Summary
Obesity-related complications such as non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D) are well-established risk factors for the development of hepatocellular carcinoma (HCC). This review provides insights into the molecular mechanisms that underlie the role of steatosis, hyperinsulinemia and hepatic inflammation in HCC development and progression. We focus on recent findings linking intracellular pathways and transcription factors that can trigger the reprogramming of hepatic cells. In addition, we highlight the role of enzymes in dysregulated metabolic activity and consequent dysfunctional signalling. Finally, we discuss the potential uses and challenges of novel therapeutic strategies to prevent and treat NAFLD/T2D-associated HCC.
Keywords: Hepatocellular carcinoma, obesity, non-alcoholic fatty liver disease, type 2 diabetes, hepatocyte transformation
Key points.
-
•
NAFLD and T2D are among the fastest growing aetiologies in HCC development.
-
•
In NAFLD and T2D, hyperinsulinemia, dysregulated glucose homeostasis and increased lipid accumulation can activate pathways that promote hepatic tumour development.
-
•
Hepatic inflammation, oxidative stress and insulin resistance are important hallmarks of NAFLD/T2D-related HCC.
-
•
Antidiabetic drugs, like metformin and thiazolidinediones, reduce HCC risk, yet their therapeutic effect can be contradictory in advanced stages.
-
•
The stratification of patients with HCC in clinical trials should consider the presence of diabetes, due to its impact on incidence and prognosis.
Introduction
Liver cancer is an aggressive and treatment-resistant pathology that represents the third most common cause of cancer-related death.1 Primary liver cancer includes hepatocellular carcinoma (HCC, comprising 75%-85% of cases) intrahepatic cholangiocarcinoma (comprising 10%-15% of cases), and other rare types. The highest HCC incidence and mortality are observed in Asia and Africa, but incidence and mortality are also increasing worldwide, especially in Europe and the US.2
Non-alcoholic fatty liver disease (NAFLD) is a risk factor that contributes to HCC development. NAFLD can progress to non-alcoholic steatohepatitis (NASH) in 20-30% of cases, and approximately 20-25% of NASH cases progress to cirrhosis,3 which is the strongest risk factor for HCC development. NAFLD is the leading cause of chronic liver disease worldwide.4,5 There is an unmet need to accurately identify metabolic risk factors that can better predict advanced stages of the disease and related complications.6
Diabetes is a metabolic disorder characterised by impaired regulation of glucose and insulin levels. The prevalence is exceptionally high, with an estimated 463 million affected patients in 2019, accounting for 9.3% of the adult human population.7 There are three major forms: autoimmune type 1 diabetes, insulin resistance-associated type 2 diabetes (T2D), and monogenic forms of diabetes. However, this classification is currently under re-evaluation.8 T2D is considered a metabolic risk factor for the development of NAFLD, advanced fibrosis, and HCC.4,6 Simon et al. demonstrated in two well-characterized cohorts that T2D is an independent risk factor for HCC development.6 T2D is significantly associated with severe liver disease,9 furthermore patients with advanced NAFLD (NASH with severe fibrosis) have a higher incidence of T2D.4,10 It is unclear whether NAFLD drives T2D, or if hyperglycaemia/hyperinsulinemia pushes NAFLD towards an advanced stage, indicating that the pathological processes are most likely intertwined. Therefore, the underlying mechanisms by which NAFLD/T2D can promote HCC development are not completely understood.
In this review, we discuss the pathogenic pathways activated by nutrient overload and the intracellular mechanisms that lead to aberrant signalling and hepatocyte reprogramming. We also review the involvement of inflammation in the transition from NAFLD/T2D to HCC. Finally, we will discuss current and new therapeutics for treating HCC and emerging technologies that will accelerate the translational process.
Risk factors involved in HCC progression in NAFLD and T2D
Genetics
To date, no genome-wide association studies have defined the genetic variations associated with NAFLD-HCC risk, in either the presence or absence of cirrhosis. However, single-nucleotide polymorphisms (SNPs) in genes that promote fat accumulation in hepatocytes have been identified as genetic risk factors in NAFLD, T2D, and HCC.[11], [12], [13] For example, the rs738409 polymorphism in phospholipase domain-containing 3 (PNPLA3) and the rs58542926 polymorphism in transmembrane 6 superfamily member 2 (TM6SF2) have been strongly associated with early steatosis and more advanced NAFLD and NASH.14 PNPLA3 rs738409 was also found at a higher frequency in a cohort of patients with HCC and T2D.15 Furthermore, the TM6SF2 rs58542926 polymorphism was associated with fatty liver and higher T2D risk in a genome-wide association study of >300,000 participants.16 However, in other studies, the PNPLA3 polymorphism was linked to an increase in liver fat, but it was not found to be associated with insulin resistance.17
More recently, a polygenic risk score has been developed to predict HCC in patients with obesity-related metabolic disorders and to improve HCC risk stratification.18 This polygenic risk score combines SNPs in PNPLA3 and TM6SF2, with other SNPs in MBOAT7 (membrane bound O-acyltransferase domain containing 7), GCKR (glucokinase regulator) and HSD17B13 (17β-hydroxysteroid dehydrogenase type 13).18
Nuclear receptor coactivator 5 (NCOA5), also known as coactivator independent of AF2, is a coregulator of oestrogen receptor α-mediated transcription. Reduced NCOA5 expression has been associated with HCC in patients with T2D.19 Remarkably, heterozygous deletion of the Ncoa5 gene in mice led to HCC through its effects on hepatic interleukin (IL)-6 expression.19,20
Carbohydrates, diabetes development and progression to HCC
An excessive intake of simple carbohydrates is associated with obesity and metabolic syndrome.21 It was estimated that a 20% reduction in added sugar intake by 2035 will reduce the prevalence of obesity, T2D, coronary heart disease as well as liver complications such as hepatic steatosis, NASH, cirrhosis and HCC in the US.22
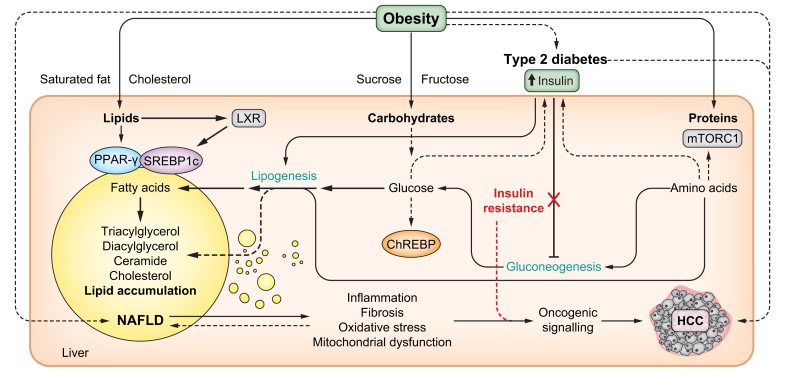
Fructose is a simple sugar whose intake has dramatically increased, owing to the consumption of sweetened beverages as part of western diets. Wali et al. showed that lipogenesis was strongly induced in mice fed a 50:50 mixture of fructose and glucose.23 Similarly, in healthy individuals, co-ingestion of fructose and glucose led to an increase in lipogenesis.23 In addition, improved cardiometabolic health was observed when mice were fed low-protein diets containing resistant starch, instead of native starch.23 These findings indicated that the type of carbohydrate and protein availability in the diet are important (Fig. 1).
Fig. 1.
Obesity and overnutrition expose the liver to an overload of energetic fuels (lipids, carbohydrates and proteins) that can affect hepatic energy metabolism with pathological consequences.
Several transcription factors are known to act as sensors of nutrients, such as PPAR-γ, which is activated by lipophilic ligands such as PUFAs; SREBP1c, which is activated by LXR in response to insulin, PUFAs and oxysterols; ChREBP, which is activated by glucose-6-phosphate; or mTORC1, which is activated in response to amino acids. These and other transcription factors regulate the expression of enzymes and signalling proteins required to execute and coordinate major metabolic pathways. The consequence of chronic aberrant activation of these transcription factors, associated with hyperinsulinemia during T2D predispose the liver to steatosis, inflammation, fibrosis, oxidative stress and mitochondrial dysfunction. These factors facilitate oncogenic transformation and HCC development. Selective insulin resistance confers liver resistance to the inhibitory action of insulin on gluconeogenesis, while the sensitivity of the liver to the stimulatory effect of insulin over lipogenesis remains. Different types of nutrients provided by the diet can accelerate metabolic dysfunction, including nutrients that are abundant in industrialised highly palatable and caloric foods such as saturated fat, cholesterol, sucrose and fructose. ChREB, carbohydrate-responsive element-binding protein; HCC, hepatocellular carcinoma; LXR, liver X receptor; mTORC1, mechanistic target of rapamycin complex 1; NAFLD, non-alcoholic fatty liver disease; PUFA, polyunsaturated fatty acid; T2D, type 2 diabetes; PPAR-γ, peroxisome proliferator-activated receptor gamma; SREBP1c, sterol regulatory element-binding protein 1c.
Softic et al. demonstrated that mice fed a high-fat diet (HFD) supplemented with fructose developed a more severe metabolic phenotype, compared to mice on a HFD supplemented with glucose.24 Fructose supplementation led to obesity, glucose intolerance and impaired insulin signalling. Sterol regulatory element binding protein-1c (SREBP1c), a master lipogenic regulator, gene expression and downstream lipogenesis genes were also activated.24 Fructose is metabolised by fructokinase (ketohexokinase), the first enzyme in fructose metabolism. In hepatocytes, fructokinase stimulation induces lipogenesis and fat accumulation.25 Mice on a high-fructose diet exhibited increased lipogenesis and developed NASH and HCC.25 Accordingly, loss of fructose metabolism has been observed in HCC patient samples, and ketohexokinase overexpression in liver cancer cells leads to decreased fructose flux through glycolysis.26
Ketogenic diet, protein intake and liver dysfunction
A ketogenic diet limits carbohydrate intake, which results in low glucose levels, thus reducing lipogenesis.27 Ketogenesis leads to ketone body production, which represents an energy source in a state of nutrient deprivation, such as prolonged fasting and starvation. Clinical trials have shown the benefits of the ketogenic diet for weight loss; however, its use remains controversial due to reports showing a worsened metabolic outcome.28 Caloric restriction can slow down the ageing process by activating reprogramming of liver metabolism. Mice subjected to high energy intake, or high caloric intake, showed an increase in proteins involved in nutrient metabolism, including glycolysis, gluconeogenesis, tricarboxylic acid cycle, lipogenesis, β-oxidation, amino acid metabolism and ketogenesis.29 Low energy intake was instead associated with RNA metabolism and upregulation of splicing. Metformin, rapamycin and resveratrol, known for prolonging lifespan in animal models, play a role in reverting changes induced by high energy and macronutrient intake. Through mTOR inhibition, these agents lead to a reduction in proteins and downstream splicing pathways.29 Interestingly, mice fed a low-protein diet showed improved metabolic health with increased mitochondrial activity.30 Conversely, excessive protein intake increases mitochondrial function and is also associated with oxidative stress,29 which accelerates the ageing process and contributes to HCC development.31 The “right” balance between protein, carbohydrate and fat intake in health and disease is still controversial. Evaluation in the obese population and in patients with T2D under treatment will help to clarify the real incidence in HCC.
Insulin resistance and lipid metabolism
The liver is a key regulator of glucose and lipid metabolism. Excessive hepatic lipid accumulation and increased glucose production characterise NAFLD and T2D.32 In several epidemiological studies, both NAFLD and T2D have been identified as significant risk factors for the development of HCC.33,34 Hepatic insulin resistance significantly contributes to T2D through fat-induced dysfunctional signalling of the insulin receptor (IR). Dysregulated IR signalling leads to aberrant downstream activation of phosphatidylinositol 3-kinase (PI3K)/AKT, which prevents insulin from inhibiting gluconeogenesis in the liver (Fig. 1). Insulin resistance induces elevated circulating insulin levels, which stimulate increased insulin-like growth factor-1 production, consequently upregulating proliferation and preventing apoptosis in hepatocytes, and thereby contributing to HCC.35 Hyperinsulinemia also activates insulin receptor substrate-1 which is associated with HCC development.36
Daily lipid overload with inadequate mitochondrial function contributes to the increased production of diacylglycerols (DAGs) and ceramides, which promote insulin resistance, NAFLD and eventual HCC development.17,37,38 The degradation of ceramides is associated with improved insulin sensitivity and decreased inflammation.39 Several studies have found that DAG accumulation can lead to hepatic insulin resistance via activation of protein kinase C (PKC). Phosphorylation of the insulin receptor by PKC was found to impair insulin signalling.40 Additionally, increased hepatic DAG content in humans was linked to hepatic insulin resistance, which was also associated with PKC activation.41 This DAG-PKC axis was found to be the strongest predictor of insulin resistance in obese patients. Dysfunctional insulin signalling can, in turn, increase lipid accumulation through a mechanism known as selective insulin resistance.42 Dysfunctional insulin signalling contributes to de novo lipogenesis (DNL) and increased hepatic fat accumulation promotes insulin resistance, leading to a vicious cycle linked to the progression of T2D and advanced NAFLD.
Hepatic steatosis occurs when fatty acid uptake and DNL are elevated over fatty acid oxidation and secretion. DNL was found to be positively associated with hepatic saturated fatty acid (SFA) content; both DNL and SFA levels are elevated in patients with NAFLD and T2D.43 In addition, SFA content was negatively correlated with hepatic insulin sensitivity43 and dysregulation of lipid metabolism correlates with the progression of liver disease to HCC.44 Thus, lipid metabolism can be drastically reprogrammed in malignant hepatic cells.
Several lipogenic enzymes, such as ACLY (ATP-citrate lyase) and ACACA (acetyl-CoA carboxylase alpha), are upregulated in liver cancer.45 Specifically, fatty acid synthase (FASN), a key enzyme in lipogenesis, is upregulated in patients with HCC and may be an important driver of cancer development.46,47 Indeed, in an HCC mouse model, deletion of Fasn prevented hepatocarcinogenesis in mice with oncogenic overexpression of c-Met/AKT and AKT alone.47 Additionally, FASN inhibition was found to suppress HCC formation in c-Myc-overexpressing tumours.48 However, FASN was also found to be dispensable in a murine HCC model, with c-Met and β-catenin overexpression.49 These studies highlight that the role of FASN and DNL in hepatocarcinogenesis is oncogene dependent, which has important implications for the design of targeted treatment options.
Peroxisome proliferator-activated receptor (PPAR)-γ is a nuclear receptor protein that plays a key role in the regulation of lipid metabolism. In the liver, PPAR-γ is an early contributor to NAFLD development (Fig. 1), where it increases steatosis by upregulating DNL and free fatty acid uptake.50 PPAR-γ was found to be elevated in the livers of obese patients with NAFLD.51,52 Paradoxically, PPAR-γ has also been found to suppress tumorigenesis, inducing PI3K/AKT-mediated apoptosis and cell cycle arrest in HCC .53 Thus, PPAR-γ agonists have been investigated in clinical trials as potential therapeutic agents for HCC,54,55 but their use remains controversial given their adverse metabolic effects.
Obesity and T2D have also been linked to expression of the lipogenic regulator SREBP1.56 Mammalian target of rapamycin complex (mTORC)1 and mTORC2 increase SREBP1 transcription and are major upstream regulators of lipogenesis. By activating SREBP1, mTORC1 is responsible for lipid synthesis during insulin resistance, and thereby contributes to hepatic steatosis.56 SREBP1 is elevated in liver tumour tissue57 and its inhibition has also been proposed as a therapeutic strategy for HCC.58,59
Free fatty acid synthesis is increased in tumoral cells for membrane support and energy production, promoting cancer growth and metastasis.45 In a study investigating the lipidomic profile of patients with NAFLD-associated HCC, a decrease in unsaturated fatty acids and acylcarnitines was found in the blood, with an increase in fatty acid transporters in tumours.60 Patients with HCC had decreased levels of free carnitines and increased levels of long-chain acylcarnitines. Notably, low serum levels of acetylcarnitine were identified as a strong candidate biomarker for HCC development.61 In a proteomic and lipidomic study of mice and humans, lipid-modifying enzymes were found to convert SFAs to monounsaturated fatty acids in HCC, and an increased ratio of long-chain n6 to n3 polyunsaturated fatty acids is associated with higher HCC risk in patients with NASH.62
Overall, these findings highlight the important role of altered insulin resistance and lipid metabolism in liver disease and indicate that they may be crucial drivers in the progression from NAFLD and T2D to HCC and early disease diagnosis.
Hepatic inflammation, a key component of NAFLD/T2D-related HCC development
Inflammatory pathways
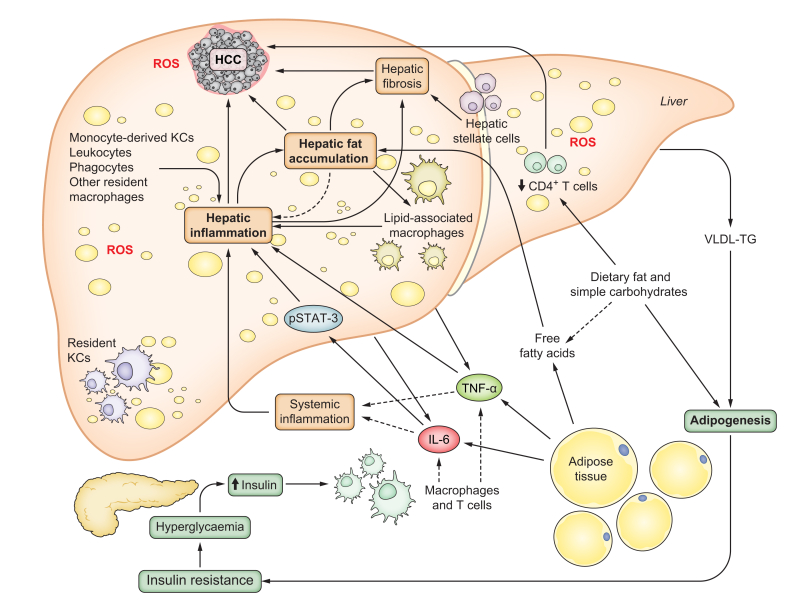
The liver is well known for its role in metabolism and detoxification, yet it also plays an essential role in the body’s immune response. Almost all subsets of leukocytes and phagocytes can be present in the liver, while the largest population of hepatic immune cells are Kupffer cells (liver-resident macrophages).63 Hepatic cells must maintain a balance of tolerance to immune cells during normal physiological function, while also remaining protected against foreign pathogens and tissue damage. Consequently, the hepatic immune cell population can be significantly altered in NAFLD, T2D and HCC64 (Fig. 2).
Fig. 2.
NAFLD and T2D are characterised by increased hepatic inflammation and oxidative stress, contributing to HCC development.
The population of resident and recruited immune cells in the liver is dynamic during the progression of NASH. Different cell types can participate in the inflammatory response. Pro-inflammatory cytokines such as IL-6 and TNF-α, are released by different tissue sources and drive an inflammatory response, including oncogenic STAT3 activation. An excess of dietary fats and simple carbohydrates favour body and hepatic fat accumulation and alters specific immune cell populations in the liver. Tumour suppressive CD4+ T cells are depleted by nutrient overload and ROS-dependent mechanisms. Hepatic resident KCs are also depleted in NAFLD and replaced by two subsets of pro-inflammatory recruited macrophages: monocyte-derived KCs and hepatic lipid-associated macrophages. These macrophages co-localize with fibrotic liver regions with activated hepatic stellate cells. In association with hepatic steatosis and inflammation, continuously high ROS levels lead to oxidative stress and liver damage that is strongly associated with HCC development. CD4, cluster of differentiation 4; HCC, hepatocellular carcinoma; IL-6, interleukin-6; KCs, Kupffer cells; NAFLD, non-alcoholic fatty liver disease; ROS, reactive oxygen species; STAT-3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor alpha; T2D, type 2 diabetes.
NAFLD and T2D are characterised by chronic low-grade inflammation, which has been linked to HCC development (Fig. 2). In patients with HCC, pro-inflammatory cytokines such as IL-6, tumour necrosis factor-α (TNF-α), and C-reactive protein levels are elevated, suggesting enhanced inflammation and insulin resistance.65,66 Chemokines have also been linked to NAFLD and HCC progression. Patients with NAFLD-associated HCC were found to have higher plasma levels of IL-8, IL-13, CCL-3, CCL-4, and CCL-5, which was correlated with activated circulating monocytes, compared to those with NAFLD without HCC.67
Cytokines can promote both immune tolerance and inflammation in the liver microenvironment. In hepatocytes, IL-6 and TNF-α can activate several signalling pathways linked to inflammation, steatosis and oncogenesis. In a mouse model of obesity, elevated expression of IL-6 and TNF-α promoted liver fat accumulation and inflammation.68 Furthermore, this inflammatory response and steatosis induced oncogenic STAT3 activation and promoted HCC development.68
NF-κB
NF-κB signalling is linked to increased insulin resistance in obesity and T2D models, where it is induced by low-grade inflammation.69,70 Patients with HCC were found to have elevated NF-kB activity.70 When the NF-kB-activating kinase IKKβ (inhibitor of NF-kB kinase subunit beta) was constitutively activated in hepatocytes, mice exhibited hyperglycaemia as well as hepatic and systemic insulin resistance.69 An increase in pro-inflammatory cytokines and inflammatory signalling was also found in IKKβ-activated mice. Thus, NF-κB activation in hepatocytes can lead to a diabetic phenotype. Interestingly, the hepatocytic IKK:NF-κB axis also regulates lipogenesis and cholesterol synthesis, independent of its central role in inflammation.71
Induction of NF-κB by obesity-associated inflammation can also lead to insulin resistance via a phosphotyrosine signalling-mediated mechanism. Activated NF-κB led to overexpression of hepatic tyrosine phosphatase PTPR-γ in obesity/T2D mouse models.72 This elevated PTPR-γ activity was linked to significant inflammation and insulin resistance in mice and humans. Upon PTPR-γ loss in mouse models, glucose production was decreased and hepatic insulin signalling was enhanced.72 Thus, the NF-κB/PTPR-γ axis affects hepatic metabolism, which is dysregulated by obesity-associated inflammation and can contribute to HCC.
NF-κB has pro-tumorigenic properties, with its activation promoting HCC cell proliferation, survival, and invasion. NF-κB can also activate stromal and immune cells, enhancing inflammation and fibrosis.70 Paradoxically, loss of NF-κB has also been found to significantly promote HCC development.70,73,74 NF-kB-activating kinase IKKβ can prevent liver tumorigenesis by suppressing hepatocyte cell death and proliferation. In a late-stage HCC mouse model, Ikkb-knockout mice showed a significant increase in tumour number and size.75 IKKβ was identified as a negative regulator of HCC development through reactive oxygen species (ROS)-mediated signal transducer and activator of transcription (STAT)3 signalling.75 It is not uncommon for both the hyperactivation and inactivation of pathways to result in similar outcomes in biology, albeit through different mechanisms. As a key mediator in inflammation and survival, understanding the context-dependent role of NF-κB in liver disease requires further investigation.
JAK-STAT
The Janus kinase (JAK)-STAT pathway is a key regulator in inflammation, insulin resistance, T2D, and HCC. Cytokines and growth factors activate JAK-STAT signalling, which leads to the expression of downstream gene targets involved in cell proliferation, survival, stress and immune responses.76
As previously described, STAT3 has been found to be constitutively activated in HCC tumours and induced by pro-inflammatory IL-6.77,78 This transcription factor is involved in tumour initiation and promotion; furthermore, phosphorylated STAT3 was found in 60% of human HCC cases and is associated with more aggressive tumours.79
JNK
In obesity, the c-Jun N-terminal kinase (JNK) family acts as a critical regulator in insulin resistance and NASH. Elevated c-Jun-JNK activity has been identified in the livers of obese patients, and was subsequently linked to hepatic insulin resistance and steatosis. JNK1 and JNK2 were found to negatively regulate insulin sensitivity and glucose uptake in HFD-fed mice.80 We found that hepatic fat accumulation activated JNK signalling, which leads to an increase in the expression of the BCL-2 (B-cell lymphoma 2) family member BIM (Bcl-2 interacting mediator of cell death).81 In a liver-specific Bim knockout mouse model, insulin sensitivity was improved while hepatic steatosis was reduced.81 BCL-2 proteins are important modulators of cell survival and are often dysregulated in cancer, including HCC.82
In addition to its role in liver steatosis, JNK signalling can promote tumour initiation in HCC. The activation of oncogenic c-Myc by JNK led to the downregulation of tumour suppressor p21 in hepatocytes.83 However, the pro-tumorigenic role of JNK seems to depend on its ability to induce expression of the pro-inflammatory cytokines IL-6 and TNF-α by non-parenchymal cells.84 This association between activated JNK signalling, inflammation and HCC development has been identified as an attractive therapeutic target.
Kupffer cells
Hepatic-resident Kupffer cells are considered pro-inflammatory drivers in the development of T2D/NAFLD-related HCC. However, it was recently revealed that resident Kupffer cells were depleted in NAFLD and were instead replaced by two subsets of pro-inflammatory recruited and activated macrophages: monocyte-derived Kupffer cells and hepatic lipid-associated macrophages.64 The latter subset was activated in obesity and able to metabolise lipids (Fig. 2). Lipid-associated macrophages were also found to be frequently accumulated in liver regions with increased pro-fibrotic Desmin, produced by hepatic stellate cells.64 Interestingly, another study demonstrated that when the insulin signalling pathway was inhibited, macrophages showed an anti-inflammatory phenotype, with lower expression of IL-6, IL-1β, and TNF-α.85 This altered macrophage heterogeneity highlights that Kupffer cell lineage and activation is important in NAFLD and HCC. Subsets of recruited and activated macrophages may be responsible for increased inflammation and fibrosis in the progression of NAFLD to HCC.
T cells
Adaptive immunity has also been shown to play a role in HCC development. In diet-induced mouse models, there was a liver-specific loss of CD4+ T cells but not CD8+ T cells.86 Excessive lipid accumulation in hepatocytes led to linoleic acid secretion and induced ROS-mediated CD4+ T-cell death. This hepatic depletion of CD4+ lymphocytes was strongly associated with increased tumorigenesis.86 However, IR knockout in T cells led to reduced production of pro-inflammatory cytokines upon activation and diminished cytotoxicity.87
The role of T cells has also been explored in NASH, with a specific subset of auto-aggressive CXCR6+ CD8 T cells identified in preclinical models and patients with NASH.88 These liver-resident T cells were reprogrammed and activated by metabolic stimuli, mediating liver damage.88 NASH-HCC has been reported to be associated with worse outcomes in patients treated with PD-L1/PD-1 immunotherapy due to expansion of activated CD8+ killer T cells.89 These findings highlight a potential role of activated CD8+ T cells in HCC progression, which has implications for immunotherapy.89 Leslie et al. showed in preclinical NASH-HCC models that antagonism of CXCR2, a chemokine receptor that is exclusively expressed on neutrophils in mice and humans, resulted in efficient tumour clearance and increased survival when combined with anti-PD-1 blockade.90 This work demonstrated that sensitisation of NASH-HCC may be beneficial to improve the efficacy of systemic treatments.
DNA methylation, oxidative stress and hepatocyte reprogramming
DNA methylation
NAFLD and T2D are multifactorial diseases influenced by hereditary genetics and environmental factors, which can induce hepatic epigenetic alterations.91 In patients with NAFLD, epigenetic changes are known to promote liver fibrosis.92 Thus, DNA methylation can occur in tissues undergoing metabolic reprogramming, which involves pathways such as insulin signalling and secretion, adipocyte differentiation, mitochondrial function, lipid and glucose homeostasis, and inflammation.93
DNA methylation signatures have also been identified in patients with NASH.94 NASH hepatic methylation can be reversible, as seen in liver biopsies of obese individuals who underwent bariatric surgery to lose weight.95 Interestingly, a DNA methylation signature obtained from the peripheral blood of patients with NASH showed epigenetic age acceleration correlating with increased liver fibrosis.96 Whether this DNA methylation profile is related to HCC development warrants further research.
In a large-scale, multi-omics study of patients with HCC, alterations by hypermethylation and mutation were observed in metabolic reprogramming genes.97 Notably, carbamoyl-phosphate synthase 1 (CPS1), a urea cycle enzyme, was found to be hypermethylated in HCC, correlating with a reduction in CPS1 mRNA levels.97 CPS1 deficiency induced excess ammonia and activated fatty acid oxidation, which provides ATP for proliferation in HCC cells.98 The methylation profile was also analysed to identify differentially methylated genes in T2D and HCC, among which CDKN1A was found as a potential diagnostic and prognostic marker in HCC.99 Additional studies in large patient cohorts are required to understand the potential role of epigenetic modifications related to NAFLD/T2D in the development of HCC.
Oxidative stress
NAFLD and T2D are characterised by an increase in hepatic fat accumulation and chronic low-grade inflammation, leading to excessive ROS levels. This increase in ROS leads to oxidative stress and liver damage which has been strongly implicated in HCC (Fig. 2). The effect of oxidative stress in obesity and HCC was covered extensively in our recent review.31
Mitochondria are involved in ROS production through their activity in energy metabolism and oxidative phosphorylation. Thus, oxidative stress induced by dysfunctional mitochondrial activity in obesity has been identified as a driver of liver pathophysiology. Using high-resolution respirometry, mitochondrial respiration has been quantified in liver biopsies of obese, insulin-resistant patients, with or without NAFLD/NASH, and compared to lean/healthy patients.100 The hepatic mitochondrial respiration rate was shown to be higher in obese, insulin-resistant patients compared to lean controls.100 However, among patients with obesity and insulin resistance, those with NASH were found to have a lower hepatic respiration rate than those without NASH. Furthermore, the patients with NASH had significantly increased hepatic insulin resistance, hepatic oxidative stress, and inflammation.100 Loss of this increased hepatic mitochondrial respiration in patients with NASH results in elevated oxidative stress, driving disease progression to HCC.31
Protein tyrosine phosphatases (PTPs) are a protein family that has been identified as a key regulator in oxidative stress and insulin resistance. PTPs contain a catalytic cysteine in their phosphatase domain that is highly susceptible to oxidation by ROS. We demonstrated that a HFD induced oxidative stress in obesity, which led to prominent PTP oxidation in the liver.42 PTPN2 (TCPTP) was inactivated, leading to an increase in lipogenesis and insulin-STAT5 signalling. This enhanced expression of STAT5 promoted insulin-like growth factor-1 production in the liver, increasing insulin resistance and the progression to T2D.42 Moreover, PTPN2 inactivation in the liver contributes to NASH and HCC development through STAT1 and STAT3-dependent mechanisms, respectively.78
Oxygen availability, oxidative stress, inflammation, and various nutrients can differentially affect hepatocyte signalling between the portal and central vein. Importantly, liver zonation can affect metabolic reprogramming in various hepatic regions.32 Although oxidative stress promotes cancer development, tumour cells can also utilise antioxidant systems for survival. Thioredoxin reductase-1 (TrxR1), an antioxidant protein, was found to be significantly overexpressed in human HCC samples.101 NRF-2, a key transcription factor in oxidative stress regulation, was shown to upregulate TrxR1 expression in HCC cell lines.101 Treatment with a TrxR1 inhibitor in vitro and in vivo exhibited potent anti-tumour effects and increased sensitivity to sorafenib treatment. Taken together, these studies highlight the potential oncogenic role of antioxidant systems in HCC, which may guide better treatment options for patients with a high antioxidant profile.
Metabolic-driven hepatocyte reprogramming
An increase in insulinemia, hepatic gluconeogenesis, and lipogenesis, with excessive lipid accumulation, represent the hallmarks of NAFLD and T2D, and have been found to alter hepatocyte function (Fig. 3). In obesity, fasting insulin concentration and insulin secretion are increased in response to meals .102 The hyperinsulinemia associated with obesity has been shown to increase cancer-related mortality, including HCC-related mortality.103 Insulin signalling modulates proliferation, survival and differentiation through RAS, AKT and PI3K, which are frequently mutated genes in HCC and are considered therapeutic targets.104 Moreover, PTEN, a well-known HCC tumour suppressor, negatively regulates insulin signalling and is also frequently mutated in liver cancers.105
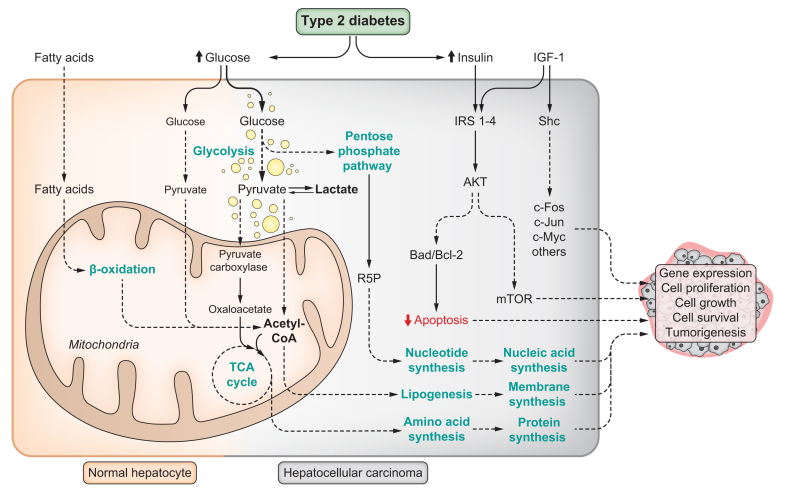
Fig. 3.
Hepatocyte transformation in NAFLD/T2D.
Normal hepatocytes prefer β-oxidation of fatty acids as a source of energy, as well as relatively low glucose uptake and oxidation. This is especially true during fasting conditions and this preference is influenced by hepatic zonation. Transformed hepatocytes exhibit high glucose uptake and rely on aerobic glycolysis as a source of energy. Pyruvate is preferentially converted into lactate, instead of being oxidised in the mitochondria. High fat intake rewires hepatocyte energy metabolism to favour glucose uptake and its utilisation as a source of energy through aerobic glycolysis and lactate production. Glucose can be used as a carbon source for biosynthetic reactions in rapidly growing tissues, as well as in cell signalling and maintenance of the redox state. High glucose uptake also sustains the substrate requirement for the pentose phosphate pathway. This is important for ribulose-5-phosphate synthesis, which is required for nucleotide biosynthesis and nucleic acid replication. Pyruvate carboxylase is induced by high-fat intake, favouring the entrance of pyruvate into the TCA cycle as oxaloacetate to maintain anaplerotic reactions required for amino acid biosynthesis. Acetyl-CoA is converted into fatty acids through lipogenesis. The pool of intracellular fatty acids (from lipogenesis and extrahepatic tissues) is used for phospholipid biosynthesis to build biological membranes. Hyperinsulinemia associated with T2D, in combination with the action of the hepatokine IGF-1, can lead to dysfunctional signalling pathways involved in cell survival, apoptosis, and stress responses. Together, these cellular and metabolic changes can represent advantages for cancer cells, allowing them to sustain rapid growth and proliferation. Bad, Bcl-2-associated agonist of cell death; Bcl-2, B-cell lymphoma 2; HCC, hepatocellular carcinoma; IGF-1, insulin-like growth factor 1; IRS1-4, insulin receptor substrate 1-4; mTOR, mammalian target of rapamycin; NAFLD, non-alcoholic fatty liver disease; Shc, Src homology 2 domain-containing transforming protein; T2D, type 2 diabetes; TCA, tricarboxylic acid.
In a streptozotocin-induced diabetic rodent model, transplanted pancreatic islets of Langerhans induced hepatocellular neoplasms.106 This method for treating type 1 diabetes involved transplanting functional islets into the liver via the portal vein. However, the rats developed liver tumours which may have been a consequence of increased insulin secretion and subsequent growth stimulation from the transplanted islets.106
Mature hepatocytes maintain plasticity through Hedgehog, Hippo-YAP-TAZ and Notch, which are activated during obesity and hyperinsulinemia to cope with chronic insults.107,108 Notch-mediated signalling can reprogramme hepatocytes to cholangiocytes or progenitors in chronic liver injury.107 Notch overexpression in mature mouse hepatocytes led to the expression of biliary markers SOX9 (SRY-box transcription factor 9) and osteoponin, which are normally absent in hepatocytes. Consistently, feeding mice a methionine- and choline-deficient diet (a NASH mouse model) resulted in SOX9 induction, steatohepatitis and biliary trans-differentiation.107 Loss of hepatocyte identity plays a role in the transformation process into cancer cells and in dedifferentiation into precursor cells that can later develop into malignant cells. Proteomics data from mouse NASH livers revealed a downregulation in hepatocyte identity genes, suggesting their importance in disease progression.109 ELF3 and GLIS2 were found to play a role in NASH.109 These transcription factors regulate the activation of hepatokines, such as Spp1 (secreted phosphoprotein 1) and Ctgf (alias Ccn2), which regulate the crosstalk between hepatic cells to induce NASH progression. Spp1 and Ctgf likely contribute to the activation of hepatic stellate cells and fibrosis.109 Stellate cells function as a signalling hub and secrete growth factors, cytokines, and chemokines named “stellakines”. These secreted factors have been shown to be increased in NASH, indicating their potential contribution to disease progression and HCC development.32
Hyperglycaemia can provide additional “fuel” to cancer cells, enabling them to maintain rapid proliferation (Fig. 3). Glucose metabolism was investigated in non-transformed livers from mice on a short-term HFD,45 which was shown to increase glucose uptake by 35%. Lactate production was increased in these mice, which recapitulated the high lactate phenotype in obese patients. The tricarboxylic acid cycle is central to energy metabolism (Fig. 3). Glycolysis-derived pyruvate can enter the tricarboxylic acid cycle after being converted into acetyl-CoA, or as oxaloacetate through pyruvate carboxylase (PC). Mice fed a HFD had increased levels of hepatic pyruvate, malate and citrate.45 HFD increased the pentose phosphate pathway and serine biosynthesis, as well as PC activity, suggesting that a fat-rich diet could induce an increase in glucose uptake similar to the tumoral state.45 Indeed, HFD intake increased liver tumours in diethylnitrosamine-injected mice.45 Serine biosynthesis and mitochondrial PC activity were elevated in HCC tissue from diethylnitrosamine-injected mice compared to liver tissue from mice fed a control diet.45
In summary, obesity and T2D can increase cancer hallmarks in non-transformed livers, suggesting that hyperinsulinemia, dysregulated glucose homeostasis and an increase in lipids can activate pathways that promote hepatic tumour development.
Novel disease models and therapeutic possibilities
Stem cell differentiation and somatic cell reprogramming to mimic human pathology
Novel methods are required to understand the pathophysiological connection between NAFLD/T2D and HCC. Studies in liver disease have mainly relied on human tissue/biopsies from donors, animal models, in vitro cell lines, and cultured primary hepatocytes. However, all these models have major limitations.
One recent development in modelling human liver function is induced pluripotent stem cells (iPSCs).[110], [111], [112] iPSCs can be derived from somatic cells of individuals with different pathologies, including patients with HCC with or without diabetes. iPSCs are then differentiated into hepatocyte-like cells (HLCs), which can be expanded and maintained in culture.113 They do not de-differentiate and maintain genomic and physiological similarities to human hepatocytes. Indeed, biobanking of patient iPSCs has aided in the acceleration of precision medicine. These patient-derived stem cell models can be used to predict a patient’s response to drug treatments.
Several studies have developed methods to differentiate human iPSCs to a hepatic cell fate. This differentiation protocol requires various growth factors such as activin A, fibroblast growth factor 2, bone morphogenetic protein 4, hepatocyte growth factor, and oncostatin M, and specific culture conditions to generate mature hepatocytes.110 Moreover, a hepatic-like phenotype can be achieved in somatic cells via the ectopic expression of native liver-enriched transcription, bypassing the intermediate pluripotent state.114 These “artificial” hepatocytes are amenable to CRISPR/Cas gene editing and useful for large-scale high-throughput screening and toxicology studies.
Organoids
While iPSC-derived HLCs have shown promise as an improved model of hepatocyte function, this method has been criticised due to the monolayer cell culture condition. Recent developments have demonstrated that 3D cell culture can more accurately simulate the cell’s environment by allowing cell-cell, cell-extracellular matrix, and mechanical interactions.115 Extracellular matrix components such as collagen, laminin, and fibronectin have been used in 3D culture to mimic the liver microenvironment. 3D culture methods have been developed using synthetic scaffolds or spontaneous hepatic organoids. Moreover, co-culture of HLCs with other cell types also allows for better modelling of liver physiology.
Steatosis can be induced in iPSC-derived human liver organoids with free fatty acid treatment.116 Liver organoids treated with antidiabetic drugs, L-carnitine and metformin, showed reduced fat accumulation.116 Organoids have also been generated from patient liver cancer cells to investigate their use as models in HCC.117 Liver cancer organoids were found to reflect patient-specific histological architecture and gene expression; furthermore, they developed tumours when transplanted in vivo.117
The use of iPSCs and organoids has great potential to advance personalised medicine for NAFLD and T2D. Additionally, genomic analysis of human iPSCs and organoids can identify genetic variants that may be diagnostic biomarkers or confer drug resistance. iPSCs and organoids have been proposed as important tools in regenerative medicine. Using gene editing, these in vitro models could enable the repair of mutations in genetic diseases prior to transplantation back into patients. Moreover, iPSCs and organoids can be genetically modified to be HLA-matched to patients, thereby preventing organ rejection. Although iPSCs and organoids have limitations, such as high cost and poor reproducibility, this technology holds great promise.
Pharmacological therapies and clinical trials
The dramatically rising incidence of NAFLD, T2D and HCC means that effective therapeutic options for patients are an urgent clinical need. Given that T2D is a known risk factor for HCC, several studies have investigated antidiabetic treatments for liver cancer. Increasing evidence suggests that diabetic agents may also be attractive therapies and play a relevant role in the management of patients with HCC. The effects of antidiabetic drugs in HCC can be evaluated at two main levels: chemoprevention and treatment (Table 1).
Table 1.
The effects of antidiabetic drugs in HCC can be evaluated at two main levels: Chemoprevention and treatment.
| Study | Phase/type | Identifier | Number of patients | Study drug | Diabetes mellitus | Number centres/countries | Year | Primary endpoint | Conclusions | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemoprevention | ||||||||||
| Evans JM et al. | Retrospective case-control study | n.a. | Cases (983) – Controls (1,846) | Metformin | T2D | Population databases/Tayside-Scotland | 2005 | Odds ratio | Metformin may reduce the risk of cancer in patients with T2D. The unadjusted odds ratio was 0.86 (95% CI 0.73 to 1.02). The unadjusted odds ratio for any exposure to metformin since 1993 was 0.79 (0.67 to 0.93). | 146 |
| ADOPT | III | NCT00279045 | Rosiglitazone (1,456) vs. metformin (1,454) vs. glyburide/glibenclamide (1,441) | Metformin, TZD, sulfonylurea | T2D | 490 centres/US, Europe/hospital based | 2006 | Monotherapy failure | Post hoc analysis of occurrence of HCC in patients enrolled in OADM monotherapy trial. HCC total: 4. HR metformin vs. rosiglitazone: 0.92 (95% CI 0.63–1.35); metformin vs. glibenclamide: 0.78 (95% CI 0.53–1.14) | 147 |
| RECORD | III | NCT00379769 | Rosiglitazone (2,220) vs. Metformin (1,122) vs. Sulfonylurea (1,105) | Metformin, TZD, sulfonylurea | T2D | 447 centres/US, Australia/hospital based | 2007 | Cardiac outcomes, regulation of glycaemia | Post hoc analysis of HCC incidence in cardiovascular outcomes study of OADM, with addition of another “rescue” OADM as needed for glycaemic control. HCC total: 4. On background of sulfonylurea: metformin vs. rosiglitazone HR 1.22 (95% CI 0.86–1.74). On background of metformin: sulfonylurea vs. rosiglitazone HR 1.33 (95% CI 0.94–1.88) | 100 |
| Donadon V et al. | Retrospective case-control study | n.a. | HCC cases (610) - matched liver cirrhosis (618) - controls (1,696) | Metformin | T2D | 1 centre/Italy | 2010 | To explore the relationships among T2D, antidiabetic therapy and HCC risk. | T2D is an independent risk factor for HCC and pre-exists to HCC occurrence. Metformin was associated with a significant reduction of risk for HCC vs. controls vs. cirrhosis cases when compared with sulfonylurea and insulin therapy (OR 0.15; CI 0.04–0.50; p = 0.005 and OR 0.16; CI 0.06–0.46; p = 0.0006 respectively) | 148 |
| Chang CH et al. | Retrospective case-control study | n.a. | T2D (606,583). A total of 10,741 liver cancer cases, 7,200 colorectal cancer cases, and 70,559 diabetic controls were included. | TZD | T2D | Population databases/Taiwan | 2012 | To assess the association between TZDs (both pioglitazone and rosiglitazone) and the occurrences of liver, colorectal, lung, and urinary bladder cancers. | The use of pioglitazone and rosiglitazone is associated with a decreased liver cancer incidence in diabetic patients. A significantly lower risk of liver cancer incidence was found for any use of rosiglitazone (OR 0.73, 95% CI 0.65-0.81) or pioglitazone (OR 0.83, 95% CI 0.72-0.95), respectively. | 136 |
| Singh S et al. | Meta-analysis | n.a. | Ten studies reporting 22,650 cases of HCC in 334,307 patients | Metformin, TZD, sulfonylurea, insulin | T2D | Multicentre | 2013 | Risk of incident HCC | Meta-analysis of observational studies showed a 50% reduction in HCC incidence with metformin use, but an increase in HCC incidence with sulfonylurea or insulin use. TZD did not modify the risk of HCC. | 128 |
| Zhang H et al. | Meta-analysis | n.a. | Seven studies reporting 562 cases of HCC in 16,549 patients | Metformin | T2D | Population databases/China | 2013 | To determine the association between metformin use and HCC among diabetic patients. | Metformin treatment was associated with reduced risk of HCC in diabetic patients (RR 0.24, 95% CI 0.13–0.46, p <0.001). | 129 |
| Lai SW et al. | Retrospective cohort study | n.a. | Controls diabetics on TZD treatment (23,580)/case diabetics on TZD treatment (23,580) | TZD | T2D | Taiwan | 2020 | Risk of incident HCC | There was a negative association in a duration-dependent manner between the risk of HCC and TZD use among T2D patients who had risk factors for HCC | 137 |
| Vilar-Gomez E et al. | Cohort | n.a. | No T2D (87) vs. T2D (212) | Metformin, sulfonylurea, insulin | T2D and NASH cirrhosis | Six centres/Europe, Asia, Australia, Cuba | 2021 | To determine the influence of T2D, hyperglycaemia, and ADMs on outcomes of HCC, liver decompensation, and death | Metformin significantly reduced the risk of hepatic decompensation and HCC only in subjects with HbA1c levels greater than 7.0% (Ahr 0.97; 95% CI 0.95–0.99 and aHR 0.67; 95% CI 0.43–0.94, respectively) | 149 |
| Kaplan DE et al. | Retrospective cohort study | n.a. | 74 984 diabetics; 40,368 with T2D before cirrhosis. 11 114 had active utilization of metformin. | Metformin | T2D | Population databases/US | 2021 | To investigate the impact of metformin exposure on mortality, hepatic decompensation, and HCC in individuals diagnosed with cirrhosis with a pre-existing diagnosis of diabetes mellitus | Metformin use in patients with cirrhosis and diabetes appears safe and is associated independently with reduced overall, but not liver-related, mortality, hepatocellular carcinoma, or decompensation after adjusting for concomitant statin and angiotensinogen-converting enzyme inhibitor/angiotensin-2–receptor blocker exposure. | 150 |
| Yen FS et al. | Observational case-control study | n.a. | 2,828 paired propensity score matched DPP-4 inhibitor users and nonusers T2D with compensated liver cirrhosis | DPP-4 inhibitors | T2D | Population databases/Taiwan | 2021 | To assess the outcomes of all-cause mortality, HCC, major adverse cardiovascular events, decompensated cirrhosis, and hepatic failure. | DPP-4 inhibitor users were associated with higher risks of decompensated cirrhosis and hepatic failure than did non-users among patients with T2D and compensated liver cirrhosis. Risk of all-cause mortality, HCC, and major cardiovascular events between DPP-4 inhibitor users and nonusers were not statistically different. | 151 |
| Li Q et al. | Meta-analysis | n.a. | Seven studies reporting 562 cases of HCC in 16,549 patients | Metformin | T2D | Multicentre | 2022 | To evaluate the relationship between metformin therapy and HCC survival and risk. | Metformin in T2D patients is significantly associated with reduced risk and all-cause mortality of HCC (OR/RR 0.59, 95% CI 0.51–0.68, I2 = 96.5%, p <0.001). | 130 |
| Kramer JR et al. | Cohort | n.a. | T2D and NAFLD (85,963) | Metformin, sulfonylurea, insulin | T2D and NAFLD | 130 centres/US | 2022 | Risk of incident HCC | Use of metformin was associated with a reduced risk of HCC compared with no medication, 22% lower risk of HCC (HR 0.77; 95% CI 0.65–0.90; p = 0.001), whereas use of combination therapy was associated with increased risk (HR for insulin and metformin, 1.53; 95% CI 1.26–1.86; p <0.0001; HR for insulin, metformin, and sulfonylureas, 1.71; 95% CI 1.41–2.08; p <0.0001). | 152 |
| Hendryx M et al. | Retrospective cohort study | n.a. | 3,185 patients with HCC and pre-existing diabetes, 137 (4.3%) patients used SGLT2 inhibitors. | Sodium-glucose cotransporter 2 (SGLT2) inhibitors | T2D | SEER-Medicare dataset/US | 2022 | aHRs for mortality | SGLT2 inhibitor initiation was associated with improved overall survival of patients with HCC and pre-existing type 2 diabetes compared with no SGLT2 inhibitor use (HR 0.68, 95% CI 0.54–0.86). | 139 |
| Treatment HCC | ||||||||||
| Chen TM et al. | Retrospective cohort study | n.a. | No T2D RFA (82)/T2D RFA with metformin (21)/T2D without metformin (32) | Metformin, sulfonylurea, insulin | T2D 32.3% | 1 centre/Taiwan | 2011 | OS | Metformin users among patients with T2D and HCC undergoing RFA had favourable OS compared to patients with T2D not receiving metformin treatment. | 153 |
| Bhat M et al. | Retrospective cohort study | n.a. | No T2D (438)/T2D not on metformin (207)/T2D on metformin (56) | Metformin | T2D 37.5% | 1 centre (part Bridge cohort) | 2014 | OS | This study demonstrates no survival benefit with metformin in patients with T2D and HCC. | 154 |
| Jang WI et al. | Retrospective cohort study | n.a. | SBRT without T2D (169)/SBRT T2D not on metformin (29)/SBRT T2D on metformin (19) | Metformin | T2D 22% | Four centres/Korea | 2015 | OS | The use of metformin in patients with HCC receiving radiotherapy was associated with higher OS. In the propensity score-matched cohort (n = 76), the OS rate of the metformin group was higher than that of the non-metformin group (2-year, 76% vs. 37%, p = 0.022). | 155 |
| Seo YS et al. | Retrospective cohort study | n.a. | Curative resection T2D on metformin (533)/curative resection T2D not on metformin (218) | Metformin | T2D | National database (NHIS and KKRC)/Korea | 2016 | OS | In patients treated with curative hepatic resection, metformin use was associated with improvement of HCC-specific mortality and reduced occurrence of retreatment events. | 156 |
| Casadei Gardini A et al. | Retrospective cohort study | n.a. | Sorafenib (51) vs. sorafenib+metformin (31) vs. sorafenib+insulin (11) | Multiple kinase inhibitor, metformin, insulin | T2D 42.5% | 1 centre/Italy | 2015 | PFS, OS | The result of greater tumour aggressiveness is described and resistance to sorafenib in patients treated with metformin. | 132 |
| Casadei Gardini A et al. | Prospective cohort study | n.a. | Sorafenib (193) vs. sorafenib+metformin (52) vs. sorafenib+insulin (34) | Multiple kinase inhibitor, metformin, insulin | T2D 30.0% | 1 centre/Italy | 2017 | PFS, OS | In patients with HCC undergoing chronic treatment with metformin, the use of sorafenib was associated with poor PFS and OS (1.9 and 6.6 months, respectively) compared to 3.7 months and 10.8 months, respectively, for patients without T2D and 8.4 months and 16.6 months, respectively, for patients on insulin (p <0.0001). | 131 |
| Chung YK et al. | Retrospective case-control study | n.a. | Recurrence after LR: sorafenib+metformin (40)/sorafenib+insulin (23)/sorafenib control (241); propensity score matching control (40) Recurrence after LT: sorafenib+metformin (14)/sorafenib+insulin (17)/sorafenib control (43); propensity score matching (28) |
Multiple kinase inhibitor, metformin, insulin | T2D | 1 centre/Korea | 2018 | OS | Absence of synergistic antitumor effects of metformin. | 133 |
| Schulte L et al. | Retrospective case-control study | n.a. | 5,093 patients with HCC, 1,917 patients (37.6%) were diagnosed with T2D, of whom 338 (17.6%) received treatment with metformin | Metformin | T2D (37.6%) | 3 centres/Germany, Austria | 2019 | OS | In the matched cohorts, mOS remained significantly longer in metformin-treated patients (22 vs. 16 months, p = 0.021). Co-treatment of metformin and sorafenib was associated with a survival disadvantage. | 127 |
| El Shorbagy S et al. | RCT | n.a. | Sorafenib+metformin (40) vs. sorafenib (40) | Multiple kinase inhibitor, metformin | T2D 60% | 2 centres/Egypt | 2021 | OS, TDP, Safety | No superior efficacy of adding metformin to sorafenib in HCC treatment. | 134 |
| Cho YY et al. | Retrospective cohort study | n.a. | 1,566 patients with unresectable HCC who received sorafenib. Long-term survivor group (survival more than 2 years, n = 257) or a control group (n = 1,309). | Multiple kinase inhibitor | Presence T2D analysed but percentage by groups not reported | 9 centres/Korea | 2021 | Clinical characteristics of long-term survivors after sorafenib treatment. | The prognostic factors predicting long-term survival were metformin use (aHR 3.464; p <0.001), hand-foot skin reaction (aHR 1.688; p = 0.003), and concomitant treatment with chemoembolization or radiotherapy (aHR 2.766; p <0.001). | 135 |
| Systemic therapy for HCC | ||||||||||
| First-line | ||||||||||
| SHARP | III | NCT00105443 | Sorafenib (299) vs. placebo (303) | Multiple kinase inhibitor: VEGFR, KIT, RET, FLT-3, PDGFR-β, RET/PTC, MAPK | Not specified | 178 centres/23 countries | 2008 | OS | Sorafenib improves survival compared with placebo | 118 |
| Asia-Pacific | III | NCT00492752 | Sorafenib (149) vs. placebo (75) | Multiple kinase inhibitor: VEGFR, KIT, RET, FLT-3, PDGFR-β, RET/PTC, MAPK | Not specified | 23 centres/China, South Korea and Taiwan | 2009 | OS | Sorafenib improves survival compared with placebo | 157 |
| REFLECT | III | NCT01761266 | Lenvatinib (478) vs. sorafenib (476) | Multiple kinase inhibitor: VEGFR 1-3, FGFR 1–4, PDGFR α, RET, KIT | Not specified | 154 centres/24 countries | 2018 | OS | Lenvatinib is non-inferior compared with sorafenib | 119 |
| IMbrave 150 | III | NCT03434379 | Atezolizumab + bevacizumab (336) vs. sorafenib (165) | Checkpoint inhibitor + Antiangiogenic: Anti-PD-L1 antibody + Anti VEGFA antibody | Not specified∗ | 111 centres/17 countries | 2020 | OS, PFS (co-primary) | Atezolizumab plus bevacizumab improve overall survival compared with sorafenib | 120 |
| HIMALAYA | III | NCT03298451 | Durvalumab + tremelimumab (Stride 393) vs. sorafenib (389) | Checkpoint inhibitor + checkpoint inhibitor: Anti-PD-1 antibody + Anti-CTLA-4 antibody | Not specified | 181 centres/16 countries | 2022 | OS | Durvalumab plus tremelimumab improve overall survival compared with sorafenib | 158 |
| COSMIC-312 | III | NCT03755791 | Atezolizumab + cabozantinib (432) vs. sorafenib (217) | Checkpoint inhibitor + multiple kinase inhibitor: Anti-PD-L1 antibody + VEGFR, MET, TAM family receptors (TYRO3, AXL, MER | Not specified∗ | 178 centres/32 countries | 2022 | PFS, OS (dual) | Atezolizumab plus cabozantinib improve progression-free survival compared with sorafenib | 159 |
| CheckMate 459 | III | NCT02576509 | Nivolumab (371) vs. sorafenib (372) | Checkpoint inhibitor: Anti-PD-1 antibody | Not specified∗ | 22 countries | 2022 | OS | Nivolumab does not improve survival compared with sorafenib | 160 |
| Second-line | ||||||||||
| RESORCE | III | NCT01774344 | Regorafenib (379) vs. placebo (194) | Protein kinase inhibitor: RAF-1, RET, BRAFV600E, VEGFR, TIE-2, PDGFR, FGFR, EGFR, CSF1R, c-kit | Not specified. NASH: 25 (7%) vs. 13 (7%) |
152 centres/21 countries | 2017 | OS | Regorafenib improves survival compared with placebo | 121 |
| CELESTIAL | III | NCT01908426 | Cabozantinib (470) vs. placebo (237) | Multiple kinase inhibitor: VEGFR, MET, TAM family receptors (TYRO3, AXL, MER) | Not specified. NASH: 43 (9%) vs. 23 (10%) |
95 centres/19 countries | 2018 | OS | Cabozantinib improves survival compared with placebo | 122 |
| REACH-2 | III | NCT02435433 | Ramucirumab (197) vs. placebo (95) | Monoclonal antibody: Anti VEGFR-2 | Not specified. NASH: 19 (10%) vs. 4 (4%) |
92 centres/22 countries | 2019 | OS | Ramucirumab improves survival compared to placebo with AFP ≥400 ng/ml | 123 |
| KEYNOTE-224 | II | NCT02702414 | Pembrolizumab (104) | Checkpoint inhibitor: Anti-PD-1 antibody | Not specified∗ | 47 centres/10 countries | 2018 | ORR | Pembrolizumab approved by the US FDA | 161 |
| CheckMate-040 | I/II | NCT01658878 | Nivolumab + ipilimumab (140) | Checkpoint inhibitor + checkpoint inhibitor: Anti-PD-1 antibody + Anti-CTLA-4 antibody | Not specified∗ | 31 centres/10 countries | 2020 | Safety, tolerability, ORR | Nivolumab and ipilimumab approved by the FDA | 162 |
| KEYNOTE-240 | III | NCT02702401 | Pembrolizmab (278) vs. placebo (135) | Checkpoint inhibitor: Anti-PD-1 antibody | Not specified∗ | 119 centres/29 countries | 2020 | PFS, OS (co-primary | Pembrolizumab does not improve survival and progression-free survival compared with placebo | 163 |
| KEYNOTE-394 | III | NCT03062358 | Pembrolizumab (300) vs. placebo (153) | Checkpoint inhibitor: Anti-PD-1 antibody | Not specified | Asia | 2022 | OS | Pembrolizumab improves survival compared with placebo in Asia | 164 |
Information on the presence of diabetes mellitus in clinical trials for the treatment of advanced HCC is very scarce. AFP, alpha-fetoprotein; CTLA-4, cytotoxic T-Lymphocyte antigen 4; ICI, immune checkpoint inhibitors; LR, liver resection; LT, liver transplantation; n.a., not applicable; OADM, oral antidiabetic medications; ORR, objective response rate; OS, overall survival; PD-1, programmed cell death 1; PFS, progression-free survival; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy; T2D, type 2 diabetes; TDP, time to disease progression; TKI, tyrosine kinase inhibitors.
Patients with controlled type 1 diabetes mellitus who are on an insulin regimen were eligible for the study.
Sorafenib was the first systemic therapy that was found to be effective in a clinical trial for advanced HCC.118 For a decade, it was the only approved first-line treatment for patients with HCC. Recently, several new effective treatments have been approved as first- and second-line therapies.[118], [119], [120], [121], [122], [123] These include several kinase inhibitors, VEGF (vascular endothelial growth factor) inhibitors, and immune-checkpoint inhibitors. However, there is still a concern regarding adverse events associated with systemic therapies. Developing alternative strategies to improve patient’s quality of life will be crucial in the advancement of HCC treatments.
Given the role of lipogenesis in NAFLD-associated HCC, FASN inhibitors have also been proposed as promising treatments. As mentioned previously, FASN was found to inhibit HCC formation in oncogenic mouse models.46,47 In a preclinical study, FASN inhibitors were found to improve efficacy in combination HCC treatments.124 Additionally, the use of a FASN inhibitor (TVB-2640) in a NASH clinical trial showed efficacy in decreasing liver fat and improving biochemical biomarkers.125 These findings provide support for investigating FASN inhibitors in NAFLD-associated HCC.
The number of patients with diabetes is generally not specified in clinical trials (Table 1). In three studies on second-line drugs, patients with diabetes are indirectly referred to as subgroups with NASH.[121], [122], [123] The classification of therapeutic groups should consider both the presence of diabetes and the level of metabolic control. This could optimise the efficacy of systemic treatments.
Metformin is an insulin sensitiser that reduces hepatic gluconeogenesis and hyperinsulinemia. It activates the AMPK pathway via inhibition of mitochondrial respiration, which increases insulin sensitivity.126 Activation of AMPK also leads to downstream inhibition of mTOR, which plays a key role in proliferation and immune activation in cancer. In patients with HCC and T2D, metformin treatment prolonged overall survival.127 Metformin was found to reduce HCC risk in a network meta-analysis of clinical studies (Table 1).[128], [129], [130] It should be considered that metformin was also associated with a poor response to sorafenib treatment in patients with HCC,127,131,132 but opposite results have also been reported.[133], [134], [135]
Thiazolidinediones (TZDs) are another class of antidiabetic drugs that activate PPARs, key regulators of glucose metabolism and insulin sensitivity.136 The anti-tumoral role of these drugs has also been noted, with evidence that they are involved in cell growth arrest, apoptosis induction, and preventing cell invasion. Indeed, the TZDs pioglitazone and rosiglitazone were associated with a reduction in liver cancer incidence in patients with T2D.136 Like metformin, TZDs were found to reduce HCC risk.127,137 However, TZDs have also been linked to an increased risk of cardiovascular events in patients with cirrhosis.138 Further studies with TZDs as a treatment for patients with HCC and T2D are required to determine its potential efficacy.
Several other classes of antidiabetic drugs have been proposed as potential therapeutics in HCC (Table 1). A recent epidemiological study found that sodium-glucose cotransporter-2 inhibitors were associated with improved overall survival in patients with HCC and T2D.139 GLP-1 receptor agonists have also been investigated in vitro and in vivo in HCC models. However, large scale studies on patients are required to determine the beneficial effects of these drugs.
Future directions
The complexity of the pathogenic pathways involved in NAFLD/T2D-related HCC remains to be elucidated. Determining the aetiopathogenesis of T2D in both NAFLD and non-NAFLD scenarios will impact the understanding of HCC initiation and progression (e.g., in patients with T2D without NAFLD but with an active hepatitis C or B virus infection, and non-abusive alcohol consumption). Recently, it was recommended that the term NAFLD be replaced by metabolic dysfunction-associated fatty liver disease (or MAFLD) to downplay the importance of alcohol in the definition and to emphasise the metabolic risk factors that underlie its pathology.140,141 Considering this non-exclusive diagnosis, the development of HCC should also be analysed based on the response to changes in diet and anti-diabetogenic drug treatment.
Mouse models cannot fully recapitulate mechanisms of human disease progression, as was observed in a comparative study between patients with NAFLD/NASH and experimental mice.142 New disease models and therapeutic treatments can aid in the understanding of T2D as a risk factor for HCC. The development of 3D organoids derived from iPSCs or organoids derived from cancer cells of patients with diabetes that can recapitulate human genetic expression will further our understanding of disease biology. New emerging technologies, including single-cell RNA sequencing, spatial transcriptomics, and advanced metabolomics, are also promising for the study of hepatic cell networks, cellular heterogeneity, and cancer clonal evolution.[143], [144], [145] These single-cell technologies will deliver new insights into disease-associated reprogramming and further our understanding of the pathological mechanisms linking NAFLD/T2D and HCC.
Conclusions
Dietary intake is crucial in the maintenance of metabolic health. Increased dietary fat and simple sugars are major inducers of altered metabolism, which includes increased lipogenesis, lipid accumulation and insulin resistance. The key responsible regulators usually involve transcription factors or upstream components controlling lipid metabolism. Obesity, NAFLD and T2D promote liver inflammation and increase oxidative stress, which accelerates oxidative cell death and promotes HCC. Therefore, HCC shares common altered metabolic pathways with NAFLD/T2D, suggesting the involvement of dysregulated lipidaemia and insulinemia in tumorigenesis promotion. The molecular pathways leading to the transition from a high-fat, insulin-resistant, inflammatory liver to tumorigenesis are not well understood. Yet some of the emerging key players have been described in this review, highlighting our current understanding of NAFLD/T2D-associated HCC. The use of innovative technologies such as 3D organoids will increase our understanding of these diseases and reveal novel therapeutic targets.
Financial support
This work was supported by a European Research Council (ERC) Consolidator grant METAPTPs (GA817940), FNRS-WELBIO grant (35112672), FNRS-PDR grant (40007740), TELEVIE grant (40007402), and ULB Foundation. BRM was supported by the “Miguel Servet Type I” program (CP19/00098, ISCIII, Spain; co-funded by the Fondo Europeo de Desarrollo Regional-FEDER). ST is supported by a TELEVIE scholarship. ENG is a Research Associate of the FNRS, Belgium.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Manuscript concept: ENG; drafting of the manuscript: ST, ML EG, CJLT, BRM, ENG. ST and ML equally contributed to this manuscript.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100811.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal A.J., Harrison S.A., Ratziu V., Abdelmalek M.F., Diehl A.M., Caldwell S., et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology. 2019;70:1913–1927. doi: 10.1002/hep.30664. [DOI] [PubMed] [Google Scholar]
- 4.Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., Abdelmalek M.F., Caldwell S., Barb D., et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang D.Q., Singal A.G., Kono Y., Tan D.J.H., El-Serag H.B., Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977 e962. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon T.G., King L.Y., Chong D.Q., Nguyen L.H., Ma Y., VoPham T., et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: results from two prospective cohort studies. Hepatology. 2018;67:1797–1806. doi: 10.1002/hep.29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. 9(th) edition. [DOI] [PubMed] [Google Scholar]
- 8.Ahlqvist E., Storm P., Karajamaki A., Martinell M., Dorkhan M., Carlsson A., et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis H., Craig D., Barker R., Spiers G., Stow D., Anstee Q.M., et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y.L., Patman G.L., Leathart J.B., Piguet A.C., Burt A.D., Dufour J.F., et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y.L., Reeves H.L., Burt A.D., Tiniakos D., McPherson S., Leathart J.B., et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang S., Zhang J., Mei T.T., Guo H.Q., Wei X.H., Zhang W.Y., et al. Association of TM6SF2 rs58542926 T/C gene polymorphism with hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2019;19:1128. doi: 10.1186/s12885-019-6173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anstee Q.M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D., et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort(☆) J Hepatol. 2020;73:505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ueyama M., Nishida N., Korenaga M., Korenaga K., Kumagai E., Yanai H., et al. The impact of PNPLA3 and JAZF1 on hepatocellular carcinoma in non-viral hepatitis patients with type 2 diabetes mellitus. J Gastroenterol. 2016;51:370–379. doi: 10.1007/s00535-015-1116-6. [DOI] [PubMed] [Google Scholar]
- 16.Liu D.J., Peloso G.M., Yu H., Butterworth A.S., Wang X., Mahajan A., et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49:1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luukkonen P.K., Zhou Y., Sadevirta S., Leivonen M., Arola J., Oresic M., et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Bianco C., Jamialahmadi O., Pelusi S., Baselli G., Dongiovanni P., Zanoni I., et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775–782. doi: 10.1016/j.jhep.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S., Li A., Liu F., Chen F., Williams M., Zhang C., et al. NCOA5 haploinsufficiency results in glucose intolerance and subsequent hepatocellular carcinoma. Cancer Cell. 2013;24:725–737. doi: 10.1016/j.ccr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhar D., Seki E., Karin M. NCOA5, IL-6, type 2 diabetes, and HCC: the deadly quartet. Cell Metab. 2014;19:6–7. doi: 10.1016/j.cmet.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Te Morenga L., Mallard S., Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346 doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 22.Vreman R.A., Goodell A.J., Rodriguez L.A., Porco T.C., Lustig R.H., Kahn J.G. Health and economic benefits of reducing sugar intake in the USA, including effects via non-alcoholic fatty liver disease: a microsimulation model. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wali J.A., Milner A.J., Luk A.W.S., Pulpitel T.J., Dodgson T., Facey H.J.W., et al. Impact of dietary carbohydrate type and protein-carbohydrate interaction on metabolic health. Nat Metab. 2021;3:810–828. doi: 10.1038/s42255-021-00393-9. [DOI] [PubMed] [Google Scholar]
- 24.Softic S., Gupta M.K., Wang G.X., Fujisaka S., O'Neill B.T., Rao T.N., et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017;127:4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todoric J., Di Caro G., Reibe S., Henstridge D.C., Green C.R., Vrbanac A., et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab. 2020;2:1034–1045. doi: 10.1038/s42255-020-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tee S.S., Kim N., Cullen Q., Eskandari R., Mamakhanyan A., Srouji R.M., et al. Ketohexokinase-mediated fructose metabolism is lost in hepatocellular carcinoma and can be leveraged for metabolic imaging. Sci Adv. 2022;8 doi: 10.1126/sciadv.abm7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoli A., Bianco A., Grimaldi K.A., Lodi A., Bosco G. Long term successful weight loss with a combination biphasic ketogenic Mediterranean diet and Mediterranean diet maintenance protocol. Nutrients. 2013;5:5205–5217. doi: 10.3390/nu5125205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe M., Tozzi R., Risi R., Tuccinardi D., Mariani S., Basciani S., et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes Rev. 2020;21 doi: 10.1111/obr.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Couteur D.G., Solon-Biet S.M., Parker B.L., Pulpitel T., Brandon A.E., Hunt N.J., et al. Nutritional reprogramming of mouse liver proteome is dampened by metformin, resveratrol, and rapamycin. Cell Metab. 2021;33:2367–2379 e2364. doi: 10.1016/j.cmet.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Solon-Biet S.M., McMahon A.C., Ballard J.W., Ruohonen K., Wu L.E., Cogger V.C., et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahma M.K., Gilglioni E.H., Zhou L., Trepo E., Chen P., Gurzov E.N. Oxidative stress in obesity-associated hepatocellular carcinoma: sources, signaling and therapeutic challenges. Oncogene. 2021;40:5155–5167. doi: 10.1038/s41388-021-01950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong X., Kuang H., Liu T., Lin J.D. A single-cell perspective of the mammalian liver in health and disease. Hepatology. 2020;71:1467–1473. doi: 10.1002/hep.31149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J.D., Ahmed F., Mara K.C., Addissie B.D., Allen A.M., Gores G.J., et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. 2020;71:907–916. doi: 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander M., Loomis A.K., van der Lei J., Duarte-Salles T., Prieto-Alhambra D., Ansell D., et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantarini M.C., de la Monte S.M., Pang M., Tong M., D'Errico A., Trevisani F., et al. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka S., Mohr L., Schmidt E.V., Sugimachi K., Wands J.R. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598–604. doi: 10.1002/hep.510260310. [DOI] [PubMed] [Google Scholar]
- 37.Roden M., Shulman G.I. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 38.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019;29:174–182 e175. doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targher G., Corey K.E., Byrne C.D., Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 40.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G., et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ter Horst K.W., Gilijamse P.W., Versteeg R.I., Ackermans M.T., Nederveen A.J., la Fleur S.E., et al. Hepatic diacylglycerol-associated protein kinase cepsilon translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep. 2017;19:1997–2004. doi: 10.1016/j.celrep.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurzov E.N., Tran M., Fernandez-Rojo M.A., Merry T.L., Zhang X., Xu Y., et al. Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab. 2014;20:85–102. doi: 10.1016/j.cmet.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roumans K.H.M., Lindeboom L., Veeraiah P., Remie C.M.E., Phielix E., Havekes B., et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat Commun. 2020;11:1891. doi: 10.1038/s41467-020-15684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul B., Lewinska M., Andersen J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broadfield L.A., Duarte J.A.G., Schmieder R., Broekaert D., Veys K., Planque M., et al. Fat induces glucose metabolism in nontransformed liver cells and promotes liver tumorigenesis. Cancer Res. 2021;81:1988–2001. doi: 10.1158/0008-5472.CAN-20-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu J., Che L., Li L., Pilo M.G., Cigliano A., Ribback S., et al. Co-activation of AKT and c-Met triggers rapid hepatocellular carcinoma development via the mTORC1/FASN pathway in mice. Sci Rep. 2016;6 doi: 10.1038/srep20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L., Pilo G.M., Li X., Cigliano A., Latte G., Che L., et al. Inactivation of fatty acid synthase impairs hepatocarcinogenesis driven by AKT in mice and humans. J Hepatol. 2016;64:333–341. doi: 10.1016/j.jhep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia J., Che L., Cigliano A., Wang X., Peitta G., Tao J., et al. Pivotal role of fatty acid synthase in c-MYC driven hepatocarcinogenesis. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Che L., Pilo M.G., Cigliano A., Latte G., Simile M.M., Ribback S., et al. Oncogene dependent requirement of fatty acid synthase in hepatocellular carcinoma. Cell Cycle. 2017;16:499–507. doi: 10.1080/15384101.2017.1282586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasenfuss S.C., Bakiri L., Thomsen M.K., Williams E.G., Auwerx J., Wagner E.F. Regulation of steatohepatitis and PPARgamma signaling by distinct AP-1 dimers. Cell Metab. 2014;19:84–95. doi: 10.1016/j.cmet.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.M., Muratalla J., Karimi S., Diaz-Ruiz A., Frutos M.D., Guzman G., et al. Hepatocyte PPARgamma contributes to the progression of non-alcoholic steatohepatitis in male and female obese mice. Cell Mol Life Sci. 2023;80:39. doi: 10.1007/s00018-022-04629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettinelli P., Videla L.A. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96:1424–1430. doi: 10.1210/jc.2010-2129. [DOI] [PubMed] [Google Scholar]
- 53.Yu J., Shen B., Chu E.S., Teoh N., Cheung K.F., Wu C.W., et al. Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. Hepatology. 2010;51:2008–2019. doi: 10.1002/hep.23550. [DOI] [PubMed] [Google Scholar]
- 54.Katoch S., Sharma V., Patial V. Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma: experimental and clinical scenarios. World J Gastroenterol. 2022;28:3535–3554. doi: 10.3748/wjg.v28.i28.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubois V., Eeckhoute J., Lefebvre P., Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127:1202–1214. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimano H., Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710–730. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 57.Li C., Yang W., Zhang J., Zheng X., Yao Y., Tu K., et al. SREBP-1 has a prognostic role and contributes to invasion and metastasis in human hepatocellular carcinoma. Int J Mol Sci. 2014;15:7124–7138. doi: 10.3390/ijms15057124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C., Tong Y., Wen Y., Cai J., Guo H., Huang L., et al. Hepatocellular carcinoma-associated protein TD26 interacts and enhances sterol regulatory element-binding protein 1 activity to promote tumor cell proliferation and growth. Hepatology. 2018;68:1833–1850. doi: 10.1002/hep.30030. [DOI] [PubMed] [Google Scholar]
- 59.Li N., Zhou Z.S., Shen Y., Xu J., Miao H.H., Xiong Y., et al. Inhibition of the sterol regulatory element-binding protein pathway suppresses hepatocellular carcinoma by repressing inflammation in mice. Hepatology. 2017;65:1936–1947. doi: 10.1002/hep.29018. [DOI] [PubMed] [Google Scholar]
- 60.Lewinska M., Santos-Laso A., Arretxe E., Alonso C., Zhuravleva E., Jimenez-Aguero R., et al. The altered serum lipidome and its diagnostic potential for Non-Alcoholic Fatty Liver (NAFL)-associated hepatocellular carcinoma. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Y., Li N., Gao L., Xu Y.J., Huang C., Yu K., et al. Acetylcarnitine is a candidate diagnostic and prognostic biomarker of hepatocellular carcinoma. Cancer Res. 2016;76:2912–2920. doi: 10.1158/0008-5472.CAN-15-3199. [DOI] [PubMed] [Google Scholar]
- 62.Muir K., Hazim A., He Y., Peyressatre M., Kim D.Y., Song X., et al. Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma. Cancer Res. 2013;73:4722–4731. doi: 10.1158/0008-5472.CAN-12-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson M.W., Harmon C., O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Remmerie A., Martens L., Thone T., Castoldi A., Seurinck R., Pavie B., et al. Osteopontin expression identifies a subset of recruited macrophages distinct from kupffer cells in the fatty liver. Immunity. 2020;53:641–657 e614. doi: 10.1016/j.immuni.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aleksandrova K., Boeing H., Nothlings U., Jenab M., Fedirko V., Kaaks R., et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60:858–871. doi: 10.1002/hep.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duan Y., Pan X., Luo J., Xiao X., Li J., Bestman P.L., et al. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.880298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ponziani F.R., Bhoori S., Castelli C., Putignani L., Rivoltini L., Del Chierico F., et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 68.Park E.J., Lee J.H., Yu G.Y., He G., Ali S.R., Holzer R.G., et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J., et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luedde T., Schwabe R.F. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heida A., Gruben N., Catrysse L., Koehorst M., Koster M., Kloosterhuis N.J., et al. The hepatocyte IKK:NF-kappaB axis promotes liver steatosis by stimulating de novo lipogenesis and cholesterol synthesis. Mol Metab. 2021;54 doi: 10.1016/j.molmet.2021.101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenachot X., Ramadori G., Ioris R.M., Veyrat-Durebex C., Altirriba J., Aras E., et al. Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat Commun. 2017;8:1820. doi: 10.1038/s41467-017-02074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luedde T., Beraza N., Kotsikoris V., van Loo G., Nenci A., De Vos R., et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Schneider A.T., Gautheron J., Feoktistova M., Roderburg C., Loosen S.H., Roy S., et al. RIPK1 suppresses a TRAF2-dependent pathway to liver cancer. Cancer Cell. 2017;31:94–109. doi: 10.1016/j.ccell.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 75.He G., Yu G.Y., Temkin V., Ogata H., Kuntzen C., Sakurai T., et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gurzov E.N., Stanley W.J., Pappas E.G., Thomas H.E., Gough D.J. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016;283:3002–3015. doi: 10.1111/febs.13709. [DOI] [PubMed] [Google Scholar]
- 77.He G., Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grohmann M., Wiede F., Dodd G.T., Gurzov E.N., Ooi G.J., Butt T., et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018;175:1289–1306 e1220. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calvisi D.F., Ladu S., Gorden A., Farina M., Conner E.A., Lee J.S., et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Singh R., Wang Y., Xiang Y., Tanaka K.E., Gaarde W.A., Czaja M.J. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Litwak S.A., Pang L., Galic S., Igoillo-Esteve M., Stanley W.J., Turatsinze J.V., et al. JNK activation of BIM promotes hepatic oxidative stress, steatosis, and insulin resistance in obesity. Diabetes. 2017;66:2973–2986. doi: 10.2337/db17-0348. [DOI] [PubMed] [Google Scholar]
- 82.Strasser A., Vaux D.L. Cell death in the origin and treatment of cancer. Mol Cell. 2020;78:1045–1054. doi: 10.1016/j.molcel.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Hui L., Zatloukal K., Scheuch H., Stepniak E., Wagner E.F. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Das M., Garlick D.S., Greiner D.L., Davis R.J. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ieronymaki E., Theodorakis E.M., Lyroni K., Vergadi E., Lagoudaki E., Al-Qahtani A., et al. Insulin resistance in macrophages alters their metabolism and promotes an M2-like phenotype. J Immunol. 2019;202:1786–1797. doi: 10.4049/jimmunol.1800065. [DOI] [PubMed] [Google Scholar]
- 86.Ma C., Kesarwala A.H., Eggert T., Medina-Echeverz J., Kleiner D.E., Jin P., et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai S., Clemente-Casares X., Zhou A.C., Lei H., Ahn J.J., Chan Y.T., et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018;28:922–934 e924. doi: 10.1016/j.cmet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 88.Dudek M., Pfister D., Donakonda S., Filpe P., Schneider A., Laschinger M., et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 89.Pfister D., Nunez N.G., Pinyol R., Govaere O., Pinter M., Szydlowska M., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leslie J., Mackey J.B.G., Jamieson T., Ramon-Gil E., Drake T.M., Fercoq F., et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut. 2022;71:2093–2106. doi: 10.1136/gutjnl-2021-326259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirchner H., Sinha I., Gao H., Ruby M.A., Schonke M., Lindvall J.M., et al. Altered DNA methylation of glycolytic and lipogenic genes in liver from obese and type 2 diabetic patients. Mol Metab. 2016;5:171–183. doi: 10.1016/j.molmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brunt E.M., Wong V.W., Nobili V., Day C.P., Sookoian S., Maher J.J., et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 93.Ling C., Bacos K., Ronn T. Epigenetics of type 2 diabetes mellitus and weight change - a tool for precision medicine? Nat Rev Endocrinol. 2022;18:433–448. doi: 10.1038/s41574-022-00671-w. [DOI] [PubMed] [Google Scholar]
- 94.Tian Y., Arai E., Makiuchi S., Tsuda N., Kuramoto J., Ohara K., et al. Aberrant DNA methylation results in altered gene expression in non-alcoholic steatohepatitis-related hepatocellular carcinomas. J Cancer Res Clin Oncol. 2020;146:2461–2477. doi: 10.1007/s00432-020-03298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahrens M., Ammerpohl O., von Schonfels W., Kolarova J., Bens S., Itzel T., et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 96.Loomba R., Gindin Y., Jiang Z., Lawitz E., Caldwell S., Djedjos C.S., et al. DNA methylation signatures reflect aging in patients with nonalcoholic steatohepatitis. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cancer Genome Atlas Research Network Electronic address wbe, cancer genome atlas research N. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169:1327–1341 e1323. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu T., Luo G., Lian Q., Sui C., Tang J., Zhu Y., et al. Discovery of a carbamoyl phosphate synthetase 1-deficient HCC subtype with therapeutic potential through integrative genomic and experimental analysis. Hepatology. 2021;74:3249–3268. doi: 10.1002/hep.32088. [DOI] [PubMed] [Google Scholar]
- 99.Wei H., Wang J., Li W., Ma R., Xu Z., Luo Z., et al. The underlying pathophysiology association between the Type 2-diabetic and hepatocellular carcinoma. J Cell Physiol. 2019;234:10835–10841. doi: 10.1002/jcp.27919. [DOI] [PubMed] [Google Scholar]
- 100.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F., et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Lee D., Xu I.M., Chiu D.K., Leibold J., Tse A.P., Bao M.H., et al. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology. 2019;69:1768–1786. doi: 10.1002/hep.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polonsky K.S., Given B.D., Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang A.M.Y., Wellberg E.A., Kopp J.L., Johnson J.D. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab J. 2021;45:285–311. doi: 10.4093/dmj.2020.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janku F., Kaseb A.O., Tsimberidou A.M., Wolff R.A., Kurzrock R. Identification of novel therapeutic targets in the PI3K/AKT/mTOR pathway in hepatocellular carcinoma using targeted next generation sequencing. Oncotarget. 2014;5:3012–3022. doi: 10.18632/oncotarget.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao Y.J., Ping X.L., Zhang H., Chen F.F., Lee P.K., Ahsan H., et al. PTEN/MMAC1 mutations in hepatocellular carcinomas. Oncogene. 1999;18:3181–3185. doi: 10.1038/sj.onc.1202659. [DOI] [PubMed] [Google Scholar]
- 106.Dombrowski F., Bannasch P., Pfeifer U. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in streptozotocin diabetic rats. Am J Pathol. 1997;150:1071–1087. [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu C., Tabas I., Schwabe R.F., Pajvani U.B. Maladaptive regeneration - the reawakening of developmental pathways in NASH and fibrosis. Nat Rev Gastroenterol Hepatol. 2021;18:131–142. doi: 10.1038/s41575-020-00365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang X., Zheng Z., Caviglia J.M., Corey K.E., Herfel T.M., Cai B., et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Loft A., Alfaro A.J., Schmidt S.F., Pedersen F.B., Terkelsen M.K., Puglia M., et al. Liver-fibrosis-activated transcriptional networks govern hepatocyte reprogramming and intra-hepatic communication. Cell Metab. 2021;33:1685–1700 e1689. doi: 10.1016/j.cmet.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Sullivan G.J., Hay D.C., Park I.H., Fletcher J., Hannoun Z., Payne C.M., et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szkolnicka D., Hay D.C. Concise review: advances in generating hepatocytes from pluripotent stem cells for translational medicine. Stem Cells. 2016;34:1421–1426. doi: 10.1002/stem.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heslop J.A., Duncan S.A. The use of human pluripotent stem cells for modeling liver development and disease. Hepatology. 2019;69:1306–1316. doi: 10.1002/hep.30288. [DOI] [PubMed] [Google Scholar]
- 113.Hu W., Lazar M.A. Modelling metabolic diseases and drug response using stem cells and organoids. Nat Rev Endocrinol. 2022;18:744–759. doi: 10.1038/s41574-022-00733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rombaut M., Boeckmans J., Rodrigues R.M., van Grunsven L.A., Vanhaecke T., De Kock J. Direct reprogramming of somatic cells into induced hepatocytes: cracking the Enigma code. J Hepatol. 2021;75:690–705. doi: 10.1016/j.jhep.2021.04.048. [DOI] [PubMed] [Google Scholar]
- 115.Blaszkiewicz J., Duncan S.A. Advancements in disease modeling and drug discovery using iPSC-derived hepatocyte-like cells. Genes (Basel) 2022;13 doi: 10.3390/genes13040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mun S.J., Ryu J.S., Lee M.O., Son Y.S., Oh S.J., Cho H.S., et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71:970–985. doi: 10.1016/j.jhep.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 117.Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarro L.M., Bradshaw C.R., et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 119.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 120.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 121.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 122.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu A.X., Kang Y.K., Yen C.J., Finn R.S., Galle P.R., Llovet J.M., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 124.Wang H., Zhou Y., Xu H., Wang X., Zhang Y., Shang R., et al. Therapeutic efficacy of FASN inhibition in preclinical models of HCC. Hepatology. 2022;76:951–966. doi: 10.1002/hep.32359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Loomba R., Mohseni R., Lucas K.J., Gutierrez J.A., Perry R.G., Trotter J.F., et al. TVB-2640 (FASN inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterology. 2021;161:1475–1486. doi: 10.1053/j.gastro.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 126.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schulte L., Scheiner B., Voigtlander T., Koch S., Schweitzer N., Marhenke S., et al. Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int. 2019;39:714–726. doi: 10.1111/liv.14048. [DOI] [PubMed] [Google Scholar]
- 128.Singh S., Singh P.P., Singh A.G., Murad M.H., Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–891. doi: 10.1038/ajg.2013.5. ; quiz 892. [DOI] [PubMed] [Google Scholar]
- 129.Zhang H., Gao C., Fang L., Zhao H.C., Yao S.K. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78–87. doi: 10.3109/00365521.2012.719926. [DOI] [PubMed] [Google Scholar]
- 130.Li Q., Xu H., Sui C., Zhang H. Impact of metformin use on risk and mortality of hepatocellular carcinoma in diabetes mellitus. Clin Res Hepatol Gastroenterol. 2022;46 doi: 10.1016/j.clinre.2021.101781. [DOI] [PubMed] [Google Scholar]
- 131.Casadei Gardini A., Faloppi L., De Matteis S., Foschi F.G., Silvestris N., Tovoli F., et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: validation study and biological rationale. Eur J Cancer. 2017;86:106–114. doi: 10.1016/j.ejca.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 132.Casadei Gardini A., Marisi G., Scarpi E., Scartozzi M., Faloppi L., Silvestris N., et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin Pharmacother. 2015;16:2719–2725. doi: 10.1517/14656566.2015.1102887. [DOI] [PubMed] [Google Scholar]
- 133.Chung Y.K., Hwang S., Song G.W., Lee Y.J., Kim K.H., Ahn C.S., et al. Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation. Ann Hepatobiliary Pancreat Surg. 2018;22:297–304. doi: 10.14701/ahbps.2018.22.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.El Shorbagy S., abuTaleb F., Labib H.A., Ebian H., Harb O.A., Mohammed M.S., et al. Prognostic significance of VEGF and HIF-1 alpha in hepatocellular carcinoma patients receiving sorafenib versus metformin sorafenib combination. J Gastrointest Cancer. 2021;52:269–279. doi: 10.1007/s12029-020-00389-w. [DOI] [PubMed] [Google Scholar]
- 135.Cho Y.Y., Yu S.J., Lee H.W., Kim D.Y., Kang W., Paik Y.H., et al. Clinical characteristics of long-term survivors after sorafenib treatment for unresectable hepatocellular carcinoma: a Korean national multicenter retrospective cohort study. J Hepatocell Carcinoma. 2021;8:613–623. doi: 10.2147/JHC.S304439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chang C.H., Lin J.W., Wu L.C., Lai M.S., Chuang L.M., Chan K.A. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55:1462–1472. doi: 10.1002/hep.25509. [DOI] [PubMed] [Google Scholar]
- 137.Lai S.W., Lin C.L., Liao K.F. Association of hepatocellular carcinoma with thiazolidinediones use: a population-based case-control study. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yen F.S., Wei J.C.C., Chiu L.T., Hsu C.C., Hou M.C., Hwu C.M. Thiazolidinediones were associated with higher risk of cardiovascular events in patients with type 2 diabetes and cirrhosis. Liver Int. 2021;41:110–122. doi: 10.1111/liv.14714. [DOI] [PubMed] [Google Scholar]
- 139.Hendryx M., Dong Y., Ndeke J.M., Luo J. Sodium-glucose cotransporter 2 (SGLT2) inhibitor initiation and hepatocellular carcinoma prognosis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0274519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 141.Eslam M., Sanyal A.J., George J., International Consensus P MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014 e1991. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 142.Febbraio M.A., Reibe S., Shalapour S., Ooi G.J., Watt M.J., Karin M. Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 2019;29:18–26. doi: 10.1016/j.cmet.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu Y., Yang A., Quan C., Pan Y., Zhang H., Li Y., et al. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun. 2022;13:4594. doi: 10.1038/s41467-022-32283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saviano A., Henderson N.C., Baumert T.F. Single-cell genomics and spatial transcriptomics: discovery of novel cell states and cellular interactions in liver physiology and disease biology. J Hepatol. 2020;73:1219–1230. doi: 10.1016/j.jhep.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guilliams M., Bonnardel J., Haest B., Vanderborght B., Wagner C., Remmerie A., et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–396 e338. doi: 10.1016/j.cell.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Evans J.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Home P.D., Kahn S.E., Jones N.P., Noronha D., Beck-Nielsen H., Viberti G., et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A diabetes outcome progression trial) and RECORD (rosiglitazone evaluated for cardiovascular outcomes and regulation of glycaemia in diabetes) clinical trials. Diabetologia. 2010;53:1838–1845. doi: 10.1007/s00125-010-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Donadon V., Balbi M., Mas M.D., Casarin P., Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 149.Vilar-Gomez E., Calzadilla-Bertot L., Wong V.W., Castellanos M., Aller-de la Fuente R., Eslam M., et al. Type 2 diabetes and metformin use associate with outcomes of patients with nonalcoholic steatohepatitis-related, child-pugh A cirrhosis. Clin Gastroenterol Hepatol. 2021;19:136–145 e136. doi: 10.1016/j.cgh.2020.04.083. [DOI] [PubMed] [Google Scholar]
- 150.Kaplan D.E., Serper M., John B.V., Tessiatore K.M., Lerer R., Mehta R., et al. Effects of metformin exposure on survival in a large national cohort of patients with diabetes and cirrhosis. Clin Gastroenterol Hepatol. 2021;19:2148–2160 e2114. doi: 10.1016/j.cgh.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 151.Yen F.S., Wei J.C., Yip H.T., Hwu C.M., Hou M.C., Hsu C.C. Dipeptidyl peptidase-4 inhibitors may accelerate cirrhosis decompensation in patients with diabetes and liver cirrhosis: a nationwide population-based cohort study in Taiwan. Hepatol Int. 2021;15:179–190. doi: 10.1007/s12072-020-10122-1. [DOI] [PubMed] [Google Scholar]
- 152.Kramer J.R., Natarajan Y., Dai J., Yu X., Li L., El-Serag H.B., et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75:1420–1428. doi: 10.1002/hep.32244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen T.M., Lin C.C., Huang P.T., Wen C.F. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858–865. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 154.Bhat M., Chaiteerakij R., Harmsen W.S., Schleck C.D., Yang J.D., Giama N.H., et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:15750–15755. doi: 10.3748/wjg.v20.i42.15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jang W.I., Kim M.S., Lim J.S., Yoo H.J., Seo Y.S., Han C.J., et al. Survival advantage associated with metformin usage in hepatocellular carcinoma patients receiving radiotherapy: a propensity score matching analysis. Anticancer Res. 2015;35:5047–5054. [PubMed] [Google Scholar]
- 156.Seo Y.S., Kim Y.J., Kim M.S., Suh K.S., Kim S.B., Han C.J., et al. Association of metformin use with cancer-specific mortality in hepatocellular carcinoma after curative resection: a nationwide population-based study. Medicine (Baltimore) 2016;95:e3527. doi: 10.1097/MD.0000000000003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 158.Abou-Alfa G.K., Lau G., Kudo M., Chan S.L., Kelley R.K., Furuse J., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 159.Kelley R.K., Rimassa L., Cheng A.L., Kaseb A., Qin S., Zhu A.X., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 160.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 161.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 162.Yau T., Kang Y.K., Kim T.Y., El-Khoueiry A.B., Santoro A., Sangro B., et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Finn R.S., Ryoo B.Y., Merle P., Kudo M., Bouattour M., Lim H.Y., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 164.Qin S., Chen Z., Fang W., Ren Z., Xu R., Ryoo B.-Y., et al. Pembrolizumab Versus Placebo as Second-Line Therapy in Patients From Asia With Advanced Hepatocellular Carcinoma: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2023;41:1434–1443. doi: 10.1200/JCO.22.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.