Abstract
This study aimed to determine whether the lotus leaf extract (LLE) had the effect of treating salpingitis in laying hens. First, the salpingitis model was established by the method of bacterial infection. Differential genes between salpingitis and healthy laying hens were identified by transcriptome sequencing, and GO and KEGG enrichment analyses were performed. Groups of treatment of antibiotics and LLE were established to verify the feasibility of the lotus leaf extract in treating salpingitis. Furthermore, the active component and pharmacological effects of LLE were identified using the UPLC-Q-TOF-MS and network pharmacology technique. At last, the mechanism of LLE treating salpingitis was further evaluated by DF-1 cells infected with bacteria. The results showed that LLE significantly reduced the levels of TLR4 and IFN-γ (P < 0.05), accelerated the levels of IgA and IgG (P < 0.05), regulated the levels of SOD and MDA (P < 0.05) in laying hens with salpingitis. A total of 1,874 differential genes were obtained according to the transcriptome sequencing. It was revealed a significant role in cell cycle and apoptosis by enrichment analysis. In addition, among the 28 components identified by UPLC-Q-TOF-MS, 20 components acted on 58 genes, including CDK1, BIRC5, and CA2 for treating salpingitis. After bacterial infection, cells were damaged and unable to complete the normal progression of the cell cycle, leading to cell cycle arrest and further apoptosis formation. However, with the intervention of LLE, bacterial infection was resisted. The cells proliferation was extensively restored, and the expression of NO was increased. The addition of LLE significantly decreased cell apoptosis. The G1 phase increased, the S phase and the G2 phase decreased in the model group; after the intervention of LLE, the G1 phase gradually returned to the average level, and G2 and S phases increased. The mRNA expression levels of BIRC5, CDK1, and CA2 were consistent with the predicted results in network pharmacology. At the same time, the mRNA expression levels of Caspase-3 and Caspase-7 were reduced after added with LLE. The mRNA expression levels of TNF-α, TRADD, FADD, Caspase-8, Caspase-10, and Caspase-9 (P < 0.05), which would inhibit death receptor activation and decrease the apoptotic cascade, were upregulated after bacterial infection. However, the results in LLE groups were downregulated (P < 0.05). Meanwhile, the mRNA expression levels of BCL-2 in LLE groups were increased significantly compared with it in model group (P < 0.05). Notably, LLE administration inhibited apoptosis and regulated the cell cycle distribution in the salpingitis induced by bacterial infection. These results indicated that the LLE attenuated bacterial-induced salpingitis by modulating apoptosis and immune function in laying hens.
Key words: lotus leaf extract, salpingitis, apoptosis, death receptor signaling pathways
INTRODUCTION
Salpingitis of laying hens is a common disease in breeding laying hens. Its clinical features frequently include oviductal inflammation, broken shell eggs, oophoritis, and peritonitis (Jordan et al., 2005). In severe cases, yolk accumulation and a large amount of white cheese-like secretion may be observed during the autopsy, leading to blockage and necrosis of fallopian tubes (Gretarsson et al., 2022). The causes of the disease are diverse and include poor hygiene in poultry houses (Abrahamsson et al., 1998; Saraiva et al., 2021), contamination of the cloaca by bacteria, and invasion into the oviduct (Poulsen et al., 2020; Yang et al., 2020), excessive egg production (Wang et al., 2020a), and lack of vitamins in feed (Ozaki et al., 2018). The disease is easy to break out on a large scale, with a high incidence rate, resulting in a decline in production performance, thus causing incalculable losses to the chicken industry.
Traditional Chinese herbal medicine can regulate the body's metabolism, immunity, and intestinal flora (Liang et al., 2013; Qi et al., 2017; Zhang et al., 2021). Under the large environment of banning the addition of antibiotics in livestock feed, Chinese herbal medicine has gradually moved into the stage of the new era (Gao et al., 2022). However, due to the uncertainty of the ingredients and the unclear pharmacological effects of various components, the progress of the popularization and application of Chinese herbal medicine in clinic has been hindered (Li et al., 2022). Lotus (Nelumbo nucifera gain) is a plant of Nymphaea, and the lotus leaf is its dry leaf and a homology of medicine and food. In the Chinese Pharmacopoeia, it was recorded to clear away heat, nourishes Yang, and stop bleeding. In addition, existing studies have shown that lotus leaf has antioxidant, anti-inflammatory, and antitumor effects (Cho et al., 2017; Song et al., 2020; Wang et al., 2023). However, research has yet to point out the feasibility of LLE in treating salpingitis in laying hens.
In this study, the effect of LLE for treating salpingitis in laying hens was verified by in vivo experiments. Method of transcriptome sequencing identifies the differential genes in laying hens with salpingitis. UPLC-Q-TOF-MS method was used to analyze the composition of LLE. The mechanism of LLE treating salpingitis in laying hens was predicted based on network pharmacology. Deeply, the mechanism was verified using vitro flow cytometry and real-time PCR methods in DF-1 cells. This study would provide a theoretical basis for the clinical development of LLE to prevent and treat salpingitis in laying hens.
MATERIALS AND METHODS
Ethics Statement
Animal experiments in this study were performed according to the Regulations on Administration of Animal Experiments (Ministry of Science and Technology of China, Approval No. 2006-398) and approved by the Animal Ethics Committee of Yangtze University (Jingzhou, Hubei, China).
Preparation of LLE
The LLE was prepared using a previous method reported by Cheng et al. (2021).
Animals, Experimental Design, and Management
The experiment was carried out according to the Chinese guidelines for Animal Welfare and approved by the Animal Ethics Committee of Yangtze University. A total of 100 healthy 500-day-old Helen Brown laying hens (Hubei Fuqiang Poultry Culture Co., Ltd., Hubei, China) were maintained in individual cages (400 mm × 350 mm × 320 mm). Cages were randomly placed in a ventilated room with a temperature of 26°C ± 2°C, a humidity of 60 ± 10%, and light of 16 h/d. Diets and water were offered daily for ad libitum intake. The diets were laying hens' feed produced by Charoen Pokphand Group in China (Table 1). Drinking water was changed once in 8 h. After adaption for 7 d, 100 laying hens were randomly divided into 5 groups as follows: blank group (without any treatment), Amoxicillin group (250 mg/kg Amoxicillin), low-dose LLE group (100 mg/kg LLE), medium-dose LLE group (125 mg/kg LLE), and high-dose LLE group (250 mg/kg LLE). To establish the salpingitis model, the laying hens except in the blank group were injected with suspension containing 0.66 mL of Staphylococcus aureus and 1.32 mL of Escherichia coli (1 × 108 CFU/mL, 1:2) via oviducts. Three laying hens were randomly sampled in the blank and model groups on d 7 to evaluate whether the model establishment was successful. Oviduct tissue was collected for transcriptome sequencing, and the remaining laying hens were treated according to drug dose. After 7 d, blood samples were obtained as quickly as possible from the wing vein, immediately transferred to the laboratory, and allowed to clot at 37°C for 2 h. Serum separated by centrifugation at 4,000 × g for 15 min at 4°C. Serum samples were stored at −20°C to analyze immune indicators and inflammatory factors further. The laying hens were then euthanized by cervical dislocation. The liver and ovaries tissue were collected and stored at −80°C for further analysis.
Table 1.
Dietary composition and nutrient levels of the basal diets.
| Ingredient | Content (%) | Nutrient levels | Content (%) |
|---|---|---|---|
| Corn | 55.42 | Crude protein | 16.5 |
| Conifer | 37.1 | Calcium | 4.20 |
| Selaginella | 2.5 | Total phosphorus | 0.5 |
| Calcium hydrogen phosphate | 0.25 | Crude fiber | 7.0 |
| Stone powder | 3.73 | Crude ash | 15.0 |
| Sodium chloride | 0.4 | Egg + cystine | 0.65 |
| Sodium chloride | 0.3 | Water content | 14.0 |
| Methionine hydroxyl analog | 0.3 | ||
| Total | 100 |
The above analytical error was performed under the allowable error as determined by the feed test results of GB/t18823.
Determination of Cytokine, Immunoglobulin Levels, and Antioxidant Index
The serum levels of TLR4 (MM-34115O1), IFN-γ (SEKCN-016), IL-10 (SEKCN-0097), IL-4 (SEKCN-0008), IgA (SEKCN-0018), and IgG (SEKCN-0126) were measured using sandwich ELISA kits (Solaibao, Beijing, China). After being weighed, the liver's tissue was added with 9 times amount of phosphate-buffered saline (PBS) for homogenization. The antioxidant levels were measured using SOD (A001-3-2) and MDA (A003-1) kits (Nanjing Jiancheng, Nanjing, China).
Detection of Constituents in LLE by UPLC-Q-TOF-MS
LLE (1.25 g) was dissolved in 7.5 mL methanol and sonicated for 60 min. The solution was complemented to 7.5 mL with methanol and centrifugated at 4,000 rpm/min for 10 min. One milliliter of the supernatant was collected, diluted 10,000 fold, and filtered by a 0.22 μm micropore filter membrane. A total of 0.5 mL filter liquor was taken for detection by ultrapore liquid chromatography (UPLC) system (AB Sciex Framingham, Massachusetts). The triple 5600 Plus high-resolution tandem mass spectrometer (AB Sciex Framingham, Massachusetts) was used to analyze positive and negative ion modes. The detection system was using a Phenomenex Kinetex C18 2.6 μM column, 0.1% formic acid in water as mobile phase A and acetonitrile as mobile phase B. Elution rate was 0.3 mL/min, the column temperature was 35°C, the collection time was 30 min, and the injection volume was 4 μL. The gradient elution procedure was 0 to 2 min, 0 to 6% B; 2 to 10 min, 6 to 60% B; 10 to 13 min, 60 to 80% B; 13 to 15 min, 80 to 95% B; 15 to 18 min, 95 to 100% B; 18 to 20 min, 100% B; 20 to 22 min, 100 to 6% B; 22 to 30 min, 6% B.
Use Triple Tof 5600+ system (SCIEXAB Sciex Framingham, Massachusetts) to detect the components of LLE after elution. ESI electric spray source was used, scanning range: TOF MS m/z 100 to 1,000; TOF MS/MS m/z 50 to 1,000, CDS automatic correction and positive and negative ion source parameters: spray voltage (ISVF): 5,500 V/–4,500 V; curtain air (CUR): 35 psi; ion source temperature (TEM): 550°C; atomizing gas Gas1: 55 psi; auxiliary gas Gas2: 55 psi; DP voltage: 70 V/–70; CE energy: 35 ± 15 V/–35 ± 15.
The mass spectrum data of LLE will be obtained using MasterView. The TCM MS/MS Library list was imported, matched, and retrieved the secondary spectrum library. The MasterView software was used to predict the molecular formula of completely unknown components by built-in peak extraction and Formula Finder functions, and match it with the ChemSpider database.
RNA Extraction and Transcriptome Sequencing of Oviduct Tissue
Total RNA was extracted using the Trizol (Invitrogen, Thermo Fisher Scientific, California), and RNA purity and integrity were monitored by NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and a Bioanalyzer 2100 system (Agilent Technologies, California). Oligo (dT)-attached magnetic beads were used to purify mRNA. Purified mRNA was fragmented into small pieces with fragment buffer at the appropriate temperature. Then first-strand cDNA was generated using random hexamer-primed reverse transcription, followed by second-strand cDNA synthesis and purification using AMPure XP Beads. Afterward, A-Tailing Mix and RNA Index Adapters were added by incubating to end cDNA repair. PCR amplified the cDNA fragments obtained from previous steps, and Ampure XP Bead refined products to get the final library. After the library was constructed, Qubit 2.0 was used for preliminary quantification, and the library was diluted to 1.5 ng/μL. Agilent 2100 BioAnalyzer was used to detect the insert size of the library. qRT-PCR was used to accurately quantify the effective concentration to ensure its quality of the library. After library detection was qualified, DNBS (DNA nano ball) were prepared and loaded onto the sequencing chip for sequencing using the MGI high-throughput sequencer.
Transcriptome Analysis
After the alignment of clean reads to the reference genome using hisat2 (v2.1.0), postquality control sequences were aligned to the reference transcript sequence using bowtie2 (v2.3.5). DESeq2 (V1.22.2) was used for differential expression significance analysis, and the screening threshold was false discovery rate (FDR) <0.05, log2FC (fold change) >1, or <−1.
Gene ontology (GO) is divided into 3 ontology, containing molecular function, biological processes, and cellular component. Hypergeometric distribution was used for GO enrichment analysis, and the GO term with Q value ≤0.05 was selected as the significantly enriched GO entry.
Kyoto Encyclopedia of Genes and Genomes (KEGG) is the central public database on Pathway, annotated with KOBAS (V3.0). Pathways with a Q value ≤0.05 were significantly enriched in differentially expressed genes. Pathway enrichment analysis was performed using R software combined with self-written scripts and BH correction.
Network Pharmacology of LLE on Prevention and Treatment of Salpingitis in Laying Hens
Constituents obtained by mass spectrometry were screened in the TCMSP database (https://old.tcmspe.com/tcmsp.php) (Ru et al., 2014). The used conditions were OB ≥30 and DL ≥0.18. Swiss ADME was further used for screening components that do not exist in TCMSP. The screening principle was Lipinski's “Rule of Five (Lipinski, 2016).” After obtaining the corresponding targets of all components, map them with the differential genes obtained by transcriptome sequencing. The network of drug targets was constructed by Cytoscape software (v3.9), and the drug disease consensus targets were performed by GO enrichment analysis and KEGG enrichment analysis.
Cell Proliferation, MOI, and NO Determination
DF-1 cells (Ploysay, Wuhan, China) were passed for 3 generations before experiments and then cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. Cells suspension was diluted to 1 × 105/mL and seeded into 96 well plates. A total of 1,000 μg/mL LLE aqueous solution filtered by 0.22 μm filter membrane was diluted to a series of concentrations using DMEM medium without serum. The different concentrations of LLE were added after the cells adhered. After incubation for 20 h at 37°C in a 5% CO2 incubator, 20 μL MTT solution was added into each well, and continued incubation for 4 h. The 490 nm reading was selected on a microplate reader to determine the effect of LLE on the viability of DF-1 cells.
Similarly, after the cells were inoculated into the 96-well plate, the suspension containing Escherichia coli and Staphylococcus aureus (1 × 108 CFU/mL, 1:2) was diluted to MOI = 1,25,50,100 and 200 using DMEM. Then immediately added to the cell culture plate. After 1 h coinfection, the multiplicity of infection was determined by inverted microscopy.
After determining the above conditions, 5 μg/mL, 15 μg/mL, and 25 μg/mL LLE were added into DF-1 cells for 24 h. Escherichia coli and Staphylococcus aureus (MOI = 200) coinfection was added for 1 h. The supernatant was collected to determine the NO content by commercial kit (s0021s, Beyotime, Sichuan, China).
Detection of Apoptosis and Cell Cycle by Flow Cytometry
At the end of the infection, apoptosis and cell cycle was assessed by propidium iodide staining and flow cytometry (Riccardi and Nicoletti, 2006), and data analysis and graphical analysis were performed using flow Jo software (v10.8).
Real-Time PCR for Apoptosis and Cell Cycle-Related mRNA Expression
Total RNA was extracted using the RNA Extraction Kit (25017 KD1; Axygen Scientific Inc,California), and the RNA concentration was measured using a microspectrophotometer (nanodrop2000, Thermo Fisher Scientific, Massachusetts). A reverse transcription Kit (rk20429; Abconal, Wuhan, China) was used for synthesizing complementary DNA (cDNA) following the manufacturer's recommended protocol. PCR primer sequences (Table 2) were synthesized by Shanghai SANGON bio (Shanghai, China). SYBR Green fast qPCR mix (RK21203; Abclonal, Wuhan, China) was used with the following program: 95°C for 3 min, 30 cycles of 95°C for 5 s, 60°C for 30 s. Internal controls used the comparative threshold cycle (2−ΔΔCt) method to process the data.
Table 2.
Primers for real-time quantitative PCR.
| Gene | Primer sequence (5′–3′) |
|---|---|
| TNF | F: CCGCCCAGTTCAGATGAGTT R: CAACCAGCTATGCACCCCA |
| Caspase-3 | F: AAGGCTCCTGGTTTATTCA R: CTGCCACTCTGCGATTTA |
| Caspase-8 | F: TCTAGCTTCCTACCTGGGCT R: AACGTCCGGCATTGTAGTTTC |
| BCL-2 | F: TTCCGTGATGGGGTCAACTG R: CACAAAGGCATCCCATCCTC |
| Caspase-7 | F: AATGAACACGGAAAACAACT R: GCATAGAGACTACGCAAGGA |
| Caspase-9 | F: CGAAGGAGCAAGCACGACA R: CAGGTTGGACTGGGATGGAC |
| Caspase-10 | F: ATGGCTTGGTCTGGATGT R: CTTGGCAGTGAAGTAGGT |
| FADD | F: ATGGATCCCTTCCTGGCTCT R: TCAGTTGCTGCTCCATGAGG |
| TRADD | F: ACTCCCCATCTCTACCTTC R: ACCCACTTGCTTCCACTTC |
| BIRC5 | F: CAGTGCTTCTTCTGCCTCAA R: CGCCCTTGGCTACATCTTC |
| CDK1 | F: AAGTGAGGAGGAAGGTG R: AATGGCAGAAGACAATAC |
| CA2 | F: CCGTCGTAGGCATCTTCA R: AGTCAGGGAGCCAGGGTA |
| GADPH | F: TCGGAGTCAACGGATTTGGC R: TTCCCGTTCTCAGCCTTGAC |
Statistical Analysis
All the above experiments were performed with more than 3 biological replicates, and all the data have been presented as means ± SD, and statistical methods were performed using one-way ANOVA followed by Tukey's multiple comparisons to determine the significant differences between groups further. All the data were plotted using IBM SPSS 25 analysis and GraphPad 8.0, and the statistical significance was graded as P < 0.05, P < 0.01, and P < 0.001.
RESULTS
Evaluation of LLE in the Treatment of Salpingitis in Laying Hens
Effects of LLE on Inflammatory and Immune Factors in Laying Hens With Salpingitis
As shown in Figure 1A, although the level of TLR4 in the low-dose LLE group was significantly higher than that in the blank group (P < 0.05), as the dose of LLE increased, the level of TLR4 gradually returned to the average level of normal laying hens, with no significant difference compared to that in the Amoxicillin group (P > 0.05). In addition, as shown in Figure 1B, the level of IFN- γ in Amoxicillin group and the LLE groups were higher than that of the blank group (P < 0.05). It was worth noting that as the dose of LLE increased, the level of IFN- γ showed a dose-dependent downward trend.
Figure 1.
Effects of LLE on serum inflammatory factors, immunity, and hepatic antioxidation in laying hens with salpingitis, they were grouped in the order of blank, Amoxicillin, low-, medium-, and high-dose groups of lotus leaf extract. (A) Serum TLR4, (B) serum IFN-γ Levels, (C) serum IL-10 levels, (D) serum IL-4 level, (E) serum IgG levels, (F) serum IgG levels, (G) liver MDA levels, (H) liver SOD levels. All experiments were repeated more than 3 times and presented as mean ± SD (significant differences (P < 0.05) between groups with different superscripts a, b, c, and d).
As shown in Figure 1C, the level of IL-10 in the Amoxicillin group was significantly higher than that in the blank group (P < 0.05). There was no statistically significant difference of the level of IL-10 between the LLE groups and the blank group (P > 0.05). The level of IL-4 was shown in Figure 1D. There was no statistically significant difference in IL-4 levels between the Amoxicillin group and the high-dose LLE group compared to the blank group (P > 0.05). Although there were significant differences between the low and medium doses of the LLE groups and the blank group (P < 0.05), there was a trend toward a dose-dependent recovery.
The effects of LLE on immune indicators in laying hens with salpingitis were shown in Figure 1E and F. The levels of IgG and IgA in the Amoxicillin group and the high-dose group were significantly higher than those in the blank group (P < 0.05). There was no significant difference between the low-dose and medium-dose LLE groups and the blank group (P > 0.05), but there was a dose-dependent upward trend.
Effect of LLE on Hepatic Antioxidant Levels in Laying Hens With Salpingitis
MDA expression level was shown in Figure 1G. Compared with the blank group, the level of MDA in the Amoxicillin group and the LLE high-dose group recovered to normal levels (P > 0.05), whereas that in the LLE low- and middle-dose groups gradually recovered with dose supplementation, despite their differences from the blank group (P < 0.05). SOD expression level was shown in Figure 1H. The level of SOD in medium and high doses of LLE was significantly increased compared to that in the blank group and Amoxicillin group (P < 0.05).
Mechanism of Treating Salpingitis With LLE in Laying Hens
Identification of Constituents in LLE
Seen in Figure 2A and B, these were the total ion flow diagram of LLE in both positive and negative ion modes after UPLC separation. It could be seen that in the positive and negative ion mode, the component peak time of LLE was within 22 min, indicating that the compounds in LLE were basically detected. In addition, under the separation of organic phase, the compounds in LLE could be well separated. Subsequently, mass spectrometry data were extracted for matching the traditional Chinese medicine component atlas library provided by SCIEX OS software, and identified the mismatched components by using built-in peak extraction and molecular formula fitting. Twenty-eight components in LLE were identified, which were shown in Table 3.
Figure 2.
Total ion chromatograms of LLE in both positive and negative ion modes. (A) Positive ion mode of lotus leaf extract, (B) negative ion mode of lotus leaf extract, the abscissa represents time, and the ordinate represents intensity. Scanning range: TOF MS m/z 100 to 1,000; TOF MS/MS m/z 50 to 1,000.
Table 3.
Compounds in lotus leaf extract.
| No. | tR/min | Mode of acquisition | Molecular formula | m/z | MS2 | Error/ppm | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 0.76 | [M−H] − | C6H12O6 | 179.0564 | 179.0575 | 3.35 | D-Tagatose |
| 2 | 0.74 | [2M+AcO-H]− | C15H20N4O8 | 827.269 | 161.0458, 341.1082, 503.1586, 665.2157 | 1.21 | Maltopentaose |
| 3 | 0.75 | [M−H]− | C25H14N2 | 341.1089 | 341.0942, 341.0656 | 3.22 | Melibiose |
| 4 | 0.75 | [2M+AcO-H]− | C8H14O7 | 503.1618 | 503.1577, 503.1607 | 2.38 | Maltotriose |
| 5 | 0.76 | [M+Cl] − | C25H14N2 | 377.0857 | 221.0667 | 0.8 | Isomaltose |
| 6 | 0.79 | [M+H]+ | C17H19NO3 | 286.1386 | 213.1 | −1.82 | Machiline |
| 7 | 0.79 | [M+H]+ | C15H14O7 | 307.0739 | 123.1,213.1,277.1 | −2.4 | (+)-Leucocyanidin |
| 8 | 0.8 | [M+H]+ | C18H17NO2 | 280.1291 | 213.1 | −1.48 | Remerin |
| 9 | 0.84 | [M−H]− | C3H6O4 | 105.0193 | 56.9996, 75.0104, 105.0217 | 6.67 | Glyceric acid |
| 10 | 0.88 | [M−H]− | C5H8O3 | 115.0399 | 55.0191, 69.0338, 71.2513 | 9.56 | maleic acid |
| 11 | 0.96 | [M−H2O-H]− | C4H8O5 | 117.0194 | 55.0189, 73.0291, 99.9256, 117.0232 | 5.13 | Amber Acid |
| 12 | 5.96 | [M−H]− | C22H16N4O8 | 463.0886 | 463.0875, 300.0273 | 0.86 | Isoquercitrin |
| 13 | 5.99 | [M+H]+ | C15H10O7 | 303.0517 | 56.9648, 113.028, 157.0558 | 5.8 | quercetin |
| 14 | 6.06 | [M+H]+ | C15H10O6 | 287.056 | 287.0137, 287.056 | 3.4 | kaempferol |
| 15 | 6.17 | [M+H]+ | C19H23NO3 | 314.1796 | 171.6,180.1,206.6 | 1.44 | Armepavine |
| 16 | 6.25 | [M+H]+ | C18H19NO2 | 282.1506 | 189.0701, 190.0781, 191.0857 | 6.3 | o-Nornuciferine |
| 17 | 6.64 | [M+H]+ | C30H50O | 427.394 | 114.1,209.2,228.2 | 1.2 | Cycloartenol |
| 18 | 6.64 | [M+H]+ | C15H14O8 | 323.0685 | 114.1,209.2,228.2,293.7 | −2.36 | Leucodelphinidin |
| 19 | 6.65 | [M+H]+ | C29H50O | 415.4005 | 415.7658, 416.2868 | 1.69 | Sitosterol |
| 20 | 7.23 | [M+H]+ | C16H12O7 | 317.0673 | 84.9581, 111.0101, 157.0543 | 5.5 | Isorhamnetin |
| 21 | 7.63 | [M+H]+ | C19H21NO2 | 296.166 | 179.0851, 219.0813, 250.1 | 5.1 | Nuciferin |
| 22 | 10.78 | [M−H]− | C15H10O5 | 269.0458 | 269.0445 | 4.46 | Emodin |
| 23 | 13.58 | [M−H]− | C14H28O2 | 227.2023 | 227.2017, 227.708, 227.5187, 227.8244 | 3.08 | Myristic acid |
| 24 | 14.95 | [M−H]− | C11H16O | 163.1127 | 65.0023, 91.0194, 119.0465, 163.001 | 7.97 | p-Coumaric acid |
| 25 | 15.25 | [M−H]− | C18H32O2 | 279.2334 | 279.2327 | 2.15 | Linoleic acid |
| 26 | 16.09 | [M−H]− | C18H34O2 | 281.249 | 281.2486, 281.2487 | 3.56 | Oleic acid |
| 27 | 22.03 | [M+H]+ | C11H10N2O10 | 331.0399 | 331.102,331.0371,331.214,331.9116 | 0.30 | Carnosol |
| 28 | 22.19 | [M−H]− | C8H22N6O6 | 297.1534 | 297.1528 | 2.02 | Fumigaclavine A |
tR/min: retention time; m/z: first-order mass spectrometry; MS2: secondary mass spectrometry.
Transcriptome Data
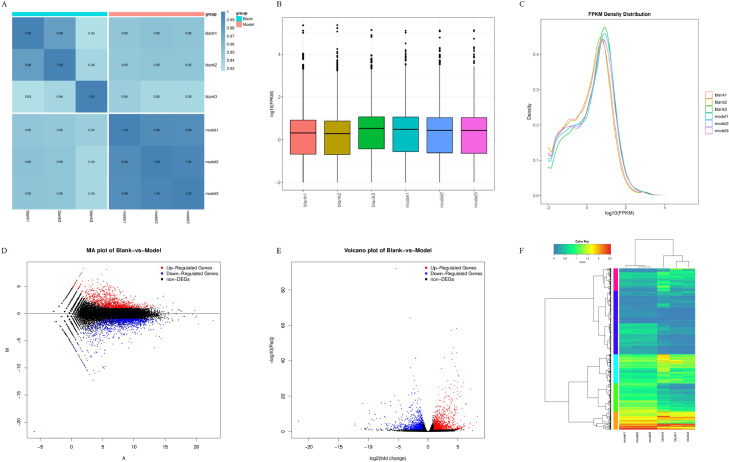
Results of transcriptome sequencing between model and blank groups were shown in Table 4. The number of sequences was more than 2.5 million in each sample, the number of clean bases was more than 7.5 GB, the average content of sample GC was about 47.1%, and the average percentages of Q20 and Q30 bases were 98.25% and 94.83%. These results demonstrated the sequencing data's quality and supported subsequent analysis. In addition, the correlation of gene expression level among samples is an important indicator to test whether experimental reliability and sample selection are reasonable. The closer the correlation coefficient is to 1, the higher the similarity of expression patterns between samples. As shown in Figure 3A, the correlation coefficients of the pieces were all above 0.93. Meanwhile, the overall expression profiles among samples were compared by expression quantity density profiles and boxplots, which were shown in Figure 3B and C. These results also illustrated the higher similarity between the samples.
Table 4.
Transcriptome sequencing results.
| Sample | Clean reads pairs | Clean base (bp) | Length | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| model1 | 34,777,362 | 10,433,208,600 | 150 | 98.2 | 94.7 | 47.1 |
| model2 | 34,698,050 | 10,409,415,000 | 150 | 98.5 | 95.6 | 47.3 |
| model3 | 37,384,853 | 11,215,455,900 | 150 | 98.1 | 94.5 | 47.6 |
| blank1 | 36,736,749 | 11,021,024,700 | 150 | 98.2 | 94.6 | 46.8 |
| blank2 | 40,507,194 | 12,152,158,200 | 150 | 98.2 | 94.7 | 46.8 |
| blank3 | 26,568,936 | 7,970,680,800 | 150 | 98.3 | 94.9 | 47 |
Sample name, clean reads pairs: filtered sequencing data, subsequent bioinformatic analysis is based on clean reads; clean base (BP): the number of floors sequenced multiplied by the length of the sequenced sequence; length: reads size; Q20 (%): the percentage of bases with a base quality value greater than or equal to 20 over the population bases; Q30 (%): the percentage of grounds with a base quality value greater than or equal to 30 over the population bases; GC (%): sum of numbers of bases G and C as a percentage of the total number of bases.
Figure 3.
Results of oviduct sequencing in laying hens infected with mixed bacteria. (A) Heatmap of gene expression level correlations across all samples, with red representing the model group and blue representing the blank group, (B) Fpkm density profiles of genes/transcripts in different samples with different colors representing different samples, (C) Fpkm boxplots of genes/transcripts in different samples with different colors representing different samples, (D) Differential gene Ma plot with black representing normal genes, red representing upregulated genes, and blue representing downregulated genes, (E) differential gene volcano plot with black representing normal genes, red representing upregulated genes, and blue representing downregulated genes, (F) differential gene expression clustering heatmap with abscissa representing samples and ordinate representing relatedness. Different colors distinguish gene expression distributions.
Identification of Differentially Expressed Genes
Genes with significantly different expression levels (P < 0.05) were called DEGs by analyzing the sequencing data of the 6 samples. To visually visualize the distribution of FDR and fold difference FC values of all genes between the 2 groups, a Ma plot and volcano plot were drawn for each group of sample comparison shown in Figure 3D and E. In addition, to visually reflect the high and low gene expression levels and expression patterns of the 6 samples from the entire plot, a clustered heatmap was constructed for the differential gene expression shown in Figure 3F. These results were shown that a total of 1,874 DEGs were identified between salpingitis and blank groups, of which 925 were upregulated, and 949 were downregulated.
Go and KEGG Enrichment Analysis
To further express the functions of the identified differential genes, the enrichment analysis of GO and KEGG pathways was performed, and the results were shown in Figure 4A and B. In Figure 4A, 7,424 GO functions were enriched, including cellular, metabolic, biological, etc. KEGG enrichment results in Figure 4B was shown that the 212 associated pathways were divided into 5 branches: transport and catalysis, cell growth and death. The enriched signaling pathway was a cell cycle among the upregulated differential genes, while the downregulated differential genes corresponded to apoptosis, as shown in Figure 4C and D.
Figure 4.
Differential gene-based GO and KEGG enrichment analysis. (A) The vertical axis represents the GO item name, the Q value is sorted from small to large, the horizontal axis represents the richness factor, and the size of the column represents how many differentially expressed genes are in this GO item. (B) The ordinate is the name of the KEGG metabolic pathway, and the abscissa is the number of genes annotated to the pathway. The genes were divided into 5 branches according to the involved KEGG metabolic pathways: cellular process, environmental information processing, genetic information processing, metabolism, and organic system. (C) KEGG pathway enrichment among upregulated genes. (D) KEGG pathway enrichment among downregulated genes.
Network Pharmacology of LLE in Treating Salpingitis in Laying Hens
After mapping the differentially sequenced genes of the salpingitis layer transcriptome with the genes acting on LLE components, it was found that there were 59 shared core genes as shown in Figure 5A. Based on these 59 core genes, a network diagram of LLE components acting on salpingitis targets was constructed shown in Figure 5B. From Figure 5B, 20 components, including quercetin, kaempferol, and so on, mainly affected PTGS1, CA2, CDK1, and other marks. In addition, the 59 genes were further analyzed by GO enrichment shown in Figure 5C. The findings were mainly focused on cell division, the G2/M transition of the mitotic cell cycle, and the positive regulation of the apoptotic process. Similarly, KEGG enrichment analysis results was shown in Figure 5D, and the inflammation pathways in the oviduct of laying hens treated with LLE were mainly cell cycle, progesterone-mediated oocyte formation, cell sensation, and so on. Notably, the signaling pathways of cell cycle and apoptosis were consistent with the transcriptome sequencing results. Therefore, apoptosis and the cell cycle would be essential research objectives in the following experiment.
Figure 5.
Network pharmacology of LLE for treating oviduct inflammation in laying hens. (A) Drug action target vs. disease target Venn diagram, blue represents lotus leaf extract, red represents transcriptome differential genes of laying hens' salpingitis. (B) Drug component action disease target network, hexagons represent drug components and circles represent genes. According to the degree ranking, the larger the degree, the larger the feature. (C) Go enrichment analysis plot based on drug action disease targets. (D) KEGG enrichment analysis plot of disease targets based on drug effects.
Verification of the Mechanism of LLE in Treating Salpingitis
Cell Proliferation, MOI, and NO Assay Results
The proliferation experiment of LLE at different concentrations on DF-1 cells was shown in Figure 6A. Compared with the blank group, there was no significant difference at the concentration of 7.8125 μg/mL, 15.625 μg/mL, 31.25 μg/mL, and 62.5 μg/mL (P > 0.05), but 62.5 μg/mL LLE showed a downward trend. To facilitate the subsequent cell test, 5 μg/mL, 15 μg/mL, and 25 μg/mL LLE were selected for the following test. Figure 6B showed the bacterial infection of MOI. Under the 40× microscope, it could be seen that the cell morphology in the field of vision was changed, and mass bacteria were growing when MOI = 200, compared with the blank group. Therefore, MOI = 200 was selected as the bacterial infection condition.
Figure 6.
Cell viability, multiplicity of infection, and nitric oxide situation. (A) Cell proliferation situation, the abscissa represents drug concentration, and the ordinate represents absorbance. (B) For bacterial infection, a 40 × microscope was used. (C) For the NO case, the abscissa represents the drug concentration, and the ordinate represents the unit (μg/mL). All experiments were repeated more than 3 times and presented as mean ± SD (significant differences (P < 0.05) between groups with different superscripts a, b, c, and d).
The change of NO content was shown in Figure 6C. The NO content in the model group was significantly lower compared with the blank group (P < 0.05). While, the NO content in 15 μg/mL, and 25 μg/mL of LLE groups was increased, which was similar to that in blank group (P > 0.05).
DF-1 Cell Apoptosis and Cycle
The cell apoptosis in each group was shown in Figure 7A. Compared with the blank group, the apoptosis of the model group was significantly expedited (P < 0.01). Furthermore, the result in the 5 μg/mL LLE group had no significant inhibitory effect on cell apoptosis (P > 0.05), but the LLE 15 μg/mL and 25 μg/mL significantly decreased cell apoptosis (P < 0.05).
Figure 7.
Apoptosis and cycle were detected by flow cytometry. (A) Apoptosis situation, (B) cell cycle situation. All experiments were repeated more than 3 times and presented as mean ± SD, (significant differences (P < 0.05) between groups with different superscripts a, b, c, and d).
The cell cycle results after infection and treatment were shown in Figure 7B. In the model group, the G1 phase was increased, and the S phase and the G2 phase were decreased, indicating that the cells were arrested in the G1 phase. After the intervention of LLE, the G1 phase gradually returned to the average level, and G2 and S phases increased.
Fluorescent Quantitative PCR Detection of Relevant Genes
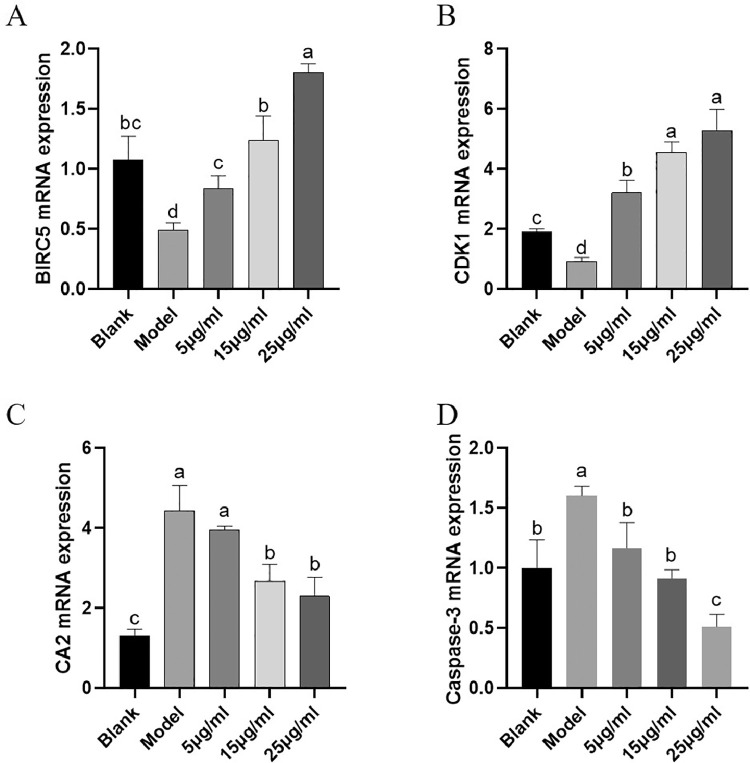
The results of cell apoptosis and cell cycle relevant genes mRNA expression were shown in Figure 8 to further validate the results of network pharmacology and transcriptome sequencing. The mRNA expression levels of BIRC5 (Figure 8A), CDK1 (Figure 8B), CA2 (Figure 8C), and the apoptosis key gene Caspase-3 (Figure 8D) were randomly measured. The mRNA expression level of BIRC5 and CDK1 in model group was significantly decreased compared with that in blank group (P < 0.05), but increased after administration with LLE, especially in 25 μg/mL LLE group, the mRNA expression level of BIRC5 and CDK1 was highest (P < 0.05). Compared with that in blank group, the mRNA expression level of CA2 was significantly higher in model group (P < 0.05). It was significantly lower in 15 μg/mL and 25 μg/mL LLE group than that in model group (P < 0.05). While the mRNA expression level of Caspase-3 was significantly increased after bacterial infection (P < 0.05), and significantly decreased after administration of LLE (P < 0.05).
Figure 8.
qPCR verifies transcriptome and network pharmacology results. (A) BIRC5 mRNA expression, (B) CDK1 mRNA expression, (C) CA2 mRNA expression, (D) Caspase-3 mRNA expression. All experiments were repeated more than 3 times and expressed as means ± SD (significant differences (P < 0.05) between groups with different superscripts a, b, c, and d).
In addition, the results in Figure 9 showed the apoptosis pathway by which bacterial infection leads to a lag in the cell cycle. After bacterial coinfection, the mRNA expression level of TNF-α (Figure 9A), FADD (Figure 9B), TRADD (Figure 9C), Caspase-7 (Figure 9E), Caspase-8 (Figure 9F), Caspase-9 (Figure 9G), and Caspase-10 (Figure 9H) in the model group were significantly increased compared with those in the blank group (P < 0.05), and the mRNA expression level of BCL-2 (Figure 9D) was significantly decreased (P < 0.05). After advanced stimulation by LLE, the mRNA expression level in all of these genes was restored, especially in 15 μg/mL and 25 μg/mL LLE group (P < 0.05).
Figure 9.
The expression of apoptosis pathway-related mRNA was detected by qPCR. (A) TNF-α mRNA expression, (B) FADD mRNA expression, (C) TRADD mRNA expression, (D) BCL-2 mRNA expression, (E) Caspase-7 mRNA expression, (F) Caspase-8 mRNA expression, (G) Caspase-9 mRNA expression, (H) Caspase-10 mRNA expression. All experiments were repeated more than 3 times and presented as mean ± SD (significant differences (P < 0.05) between groups with different superscripts a, b, c, and d).
DISCUSSION
Salpingitis, a common disease of laying hens in large-scale breeding mode, is mainly caused by external gram-negative bacteria such as Escherichia coli and Salmonella invading the fallopian tubes through cloaca (Landman and Cornelissen, 2006; Ozaki et al., 2018). When gram-negative bacteria infect the fallopian tubes of laying hens, the secretion of proinflammatory cytokines increases dramatically under the stimulation of LPS released during bacterial death and reproduction (Liu et al., 2023). Therefore, anti-inflammatory was considered as a commonly therapies. In order to determine the effectiveness of LLE treating salpingitis, the expression of inflammatory-related and immune-enhancing factors in the oviduct was detected.
The pathogenic pathogen-associated molecular, such as LPS, influence TLRs to initiate activation of nuclear factor-κB (NF-κB) in macrophages and subsequent inflammatory responses (Kamimura et al., 2017; Sutton et al., 2022; Tong et al., 2022). The TLR stimulation increases production of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 (Al-Zghoul and Mohammad Saleh, 2020). Gram-negative bacteria, e.g. Salmonella, produces LPS, which stimulates TLR4 as the most sensitive TLR to trigger the innate immune response of immune cells (Alexander and Rietschel, 2001). TLR4 then recruits adaptor proteins and activates 2 distinct signaling pathways (the MyD88-dependent pathway and the TRIF-dependent pathway), which are all mediate the NF-κB activation (Kawai and Akira, 2010). Activation of NF-κB subsequently induces the expression of related inflammatory genes, thereby promoting the synthesis and release of cytokines and inflammatory mediators, such as IL-4 and IL-6, leading to inflammatory response (Liu et al., 2022). IFN-γ is a proinflammatory cytokine that is responsible for increasing the expression of major histocompatibility complex antigens and provides host defense against intracellular pathogens such as gram-negative bacteria (Benbernou and Nauciel, 1994; Adhikari et al., 2018). In this study, the expression of TLR4 and IFN-γ was downregulated in Amoxicillin- and LLE-supplemented group laying hens compared with the blank group, showing a lower inflammatory response. Meanwhile, upregulation of anti-inflammatory cytokine IL-10 and IL-4 might inhibit the induced inflammation. Our previous studies had found that Nuciferine, a major bioactive component of lotus leaves, could improve intestinal inflammation induced by DSS (Zhu et al., 2022). Thus, it was concluded that LLE-injected could inhibit the expression of inflammation-related genes through the TLR4/NF-κB signaling pathway, thereby alleviating oviduct inflammation in mixed bacteria-challenged laying hens.
The mucosa of oviduct of hens are susceptible to pathogens generally causing inflammation of mucosa, resulting in a deterioration of the health of the host animal, reduced egg production and bacterial contamination of eggs (Liu et al., 2022). Therefore, the detection of immune-related indexes expression in the oviduct can evaluate the health status of the oviduct more comprehensively. Immune cells and immune active molecules play important roles in the immune regulation system. Traditional Chinese medicine and its active ingredients increase the immunoglobulin and anti-inflammatory cytokine contents in the body's inflammatory response and decrease mRNA expression levels of proinflammatory cytokines in tissue, playing an important role in stabilizing the immune defense system (Wang et al., 2021a, 2022; Yang et al., 2021). Immunoglobulin harbors antibody activity, and its chemical structure, which is similar to that of globulin, mainly includes IgA and IgG. IgG is the most important part of the immune response, playing a critical role in the immune response against infection (Manangi et al., 2015). IgA is the main exocrine immunoglobulin (Sterlin et al., 2020). Furthermore, immunoglobulin is the basis of humoral immunity (Victora and Nussenzweig, 2022). In the current study, the levels of IgA and IgG were elevated compared with the blank group. Various pharmacological researches were also reported that the lotus leaf had anti-inflammatory, antibacterial, elevates human immune capacity, and antioxidant effects (Song et al., 2019; Li et al., 2021; Xu et al., 2022).
The release of free radicals from proinflammatory cytokines was reduced, and the oxidative stress cascade is alleviated (Jeon et al., 2020). The antioxidative enzyme system is the first line of antioxidant defense and a little alteration in the activity of the antioxidative enzyme can change the balance between the production of ROS and the antioxidant system (Ibtisham et al., 2019). SOD activity and MDA concentration are the main parameters to assess the oxidative status of birds (Wang et al., 2008). In the present study, MDA concentration was downregulated, while, SOD activity was enhanced in Amoxicillin- and LLE-supplemented group compared to the blank group. Therefore, well regulation of mentioned parameters showed that the antioxidant status of LLE-supplemented group was improved. Furthermore, it has further prevented bacterial infection by supplementation with quercetin, kaempferol, etc. (Heinz et al., 2010; Xu et al., 2023).
Lotus leaves, to be used as traditional medicines for a long history, have also attracted increasing attention for functional food development owing to their promising health benefits, including antioxidant, hypoglycemic, immunomodulatory, hepatoprotective, antiproliferative, and antiobesity effects, as well as modulation of colonic microbiota (Chen et al., 2019; Wang et al., 2020b, 2021b). Like other traditional Chinese medicine, lotus leaves are also characterized by multiple components, targets, and pathways for treating diseases. Although there is evidence that lotus leaves may provide an anti-inflammatory and immunoenhancement effect on the chicken, the effect and specific mechanism (active components, specific targets, and pathways) on salpingitis of laying hens is still unclear (Cheng et al., 2021; Zhu et al., 2022). With the development of technology, different hyphenated chromatography techniques are more and more applied to the analysis of components, especially liquid chromatography-mass spectrometry (UPLC-Q-TOF-MS) (Abdullah AlFaris et al., 2020; Feng et al., 2021). Owing to the diversity of chemical components within a TCM and the complexity of its mechanisms of action, chromatographic technology has emerged as the main analytical method for the quality control of TCM. Network pharmacology is a new research method for analyzing drug intervention in diseases. Based on existing databases, the intersection targets of drugs and diseases can be found. By analyzing these intersection targets, the core targets and specific mechanisms can be predicted (Cheng et al., 2022; Nogales et al., 2022). However, the development of network pharmacology in animal medicine still needs to be improved due to the missing disease-related genes. In the present study, the constituents of LLE were identified by UPLC-Q-TOF-MS, including 28 prototype compounds. Subsequently, transcriptome sequencing of disease sites in salpingitis laying hens was performed to analyze disease-contributing genes. On the basis of above results, a network pharmacological analysis was conducted to explore the information of targets and metabolic pathways associated with the compounds of LLE. The results showed that 28 active compounds, including quercetin, kaempferol, stigmasterol, and others, were identified in the LLE. This result was consistent with previous reports (Uddin et al., 2014; Li et al., 2021; Tungmunnithum et al., 2022). The 1,874 disease genes contributed by transcriptome sequencing were found to share 59 of the same genes after being combined with network pharmacology and 20 with drugs acting on these genes, the results showing the feasibility of LLE in a pharmacological approach.
Bacterial infection can cause cell cycle arrest and induce apoptosis (Grasso and Frisan, 2015; Robinson and Aw, 2016). Results of flow cytometry were indicated that the cell cycle was blocked in the S/G2 phase 1 h after bacterial infection and further induced apoptosis. In the present study, with the intervention of LLE, the cell cycle was gradually recovered to normal, and the apoptosis was reduced. This may be due to the effect of the active component of LLE, Armepavine, acting on iNOS, which would have promoted NO release from DF-1. Activation of the NO release may have led to upregulation of survival transcription factors that interacted with other components to mitigate apoptosis (Richter, 1998).
Increasing evidences indicate that apoptosis is correlated with the development of inflammation and it could be as a therapeutic strategy for inflammatory disorders (Li et al., 2023). Woods et al. had reported on the efficacy of the naturally occurring cytokine, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), to promote cell death in aGCTs under specific conditions, and healthy granulosa cells were inherently resistant to TRAIL-induced apoptosis (MacDonald et al., 2018). Following activation through TRAIL-binding, the intracellular death domain links with Fas-associated death domain (FADD) protein, resulting in the formation of the apoptosis-inducing signaling complex (DISC) (Peter, 2011), subsequently leads to activation of Caspase-8, which cleaves bid and triggers the release of cytochrome c from the mitochondria into the cytosol (Deveraux et al., 1998), triggering the caspase cascade that ultimately results in cellular apoptosis. However, the ability of TRAIL to readily induce apoptosis is diminished, as the TRAIL-induced caspase cascade is opposed by prosurvival proteins, namely the inhibitor of apoptosis (IAP) family, which includes Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5). Notably, BIRC5 is a potent inhibitor of caspase activation, and when present, actively promotes cell survival through inhibition of caspase-induced apoptosis (Konopleva et al., 1999). CDK1, a cyclin-dependent kinase, is famous for its cell cycle regulation function (Enserink and Chymkowitch, 2022). When CDK1 is inhibited, it often causes cell apoptosis (Yi et al., 2021). Our results indicated that the coinfection of Escherichia coli and Staphylococcus aureus with DF-1 led to the downregulation of BIRC5 and CDK1. However, with the intervention of LLE, BIRC5 and CDK1 showed a sharp dose-dependent increase, which may be related to cell proliferation. Carbonic anhydrase 2 is involved in diverse biological processes, reported that in high glucose (Hg)-induced renal tuberculosis injury, and the use of CA2 inhibitors could reduce the picitamol (PIC)-induced epithelial HK-2 apoptosis (Zhang et al., 2023). In the present study, CA2 mRNA levels were upregulated significantly after bacterial infection and were found to be restored after treatment with LLE.
Caspases occupy a crucial status in the apoptosis (Van Opdenbosch and Lamkanfi, 2019). Li et al. had reported that sialic acid repressed the expressions of apoptosis-related gene including Caspase-3 and Caspase-9 in RAW264.7 cells (Li et al., 2023). BCL-2 family proteins modify the mitochondrial-dependent apoptosis, which is the antiapoptotic protein. The release of Cyt-c from mitochondrial membrane into the cytosol caused the cell death, which was mediated by abnormal expressions of BCL-2 family genes (Ow et al., 2008). The BCL-2/Bax ratio-mediated apoptotic pathway could activate Caspase-9, thereby regulating the activation of Caspase-3 and inducing the cell apoptosis. Caspase-3, one of the star genes of apoptosis, can cut many key cell proteins, thus causing cell apoptosis and cascade reaction (Porter and Jänicke, 1999). It was found that bacterial infection promoted the expressions of Caspase-7, Caspase-8, Caspase-9, Caspase-10, and Caspase-3 and declined the level of BCL-2. However, LLE treatment notably increased the BCL-2 expression as well as suppressed the expressions of Caspase-7, Caspase-8, Caspase-9, Caspase-10, and Caspase-3. Furthermore, the upstream regulatory genes of apoptosis, TNF-α, FADD, and TRADD, were also promoted the expressions after bacterial infection, however LLE treatment notably suppressed their expressions. LLE might possess significant antiapoptosis effects by the activation of the caspase cascade reaction pass-through the TNF signal pathway mediated by FADD and TRADD. This was consistent with the research of Micheau and Tschopp (Micheau and Tschopp, 2003).
CONCLUSIONS
In conclusion, these results suggested that LLE has potential as a therapeutic compound for the treatment of salpingitis in laying hen. Furthermore, we also found that LLE contained 28 components using UPLC-Q-TOF-MS technique, and 1,874 differential genes provided by transcriptome sequencing. Network pharmacology showed that 20 active components of LLE act on 58 disease genes, which could treat salpingitis in laying hens. Nevertheless, the reasons for salpingitis were complicated and multifaceted, so the alleviated effect of LLE on salpingitis of laying hens needed to be further explored.
ACKNOWLEDGMENTS
Research was funded by grants from the National Natural Science Foundation of China (Grant No. 31602099), and Jingzhou Civic Science and Technology Projects (Grant No. 202254-07CC). This study was also partially supported by the unveiled project of “Advanced Technology Integration Demonstration Base Construction and Targeted Research” in the pilot County of Shishou (Grant No. SS202311).
DISCLOSURES
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my coauthors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have been approved the manuscript that is enclosed.
REFERENCES
- Abdullah AlFaris N., Zaidan Altamimi J., Alothman Z.A., Wabaidur S.M., Ghafar A.A., Saleh Aldayel T. Development of a sensitive liquid-liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry method for the analysis of carbaryl residues in fresh vegetables sold in Riyadh. J. King Saud. Univ. Sci. 2020;32:2414–2418. [Google Scholar]
- Abrahamsson P., Fossum O., Tauson R. Health of laying hens in an aviary system over five batches of birds. Acta Vet. Scand. 1998;39:367–379. doi: 10.1186/BF03547785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari P., Cosby D.E., Cox N.A., Franca M.S., Williams S.M., Gogal R.M., Ritz C.W., Kim W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poult. Sci. 2018;97:2525–2533. doi: 10.3382/ps/pey101. [DOI] [PubMed] [Google Scholar]
- Alexander C., Rietschel E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- Al-Zghoul M.B., Mohammad Saleh K.M. Effects of thermal manipulation of eggs on the response of jejunal mucosae to posthatch chronic heat stress in broiler chickens. Poult. Sci. 2020;99:2727–2735. doi: 10.1016/j.psj.2019.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbernou N., Nauciel C. Influence of mouse genotype and bacterial virulence in the generation of interferon-gamma-producing cells during the early phase of Salmonella typhimurium infection. Immunology. 1994;83:245–249. [PMC free article] [PubMed] [Google Scholar]
- Chen G., Zhu M., Guo M. Research advances in traditional and modern use of Nelumbo nucifera: phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 2019;59:S189–S209. doi: 10.1080/10408398.2018.1553846. [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhang M., Zheng Y., Wang J., Wang Q. Integrative analysis of network pharmacology and proteomics to identify key targets of Tuomin-Zhiti-Decoction for allergic rhinitis. J. Ethnopharmacol. 2022;296 doi: 10.1016/j.jep.2022.115448. [DOI] [PubMed] [Google Scholar]
- Cheng L., Zhang W., Jin Q., Zhu Y., Chen R., Tian Q., Yan N., Guo L. The effects of dietary supplementation with lotus leaf extract on the immune response and intestinal microbiota composition of broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B.O., Che D.N., Yin H.H., Shin J.Y., Jang S.I. Diospyros lotus leaf and grapefruit stem extract synergistically ameliorate atopic dermatitis-like skin lesion in mice by suppressing infiltration of mast cells in skin lesions. Biomed. Pharmacother. 2017;89:819–826. doi: 10.1016/j.biopha.2017.01.145. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Roy N., Stennicke H.R., Van Arsdale T., Zhou Q., Srinivasula S.M., Alnemri E.S., Salvesen G.S., Reed J.C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink J.M., Chymkowitch P. Cell cycle-dependent transcription: the cyclin dependent kinase Cdk1 is a direct regulator of basal transcription machineries. Int. J. Mol. Sci. 2022;23:1293. doi: 10.3390/ijms23031293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Gao M., Zhai Y., Li X., Wang Y., Xie T., Yao W., Shan J., Zhang L., Ding A. A novel strategy based on targeted cellular metabolomics for quantitatively evaluating anti-aging effect and screening effective extracts of Erzhi Wan. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021;1178 doi: 10.1016/j.jchromb.2021.122857. [DOI] [PubMed] [Google Scholar]
- Gao J., Yang Z., Zhao C., Tang X., Jiang Q., Yin Y. A comprehensive review on natural phenolic compounds as alternatives to in-feed antibiotics. Sci. China Life Sci. 2022,:1518–1534. doi: 10.1007/s11427-022-2246-4. [DOI] [PubMed] [Google Scholar]
- Grasso F., Frisan T. Bacterial genotoxins: merging the DNA damage response into infection biology. Biomolecules. 2015;5:1762–1782. doi: 10.3390/biom5031762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretarsson P., Kittelsen K., Moe R.O., Vasdal G., Toftaker I. End of lay postmortem findings in aviary housed laying hens. Poult. Sci. 2022;102 doi: 10.1016/j.psj.2022.102332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S.A., Henson D.A., Austin M.D., Jin F., Nieman D.C. Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial. Pharmacol. Res. 2010;62:237–242. doi: 10.1016/j.phrs.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibtisham F., Nawab A., Niu Y., Wang Z., Wu J., Xiao M., An L. The effect of ginger powder and Chinese herbal medicine on production performance, serum metabolites and antioxidant status of laying hens under heat-stress condition. J. Therm. Biol. 2019;81:20–24. doi: 10.1016/j.jtherbio.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Jeon Y.-D., Lee J.-H., Lee Y.-M., Kim D.-K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109847. [DOI] [PubMed] [Google Scholar]
- Jordan F.T.W., Williams N.J., Wattret A., Jones T. Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock. Vet. Rec. 2005;157:573–577. doi: 10.1136/vr.157.19.573. [DOI] [PubMed] [Google Scholar]
- Kamimura T., Isobe N., Yoshimura Y. Effects of inhibitors of transcription factors, nuclear factor-kappaB and activator protein 1, on the expression of proinflammatory cytokines and chemokines induced by stimulation with Toll-like receptor ligands in hen vaginal cells. Poult. Sci. 2017;96:723–730. doi: 10.3382/ps/pew366. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Konopleva M., Zhao S., Xie Z., Segall H., Younes A., Claxton D.F., Estrov Z., Kornblau S.M., Andreeff M. Apoptosis. Molecules and mechanisms. Adv. Exp. Med. Biol. 1999;457:217–236. [PubMed] [Google Scholar]
- Landman W.J., Cornelissen R.A. Escherichia coli salpingitis and peritonitis in layer chickens: an overview. Tijdschrift diergeneeskunde. 2006;131:814–822. [PubMed] [Google Scholar]
- Li N., Amatjan M., He P., Zhang B., Mai X., Jiang Q., Xie H., Shao X. Integration of network pharmacology and intestinal flora to investigate the mechanism of action of Chinese herbal formula in attenuating adenine and ethambutol hydrochloride-induced hyperuricemic nephropathy in rats. Pharm. Biol. 2022;60:2338–2354. doi: 10.1080/13880209.2022.2147551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., He Y., Yang Y., Gou Y., Li S., Wang R., Zeng S., Zhao X. Antioxidant and inflammatory effects of Nelumbo nucifera Gaertn. leaves. Oxid. Med. Cell Longev. 2021;2021 doi: 10.1155/2021/8375961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Xie T., Guo T., Hu Z., Li M., Tang Y., Wu Q., Luo F., Lin Q., Wang H. Sialic acid exerts anti-inflammatory effect through inhibiting MAPK-NF-κB/AP-1 pathway and apoptosis in ulcerative colitis. J. Funct. Foods. 2023;101 [Google Scholar]
- Liang X., Yamazaki K., Kamruzzaman M., Bi X., Panthee A., Sano H. Effects of Chinese herbal medicine on plasma glucose, protein and energy metabolism in sheep. J. Anim. Sci. Biotechnol. 2013;4:51. doi: 10.1186/2049-1891-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C.A. Rule of five in 2015 and beyond: target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016;101:34–41. doi: 10.1016/j.addr.2016.04.029. [DOI] [PubMed] [Google Scholar]
- Liu Z.P., Chao J.R., Xu P.T., Lv H.Y., Ding B.Y., Zhang Z.F., Li L.L., Guo S.S. Lonicera flos and Cnicus japonicus extracts improved egg quality partly by modulating antioxidant status, inflammatory-related cytokines and shell matrix protein expression of oviduct in laying hens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhao L., Zhao Z., Wu Y., Cao J., Cai H., Yang P., Wen Z. Rubber (Hevea brasiliensis) seed oil supplementation attenuates immunological stress and inflammatory response in lipopolysaccharide-challenged laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J.A., Kura N., Sussman C., Woods D.C. Mitochondrial membrane depolarization enhances TRAIL-induced cell death in adult human granulosa tumor cells, KGN, through inhibition of BIRC5. J. Ovarian Res. 2018;11:89. doi: 10.1186/s13048-018-0463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manangi M.K., Vazques-Añon M., Richards J.D., Carter S., Knight C.D. The impact of feeding supplemental chelated trace minerals on shell quality, tibia breaking strength, and immune response in laying hens. J. Appl. Poult. Res. 2015;24:316–326. [Google Scholar]
- Micheau O., Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Nogales C., Mamdouh Z.M., List M., Kiel C., Casas A.I., Schmidt H.H.H.W. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022;43:136–150. doi: 10.1016/j.tips.2021.11.004. [DOI] [PubMed] [Google Scholar]
- Ow Y.-L.P., Green D.R., Hao Z., Mak T.W. Cytochrome c: functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Yonehara K., Murase T. Virulence of Escherichia coli isolates obtained from layer chickens with colibacillosis associated with pericarditis, perihepatitis, and salpingitis in experimentally infected chicks and embryonated eggs. Avian Dis. 2018;62:233–236. doi: 10.1637/11685-060717-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Peter M.E. Apoptosis meets necrosis. Nature. 2011;471:310–312. doi: 10.1038/471310a. [DOI] [PubMed] [Google Scholar]
- Porter A.G., Jänicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Poulsen L.L., Kudirkiene E., Jørgensen S.L., Djordjevic S.P., Cummins M.L., Christensen J.P., Christensen H., Bisgaard M., Thøfner I. Whole genome sequence comparison of avian pathogenic Escherichia coli from acute and chronic salpingitis of egg laying hens. BMC Vet. Res. 2020;16:148. doi: 10.1186/s12917-020-02369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Gao F., Hou L., Wan C. Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 2017;45:1157–1167. doi: 10.1142/S0192415X1750063X. [DOI] [PubMed] [Google Scholar]
- Riccardi C., Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protocols. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Richter C. Nitric oxide and its congeners in mitochondria: implications for apoptosis. Env. Health Persp. 1998;106(Suppl. 5):1125–1130. doi: 10.1289/ehp.98106s51125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K.S., Aw R. The commonalities in bacterial effector inhibition of apoptosis. Trends Microbiol. 2016;24:665–680. doi: 10.1016/j.tim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva S., Saraiva C., Oliveira I., Stilwell G., Esteves A. Effects of age, weight, and housing system on prevalence of dead on arrival and carcass condemnation causes in laying hens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.-R., Han A.-R., Lim T.-G., Lee E.-J., Hong H.-D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019;128:546–555. doi: 10.1016/j.ijbiomac.2019.01.131. [DOI] [PubMed] [Google Scholar]
- Song Y.-R., Han A.-R., Park S.-G., Cho C.-W., Rhee Y.-K., Hong H.-D. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020;153:169–179. doi: 10.1016/j.ijbiomac.2020.02.252. [DOI] [PubMed] [Google Scholar]
- Sterlin D., Fadlallah J., Slack E., Gorochov G. The antibody/microbiota interface in health and disease. Mucosal. Immunol. 2020, 3-11;13 doi: 10.1038/s41385-019-0192-y. [DOI] [PubMed] [Google Scholar]
- Sutton K., Balic A., Kaspers B., Vervelde L. In: Pages 167–195 in Avian Immunology. 3rd ed. Kaspers B., Schat K.A., Göbel T.W., Vervelde L., editors. Academic Press; Boston, MA: 2022. Chapter 8.1 - Macrophages and dendritic cells. [Google Scholar]
- Tong Y., Yu C., Xie Z., Zhang X., Yang Z., Wang T. Trans-anethole ameliorates lipopolysaccharide-induced acute liver inflammation in broilers via inhibiting NF-κB signaling pathway. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungmunnithum D., Drouet S., Hano C. Flavonoids from sacred lotus Stamen extract slows chronological aging in yeast model by reducing oxidative stress and maintaining cellular metabolism. Cells. 2022, 599;11 doi: 10.3390/cells11040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin G., Rauf A., Siddiqui B.S., Muhammad N., Khan A., Shah S.U.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine. 2014;21:954–959. doi: 10.1016/j.phymed.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., Nussenzweig M.C. Germinal centers. Annu. Rev. Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- Wang Z., Cheng Y., Zeng M., Wang Z., Qin F., Wang Y., Chen J., He Z. Lotus (Nelumbo nucifera Gaertn.) leaf: a narrative review of its Phytoconstituents, health benefits and food industry applications. Trends Food Sci. Technol. 2021;112:631–650. [Google Scholar]
- Wang M., Hu W.-J., Wang Q.-H., Yang B.-Y., Kuang H.-X. Extraction, purification, structural characteristics, biological activities, and application of the polysaccharides from Nelumbo nucifera Gaertn. (lotus): a review. Int. J. Biol. Macromol. 2023;226:562–579. doi: 10.1016/j.ijbiomac.2022.12.072. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li J., Xie Y., Zhang H., Jin J., Xiong L., Liu H. Effects of a probiotic-fermented herbal blend on the growth performance, intestinal flora and immune function of chicks infected with Salmonella pullorum. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-j., Liang Y.-x., Hu F.-l., Sun Y.-f., Zou M.-y., Luo R.-l., Peng X.-l. Chinese herbal formulae defend against Mycoplasma gallisepticum infection. J. Integr. Agric. 2022;21:3026–3036. [Google Scholar]
- Wang C., Pors S.E., Christensen J.P., Bojesen A.M., Thøfner I. Comparison and assessment of necropsy lesions in end-of-lay laying hens from different housing systems in Denmark. Poult. Sci. 2020;99:119–128. doi: 10.3382/ps/pez569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Z., Xu C.L., An Z.H., Liu J.X., Feng J. Effect of dietary bovine lactoferrin on performance and antioxidant status of piglets. Anim. Feed Sci. Technol. 2008;140:326–336. [Google Scholar]
- Wang Y., Yao W., Li B., Qian S., Wei B., Gong S., Wang J., Liu M., Wei M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020;52:1959–1975. doi: 10.1038/s12276-020-00534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Fang J., Ou D., Xu J., Deng X., Chi G., Feng H., Wang J. Therapeutic potential of kaempferol on Streptococcus pneumoniae infection. Microbes Infect. 2023;25 doi: 10.1016/j.micinf.2022.105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Wang X., Wang B., Tang Y., Qin Z., Yin S., Liu Z., Sun H. Carbonized lotus leaf/ZnO/Au for enhanced synergistic mechanical and photocatalytic bactericidal activity under visible light irradiation. Colloids Surfaces B Biointerf. 2022;215 doi: 10.1016/j.colsurfb.2022.112468. [DOI] [PubMed] [Google Scholar]
- Yang L., Luo H., Tan D., Zhang S., Zhong Z., Wang S., Vong C.T., Wang Y. A recent update on the use of Chinese medicine in the treatment of inflammatory bowel disease. Phytomedicine. 2021;92 doi: 10.1016/j.phymed.2021.153709. [DOI] [PubMed] [Google Scholar]
- Yang X., Xia Y.-H., Wang J.-Y., Li Y.-T., Chang Y.-F., Chang H.-T., Liu H.-Y., Chen L., Wang C.-Q. The role of GtxA during Gallibacterium anatis infection of primary chicken oviduct epithelial cells. Mol. Cell Probes. 2020;53 doi: 10.1016/j.mcp.2020.101641. [DOI] [PubMed] [Google Scholar]
- Yi Y.-C., Liang R., Chen X.-Y., Fan H.-N., Chen M., Zhang J., Zhu J.-S. Dihydroartemisinin suppresses the tumorigenesis and cycle progression of colorectal cancer by targeting CDK1/CCNB1/PLK1 signaling. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.768879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang H.-Y., Tian J.-X., Lian F.-M., Li M., Liu W.-K., Zhen Z., Liao J.-Q., Tong X.-L. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110857. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Q., Li F., Li S., Lin H., Huo Y. Piceatannol protects against high glucose-induced injury of renal tubular epithelial cells via regulating carbonic anhydrase 2. Nephron. 2023:1–14. doi: 10.1159/000529212. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhao Q., Huang Q., Li Y., Yu J., Zhang R., Liu J., Yan P., Xia J., Guo L., Liu G., Yang X., Zeng J. Nuciferine regulates immune function and gut microbiota in DSS-induced ulcerative colitis. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.939377. [DOI] [PMC free article] [PubMed] [Google Scholar]