Abstract
We report a rare case of native tricuspid valve infective endocarditis caused by Neisseria mucosa/sicca, a gram-negative diplococcus which colonizes the upper respiratory tract. A female in her late 20 s with a history of injection drug use (IDU) who recently completed treatment for methicillin-sensitive Staphylococcus aureus (MSSA) native tricuspid valve infective endocarditis presented to the hospital with a 6-week history of increasing chest pain, shortness of breath and night sweats. Blood cultures grew Neisseria mucosa/sicca species in 3 of 3 sets. Transthoracic echocardiogram showed a large 3 cm × 2.2 cm vegetation on the tricuspid valve with severe regurgitation. The patient was initially treated with ceftriaxone and gentamicin. Her case was complicated by ongoing septic pulmonary emboli ultimately require pulmonary endarterectomy and repair of her tricuspid valve. We hope this case highlights a rare but known cause of infective endocarditis especially in patients with a history of IDU who may lick their needles, which predisposes those individuals to intravenous introduction of oral bacteria.
Keywords: Infective endocarditis, Neisseria, Septic pulmonary emboli, Tricuspid valve, Intravenous drug use
Highlights
-
•
Infective endocarditis should be managed with ID, cardiology, and cardiothoracic surgery for optimal management.
-
•
N. mucosa/sicca show good sensitivity and response to treatment with beta-lactams, aminoglycosides and fluoroquinolones.
-
•
Licking needles prior to drug injection can introduce mouth flora species into the bloodstream, a source for rare cases of IE.
Introduction
Infective endocarditis is a disease that poses significant morbidity and mortality. Predisposing risk factors include underlying structural heart disease and factors which increase the risk of bacterial translocation to the blood, such as poor dentition and injection drug use (IDU). Infective endocarditis is most commonly caused by gram-positive organisms including Staphylococcus aureus, coagulase-negative staphylococcus, streptococci species, and enterococci (Holland 2016). Rarer pathogens include fungi and gram-negative species including the HACEK organisms. Patients who inject drugs and also lick their needles are at increased risk for oral flora being a causative agent, and so this should be ascertained in the initial history taking of these individuals. Rapid identification of the pathogen is critical both in guiding optimal antimicrobial therapy, duration and for determining prognosis.
We report a very rare case of Neisseria mucosa/sicca species native tricuspid valve infective endocarditis. No treatment guidelines exist for this species, and only a few cases are reported in the English literature.
Case presentation
A female in her late 20′s with past medical history of active IDU, high-risk sexual activity, untreated chronic hepatitis C, and previous methicillin-sensitive Staphylococcus aureus (MSSA) native tricuspid valve infective endocarditis (IE) complicated by septic pulmonary emboli which was successfully treated with a 6-week course of IV oxacillin 5 months prior, was admitted with a 6-week history of worsening pleuritic chest pain, shortness of breath with minimal exertion and night sweats. She denied a cough, hemoptysis, lower limb swelling, or orthopnea. Preceding her symptoms, she had relapsed with intravenous opioids and was subsequently detained in a correctional facility. She did recall licking her needles to help clean them for reuse. Per the patient, she had received an unknown short treatment of intramuscular antibiotics while in the facility for an unknown infection. Her symptoms however continued and so she presented to the hospital the day following discharge from the correctional facility.
In the emergency department, the patient was febrile with a temperature of 102 °F, tachycardic with a heart rate of 140 beats per minute, BP of 139/99 mmHg, and saturating 99 % SpO2 on room air with a respiratory rate of 20 breaths per minute. On physical exam, she appeared uncomfortable but was otherwise alert and oriented. Breath sounds were clear on auscultation, hearts sound s1 and s2 were present without additional sounds or murmurs, the abdomen was soft and non-tender, there was no vascular/immunologic phenomena of IE, and there was no lower limb edema.
From her laboratory investigations, her initial white cell count was 12.9 k/mm3, hemoglobin was 9.1 gm/dl, platelet count of 224 k/mm3. Her electrolytes were in the reference range and there was no evidence of renal impairment with creatinine and BUN 0.5 mg/dL and 14 mg/dL, respectively. CRP was high at 19.7 mg/dL. Her chest x-ray showed multifocal bilateral airspace opacities somewhat nodular in appearance which were worse than her radiograph from previous admission (Fig. 1A). Her HIV test was negative. A transthoracic echocardiogram (TTE) showed a large 3 cm × 2.2 cm vegetation on the tricuspid valve complicated by severe tricuspid regurgitation, a small pericardial effusion, and estimated pulmonary arterial pressures of 25 – 30 mmHg (Fig. 1B). All 3 sets of initial blood cultures drawn were identified as Neisseria mucosa/sicca species via matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF). Antibacterial sensitivities were not available as the organism failed to grow for susceptibility testing.
Fig. 1.
A) Chest radiograph on initial admission showed multifocal airspace opacities concerning for pneumonia or septic emboli. B) Transthoracic echo showed 3 cm × 2.2 cm vegetation on the tricuspid valve complicated by severe tricuspid regurgitation.
On admission, the patient was started on IV crystalloid fluids, vancomycin and azithromycin for concern of IE vs multifocal pneumonia. Infectious diseases (ID) were consulted who recommended continuing vancomycin for concern for IE and stopping azithromycin. Vancomycin was later changed to ceftriaxone 2 g daily and gentamicin 200 mg daily after the identification of the Neisseria mucosa/sicca species.
The patient went on to develop hypoxia at rest which was relieved with 2–4 L of oxygen via nasal prong, her blood pressure remained stable but tenuous which was managed with IV fluid support. Cardiothoracic surgery was consulted and opined that while the patient had several indications for valve replacement including severe tricuspid regurgitation, a large vegetation, and evidence of septic emboli, she was not in right-sided heart failure and was stable with current medical management. As such surgery was deferred given the concern for future IDU relapse. Addiction services were also consulted and the patient opted in for services/counseling and was started on 20 mg methadone daily to relieve cravings.
The patient tolerated antibiotics well and repeat blood cultures were negative on hospital day 4. She remained hemodynamically stable without symptoms of heart failure or shock, and her hypoxia resolved. Discharge was prolonged due to a self-limiting fever on day 13 with a negative infectious workup, and patient placement concerns. Ultimately, the patient was discharged on hospital day 19. Gentamicin was discontinued, and she completed a total of 4 weeks of intravenous ceftriaxone via a peripherally inserted central catheter with outpatient ID, cardiology, and addiction service follow-up.
At follow-up as an outpatient with ID the patient was noted to be experiencing occasional low-grade temperatures with mild tachycardia and leukocytosis. Repeat blood cultures were negative. She was scheduled for a follow-up TTE to monitor the size of her vegetation and further follow-up post-completion of antibiotics.
The patient represented to the hospital 3 days post-completion of her antibiotics with worsening chest pain and shortness of breath. Repeat TTE showed persistent severe tricuspid regurgitation but no evidence of the previous vegetation. Computer tomography pulmonary angiography was consistent with ongoing multiple septic pulmonary emboli. She was started on IV vancomycin and piperacillin-tazobactam, however, this was stopped after multiple sets of blood cultures failed to grow. She ultimately was discharged on apixaban with pulmonary follow-up, requiring 4 L of oxygen in the community.
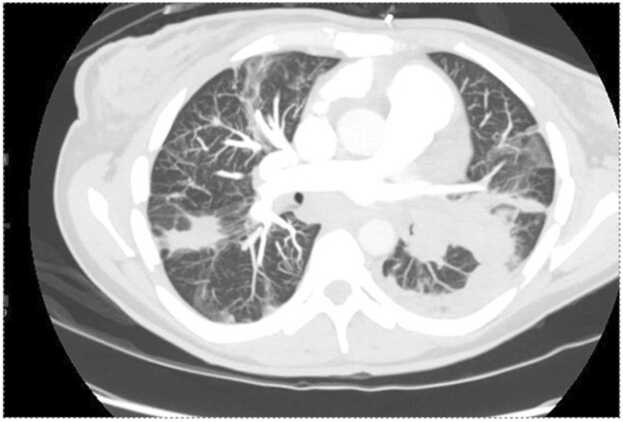
The patient represented two weeks later with hemoptysis and shortness of breath. She reported good compliance with her apixaban, and maintained sobriety on methadone 80 mg daily. Her TTE was again repeated and showed no vegetation, moderately reduced ejection fraction, and evidence of right heart strain with severe tricuspid regurgitation. Computer tomography pulmonary angiography showed only minimal improvement of extensive pulmonary artery emboli involving the left proximal pulmonary artery, the entire left lower pulmonary artery, and multifocal involvement of the right pulmonary artery (Fig. 2). There was also an increase in the size of consolidating opacities in the left upper and lower lobes consistent with worsening pulmonary infarcts. Based on the patient's extensive clot burden, inability to tolerate anticoagulation due to hemoptysis, and sustained sobriety, cardiothoracic surgery recommended pulmonary artery endarterectomy with concurrent tricuspid valve repair. The patient was transferred out of the hospital network for the procedure with follow-up pending.
Fig. 2.
Computer tomography pulmonary angiography showed diffuse pulmonary emboli and increased consolidative opacity in the left upper lung when compared to previous imaging, concerning for evolving pulmonary infarcts.
Discussion
We present a case of native tricuspid valve Neisseria sicca/mucosa infective endocarditis complicated by septic pulmonary emboli in a young female with a background of IDU and previous MSSA tricuspid infective endocarditis.
Neisseria mucosa and sicca species are gram-negative diplococci that colonize the upper respiratory tract. While typically considered a non-pathogenic species, rare cases of pneumonia, meningitis, and osteomyelitis have been reported [1], [2], [3]. While a less common causative agent than its related Neisseria gonorrhea and meningitidis, cases of infective endocarditis secondary to N. mucosa and sicca species have also been described [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. These cases typically are associated with underlying valve disease or IDU which reflects the species relatively low pathogenic potential in immunocompetent individuals.
Our patient had significant risk factors for her presentation including ongoing IDU, previous IE, poor dentition, and the fact that she licked her needles to clean them placing her at considerable risk for the introduction of oral flora into her bloodstream with adherence to an already damaged tricuspid valve. Her case was complicated by a significant vegetation causing severe tricuspid regurgitation and multiple septic pulmonary emboli. This is not uncommon amongst N. mucosa/sicca IE cases with previously reported rates of septic emboli occurring in 69 % of reviewed cases [4].
In this case, we were unable to obtain sensitivities, and treatment was based on a review of previously reported cases. The patient received 19 days of gentamicin while she was in hospital and a total of 4 weeks of IV ceftriaxone. Case reports demonstrate good efficacy of beta-lactams including penicillin and ampicillin [5], [6], [7], as well as with other combinations of beta-lactams with aminoglycosides and fluoroquinolones [8], [9], [10], [11]. Our patient had a good response to the above regimen, with persistent clearance of her vegetation and blood cultures.
IDU associated IE necessitates holistic management, as individuals who continued to use injected drugs are at increased risk of morbidity and mortality. A successful harm reduction strategy includes both engagement with opioid maintenance therapy programs such as methadone or buprenorphine, and education on harm-reduction strategies including skin and needle hygiene as well as safe-injection sites. Furthermore, addiction services should be involved during initial inpatient management, as this has been shown to increase follow-up with opioid maintenance programs and to reduce morbidity and mortality in IDU-associated IE [12]. Addiction services, both inpatient and outpatient, were key in our patient's case, and her successful abstinence ended up being an important factor in her candidacy for surgery.
Surgery was initially considered in our patient given the size of her vegetation, significant tricuspid regurgitation, and septic emboli; however, ultimately was deferred given concerns for potential IDU relapse and the patient's stability during her initial hospitalizations. Unfortunately, despite the completion of antibiotics with clearance of both her vegetation and blood cultures, she continued to present with ongoing septic pulmonary arterial emboli. At this point, surgery was offered to the patient given her worsening right heart strain and ongoing pulmonary artery emboli. This case highlights the challenge of determining surgical candidacy in individuals who continue to use IV drugs. On one hand, surgery may be a necessary step for recovery in the setting of structural damage to the heart. On the other hand, any relapse in IV drug use with a metallic valve in situ would pose significant morbidity and mortality. These situations challenge the physician’s ethical balance between beneficence and non-maleficence. Our patient’s case highlights the role of continuing multidisciplinary discussions in the setting of persistent complications, as one's candidacy for surgery is not a static variable.
Ethical approval
Ethical approval was not saught, asno study was conducted.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
None.
CRediT authorship contribution statement
John Szendrey - contributed to patient care, writing/review, literature search and approval of final submission. Aleezay Asghar - contributed to patient care, writing/review, literature search and approval of final submission. Nassim Mokraoui - contributed to patient care, writing/review, literature search and approval of final submission. Durane Walker - contributed to patient care, writing/review, literature search and approval of final submission.
Declaration of Competing Interest
The authors have no competing interests to report.
Acknowledgments
We would like to knowledge all of the healthcare providers involved in the patient’s care.
Contributor Information
John A. Szendrey, Email: john.szendrey@baystatehealth.org.
Aleezay Asghar, Email: aleezay.asghar@baystatehealth.org.
Nassim Mokraoui, Email: nasmokra@gmail.com.
Durane Walker, Email: durane.walker@baystatehealth.org.
References
- 1.Doern G.V., Blacklow N.R., Gantz N.M., et al. Neisseria sicca osteomyelitis. J Clin Microbiol. 1982;16(3):595–597. doi: 10.1128/jcm.16.3.595-597.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartin J.S. Neisseria sicca meningitis in a woman with nascent pernicious anemia. Am J Med. 2000;109(2):175–176. doi: 10.1016/s0002-9343(00)00438-1. [DOI] [PubMed] [Google Scholar]
- 3.Gris P., Vincke G., Delmez J.P., et al. Neisseria sicca pneumonia and bronchiectasis. Eur Respir J. 1989;2(7):685. [PubMed] [Google Scholar]
- 4.Pilmis B., Lefort A., Lecuit M., Join-Lambert O., Nassif X., Lortholary O., Charlier C. Endocarditis due to Neisseria mucosa: case report and review of 21 cases: a rare and severe cause of endocarditis. J Infect. 2014;68(6):601. doi: 10.1016/j.jinf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Epelbaum S., Laurent C., Morin G., Laurans G., Piussan C. Neisseria mucosa endocarditis complicated by intracerebral aneurysm. Arch Fr Pedia. 1993;50(3):231–233. [PubMed] [Google Scholar]
- 6.Davis C.L., Towns M., Henrich W.L., Melby K. Neisseria mucosus endocarditis following drug abuse. Case report and review of the literature. Arch Intern Med. 1983;143(3):583–585. [PubMed] [Google Scholar]
- 7.Locke M., Smith A., Epstein L.M., Niknam N., Boparai R. Pacemaker lead-related endocarditis with Neisseria sicca. BMJ Case Rep Cp. 2022;15(7) doi: 10.1136/bcr-2022-249795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuduri A.A.D.C., Palombarani S.A., Pallone E.C., Almuzara M.N. Neisseria mucosa endocarditis in a native valve. Enferm Infecc Microbiol Clin. 2007;25(8):553–555. doi: 10.1157/13109994. [DOI] [PubMed] [Google Scholar]
- 9.Debellemanière G., Chirouze C., Hustache-Mathieu L., Fournier D., Biondi A., Hoen B. Neisseria sicca Endocarditis Complicated by Intracranial and Popliteal Aneurysms in a Patient with a Bicuspid Aortic Valve. Case Rep Infect Dis. 2013;2013 doi: 10.1155/2013/895138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Vélez R., Fortun J., de Pablo C., Beltran J.M. Native-valve endocarditis due to Neisseria sicca. Clin Infect Dis. 1994;18(4):660–661. doi: 10.1093/clinids/18.4.660. [DOI] [PubMed] [Google Scholar]
- 11.Jeurissen A., Stroy J.P., Wielenga R.P., Andriesse G.I. Severe infective endocarditis due to Neisseria Sicca: case report and review of literature. Acta Clin Belg. 2006;61(5):256–258. doi: 10.1179/acb.2006.043. [DOI] [PubMed] [Google Scholar]
- 12.Bahjia A., Yanagawac B., Lamba W. Harm reduction for injection drug users with infective endocarditis: a systematic review. Can J Addict. 2020;11(2):13–22. [Google Scholar]