P value of 0.5 in the log-rank test were entered into parametric analysis with the log-normal model. Gestational age greater than 34 weeks (βc) = 1.04; 95% CI: 0.53, 1.56), Gestational age from 32 to 34 weeks (βc = 0.57; 95% CI: 0.19,0 0.94), had no respiratory distress syndrome (βc = 0.85; 95% CI: 0.49, 1.22), high body mass index (βc = −1.34; 95% CI: −1.87, −0.80), non-union marital status (βc = −0.71; 95% CI: −1.34, −0.09), multiple pregnancies (βc = −0.66; 95% CI: −0.99–0.32), multiparous (βc = 0.35; 95% CI: 0.01, 0.69), hypothermia (βc = −1.19; 95% CI: −1.76, −0.62), KMC is not given (βc = −1.9; 95% CI: −2.34, −1.41) and non-cephalic presentation (βc = −1.23; 95% CI: −1.99,-0.46) were significant predictors for the survival of preterm neonates (Table 5).
Table 5.
Log normal model of predictors of Preterm survival among preterm neonates’
| Variables | Category | Outcome |
Unadjusted beta coefficient (95% CI) | Adjusted beta coefficient (95% CI) | |
|---|---|---|---|---|---|
| Death | Censored | ||||
| Marital status | Union | 120 | 316 | 1 | 1 |
| Not union | 12 | 8 | −0.94 (-1.72, −0.16) | −0.71(-1.34, -0.09) | |
| The plurality of the neonate | Singleton | 76 | 260 | 1 | 1 |
| Multiple | 56 | 64 | −0.95 (-1.35, −0.55) | −0.66(-0.99–0.32) | |
| Presentation | Cephalic | 124 | 316 | 1 | 1 |
| Non-cephalic | 8 | 8 | −1.3 (-2.13, −0.41) | −1.23(-1.99,-0.46) | |
| Neonate sex | Male | 92 | 148 | 1 | 1 |
| Female | 40 | 176 | 0.93 (0.54, 1.32) | 0.07 (-0.25, 0.40) | |
| Parity | Null Para | 40 | 132 | 1 | 1 |
| Multiparous | 92 | 192 | −0.45 (-.85, −0.052) | 0.35(0.01, 0.69) | |
| Pre-pregnancy maternal body mass index | <18.5 kg/m2 | 12 | 76 | 1 | 1 |
| 18.5–24.99 kg/m2 | 64 | 156 | −0.83 (-1.43, −0.23) | −0.9(-1.4, -0.36) | |
| ≥ 25 kg/m2 | 56 | 92 | −1.21 (-1.83, −0.59) | −1.34(-1.87, -0.80) | |
| Respiratory distress syndrome | Yes | 96 | 20 | 1 | 1 |
| No | 36 | 304 | 2 (1.6, 2.4) | 0.85(0.49, 1.22) | |
| Gestational age | Less than 32 weeks | 52 | 24 | 1 | 1 |
| 32–34 weeks | 64 | 132 | 1.48 (1.04, 1.92) | 0.57(0.18, 0.96) | |
| >34 weeks | 16 | 168 | 1.98 (1.43, 2.53) | 1.04(0.53, 1.56) | |
| Hypothermia | Yes | 6 | 40 | 1 | 1 |
| No | 126 | 284 | −1.49 (-2.17, −0.65) | −1.19(-1.76, -0.62) | |
| Perinatal asphyxia | Yes | 28 | 16 | 1 | 1 |
| No | 104 | 308 | 1.1 (0.53, 1.59) | −0.35 (-0.78, 0 .08) | |
| Resuscitated at birth | Yes | 24 | 12 | 1 | 1 |
| No | 108 | 312 | 1.5 (0.92, 2.1) | 0.33 (-0.19, 0.86) | |
| Antenatal corticosteroid administered | Yes | 20 | 117 | 1 | 1 |
| No | 112 | 207 | −0.82 (-1.27, −0.36) | −0.06 (-0.43, 0.32) | |
| Birth weight | ≥ 2500 g | 6 | 28 | 1 | 1 |
| 1500–2499 g | 42 | 264 | −0.06 (-.80, 0.92) | 0.39 (-0.31, 1.1) | |
| <1500 g | 84 | 32 | −1.5 (-2.35, −0.60) | 0.21 (-0.53, 0.94) | |
| kangaroo mother care | Yes | 9 | 101 | 1 | 1 |
| No | 123 | 223 | −2.13 (-2.62, −1.64) | −1.9(-2.34, -1.41) | |
Bold - significant.
The survival time for preterm neonates born at a gestational age of greater than 34 weeks was accelerated by a factor of 2.8 compared to neonates born with a gestational age of fewer than 32 weeks (βc = 1.04; 95% CI: 0.53, 1.56). The survival time for Preterm neonates who were born with a gestational age of between 32 and 34 weeks was increased by a factor of 1.77 compared to neonates born with a gestational age of fewer than 32 weeks (βc = 0.57; 95% CI: 0.19,0 0.94). Neonates without respiratory distress syndrome were 2.34 times more likely to survive than those with respiratory distress syndrome (βc = 0.85; 95% CI: 0.49, 1.22). The survival time for Preterm neonates whose mothers' pre-pregnancy BMI was greater than twenty-five kg/m2 was accelerated by a factor of 0.26 compared to those whose mothers' BMI was less than 18.5 kg/m2 (βc = −1.34; 95% CI: −1.87, −0.80). The survival time for Preterm neonates whose mothers were non-union marital status was accelerated by a factor of 0.49 compared to preterm neonates whose mothers were married status (βc = −0.71; 95% CI: −1.34, −0.09). Multiple preterm neonates' survival time was increased by a factor of 0.52 compared to singleton preterm neonates (βc = −0.66; 95% CI: −0.99–0.32). The survival time of preterm neonates born from multipara mothers’ was increased by a factor of 1.4 compared to those null-para mothers (βc = 0.35; 95% CI: 0.01, 0.69). The survival time for normothermic preterm neonates was accelerated by a factor of 0.30 compared to hypothermic preterm neonates (βc = −1.19; 95% CI: −1.76, −0.62). The survival time for preterm neonates who were not getting KMC was accelerated by a factor of 0.15 compared to those who got KMC (βc = −1.9; 95% CI: −2.34, −1.41). The survival time for preterm neonates born with non-cephalic presentation had accelerated by a factor of 0.29 compared to those born with cephalic presentation (βc = −1.23; 95% CI: −1.99,-0.46).
4. Discussion
This study showed the outcome of preterm infants and its predictors in South Gondar zone public hospitals. The overall preterm survival rate was 71.1% (95% CI: 66.7, 75.1). In the final model, gestational age, respiratory distress syndrome, pre-pregnancy body mass index, marital status, parity, the plurality of neonates, not given KMC, hypothermia, and presentation were significant predictors of preterm neonates’ survival.
The overall preterm neonatal survival rate was 71.1% (95% CI: 66.7, 75.1). It was in line with studies done in Addis Ababa, Ethiopia, 69.3% [36]. In contrast, it is lower than a study conducted in Gondar university comprehensive specialized hospital, Ethiopia, 74.8% [23]. This discrepancy might be due to these studies being in a comprehensive specialized hospital, in which the service provided for the maternal and neonatal population might be better than in the primary hospital. This study's overall preterm neonatal survival rate was higher than in Jimma, Ethiopia, at 65.1% [24], and Mizan Tepi University Teaching Hospital, Ethiopia [27]. The difference might be a time difference. Currently, the government of Ethiopia gives more attention to neonatal health than before to achieve the Sustainable Development Goals by 2030. Every year, the government of Ethiopia boosts the number of trained health professionals, which improves the care of preterm neonates.
This study showed that the survival time for preterm neonates born at a gestational age greater than 34 weeks was higher than those born with a gestational age of fewer than 32 weeks. This finding was similar to a study conducted in Jimma, Ethiopia [24], and a prospective study in Nigeria [37]. The possible justification might be that as gestational age increases, better physiological and immunological maturity occurs.
In the present study, preterm neonates without respiratory distress syndrome had less hazard of death than preterm neonates with respiratory distress syndrome. This might be because preterm neonates with respiratory distress syndrome might be prone to severe infection and other comorbidities, such as pulmonary hemorrhage, that upsurge the mortality hazard. This study is consistent with a study conducted in Gondar and Jimma, Ethiopia [23,24].
Obese mothers have a high risk for chronic illness, leading to abnormal placentation and perinatal health problems, including neonatal death. In this study, Preterm neonates whose mothers' pre-pregnancy BMI was greater than and equal to twenty-five kg/m2 were at a higher risk of death than those whose mothers’ BMI was less than 18.5 kg/m2. This is in line with another study that revealed that a rise in maternal pre-pregnancy BMI was associated with diminished odds of infant survival [31].
In this study, preterm neonates with multiple pregnancies had a high risk of death than singleton pregnancy status. This contrasts with a Western Uganda [22] study that showed singleton deliveries had higher mortality hazards than multiple gestations. This might be because multiple gestations are mostly associated with lower birth weight which may be disposed to metabolic disorders such as hypoglycemia, hypothermia, and other complications which increase the hazard of death of preterm neonates.
In this study, preterm neonates born to non-union mothers had a higher hazard of death than preterm neonates delivered to married mothers. This is similar to a study done in Brazil that found that unmarried women had a greater chance of neonatal death when compared to married mothers [38]. This might be because neonates whose mother is widowed or divorced have a higher risk of death because of more significant economic and psycho-social difficulties related to chronic sorrow. Female-headed households are more likely to experience poverty, which is associated with an increased risk of infant death [39]. Additionally, evidence shows the presence of a partner is a protective factor for vertical transmission of infections such as congenital syphilis that expose the neonates to adverse neonatal outcomes [40].
In this study, having a non-cephalic presentation has a high risk of death. This is similar to a study done in southwest Ethiopia [27]. This might be a non-cephalic presentation in preterm neonates with a greater risk for asphyxia that further complicates and leads to death. In addition to this, the procedures made to deliver the baby for non-cephalic presentation, such as breech or shoulder presentation, might lead the baby to ulceration or injuries which lead to sepsis or other complications that increase the risk of death of preterm neonates, Preterm neonates' born from null-para mothers had a high risk of death compared to those born from multipara mothers’. This is similar to other existing literature [[41], [42], [43]]. This might be due to the fact that neonates born to multiparous women have been found to have a higher birth weight [42]. Additionally, evidence shows null parity has been associated with stillbirth and intrapartum-related neonatal mortality [44,45].
Hypothermic preterm neonates had more risk of death than normothermic neonates. This is similar to a study done at Mizan Tepi University Teaching Hospital [27] in Brazil [46], Jigjiga University Referral Hospital [47], Malawi [26], and five hospitals in Ethiopia [48]. Large body surface area and less subcutaneous fat accumulation may prone preterm neonates to hypothermia. Hypothermia mainly relates to hypoxia, hypoglycemia, respiratory and metabolic acidosis, and inhibition of surfactant production. Due to these, the risk of death in preterm neonates increases.
In this study, kangaroo mother care decreased the risk of preterm neonate death. This is in line with studies done in Mizan Tepi University Teaching Hospital, Ethiopia [27], in northern Ethiopia [49], and meta-analyses, and Systematic Review studies which showed that KMC substantially reduces neonatal mortality among preterm babies [50,51]. This might be due to KMC's increased breastfeeding, weight gain, and psycho-social outcomes such as bonding and maternal satisfaction. Additionally, KMC reduced infections, apnea, prematurity, early recognition of illness, and length of hospital stay.
4.1. Strengths and limitations of the study
The multicenter, prospective cohort study design that enables the collection of highly qualified, more complete, and representative data is the strength of this study. However, this study is limited to only the short-term neonatal outcome. It does not look at the long-term morbidity and mortality of these babies. Neurological and cardiovascular morbidity is not included as it needs additional sophisticated investigation unavailable in the study setting. Some maternal and neonatal predictors, such as PROM, and institutionally related predictors such as patient-provider ratio, and good laboratory facility did not address appropriately.
5. Conclusions and recommendations
5.1. Conclusion
In this study, the preterm survival rate was low. Gestational age greater than 34 weeks, no respiratory distress syndrome, and multiparous mothers were positively associated with the survival of preterm neonates. Whereas high pre-pregnancy maternal body mass index, non-union marital status of mothers, multiple pregnancies, hypothermia, not getting Kangaroo mother care, and non-cephalic presentation were negatively associated with the survival of preterm neonates.
5.2. Recommendations
-
➢
For Healthcare providers and stakeholders
-
✓
Obstetric and neonatal care should be improved
-
✓
Special attention should be given to caring for preterm babies to enhance their survival, especially for multiple pregnancies, non-cephalic presentation, hypothermia, neonates with RDS, and very and moderate preterm neonates.
-
✓
Focus on Prevention, early diagnosis & management of neonatal comorbidities.
-
✓
Should apply KMC for all stable preterm neonates.
Author contribution statement
Habtamu Shimels: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. </p>
Fentaw Teshome Dagnaw; Solomon Demis; Binyam Minuye Birhane; Melkalem Mamuye Azanaw; Ermias Sisay Chanie; Worku Necho Asferie; Metsihet Tariku Fetene; Ayenew Mose; Demeke Mesfin Belay; Demewoz Kefale; Amare Kassaw; Mulu Tiruneh; Aragaw Tesfaw; Birara Aychew Tilaye; Getachew Arage; Alemwork Baye Kebede; Sofonyas Abebaw Tiruneh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. </p>
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization . New global estimates on preterm birth published. 2018. https://www.who.int/news/item/17-11-2018-new-global-estimates-on-preterm-birth-published Geneva. [Google Scholar]
- 2.Patel R.M. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 2016;33(3):318–328. doi: 10.1055/s-0035-1571202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howson C.P., Kinney M.V., Lawn J.E., editors. March of Dimes, PMNCH, Save the children, WHO. Born Too Soon: The global action report on preterm birth. World Health Organization; Geneva: 2012. https://apps.who.int/iris/handle/10665/44864 [Google Scholar]
- 4.Lumley J. Session 1-importance of PTB-Defining the problem of PTB: review of epidemiology. BJOG-an International Journal of Obstetrics and Gynaecology-Supplements. 2003;(20):3–7. [PubMed] [Google Scholar]
- 5.Goldenberg R.L. The management of preterm labor. Obstet. Gynecol. 2002;100(5):1020–1037. doi: 10.1016/s0029-7844(02)02212-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck S., Wojdyla D., Say L., Betran A.P., Merialdi M., Requejo J.H., et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howson C.P., Kinney M.V., McDougall L., Lawn J.E. Born too soon: preterm birth matters. Reprod. Health. 2013;10(1):1–9. doi: 10.1186/1742-4755-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharrow D., Hug L., You D., Alkema L., Black R., Cousens S., et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Global Health. 2022;10(2):e195–e206. doi: 10.1016/S2214-109X(21)00515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You D., Hug L., Ejdemyr S., Idele P., Hogan D., Mathers C., et al. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet. 2015;386(10010):2275–2286. doi: 10.1016/S0140-6736(15)00120-8. [DOI] [PubMed] [Google Scholar]
- 11.WHO . Global strategy for women’s, children’s and adolescents’ health. 2016. https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/mca/number-of-neonatal-deaths---by-cause [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham F.G.B.S., Hauth J.C., Rouse D.J., Spong C.Y., et al. 23rd ed. McGraw-Hill Professional; New York City: 2010. Williams Obstetrics. [Google Scholar]
- 13.Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E., et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 14.Marchant T., Willey B., Katz J., Clarke S., Kariuki S., Ter Kuile F., et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9(8) doi: 10.1371/journal.pmed.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNICEF. Maternal . 2015. Newborn Health Disparities in Ethiopia.https://data.unicef.org/wpcontent/uploads/country_profiles/Ethiopia/country%20profile_ETH.pdf [Google Scholar]
- 16.Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF . EPHI and ICF; Rockville, Maryland, USA: 2021. Ethiopia Mini Demographic and Health Survey 2019: Final Report.https://dhsprogram.com/pubs/pdf/FR363/FR363.pdf [Google Scholar]
- 17.Boyle E.M., Johnson S., Manktelow B., Seaton S.E., Draper E.S., Smith L.K., et al. Neonatal outcomes and delivery of care for infants born late preterm or moderately preterm: a prospective population-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2015;100(6):F479. doi: 10.1136/archdischild-2014-307347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrestha S., Dangol S.S., Shrestha M., Shrestha R.P. The outcome of preterm babies and associated risk factors in a hospital. JNMA; a journal of the Nepal Medical Association. 2010;50(180):286–290. [PubMed] [Google Scholar]
- 19.Lin P.W., Stoll B.J. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 20.Platt M.J. Outcomes in preterm infants. Publ. Health. 2014;128(5):399–403. doi: 10.1016/j.puhe.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Dagnachew T., Yigeremu M. Survival of preterm neonates and its determinants in teaching hospitals of Addis Ababa University. J. Women's Health Care. 2019;8:2. doi: 10.4172/2167-0420.1000461. [DOI] [Google Scholar]
- 22.Egesa W.I., Odong R.J., Kalubi P., Ortiz Yamile E.A., Atwine D., Turyasiima M., Kiconco G., Maren M.B., Nduwimana M., Ssebuufu R. Preterm neonatal mortality and its determinants at a tertiary hospital in western Uganda: a prospective cohort study. Pediatr. Health Med. Therapeut. 2020 Oct 7;11:409–420. doi: 10.2147/PHMT.S266675. PMID: 33117056; PMCID: PMC7548335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yehuala S., Ayalew S., Teka Z. Survival analysis of premature infants admitted to neonatal intensive care unit (NICU) in NorthwestNorthwest Ethiopia using semi-parametric frailty model. J. Biometrics Biostat. 2015;6:223. doi: 10.4172/2155-6180.1000223. [DOI] [Google Scholar]
- 24.Wesenu M., Kulkarni S., Tilahun T. Modeling determinants of time-to-death in premature infants admitted to neonatal intensive care unit in Jimma University Specialized Hospital. Annals of Data Science. 2017;4(3):361–381. [Google Scholar]
- 25.Mengesha H.G., Wuneh A.D., Lerebo W.T., Tekle T.H. Survival of neonates and predictors of their mortality in Tigray region, Northern Ethiopia: prospective cohort study. BMC Pregnancy Childbirth. 2016;16(1):1–13. doi: 10.1186/s12884-016-0994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phoya F., Langton J., Dube Q., Iroh Tam P.Y. Association of neonatal hypothermia with morbidity and mortality in a tertiary hospital in Malawi. J. Trop. Pediatr. 2020;66(5):470–478. doi: 10.1093/tropej/fmz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bereka B., Demeke T., Fenta B., Dagnaw Y. Survival status and predictors of mortality among preterm neonates admitted to mizan Tepi university teaching hospital, South west Ethiopia. Pediatr. Health Med. Therapeut. 2021;12:439–449. doi: 10.2147/PHMT.S319774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iams J.D., Romero R., Culhane J.F., Goldenberg R.L. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet (London, England) 2008;371(9607):164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 29.Griffin J.B., Jobe A.H., Rouse D., McClure E.M., Goldenberg R.L., Kamath-Rayne B.D. Evaluating WHO-recommended interventions for preterm birth: a mathematical model of the potential reduction of preterm mortality in sub-Saharan Africa. Glob. Health Sci. Pract. 2019;7(2):215–227. doi: 10.9745/GHSP-D-18-00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbasi K.A. Neonatal disease profile in Larkana before and after establishment of neonatal ward. JPMA. The Journal of the Pakistan Medical Association. 1995 Sep;45(9):235–236. PMID: 8683827. [PubMed] [Google Scholar]
- 31.Chawla S., Laptook A.R., Smith E.A., Tan S., Natarajan G., Wyckoff M.H., et al. In-hospital mortality and morbidity among extremely preterm infants in relation to maternal body mass index. J. Perinatol. 2021;41(5):1014–1024. doi: 10.1038/s41372-020-00847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong X., Xu F., Wu R., et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. 2016;16:174. doi: 10.1186/s12887-016-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gebremedhin D., Berhe H., Gebrekirstos K. Risk factors for neonatal sepsis in public hospitals of mekelle city, North Ethiopia, 2015: unmatched case-control study. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A., Soujanya S., Madhava K. Neonatal outcomes as per gestational age in late preterm births: a retrospective study. International Journal of Contemporary Pediatrics. 2017;4(1):5–8. [Google Scholar]
- 35.Desta B.N., Assefa N., Damte T.D., Hordofa L.O. Neonatal mortality and its risk factors in eastern Ethiopia: a prospective cohort study in Kersa health and demographic surveillance system (Kersa HDSS) Mortality. 2016;13(18):19. [Google Scholar]
- 36.Temesgen Merertu, Worku Bogale, Regassa Yonas, Mekasha Amha. Survial of preterm infants admitted to tikur anbessa hospital nicu. Addis Ababa. Ethiopian Journal of Pediatrics and Child Health. 2014;10:10. http://ejol.aau.edu.et/index.php/EJPCH/article/view/1001 [Google Scholar]
- 37.Osuorah C., Ifediora C., Asinobi I., Ekwochi U., Agwu S., Ndu I., et al. Determinants of survival in low birth weight infants at a tertiary healthcare facility in South Eastern Nigeria. Chidiebere ODI, et al. The Low-birth weight Infants: Pattern of Morbidity and Mortality in a Tertiary Healthcare Facility in the South Eastern Nigeria. Ann Med Health Sci Res. 2018. 2017;8:4–10. [Google Scholar]
- 38.Migoto M.T., Oliveira R.P., Silva A.M.R., Freire M.H.S. Early neonatal mortality and risk factors: a case-control study in Paraná State. Rev Bras Enferm [Internet] 2018;71(5):2527–2534. doi: 10.1590/0034-7167-2016-0586. [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Robinson D., Lai E.T.C., Wickham S., et al. Assessing the impact of rising child poverty on the unprecedented rise in infant mortality in England, 2000–2017: time trend analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domingues R.M.S.M., Saraceni V., Hartz Z.M.A., Leal M.C. Congenital syphilis: a sentinel event in antenatal care quality. Rev Saúde Pública[Internet] 2013 doi: 10.1590/S0034-89102013000100019. cited 2016 Sep 26];47(1). Available from: [DOI] [PubMed] [Google Scholar]
- 41.Bai J, wong FW, bauman A, mohsin M. Parity and pregnancy outcomes. Am. J. Obstet. Gynecol. 2002;186(2):274–278. doi: 10.1067/mob.2002.119639. [DOI] [PubMed] [Google Scholar]
- 42.Kozuki N., Lee A.C., Silveira M.F., Sania A., Vogel J.P., Adair L., et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Publ. Health. 2013;13(3):1–10. doi: 10.1186/1471-2458-13-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garces A., Perez W., Harrison M.S., Hwang K.S., Nolen T.L., Goldenberg R.L., et al. Association of parity with birthweight and neonatal death in five sites: the Global Network's Maternal Newborn Health Registry study. Reprod. Health. 2020;17(Suppl 3):182. doi: 10.1186/s12978-020-01025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee A.C., Mullany L.C., Tielsch J.M., Katz J., Khatry S.K., LeClerq S.C., Adhikari R.K. Darmstadt GL: community-based stillbirth rates and risk factors in rural Sarlahi, Nepal. Int. J. Gynecol. Obstet. 2011;113(3):199–204. doi: 10.1016/j.ijgo.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Fretts R. Stillbirth epidemiology, risk factors, and opportunities for stillbirth prevention. Clin. Obstet. Gynecol. 2010;53(3):588–596. doi: 10.1097/GRF.0b013e3181eb63fc. [DOI] [PubMed] [Google Scholar]
- 46.de Almeida M.F., Guinsburg R., Sancho G.A., Rosa I.R., Lamy Z.C., Martinez F.E., et al. Hypothermia and early neonatal mortality in preterm infants. J. Pediatr. 2014;164(2):271–275 e1. doi: 10.1016/j.jpeds.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 47.Ibrahim A.M., Farah A.M., Osman M.O., Hashi A. The effect of admission hypothermia for neonatal death among neonates admitted to neonatal intensive care unit at sheik hassan yabare Jigjiga university referral hospital in Jigjiga city, Somali region, eastern Ethiopia. Res. Rep. Neonatol. 2021;11:43–55. [Google Scholar]
- 48.Demtse A.G., Pfister R.E., Nigussie A.K., McClure E.M., Ferede Y.G., Tazu Bonger Z., et al. Hypothermia in preterm newborns: impact on survival. Glob Pediatr Health. 2020;7 doi: 10.1177/2333794X20957655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girma B., Nigussie J. Magnitude of preterm hospital neonatal mortality and associated factors in northern Ethiopia: a cross-sectional study. BMJ Open. 2021;11(12) doi: 10.1136/bmjopen-2021-051161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn J.E., Mwansa-Kambafwile J., Horta B.L., Barros F.C., Cousens S. 'Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications. Int. J. Epidemiol. 2010 Apr;39(1):i144–i154. doi: 10.1093/ije/dyq031.PMID:20348117. PMCID: PMC2845870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conde-Agudelo A., Díaz-Rossello J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2016 Aug 23;2016(8) doi: 10.1002/14651858.CD002771.pub4. CD002771. PMID: 27552521; PMCID: PMC6464509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.