The story of the Phase III ADAURA trial remains a fantastic read: three years of osimertinib therapy given in the adjuvant setting continues to show benefit to patients with early-stage non-small cell lung cancer (NSCLC) harboring an epidermal growth factor receptor mutation (EGFRm) (1).
In 2020, the preliminary results of ADAURA were presented (2). The independent data monitoring committee (IDMC) had noted a marked difference in disease recurrence among patients receiving placebo compared with those receiving osimertinib. As such, the trial was unblinded to the sponsor. At this time, all patients had been randomized and all had received at least one year of treatment. The trial data were only 33% mature, although the pre-planned, protocol-specified endpoint of disease-free survival (DFS) was to be reviewed at 50% maturity. Nonetheless, formal statistical testing was applied to the preliminary results based on recommendation by the IDMC.
The initial analysis showed that for the primary endpoint of DFS among patients with resected stage II/IIIA disease, the hazard ratio (HR) was 0.17 (2). Similarly, in the overall population of patients with resected stage IB–IIIA disease, the HR was 0.20. What does this mean? It means that a patient randomized to placebo in this trial had an 80% higher chance of disease recurrence than a patient randomized to osimertinib in the study period. Based on adjuvant trials conducted to-date in multiple tumor types, we know that the absolute benefit of adjuvant therapy is greatest in advanced disease stages. This was also the case in ADAURA: HRs for DFS in stages IB, II, and IIIA were 0.39, 0.17, and 0.12, respectively. An early snapshot of the data also showed a difference in overall survival (OS).
Now to address the updated result of ADAURA, the subject of this editorial (1). At the time of this analysis, the trial data were at 50% maturity, the target pre-specified before early unblinding. Median follow-up was 44.2 months (osimertinib) and 19.6 months (placebo) for patients with stage II–IIIA disease. As statistical testing was already performed, it was not repeated. Compared with the preliminary results, the HR for DFS among patients with resected stage II/III disease was slightly higher, at 0.23, as was the HR for stage IB–IIIA disease (0.27) (Figure 1). Stated simply, there was a 73% higher risk of relapse for patients treated with placebo than those treated with osimertinib in the full population. Surprisingly, some oncologists have been disappointed with these updated results. I believe this is only because they are making unfair comparisons to the preliminary findings—in the overall study population, the benefit of adjuvant osimertinib continues to shine over placebo and is an outstanding clinical result.
Figure 1.
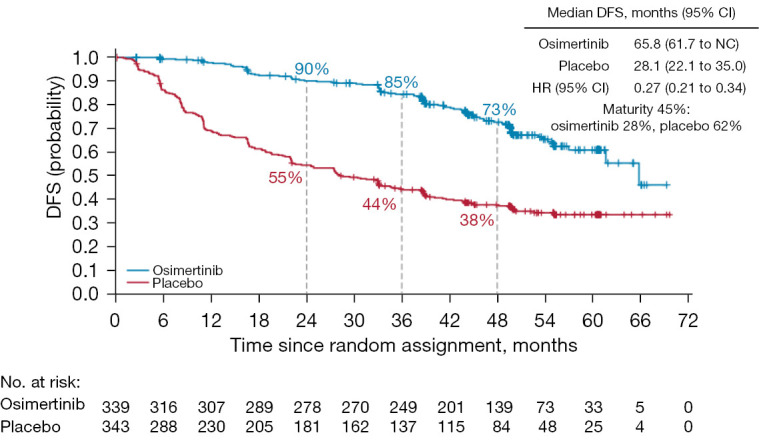
Updated Kaplan-Meier analysis of DFS among patients with stage IB-IIIA NSCLC (per investigator assessment). Tick marks indicate censored data. Source from (1). DFS, disease-free survival; CI, confidence interval; HR, hazard ratio; NC, not calculated; NSCLC, non-small cell lung cancer.
Review of the shape of the updated DFS curves reveals a change in the slope of the osimertinib curve at 36 months. This is the time at which delivery of adjuvant osimertinib was completed. This observation begs the question: does three years of osimertinib therapy just prolong time to recurrence, rather than provide a cure? In the advanced stage IV setting, use of osimertinib is considered palliative, not curative. Has any tyrosine kinase inhibitor cured a solid malignancy in the adjuvant setting? The answer is yes, in the setting of primary gastrointestinal stromal tumors (GIST) (3). When provided for three years after resection, adjuvant imatinib improved recurrence-free survival, and more importantly, OS. So why do we believe this cannot occur in lung cancer?
Interestingly, earlier trials of adjuvant imatinib in GIST were negative when therapy was provided for only two years (4). Similarly, in the CTONG 1104 trial of adjuvant gefitinib, DFS was significant but OS was not after two years of therapy (5). In ADAURA, was three years of adjuvant osimertinib therapy adequate to provide an OS benefit? We have heard through a press release that the answer is yes (6). Still, the OS analysis was performed at only 20% maturity and, unfortunately, we are told this is the final evaluation. We may never know whether the shape and slope of the survival curve will flatten. A missed opportunity.
In addition to the DFS benefit of osimertinib in ADAURA, the low rate of central nervous system (CNS) recurrence must be acknowledged, as this finding is one of the most compelling reasons to use osimertinib in the adjuvant setting. In the preliminary analysis, 98% of patients in the osimertinib group were alive without CNS disease, compared with 85% in the placebo group (2). In the updated evaluation, the results continued to favor osimertinib, with 93% of osimertinib-treated patients alive without CNS disease (still 85% with placebo) (1). HRs for CNS DFS were 0.24 and 0.36 for resected stage II/IIIA and stage IB–IIIA disease, respectively. Still, it is important to recognize that regular CNS imaging was not mandated in the trial protocol—such imaging was left to the discretion of the investigator and only required if a patient presented with suspicious symptoms. As such, strong conclusions regarding CNS protection/prevention by adjuvant osimertinib cannot be made. Another missed opportunity.
How do we define cure? In the world of thoracic oncology, most would define it as disease-free status at five years. Will the ADAURA survival data ultimately be reviewed again and hit this mark? We don’t know. And will osimertinib-treated patients eventually experience recurrence, if they do not die first of other causes? I believe that some patients who receive adjuvant osimertinib will remain disease free for their lifetime. I may be proven wrong—only time and subsequent trials will answer the question of whether we can we finally cure lung cancer that harbors an EGFRm.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-222/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-222/coif). The author has no conflicts of interest to declare.
References
- 1.Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol 2023;41:1830-40. 10.1200/JCO.22.02186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 3.Joensuu H, Eriksson M, Sundby Hall K, et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients With High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol 2020;6:1241-6. 10.1001/jamaoncol.2020.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casali PG, Le Cesne A, Velasco AP, et al. Final analysis of the randomized trial on imatinib as an adjuvant in localized gastrointestinal stromal tumors (GIST) from the EORTC Soft Tissue and Bone Sarcoma Group (STBSG), the Australasian Gastro-Intestinal Trials Group (AGITG), UNICANCER, French Sarcoma Group (FSG), Italian Sarcoma Group (ISG), and Spanish Group for Research on Sarcomas (GEIS)(☆). Ann Oncol 2021;32:533-41. 10.1016/j.annonc.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Wu YL, Zhong W, Wang Q, et al. Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): A randomized, Phase III trial (CTONG 1104). J Clin Oncol 2017;35:8500. 10.1200/JCO.2017.35.15_suppl.8500 [DOI] [Google Scholar]
- 6.AstraZeneca. Tagrisso demonstrated strong overall survival benefit in the ADAURA Phase III trial for adjuvant treatment of patients with early-stage EGFR-mutated lung cancer. 9 March 2023. Accessed: 17 March 2023. Available online: https://www.astrazeneca.com/media-centre/press-releases/2023/tagrisso-demonstrated-strong-overall-survival-benefit-in-the-adaura-phase-iii-trial.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


