Abstract
Bone disorders are major health issues requiring specialized care; however, the traditional bone grafting method had several limitations. Thus, bone tissue engineering has become a potential alternative. In therapeutic treatments, using fetal bovine serum (FBS) as a culture supplement may result in the risk of contamination and host immunological response; therefore, human platelet lysate (hPL) has been considered a viable alternative source. This study attempted to compare the effectiveness and safety of different culture supplements, either FBS or hPL, on the osteoblastic differentiation potential of mesenchymal stem cells derived from human amniotic fluid (hAF-MSCs) under a three-dimensional gelatin scaffold. The results indicate that hAF-MSCs have the potential to be used in clinical applications as they meet the criteria for mesenchymal stem cells based on their morphology, the expression of a particular surface antigen, their proliferation ability, and their capacity for multipotent differentiation. After evaluation by MTT and Alamar blue proliferation assay, 10% of hPL was selected. The osteogenic differentiation of hAF-MSCs under three-dimensional gelatin scaffold using osteogenic-induced media supplemented with hPL was achievable and markedly stimulated osteoblast differentiation. Moreover, the expressions of osteoblastogenic related genes, including OCN, ALP, and COL1A1, exhibited the highest degree of expression under hPL-supplemented circumstances when compared with the control and the FBS-supplemented group. The induced cells under hPL-supplemented conditions also presented the highest ALP activity level and the greatest degree of calcium accumulation. These outcomes would indicate that hPL is a suitable substitute for animal derived serum. Importantly, osteogenic differentiation of human amniotic fluid derived mesenchymal stem cells using hPL-supplemented media and three-dimensional scaffolds may open the door to developing an alternative construct for repairing bone defects.
Keywords: Human amniotic fluid mesenchymal stem cells, Osteogenic differentiation, Osteoblast-like cells, Human platelet lysate, Scaffold, Tissue engineering
1. Introduction
Bone defects and bone degenerative disorders require specialized treatment. The use of autologous bone grafts is generally regarded as the gold standard in orthopedic reconstruction. However, this method is an invasive technique with several drawbacks, including donor site mobility, pain, discomfort, risk of infection, and potential fracture at the donor site [1,2]. Therefore, many studies have attempted to identify an appropriate alternative technique to improve the properties of bone grafting, including cell-based therapy or bone tissue engineering [3]. Three crucial components serve as the cornerstone of successful tissue engineering, namely osteogenic cells, osteoconductive scaffolds, and osteoinductive molecules [4].
The first main component of this process is the cell itself. The cells, which may be part of an engineered tissue, should possess immunocompatibility and the ability of self-renewal and differentiation, which are some of the most promising attributes of stem cells. In this light, mesenchymal stem cells (MSCs) have received considerable interest in a variety of clinical domains [5,6]. Mesenchymal stem cells can come from a variety of potential origins [7,8]. MSCs were initially isolated from bone marrow, although they have been associated with certain disadvantages. These would include the involvement of the invasive bone marrow aspiration procedure that can be both painful and have a high risk of infection. It has also been determined that the proliferation and differentiation capacities of these MSCs are affected by an increase in age [9]. Nevertheless, mesenchymal stem cells can also be acquired from umbilical cord blood and adipose tissue, but these sources provide fewer numbers of MSCs than bone marrow [10]. The above-mentioned sources of MSCs seem to have some limitations as alternative stem cell sources for regenerative medicine. Human amniotic fluid (hAF) has become an interesting source of mesenchymal stem cells because it contains a highly abundant mesenchymal stem cell population that can be easily obtained with a minimally harmful collection procedure and little ethical controversy [11,12]. Remarkably, human amniotic fluid-derived mesenchymal stem cells (hAF-MSCs) exhibit both embryonic and adult stem cell characteristics [[13], [14], [15]]. In addition, they are known to express pluripotency markers [14,15]; moreover, under specific culture conditions they exhibit the potential to differentiate into a variety of cell lineages derived from all three germ layers, such as adipogenic, chondrogenic, osteogenic [11,14,[16], [17], [18], [19]], myogenic [11,19], neurogenic [11,[18], [19], [20]], cardiogenic [19,21], hepatogenic [19,22], and vascular endothelial layers [19,23,24]. Additionally, hAF-MSCs reveal hypoimmunogenic properties and do not develop into teratomas [15,25].
Not only are cells the most important components of tissue engineering, but the scaffolds are known to serve as the extracellular matrix (ECM) for the formation of new bones in defective areas. The material that is used for bone grafts should provide three-dimensional support for cell migration, proliferation, and differentiation [26,27]. Biodegradable polymers generated from natural sources have been studied and utilized as bone graft material in a variety of orthopedic applications [28]. One of the protein-derived material scaffolds is the gelatin sponge, which is frequently utilized in wound care and surgery. Moreover, under physiological settings, gelatin does not exhibit any antigenicity or immunogenicity capabilities [29]. For this reason, gelatin has been determined to be the appropriate type of material for use in tissue engineering.
Regarding osteoinductive molecules, treatment with MSCs, however, often requires an in vitro cell expansion step to achieve therapeutic treatment. In the cultivation process, the most common and most widely used cell culture medium supplement is fetal bovine serum (FBS) [30]; however, its drawback is its endotoxin content which can increase the risk of contamination and the possibility of triggering a host immunological response [[31], [32], [33]]. Recently, human platelet lysate (hPL) has emerged as a useful substitute for FBS [32,33]. It has several advantages over FBS, including the ease with which it can be produced from human sources and the presence of numerous bioactive substances, such as growth factors and cytokines, which interact to support the cell growth, behavior, and differentiation of MSCs [34,35]. Moreover, hPL has been proven to be a productive alternative without introducing the risk of immune reactions or xenogeneic infections [[31], [32], [33]]. Some of the growth factors in hPL have been reported to influence bone formation, either intramembranous type and/or endochondral ossification type [36] and have also been documented in promoting the osteogenic differentiation of MSCs [37,38].
Taken together, hAF-MSCs cultured in a three-dimensional system with hPL supplements appear to be potentially useful components in therapeutic and tissue engineering applications. The objective of this present study was to compare the effectiveness and safety of different culture supplements, either FBS or hPL, on the osteoblastic differentiation potential of hAF-MSCs under conditions of a biomimetic three-dimensional system in order to manufacture an ideal bone substitute. This in vitro study could, therefore, be the first stage in the successful development of an alternative treatment for bone tissue regeneration.
2. Materials and methods
2.1. Cell samples preparation
Human amniotic fluid (hAF) cell samples were collected from the Human Genetic Laboratory, Department of Anatomy, Faculty of Medicine, Chiang Mai University. All samples were back-up specimens obtained from an amniocentesis procedure in prenatal diagnosis during the 16th–22nd weeks of the gestational period with a normal karyotype. All experiments were granted approval from the Research Ethical Committee of the Faculty of Medicine, Chiang Mai University, October 15, No. ANA-2564-08540.
2.2. Isolation and expansion of human amniotic fluid-derived mesenchymal stem cells (hAF-MSC)
For the culture and isolation process, the reserved flasks of hAF cultured in expansion medium (BIOAMF-3TM Complete Medium) (Biological Industries, Kibbutz Beit Haemek, Israel) were washed with sterile phosphate buffer saline (PBS) (Amresco®, Ohio, USA), and the culture was substituted with basal growth medium containing Dulbecco's Modified Eagle Medium (DMEM) – high glucose (Gibco,USA), 40 mg/ml gentamycin, and 10,000 U/ml Pen Strep (penicillin and streptomycin) (Gibco, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Massachusetts, USA) in 25 cm2 culture flasks (Corning Incorporated, NY, USA) at 37 °C and 5% CO2 in a humidified incubator. On the following day, the non-adherent cells were gently removed, while the adherent ones remained in the culture. The culture medium was changed every three days. The hAF cell samples were observed under a DMi1 inverted phase contrast microscope (Leica Microsystems, USA). When the adherent cells reached 80%–90% confluency, they were sub-cultured using 0.25% trypsin-EDTA (Gibco® by Life technologies, USA). The cells were then re-expanded in 25 cm2 culture flasks under the same conditions at a 1:2 ratio. The 2nd - 4th passage of the hAF samples were utilized for all the further experiments.
Subsequently, the hAF cells were then evaluated in terms of MSC characteristics based on their morphology, their surface marker expressions, their proliferation ability, and their differentiation potential as described by the International Society for Cellular Therapy (ISCT) [8].
2.3. Flow cytometry analysis
The hAF samples (n = 3) were characterized by measuring the expression of MSC specific surface protein markers. The cells in the 2nd passage were trypsinized with 0.25% trypsin-EDTA and centrifuged at 3700 rpm for 6 min. Then, the cell pellets were washed twice with 1% bovine serum albumin in PBS (1% BSA-PBS) and centrifuged twice at 3700 rpm for 6 min. After that, the non-specific bindings were blocked by 10% human AB – serum in 1% BSA-PBS for 30 min on ice. Next, the cells were incubated with fluorescent-conjugated monoclonal antibodies: phycoerythrin (PE)-conjugated mouse anti-human CD31, CD45, CD73, CD117, and HLA-DR (Immuno Tools GmbH, Friesoythe, Germany); fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD34, CD44, HLA-ABC (Immuno Tools GmbH, Friesoythe, Germany), and mouse anti-human fibroblast surface protein markers (Merck Millipore, Massachusetts, USA); and Alexa fluor-conjugated mouse anti-human Oct-4 (Merck Millipore, Massachusetts, USA). PE mouse isotype control, FITC mouse isotype control (BioLegend, San Diego, USA), and Alexa fluor mouse isotype control (Invitrogen, Massachusetts, USA) were used as negative controls. At the final stage, the cells were washed and re-suspended in 1% BSA-PBS and the fluorescent expression was detected by DxFLEX Flow Cytometer (Beckman Coulter, California, United States). They were then analyzed by Cytexpert for DxFLEX software (Beckman Coulter). Descriptive analysis was then employed and results were presented as mean ± SEM values.
2.4. Alamar blue cell proliferation assay
To confirm that one of the MSC properties involves cell proliferation, Alamar blue was employed to quantitatively measure cell proliferation by monitoring the oxidation-reduction (REDOX) environment that can be found in living cells. The active ingredient of Alamar blue is non-fluorescent resazurin, which is non-toxic and stable in culture medium. Alamar blue; therefore, it can be reduced by NADPH, FADH, FMNH, NADH, as well as cytochromes. It changes from the oxidized, non-fluorescent, blue state to the reduced, fluorescent, pink state of resorufin. An increase in cell viability can be reflected in cell division, which can then be used to evaluate cell proliferation. The hAF cell samples at the 2nd passage (n = 3) were plated in triplicate into 24 well-plates in 5 wells/sample at a density of 5 × 103 cells/well and cultured in an incubator at 37 °C, 5% CO2, and 95% humidity with basal growth medium for 24 h. After that, the basal medium was discarded and 100 μl of 10% (v/v) alamar blue (Sigma,USA) in DMEM was added to the wells, including the wells that served as negative control and they all were incubated for 4 h. After that, 100 μl of alamar blue solution in each well was transferred to a 96-well plate for optical density (OD) measurement using a spectrophotometer plate reader (Synergy H4 Hybrid Microplate Reader, BioTek, Vermont, USA) at wavelengths of 540 and 630 nm. The cells were then continuously cultured under the same conditions, and an Alamar blue cell proliferation assay was performed every other day until day 21 of the culturing period. The data were analyzed to obtain the percentage reduction of Alamar blue which was calculated as follows:
Sx = Alamar blue fluorescence signal of the sample at day x.
Scontrol = Signal from the control (the culture medium supplemented with 10 vol % alamar blue).
S100% = Reduced form of alamar blue produced by autoclaving controls.
2.5. Tri-lineage differentiation assay
One of the criteria for defining a mesenchymal stem cell is that the cells should be differentiated into osteoblasts, chondroblasts, and adipocytes under appropriate circumstances. To confirm the criteria, each hAF sample (n = 3) was split into three groups, each with 1 × 104 cells/well in triplicate in 24-well culture plate, and the differentiation media was then applied. The culture plates were maintained in 5% CO2 at 37 °C in a highly humidified incubator for 21 days with medium replacement occurring every three days.
For osteogenic differentiation, the seeded cells were cultured with osteogenic induced medium consisting of basal growth medium supplemented with 50 μg/ml ascorbic acid, 10−7 M dexamethasone, and 10 mM β-glycerophosphate. Osteogenic differentiated cells were determined by Alizarin Red S staining of the calcified extracellular matrix deposition.
For chondrogenic differentiation, the seeded cells were cultured by adding chondrogenic induced medium consisting of basal growth medium supplemented with 25 μg/ml insulin, 10−7 M dexamethasone, and 25 μg/ml ascorbic acid. S-GAG formations in ECM were then determined by Safranin O staining.
For adipogenic differentiation, the seeded cells were cultured with adipogenic induced medium consisting of basal growth medium supplemented with 60 μm Indomethacin, 10−6 M dexamethasone, and 5 μg/ml insulin. After a specific period of cultivation, formations of intracellular lipid droplets were monitored using Oil Red O staining.
2.6. Human platelet lysate (hPL) preparation
The pooled human platelet concentrate (hPC) was obtained by pooling 15 platelet apheresis collections from the Laboratory of Blood Bank Section, Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University. All pooled hPC underwent three cycles of being frozen and then thawed at −80 °C and 37 °C, respectively. The remaining cellular fragments were eliminated by centrifugation at 4000 rpm for 20 min. After that, the supernatant was filtered using a 0.2 μm filter (Corning Incorporated, NY, USA) and 4U/ml of heparin was added to prevent gel formation. The final stock of hPL was kept at −20 °C.
To determine the impact of utilizing different serum types on cell viability and to evaluate the optimal concentration of hPL that was used as a supplement in the basal growth medium, the isolated hAF-MSCs were assessed using two different assays: Methylthiazol tetrazolium (MTT) and Alamar blue proliferation analysis.
2.7. Methylthiazol tetrazolium (MTT) assay
To evaluate the effect of hPL on the viability of hAF-MSCs, Methylthiazol tetrazolium (MTT) was performed. In brief, 3 × 103 hAF-MSCs (n = 3) were seeded into a 96 well-plate with 100 μl culture medium for 24 h. After that, various concentrations of hPL (2.5% hPL, 5% hPL, 10% hPL, 20% hPL, and 40% hPL) supplemented in 100 μl of basal growth medium were added to each well and they were incubated for 24, 48, 72, and 96 h. Out of seven wells, five wells contained various percent-concentrations of hPL supplements, one contained only high-glucose DMEM as a positive control, and the remaining one contained 10% DMSO (Sigma-Aldrich, USA) as a negative control. After the incubation period, the culture medium was replaced by MTT working solution (final concentration of 0.5 mg/ml in PBS) (Invitrogen, Massachusetts, USA) and further incubated for 4 h. The formed formazan crystals were solubilized by adding 50 μL of DMSO in each well for 10 min. The purple color intensity of the dissolved formazan crystals was quantified using a spectrophotometer plate reader at 540 nm. The experiment was performed in three replicates. The percentage of cell viability was calculated using the following formula:
2.8. Alamar blue proliferation assay of hPL
To confirm the effect of hPL in the prolonged cell culture, Alamar blue proliferation analysis (n = 3) was performed in triplicate, as has been previously described in the Alamar blue proliferation assay procedure.
2.9. Osteogenic induction
The osteoblastic differentiation potential of hAF-MSCs (n = 5) was examined and the effect of different supplements (hPL or FBS) on three-dimensional culture conditions was evaluated. Before plating and scaffolding the cells, the 5 mm2 diameter sterilized gelatin scaffolds (SPONGOSTAN™ Standard, Ferrosan Medical Devices, Denmark) were pre-wetted with basal growth medium for 5 min. Then, 10 μl of DMEM containing 3 × 105 cells was seeded on the top of the scaffold, placed in a 24-well culture plate, and immediately incubated for 2.30 h at 37 °C and in a 5% CO2 high humidify incubator for primary cell attachment. After that, an additional of each culture mediums were then added into each well.
In the control group, cells were treated with 1 ml basal growth medium (DMEM-high glucose, Penstrep and gentamycin). The other two induced groups were treated with 1 ml osteogenic-induced medium consisting of DMEM-high glucose, Penstrep, gentamycin supplemented with 50 μg/ml ascorbic acid (Sigma-Aldrich, China), 10−7 M dexamethasone (Sigma-Aldrich, USA), and 10 mM β-glycerophosphate (Sigma-Aldrich, USA). These two induced groups were then supplemented with either FBS or hPL. The optimal percentage of hPL established by the MTT assay and Alamar blue proliferation assay was used to investigate the osteogenic-induced group. The entire plate was continuously cultured under conditions of 37 °C, 95% humidity, and 5% CO2 for 21 days. Refreshment of the culture medium was carried out every three days.
2.10. Osteogenic specific gene expression analysis by reverse transcription quantitative PCR (RT-qPCR)
After 14 days of osteogenic differentiation (n = 5), total RNA was extracted using an Illutra RNAspin Mini RNA Isolation kit (GE Healthcare, UK) and RNA concentration was measured using a Nanodrop spectrophotometer (Nanodrop Technologies, Montchanin, USA). The first strand of complementary DNA (cDNA) was synthesized using a TetrocDNA synthesis kit (Bioline, USA) according to the manufacturer's instructions. RT-qPCR was achieved by using a SensiFAST™ SYBR® No-ROX Kit (Bioline, USA) on 7500 FastReal-Time PCR System (Applied Biosystems, USA). The osteoblastogenic gene expression levels of RUNX2, OCN, ALP, and COL1A1 were analyzed using the method. The expression level of each gene was assessed in duplicate, and the mean CT value was used for normalization relative to the expression of GAPDH, which is considered a housekeeping gene. Gene specific primer sequences (Life science AP, Thailand) are presented in (Table 1).
Table 1.
Real-time PCR primer sequences.
| Primer | Real-time PCR primer sequences | |
|---|---|---|
| Runt-related transcription factor 2 (RUNX2) | Forward 5′-3′ Reverse 5′-3′ |
ATCCAGCCACCTTCACTTACACC GGGACCATTGGGAACTGATAGG |
| Osteocalcin (OCN) | Forward 5′-3′ Reverse 5′-3′ |
GAAGCCCAGCGGTGCA CACTACCTCGCTGCCCTCC |
| Alkaline phosphatase (ALP) | Forward 5′-3′ Reverse 5′-3′ |
CATGGCTTTGGGCAGAAGGA CTAGCCCCAAAAAGAGTTGCAA |
| Type I collagen (COL1A1) | Forward 5′-3′ Reverse 5′-3′ |
TGTGGATGCCTCTTGGGTATC TTTTGGCCATCTCTTCCTTCA |
| GAPDH | Forward 5′-3′ Reverse 5′-3′ |
GAAGGTGAAGGTCGGAGTC GAAGATGGTGATGGGATTTC |
2.11. Alkaline phosphatase (ALP) activity assay
Alkaline phosphatase (ALP) is widely recognized as a biochemical indicator of osteoblast mineralization activity. ALP can hydrolyze p-nitrophenyl phosphate (pNPP) into p-nitrophenol (pNP) and phosphate, which then appears yellow in color. In this study, at days 14 and 21 after seeding, ALP activity was evaluated (n = 5). Briefly, the scaffolds with cells were prewashed two times with sterile PBS, and then 200 μl pNPP substrate solution (Sigma, USA) was added to each well and they were incubated at 37 °C for 30 min. The yellow product was aspirated to a fresh 96 well-reading plate in duplicate and the reaction was stopped by adding 50 μL of 3 M NaOH. An absorbance wavelength of 405 nm was achieved using a spectrophotometer. To established standards, standard P-nitrophenol (P-nitrophenol 0.1 M in NaOH 1 M) (Sigma, USA) was prepared and 10-fold stock solution was diluted with NaOH 1 M from 1000 μM. Ultimately, only NaOH remained. The standard solution was then moved to a 96 well-reading plate in duplicate. The mean OD value of the pNP standard was plotted as a standard curve to establish a linear formula in order to analyze the ALP concentration obtained from the cell samples.
The rest of the cells were normalized for the total amount of protein content using Bradford protein assay. A spectrometer set at a wavelength of 620 nm was used to analyze the absorbance in this process.
The data were calculated as follows:
Sample ALP = absorbance of sample.
Total protein = absorbance of Bradford assay from the same sample.
2.12. Paraffin section
After 21 days of hAF-MSCs cultivation within the gelatin scaffold (n = 5), the cells were firstly washed with cold PBS and fixed in 4% paraformaldehyde in PBS at 4 °C for 4 h. Then, seeded cells were washed three times with distilled water and dehydrated with ethanol series at concentrations of 50%, 70%, 80%, 90% each for 5 min and 95% overnight. After that, they were immersed twice in 100% ethanol in plastic cassettes for 1 h at room temperature. After the dehydration period, the tissues were cleared by being immersed twice in xylene (AnalaR NORMAPUR®, France) for 1 h on a shaker at room temperature. The hAF-MSCs tissues were infiltrated and embedded in a mixture of paraffin and xylene at a ratio 2:1, 1:1, and pure paraffin at 60 °C for 30 min each, respectively. The scaffold-embedding process was performed at the embedding center (HESTION, Australia). For the purposes of sectioning, the embedded paraffin was cut into 5 μm serial sections using a microtome (Microm HM325 Microtome, GMI Inc, USA) and transferred onto glass slides for further analysis.
2.13. Alizarin red S staining
On the 21st day, alizarin red S staining was used to observe the osteogenic mineralization in ECM of the hAF-MSCs on the scaffold under all conditions (non-induced, induced with FBS or hPL) (n = 5). Before proceeding with the staining protocol, sectioned slides were deparaffinized and rehydrated. To deparaffinize the specimens, Xylene was used twice for 5 min each time. To rehydrate the specimens, they were immersed in sequences of alcohol series at 100% and 95% twice, and 80% once, each for 2 min. After that, the sectioned slides were washed with distilled water. Alizarin Red S staining solution was then added. Subsequently, the sample slides were placed on the shaker for 20 min at room temperature. Later, they were rinsed three times with distilled water until cell debris and excess dye were washed out. Finally, the alizarin red-stained area was examined under a DMi1 inverted phase contrast microscope.
2.14. Statistical analysis
Data were analyzed with descriptive analysis and the statistical differences among the three groups of hAF-MSCs were determined using one way analysis of variance (one-way ANOVA) followed by post hoc multiple comparison test using SPSS version 26.0 (IBM, New York, USA). All values are presented as mean ± SEM values and significance is considered when the p-value was less than 0.05 (p < 0.05).
3. Results
3.1. Cell morphology
Human amniotic fluid (hAF) cell samples were obtained from the Human Genetic Laboratory, Department of Anatomy, Faculty of Medicine, Chiang Mai University. All samples were obtained via the amniocentesis procedure in prenatal diagnosis and illustrated in normal karyotype (46,XX or 46,XY).
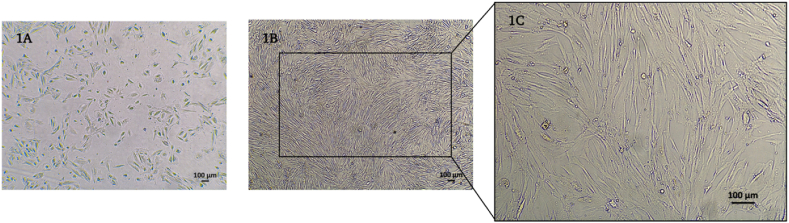
The hAF cells demonstrated the capacity for cell adhesion and expansion on the culture surface under favorable conditions throughout the cultivation period. Initially, the hAF cells displayed a heterogeneous cell population comprising both epithelioid and spindle-shaped cells (Fig. 1A). Upon subculturing, the non-adherent cells were removed, revealing a homogeneous population of fibroblast-like cells that exhibited the characteristic morphology of mesenchymal stem cells (Fig. 1B and C).
Fig. 1.
Morphology of hAF cells at the 1st passage (A) and hAF cells at the 2nd passage (B) with 4× magnification (1B) and 10× magnification (1C).
3.2. Cell characterization according to flow cytometry analysis
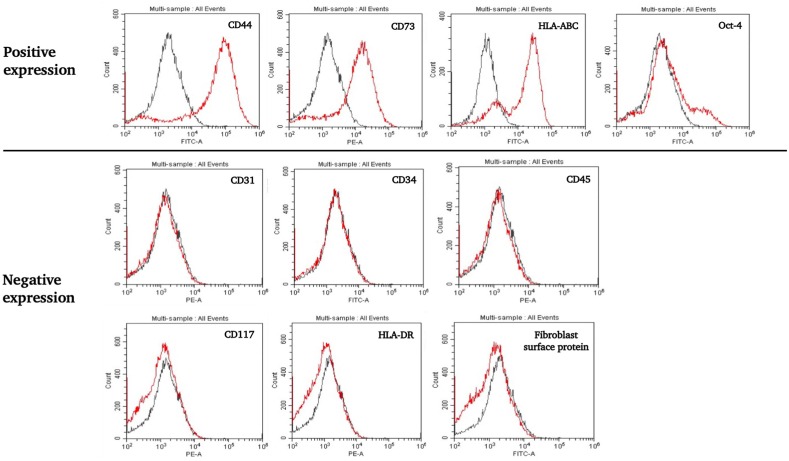
The 2nd passage of cultured hAF cells (n = 3) was characterized by using flow cytometry analysis in order to detect the MSC antigenic phenotype at the cell surface. The results indicate that the hAF cells exhibited positive expression of MSC markers including CD44 (99.92 ± 0.03), CD73 (99.87 ± 0.02), HLA-ABC (99.68 ± 0.03), and Oct-4 (6.91 ± 0.28), respectively. On the other hand, the cells were negative for CD31 (0.38 ± 0.13), CD34 (0.41 ± 0.24), CD45 (0.47 ± 0.16), CD117 (4.2 ± 2.25), HLA-DR (0.38 ± 0.14), and the fibroblast surface protein (0.28 ± 0.08), respectively (Fig. 2).
Fig. 2.
Flow cytometry histogram of the expressions of the cell surface protein markers of hAF cells during the 2nd passage. The hAF cells exhibited a positive degree of expression of CD44, CD73, HLA-ABC, and Oct-4. On the other hand, the cells were found to be negative for CD31, CD34, CD45, CD117, HLA-DR, and fibroblast surface protein markers.
3.3. Cell proliferation analysis by alamar blue assay
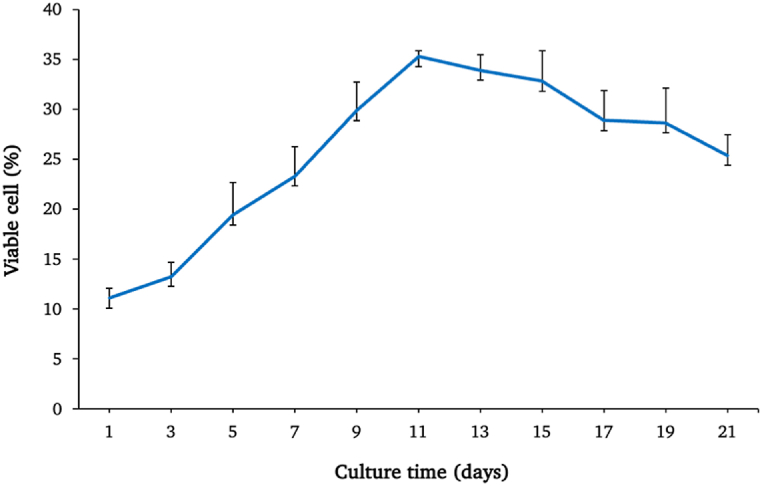
The hAF cells (n = 3) were investigated for proliferation rate by Alamar blue assay over 21 days of cultivation. After plotting the line graph, the early phase of cultivation indicated a slight increase in cell growth. Then, from day 3 to day 11, the proliferation rates increased relatively sharply and reached the highest peak on day 11, which was representative of the exponential phase of cell growth. After that, the cells stabilized before beginning to decrease in numbers continuously (Fig. 3).
Fig. 3.
The growth curve and viability of the hAF cells over 21 days. Data are presented as mean ± SEM values.
3.4. Tri-lineage differentiation
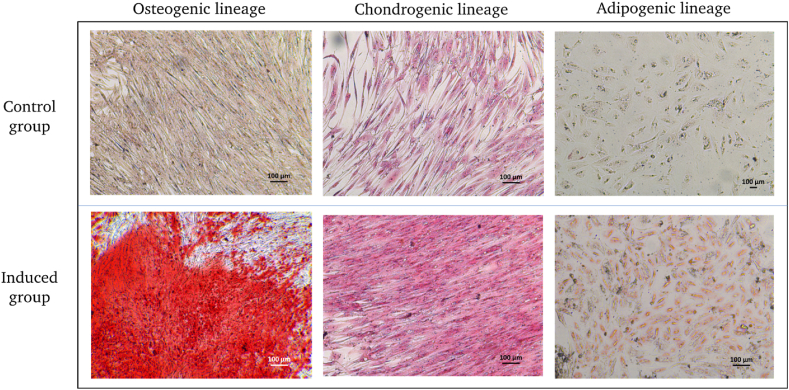
The multipotent differentiation capacity of hAF was examined by culturing the cells under induced medium. The terminal differentiation of hAF was confirmed by histological staining at a 21-day time point. Osteogenic differentiation was confirmed by the mineralization of calcium, as can be seen by Alizarin red S staining. For chondrogenic differentiation, s-GAG development in ECM of the cells was verified as can be seen by the Safranin O staining. For adipogenic induction, the intracytoplasmic vacuoles in the cytoplasm were stained positive with Oil red O staining (Fig. 4).
Fig. 4.
Accordingly, hAF differentiation potential assay after 21 days of specific induction.
3.5. Methylthiazole tetrazolium (MTT) assay for optimal hPL concentration
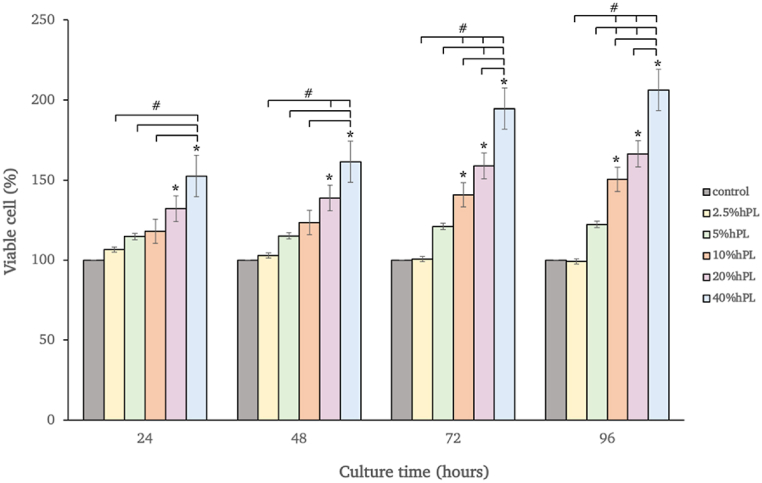
Prior to the osteogenic inducing procedure, the optimal percentage of hPL supplemented in the medium was evaluated by MTT assay. The hAF-MSCs were treated with DMEM-high glucose and supplemented with various percentages of human platelet lysate (hPL) including 2.5%, 5%, 10%, 20%, or 40% and incubated for 24, 48, 72, and 96 h, respectively. The results showed an increase in cell viability for all hPL-treated groups when compared with the control group at all time points. The 20% and 40% hPL treated group displayed a higher degree of statistical significance on cell viability when compared with the control group at all time points. Moreover, after 72 h of cultivation, 10% hPL became significantly higher than the control group (p < 0.05). At 96 h, the hPL treated cells at concentrations of 10%, 20%, and 40% displayed a higher degree of statistical significance when compared with 2.5% and 5% hPL. However, no statical significance was observed between concentrations of 10% and 20% hPL (Fig. 5).
Fig. 5.
Viability of hAF-MSCs exposed to hPL (2.5–40%) in DMEM for 24, 48,72, and 96 h. The cells cultured in pure DMEM were used as the control. Data are presented as mean ± SEM values. * Denoted for statistical significance when compared with the control group (p < 0.05) # Denoted for statistical significance among the groups (p < 0.05).
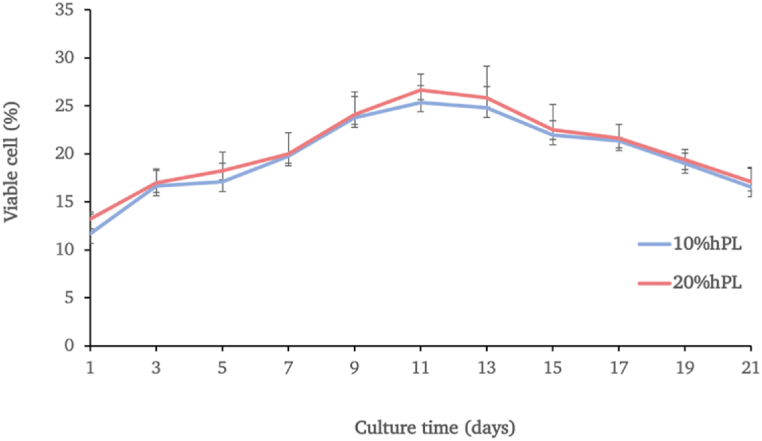
3.6. Prolonged effect of hPL cultured cells by alamar blue proliferation assay
The hAF cells were cultured in parallel in cultured media supplemented with either 10% or 20% hPL for 21 days. The growth characteristics of the paired samples (n = 3) indicated no significant differences between conditions but demonstrated a similar pattern over 21 days of cultivation. The cells cultured in both conditions exhibited a slight increase in cell growth and entered into the exponential phase of cell growth with no significant differences in percentage of cell viability. After that, the cell numbers slightly decreased and proceeded to decrease continuously (Fig. 6).
Fig. 6.
The growth curve and viability of the hAF cells cultured in media supplemented with either 10% hPL or 20% hPL oveer 21 days. Data are presented as mean ± SEM values.
3.7. Reverse transcription quantitative PCR (RT-qPCR)
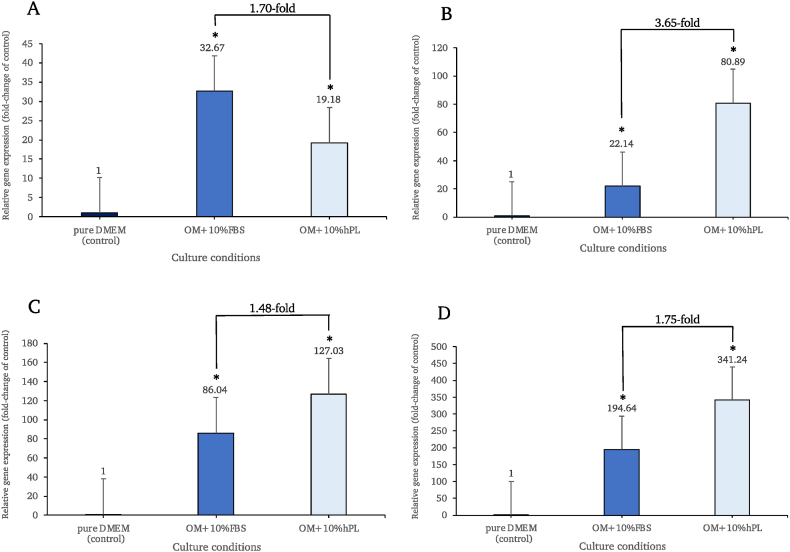
The RT-qPCR results revealed significantly higher expression levels of all genes in the osteogenic-induced groups supplemented with either 10% FBS or 10% hPL compared to the control group (p < 0.05) (Fig. 7). The expression levels of RUNX2 (Fig. 7A), OCN (Fig. 7B), ALP (Fig. 7C) and COL1A1 (Fig. 7D) were increased in FBS-supplemented induced cells when compared to non-induced control cells (32.67-fold, 22.14-fold, 86.04-fold and 194.64-fold, respectively). Moreover, the expression of these genes was similarly elevated in the induced cells that had received hPL supplementation. (19.18-fold, 80.89-fold, 127.03-fold and 341.24-fold, respectively). Interestingly, the expression levels of OCN, ALP and COL1A1 were up-regulated more in the induced cells cultured in hPL than in those of FBS (3.65, 1.48 and 1.75 times higher expression, respectively).
Fig. 7.
Expression levels of osteoblastogenic related genes, including RUNX2 (A), OCN (B), ALP (C), and COL1A1 (D), were normalized to GAPDH and were relative to the control group on day 14. * Denoted for statistical significance when compared with the control group (p < 0.05).
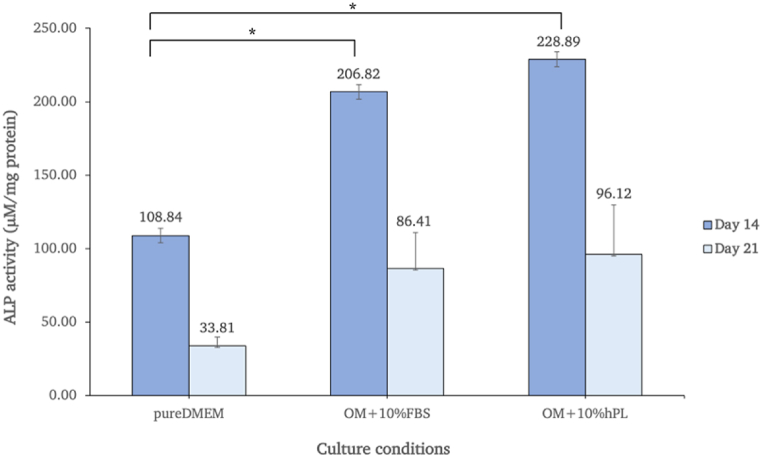
3.8. Alkaline phosphatase (ALP) activity assay
The ALP activity assay was carried out after cells had been cultured for 14 and 21 days. The ALP activity of each sample was normalized to the amount of protein in the sample. The findings demonstrated that the activity levels were higher in both osteogenic-induced conditions (10% FBS and 10% hPL) than in the control group on every measuring day. On day 14 of the culture period, the induced groups demonstrated the highest level of ALP activity, which was significantly greater than that observed in the control group (p < 0.05). After that, the activity levels began to fall at the end of culturing phase (day 21). Furthermore, the cells treated with 10% hPL exhibited a greater increase in ALP activity compared to those treated with 10% FBS (Fig. 8).
Fig. 8.
ALP activity of hAF-MSCs cultivated for 14 and 21 days under different culture medium conditions.
One-way ANOVA was applied to interpret the difference between groups (p < 0.05).
3.9. Alizarin red S staining
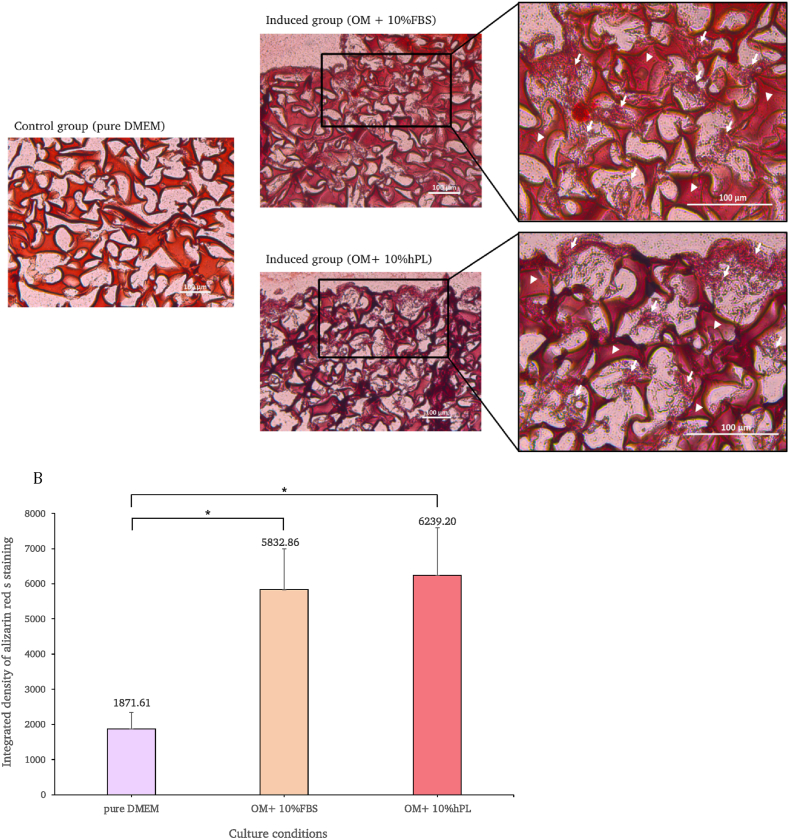
On day 21, the scaffolds were sectioned and alizarin red s staining was carried out on all cultivated cells.
The results indicate that both osteogenic-induced groups possessed robust osteogenic features, reflected by the greater apparent degree of red staining of calcium that had accumulated in the scaffold pore when compared with the control group (Fig. 9A). The quantification of staining intensity was analyzed using ImageJ software and the results revealed a statistically significant difference between the induced groups and the control group. By measuring the degree of calcium deposition, it was found that both osteogenic-induced groups revealed significantly greater red staining intensity than the control group (p < 0.05). Interestingly, when taking the two induced groups into consideration, the staining intensity was greater under hPL-supplemented conditions than under FBS-supplemented conditions. However, the data indicate no statically significant difference between these two groups (Fig. 9B).
Fig. 9.
Calcium deposition of the control group and the osteogenic-induced group under scaffold culture conditions was identified with alizarin red S staining. Arrows are used to represent deposited calcium, while arrowheads are used to represent the scaffold (A). In the graph, the staining intensities of the cells cultured with pure DMEM, and the osteogenic-induced medium supplemented with 10% FBS and 10% hPL under scaffold culture conditions, are compared. One-way ANOVA was used to identify differences between the groups and the conditions (p < 0.05) (B). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Amniotic fluid (AF) is a clear-yellowish liquid that surrounds the fetus inside the amniotic cavity in the amnion or amniochorionic membrane. The widely used method to culture amniotic fluid cells is an ultrasound-guided transabdominal puncture, which is considered a less invasive technique [11,12]. The component in AF is mainly water and composed of heterogeneity of suspending cells. The AF cells can be classified mainly based on morphological and growth characteristics to generate three types of cells, including epitheloid (E-type) cells, amniotic fluid (AF-type) cells, and fibroblastic (F-type) cells [39]. In this study, the morphology of cells in the early period of cultivation revealed heterogeneity of cell populations. However, the non-adherent cells were removed during the 2nd passage of subculture, where they revealed the homogenous fibroblast-like cell shape that is typical of MSCs [11]. They also demonstrated the ability to adhere to and expand on the surface of the plastic culture. The previous studies examined the three types of AF cells and discovered that F-type cells had the greatest growth potential and were the easiest type to subculture [14,19,40].
The hAF-MSCs meet the criteria for mesenchymal stem cells as defined by the International Society for Cellular Therapy based on their morphology, expression of a particular surface antigen, proliferation ability, and capacity for multipotent differentiation [8].
The hAF-MSCs possess the expression of the following MSC biomarkers, including CD44 and CD73 [14,41]. On the other hand, the cells showed negative for other markers, including the platelet endothelial cells adhesion marker (CD31), hematopoietic cell surface marker (CD34 and CD45) and amniotic fluid-derived stem cells marker (CD117) [40,42]. The hAF-derived cells showed negative expression for CD117, also known as c-Kit or stem cell factor receptor. CD117 is a cell surface receptor that plays a role in cell survival, proliferation, and differentiation [43]. Furthermore, CD117 has been used as a marker to distinguish between AF-MSCs and amniotic fluid stem cells [14]. According to previous studies investigating the expression of this marker, it has been reported that CD117 is not detectable in the hAF cell population derived from the 2nd to 3rd trimester [21,[44], [45], [46]]. This aligns with our findings, where we observed no expression of the CD117 marker in the hAF-derived cells. Moreover, hAF-MSCs revealed hypoimmunogenic properties [15] as they uncovered the expression of HLA-ABC markers corresponding to MHC class I and the lack of expression of HLA-DR markers corresponding to MHC class II. MHC class I molecules were found on the cell surface of all nucleated cells in the bodies of vertebrates, while MHC class II molecules were found in only antigen presenting cells under inflammatory conditions. Due to the positive HLA-ABC expression and negative HLA-DR expression, hAF-MSCs had a significantly lower risk of allograft rejection, making them safe to be utilized in human transplantation [14,47,48]. Interestingly, the hAF-MSCs exhibited embryonic stem cell characteristics as they expressed the pluripotent marker Oct-4, which is essential for the self-renewal of stem cells [[13], [14], [15]] in agreement with earlier studies [24,49]. Oct-4 expression is not a defining characteristic of mesenchymal stem cells. Therefore, in our samples of hAF-MSCs as adult stem cells with multipotent potency, may be observed the low levels of Oct-4 expression in subpopulations of cells. It is possible to infer that the isolated hAF-MSCs exhibit multipotent potency with some pluripotent potency compared to other adult stem cells and may have the potential to be used in clinical applications based on the results of cell surface protein expressions and immunoprivileged features of the cell.
Consequently, the finding of this study was that the hAF-MSCs were also able to replicate themselves and maintain their self-renewal ability, as shown by the growth curve of the proliferation assay. The hAF-MSCs growth curve demonstrated a similar pattern to that of the previous findings, which demonstrated a short log phase before achieving its highest peak around days 11–13 and remaining plateaued afterward [21,23,45,46]. There is proof that the telomerase reverse transcriptase (TERT) in AF-MSCs shortens telomeres during cell division, causing hAF-MSCs to remain in a stable state following division [50]. Nevertheless, they were able to maintain their capacity for self-renewal.
In term of capacity for multipotent differentiation, the hAF in this study provided the evidence that they can differentiate into osteogenic, chondrogenic, and adipogenic lineages in accordance with MSCs properties, which are consistent with many previous studies [9,15,45,46].
One of the key components of tissue engineering is a scaffold that simulates the extracellular matrix's (ECM) role in bone regeneration by supporting cellular communication, oxygen and nutrient transportation, and waste elimination [26,27]. Fabricating scaffolds has made use of a variety of materials, including bioceramics and synthetic and natural polymers. However, the drawbacks of the synthetic materials, such as the emission of small particles during their degradation that may trigger inflammatory reactions, are a point of concern [[51], [52], [53]]. As bone graft materials, naturally derived materials outperform synthetic materials due to their good biocompatibility, and high affinity and compatibility with the other matrix proteins [54]. Moreover, biodegradable polymers from natural sources have been researched and used as bone graft or cartilage graft materials to repair defects in a range of orthopedic and dental applications [28]. Gelatin, a natural biopolymer generated from animal collagen, has shown to have a chemical structure similar to collagen. When compared to collagen, its simplicity of extraction and preparation results in a cheaper and higher quantity of production. Furthermore, gelatin does not produce hazardous byproducts during their degradation and does not express any antigenicity or immunogenicity in physiological conditions [29,52,55]. Moreover, gelatin contains RGD sequences that act as a location for cell attachment and increase the binding affinity of the integrin molecule on cell membrane [56]. In many earlier studies, gelatin was used as a component of the scaffold, demonstrating not only its ability to support cell adhesion but also its propensity to imitate real bone tissue and encourage osteogenesis both in vitro and in vivo [45,[57], [58], [59], [60]].
In this study, the apheresis technique was used to obtain the platelet concentrates (PC). Several studies state that this method has considerable benefits owing to the high platelet concentration and low leukocyte contamination levels that are related to it [[61], [62], [63]]. Moreover, the release of the growth factors from PC was accomplished using the freeze and thaw technique. Many studies have verified that this method is fast and effective with no contaminants caused by chemical activators [64,65].
In addition, the MTT results revealed that after 72 h of cultivation, cell viability was significantly greater in the 10%, 20%, and 40% hPL-supplemented groups than in the control group; however, there was no significant difference in cell viability in the 10% and 20% hPL groups. The results indicated that a minimum hPL concentration can effectively increase cell viability. So, from the MTT results, the hPL concentration between 10% and 20% appeared to be the optimal percentage to supplement in the culture media. After undergoing alamar blue proliferation analysis in order to confirm the effect of 10% and 20% hPL-supplemented media in prolonged cell culture, it was found that the hAF-MSCs could proliferate under hPL-supplemented conditions, and there were no significant differences in terms of proliferation capacity between 10% and 20% hPL-supplemented media, in accordance with previous studies [24,66]. Several earlier studies on the impacts of hPL on various sources of MSC, such as stem cells from bone marrow or dental tissue, discovered that the optimal concentration of hPL varied from 5% to 10% depending on the MTT experiment of each study [38,67,68]. Based on the obtained data from this study, the minimum effective concentration at 10% hPL was selected in order to investigate the ability of hAF-MSCs to differentiate into osteoblasts.
In this study, osteogenic differentiation of hAF-MSCs using osteogenic-induced media supplemented with hPL was achievable. Following 14 days of induction, the functionality of differentiated cells toward osteoblastic-like cells was evaluated by measuring the expression of osteoblastogenic-specific genes, including levels of RUNX2, OCN, ALP, and COL1A1 which are crucial for the process of bone mineralization. RUNX2 can regulate osteogenesis through activating genes of many osteogenic-related signaling pathways [69]. Osteoblasts primarily secrete osteocalcin (OCN), which has a strong affinity to bind Ca2+. Osteoblasts also release membrane-bounded matrix vesicles containing alkaline phosphatase (ALP). These enzymes have the ability to remove PO4− ions from various macromolecules in the bone matrix. The high local concentrations of both Ca2+ and PO4− ions can accumulate and mineralize to calcium hydroxyapatite crystals. These crystals develop and become embedded in the gap junction of the collagen fibers (COL1A1) [70]. RT-qPCR results demonstrated that the expressions of four osteoblastogenic-related genes were higher in both the FBS and hPL-supplemented osteogenic-induced groups when compared to the non-induced group. Interestingly, when compared to the control and FBS conditions, the hPL showed a high capacity to produce functional osteoblasts by showing the highest expression of OCN, ALP, and COL1A1, with the greatest ALP activity level. In addition, the calcium ions were bound and deposited in the ECM, and the group that had received hPL supplementation for the osteogenic-induced condition also showed the highest level of intensity as determined by the alizarin red S staining procedure. However, the expression of RUNX2 exhibited a distinct pattern compared to other genes in the hPL-supplemented group. This disparity could be attributed to the fact that RUNX2 plays a role in initiating early osteoprogenitor differentiation and proliferation [71], while other genes such as OCN, COL1A1, and ALP are expressed at a later stage. However, since our RT-qPCR analysis specifically focused on the expression of osteogenic-specific genes on day 14. Therefore, it is possible that the level of RUNX2 was relatively low compared to the other genes, which exhibited increased expression at that particular time point.
These findings indicate that hPL, in particular, contains the platelet's alpha granules, composed of a diverse range of growth factors [34,35,72] that affect bone in many ways [34,35,37,38]. There is evidence that hPL factors can regulate osteogenesis through RUNX2-and OSX-independent pathways [37,73] in order to form bone cells and express certain osteogenic genes, respectively. Some growth factors, including BMP2-4-6, TGF-1, bFGF, IGF, PF-4, Interleukin-1, and osteonectin, have an osteo-inductive action [72,74,75]. Similarly, various growth factors in hPL play a role in bone formation during human development, both intramembranous and endochondral types [36]. Moreover, a study by Du et al. revealed that hPL possesses a molecular mechanism that controls the metabolism and activities of MSCs, encouraging MSC proliferation. This indicates that it can be a useful biological material for stem cell engineering and regenerative medicine [76]. Similarly, Caas-Arboleda et al. verified that hPL is an appropriate supply of factors that support the survivability, stability, and potency of WJ-MSC, offering significant possibilities for the development of effective regenerative medicine [77].
The effects of hPL as a medium supplement on various MSC sources and various biomimetic scaffold materials have been the subject of many previous studies. The study by Jafar et al. investigating osteogenic differentiation of SCAP and PDLSCs cultured on PLGA scaffolds in culture medium containing 5% hPL showed significant expressions of bone markers, calcium deposition, and ALP activity during the osteogenic differentiation period [38]. Similarly, based on Atari et al. and Chen et al. DPSCs seeded on hydroxyapatite-tricalcium phosphate (HA/TCP) or on Cell Carrier glass scaffold in culture medium containing 5% hPL significantly promoted the osteogenic differentiation of DPSCs, as indicated by measurement of ALP activity and calcium mineralization [78,79]. Furthermore, Kirsch et al. and Santo et al. revealed that the addition of hPL to the 3D culture system enabled the effective osteogenic differentiation of hAD-MSCs [80,81]. Despite the differences in MSC sources and scaffold materials, the majority of the researchers agreed that the culture in three-dimensional system with hPL have similar capability to support in the osteogenic differentiation of MSCs.
5. Conclusion
This present study has revealed that hAF-MSCs fulfilled the characteristics of MSCs, including the expression of MSCs specific markers, their proliferation ability, and their capacity for multipotent differentiation. They could then be potentially differentiated into osteoblast-like cells. Furthermore, the results of this study indicate that 10% hPL could be used as an efficient supplement for animal-free cell culture, which supports the ability of hAF-MSCs to differentiate into osteoblasts when seeded in a three-dimensional scaffold. It appears that a cell, a scaffold, and a growth supplement complete the optimum combination required in the healing of bone defects, paving the way for the creation of an alternate construct for repairing bone defects. Notably, they could also be significantly beneficial for use in the future development of other important medications.
Author contribution statement
Kantirat Yaja: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sirinda Aungsuchawan, Suteera Narakornsak: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Peraphan Pothacharoen, Rungusa Pantan, Waleephan Tancharoen: Conceived and designed the experiments.
Funding statement
Sirinda Aungsuchawan was supported by Faculty of Medicine, Chiang Mai University and Faculty of Medicine, Chiang Mai University ANA-2564-08540. Kantirat Yaja was supported by CMU Presidential Scholarship for PhD program in Anatomy, Faculty of Medicine, Chiang Mai University and Faculty of Medicine, Chiang Mai University ANA-2564-08540.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18599.
Contributor Information
Sirinda Aungsuchawan, Email: sirinda.a@cmu.ac.th.
Suteera Narakornsak, Email: suteera.n@cmu.ac.th.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mistry A.S., Mikos A.G. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 3.Waese E.Y., Kandel R.A., Stanford W.L. Application of stem cells in bone repair. Skeletal Radiol. 2008;37:601–608. doi: 10.1007/s00256-007-0438-8. [DOI] [PubMed] [Google Scholar]
- 4.Khaled E.G., Saleh M., Hindocha S., Griffin M., Khan W.S. Tissue engineering for bone production- stem cells, gene therapy and scaffolds. Open Orthop. J. 2011;5(Suppl 2):289–295. doi: 10.2174/1874325001105010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxson S., Lopez E.A., Yoo D., Danilkovitch-Miagkova A., Leroux M.A. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waese E.Y.L., Kandel R.R., Stanford W.L. Application of stem cells in bone repair. Skeletal Radiol. 2008;37:601–608. doi: 10.1007/s00256-007-0438-8. [DOI] [PubMed] [Google Scholar]
- 7.Rastegar F., Shenaq D., Huang J., Zhang W., Zhang B.Q., He B.C., et al. Mesenchymal stem cells: molecular characteristics and clinical applications. World J. Stem Cell. 2010;2:67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement, Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Pipino C., Pandolfi A. Osteogenic differentiation of amniotic fluid mesenchymal stromal cells and their bone regeneration potential. World J. Stem Cell. 2015;7:681–690. doi: 10.4252/wjsc.v7.i4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cell. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 11.Savickiene J., Treigyte G., Baronaite S., Valiuliene G., Kaupinis A., Valius M., et al. Human amniotic fluid mesenchymal stem cells from second- and third-trimester amniocentesis: differentiation potential, molecular signature, and proteome analysis. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/319238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdulrazzak H., Moschidou D., Jones G., Guillot P.V. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J. R. Soc. Interface. 2010;7(Suppl 6):S689–S706. doi: 10.1098/rsif.2010.0347.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi B., Merlo B., Colleoni S., Iacono E., Tazzari P.L., Ricci F., et al. Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem Cell Rev Rep. 2014;10:712–724. doi: 10.1007/s12015-014-9525-0. [DOI] [PubMed] [Google Scholar]
- 14.De Coppi P., Bartsch G., Siddiqui M.M., Xu T., Santos C.C., Perin L., et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 15.Siegel N., Rosner M., Hanneder M., Valli A., Hengstschläger M. Stem cells in amniotic fluid as new tools to study human genetic diseases. Stem Cell Rev. 2007;3:256–264. doi: 10.1007/s12015-007-9003-z. [DOI] [PubMed] [Google Scholar]
- 16.In 't Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., Noort W.A., Claas F.H., Willemze R., et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 17.Tsai M.S., Lee J.L., Chang Y.J., Hwang S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004;19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 18.Kim J., Lee Y., Kim H., Hwang K.J., Kwon H.C., Kim S.K., et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J., Wang D., Liang T., Guo Q., Zhang G. Amniotic fluid-derived mesenchymal stem cells: characteristics and therapeutic applications. Arch. Gynecol. Obstet. 2014;290:223–231. doi: 10.1007/s00404-014-3231-7. [DOI] [PubMed] [Google Scholar]
- 20.Ochiai D., Masuda H., Abe Y., Otani T., Fukutake M., Matsumoto T., et al. Human amniotic fluid stem cells: therapeutic potential for perinatal patients with intractable neurological disease. Keio J. Med. 2018;67:57–66. doi: 10.2302/kjm.2017-0019-IR. [DOI] [PubMed] [Google Scholar]
- 21.Markmee R., Aungsuchawan S., Tancharoen W., Narakornsak S., Pothacharoen P. Differentiation of cardiomyocyte-like cells from human amniotic fluid mesenchymal stem cells by combined induction with human platelet lysate and 5-azacytidine. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Liu D.Q., Li B.W., Guan L.D., Yan Z.F., Li Y.L., et al. Human amniotic fluid-derived stem cells can differentiate into hepatocyte-like cells in vitro and in vivo, in Vitro Cell. Dev Biol Anim. 2011;47:601–608. doi: 10.1007/s11626-011-9450-3. [DOI] [PubMed] [Google Scholar]
- 23.Tancharoen W., Aungsuchawan S., Pothacharoen P., Markmee R., Narakornsak S., Kieodee J., et al. Differentiation of mesenchymal stem cells from human amniotic fluid to vascular endothelial cells. Acta Histochem. 2017;119:113–121. doi: 10.1016/j.acthis.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Tancharoen W., Aungsuchawan S., Pothacharoen P., Bumroongkit K., Puaninta C., Pangjaidee N., et al. Human platelet lysate as an alternative to fetal bovine serum for culture and endothelial differentiation of human amniotic fluid mesenchymal stem cells. Mol. Med. Rep. 2019;19:5123–5132. doi: 10.3892/mmr.2019.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadia S., Alessia P., Marina P., Federica B., Massimo M., Valentina R., et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339–346. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- 26.Chocholata P., Kulda V., Babuska V. Fabrication of scaffolds for bone-tissue regeneration. Materials. 2019;12 doi: 10.3390/ma12040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C., Tan A., Pastorin G., Ho H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013;31:654–668. doi: 10.1016/j.biotechadv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Damien C.J., Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J. Appl. Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 29.Sela M., Arnon R. Studies on the chemical basis of the antigenicity of proteins. 1. Antigenicity of polypeptidyl gelatins. Biochem. J. 1960;75:91–102. doi: 10.1042/bj0750091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gstraunthaler G., Lindl T., van der Valk J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65:791–793. doi: 10.1007/s10616-013-9633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnouf T., Strunk D., Koh M.B., Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 32.Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus. Med. Hemotherapy. 2013;40:326–335. doi: 10.1159/000354061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemeda H., Giebel B., Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Schallmoser K., Bartmann C., Rohde E., Reinisch A., Kashofer K., Stadelmeyer E., et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 35.Schallmoser K., Strunk D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol. Biol. 2013;946:349–362. doi: 10.1007/978-1-62703-128-8_22. [DOI] [PubMed] [Google Scholar]
- 36.Solheim E. Growth factors in bone. Int. Orthop. 1998;22:410–416. doi: 10.1007/s002640050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chevallier N., Anagnostou F., Zilber S., Bodivit G., Maurin S., Barrault A., et al. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2010;31:270–278. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 38.Jafar H., Abuarqoub D., Ababneh N., Hasan M., Al-Sotari S., Aslam N., et al. hPL promotes osteogenic differentiation of stem cells in 3D scaffolds. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bossolasco P., Montemurro T., Cova L., Zangrossi S., Calzarossa C., Buiatiotis S., et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- 40.Rosner M., Schipany K., Shanmugasundaram B., Lubec G., Hengstschläger M. Amniotic fluid stem cells: future perspectives. Stem Cell. Int. 2012;2012 doi: 10.1155/2012/741810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.T L.R., Sánchez-Abarca L.I., Muntión S., Preciado S., Puig N., López-Ruano G., et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cananzi M., De Coppi P. CD117(+) amniotic fluid stem cells: state of the art and future perspectives. Organogenesis. 2012;8:77–88. doi: 10.4161/org.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miettinen M., Lasota J. Kit (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 44.Vadasz S., Jensen T., Moncada C., Girard E., Zhang F., Blanchette A., et al. Second and third trimester amniotic fluid mesenchymal stem cells can repopulate a de-cellularized lung scaffold and express lung markers. J. Pediatr. Surg. 2014;49:1554–1563. doi: 10.1016/j.jpedsurg.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Laowanitwattana T., Aungsuchawan S., Narakornsak S., Markmee R., Tancharoen W., Keawdee J., et al. Osteoblastic differentiation potential of human amniotic fluid-derived mesenchymal stem cells in different culture conditions. Acta Histochem. 2018;120:701–712. doi: 10.1016/j.acthis.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Narakornsak S., Poovachiranon N., Peerapapong L., Pothacharoen P., Aungsuchawan S. Mesenchymal stem cells differentiated into chondrocyte-Like cells. Acta Histochem. 2016;118:418–429. doi: 10.1016/j.acthis.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Rockett J.C., Darnton S.J., Crocker J., Matthews H.R., Morris A.G. Expression of HLA-ABC, HLA-DR and intercellular adhesion molecule-1 in oesophageal carcinoma. J. Clin. Pathol. 1995;48:539–544. doi: 10.1136/jcp.48.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X., Zhang Z., Feng J.Q., Dusevich V.M., Sinha K., Zhang H., et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phermthai T., Odglun Y., Julavijitphong S., Titapant V., Chuenwattana P., Vantanasiri C., et al. A novel method to derive amniotic fluid stem cells for therapeutic purposes. BMC Cell Biol. 2010;11:79. doi: 10.1186/1471-2121-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trohatou O., Anagnou N.P., Roubelakis M.G. Human amniotic fluid stem cells as an attractive tool for clinical applications. Curr. Stem Cell Res. Ther. 2013;8:125–132. doi: 10.2174/1574888x11308020003. [DOI] [PubMed] [Google Scholar]
- 51.Petrochenko P., Narayan R.J. Novel approaches to bone grafting: porosity, bone morphogenetic proteins, stem cells, and the periosteum. J. Long Term Eff. Med. Implants. 2010;20:303–315. doi: 10.1615/jlongtermeffmedimplants.v20.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoque M.E., Nuge T., Tshai K.Y., Nordin N., Prasad V. Gelatin based scaffolds for tissue engineering – a review. Polym. Res. J. 2015;9:15–32. [Google Scholar]
- 53.Campana V., Milano G., Pagano E., Barba M., Cicione C., Salonna G., et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014;25:2445–2461. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohanizadeh R., Swain M.V., Mason R.S. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J. Mater. Sci. Mater. Med. 2008;19:1173–1182. doi: 10.1007/s10856-007-3154-y. [DOI] [PubMed] [Google Scholar]
- 55.Chen S., Zhang Q., Nakamoto T., Kawazoe N., Chen G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng. C Methods. 2015;22:189–198. doi: 10.1089/ten.TEC.2015.0281. [DOI] [PubMed] [Google Scholar]
- 56.DeVolder R., Kong H.J. Hydrogels for in vivo-like three-dimensional cellular studies. Wiley Interdiscip Rev Syst Biol Med. 2012;4:351–365. doi: 10.1002/wsbm.1174. [DOI] [PubMed] [Google Scholar]
- 57.Chen W., Zhou H., Weir M.D., Tang M., Bao C., Xu H.H. Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement-chitosan-RGD scaffold for bone repair. Tissue Eng. 2013;19:915–927. doi: 10.1089/ten.tea.2012.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y., Jiang Y., Liu Q., Gao T., Feng J.Q., Dechow P., et al. Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng. 2013;19:1754–1763. doi: 10.1089/ten.tea.2012.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamalpoor Z., Soleimani M., Taromi N., Asgari A. Comparative evaluation of morphology and osteogenic behavior of human Wharton's jelly mesenchymal stem cells on 2D culture plate and 3D biomimetic scaffold. J. Cell. Physiol. 2019;234:23123–23134. doi: 10.1002/jcp.28876. [DOI] [PubMed] [Google Scholar]
- 60.Yang S.-H., Hsu C.-K., Wang K.-C., Hou S.-M., Lin F.-H. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein—a viable scaffold for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005;74B:468–475. doi: 10.1002/jbm.b.30200. [DOI] [PubMed] [Google Scholar]
- 61.Singh R.P., Marwaha N., Malhotra P., Dash S. Therapeutic efficacy of different types of platelet concentrates in thrombocytopenic patients. Indian J Hematol Blood Transfus. 2008;24:16–22. doi: 10.1007/s12288-008-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh R.P., Marwaha N., Malhotra P., Dash S. Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J. Transfus. Sci. 2009;3:86–94. doi: 10.4103/0973-6247.53882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmermann R., Jakubietz R., Jakubietz M., Strasser E., Schlegel A., Wiltfang J., et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–1224. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]
- 64.Strandberg G., Sellberg F., Sommar P., Ronaghi M., Lubenow N., Knutson F., et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57:1058–1065. doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 65.Rauch C., Feifel E., Amann E.-M., Spötl H., Schennach H., Pfaller W., et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28:305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 66.Fekete N., Gadelorge M., Fürst D., Maurer C., Dausend J., Fleury-Cappellesso S., et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abuarqoub D., Awidi A., Abuharfeil N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Arch. Oral Biol. 2015;60:1545–1553. doi: 10.1016/j.archoralbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Lucarelli E., Beccheroni A., Donati D., Sangiorgi L., Cenacchi A., Del Vento A.M., et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 69.James A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica. 2013;2013 doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murshed M. Mechanism of bone mineralization. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komori T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 72.van den Dolder J., Mooren R., Vloon A.P., Stoelinga P.J., Jansen J.A. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12:3067–3073. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- 73.Kärner E., Bäckesjö C.M., Cedervall J., Sugars R.V., Ahrlund-Richter L., Wendel M. Dynamics of gene expression during bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Biochim. Biophys. Acta. 2009;1790:110–118. doi: 10.1016/j.bbagen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Koch H., Jadlowiec J.A., Campbell P.G. Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cell. Dev. 2005;14:621–631. doi: 10.1089/scd.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 75.Marx R.E., Carlson E.R., Eichstaedt R.M., Schimmele S.R., Strauss J.E., Georgeff K.R. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 76.Du P., Tao X., Liu K., Lin J., Shi Y., Park K., et al. Human platelet lysate (hPL) alters the lineage commitment and paracrine functions of human mesenchymal stem cells via mitochondrial metabolism. Appl. Mater. Today. 2022;26 [Google Scholar]
- 77.Cañas-Arboleda M., Beltrán K., Medina C., Camacho B., Salguero G. Human platelet lysate supports efficient expansion and stability of wharton's jelly mesenchymal stromal cells via active uptake and release of soluble regenerative factors. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atari M., Gil-Recio C., Fabregat M., García-Fernández D., Barajas M., Carrasco M.A., et al. Dental pulp of the third molar: a new source of pluripotent-like stem cells. J. Cell Sci. 2012;125:3343–3356. doi: 10.1242/jcs.096537. [DOI] [PubMed] [Google Scholar]
- 79.Chen B., Sun H.H., Wang H.G., Kong H., Chen F.M., Yu Q. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials. 2012;33:5023–5035. doi: 10.1016/j.biomaterials.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 80.Kirsch M., Rach J., Handke W., Seltsam A., Pepelanova I., Strauß S., et al. Comparative analysis of mesenchymal stem cell cultivation in fetal calf serum, human serum, and platelet lysate in 2D and 3D systems. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.598389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santos S.C., Custódio C.A., Mano J.F. Photopolymerizable platelet lysate hydrogels for customizable 3D cell culture platforms. Advanced healthcare materials. 2018;7 doi: 10.1002/adhm.201800849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.