Abstract
Introduction and importance
Isthmocele is identified as an iatrogenic defect in the myometrium of the anterior uterine wall at the site of a previous cesarean scar due to defective tissue healing. Patients may have varied symptoms including abnormal uterine bleeding (AUB) and pelvic pain.
Herein, we report a rare case of a large isthmocele that manifested with secondary amenorrhea; which was not reported in the medical literature previously.
Case presentation
A 30-year-old Syrian woman, G5P5, came to our clinic with a complaint of secondary amenorrhea that began two years ago. She was treated symptomatically with progesterone with no response. She has had five cesarean sections. Ultrasonography findings suggested a large uterine niche. Trans-Abdominal niche repair was the obtained technique, depending on the drainage of the isthmocele, excising the fibrotic tissue from the edges and re-approximating them. On follow-up, menstruation returned to normal.
Clinical discussion
Isthmocele can be, radiologically, defined as a hypoechoic or anechoic, triangular area at the scar site. Its pathophysiology is still unknown. Although, an isthmocele can be diagnosed using a variety of imaging techniques like ultrasonography (US), magnetic resonance imaging (MRI), sonohysterography, and hysteroscopy; transvaginal ultrasound (TVUS) is the first method described for assessing it.
The goal of isthmocele treatment is to alleviate symptoms.
Conclusion
We recommend that health awareness campaigns alert people to the need to see a specialist doctor in the context of a serious complaint. For the uterine niche, many risk factors can be avoided to reduce its probability.
Keywords: Cesarean, Isthmocele, Niche, Amenorrhea, Case report
Highlights
-
•
For the uterine niche, many risk factors can be avoided to reduce its probability.
-
•
The main symptom was secondary amenorrhea which was not reported.
-
•
For doctors; electing the appropriate indication for cesarean.
1. Introduction
Cesarean section (CS) is one of the most common surgical operations worldwide and its rate is growing dramatically to form one-third of all deliveries (1). With this uncontrolled increase in CS deliveries, the early and late complications of this procedure also increase. One of these complications is the isthmocele. Isthmocele or uterine niche, or cesarean scar defect (CSD) is identified as an iatrogenic defect in the myometrium of the anterior uterine wall at the site of previous cesarean scar due to defective tissue healing. Sometimes it is described as myometrial thinning (2). The prevalence of isthmocele varies due to diagnostic techniques. Tulandi et al. have reported that in transvaginal ultrasound (TVUS) examination, the prevalence ranges between 24 % to 70 %, while in sonohysterography (SHG) examination the prevalence ranges from 56 % to 84 % (3,4). The etiology is still unclear but many risk factors were determined such as multiple CS (3), long duration of labor, cervical dilatation, stage of the presenting part, and low uterine incision (1). Patients may have varied symptoms including abnormal uterine bleeding (AUB), pelvic pain, post-menstrual spotting, and infertility, though many women may be asymptomatic and diagnosed incidentally (2,5).
The diagnosis is made by sonography (transvaginal and transabdominal), saline instillation SHG, or magnetic resonance imaging (MRI) (4,5). The optimal treatment differs from one patient to another. Treatment options range widely from medical treatment to hysterectomy (5).
This manuscript has been reported in line with SCARE's 2020 Criteria (6).
Herein, we report a rare case of a large isthmocele that manifested with secondary amenorrhea; which was not reported in the medical literature previously.
2. Presentation of case
A 30-year-old Syrian woman, G5P5, came to our private clinic with a complaint of secondary amenorrhea and chronic pelvic pain. Her complaint began after the last cesarean section which was two years ago. Pelvic pain was moderate, colic, and non-responsive to analgesics. For secondary amenorrhea, she was treated symptomatically with progesterone to perform withdrawal bleeding; without undergoing any diagnostic tests; but with no response. She is a smoker with no medical history. She has no family history of a similar problem. She has had five cesarean sections. Physical examination and laboratory tests were unremarkable.
TVUS of the pelvis showed a cystic structure with a thick wall and turbid content on the front face of the uterus at the site of the CS scar, behind the bladder. The structure measured (57 × 38 × 32) mm and connected with the uterine cavity by a 10 mm duct. The residual myometrium (RMT) was <3 mm. These findings suggested a large uterine niche at the site of the CS. No additional investigations were obtained.
As the patient underwent failed medical treatment previously, surgery was the treatment of choice. The hysteroscopic and laparoscopic approaches were excluded due to three factors: the expected adhesions due to five past CS, high costs, and low resources in our country after the war. On the other hand, laparotomy was preferred due to the large size of the defect and RMT < 3 mm.
Trans-Abdominal niche repair was the obtained technique. First, Hegar's dilator and intracervical Foley catheter were applied by US guidance to achieve better identification of the defect.
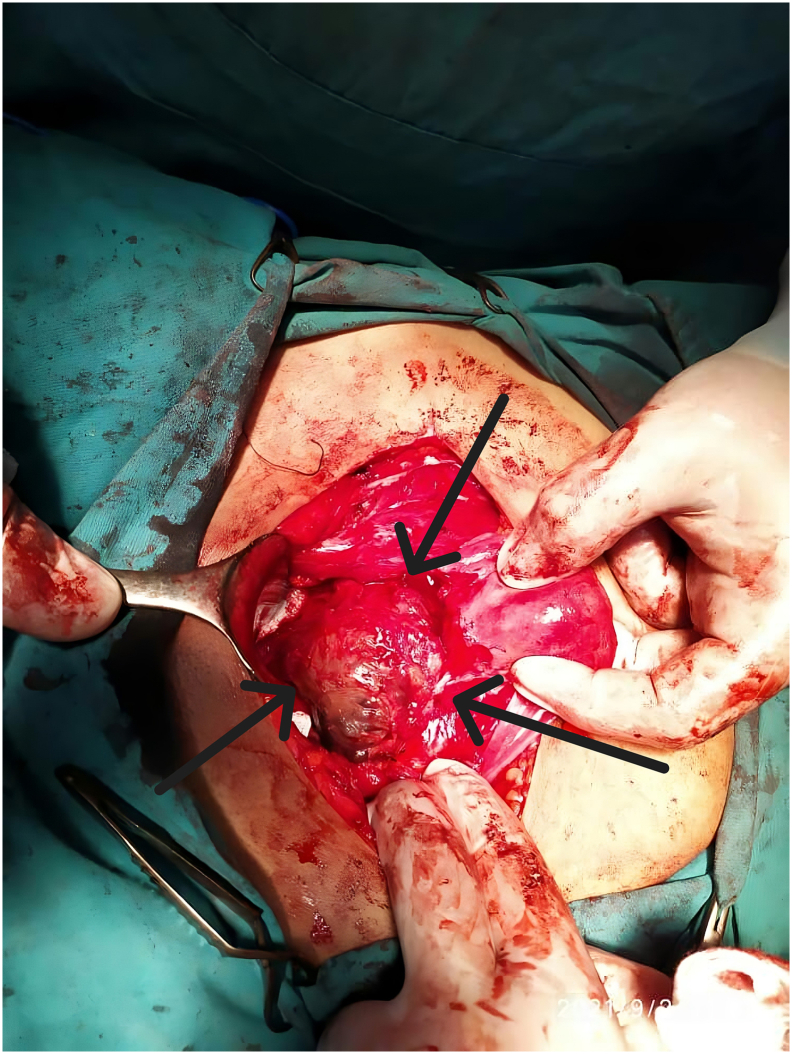
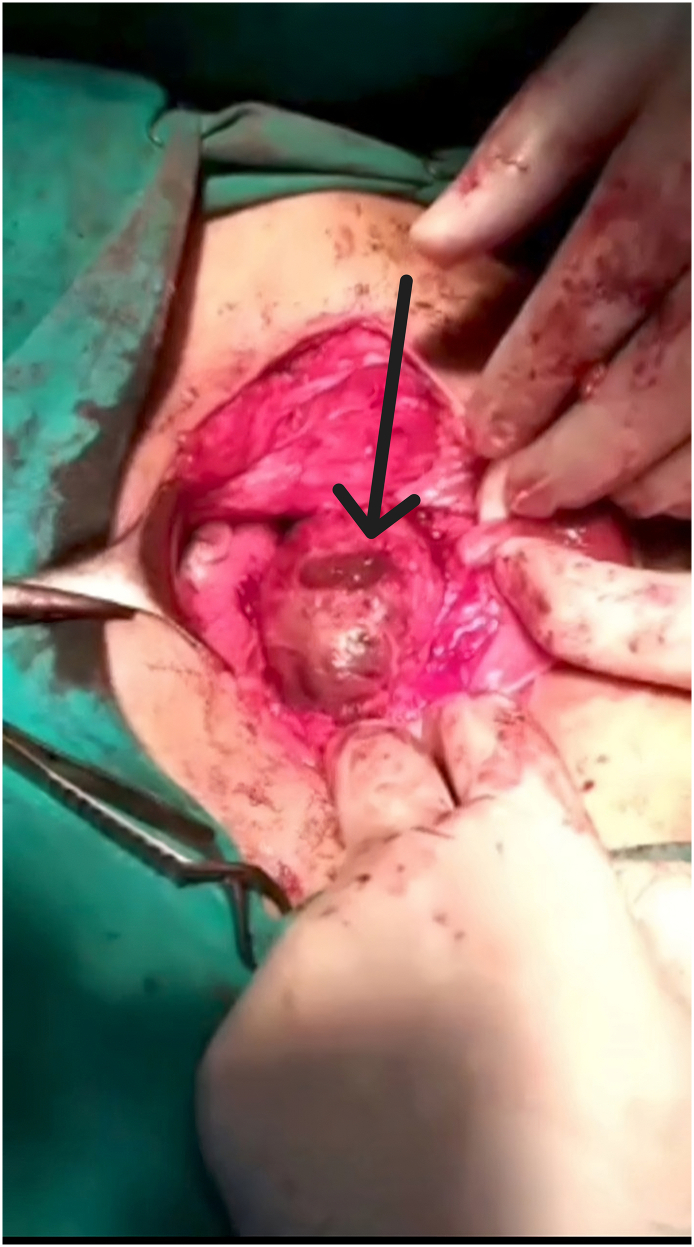
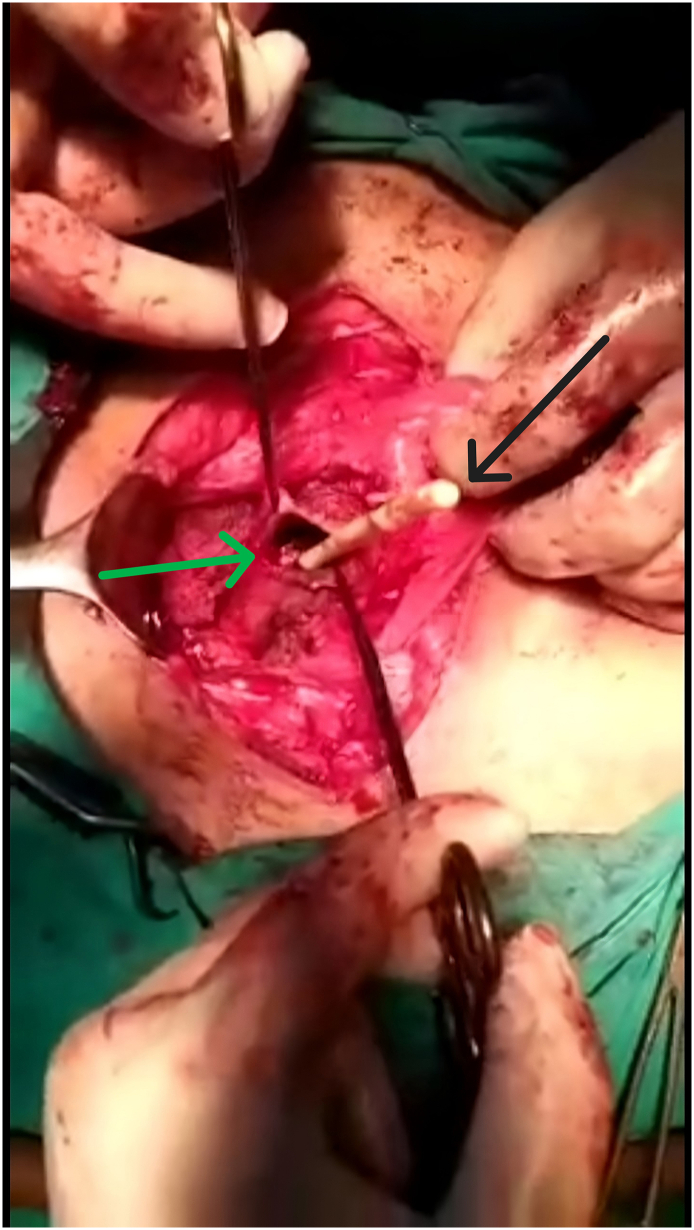
After the abdomen was opened, the bladder was dissected from the anterior face of the uterus where the isthmocele was located (Fig. 1). The isthmocele was drained (Fig. 2, Fig. 3) by a longitudinal incision. Fibrotic tissue from the edges was excised and reapproximated in 2 Layers.
Fig. 1.
A large isthmocele that measures 5 cm in diameter, before drainage.
Fig. 2.
The discharge of the isthmocele content after a longitudinal incision on it.
Fig. 3.
The green arrow indicates the isthmocele margin. The black arrow demonstrates a Foley catheter inside the isthmocele. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
On follow-up, pelvic pain was resolved and menstruation returned to normal two months after the operation. The RMT significantly increased.
3. Discussion
While the optimal CS rate recommended by The World Health Organization (WHO) is around 15 % (7), the current rate is increasing rapidly with prevalence ranging between 6 and 27.2 % according to a study including data from 150 countries (8,9).
Although Cesarean incisions recover well, it has complication as any other surgical procedure (10).
CSD is one of the complications which is also called niche, isthmocele, diverticulum, or pouch. Poidevin in 1961, first described isthmocele (11).
Radiologically, isthmocele can be defined as a hypoechoic or anechoic, triangular area at the scar site (4,12).
The pathophysiology of the uterine niche is still unknown, although several risk factors were reported (1,7,13).
Vervoort et al. have reported four hypotheses about the etiology. The first hypothesis focuses on the site of the hysterotomy. This hypothesis proposes that the lower uterine incision on its cervical part is associated with a higher incidence of isthmocele. This is explained by the presence of mucous glands in this part where the incision was made. These glands produce mucus during the healing time, which could dilate the sutured rims of the myometrium. This can be seen in cases of longer active labor prior to emergency cesarean (7).
The second hypothesis concerns the surgical technique. The invalid closure of the deeper muscular layer or an incomplete closure could be responsible for CSD formation and development. The third hypothesis proposes that impaired wound healing, and attracting the rims of the wound due to early adhesion formation between the hysterotomy scar and the anterior abdominal wall are the essential causes of isthmocele (7,14).
The fourth hypothesis relates to patient factors, such as individual/genetic predisposition, and post-operative infection (7). The details for the past five cesarean sections of our patient; the indication, location of the incision, and uterine closure technique; were not available, so we could not favor one hypothesis over another.
The principal risk factor is multiple CS (3), as in our case. There are several other risk factors such as duration of labor, cervical dilatation, stage of the presenting part, and low uterine incision that accompany lesser vascularized myometrium resulting in inadequate healing (1,15). Retroflexed Uterus is also an important risk factor, as gravity increases the counteracting forces on the uterus (1,7). The uterus in our case was anteflexed.
In some cases, the symptoms could be present because of the size of the defect (3).
Abnormal uterine bleeding (AUB) is the most common symptom which usually presents as postmenstrual bleeding (3). But, in our case, the main symptom was secondary amenorrhea which was not reported in the medical literature.
Accumulation of blood and menstrual debris within the defect may be predisposed by the presence of an isthmocele, which is associated with decreased uterine contractility due to fibrotic tissue around the scar. This accumulation slows menstrual flow leading to AUB (4,16).
Morris (17) suggests, based on the pathology findings of free erythrocytes in the scar tissue, that there was a recent hemorrhage and that the blood could also have been produced in situ, causing intermittent spotting. No matter what the source, the presence of blood in the isthmocele is likewise connected with a higher mucus emission, which could add to postmenstrual AUB (18).
Patients with CSD may also suffer from a significant problem in the form of infertility. The lower ripeness rate may be connected with the constancy of menstrual blood in the pocket, which influences the cervical mucus, as well as sperm motility and implantation (1,19).
Several symptoms were recorded in the literature, such as dysmenorrhea and pelvic pain (13).
Usually, many obstetric complications occur during pregnancy, associated with the presence of an isthmocele. These obstetric complications include uterine rupture, placenta previa, and scar dehiscence (20).
A cesarean scar ectopic pregnancy, which occurs in almost 1 in 1,886 to 2,216 pregnancies, is another complication that has been reported (19).
The walls of the isthmocele may rupture during the development of the fetus and the gestational sac, resulting in the known severe complications of an ectopic pregnancy (13,19).
Until now, there are no clear criteria for the diagnosis of isthmocele (3,13,21).
The anterior uterine wall can be evaluated and an isthmocele can be diagnosed using a variety of imaging techniques like US, MRI, SHG, hysterography, and hysteroscopy (22).
The first and most common method described for assessing the integrity of the uterine wall in non-pregnant patients is TVUS (13,22), as in our case.
Because postmenstrual bleeding is the primary symptom, the early proliferative phase best demonstrates the blood inside the isthmocele, making its identification possible even without saline infusion (3).
On TVUS, the defect has been identified as either a deformity (wedge, shape, concavity, or sacculation) on the anterior isthmus or an anechoic triangle defect in the myometrium with the base communicating with the uterine cavity (23,24).
Using six shapes to describe the defect, Bij de Vaate et al. (4) proposed a more systematic classification: droplet, cyst, semicircle, rectangle, circle, and triangle.
In the evaluation of isthmocele, the most useful discriminating measurement is the residual myometrium thickness (25).
In addition, patients who have had two or more previous CSs have a scar that is thinner. The scar is thicker in those who had their last CS more than two years ago (26).
The goal of isthmocele treatment is to alleviate symptoms. As a result, cases without symptoms should not be treated (27).
Medical treatment is an option, though surgery is the most common choice. Surgical options include laparotomy, vaginal repair, hysteroscopy, and laparoscopy (including robotic laparoscopy) (28).
For symptomatic ladies who would rather not get pregnant and favor a conservative treatment, oral contraceptive pills could be the chance of choice (29).
The leftover myometrial thickness is the principal parameter to carry out hysteroscopy. Furthermore, if the myometrium thickness at the site of the defect is <3 mm, the hysteroscopic approach may result in bladder injury and uterine perforation (30).
If there are symptoms and a desire to preserve fertility, a trans-abdominal approach (laparotomy, laparoscopic, robotic) has been recommended for large defects (RM <3 mm) (5).
In trans-abdominal isthmocele repair, the edges of the isthmocele are cut off to remove the scar tissue, and two-layer sutures are used to close the defect (3).
The trans-abdominal approach makes it easier to see where the problem is, which makes it possible to repair it and makes the myometrium thicker (14).
In a retrospective study, Zhang found that the transvaginal repair and the laparoscopic approach produced comparable outcomes. Moreover, It was reported that the transvaginal isthmocele repair was less expensive, required less time to perform, and was comparable to laparoscopy in terms of effectiveness (31). For our patient, laparoscopy could not be conducted due to high costs and low resources. Also, the transvaginal approach was not obtained due to the large size of the isthmocele and our available surgical abilities on the trans-abdominal approach are more qualified than the transvaginal ones.
Hysterectomy is the definitive treatment for symptomatic patients who have finished their reproductive life (22).
4. Conclusion
Symptomatic treatment is a common act in developing countries. Maybe, it is acceptable due to expensive diagnostic tests in these countries, but to a specific limit. Long symptomatic management with no response can aggravate small problems which can be resolved with lesser interventions. To limit this problem, we recommend that health awareness campaigns alert people to the need to see a specialist doctor in the context of a serious or long complaint.
In addition, for the uterine niche, some risk factors can be avoided by the patient and the doctor. For the patient, cesarean planning is effective. For doctors; electing the appropriate indication for cesarean, the best site for uterine incision, and the best technique for uterine closure; all these points can reduce uterine niche probability.
Abbreviations
- CSD
Cesarean scar defect
- CS
Cesarean section
- TVUS
Transvaginal ultrasound
- SHG
Sonohysterography
- AUB
Abnormal uterine bleeding
- MRI
Magnetic resonance imaging
- US
Ultrasound
- RMT
Residual myometrium
- WHO
World Health Organization
CRediT authorship contribution statement
Basel Al-Ghotani: contributed to drafting, reviewing, and editing.
Nafiza Martini: contributed to drafting, reviewing, and editing.
Ebaa Alabdallah: contributed to drafting, reviewing, and editing.
Ieman Alawad: contributed to data collecting, reviewing and editing.
Khaled Hussien: is the mentor. Contributed to reviewing and Supervising.
All authors read and approved the final manuscript.
Sources of funding
Not applicable.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer reviewed.
Registration of research studies
N/A.
Ethics approval
Ethical approval for this study was provided by the Ethical Committee of Damascus University (ID number: 4369), Damascus, Syria on 30 June 2022.
Declaration of competing interest
All the authors declared that they have no conflicts of interest.
Acknowledgments
Acknowledgement
We wish to show our appreciation to Stemosis for Scientific Research, a Syria-based scientific research youth association managed by Dr. Nafiza Martini, for the scientific environment they provided. The authors thank Dr. Eyad Abdullah, Dr. Marwan Aljajeh, and Dr. Hazem Kamil for their technical help and consultation.
Guarantor
Dr. Khaled Hussien.
Data availability
All the relevant patient data and clinical history is provided within this article.
References
- 1.Bij de Vaate A.J., van der Voet L.F., Naji O., Witmer M., Veersema S., Brölmann H.A., et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet. Gynecol. 2014;43(4):372–382. doi: 10.1002/uog.13199. [DOI] [PubMed] [Google Scholar]
- 2.Kremer T.G., Ghiorzi I.B., Dibi R.P. Isthmocele: an overview of diagnosis and treatment. Rev. Assoc. Med. Bras. (1992) 2019;65(5):714–721. doi: 10.1590/1806-9282.65.5.714. [DOI] [PubMed] [Google Scholar]
- 3.Tulandi T., Cohen A. Emerging manifestations of cesarean scar defect in reproductive-aged women. J. Minim. Invasive Gynecol. 2016;23(6):893–902. doi: 10.1016/j.jmig.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Bij de Vaate A.J., Brölmann H.A., van der Voet L.F., van der Slikke J.W., Veersema S., Huirne J.A. Ultrasound evaluation of the cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet. Gynecol. 2011;37(1):93–99. doi: 10.1002/uog.8864. [DOI] [PubMed] [Google Scholar]
- 5.Kulshrestha V., Agarwal N., Kachhawa G. Post-caesarean niche (Isthmocele) in uterine scar: an update. J. Obstet. Gynaecol. India. 2020;70(6):440–446. doi: 10.1007/s13224-020-01370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Vervoort A.J., Uittenbogaard L.B., Hehenkamp W.J., Brölmann H.A., Mol B.W., Huirne J.A. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Human Reprod. (Oxford, England) 2015;30(12):2695–2702. doi: 10.1093/humrep/dev240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indraccolo U., Scutiero G., Matteo M., Indraccolo S.R., Greco P. Cesarean section on maternal request: should it be formally prohibited in Italy? Annali dell’Istituto superiore di sanita. 2015;51(2):162–166. doi: 10.4415/ANN_15_02_15. [DOI] [PubMed] [Google Scholar]
- 9.Betrán A.P., Ye J., Moller A.B., Zhang J., Gülmezoglu A.M., Torloni M.R. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Spiezio Sardo A., Saccone G., McCurdy R., Bujold E., Bifulco G., Berghella V. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet. Gynecol. 2017;50(5):578–583. doi: 10.1002/uog.17401. [DOI] [PubMed] [Google Scholar]
- 11.Poidevin L.O. The value of hysterography in the prediction of cesarean section wound defects. Am. J. Obstet. Gynecol. 1961;81:67–71. doi: 10.1016/s0002-9378(16)36308-6. [DOI] [PubMed] [Google Scholar]
- 12.Naji O., Abdallah Y., Bij De Vaate A.J., Smith A., Pexsters A., Stalder C., et al. Standardized approach for imaging and measuring cesarean section scars using ultrasonography. Ultrasound Obstet. Gynecol. 2012;39(3):252–259. doi: 10.1002/uog.10077. [DOI] [PubMed] [Google Scholar]
- 13.Tower A.M., Frishman G.N. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J. Minim. Invasive Gynecol. 2013;20(5):562–572. doi: 10.1016/j.jmig.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Sipahi S., Sasaki K., Miller C.E. The minimally invasive approach to the symptomatic isthmocele - what does the literature say? A step-by-step primer on laparoscopic isthmocele - excision and repair. Curr. Opin. Obstet. Gynecol. 2017;29(4):257–265. doi: 10.1097/GCO.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 15.Wang C.B., Chiu W.W., Lee C.Y., Sun Y.L., Lin Y.H., Tseng C.J. Cesarean scar defect: correlation between cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet. Gynecol. 2009;34(1):85–89. doi: 10.1002/uog.6405. [DOI] [PubMed] [Google Scholar]
- 16.Thurmond A.S., Harvey W.J., Smith S.A. Cesarean section scar as a cause of abnormal vaginal bleeding: diagnosis by sonohysterography. J. Ultrasound Med. 1999;18(1):13–16. doi: 10.7863/jum.1999.18.1.13. quiz 7–8. [DOI] [PubMed] [Google Scholar]
- 17.Morris H. Surgical pathology of the lower uterine segment caesarean section scar: is the scar a source of clinical symptoms? Int. J. Gynecol. Pathol. 1995;14(1):16–20. doi: 10.1097/00004347-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Florio P., Filippeschi M., Moncini I., Marra E., Franchini M., Gubbini G. Hysteroscopic treatment of the cesarean-induced isthmocele in restoring infertility. Curr. Opin. Obstet. Gynecol. 2012;24(3):180–186. doi: 10.1097/GCO.0b013e3283521202. [DOI] [PubMed] [Google Scholar]
- 19.Setubal A., Alves J., Osório F., Guerra A., Fernandes R., Albornoz J., et al. Treatment for uterine Isthmocele, a pouchlike defect at the site of a cesarean section scar. J. Minim. Invasive Gynecol. 2018;25(1):38–46. doi: 10.1016/j.jmig.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Futyma K., Gałczyński K., Romanek K., Filipczak A., Rechberger T. When and how should we treat cesarean scar defect - isthmocoele? Ginekol. Pol. 2016;87(9):664–668. doi: 10.5603/GP.2016.0063. [DOI] [PubMed] [Google Scholar]
- 21.Raimondo G., Grifone G., Raimondo D., Seracchioli R., Scambia G., Masciullo V. Hysteroscopic treatment of symptomatic cesarean-induced isthmocele: a prospective study. J. Minim. Invasive Gynecol. 2015;22(2):297–301. doi: 10.1016/j.jmig.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Marotta M.L., Donnez J., Squifflet J., Jadoul P., Darii N., Donnez O. Laparoscopic repair of post-cesarean section uterine scar defects diagnosed in nonpregnant women. J. Minim. Invasive Gynecol. 2013;20(3):386–391. doi: 10.1016/j.jmig.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Fabres C., Aviles G., De La Jara C., Escalona J., Muñoz J.F., Mackenna A., et al. The cesarean delivery scar pouch: clinical implications and diagnostic correlation between transvaginal sonography and hysteroscopy. J. Ultrasound Med. 2003;22(7):695–700. doi: 10.7863/jum.2003.22.7.695. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 24.Roberge S., Boutin A., Chaillet N., Moore L., Jastrow N., Demers S., et al. Systematic review of cesarean scar assessment in the nonpregnant state: imaging techniques and uterine scar defect. Am. J. Perinatol. 2012;29(6):465–471. doi: 10.1055/s-0032-1304829. [DOI] [PubMed] [Google Scholar]
- 25.Donnez O., Donnez J., Orellana R., Dolmans M.M. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil. Steril. 2017;107(1) doi: 10.1016/j.fertnstert.2016.09.033. (289-96.e2) [DOI] [PubMed] [Google Scholar]
- 26.Indraccolo U., Scutiero G., Matteo M., Mastricci A.L., Barone I., Greco P. Correlations between sonographically measured and actual incision site thickness of lower uterine segment after repeated caesarean section. Minerva Ginecol. 2015;67(3):225–229. [PubMed] [Google Scholar]
- 27.Di Spiezio Sardo A., Zizolfi B., Calagna G., Giampaolino P., Paolella F., Bifulco G. Hysteroscopic isthmoplasty: step-by-step technique. J. Minim. Invasive Gynecol. 2018;25(2):338–339. doi: 10.1016/j.jmig.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Rock JAaJ H.W. In: Te Linde’s operative gynecology. 10th ed. Rock John A., Jones Howard W., III, editors. c2008. Wolters Kluwer / Lippincott Williams & Wilkins; United States: Philadelphia: 2008. [Google Scholar]
- 29.Iannone P., Nencini G., Bonaccorsi G., Martinello R., Pontrelli G., Scioscia M., et al. Isthmocele: from risk factors to management. Rev. Brasileira de Ginecologia e Obstetricia : revista da Federacao Brasileira das Sociedades de Ginecologia e Obstetricia. 2019;41(1):44–52. doi: 10.1055/s-0038-1676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abacjew-Chmylko A., Wydra D.G., Olszewska H. Hysteroscopy in the treatment of uterine cesarean section scar diverticulum: a systematic review. Adv. Med. Sci. 2017;62(2):230–239. doi: 10.1016/j.advms.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y. A comparative study of transvaginal repair and laparoscopic repair in the management of patients with previous cesarean scar defect. J. Minim. Invasive Gynecol. 2016;23(4):535–541. doi: 10.1016/j.jmig.2016.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant patient data and clinical history is provided within this article.