Abstract
The environmental non-element cadmium (Cd) is toxic to all forms of life, and it also has a negative impact on plant development and growth. In order to ascertain the effects of cadmium on tomato growth and the function of oak leaf biomass in the reduction of toxicity and translocation of cadmium in different parts of tomato genotypes, two tolerant and two sensitive tomato genotypes were exposed to cadmium stress through the availability or unavailability of oak leaf biomass. The experiment involved two factors. The first factor was the various treatment levels, including soil without Cd treatment and sodium hydroxide (NaOH) oak leaf biomass pretreatment (COC-control), soil with Cd treatment and without NaOH oak leaf biomass pretreatment (CdC), and soil with Cd treatment and NaOH oak leaf biomass pretreatment (CdOBC). The second element consists of four tomato genotypes. Comparing to control conditions, all tomato genotypes spotted significant reductions in all morphological traits under Cd stress in the presence or absence of NaOH oak leaf pretreatment. Related to CdC conditions, root length, shoot length, root fresh weight per plant, shoot fresh weight per plant, root dry weight per plant, shoot dry weight per plant, and total fruit weight per plant were significantly improved by 4.25%, 9.75%, 23.24%, 10.10%, 28.10%, 9.08%, and 4.61%, respectively, under the availability of pretreatment of oak leaf biomass. The tolerant genotypes (Karazi and Sirin) exhibited the greatest increase in all traits evaluated, with the exception of root length, under the CdOBC condition compared to the CdC statement. Significant increases in leaf biochemical parameters were seen with the availability or absence of NaOH pretreatment of oak leaf biomass in the soil. The maximum values of proline content, soluble sugar content, antioxidant activity, and guaiacol peroxidase were stated in the presence of oak biomass under Cd conditions (CdOBC), with mean values of 1772.46 μg g −1, 687.18 μg g −1, 1025.74 μg g −1-, and 0.43 units min −1 g −1, respectively. The in vitro-tolerant genotypes exhibited the maximum values of all biochemical parameters. The concentration of cadmium in the studied tomato genotypes revealed that cadmium accumulated more in the roots than other parts. According to these outcomes, NaOH pretreatment of oak leaf biomass can be employed to diminish the hazard of cadmium absorption by edible parts.
Keywords: Solanum lycopersicum, Heavy metal, Plant biomass, Biochemical parameters, Absorption
1. Introduction
The potential for heavy metals in soil to accumulate over time and harm living species has emerged as a major environmental concern. There are currently no regulations in place that effectively limit the negative human effects on the environment and agricultural soils, where metals can be absorbed by crops during growth and concentrate to poisonous levels that are harmful to plants and pose risks of entering the food chain [1]. Cadmium gets into agricultural soils through the use of phosphate fertilizers, which contain cadmium and lead as active ingredients or as by-products [2,3]. Cadmium is classified as a highly hazardous metal since it does not play a role in biological functions in organisms; its high solubility in water makes it readily available for absorption, leading to lethal levels [4]. Even though plants can manage their metabolism processes under the availability of excess quantities of cadmium better than animals, overdose concentrations of cadmium have negative effects on plants' organs [5]. Apparent symptoms of cadmium toxicity above a critical threshold are exhibited in plants by delay in growth, browning of roots, necrosis, reduced yield, chlorosis, and finally death [6]. The abundance of cadmium in plant tissues can have a deleterious impact on a variety of physiological activities, including the uptake and translocation of minerals from roots to shoots, tissue water status, and disrupted glucose metabolism [[7], [8], [9]].
Plants have developed natural defense systems such as chelating agent synthesis, non-enzymatic and enzymatic antioxidants, and osmolyte generation to defend their metabolic processes against metal stress. Plant species respond differently to heavy metal stress depending on plant variety and heavy metal type [4]. Organic acids, amino acids, polypeptides, and peptides are all examples of naturally occurring ligands that play an important role in the detoxification of heavy metals in plant metabolism [10]. Supporting plant defense mechanisms, phytochelatins (PCs) are peptides derived from glutathione that serve to shield plant tissues from heavy metal damage and reduce free metal concentrations in plant tissues. PCs help plant cells detoxify and recover from heavy metal stress by forming stable complexes with the ions [11]. As both chelate their own carboxyl groups in their structures, the metallothioneins (MTs) and PCS processes are thought to work similarly in storing heavy metals in the vacuole and cell wall [12]. MTs form stable complexes by attaching to the ions of heavy metals. Inactivation of enzymes, blocking functional groups of metabolically important compounds, replacement or substitution of critical components, and disruption of the integrity of cell membranes may all contribute to the phytotoxicity of heavy metals [13]. Heavy metal poisoning causes an increase in reactive oxygen species (ROS) production by interfering with electron transport activities. Because of this rise in ROS, plant cells are put under oxidative stress, which can lead to damage to membranes, biomolecular degradation, the leakage of ions, and DNA breakage [14]. In return, the biochemical activity for reducing ROS generation will be increased by plants, including proline content, soluble sugar content, total phenolic content, guaiacol peroxidase activity, catalase, antioxidants, and many others [15]. Antioxidants, enzymatic and non-enzymatic, are crucial because they prevent the harmful effects of free radicals by reducing hydrogen peroxide (H2O2) to harmless water and oxygen [16].
Several methods have been developed to immobilize cadmium in the soil via the addition of organic amendments. Organic biomass contains various functional groups that impact the physicochemical parameters of the soil and aid in the immobilization of cadmium by plant parts [17]. The cellulose component of plant materials is effective for heavy metal ion biosorption [18]. Common biosorbents utilized in biosorption studies for the removal of heavy metals include roots, leaves, and seeds [19]. Because agriculturally based plant adsorbents are cost-effective, made from renewable resources, easily accessible, and mostly made up of cellulose and lignin, they provide a potential choice for the treatment of contaminated soil with heavy metals [20]. Compounds with lyophilic and lyophobic groups called surfactants can stick to surfaces. Anionic, non-ionic, cationic, and amphoteric are the four different categories of surfactants. Surfactants reduce surface tension and increase wetting power, facilitating the adsorption of heavy metals from liquids onto biomass [21].
The tomato, scientifically known as Solanum lycopersicum L., is a significant annual crop that is a member of the Solanaceae family. In terms of planting area and yield, it is the second most widely grown crop after potatoes [22,23]. Subtropical, tropical, and warm temperate zones are ideal for tomato cultivation. For a long time, this vegetable crop has been highly valued by people all over the world. Tomatoes, on the other hand, are more sensitive to environmental stressors such as cadmium, salt, drought, and flooding [24].
Ninety percent of Iraq’s total forest cover consists of oak forests, which are confined to the Kurdistan Region in the northeast of the country and a few riverine forests along the Euphrates and Tigris rivers. Ecologically, it’s important because mammals and birds rely on it for shelter and sustenance, but economically and culturally, it serves a variety of purposes as well [25]. The oak tree is useful from root to leaf. The leaf organ, in particular, contains a comparatively large quantity of bioactive compounds related to improving the plant’s antioxidant system. Different oak species' leaves have been studied chemically in a number of studies, and the existence of phenolic, flavonoid, and terpenoid compounds has been established. Significant radical scavenging, antimicrobial, and antitopoisomerase abilities were also shown [[26], [27], [28], [29], [30]]. To our knowledge, however, there is no information on the effects of NaOH oak leaf biomass pretreatment on the mechanisms involved in the control of Cd stress. This study tested the hypothesis that oak leaf biomass pretreatment with NaOH could reduce the toxicity and bioavailability of Cd in tomato plants subjected to Cd stress. Therefore, the goal of this research was to explore the effects of NaOH oak leaf biomass pretreatment on the reduction of toxicity and translocation of cadmium in various tomato plant organs.
2. Materials and methods
2.1. Plant materials and preparation of plant biomass
According to in-vitro tests (germination percentage, growth, and biomass of seedlings) of 64 tomato genotypes under Cd stress (unpublished data), two tolerant genotypes, Karazi and Sirin, and two susceptible genotypes, Super and Sewi Qaladze, were used in this study. The tomato genotypes were gathered from the Ministry of Agriculture and Water Resources' Agricultural Research Center in Kurdistan, Iraq. Mature and healthy oak leaves (Quercus aegilops Oliv.) were collected on May 5, 2021, when they were in the vegetative stage. The leaves were then dried at room temperature. The plant leaf biomass was prepared according to the procedure provided by Ref. [31], with a few modifications. Initially, the dried plant samples (leaves) were ground into fractions measuring 0.25–0.60 mm. Oak leaf biomass (200 g) was dissolved in 1 L of distilled water containing 20 g of sodium hydroxide. The suspensions were shaken for 24 h and then filtered through fine mesh to remove the water and obtain the plant biomass. The plant biomass was repeatedly washed until the pH decreased to a near-neutral condition (7.0). Then, the biomass dried at room temperature [32].
2.2. Layout of the experiment and stressing of tomato genotypes by cadmium
A factorial design with a completely randomized design (CRD) was conducted. The experiment involved two factors. The first factor was the various treatment levels, including soil (10 kg) without cadmium (CdCl2) and NaOH oak leaf biomass pretreatment (COC), soil (10 kg) containing 35 mg of cadmium per kilogram of soil and without NaOH oak leaf biomass pretreatment (CdC), and soil (10 kg) containing 35 mg of cadmium per kilogram of soil and 80 g of NaOH oak leaf biomass pretreatment (CdOBC). The second factor consists of four tomato genotypes. The experimental soil had a silty clay texture, an EC of 0.65 dS m−1, a pH of 7.4, a total nitrogen content of 16.08 g kg−1, a phosphorus content of 3.97 mg kg−1, an accessible potassium content of 0.19 meq L−1, an exchangeable phosphorus content of 0.29 mg kg−1, and a cadmium concentration of 0.10 ppm. Tomato seeds from four different genotypes were planted in plastic trays and kept in a greenhouse. Each genotype underwent three treatments. Each tomato genotype consisted of eight pots (replications) for each treatment. All treatments were arranged in a complete randomized design. The pots were placed in a greenhouse and irrigated by the drip method. The plants grew during the 2021 spring and summer seasons. During the experiment, the average daytime and overnight relative humidity in the greenhouse was 16.64/40.155, and the average temperature was 39.89/20.32 °C. The photoperiod for the plants was 14 h of natural light. During the plant’s growing stage, weeds were physically removed.
2.3. Measurement of morphological characters
At the end of maturation, all morphological measurements were documented. The plant morphological parameters were investigated, including root length (RL in cm), shoot length (SL in cm), root fresh weight per plant (RFW in g), root dry weight per plant (RDW in g), shoot fresh weight per plant (SFW in g), shoot dry weight per plant (SDW in g), and total fruit weight per plant (TFW in g). The plants were uprooted and rinsed with distilled water, and the lengths of their roots and shoots were measured using a ruler. The fresh weight was determined directly after collection, while the dry weight was measured after achieving a consistent weight after 52 h in a 78 °C oven [30].
2.4. Meaurement of biochemical parameters
The fresh leaves of four tomato genotypes were harvested one month prior to full maturity for analysis. To analyze the responses of tomato genotypes, similar protocols as displayed in our previous work [33] were used to measure: proline content (PC in μg g−1 leaf fresh weight), total phenolic content (TPC in μg gallic acid g−1 leaf fresh weight), antioxidant activity (AC in μg Trolox g−1 leaf fresh weight), soluble sugar content (SSC in μg glucose g−1 leaf fresh weight), catalase (CAT in units min−1 g−1 leaf fresh weight), and guaiacol peroxidase (GPA in units min−1 g−1 leaf fresh weight).
2.5. Cadmium determination in different organs
The collected tomato root, stem, leaf, and fruit samples were digested with a 5:1 concentrated HNO3:HClO4 solution, as stated by Ref. [34]. To find out if the digested samples were contaminated with cadmium, the concentrations of cadmium were measured four times with flame atomic absorption spectrometry (PinAAcle 900H, Perkin Elmer, USA). Cadmium concentrations were expressed in parts per million (ppm) for root, stem, leaf, and fruit.
2.6. Calculation of trait index
The following equation was used to determine the trait index [30,35]:
2.7. Calculation of cadmium concentration
After determining the cadmium concentration in roots and shoots and the percentage reduction of cadmium in roots and shoots due to the presence of the plant biomass for respective genotypes, the following formula was used [36]:
where XCdC represents the main performance of the tomato genotype under cadmium stress conditions, while XCdOBC represents the main performance of the same genotype treated with the NaOH oak leaf biomass pretreatment under cadmium stress conditions.
2.8. Statistical data analysis
Statistical tests were conducted in XLSTAT 2019, version 2.2 [37]. The treatments' and genotypes' major effects were performed using a two-way analysis of variance at p ≤ 0.01. Duncan’s new multiple range test (DNMRT) was applied to evaluate the difference between the mean values. All data were presented as mean values and standard errors. All figures were plotted using XLSTAT version 2019.2.2 software.
3. Results
3.1. Evaluation of morphological characters
In comparison to control conditions, all morphological traits significantly (p ≤ 0.01) declined in the availability of cadmium under all treatment conditions (Tables 1 and S1). The values of decreasing percentages were between −15.01 and −16.33% for root length (RL), −18.00 and −12.05% for shoot length (SL), −25.32 and −9.65% for root fresh weight (RFW), −9.47 and −5.12% for shoot fresh weight (SFW), −25.12 and 5.27% for root dry weight (RDW), −8.88 and −4.48% for shoot dry weight (SDW), and −13.85 and −10.43% for total fruit weight per plant (TFW). The highest decreasing percentage was observed in the absence of oak leaf biomass pretreated with NaOH (CdC condition). Comparing the treated tomato plants with oak leaf biomass pretreated with NaOH (CdBOC condition) to the CdC condition, an increase in all morphological traits was noticed. The values of increasing percentage were 4.25, 9.75, 23.24, 10.10, 28.10, 9.08, and 4.61% for RL, SL, RFW, SFW, RDW, SDW, and TFW, respectively (Fig. 1).
Table 1.
Changes in the percentages of tomato genotypes' morphological traits in response to cadmium stress with and without oak leaf biomass pretreated with sodium hydroxide compared to control conditions.
| Trait | CdC | CdOBC |
|---|---|---|
| Root length (%) | −15.01 ± 2.21 a | −16.33 ± 1.61 a |
| Shoot length (%) | −18.00 ± 3.03 a | −12.05 ± 3.46 b |
| Root fresh weight (%) | −25.32 ± 1.57 a | −9.65 ± 1.67 b |
| Shoot fresh weight (%) | −9.47 ± 1.87 a | −5.12 ± 1.52 b |
| Root dry weight (%) | −25.12 ± 2.94 a | −5.27 ± 3.01 b |
| Shoot dry weight (%) | −8.88 ± 0.79 a | −4.48 ± 0.62 b |
| Total fruit weight per plant (%) | −13.85 ± 1.72 a | −10.43 ± 1.38 b |
The negative values represented decreasing percentages of the investigated parameters in comparison to the control conditions. According to Duncan’s new multiple range test, different letters denote statistically significant differences (p ≤ 0.01). The values are presented by the trait index (%) ± standard error. The values represent the average of eight separate evaluations.
Fig. 1.
A sunburst chart represents the increasing percentages of different morphological characters in the tomato plants treated with oak leaf biomass pretreated with sodium hydroxide compared to the untreated plants under Cd stress conditions. RL: root length, SL: shoot length, RFW: root fresh weight, SFW: shoot fresh weight, RDW: root dry weight, SDW: shoot dry weight, TFW: total fruit weight per plant.
Except for the fresh weight of the roots, the responses to morphological characteristics were statistically different between tomato genotypes, as indicated in Tables 2 and S1. Under Cd stress conditions, all phenotypical characters of the tomato genotypes were found to decrease significantly when exposed to the various treatments. Sewi Qaladze genotype reported the highest decreased values for RL (22.24%), SFW (16.09%), SDW (9.34%), and TFW (16.00%), while Super genotype provided the highest decreased values for SL (29.88%), RFW (9.34%), and RDW (24.17%). Significant variations were seen in RFW and TFW parameters with regard to the interaction between the two categories (treatments and genotypes). The Sirin genotype had the lowest decreasing values for SL, RFW, SFW, RDW, SDW, and TFW under Cd stress in the presence of oak leaf biomass pretreated with NaOH (Tables S1 and S2).
Table 2.
Morphological responses of different tomato genotypes in the presence and absence of oak leaf biomass pretreated with sodium hydroxide under cadmium stress conditions compared to control conditions.
| Trait | Sirin | Karazi | Super | Sewi Qaladze |
|---|---|---|---|---|
| Root length (%) | −17.36 ± 2.14 bc | −13.25 ± 2.83 ab | −9.82 ± 1.15 a | −22.24 ± 1.47 c |
| Shoot length (%) | −6.20 ± 4.06 a | −11.71 ± 3.49 a | −29.88 ± 2.07 b | −12.31 ± 1.91 a |
| Root fresh weight (%) | −17.58 ± 6.17 a | −15.70 ± 3.15 a | −20.15 ± 2.84 a | −16.51 ± 3.74 a |
| Shoot fresh weight (%) | −2.81 ± 0.72 a | −6.02 ± 1.50 a | −4.26 ± 1.33 a | −16.09 ± 1.77 b |
| Root dry weight (%) | −11.73 ± 2.43 a | −12.41 ± 2.37 a | −24.17 ± 2.57 b | −12.46 ± 2.97 a |
| Shoot dry weight (%) | −5.26 ± 1.23 a | −5.29 ± 0.89 a | −6.83 ± 1.22 a | −9.34 ± 1.52 b |
| Total fruit weight per plant (%) | −5.29 ± 1.10 a | −12.31 ± 1.42 b | −14.96 ± 1.88 b | −16.00 ± 2.08 b |
In comparison to the control conditions, the negative values represented decreasing percentages of the investigated parameters. Letters in the same row with different meanings specify statistically significant differences (p ≤ 0.01) using Duncan’s new multiple range test. The values are presented by the trait index (%) ± standard error. The values shown are the averages of eight separate measurements.
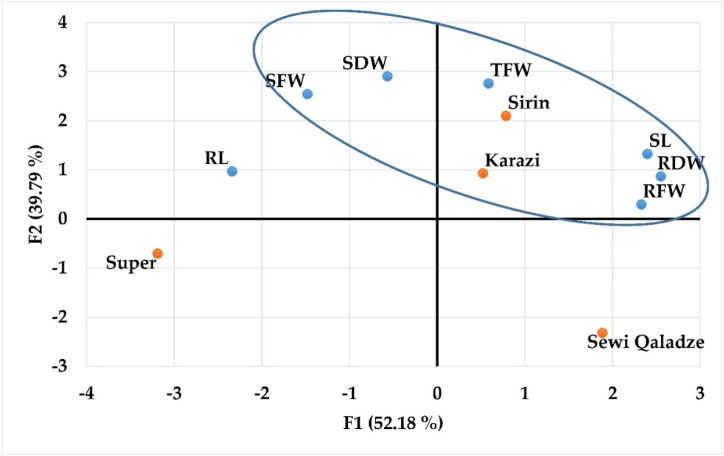
Principal component analysis (PCA) is a multivariate analytic approach used to determine the similarity of distinct genotypes. In addition, it is employed to establish the association between different characteristics. For both the CdC and CdOBC environments, seven variables related to morphological traits were analyzed using principal component analysis. The eigenvalues of the first two components were both greater than one, indicating a cumulative distribution of 91.97% (52.18% for the first component and 39.79% for the second). On the PCA plot, different distributions of the analyzed characteristics and genotypes were detected. Three major traits, SL, RFW, and RDW, were found to account for the majority of the observed variance along F1. It was SFW, SDW, and TFW, however, that contributed the most to the overall variance along F2 (Fig. 2). Regarding the dispersion of genotypes, significant patterns were identified. Sirin and Karazi genotypes placed in the upper right-quarter (blue outline) exhibited the lowest decline in SL, RFW, RDW, SFW, SDW, and TFW, while Sewi Qaladze genotypes located in the lower right-quarter revealed the most decline in these traits. Consequently, the tolerance and susceptibility grading of genotypes based on their morphological characteristics was as follows: Cd-tolerant > sensitive; Sirin > Karazi > Super > Sewi Qaladze.
Fig. 2.
A Plot from principal component analysis (PCA) showing the relationship between biomass yield and the four tomato genotypes tested.
3.2. Analysis of cadmium levels in different tomato genotypes' roots, stems, leaves, and fruit
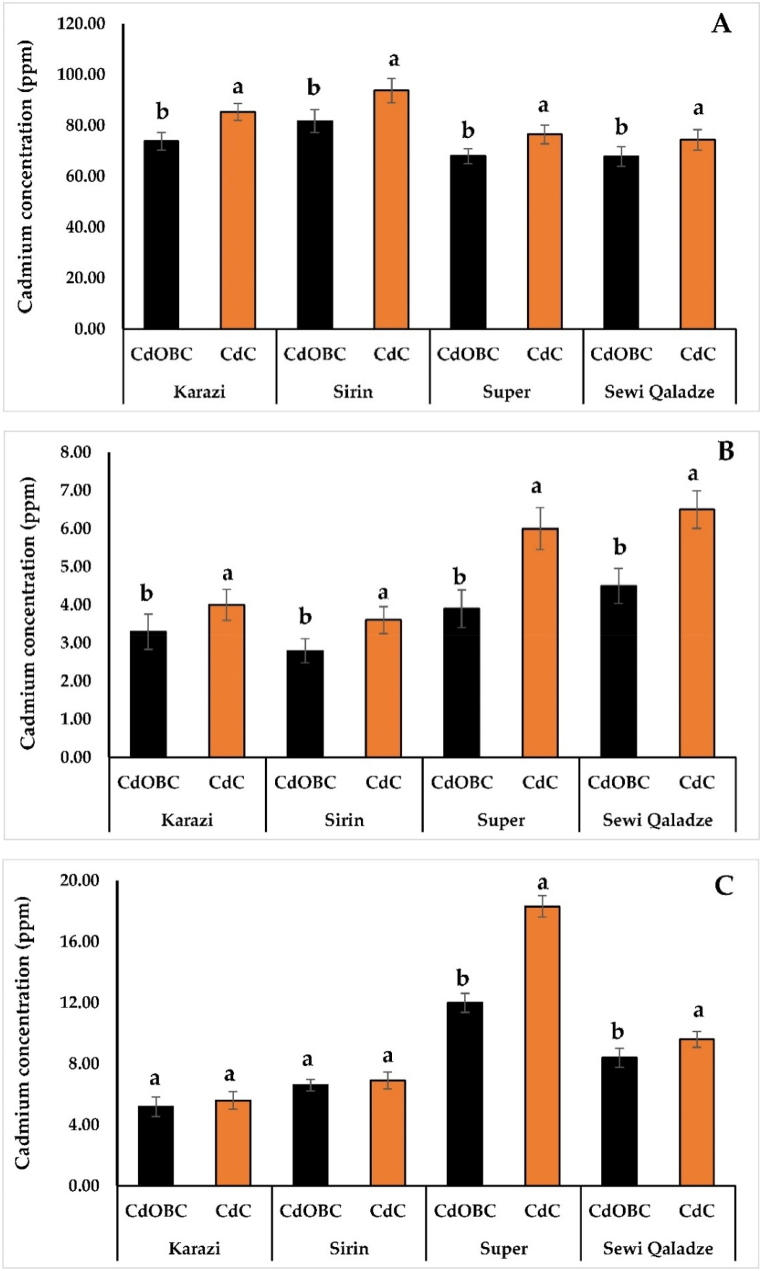
As shown in Table S3, the Cd concentrations were significantly different (p ≤ 0.01) between the CdC and CdOBC treatments in all tomato organs except for the leaf of the Karazi and Sirin genotypes. The collected roots and shoots are exposed to atomic absorption to determine cadmium accumulation in these organs in the presence and absence of oak leaf biomass pretreated with NaOH. In the presence of oak leaf biomass pretreated with NaOH, cadmium accumulation varied from 67.80 to 81.70 ppm in the root (Fig. 3A), 2.80–4.50 ppm in the stem (Fig. 3B), 5.20–12.00 ppm in the leaf (Fig. 3C), and 0.00–0.44 ppm in the fruit (Table 3). Karazi and Sirin genotypes had the highest levels of Cd absorption in their roots, whereas Super and Sewi Qaladze genotypes had the lowest values. Similar effects were reported in the absence of oak leaf biomass pretreated with NaOH for the roots. In contrast, Cd accumulations in the stems and leaves of Karazi and Sirin were lower in the presence and absence of oak leaf biomass pretreated with NaOH than those of Super and Sewi Qaladze in the presence and absence of oak leaf biomass pretreated with NaOH (Fig. 3). Regarding the cadmium content of fruit, Super and Sewi Qaladze had the greatest concentrations (Table 3). The accumulation of cadmium followed the same pattern: roots > leaves > stems > fruits.
Fig. 3.
Cadmium levels in the roots (A), stems (B), and leaves (C) of four tomato genotypes in the presence (CdOBC) and absence (CdC) of oak leaf biomass pretreated with sodium hydroxide. Bars represent means ± standard error. Using Duncan’s new multiple range test, the different letters within each genotype reveal the statistically significant difference in mean values. The values shown are the averages of three independent measurements.
Table 3.
Cadmium concentration in the fruits of four tomato genotypes in the presence (CdOBC) and absence (CdC) of oak biomass pretreated with sodium hydroxide.
| Genotypes | Treatments | Cadmium Concentration (ppm) |
|---|---|---|
| Karazi | CdOBC | 0.00 ± 0.00 ns |
| CdC | 0.00 ± 0.00 ns | |
| Sirin | CdOBC | 0.00 ± 0.00 ns |
| CdC | 0.00 ± 0.00 ns | |
| Super | CdOBC | 0.44 ± 0.08 b |
| CdC | 0.53 ± 0.09 a | |
| Sewi Qaladze | CdOBC | 0.13 ± 0.02 b |
| CdC | 0.18 ± 0.02 a |
Letters in the same row with different meanings designate statistically significant differences (p ≤ 0.01) using Duncan’s new multiple range test. The values are presented by the trait index (%) ± standard error. The values shown are the averages of eight separate measurements.
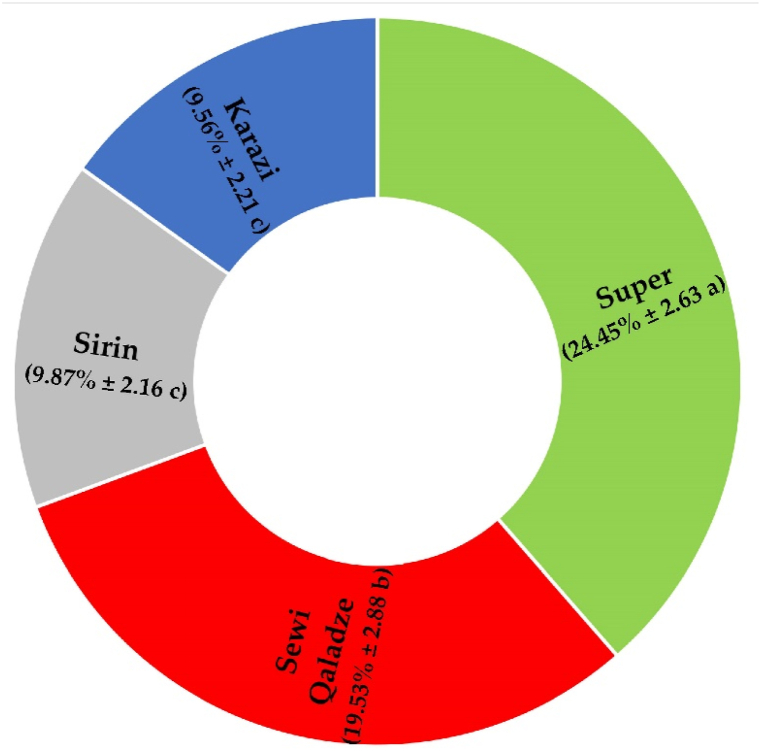
When comparing the three organs (root, leaf, and stem) in terms of Cd accumulation decrease, substantial differences were found between the root, stem, and leaf in the presence of oak leaf biomass pretreated with NaOH. The greatest reduction in Cd accumulation was documented in the stem and root of tolerant tomato genotypes, whereas it was recorded in the leaf and root of sensitive genotypes (Fig. 4). According to the reduction in organ Cd accumulation, there was a significant decrease in cadmium accumulations among tomato genotypes in the presence of oak leaf biomass pretreated with NaOH. Compared to CdC conditions, oak leaf biomass pretreated with NaOH induced 13.58, 12.90, 11.11, and 8.87% decreases in cadmium accumulations in the roots of Karazi, Sirin, Super, and Sewi Qaladze, respectively. Similarly, oak leaf biomass pretreated with NaOH reduced cadmium accumulations in stems by 17.50, 22.22, 35.00, and 30.77%, relative to CdC conditions, for Karazi, Sirin, Super, and Sewi Qaladze, respectively. Compared to CdC conditions, oak leaf biomass pretreated with NaOH provided 7.14, 4.35, 34.43, and 12.50% declines in cadmium accumulations in leaves for Karazi, Sirin, Super, and Sewi Qaladze, respectively. The four tomato genotypes showed significant differences across all organs (root, stem, leaf, and fruit). In contrast to the tolerant genotypes, which revealed Cd reduction values of 9.87% for Sirin and 9.53% for Karazi, the sensitive genotypes showed the highest reduction in Cd accumulation, with values of 24.45% for Super and 19.53% for Sewi Qaladze (Fig. 5).
Fig. 4.
Percentage reduction in cadmium accumulation in four tomato genotypes in the presence of oak leaf biomass pretreated with sodium hydroxide (CdOBC) compared to the absence of oak leaf biomass pretreated with sodium hydroxide (CdC). Bars represent means ± standard error. Different letters in each genotype indicate a statistically significant difference between the means, as ascertained by Duncan’s new multiple range test. The values shown are the averages of three independent measurements.
Fig. 5.
A sunburst graph illustrating the reduced percentage of cadmium accumulation across all organs of various tomato genotypes. The values in the chart show the percentage reduction in cadmium accumulation and the standard error. Duncan’s new multiple range test shows that genotypes with different letters have statistically significant differences in their means.
3.3. Biochemical responses of tomato genotypes of different treatments under cadmium stress conditions
The differences in cadmium tolerance between COC, CdC, and CdOBC treatments were further investigated on the leaf organs of tomato plants by using six biochemical markers. Based on ANOVA and DNMRT tests at p ≤ 0.01 for comparing the average values of different treatments in the presence of cadmium and plant biomass pretreated with NaOH, significant responses can be perceived by tomato genotypes for studied traits (Tables 4 and S4). The strongest activities of proline content (PC), soluble sugar content (SSC), antioxidant activity (AC), and guaiacol peroxidase (GPA) were stated under the presence of oak leaf biomass pretreated with NaOH under Cd conditions (CdOBC) with mean values of 1772.46 μg g −1, 687.18 μg g −1, 1025.74 μg g −1, and 0.43 units min −1 g −1, respectively, while the absence of oak leaf biomass pretreated with NaOH under Cd stress conditions documented the greatest contents of total phenolic content (TPC) and catalase (CAT) with the values of 393.78 μg g −1 and 153.80 units min −1 g −1, respectively. For all biochemical characteristics, however, the control conditions (COC) specified the minimum mean values.
Table 4.
Influence of different conditions on the accumulation of biochemical traits in cadmium-stressed tomato plants.
| Parameters | COC | CdC | CdOBC |
|---|---|---|---|
| Proline content (μg g −1) | 1274.38 ± 18.58 c | 1481.31 ± 81.25 b | 1772.46 ± 72.24 a |
| Soluble sugar content (μg g −1) | 542.58 ± 34.48 c | 633.63 ± 52.40 b | 687.18 ± 57.10 a |
| Total phenolic content (μg g −1) | 342.47 ± 5.46 c | 400.43 ± 18.30 a | 393.78 ± 7.61 b |
| Antioxidant activity (μg g −1) | 894.29 ± 6.84 c | 919.45 ± 14.95 b | 1025.74 ± 36.15 a |
| Guaiacol peroxidase (units min −1 g −1) | 0.12 ± 0.01 c | 0.29 ± 0.03 b | 0.43 ± 0.01 a |
| Catalase (units min −1 g −1) | 79.65 ± 4.70 c | 158.72 ± 12.17 a | 153.80 ± 6.69 b |
Duncan’s new multiple range test determines that different letters in the same row indicate statistically significant differences (p ≤ 0.01). The trait index (%) ± standard error was used to present the value. The values are the average of three separate assessments.
As shown in Table 5, the Karazi and Sirin genotypes had the highest mean values of biochemical parameters, while the Super and Sewi Qaladze genotypes had the lowest. The Karazi genotype showed the highest contents of SSC (822.18 μg g −1), TPC (420.37 μg g −1), and AC (1016.52 μg g −1), while the highest amounts of PC (1767.42 μg g −1), GPA (0.32 units min −1 g −1), and CAT (150.37 units min −1 g −1) were stated by the Sirin genotype. The smaller quantities of biochemical features were spotted in the Super and Sewi Qaladze genotypes. Consequently, the highest and lowest grading of genotypes based on their biochemical characteristics was as follows: high amount > low amount, Sirin > Karazi > Super > Sewi Qaladze. As a result of the interaction between the two factors, all biochemical parameters demonstrated significant modifications (Table S5). Under Cd stress, the Karazi genotype exhibited the greatest increases in SSC (950.68 g g −1) and antioxidant activity (1168.65 g g −1) in the presence of oak biomass (CdOBC), whereas the Sirin genotype displayed high increases in PC (2038.62 g g −1) and GPA (0.48 units min −1 g −1) in the presence of oak biomass (CdOBC). All biochemical characters were found to be lowest in the Super genotype when grown in COC conditions.
Table 5.
Biochemical responses of different tomato genotypes under Cd stress conditions in the presence and absence of oak biomass pretreated with sodium hydroxide.
| Traits | Karazi | Sirin | Sewi Qaladze | Super |
|---|---|---|---|---|
| Proline content (μg g −1) | 1595.28 ± 106.73 b | 1767.42 ± 99.82 a | 1357.76 ± 54.84 c | 1317.08 ± 45.48 d |
| Soluble sugar content (μg g −1) | 822.18 ± 44.81 a | 724.34 ± 18.39 b | 441.42 ± 14.54 d | 496.56 ± 7.46 c |
| Total phenolic content (μg g −1) | 420.37 ± 19.13 a | 381.49 ± 11.43 b | 350.27 ± 1.88 d | 363.45 ± 16.19 c |
| Antioxidant activity (μg g −1) | 1016.52 ± 38.57 a | 990.27 ± 32.12 b | 921.35 ± 6.43 c | 857.84 ± 6.28 d |
| Guaiacol peroxidase (units min −1 g −1) | 0.31 ± 0.05 a | 0.32 ± 0.06 a | 0.26 ± 0.03 b | 0.22 ± 0.05 c |
| Catalase (units min −1 g −1) | 137.64 ± 13.66 b | 150.37 ± 23.13 a | 130.36 ± 9.28 c | 104.52 ± 10.02 d |
Letters in the same row with different meanings indicate statistically significant differences (p ≤ 0.01) using Duncan’s new multiple range test.The trait index (%) ± standard error was used to present the value. The numbers illustrate an average of three independent estimates.
The PCA has been tested to achieve the quantitative association between biochemical traits used in this part of the investigation, which are responded by selected tomato genotypes under normal and Cd stress conditions in the presence and absence of oak leaf biomass pretreated with NaOH. In respect to biochemical traits, the analysis of PCA showed that the first two components (F1 and F2) were perfectly fitted in capturing the total differences, in which 100% of the total differences were seized. The first major component (F1) describes 88.65% of the entire variance, whereas the second major component (F2) describes 11.35% (Fig. 6). In respect of the association between different treatments and biochemical traits, CdOBC, COC, PC, SSC, and GPA were the most notable contributors to the variability along F1. The CdOBC treatment (red outline) exhibited the largest accumulation values of PC, SSC, AC, and GPA, whereas the CdC application (blue outline) revealed the greatest accumulation scores of CAT and TPC. Nevertheless, most of the diversity along F2 was caused by CdC, CAT, TPC, and AC (Fig. 6A). Concerning the distribution of tomato genotypes and biochemical traits, the first two components accounted for 97.13% of the total differences. The majority of the observed variance along F1 can be attributed to the AC, SSC, PC, and GPA characteristics. Contrarily, most of the variation in F2 was caused by the CAT and TPC characteristics (Fig. 6B). The tolerant genotypes (red and blue outlines) stated the maximum values of all biochemical traits.
Fig. 6.
Principal component analysis plot for the distribution of treatments (A) and four tomato genotypes (B) in association with biochemically tested traits under control and the presence of cadmium conditions. PC: proline content, SSC: soluble sugar content, TPC: total phenolic content, AC: antioxidant activity, GPA: guaiacol peroxidase, CAT: catalase, COC: control condition, CdC: treatment of plants with cadmium in the absence of oak biomass pretreated with sodium hydroxide, CdOBC: treatment of plants with cadmium in the presence of oak biomass pretreated with sodium hydroxide.
4. Discussion
Cd toxicity has been shown to harm plants by interfering with vital physiological processes [38]. Lipid peroxidation, membrane damage, and enzyme deactivation result from heavy metal exposure in plants because of the production of reactive oxygen species (ROS), which react with lipids, proteins, and nucleic acids [15,39,40]. In this study, tomato plants' growth and yield characteristics were dramatically affected by cadmium toxicity. These declines are caused by a combination of factors, including an increase in the production of reactive oxygen species (ROS), which damages the cell membrane and macromolecules; an inhibition of glycolysis-mediated cell division and elongation [41], a decrease in mitotic division of meristematic cells [42], a decrease in nutrient uptake and photosynthetic efficiency, which weakens their photosynthetic production capacity; and an increase in the production of ROS [38]. Cd’s negative effects on tomato plants' growth and yield have been shown in a number of studies [24,[43], [44], [45], [46]]. In comparison to the CdOBC condition, the CdC condition had larger decreasing percentages of growth and yield characteristics. The NaOH oak biomass pretreatment (CdOBC) resulted in a decrease in bioavailability and the mobilization of Cd to the roots, which in turn decreased the cytotoxic effects of Cd. One possible explanation for the enhanced metal biosorption by oak biomass pretreated with NaOH and Cd immobilization from the soil to the roots is that pretreatment removes surface impurities, disrupts cell-membrane structure, and exposes accessible binding sites for metal bioabsorption. NaOH can be used to treat oak biomass in order to remove putrefactive autolytic enzymes and the lipids and proteins that cover reactive sites [32,47,48]. During the NaOH oak biomass pretreatment, specific polymers, including polysaccharides with a high affinity for metal ions like Cd, may be released [31]. Oak biomass is quickly depleted of H+ ions when treated with sodium hydroxide, which leaves a surplus of negative charge on the cellular surface that captures even more Cd ions due to its high ionic repulsion potential [36].
The biomass of shoots and roots of the Cd-sensitive genotypes (Super and Sewi Qaladze) was more affected by Cd than that of the Cd-tolerant genotypes (Sirin and Karazi). Under CdC and CdOBC stress conditions, tomato biomass was distributed differently. Root organ accumulation of Cd had a negative effect on root morpho-physiological processes, leading to a smaller decrease in aboveground biomass. To protect its physiological and metabolic processes active in tissues like photosynthetic tissues and to reduce Cd mobility in the plant shoot system, the plant stored more Cd in the cell walls and vacuoles of the roots [[49], [50], [51]]. The production of gibberellin, which causes cell elongation in roots and shoots, may be increased by the CdOBC condition when compared to CdC conditions [52].
The cortical tissue of the root allows cadmium to enter the root easily, which is one of the causes of the deposition of Cd in the roots. Exudation (carbohydrates, amino acids, and enzymes), which are released by the root cap and rhizodermal cells and have the ability to bind cadmium, is also present on the growing root part [36,53]. Cadmium moves radially across the root layers using the same apoplastic and symplastic pathways as the other important nutrients [54]. Additionally, the decrease in pectin, lignin, and cellulose levels in roots following Cd stress may help increase the mobility and toxicity of Cd [55]. The roots of the plant initially take up cadmium, which is subsequently transferred to the above-ground sections of the plant. However, we found that, particularly in tolerant genotypes, very little Cd was transmitted to the tomato shoots. Our findings about the Cd concentration in various plant organs revealed that the tomato plant’s roots had the greatest Cd content, but the tomato fruit had the lowest Cd concentration. When comparing Cd-tolerant and sensitive genotypes, it was shown that under CdC and CdOBC conditions, the roots of tolerant genotypes had higher Cd accumulations than those of sensitive genotypes. The tomato genotypes were Super, Sewi Qaladze, Sirin, and Karazi in order of greatest to smallest Cd concentration in the stem, and Sewi Qaladze, Super, Sirin, and Karazi in sequence of highest to minimum Cd concentration in the leaf.
In order to comprehend the reactions of tolerant and sensitive genotypes to Cd stress, the biochemical tests of the leaves were conducted under CdC and CdOBC conditions. Comparing CdC and CdOBC conditions to COC conditions, a high concentration of biochemical components was reported. When exposed to Cd stress in the presence of oak leaf biomass pretreated with NaOH, tomato plants tended to accumulate high levels of PC, SSC, AC, and GPA, indicating that they used these osmoprotectant and antioxidant molecules to tolerate the Cd toxicity. In contrast, when exposed to Cd stress in the absence of oak leaf biomass pretreated with NaOH, tomato plants tended to accumulate high levels of TPC and CAT, indicating that they used these molecules to reduce the negative effects of Cd. The CdOBC treatment may enhance CO2 capture for the synthesis of lycopene, which has antioxidant properties and lessens the harm from the ROS reaction [56]. Furthermore, the use of CdOBC may have an impact on the levels of free polyphenols and antioxidant compounds, causing a reduction in the production of ROS [57].
By chelating heavy metals or neutralizing free radicals, phenolic substances can reduce the oxidative damage caused by heavy metal stress [58]. To this end, phenolic component concentrations are frequently utilized as a surrogate for plant responses to abiotic stresses. This could help explain why phenolic compound content increased in both the presence and absence of oak leaf biomass under Cd stress conditions. Increased phenolic accumulation in leaf tissue following Cd exposure demonstrates that tomato plants use phenolic chemicals as part of their defense mechanism against Cd stress [59]. Tomato plants with higher phenolic compound content in their leaves exhibited increased ROS dissolution capacity, enhanced chelation abilities toward Cd metals in root plants, and reduced translocation of Cd from the roots to the shoots [60]. It is possible that the roots will absorb this chelated Cd metal into vacuoles [61]. Plants with higher concentrations of soluble phenolic molecules, including lignin biosynthesis intermediates, are better able to withstand the toxic effects of Cd by constructing physical barriers between their cells and the metals [62]. In our research, we found that the availability of oak leaf biomass pretreated with NaOH under Cd treatment had different effects on the levels of stress-induced antioxidant substances like phenolic compounds, GPA, and CAT that may help protect cells from the damage caused by Cd [63]. Some research suggests that preventing lipid membrane peroxidation and maintaining membrane integrity can be achieved by increasing the activity of protective enzyme systems like GPA and CAT [[64], [65], [66]]. This study reported that stressed plants produced more of the osmoprotectant compounds PC and SSC in the presence and absence of oak leaf biomass pretreated with NaOH. The PC and SSC activities include osmotic regulators, metal chelators, protein stabilizers, and lipid peroxidation inhibitors. Sugars provide both energy and the carbon skeletons required by growing cells to build organic compounds [67,68]. According to the aforementioned findings, using oak leaf biomass can help solve environmental and sustainability issues brought on by the widespread use of pesticides and fertilizers. Based on the obtained data, we hypothesize that the NaOH oak leaf biomass pretreatment can alleviate Cd poisoning by accumulating high levels of PC, SSC, CAT, and AC.
5. Conclusions
It was observed that oak leaf biomass pretreatment mitigated the detrimental effects of Cd on plant biomass. It induced osmotic adjustment and preserved antioxidant production in tomato genotypes. The quantity of extractable Cd, its translocation, and its bioaccumulation in tomato genotypes were reduced by oak leaf biomass. Furthermore, it was found that tomato genotypes did not all react the same way to Cd stress. From the above outcomes, it is possible to conclude that adding oak leaf biomass to soils contaminated with cadmium can help minimize the absorption of cadmium, making this a cost-effective and strategic choice for agricultural soils to limit metal toxicity and cadmium accumulation that can reach edible components. In addition, the study revealed a simple, inexpensive, and ecologically beneficial way for remediating soil without the use of skilled employees. Additional research on the use of additional surfactants, such as Triton X-100 and cetyltrimethylammonium bromide (CTAB), is required to determine the capacity of oak biomass to reduce the toxicity and translocation of cadmium. Further investigation into the potential of oak biomass to mitigate the deleterious effects of other heavy metals on plant life is warranted.
Author contribution statement
Nawroz Abdul Razzak Tahir: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kamaran Salh Rasul: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Djshwar Dhahir Lateef: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
The authors are appreciative to the people who worked in the College of Agricultural Engineering Sciences at the University of Sulaimani for their assistance and cooperation throughout the research process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18660.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vareda J.P., Valente A.J., Durães L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J. Environ. Manag. 2019;246:101–118. doi: 10.1016/j.jenvman.2019.05.126. [DOI] [PubMed] [Google Scholar]
- 2.Suciu N.A., de Vivo R., Rizzati N., Capri E. Cd content in phosphate fertilizer: which potential risk for the environment and human health? Curr. Opin. Environ. Sci. Health. 2022;30 [Google Scholar]
- 3.Grant C.A., Sheppard S.C. Fertilizer impacts on cadmium availability in agricultural soils and crops. Hum. Ecol. Risk Assess. 2008;14(2):210–228. [Google Scholar]
- 4.Ma J.F., Shen R.F., Shao J.F. Transport of cadmium from soil to grain in cereal crops: a review. Pedosphere. 2021;31(1):3–10. [Google Scholar]
- 5.Michiel H., Ann C., Jana D., Verena I., Stéphanie V., Marijke J., Sophie H. Cadmium and plant development: an agony from seed to seed. Int. J. Mol. Sci. 2019;20(16):3971. doi: 10.3390/ijms20163971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin S., Liu H., Nie Z., Rengel Z., Gao W., Li C., Zhao P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere. 2020;30(2):168–180. [Google Scholar]
- 7.Hatamian M., Rezaei Nejad A., Kafi M., Souri M.K., Shahbazi K. Interaction of lead and cadmium on growth and leaf morphophysiological characteristics of European hackberry (Celtis australis) seedlings. Chem. Biol. Technol. Agric. 2020;7(1):1–8. [Google Scholar]
- 8.Souri M.K., Hatamian M., Tesfamariam T. Plant growth stage influences heavy metal accumulation in leafy vegetables of garden cress and sweet basil. Chem. Biol. Technol. Agric. 2019;6(1):1–7. [Google Scholar]
- 9.Guo T.R., Zhang G.P., Zhang Y.H. Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids Surf., B. 2007;57(2):182–188. doi: 10.1016/j.colsurfb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Leverrier P., Montigny C., Garrigos M., Champeil P. Metal binding to ligands: cadmium complexes with glutathione revisited. Anal. Biochem. 2007;371(2):215–228. doi: 10.1016/j.ab.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Zulfiqar U., Jiang W., Xiukang W., Hussain S., Ahmad M., Maqsood M.F., Ali N., Ishfaq M., Kaleem M., Haider F.U., Farooq N., Naveed M., Kucerik J., Brtnicky M., Mustafa A. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils; A comprehensive review. Front. Plant Sci. 2022;13:401. doi: 10.3389/fpls.2022.773815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsbrough Peter. In: Metal Tolerance in Plants: the Role of Phytochelatins and Metallothioneins. Terry N., Banuelos G.S., editors. Lewis Publishers; Boca Raton: 2000. pp. 221–233. (Phytoremediation of Contaminated Soil and Water). [Google Scholar]
- 13.El Rasafi T., Oukarroum A., Haddioui A., Song H., Kwon E.E., Bolan N., Tack F.M.G., Sebastian A., Prasad M.N.V., Rinklebe J. Cadmium stress in plants: a critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. 2022;52(5):675–726. [Google Scholar]
- 14.Berni R., Luyckx M., Xu Xuan, Legay S., Sergeant K., Hausman J.F., Lutts S., Cai G., Guerriero G. Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019;161:98–106. [Google Scholar]
- 15.Hasanuzzaman M., Bhuyan M., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9(8):681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi J., Wang J., Gong Z., Zhou J.-M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017;38:92–100. doi: 10.1016/j.pbi.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Chakresh Kumar J., Davendra Singh M., Anuj Kumar Y. Applicability of plant based biosorbents in the removal of heavy metals: a review. Environ. Process. 2016;2(3):495–523. [Google Scholar]
- 18.Yu H., Wang J., Yu J.X., Wang Y., Chi R.A. Effects of surface modification on heavy metal adsorption performance and stability of peanut shell and its extracts of cellulose, lignin, and hemicellulose. Environ. Sci. Pollut. Res. 2020;27(21):26502–26510. doi: 10.1007/s11356-020-09055-x. [DOI] [PubMed] [Google Scholar]
- 19.Madeła M., Skuza M. Towards a circular economy: analysis of the use of biowaste as biosorbent for the removal of heavy metals. Energies. 2021;14(17):5427. [Google Scholar]
- 20.Pertile E., Vaclavik V., Dvorsky T., Heviankova S. The removal of residual concentration of hazardous metals in wastewater from a neutralization station using biosorbent—a case study company Gutra, Czech Republic. Int. J. Environ. Res. Publ. Health. 2020;17(19):7225. doi: 10.3390/ijerph17197225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguirre-Ramírez M., Silva-Jiménez H., Banat I.M., Díaz De Rienzo M.A. Surfactants: physicochemical interactions with biological macromolecules. Biotechnol. Lett. 2021;43(3):523–535. doi: 10.1007/s10529-020-03054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abeer Hashem, Abd_Allah E.F., Alqarawi A.A., Al Huqail Asma A., Egamberdieva D., Wirth S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016;23(2):272. doi: 10.1016/j.sjbs.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmatizadeh R., Arvin S.M.J., Jamei R., Mozaffari H., Reza Nejhad F. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact. 2019;14(1):474–481. [Google Scholar]
- 24.Badawy I.H., Hmed A.A., Sofy M.R., Al-Mokadem A.Z. Alleviation of cadmium and nickel toxicity and phyto-stimulation of tomato plant L. by Endophytic Micrococcus luteus and Enterobacter cloacae. Plants. 2022;11(15):2018. doi: 10.3390/plants11152018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed A.A., Qadir S.A., Tahir N.A. Genetic variation and structure analysis of Iraqi valonia oak (Quercus aegilops L.) populations using conserved DNA-derived polymorphism and inter-simple sequence repeats markers. Plant Mol. Biol. Rep. 2022;41(1):1–14. [Google Scholar]
- 26.Yin P., Yang L., Li K., Fan H., Xue Q., Li X., Sun L., Liu Y. Bioactive components and antioxidant activities of oak cup crude extract and its four partially purified fractions by HPD-100 macroporous resin chromatography. Arab. J. Chem. 2019;12(2):249–261. [Google Scholar]
- 27.Sánchez-Burgos J.A., Ramírez-Mares M.V., Larrosa M.M., Gallegos-Infante J.A., González-Laredo R.F., Medina-Torres L., Rocha-Guzmán N.E. Antioxidant, antimicrobial, antitopoisomerase and gastroprotective effect of herbal infusions from four Quercus species. Ind. Crop. Prod. 2013;42:57–62. [Google Scholar]
- 28.Jong J.K., Bimal K.G., Hyeun C.S., Kyung J.L., Ki S.S., Young S.C., Taek S.Y., Ye-Ji L., Eun-Hye K., Ill-Min C. Comparison of phenolic compounds content in indeciduous Quercus species. J. Med. Plants Res. 2012;6(39):5228–5239. [Google Scholar]
- 29.Korbekandi H., Chitsazi M.R., Asghari G., Bahri Najafi R., Badii A., Iravani S. Green biosynthesis of silver nanoparticles using Quercus brantii (oak) leaves hydroalcoholic extract. Pharm. Biol. 2015;53(6):807–812. doi: 10.3109/13880209.2014.942868. [DOI] [PubMed] [Google Scholar]
- 30.Tahir N.A., Rasul K.S., Lateef D.D., Grundler F.M.W. Effects of oak leaf extract, biofertilizer, and soil containing oak leaf powder on tomato growth and biochemical characteristics under water stress conditions. Agriculture. 2022;12(12):2082. [Google Scholar]
- 31.Bello O.S., Adegoke K.A., Akinyunni O.O. Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl. Water Sci. 2017;7(3):1295–1305. [Google Scholar]
- 32.Bhatti H.N., Mumtaz B., Hanif M.A., Nadeem R. Removal of Zn(II) ions from aqueous solution using Moringa oleifera Lam. (horseradish tree) biomass. Process Biochem. 2007;42(4):547–553. [Google Scholar]
- 33.Lateef D., Mustafa K., Tahir N. Screening of Iraqi barley accessions under PEG-induced drought conditions. Life. 2021;14(1):308–332. [Google Scholar]
- 34.Chamon A.S., Gerzabek M.H., Mondol M.N., Ullah S.M., Rahman M., Blum W.E.H. Influence of cereal varieties and site conditions on heavy metal accumulations in cereal crops on polluted soils of Bangladesh. Commun. Soil Sci. Plant Anal. 2005;36(7–8):889–906. [Google Scholar]
- 35.Tahir N.A., Lateef D.D., Mustafa K.M., Rasul K.S. Under natural field conditions, exogenous application of moringa organ water extract enhanced the growth- and yield-related traits of barley accessions. Agriculture. 2022;12(9):1502. [Google Scholar]
- 36.An T., Gao Y., Kuang Q., Wu Y., Zaman Q.u., Zhang Y., Xu B., Chen Y. Effect of silicon on morpho-physiological attributes, yield and cadmium accumulation in two maize genotypes with contrasting root system size and health risk assessment. Plant Soil. 2022;477(1):117–134. [Google Scholar]
- 37.XLSTAT Statistical and Data Analysis Solution. Addinsoft; Boston, USA: 2019. [Google Scholar]
- 38.Haider F.U., Liqun C., Coulter J.A., Cheema S.A., Wu J., Zhang R., Wenjun M., Farooq M. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021;211 doi: 10.1016/j.ecoenv.2020.111887. [DOI] [PubMed] [Google Scholar]
- 39.Juan C.A., Pérez de la Lastra, Manuel José, Plou F.J., Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021;22(9):4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayem A.A., Hossain M.K., Lee S.B., Kim K., Saha S.K., Yang G.-M., Choi H.Y., Cho S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18(1):120. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalla Vecchia F., La Rocca N., Moro I., de Faveri S., Andreoli C., Rascio N. Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci. 2005;168(2):329–338. [Google Scholar]
- 42.Abbas T., Rizwan M., Ali S., Adrees M., Zia-Ur-Rehman M., Qayyum M.F., Ok Y.S., Murtaza G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018;25(26):25668–25680. doi: 10.1007/s11356-017-8987-4. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho M.E., Piotto F.A., Franco, Borges K.L., Gaziola S.A., Castro P.R., Azevedo R.A. Cadmium toxicity degree on tomato development is associated with disbalances in B and Mn status at early stages of plant exposure. Ecotoxicology. 2018;27(10):1293–1302. doi: 10.1007/s10646-018-1983-8. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho Marcia E.A., Piotto Fernando A., Gaziola Salete A., Jacomino Angelo P. Marijke Jozefczak, Ann Cuypers, Ricardo A. Azevedo, New insights about cadmium impacts on tomato: plant acclimation, nutritional changes, fruit quality and yield. Food Energy Secur. 2018;7(2) [Google Scholar]
- 45.Naciri R., Lahrir M., Benadis C., Chtouki M., Oukarroum A. Interactive effect of potassium and cadmium on growth, root morphology and chlorophyll a fluorescence in tomato plant. Sci. Rep. 2021;11(1):1–10. doi: 10.1038/s41598-021-84990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dad K., Nawaz M., Hassan R., Javed K., Shaheen A., Zhao F., Imran M., Shah S.T.H., Anwar M.F., Aurangzaib M. Impact of biochar on the growth and physiology of tomato grown in the cadmium contaminated soil. Pakistan J. Agric. Sci. 2021;34(2):454–462. [Google Scholar]
- 47.Ali Redha A. Removal of heavy metals from aqueous media by biosorption. Arab J. Basic Appl. Sci. 2020;27(1):183–193. [Google Scholar]
- 48.Muraleedharan T.R., Venkobachar C. Mechanism of biosorption of copper (II) by Ganoderma iucidum. Biotechnol. Bioeng. 1990;35(3):320–325. doi: 10.1002/bit.260350314. [DOI] [PubMed] [Google Scholar]
- 49.Yuan H.M., Huang X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016;39(1):120–135. doi: 10.1111/pce.12597. [DOI] [PubMed] [Google Scholar]
- 50.Xu L.L., Fan Z.Y., Dong Y.J., Kong J., Bai X.Y. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol. Plant. (Prague) 2015;59(1):171–182. [Google Scholar]
- 51.Cong L., Yu L., Jing T., Yanshu Z., Jinjuan F. Changes in sucrose metabolism in maize varieties with different cadmium sensitivities under cadmium stress. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong H., Lu D., Li Z., Wu J., Ning X., Lin W., Bai Z., Zheng C., Sun Y., Chi W., Zhang L., Xu X. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Commun. 2023 doi: 10.1016/j.xplc.2023.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bali A.S., Sidhu G.P.S., Kumar V. Root exudates ameliorate cadmium tolerance in plants: a review. Environ. Chem. Lett. 2020;18(4):1243–1275. [Google Scholar]
- 54.Xu L., Wang Y., Zhang F., Tang M., Chen Y., Wang J., Karanja B.K., Luo X., Zhang W., Liu L. Dissecting root proteome changes reveals new insight into cadmium stress response in radish (Raphanus sativus L.) Plant Cell Physiol. 2017;58(11):901–1913. doi: 10.1093/pcp/pcx131. [DOI] [PubMed] [Google Scholar]
- 55.Wang S., Liu Y., Kariman K., Li J., Zhang H., Li F., Chen Y., Ma C., Liu C., Yuan Y., Zhu Z., Rengel Z. Co-cropping Indian mustard and silage maize for phytoremediation of a cadmium-contaminated acid paddy soil amended with peat. Toxics. 2021;9(5):91. doi: 10.3390/toxics9050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M., Xia Q., Lv S., Tong J., Wang Z., Nie Q., Yang J. Enhanced CO2 capture for photosynthetic lycopene production in engineered Rhodopseudomonas palustris a purple nonsulfur bacterium. Green Chem. 2022;24(19):7500–7518. [Google Scholar]
- 57.Wang L., Li X., Gao F., Liu Y., Lang S., Wang C., Zhang D. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 2023;94 doi: 10.1016/j.ultsonch.2023.106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S., Wang Q., Lu H., Li J., Yang D., Liu J., Yan C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019;169:134–143. doi: 10.1016/j.ecoenv.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Chao Y.-Y., Chen C.-Y., Huang W.-D., Kao C.H. Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil. 2010;329(1):327–337. [Google Scholar]
- 60.Jiang S., Weng B., Liu T., Su Y., Liu J., Lu H., Yan C. Response of phenolic metabolism to cadmium and phenanthrene and its influence on pollutant translocations in the mangrove plant Aegiceras corniculatum (L.) Blanco (Ac), Ecotoxicol. Environ. Saf. 2017;141:290–297. doi: 10.1016/j.ecoenv.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 61.Luo J.-S., Zhang Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crops J. 2021;9(3):521–529. [Google Scholar]
- 62.Goncharuk E.A., Zagoskina N.V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium: review. Molecules. 2023;28(9):3921. doi: 10.3390/molecules28093921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jawad Hassan M., Ali Raza M., Ur Rehman S., Ansar M., Gitari H., Khan I., Wajid M., Ahmed M., Abbas Shah G., Peng Y., Li Z. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants. 2020;9(11):1575. doi: 10.3390/plants9111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao H., Guan J., Liang Q., Zhang X., Hu H., Zhang J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-89322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galić V., Mlinarić S., Marelja M., Zdunić Z., Brkić A., Mazur M., Begović L., Šimić D. Contrasting water withholding responses of young maize plants reveal link between lipid peroxidation and osmotic regulation corroborated by genetic analysis. Front. Plant Sci. 2022;13:2099. doi: 10.3389/fpls.2022.804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waheed A., Haxim Y., Islam W., Ahmad M., Ali S., Wen X., Khan K.A., Ghramh H.A., Zhang Z., Zhang D. Impact of cadmium stress on growth and physio-biochemical attributes of Eruca sativa Mill. Plants. 2022;11(21):2981. doi: 10.3390/plants11212981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turan V., Khan S.A., Mahmood-ur-Rahman, Iqbal M., Ramzani P.M.A., Fatima M. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol. Environ. Saf. 2018;161:409–419. doi: 10.1016/j.ecoenv.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 68.Shahbaz A.K., Adnan Ramzani P.M., Saeed R., Turan V., Iqbal M., Lewińska K., Abbas F., Saqib M., Tauqeer H.M., Iqbal M., Fatima M., Rahman M. Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol. Environ. Saf. 2019;173:182–191. doi: 10.1016/j.ecoenv.2019.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.