Abstract
Background
Not all non-small cell lung cancer (NSCLC) patients will benefit from immune checkpoint therapy and use of these medications carry serious autoimmune adverse effects. Therefore, biomarkers are needed to better identify patients who will benefit from its use. Here, the correlation of overall survival (OS) with baseline and early treatment period serum biomarker responses was evaluated in patients with NSCLC undergoing immunotherapy.
Methods
Patients diagnosed with NSCLC undergoing immunotherapy (n=597) at a tertiary academic medical center in South Korea were identified between January 2010 and November 2021. The neutrophil-lymphocyte ratio (NLR), C-reactive protein (CRP), and lactate dehydrogenase (LDH) levels in the survival and non-survival groups were examined at baseline and early treatment periods. Additionally, aberrant laboratory parameters at each period were used to stratify survival curves and examine their correlation with one-year OS.
Results
In the non-survival group, the NLR, CRP, and LDH levels at the early treatment period were higher than those at the baseline (P<0.001). The survival curves stratified based on aberrant laboratory findings in each period varied (log-rank test P<0.001). Multivariate Cox regression analysis revealed that having prescribed more than 3rd line of chemotherapy [hazard ratio (HR) =3.19, 95% confidence interval (CI): 1.04–9.82; P=0.043] and early treatment period CRP (HR =3.88; 95% CI: 1.55–9.72; P=0.004) and LDH (HR =4.04; 95% CI: 2.01–8.12; P<0.001) levels were significant predictors of one-year OS.
Conclusions
Early treatment period CRP and LDH levels were significant predictors of OS in patients with NSCLC undergoing immunotherapy.
Keywords: Non-small cell lung cancer (NSCLC), immunotherapy, neutrophil-lymphocyte ratio (NLR), C-reactive protein (CRP), lactate dehydrogenase (LDH)
Highlight box.
Key findings
• Patients with NSCLC who were prescribed immunotherapy, having more than 3rd line of chemotherapy and the early treatment period CRP and LDH levels were significant predictors of OS after the first immunotherapy.
What is known and what is new?
• Baseline number of line of chemotherapy before initial immunotherapy, CRP, and LDH are important predictors for OS of NSCLC patients prescribed immunotherapy.
• Early treatment period CRP and LDH are important factors for predicting OS of NSCLC prescribed immunotherapy.
What is the implication, and what should change now?
• For patients with NSCLC who were prescribed immunotherapy, clinicians should check the line of chemotherapy that the patient was treated, and the early treatment period CRP and LDH levels for predicting the OS.
Introduction
Immunotherapies can restore host immune responses against cancer, reverse immune escape, or evasion, and promote tumor cell death. Immune checkpoint inhibitors (ICIs), which block the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) axis, have been the most important development in cancer therapy in the last few decades. The blockade of PD-1 or PD-L1 alone or in combination with conventional chemotherapy is the standard first-line therapy for stage IV non-small cell lung cancer (NSCLC) (1).
Only 15.3–47.6% of patients with NSCLC respond to immunotherapy (2). Approximately 23.4% and 45% of patients experience immune-related adverse events after single immunotherapy and multiple immunotherapy (ipilimumab plus nivolumab) treatments, respectively (3). The identification of biomarkers for predicting the responder population is crucial. Previous studies have identified prognostic markers, such as PD-L1 expression (4), tumor mutational burden (5), tumor-infiltrating lymphocytes (6), and microsatellite instability-high status (7) for immunotherapy. However, these markers, except for PD-L1 expression, do not provide consistent outcomes. Additionally, none of the developed markers reflect tumor heterogeneity. Furthermore, standardized methods have not been developed to interpret the markers (8) and the detection of these biomarkers is dependent on the availability of adequate amounts of tumor tissue. Thus, there is a need to identify serum biomarkers as they enable a convenient and nearly non-invasive evaluation.
Cancer-associated inflammation is reported to be a poor survival predictor. In particular, neutrophil-lymphocyte ratio (NLR) and C-reactive protein (CRP), which are markers for systemic inflammation, have been negatively correlated with prognosis (8-12). Lactate dehydrogenase (LDH), a housekeeping enzyme that is released by rapidly growing tumors, is positively correlated with tumor burden (13). High pretreatment LDH level is associated with poor outcomes in patients with NSCLC undergoing immunotherapies (14). Combinations of these markers have been associated with poor prognosis. The lung immune prognostic index (LIPI), which comprises LDH and NLR, is divided into the following three groups: good, intermediate, and poor LIPI groups. The baseline LIPI is correlated with overall survival (OS) and progression-free survival in patients with NSCLC undergoing immunotherapy (15). In addition to pretreatment markers, the analysis of dynamics of these markers that respond to immunotherapy can predict the prognosis of patients undergoing immunotherapy. The upregulation of CRP expression levels over time is a strong indicator of an increased progression risk. Conversely, the downregulation of CRP expression is associated with improved treatment response (12). Previous study has demonstrated that the CRP responders in whom the serum CRP level decreases by >30% after immunotherapy relative to the baseline levels exhibited a good prognosis (16).
This study developed a prediction model for OS using baseline and early treatment period responses of the serum biomarkers (NLR, CRP, and LDH) in patients with NSCLC who were prescribed immunotherapies, not combined with conventional chemotherapy. Although these serum biomarkers have been previously examined, their dynamics have not been reported. We present this article in accordance with the REMARK reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-7/rc).
Methods
Study design and participants
This study retrospectively identified patients who were diagnosed with NSCLC and prescribed immunotherapies with ipilimumab, pembrolizumab, nivolumab, or atezolizumab between January 1, 2010, and November 25, 2021, at the Severance Hospital, a tertiary academic medical center in South Korea. Patients who had no recorded pathological reports and were prescribed chemotherapy in combination with immunotherapy were excluded from this study to evaluate the effect of immunotherapy alone. Additionally, patients with a survival duration of less than one year were excluded.
Data collection
Demographic, diagnosis, laboratory, pathology, treatment, and OS data were extracted from electronic medical records using a standardized data collection method. The demographic data included the age, sex, body mass index (BMI), European Cooperative Oncology Group (ECOG) and line of chemotherapy of the patients when the immunotherapy was first prescribed. Also, the initial cancer stage value was collected. The type of lung cancer was identified from the diagnosis data. From the pathology reports, the cell type of the NSCLC and the epidermal growth factor receptor (EGFR) and PD-L1 expression levels were retrieved. The laboratory data included the levels of NLR, CRP, and LDH, which were previously reported to be prognosis prediction markers of immunotherapy. The treatment data included the frequency and type of immunotherapy prescribed to the patients. The OS was defined as the time between the date of immunotherapy initiation and the date of death or last follow-up.
Definitions
The total observation period comprised 14 days before the initiation of the first immunotherapy and the first 8 weeks of immunotherapy administration. For each laboratory parameter, the mean levels within 14 days before the first immunotherapy initiation were defined as the baseline values. The early treatment period was defined as the period of 8 weeks from the first immunotherapy prescription. The mean levels of laboratory parameters within the early treatment period were considered the early treatment values. The condition was defined as aberrant if the levels of at least one of the markers were above the physiological range (NLR >4, CRP >8 mg/dL, and LDH >247 IU/L). Meanwhile, the condition was defined as physiological if the levels of parameters were within the physiological range. The patients were divided into the following two groups based on survival for 1 year: the survival and non-survival groups.
Statistical analysis
The demographic variables of patients were summarized for the total population and individual group. Categorical variables are represented as frequencies and percentages, while continuous variables are represented as mean ± standard deviation (SD). The laboratory parameter values at the baseline and early treatment period were represented as median and interquartile range (IQR) and compared using the Wilcoxon rank test. In addition, the correlation among NLR, CRP, LDH, and PD-L1 levels was investigated using a correlation matrix. The categorical and continuous variables were investigated. The OS was estimated using the Kaplan-Meier method. The correlation between the group and OS was assessed using the log-rank test. The survival curves were prepared based on each laboratory parameter that was aberrant in the baseline and early treatment period. The correlation between markers and OS was assessed using the Cox proportional hazards model using the following covariates: age, sex (female vs. male), and baseline and early treatment period levels of NLR (<4 vs. ≥4), CRP (<8 vs. ≥8 mg/dL), and LDH (<247 vs. ≥247 IU/L) as binary variables. The model developed using early treatment period laboratory parameters and that developed using only baseline laboratory parameters were compared with demographic features using the concordance index (C-index) (17), which evaluates the performance of the survival model. The sensitivity analysis was performed by changing the observation period to 2 and 3 years. Moreover, the survival was analyzed with the time-varying Cox regression model (18), which can reflect not only the baseline and the early treatment period response but also the dynamic responses for 8 weeks. The correlation between NLR, CRP, LDH, and PD-L1 levels was investigated using a correlation matrix. The categorical and continuous variables were investigated. The laboratory parameter values were imputed using the feed-forward method for developing the time-varying Cox regression model. In case the laboratory tests were not performed, the laboratory values were removed. Since EGFR-mutated NSCLC patients are known to show low benefit of ICI therapy, we conducted a subgroup analysis of patients who were identified as having EGFR mutation. For this population, we checked the OS with Kaplan-Meier method and analyzed the correlation between markers and OS using the Cox proportional hazards model for variables that were used in the analysis above. Since there could be a selection bias, we also analyzed the patients who has less than one-year window period. For the group including the patients who had less than 1 year of OS, we compared the demographic variables between the survival and non-survival groups. Also, we analyzed the correlation between the markers and OS with Cox proportional hazards models that were conducted above.
All statistical analyses were two-sided tests. Differences were considered significant at P<0.05. All statistical analyses were performed using R 4.1.0.
Ethical statement
This research was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the institutional review board of Severance Hospital, Seoul, Korea (IRB No. 2019-2129-011). Waivers of consent were granted based on general impracticability and minimal harm.
Results
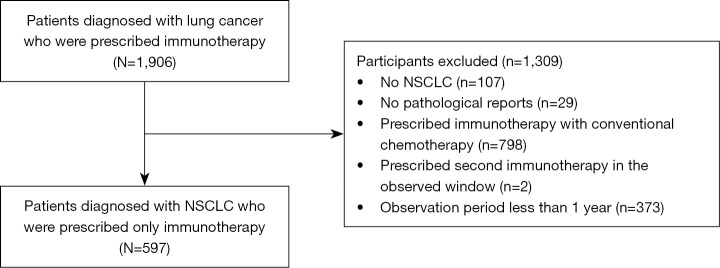
In total, 1,906 patients diagnosed with lung cancer and undergoing immunotherapy at the Severance Hospital between January 1, 2010, and November 25, 2021, were enrolled in the study. The following cases were excluded from the study: cases with non-NSCLC as determined from the pathological reports: 107 patients; cases without pathological reports: 29 patients; cases who were prescribed immunotherapy with conventional chemotherapy: 798 patients; cases who underwent two immunotherapies in the study period: 2 patients (only patients who were prescribed immunotherapy once during the study period were included); cases for whom the observation period was <1 year: 373 patients (Figure 1).
Figure 1.
A flow diagram of study participant selection criteria for the study. NSCLC, non-small cell lung cancer.
The clinical characteristics of patients are summarized in Table 1. The mean age of the study cohort was 61.2±10.2 years. Among the 597 patients included in this study, 437 (73.2%) were males. Adenocarcinoma and squamous cell carcinoma were diagnosed in 425 (71.2%) and 160 (26.8%) patients, respectively. The diagnosis was not significantly different between the survival and non-survival groups. Atezolizumab, pembrolizumab, and nivolumab were prescribed for 178 (29.8%), 207 (34.7%), and 212 (35.5%) patients, respectively. Most of the patients were stage IV (n=575, 96.3%). For line of chemotherapy, patients who had secondary line of treatment or more, were the majority in the non-survival group (P<0.001). Also, for ECOG, the non-survival group 0.8±0.9 were higher than the survival group 0.4±0.6 (P<0.001). Also, for line of chemotherapy, for groups that were prescribed with more than 2nd line, most of the patients were in the non-survival group (P<0.001).
Table 1. Baseline characteristics of the identified patients.
| Characteristics | Total (N=597) | Survival group (N=265) | Non-survival group (N=332) | P value |
|---|---|---|---|---|
| Age (years), mean (SD) | 61.2 (10.2) | 62.0 (10.0) | 60.6 (10.4) | 0.008 |
| Sex, n (%) | 0.162 | |||
| Male | 437 (73.2) | 202 (76.2) | 235 (70.8) | |
| Female | 160 (26.8) | 63 (23.8) | 97 (29.2) | |
| BMI (kg/m2), mean (SD) | 23.0 (3.3) | 23.4 (3.2) | 22.6 (3.3) | 0.003 |
| ECOG, mean (SD) | 0.6 (0.8) | 0.4 (0.6) | 0.8 (0.9) | <0.001 |
| Stage, n (%) | <0.574 | |||
| III | 2 (0.3) | 0 (0.0) | 2 (0.6) | |
| IV | 575 (96.3) | 258 (97.4) | 317 (95.5) | |
| Line of chemotherapy (count), n (%) | <0.001 | |||
| 1 | 97 (16.2) | 66 (24.9) | 31 (9.3) | |
| 2 | 255 (42.7) | 120 (45.3) | 135 (40.7) | |
| ≥3 | 201 (33.7) | 71 (26.8) | 130 (39.2) | |
| Immunotherapy type, n (%) | <0.001 | |||
| Atezolizumab | 178 (29.8) | 63 (23.8) | 115 (34.6) | |
| Pembrolizumab | 207 (34.7) | 118 (44.5) | 89 (26.8) | |
| Nivolumab | 212 (35.5) | 84 (31.7) | 128 (38.6) | |
| Histological type, n (%) | 0.509 | |||
| Adenocarcinoma | 425 (71.2) | 185 (69.8) | 240 (72.3) | |
| Squamous cell carcinoma | 160 (26.8) | 76 (28.7) | 84 (25.3) | |
| Others | 12 (2.0) | 4 (1.5) | 8 (2.4) | |
| EGFR status, n (%) | 0.003 | |||
| Wild-type | 251 (42.0) | 119 (44.9) | 132 (39.8) | |
| Mutant | 79 (13.2) | 22 (8.3) | 57 (17.2) | |
| Unknown | 267 (44.7) | 124 (46.8) | 143 (43.1) | |
| PD-L1 expression (%), mean (SD) | 36.5 (39.4) | 47.0 (40.8) | 27.6 (35.9) | <0.001 |
| Observation (days), mean (SD) | 224.1 (143.7) | 365 (0.0) | 111.6 (92.7) | <0.001 |
SD, standard deviation; BMI, body mass index; ECOG, European Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death ligand 1.
The NLR and CRP levels at the early treatment period were higher when compared with those at the baseline in the survival group (Table 2). However, the LDH levels were significantly different between the early treatment period and baseline in the non-survival group but not in the survival group. Moreover, the median NLR and CRP levels in the non-survival group were higher than those in the survival group at the baseline {median (IQR); NLR, 2.60 (1.82–3.86) vs. 4 [3–7], P<0.001; CRP, 7 mg/dL (2–26 mg/dL) vs. 29 mg/dL (6–70 mg/dL); P<0.001} (Table 2). In contrast, the median LDH levels were not significantly different between the survival and non-survival groups at the baseline [median (IQR); 224 IU/L (181–292 IU/L) vs. 216 IU/L (188–270 IU/L); P=0.9] (Table S1). At the early treatment period, the NLR, CRP, and LDH levels in the non-survival group were higher than those in the survival group (Table S1). In our correlation analysis in categorized variables, we found that early treatment period NLR, LDH were positively correlated to early treatment CRP levels, 0.468 and 0.620 respectively. Also, early treatment period CRP levels were positively correlated with baseline CRP levels with 0.781. Also, early treatment period NLR showed 0.547 of correlation with early treatment period LDH. However, with the continuous variables, there were no variables that showed correlation over 0.5 (Figure S1A,S1B).
Table 2. Comparison of laboratory parameter values between baseline and early treatment period in the survival and non-survival groups1.
| Parameters | Survival group | Non-survival group | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Early treatment period | P value | Baseline | Early treatment period | P value | ||
| NLR, median [IQR] | 2.60 [1.82–3.86] | 2.51 [1.84–3.88] | 0.8 | 4 [3–7] | 7 [4–14] | <0.001 | |
| CRP (mg/dL), median [IQR] | 7 [2–26] | 7 [2–38] | >0.9 | 29 [6–70] | 64 [29–112] | <0.001 | |
| LDH (IU/L), median [IQR] | 224 [181–292] | 205 [177–240] | 0.042 | 216 [188–270] | 320 [236–482] | <0.001 | |
1, P value was calculated using the Wilcoxon rank sum test. NLR, neutrophil-lymphocyte ratio; IQR, interquartile range; CRP, C-reactive protein; LDH, lactate dehydrogenase.
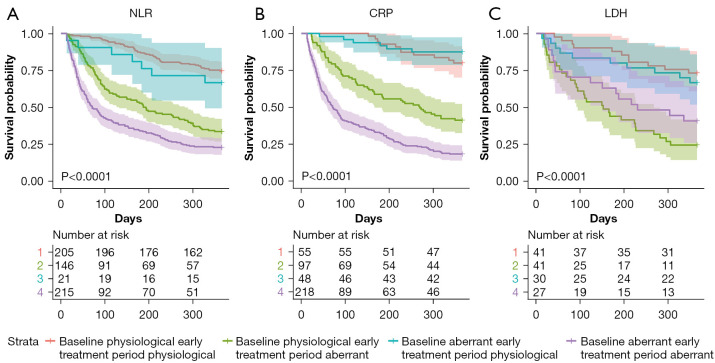
The survival curves stratified based on the aberrant levels of each laboratory parameter at the baseline and early treatment period were significantly different (log-rank test, P<0.0001). Patients with aberrant baseline and early treatment period NLR and CRP levels exhibited the poorest prognosis, followed by patients with physiological baseline NLR and CRP levels but aberrant early treatment period NLR and CRP levels and patients with physiological early treatment period NLP and CRP levels. The prognosis of patients with physiological early treatment period LDH levels was significantly better than that of patients with aberrant early treatment period LDH levels when the patients had physiological baseline LDH (log-rank test, P<0.0001). Additionally, the prognosis of patients with aberrant baseline and early treatment period LDH levels was significantly poorer than that of patients with physiological baseline and early treatment period LDH levels (log-rank test, P<0.001) (Figure 2; Table S2).
Figure 2.
Kaplan-Meier curves for OS stratified based on aberrant laboratory parameters at baseline and early treatment period in patients with non-small cell lung cancer undergoing immunotherapy only. Strata 1, 2, 3, and 4 indicate baseline physiological and early treatment period physiological, baseline physiological and early treatment period aberrant, baseline aberrant and early treatment period physiological, and baseline aberrant laboratory parameter and early treatment period aberrant laboratory parameter respectively. (A) Kaplan-Meier curves of OS stratified based on aberrant NLR at baseline and early treatment period; (B) Kaplan-Meier curves of OS stratified based on aberrant CRP at baseline and early treatment period; (C) Kaplan-Meier curves of OS stratified based on aberrant LDH at baseline and early treatment period. NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein, LDH, lactate dehydrogenase; OS, overall survival.
The OS prediction model based on age, sex, BMI, ECOG, line of chemotherapy, initial stage and the baseline and early treatment period NLR, CRP, and LDH levels are shown in Table 3. Additionally, the OS prediction model based on only baseline NLR, CRP, and LDH levels was developed (Table S3). In the baseline only prediction model, only the NLR value [hazard ratio (HR) =3.14; 95% confidence interval (CI): 2.07–4.76; P<0.001] was the significant predictor of OS. However, univariate analysis revealed that all laboratory parameters (except baseline LDH status) were significant predictors of OS when the early treatment period laboratory parameter values were included in the analysis. Multivariate analysis revealed that the early treatment period CRP (HR =3.88; 95% CI: 1.55–9.72; P=0.004) and LDH (HR =4.04; 95% CI: 2.01–8.12; P<0.001) were significant predictors of OS. The C-index and the R-squared value of the multivariate model was 0.809 [standard error (SE) =0.028] and 0.503, respectively, which was higher than that of the baseline only model [C-index =0.703 (SE =0.028); R-squared value =0.272]. The line of chemotherapy were also significant factors for predicting of OS. For 3rd line of chemotherapy, (HR =3.19; 95% CI: 1.04–9.82; P=0.043). However, the HR for stage value.
Table 3. Univariate and multivariate analyses of predictors of overall survival using the Cox proportional hazards regression model.
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 0.99 (0.98–1.00) | 0.071 | 1.00 (0.96–1.03) | 0.830 | |
| Sex | |||||
| Female | 1 | 1 | |||
| Male | 0.81 (0.64–1.03) | 0.081 | 1.50 (0.69–3.25) | 0.303 | |
| BMI | 0.93 (0.90–0.97) | <0.001 | 1.01 (0.92–1.11) | 0.817 | |
| ECOG | 1.68 (1.49–1.90) | <0.001 | 1.48 (1.06–2.04) | 0.019 | |
| Stage | |||||
| III | 1 | 1 | |||
| IV | 0.42 (0.10–1.69) | 0.222 | NA | ||
| Line of chemotherapy | |||||
| 1 | 1 | 1 | |||
| 2 | 1.93 (1.31–2.86) | 0.001 | 2.38 (0.78–7.24) | 0.127 | |
| ≥3 | 2.71 (1.83–4.01) | <0.001 | 3.19 (1.04–9.82) | 0.043 | |
| Baseline NLR levels | |||||
| Physiological | 1 | 1 | |||
| Aberrant | 2.73 (2.20–3.40) | <0.001 | 1.84 (0.92–3.68) | 0.084 | |
| Early treatment period NLR levels | |||||
| Physiological | 1 | 1 | |||
| Aberrant | 4.69 (3.53–6.23) | <0.001 | 1.30 (0.61–2.80) | 0.498 | |
| Baseline CRP levels | |||||
| Physiological | 1 | 1 | |||
| Aberrant | 2.16 (1.67–2.79) | <0.001 | 1.58 (0.84–2.94) | 0.153 | |
| Early treatment period CRP levels | |||||
| Physiological | 1 | 1 | |||
| Aberrant | 6.64 (4.40–10.02) | <0.001 | 3.88 (1.55–9.72) | 0.004 | |
| Baseline LDH levels | |||||
| Physiological | 1 | 1 | |||
| Aberrant | 0.80 (0.54–1.18) | 0.257 | 0.74 (0.41–1.37) | 0.341 | |
| Early treatment period LDH levels | |||||
| Physiological | 1 | 1 | |||
| Aberrant | 4.72 (3.49–6.38) | <0.001 | 4.04 (2.01–8.12) | <0.001 | |
HR, hazard ratio; CI, confidence interval; SD, standard deviation; BMI, body mass index; ECOG, European Cooperative Oncology Group; NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein; LDH, lactate dehydrogenase.
In the sensitivity analysis, 2- and 3-year OS prediction models also revealed that early treatment period CRP and LDH levels were significant predictors of OS (Table S4) Moreover, the time-varying Cox proportional hazards regression model revealed that NLR (HR =2.45; 95% CI: 1.74–3.45; P<0.001), CRP (HR =3.37; 95% CI: 2.19–5.17; P<0.001), and LDH (HR =3.31; 95% CI: 2.32–4.72; P<0.001) were significant predictors of OS (Table S5). The analysis of the EGFR-mutated NSCLC patients showed similar to the previous analysis, the Kaplan-Meier curve was distinguished between the groups, and the survival rate of patients with outliers detected in the early treatment period was relatively lower than that of other groups in all lab results (log-rank test, P<0.01) (Figure S2). Early treatment period LDH (HR =4.26; 95% CI: 1.41–12.81; P=0.010) was identified as a significant factor in the multivariable analysis (Table S6). The distribution of population was similar with the results that excluded the patients who had less than one-year observation window (Table S7). We also performed HR modeling for patients who survived one year or less. Early treatment period CRP and LDH remained significant factors to predict the OS, and there were no distinctive differences in the Kaplan-Meier curves (Figure S3, Table S8).
Discussion
In this study, the prognostic value of NLR, CRP, and LDH levels at baseline (before the first immunotherapy prescription) and early treatment period (within 8 weeks after the first immunotherapy prescription) was analyzed. In total, 597 patients who were diagnosed with NSCLC and prescribed only immunotherapy were selected. The baseline NLR levels and the early treatment period CRP and LDH levels were significant predictors of OS after the first immunotherapy. The performance of the OS prediction model including early treatment response was better than that of the OS prediction model including only baseline laboratory parameters.
The study population in most previous studies (12,19-21) comprised less than 500 patients. However, this study enrolled more than 500 patients who were all Asians. The time at enrolment was not the same as the time of diagnosis, but it is the time at which immunotherapy was first prescribed.
Atezolizumab was prescribed later than pembrolizumab and nivolumab. Therefore, atezolizumab was the least prescribed immunotherapeutic in the study period. According to the Korean drug insurance benefits for immunotherapy in NSCLC, pembrolizumab should be prescribed when PD-L1 expression is more than 50% after conventional cytotoxic chemotherapy treatment, while nivolumab can be prescribed when PD-L1 expression is more than 10%. Thus, nivolumab was commonly prescribed when compared with pembrolizumab. Including patients whose observation period was less than 1 year, atezolizumab was the most prescribed immunotherapeutic. This may be because atezolizumab can be prescribed irrespective of PD-L1 expression level.
The NLR, CRP, and LDH levels at the baseline were not significantly different from those at the early treatment period in the survival group. However, the NLR, CRP, and LDH levels were significantly different between the baseline and the early treatment period in the non-survival group. Patients with aberrant baseline and early treatment period NLR and CRP levels exhibited poorer prognosis than those with aberrant baseline NLR and CRP levels but early treatment physiological NLR and CRP levels. However, the LDH levels exhibited the opposite trends. This was because the CI values of LDH widely varied as the number of LDH tests was lower than that of other laboratory tests.
This study demonstrated that in addition to the response of pretreatment laboratory parameters, the response of laboratory parameters in the early treatment period can predict the prognosis of patients with NSCLC who underwent immunotherapy. However, the critical time point varied for different laboratory parameters. That is, the baseline NLR levels were significant predictors of OS. In contrast, in the early treatment period, CRP and LDH levels were significant predictors of OS. Moreover, both the C-index and R-squared values in the Cox regression model with early treatment laboratory parameter values were higher than those in the Cox regression model with baseline only values of laboratory parameters. This indicates that the prediction model with early treatment response exhibited enhanced performance and enabled comprehensive data analysis. In the time-varying model, the highest C-index was 0.800. However, the time-varying model comprised the weekly values. Thus, the practicality of the time-varying model was lesser than that of the multivariate model.
In a previous study, pretreatment NLR levels over the threshold values were predictors of short OS (HR =3.977; 95% CI: 1.227–12.889; P=0.014) in patients with renal cell carcinoma who were prescribed immunotherapy (10). The dynamic changes in the levels of NLR—baseline NLR (HR =1.515; 95% CI: 1.26–1.82), NLR levels before the second dose (HR =1.67; 95% CI: 1.39–2.00), and NLR trend (HR =1.58; 95% CI: 1.11–2.27)—were independent prognostic factors for OS in patients with solid cancer who were prescribed immunotherapy (19). Tumor cells secrete chemokines, such as interleukin-8 (IL-8) and recruit neutrophils into the tumor, resulting in tumor progression and enhanced metastatic potential (22,23). This study did not find a significant association between baseline and early treatment period NLR and OS. The HR for baseline NLR was 1.84 (95% CI: 0.92–3.68; P=0.084), although the multivariate Cox regression for baseline NLR showed a P value of 0.08, which was likely due to the small sample size. A sensitivity analysis including patients with less than one-year windows found a HR of 1.64 (95% CI: 0.99–2.70; P=0.053), which was still not a significant result, but the trend remained present.
Early treatment period CRP levels were significant predictors of OS. This was consistent with the results of a previous study, which reported that the CRP responder in whom the serum CRP levels decreased by 30% after immunotherapy relative to the baseline exhibited a good prognosis (HR =0.20; 95% CI: 0.10–0.42) (16). However, the time window for distinguishing between the responder and the non-responder was 12 weeks in the previous study, whereas it was 8 weeks in this study. The short period needed to distinguish between the responder and non-responder is important because the timely identification of non-responders can significantly contribute to the OS of patients. Elevated CRP levels can be explained by persistent enhanced inflammatory responses in tumors that suppress anti-tumor immunity and promote cancer progression through several mechanisms (24), resulting in a poor prognosis of immunotherapy.
Limited studies have examined LDH kinetics in cancer treatment. LDH kinetic analysis has advantages over baseline LDH analysis in predicting the prognosis of patients with NSCLC treated with bevacizumab (25). The absolute LDH levels and LDH ratios with longitudinal LDH were associated with the response to chemotherapy in nasopharyngeal cancer (26). Elevated LDH levels can be explained by the increased glycolytic activity in the tumor, as well as by tumor necrosis owing to high tumor burden-induced hypoxia. Glycolysis and hypoxia contribute to an immune suppressive microenvironment (27), resulting in a poor prognosis of immunotherapy.
The baseline CRP and LDH values were not significantly different between the survival and non-survival groups in this study. To examine the underlying reason, the HR changes were plotted against the laboratory parameter changes (Figure S4). In this graph, the baseline CRP and LDH levels exhibited nonconstant changes with the increase in laboratory parameter values. However, the baseline and early treatment NLR levels and early treatment CRP and LDH levels increased with only positive HR values as the laboratory parameter values increased. This explains the non-significant difference in baseline CRP and LDH levels between the survival and non-survival groups.
Laboratory tests, including complete blood count, differential testing, and CRP and LDH analyses, are inexpensive and almost routinely performed in every cycle of PD-1/PD-L1 inhibitor treatment. Thus, these laboratory tests are easily available. A prediction model can be developed by combining these laboratory tests at both baseline and early treatment periods. These parameters can serve as universally accessible predictive and prognostic markers of therapy response.
Limitations
This study had some limitations. As this was a retrospective study, the selection and information bias of the population comprising patients with diverse characteristics primarily treated based on protocol before the prescription of immunotherapy as the common standard of care for NSCLC must be considered. Additionally, information on potential time-dependent confounders, such as infections that may have affected CRP levels was not available and thus could not be included in this study. Furthermore, only OS was evaluated in this study. Future studies must include non-cancer deaths rather the progression-free survival. However, this is a large cohort study that provided useful information on the prognostic and predictive value of pretreatment and early treatment period NLR, CRP, and LDH levels, which reflect the dynamic changes in the laboratory parameters.
Conclusions
In patients with NSCLC who were prescribed immunotherapy, the early treatment period CRP and LDH levels were significant predictors of OS after the first immunotherapy.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge the support provided by the Bio-Industrial Technology Development Program (No. 20014841), funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Funding: This research was supported by a grant of the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea to MinDong Sung.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of any part of the work are appropriately investigated and resolved. This research was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the institutional review board of Severance Hospital, Seoul, Korea (IRB No. 2019-2129-011); waivers of consent were granted based on general impracticability and minimal harm.
Footnotes
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-7/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-7/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-7/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-7/coif). MS received funding from MD-PhD/Medical Scientist Training Program (the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea). The other authors have no conflicts of interest to declare.
References
- 1.Berghmans T, Dingemans AM, Hendriks LEL, et al. Immunotherapy for nonsmall cell lung cancer: a new therapeutic algorithm. Eur Respir J 2020;55:1901907. 10.1183/13993003.01907-2019 [DOI] [PubMed] [Google Scholar]
- 2.Mushti SL, Mulkey F, Sridhara R. Evaluation of Overall Response Rate and Progression-Free Survival as Potential Surrogate Endpoints for Overall Survival in Immunotherapy Trials. Clin Cancer Res 2018;24:2268-75. 10.1158/1078-0432.CCR-17-1902 [DOI] [PubMed] [Google Scholar]
- 3.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563-80. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 4.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14:847-56. 10.1158/1535-7163.MCT-14-0983 [DOI] [PubMed] [Google Scholar]
- 5.Strickler JH, Hanks BA, Khasraw M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin Cancer Res 2021;27:1236-41. 10.1158/1078-0432.CCR-20-3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paijens ST, Vledder A, de Bruyn M, et al. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol 2021;18:842-59. 10.1038/s41423-020-00565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin IH, Akce M, Alese O, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer 2019;121:809-18. 10.1038/s41416-019-0599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol 2018;23:634-40. 10.1007/s10147-018-1250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ota Y, Takahari D, Suzuki T, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol 2020;85:265-72. 10.1007/s00280-019-04023-w [DOI] [PubMed] [Google Scholar]
- 10.Jeyakumar G, Kim S, Bumma N, et al. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:82. 10.1186/s40425-017-0287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama Y, Yamada T, Chihara Y, et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Sci Rep 2020;10:17495. 10.1038/s41598-020-74573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riedl JM, Barth DA, Brueckl WM, et al. C-Reactive Protein (CRP) Levels in Immune Checkpoint Inhibitor Response and Progression in Advanced Non-Small Cell Lung Cancer: A Bi-Center Study. Cancers (Basel) 2020;12:2319. 10.3390/cancers12082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60-5. 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Li Y, Yan X, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med 2019;8:1467-73. 10.1002/cam4.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klümper N, Saal J, Berner F, et al. C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer 2022;10:e004024. 10.1136/jitc-2021-004024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Reinikainen J, Adeleke KA, et al. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018;6:121. 10.21037/atm.2018.02.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viñal D, Gutierrez-Sainz L, Martinez D, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced cancer patients receiving immunotherapy. Clin Transl Oncol 2021;23:1185-92. 10.1007/s12094-020-02509-1 [DOI] [PubMed] [Google Scholar]
- 20.Oya Y, Yoshida T, Kuroda H, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 2017;8:103117-28. 10.18632/oncotarget.21602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khunger M, Patil PD, Khunger A, et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherapy in non-small cell lung cancer patients. PLoS One 2018;13:e0197743. 10.1371/journal.pone.0197743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagerling C, Werb Z. Neutrophils: Critical components in experimental animal models of cancer. Semin Immunol 2016;28:197-204. 10.1016/j.smim.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938-45. 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Callaghan DS, O'Donnell D, O'Connell F, et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 2010;5:2024-36. 10.1097/JTO.0b013e3181f387e4 [DOI] [PubMed] [Google Scholar]
- 25.Li B, Li C, Guo M, et al. Predictive value of LDH kinetics in bevacizumab treatment and survival of patients with advanced NSCLC. Onco Targets Ther 2018;11:6287-94. 10.2147/OTT.S171566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L, Sim AYL, Wu Y, et al. Lactate dehydrogenase kinetics predict chemotherapy response in recurrent metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol 2020;12:1758835920970050. 10.1177/1758835920970050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wilpe S, Koornstra R, Den Brok M, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology 2020;9:1731942. 10.1080/2162402X.2020.1731942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as