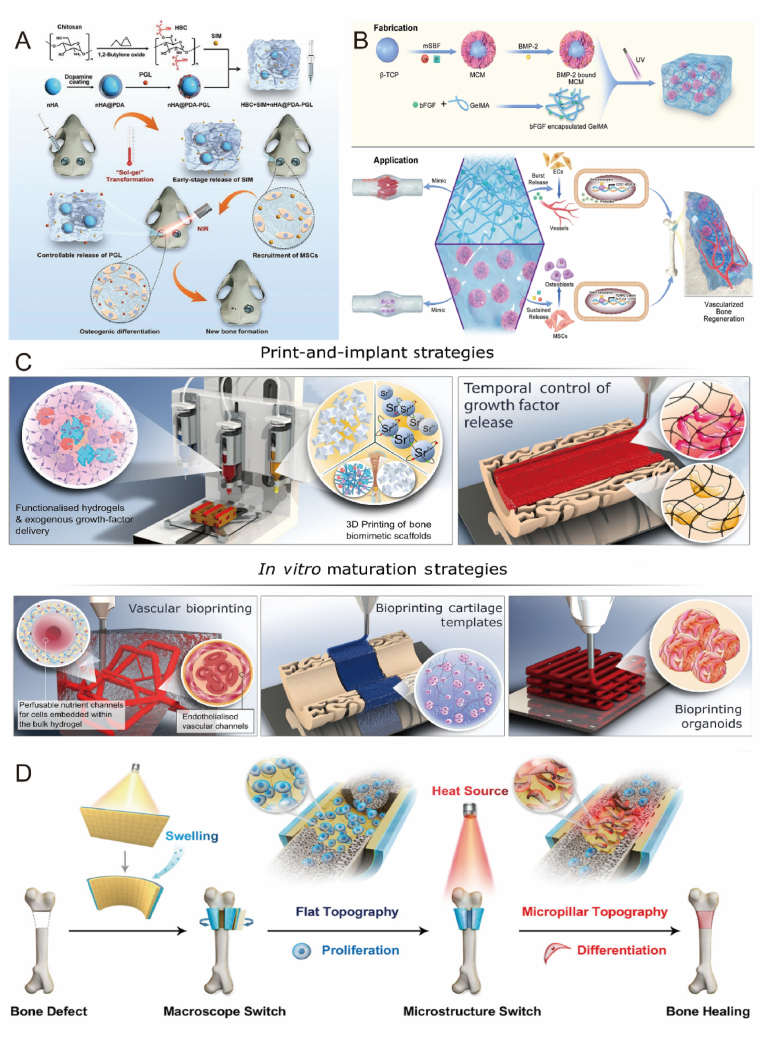
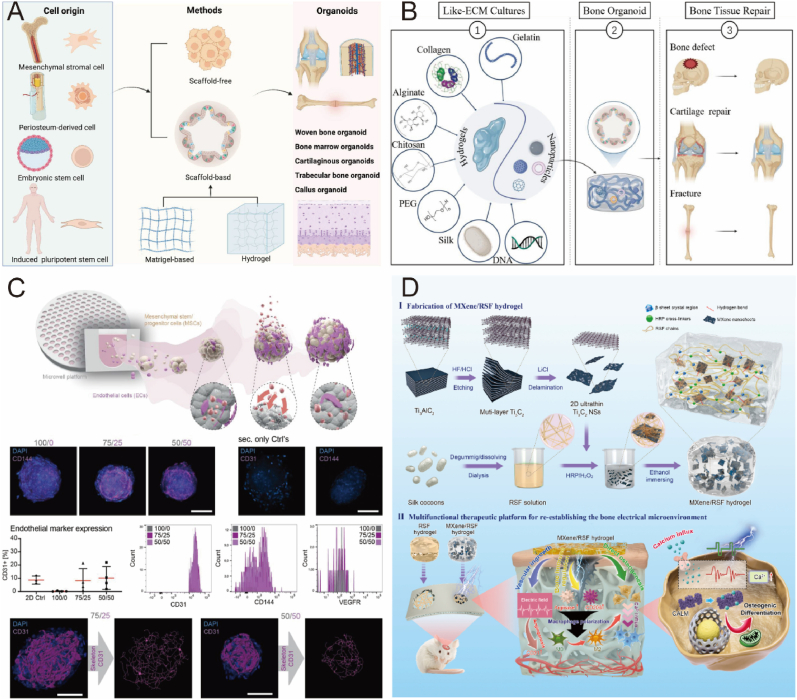
Abstract
Treatment of large bone defects represents a great challenge in orthopedic and craniomaxillofacial surgery. Traditional strategies in bone tissue engineering have focused primarily on mimicking the extracellular matrix (ECM) of bone in terms of structure and composition. However, the synergistic effects of other cues from the microenvironment during bone regeneration are often neglected. The bone microenvironment is a sophisticated system that includes physiological (e.g., neighboring cells such as macrophages), chemical (e.g., oxygen, pH), and physical factors (e.g., mechanics, acoustics) that dynamically interact with each other. Microenvironment-targeted strategies are increasingly recognized as crucial for successful bone regeneration and offer promising solutions for advancing bone tissue engineering. This review provides a comprehensive overview of current microenvironment-targeted strategies and challenges for bone regeneration and further outlines prospective directions of the approaches in construction of bone organoids.
Keywords: Bone regeneration, Physiological microenvironment, Chemical microenvironment, Physical microenvironment, Biomaterials
Graphical abstract
1. Introduction
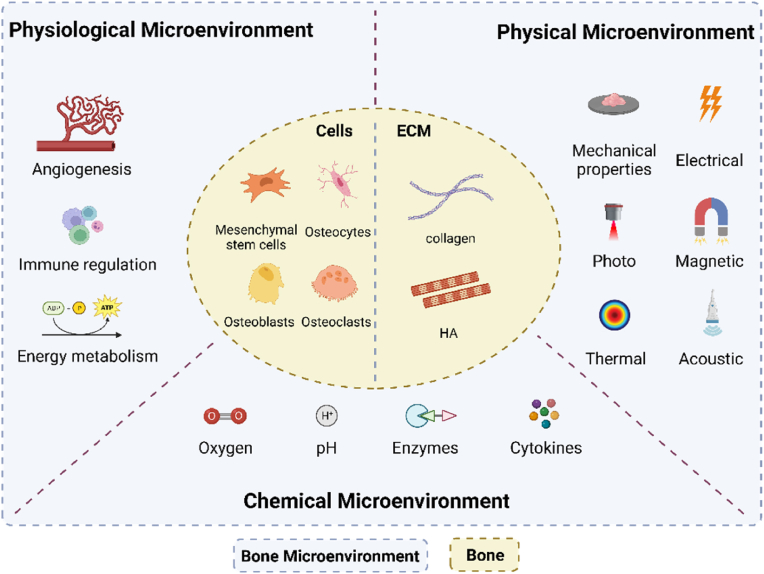
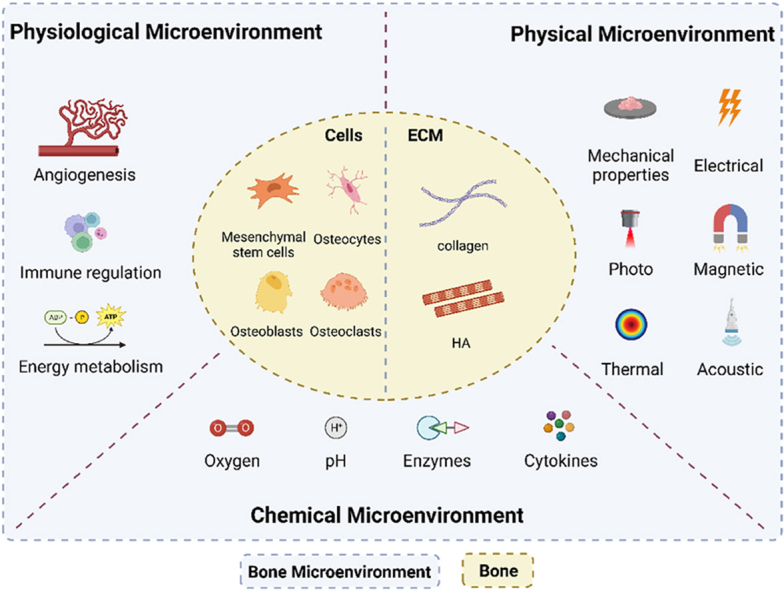
With the development of an aging society, the incidence of bone-related diseases has dramatically increased worldwide [1]. Bone has the potential to heal and regenerate, but the inability to complete bone healing on its own in large segmental bone defects (>2 cm critical size or >50% loss of bone girth) due to trauma, infection, tumor resection or developmental malformations is a serious problem in orthopedic treatment [2]. Although autologous bone grafting is the “gold standard” for clinical bone repair, it still suffers from secondary surgical injuries, severe donor area injuries and complications [3]. Bone tissue engineering to design material scaffolds, cells and signaling molecules to induce new bone tissue formation by eliminating risks associated with autografting has been a key approach in current bone repair treatments [4]. A variety of different cells in bone (including bone-associated cells, immune cells, endothelial cells, etc.) share the same microenvironment and play an important role in osteogenesis. However, traditional bone tissue engineering studies have focused only on the effects of extracellular matrix (with collagen and hydroxyapatite as the main components [5]) on osteogenesis, ignoring the effects of other cellular and non-cellular cues in the local microenvironment on osteogenesis. Bone microenvironment is a complex structural and biological system that can be distinguished into three parts according to its function and components it contains: physiological microenvironment, chemical microenvironment, and physical microenvironment (Fig. 1). Physiologically, it contains immune cells and endothelial cells, and the physiological microenvironment supports the effective functioning of bone tissue through the integration of cellular energy metabolism exhibiting a tendency to immunosuppression and protection of hematopoietic stem cell components. Recent studies have shown that M2-type macrophages can improve the physiological microenvironment at the site of injury and promote osseointegration by converting the inflammatory microenvironment into an anti-inflammatory microenvironment [6]. Chemically, nutrients such as oxygen and pH as well as various signaling molecules released by different cell types are essential for cell survival and function. Li et al. attempted to enhance the oxygen supply to bone defect areas, and the hypoxic environment was improved, showing a significant increase in osteogenic capacity [7]. Physically, appropriate external stimuli can also influence cell fate, providing new ideas for bone tissue engineering to treat bone defects. By comparing osteoblasts in static culture to those subjected to mechanical stimulation, Brady found that paracrine factors secreted after mechanical stimulation significantly enhanced migration, proliferation, and osteogenesis [8]. In conclusion, engineering of bone microenvironment has emerged as a promising research direction for the design of bone biomaterials. In this review, we elucidate the dynamic regulatory role of cellular and peripheral cues in the bone microenvironment, systematically classify the various cues of the bone microenvironment, and summarize the regulatory role of each class of cues on bone repair. Finally, we discuss the challenges faced in constructing bone microenvironments and future directions for incorporating bone organoids. This work provides solid theoretical support for the construction of advanced bone microenvironments and offers new avenues for the development of bone tissue engineering.
Fig. 1.
The diagram of bone tissue cell microenvironment. Bone microenvironment can be divided into three parts: physiological microenvironment, chemical microenvironment and physical microenvironment.
2. Traditional strategy mimicking ECM
ECM is regarded as a novel regenerative material that not only provides a physical scaffold for cells in tissues but also regulates numerous cellular processes, including growth, migration, differentiation, and morphogenesis [[9], [10], [11], [12]]. The ECM is adynamic structure that undergoes controlled remodeling continually. Maintaining its integrity and homeostasis is essential for tissue growth and organ physiology, while the loss of ECM components or structural changes may result in disease development. A key distinction between bone and other tissues lies in the high percentage of matrix components and the low percentage of cellular components in bone. Heterogeneity is one of the fundamental features of ECM, and numerous studies have shown that different physical properties of biomaterials can influence the differentiation fate and cellular behavior of stem cells [13]. Mesenchymal stem cells have multidirectional differentiation potential and can differentiate into a variety of tissue cells such as adipose, bone, cartilage, muscle, nerve, cardiac muscle, endothelium, etc. [14]. It is also the most widely studied seed cell in bone tissue engineering. Research has supported that biomaterials can achieve stem cell osteogenic differentiation by modulating nanoscale surface morphology in the absence of additional osteogenic supplements [15]. For example, Zhang et al. designed a nanocomposite membrane that mimics endogenous potentials, and a proper electrical microenvironment greatly promotes bone regeneration [16]. In this review, we focus on the field of biomaterials to modulate heterogeneous osteogenic differentiation. The composition of bone tissue ECM can be categorized into organic and inorganic components. The organic component primarily consists of collagen, while the inorganic part is mainly composed of hydroxyapatite (HA). Together, these components constitute the ECM and possess specific biological functions [17]. Mimicking the ECM of bone tissue to promote cellular function and bone regeneration is a reasonable and feasible approach [18]. In this section, the traditional ECM-based repair strategy will be discussed in detail.
2.1. Mimicking component of ECM
2.1.1. Collagen
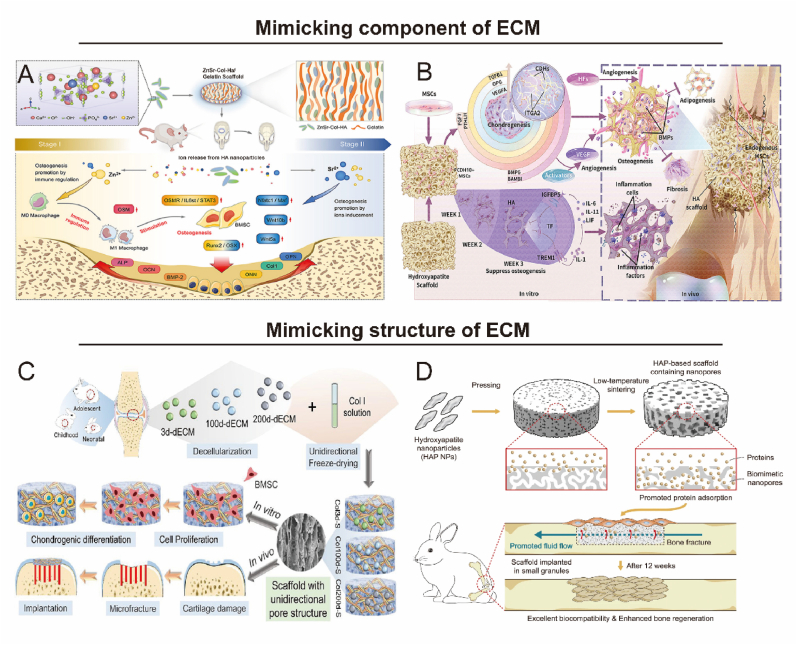
The ECM of bone tissue is abundant in collagen, which can be cross-linked via functional groups to generate materials with tailored mechanical or biological properties, making it highly applicable in tissue engineering [19,20]. Collagen scaffolds exhibit favorable biological properties, such as hydrophilicity, low antigenicity, and biodegradability [21,22]. However, singular collagen scaffolds lack sufficient mechanical strength, leading to the incorporation of other biomaterials like calcium phosphate, bioceramics, and polymers during scaffold design [23,24]. Zhong et al. developed a collagen-HA co-assembled scaffold, which exhibited strong bioactivity and increased the osteogenic differentiation potential of BMSCs with a similar composition to ECM (Fig. 2A) [25]. Xia's group further combined collagen with calcium phosphate to create a biomimetic composite coating that enhanced cellular affinity and in vivo absorption, supporting osteoblast activity and new bone formation [26].
Fig. 2.
Biomaterials mimic the component and structure of ECM for bone repair. A) Collagen-HA co-assembled scaffolds loaded with immune ions are programmed to promote osteogenic gene expression in BMSCs. Reproduced and adapted with permission [25]. Copyright 2022, Elsevier. B) Bionic HA scaffold regulates the cellular activity of MSCs and enhances the ossification process to promote bone repair. Reproduced and adapted with permission [37]. Copyright 2021, Elsevier. C) Age-specific dECM provides different local environments for BMSCs and promotes microfracture repair in vivo. Reproduced and adapted with permission [40]. Copyright 2021, American Chemical Society. D) HA scaffold with natural bone-mimicking nanopores to improve bone regeneration efficiency. Reproduced and adapted with permission [41]. Copyright 2022, Springer Nature.
Numerous membrane types have been utilized in guided bone regeneration techniques. Collagen membranes, the most prevalent, offer resorbability and in vivo degradation, minimizing inflammatory responses associated with foreign body scaffolds [27,28]. Presently, collagen membranes are frequently employed in the restoration of periodontal defects, obviating the need for secondary surgery [29]. Bio-Gide®, a prominent commercial collagen membrane, exhibits a unique bilayer structure that effectively inhibits epithelial cell infiltration into the defect site and fosters osseointegration [30,31]. To enhance the resistance to degradation and to expand the efficacy of absorbable collagen membranes, many chemical and physical cross-linking methods have been applied to manufacture cross-linked collagen fibers, including UV, glutaraldehyde, hexamethylene diisocyanate, diphenylphosphoryl azide, and enzymatic cross-linking [32]. The principle is to extract collagen into single fibers, which are then reconstituted and cross-linked [33].
2.1.2. Hydroxyapatite
HA possesses exceptional biocompatibility and mechanical properties and demonstrates stable interfacial binding within bone tissue [34,35]. HA surfaces can directly bind to new bone, support osteoblast adhesion, growth, and differentiation, and facilitate new bone deposition by means other than adjacent living bone [36]. Consequently, HA is extensively employed as a bone substitute, metal implant coating, tissue engineering scaffold, and drug delivery carrier, earning the designation of a “bioactive material.” Recent studies have revealed that HA scaffolds can function as osteogenic mediators during osteogenesis via the ZBTB16 and WNT signaling pathways [37]. This phenomenon may be attributed to the integrated microenvironment of specific bioactive materials providing an osteogenic advantage for osteoblasts (Fig. 2B). Ohgushi et al. further corroborated the interaction between HA and bone tissue, demonstrating that the HA surface can support cell differentiation and facilitate cell and bone formation [38]. HA coating combined with metal implants is a widely employed bone repair strategy. Yamada et al. successfully generated nano-polycrystalline HA on the surface of micro-coarsened titanium (Ti) [39]. During the healing phase, the HA-coated Ti significantly enhanced the strength of bone-implant integration compared to uncoated micro-roughing Ti. Thus, the osteogenic scaffold can be optimized by HA coating to improve bone-implant integration and pinpoint specific bone morphogenesis parameters.
2.2. Mimicking the structure of ECM
2.2.1. Decellularized ECM scaffold
The decellularized extracellular matrix (dECM) is the extracellular part of the tissue that is highly bioactive, low immunogenic and well biodegradable. The main advantage of dECM is that biomolecules in ECM are retained, supporting cell growth and viability. However, the decellularization process presents challenges, particularly maximizing cellular material removal while minimizing ECM damage. To evaluate dECM's potential for promoting the osteogenic process, Hashimoto et al. compared MSC differentiation on three-dimensional dECM and two-dimensional tissue culture polystyrene discs, observing significantly higher alkaline phosphatase (ALP) activity in MSCs grown in decellularized bone matrix [42]. This finding suggests that the decellularized bone matrix facilitates early osteogenic differentiation of MSCs. Furthermore, when rabbit dECM from different age groups, such as neonates, children, and adolescents, was co-cultured with BMSCs, BMSCs exhibited distinct cell morphology, roundness, and proliferation characteristics in vitro (Fig. 2C) [40]. To enhance the osteoinductivity of dECM, researchers have tried to incorporate different materials such as HA, glass-ceramics and Ti in dECM scaffolds, all of which proved to enhance osteogenic differentiation and bone repair [43,44]. These findings indicate that dECM, as a biomaterial, holds the potential for promoting bone regeneration.
2.2.2. Synthetic ECM scaffold
Hydrogels, polymeric materials with water solubility, form three-dimensional network structures through cross-linking reactions between hydrophilic polymers. Owing to their biomimetic properties, hydrogels can emulate the ECM internal structure and provide support for cells to perform physiological functions [45]. Many natural or synthetic materials are used as hydrogel base units for bone repair. In the last decade, hydrogels have been developed in various implantable forms to address different types or locations of bone diseases [46]. Implantable hydrogels are widely used due to their mechanical strength and ease of shape adjustment at pre-designed sites. In-situ injectable hydrogel scaffolds have excellent sol-gel properties allowing filling of defective sites without traditional major surgery [47]. Thus, hydrogels are considered promising candidates for bone tissue engineering. Due to the nanoscale size of natural tissues or organs, nanomaterials have superior physicochemical properties in terms of bionanotechnology, and nanoscale ECM scaffolds are emerging as a developmental direction to promote bone repair potential [48]. Li et al. introduced HA nanoparticles into bone graft materials by varying the sintering temperature to form nanoscale pore scaffolds similar to natural bone [41]. The results demonstrated that the mechanical strength, cell proliferation, and differentiation rates of the scaffolds with nanopores were significantly increased (Fig. 2D).
Electrospinning, a versatile technique facilitating the fabrication of nanofibers with controlled diameters and distinct structures, is employed to construct polymeric nanofiber scaffolds for bone tissue engineering due to its structural resemblance to tissue ECM, straightforward setup, and cost-effective operation [49]. Fibers generated through this method exhibit high homogeneity and mechanical strength, forming porous scaffolds with an elevated surface-to-volume ratio. A diverse array of synthetic biodegradable polymers and natural macromolecules have been utilized to create fibrous scaffolds. While electrospun natural polymers demonstrate enhanced hydrophilicity, synthetic polymers possess greater robustness and superior mechanical properties. Synthetic electrospun fiber scaffolds can be further functionalized to augment cellular activities by incorporating compounds or morphogens such as HA, glycosaminoglycan, and recombinant human BMP-2. Controlled release of these compounds from the scaffolds can be achieved through the meticulous blending of various synthetic biodegradable polymers. Providing both topographical and biochemical signals, the electrospun nanofibrous scaffolds may offer an optimal microenvironment that mimics native ECM for seeded cells [50]. Li et al. developed a novel nanoparticle-embedded electrospun nanofiber scaffold for controlled dual delivery of BMP-2 and DEX [51]. In vivo osteogenesis studies showed that controlled dual delivery of BMP-2 and DEX promotes calvarial bone defect repair; DEX effectively promotes early calcified bone formation, while BMP-2 facilitates long-term new bone formation. In conclusion, dual drug-loaded nanofiber scaffolds may be ideal candidates for bone tissue engineering.
2.3. Limitations of traditional strategy mimicking ECM
A primary focus of conventional bone tissue engineering is the development of biomaterials that emulate the composition and structure of the ECM to modulate bone regeneration. The method of mimicking bone ECM is superior because it is simple and similar to bone ECM. However, in many cases, the importance of the local cellular microenvironment in injury repair is often overlooked, and this simple approach of mimicking bone-like structures may lack the physiological, chemical, and physical cues that provide cells with the ability to form bone [[52], [53], [54]]. It is well known that key components such as collagen and HA provide the environment for osteogenic differentiation of stem cells in the bone structure, but natural ECM contains growth factors that are also necessary for normal bone formation. The dECM retains most of the original structure in biological tissues, yet some inactivation processes during formation irreversibly destroy the active components, such as proteases and growth factors. It has been shown that different oxygen concentrations, pH ranges, and appropriate stress stimulation can promote osteoblast proliferation and matrix secretion. All these studies suggest that we need to focus equally on the non-ECM part of the microenvironment.
Pericellular interactions in the microenvironment are dynamic, and their dynamics not only act as a reservoir for their signaling molecules but also mediate signals from other sources. These signals include a variety of factors, including cell-cell, cell-growth factor, pericellular chemical changes, and physical stimuli [55]. These dynamic processes ultimately determine the equilibrium and potential aberrations of the tissue. In traditional strategy described above, researchers focus more on the simulation of the initial infrastructure, ignoring the signals that cells must receive from the environment as they develop within or on the scaffold to achieve an ordered, specific genetic program that ultimately results in tissue/organ formation. Therefore, we believe that designing the cellular microenvironment requires more attention to the physiological, chemical and physical cues in the microenvironment rather than solely focusing on ECM features, which would be a revolutionary chance for the tissue engineering field.
3. Bone microenvironment
3.1. Composition of bone microenvironment
The bone microenvironment consists of three key components: Physiological (e.g., neighboring cells such as macrophages), chemical (e.g., oxygen, pH), and physical factors (e.g., mechanics, acoustics). These components work in concert to provide functional support for bone growth and development [56].
3.1.1. Physiological microenvironment
The physiological well-being of bone is determined not only by the dynamic equilibrium between osteoblasts and osteoclasts but also by other cells in the local microenvironment, such as immune and endothelial cells. Immune cells, encompassing lymphocytes (B and T cells), macrophages, and dendritic cells, have been demonstrated to secrete active factors that impact bone formation [57,58]. For instance, macrophages promote osteoblastogenesis by releasing interleukin-18 (IL-18) [59,60]. In the event of bone fractures, immune cells, particularly macrophages, are involved throughout the entire healing process, providing defense against pathogens and releasing a diverse array of effectors to regulate bone remodeling. The immune system also contributes to the development of pathological and chronic conditions in osteoporosis [61]. Endothelial cells, which form the blood vessel linings, along with pericytes, are crucial for bone tissue homeostasis by producing paracrine signaling molecules known as angiocrine factors [62]. Research has indicated that endothelial cells secrete several signaling molecules via paracrine interactions, such as platelet-derived growth factor (PDGF)-BB, vascular endothelial growth factor (VEGF), and BMP-2, which play an active role in the regulation of bone homeostasis [63].
3.1.2. Chemical microenvironment
The chemical microenvironment of bone contains numerous soluble factors, such as nutrients (e.g., oxygen, pH) and signaling molecules (e.g., enzymes, cytokines). Oxygen is the most easily depleted nutrient, and its insufficient supply has impeded the success of engineering intricate and sizable tissue constructs. Among soluble signaling molecules, cytokines have garnered significant attention in engineering biomimetic cellular microenvironments. BMPs, a group of structurally similar, highly conserved functional proteins, belong to the TGF-β superfamily [64]. BMP-2, a critical factor in osteogenesis, induces the differentiation of undifferentiated MSCs into chondrocytes and osteoblasts, which participate in bone and cartilage growth, development, and reconstruction processes [65]. Angiogenesis and osteogenesis are intimately connected, with VEGF performing distinct functions at various stages of bone formation, such as recruiting macrophages during the inflammatory phase to promote angiogenesis and stimulating osteoclastogenesis during the repair phase to maintain bone homeostasis [[66], [67], [68]].
3.1.3. Physical microenvironment
Except for the physiological and chemical cues mentioned above, cells interact with and respond to physical stimuli (including mechanical, photo, thermal, electrical, magnetic and acoustic stimuli) [69]. Researchers have adopted this principle to achieve efficient bone repair through the synergistic action of external field stimulation and responsive scaffolds. Mechanical stimuli can affect cell behavior through mechanotransduction. Photic stimuli (e.g., near-infrared light) can upregulate osteogenic gene expression to enhance bone regeneration [70]. Thermally responsive materials respond to temperature and serve to enhance cellular activity [71]. Electrical stimulation promotes migration, proliferation and differentiation of osteoblasts [72]. One possible mechanism is that electrical stimulation upregulates intracellular calcium concentration and subsequently regulates osteogenesis via the calmodulin pathway [73,74]. Magnetic stimulation therapy can be classified into two types: static magnetic fields (SMF) or electromagnetic fields (EMF), although the underlying biological mechanisms are still elusive, in vitro studies have shown that it can significantly enhance osteoblast differentiation [75,76]. Acoustic fields induce material deformation through acoustic radiation forces [77,78]. Although acoustic field stimulation has not been extensively utilized for engineering the bone microenvironment, it possesses considerable potential.
3.2. Importance of bone microenvironment
Over the past decade, comprehensive research has enhanced our understanding of the effects of biochemical and biophysical cues on cellular behavior. The bone microenvironment, as a highly dynamic and complex network, regulates the biological behavior of cells primarily through the following mechanisms: 1) providing physiological and biochemical signals to cells; and 2) providing physical and stimulatory signals to cells. The principal challenge in comprehending the bone microenvironment lies in adapting to its dynamic properties, where cellular feedback plays a significant role. However, the spatial and temporal variations of these cues, as well as their independent or collective actions with cells in forming intricate microenvironmental networks, remain unclear. Gaining insight into the influence of these dynamics on the regulation of cellular behavior is crucial for enhancing the development of bionanomaterials that can be employed in designing cellular microenvironments and facilitating numerous biomedical applications. Below we describe how different cues guide changes in the bone microenvironment and provide tools to interpret the microenvironment.
4. Engineered bone microenvironment
The coordinated interplay of physiological, chemical, and physical signaling in the bone microenvironment is crucial for regulating cellular processes in both developing and mature skeletons [79]. Recent progress in bone biology has resulted in a growing interest in using biomaterial scaffolds and bioreactors to engineer microenvironments that mimic natural bone functions [80]. In the following sections, we systematically review how bone biology and tissue engineering have been integrated to create controllable microenvironments at multiple levels (Table 1).
Table 1.
Microenvironment-targeted strategy.
| Types | Pathway | Materials | Function | Ref. |
|---|---|---|---|---|
| Physiological Microenvironment | Immunomodulation | Titanium implant | Micro-rough and hydrophilic surfaces promote the release of anti-inflammatory factors from macrophages | [81] |
| Polyethylene terephthalate | Macrophages adhering to hydrophilic and anionic surfaces selectively produce anti-inflammatory cytokines | [82] | ||
| Magnesium containing microspheres | Mg2+ release upregulates anti-inflammatory genes and triggers immune regulation | [83] | ||
| miR-181b exosomes | Exo-181b activates the PRKCD/AKT signaling pathway to promote M2 polarization | [84] | ||
| Angiogenesis | Sulfated chitosan scaffold | Dual-module scaffold continuously releases rhBMP-2 and VEGF, synergistically promoting osteogenesis and angiogenesis | [85] | |
| Nanofibrous gelatin-silica hybrid scaffold | Vascular-mimicking microchannel scaffold promotes rapid vascularization and bone regeneration | [86] | ||
| Energy metabolism | Bioenergetic-active material scaffold | Scaffold degradation fragments can increase mitochondrial membrane potential to accelerate bone regeneration | [87] | |
| GelMA hydrogel | Mg2+ increases cellular bioenergy levels to promote osteogenesis induction | [88] | ||
| Citrate composite support | Citrate-mediated elevation of cellular energy levels supports metabolic osteogenesis | [89] | ||
| Chemical Microenvironment | Oxygen | Liposomal/hydrogel complexes | ROS-responsive hydrogel releases oxygen to promote bone regeneration | [90] |
| PCL/nHA/CaO2 scaffold | The bionic scaffold releases oxygen continuously to promote bone defect repair | [91] | ||
| Bioactive glass/collagen–glycosaminoglycan scaffold | Co2+ mimics hypoxic signaling to activate the HIF pathway to support osteogenesis | [92] | ||
| pH | MOF@CaP nanoplatform | Nanoplatform mimics low pH environment to enhance bone regeneration and capacity | [93] | |
| Custom Titanium implant | The alkaline microenvironment mediates the osteogenic differentiation of stem cells and promotes new bone formation | [94] | ||
| Enzymes and Cytokines | Chondroitin Sulfate/Polyethylene Glycol hydrogel | Matrix metalloproteinase-mediated degradation of hydrogels regulates stem cell differentiation | [95] | |
| GelMA hydrogel | Mineralized alkaline phosphatase enhances the osteogenic differentiation potential of BMSCs | [96] | ||
| Hyaluronic acid hydrogel | Nanozymes mediate O2 production from endogenous H2O2 and provide a microenvironment for osteogenesis | [97] | ||
| Physical Microenvironment | Mechanical forces | Polydimethylsiloxane substrates | Stiff materials have a higher osteogenic potential than soft materials | [98] |
| The flow loop apparatus | Sustained low-velocity shear stress stimulates the expression of osteogenic markers in stem cells | [99] | ||
| Gelatin hydrogel | Reversibly connected, highly elastic hydrogel adapts to dynamic stresses and supports bone regeneration processes | [100] | ||
| Temperature | light-responsive poly (N-isopropylacrylamide- co -nitrobenzyl methacrylate) | Ultraviolet light stimulates the release of dexamethasone from photosensitive materials to promote bone regeneration | [101] | |
| Poly (vinyl alcohol) fibers | Thermoresponsive fibers improve the toughness of calcium phosphate cement and enhance bone repair | [102] | ||
| Biphasic calcium phosphate scaffold | Regulates drug release by changing the light source wavelength to promote bone repair | [103] | ||
| Electric field | Whitlockite scaffold | Scaffolds provide an endogenous electric field to the defect site and inhibit the activity of osteoclasts. | [104] | |
| Triboelectric nanogenerator | Mediated proliferation and differentiation of osteoblasts by electrical stimulation | [105] | ||
| Magnetic field | Poly(lactide-co-glycolide) scaffold | Magneto-thermal accelerated degradation behavior of magnetic scaffolds under alternating magnetic fields | [106] | |
| Static magnetic field | Magnetic fields can regulate the direction of osteoblast growth | [107] | ||
| Acoustic | Collagen sponge | In situ recruitment of osteogenic factors by ultrasonically shocked microbubbles | [108] | |
| Acoustically responsive scaffold/hydrogel | Pulsed ultrasound recruits BMSCs for bone repair | [109] | ||
| Programming design | Poly(aryl-ether-ether-ketone) (PEEK) implant | Programmed surface coating to release osteogenic drugs over time | [110] | |
| GelMA hydrogel | Programming a two-factor delivery system to match the bone repair healing process | [111] |
4.1. Physiological microenvironment
Maintaining the homeostasis of the physiological microenvironment of bone is paramount for preserving the vitality and functionality of bone-related cells, and is a crucial aspect of bone regeneration [18]. This review focuses on investigations concerning immune regulation, angiogenesis, and energy metabolism within the bone physiological microenvironment.
4.1.1. Immune regulation
Osteoimmunology is a specialized area of research that examines the reciprocal relationship between bone cells and the immune system [112]. Numerous factors typically categorized as immunological agents, such as interleukins (e.g., IL-6, IL-11, IL-17, and IL-23) [[113], [114], [115]], tumor necrosis factor (TNF)-α [116], RANK and its ligand RANKL [117], nuclear factor of activated T cells (NFATc1) [118] have been found to exert a significant influence on osteoclasts and osteoblasts. In particular, macrophages of the innate immune system undergo diverse polarization states, with M1 macrophages exhibiting pro-inflammatory behavior and M2 macrophages demonstrating anti-inflammatory characteristics [119]. These macrophage phenotypes and their polarization are indispensable for biomaterials to stimulate bone regeneration [120].
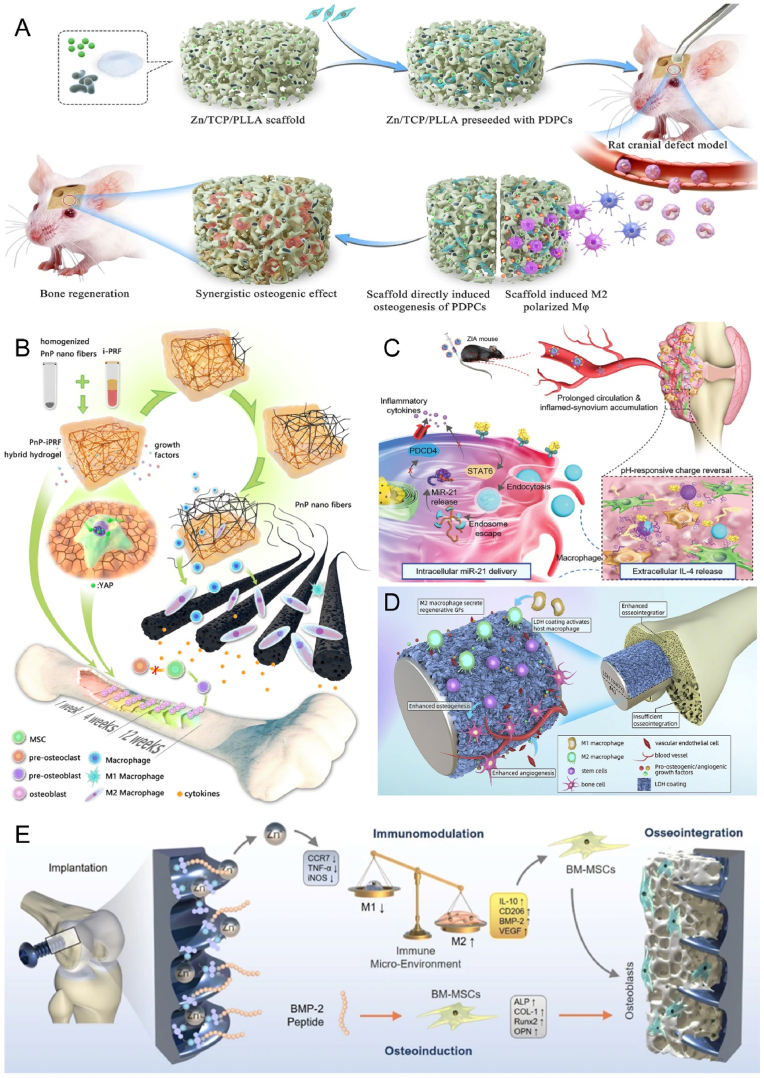
Biomaterials have been recognized as a promising approach for regulating bone regeneration by altering surface morphology [81,121], stiffness [122], porosity and pore size [123], hydrophilicity [124] and surface charge [82] through physical and chemical modifications, thus affecting macrophage polarization, phenotype and function and contributing to the M1 to M2 phenotype transition. Biomaterials can also provide bioactive molecules, including bioactive ions [83], drugs [6], cytokines [125], and microRNAs(miRNAs) [84], which can activate anti-inflammatory signaling directly or indirectly. For instance, Zinc is an essential trace element in bone formation and plays a critical role in osteogenesis and immune processes. Therefore, it is frequently used to modify biological materials to improve bone immunomodulatory capacity. Huang et al. investigated the role of Zn2+ in osteoinduction and immunomodulation by combining it with a phosphate/poly(l-lactic acid) scaffold (TCP/PLLA) [126]. Compared to a single scaffold, the sustained-release scaffold of Zn2+ showed a stronger effect on bone differentiation while inducing macrophage polarization toward the M2 phenotype, resulting in a favorable osteogenic microenvironment (Fig. 3A).
Fig. 3.
Biomaterials mimic the immune environment to promote bone repair. A) Sustained release of Zn2+ from bioactive ceramics induces M2 macrophages, further promoting periosteal-derived progenitor cells-mediated bone regeneration. Reproduced and adapted with permission [126]. Copyright 2021, Springer Nature. B) Blood-derived hybrid hydrogels promote bone healing by reprogramming the immune environment. Reproduced and adapted with permission [127]. Copyright 2022, American Chemical Society. C) Nanocomplexes deliver miR-21 and IL-4 in layers to promote macrophage polarization to the M2 phenotype. Reproduced and adapted with permission [128]. Copyright 2021, Wiley-VCH GmbH. D) Mg–Al laminated double hydroxide coating induces macrophage polarization to an M2 anti-inflammatory phenotype that exhibits good osteogenic and angiogenic potential. Reproduced and adapted with permission [129]. Copyright 2021, Elsevier. E) Dual-effect coated Ti screws modified with Zn2+ and BMP-2 co-regulate the bone immune microenvironment at the bone-implant interface. Reproduced and adapted with permission [130]. Copyright 2022, Springer Nature.
The incorporation of biomaterials with cytokines has emerged as a direct and effective strategy for promoting bone regeneration. Human blood is naturally rich in various cytokines, and extensive bone injury is often accompanied by a poor regenerative microenvironment, especially an unfavorable immune microenvironment. Studies have shown that autologous blood-derived hydrogels can create the right conditions for osteogenesis by reprogramming the skeletal immune microenvironment, showing great promise in the field of personalized regenerative medicine (Fig. 3B) [127]. To take a more refined perspective, Deng et al. selected the key cytokines IL-4 and miR-21, which inhibit inflammation and delivered them to the site of injury in a programmed manner to modulate the bone immune microenvironment (Fig. 3C) [128]. Surface modifications of bone implants, including coating (Fig. 3D) [129] and molecular click (Fig. 3E) [130] methods, have also been utilized to achieve the same effect. Among the cells of the adaptive immune system, regulatory T cells may be promising candidates for a positive regulatory effect on fracture healing. Chen et al. showed that regulatory T cell exosomes can significantly enhance bone repair, demonstrating that regulatory T cells are promising and effective therapeutic agents for bone reconstruction, but the exact mechanism still needs to be discovered [131].
Despite the obvious progress in the field of bone immunology, many questions remain. For example, the immune system shares a variety of transcription factors, signaling molecules, and membrane receptors during bone repair, and the underlying molecular mechanisms by which it promotes bone regeneration remain unclear. Therefore, it is crucial to identify the molecular mechanisms by which osteoblasts and the immune system interact. In addition, bone injury patients with concomitant autoimmune diseases are commonly seen in the clinic, and autoimmunity may affect bone healing [132]. Future research advances could personalize microenvironmental therapies to address the need for clinical treatment.
4.1.2. Angiogenesis
Bone tissue is heavily reliant on the vascular system to receive oxygen and nutrients, as it is highly vascularized. The skeletal system is known to receive a substantial amount of blood output from the heart, estimated to be 10–15%. Thus, angiogenesis serves a key function in driving the development of bone tissue. Multiple approaches have been suggested to improve angiogenesis in bone tissue regeneration, such as administering angiogenic growth factors like VEGF and FGF, selecting appropriate seed cells like stem cells or mature vascular cells, and designing three-dimensional (3D) bionic scaffolds. The effectiveness of tissue engineering repair is closely related to the design of the biomaterial, which creates an environment suitable for cell growth, adhesion and differentiation. To promote vascularized osteogenesis, scaffold materials used for tissue engineering must possess similar biological properties to natural bone. For instance, natural collagen, fibrin gels, and bone cement exhibit good osteoconductivity, but are fragile and have poor mechanical properties. In contrast, materials such as bioactive glass and polylactic acid have good degradation properties but limited hydrophilicity and histocompatibility. Given these limitations, using a single material for vascularized bone regeneration is challenging.
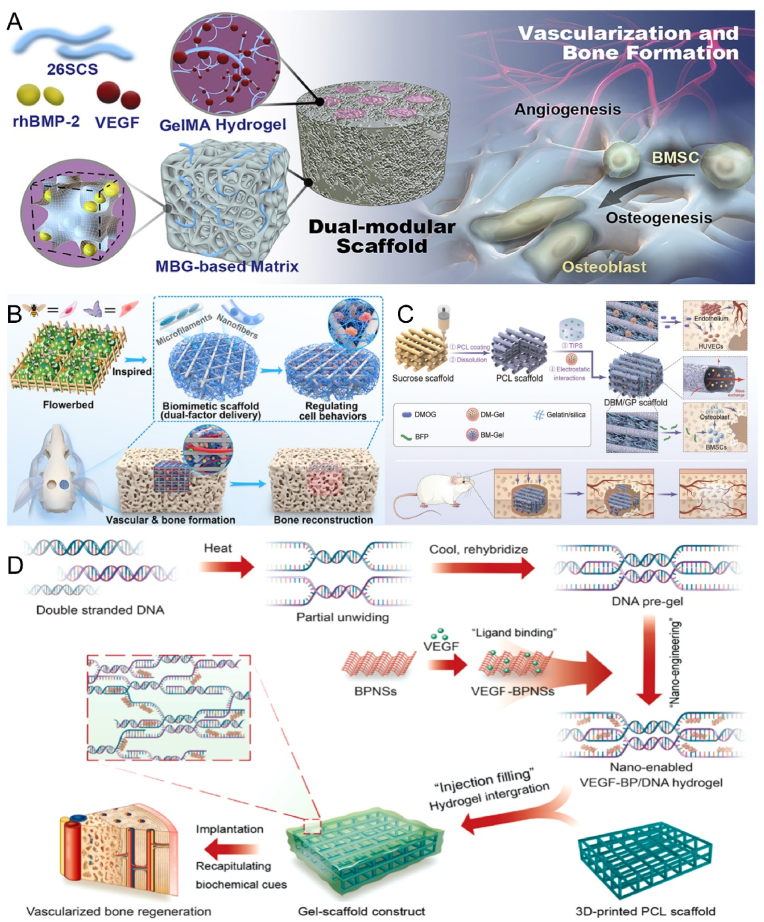
Tang et al. developed a dual modular scaffold that was designed to release different growth factors with different characteristics while maintaining their biological activity to promote angiogenesis and osteogenic capacity (Fig. 4A) [133]. Apart from the scaffold structure and composition, cytokines and ionic components also play critical roles in angiogenesis and bone regeneration. Therefore, scaffold properties can be improved by incorporating these components to enhance vascularized bone tissue engineering. Angiogenesis is mediated by a complex interplay of molecular signals involving various cytokines and ions, including VEGF, Angiopoietin (ANG), Silicon (Si), Magnesium (Mg), and Calcium (Ca). BMP-2, which is the most potent osteogenic agent, was loaded into poly(lactic acid)-glycolic acid copolymer tubes by Bouyer et al., resulting in intact defect bridging and vascularized bone tissue formation [134]. This indicates that BMP-2 has the potential to promote vascularized osteogenesis. Additionally, dimethyl oxalyl glycine (DMOG) has gained considerable attention in vascularized osteogenesis. Bionic scaffolds loaded with DMOG have been shown to exhibit excellent angiogenesis and stable bone formation both in vitro and in vivo (Fig. 4B) [135]. Ha et al. co-loaded DMOG, an osteogenic factor, and a pro-angiogenic factor into nanofibrous scaffolds with an interconnected perfusable microchannel network (Fig. 4C) [86]. The microchannel structure provided the necessary foundation for nutrient transport and improved degradation, serving as a model for in vivo pre-vascularization.
Fig. 4.
Biomaterials enhance angiogenesis to promote bone repair. A) 26SCS-functionalized bimodular scaffolds deliver BMP-2 and VEGF for synergistic osteogenesis and angiogenesis. Reproduced and adapted with permission [133]. Copyright 2020, Elsevier. B) 3D-printed two-factor delivery scaffolds sequentially release DMOG and Sr ions that match the angiogenic and osteogenic processes. Reproduced and adapted with permission [135]. Copyright 2023, American Chemical Society. C) Nanofibrous gelatin-silica hybrid scaffold with the spatiotemporal release of DMOG and bone-forming peptide to enhance angiogenesis and improve bone regeneration. Reproduced and adapted with permission [86]. Copyright 2022, Wiley-VCH GmbH. D) Dynamic DNA hydrogels loaded with black phosphorus nanosheets continuously release VEGF and promote mature vessel growth to induce osteogenesis. Reproduced and adapted with permission [136]. Copyright 2022, Elsevier.
The primary benefit of revascularization is the ability to achieve perfusion immediately after implantation, accelerating the development of the entire capillary network. In a rabbit model, it was shown that revascularized grafts enhanced the formation of new bone and capillaries. Tissue engineering commonly employs 3D printing technology as a strategy. 3D bioprinting technology enables precise localization of biomaterials, biochemistry, and living cells. Inspired by this technology, Miao et al. designed a nano-dynamic hydrogel scaffold with the aid of 3D printing. VEGF-decorated black phosphorus nanosheets (BPNSs) and DNA impart functionality to the hydrogel, enhancing angiogenesis and osteogenic activity (Fig. 4D) [136].
The greatest challenge for angiogenic strategies is that newly formed capillaries after stent implantation are transient and require continuous supplementation with exogenous nutrients [137]. Therefore, an in-depth understanding of cellular dynamics, cellular microenvironment and cell-cell interactions may guide the design of next-generation angiogenic scaffolds.
4.1.3. Energy metabolism
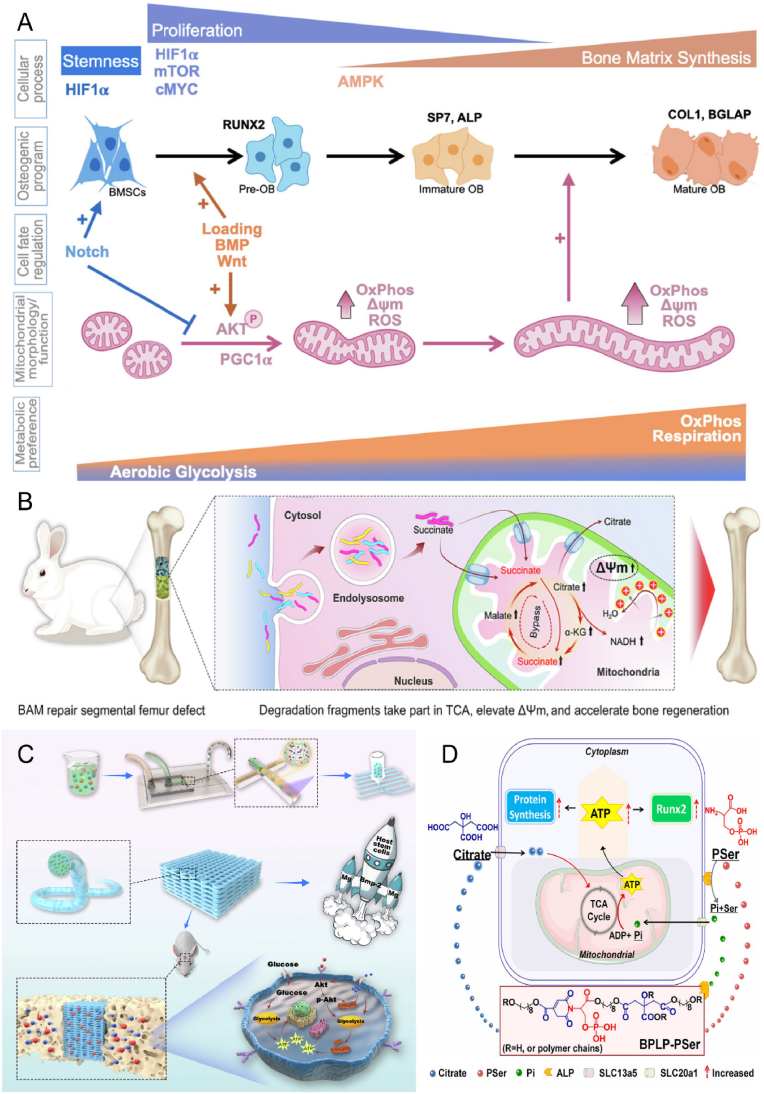
Cellular energy homeostasis involves the regulation of energy production and consumption in cells during normal physiological processes, achieved through nutrient uptake and biosynthesis (Fig. 5A) [138,139]. Two main metabolic pathways that convert nutrients to adenosine triphosphate (ATP) for energy to support biosynthetic activities are glycolysis and oxidative phosphorylation. The skeleton requires a significant amount of ATP to maintain its health, normal differentiation, and physiological functions. Cells follow a strict mechanism to regulate metabolic fluxes to maintain metabolic homeostasis [140]. This involves the regulation of gene expression, mRNA transcription and translation, and the expression of transporter proteins and metabolic enzymes in response to extracellular factors such as hormones and intertissued signals [141]. In this way, metabolic activities and pathways are regulated to support desired physiological functions. For example, osteoblast progenitors increase glucose uptake by upregulating the expression of glucose transporter protein 1 (GLUT1) in response to osteogenic signals, thereby meeting the energy requirements for osteogenic differentiation [136]. Additionally, in a hypoxic in vivo environment, undifferentiated MSCs exhibit higher glycolytic activity and lower oxidative phosphorylation activity, which suggests that cells may implement self-protective mechanisms to prevent aging caused by oxidative contingencies [142].
Fig. 5.
Biomaterials improve energy metabolism for bone repair. A) Bioenergetic regulation and signaling during osteoblast differentiation. Reproduced and adapted with permission [139]. Copyright 2022, Elsevier. B) Bioenergetically active material scaffolds that accelerate bone formation by degradation-mediated increases in mitochondrial membrane potential. Reproduced and adapted with permission [87]. Copyright 2020, American Association for the Advancement of Science. C) Mg2+ energy drive improved low-dose BMP-2-induced bone regeneration. Reproduced and adapted with permission [88]. Copyright 2022, Elsevier. D) Citrate-based biomaterials support osteogenic differentiation by providing degradation products during degradation and regulating the metabolic pathway of energy production. Reproduced and adapted with permission [89]. Copyright 2018, National Academy of Sciences.
Recent evidence indicates that modulating cellular metabolism can influence gene expression and signaling pathways to promote bone regeneration. One approach to achieve this is through the use of materials that induce material-derived cellular signaling. or instance, a bioenergetically active scaffold was designed by Liu et al. to release degradation debris in a controlled manner and produce metabolic intermediates, which enter the mitochondria and enhance the tricarboxylic acid (TCA) cycle, thereby increasing mitochondrial membrane potential and promoting bone regeneration (Fig. 5B) [87]. Similarly, ion-doped biomaterials can effectively modulate metabolism to regulate cellular function by controlling the release of metal ions that act as cofactors for metabolic enzymes or indirectly affect enzyme activity. Lin et al. demonstrated that a Mg2+-based bioenergy-driven strategy improved BMP-2-driven bone regeneration by increasing mitochondrial membrane potential and upregulating metabolic enzyme activity through the Akt signaling pathway (Fig. 5C) [88]. In addition, the regulation of metabolites, cofactors, and key substrates can influence intracellular metabolic events, with citrate from citrate-based biomaterials shown to promote osteogenic differentiation (Fig. 5D) [89]. The regulation of cellular energy metabolism may be a determinant of cell survival, proliferation, differentiation, and specific functions. Thus, understanding the specific energetic and biosynthetic requirements of different cell types is crucial for the design of effective regenerative engineering strategies.
Current research in bone tissue engineering in terms of cellular energy metabolism lags behind the neurological and cardiovascular fields. One of the main difficulties is that osteogenic differentiation exhibits a high proliferation rate in the initial stages and a synthesis and deposition phase in the later stages mainly by mechanisms. Different cellular stages require different metabolic signals and energy allocation. This would be a great challenge to match the dynamic formation process with inactive biomaterials.
4.2. Chemical microenvironment
In the bone microenvironment, cellular interactions are critical, and there exists a plethora of nutrients and signaling molecules that play significant roles in bone regeneration. This section outlines some of the essential factors, including nutrients such as oxygen and pH, as well as signaling molecules like enzymes and cytokines.
4.2.1. Oxygen
Oxygen is an indispensable molecule for the maintenance of cell viability, growth, metabolism, differentiation, and intercellular communication [143]. In healthy tissues, capillaries ensure the provision of sufficient oxygen to cells. Hypoxia arises when the distance between cells and blood vessels surpasses 100–200 μm [144]. The primary cause of tissue hypoxia is disruption of the vascular network at the injury site, which results in delayed oxygen delivery to the adjacent cells. Simultaneously, chronic hypoxia frequently contributes to widespread cell death and tissue necrosis. Notably, various skeletal cells exhibiting high metabolic activity and oxygen demand exhibit heightened sensitivity to hypoxic conditions. Consequently, it is imperative to ensure adequate oxygen supply to hypoxic tissues and regulate cellular metabolism to adapt to the hypoxic environment [145].
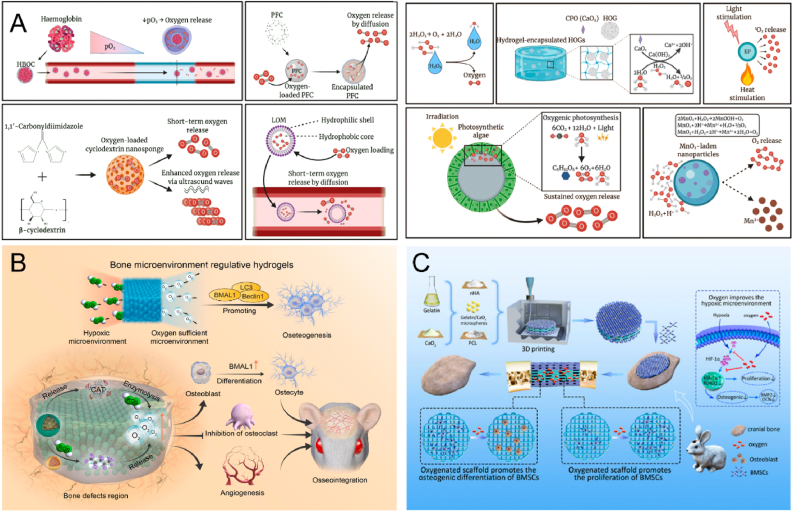
Tissue engineering facilitates in situ oxygen production by incorporating oxygen-generating components into biological materials. Oxygen production based on hemoglobin and peroxides has achieved remarkable results, but emerging technologies such as oxygen microbubbles, nanosponges and photosynthetic algae are equally worthy of in-depth study (Fig. 6A). Sun et al. developed an innovative composite hydrogel material capable of converting ROS to O2, with the capacity to accelerate O2 production in response to excess ROS based on the requirements of the affected region [90]. This hydrogel demonstrates effective oxygen generation capacity, promoting angiogenesis, inhibiting osteoclast differentiation, and enhancing osteoblast differentiation under hypoxic conditions (Fig. 6B). Wang et al. incorporated CaO2 in 3D printed scaffolds that exhibited good cytocompatibility and oxygen release, thus significantly improving cell survival and growth under hypoxic conditions (Fig. 6C) [91].
Fig. 6.
Biomaterials release oxygen to promote bone repair. A) Oxygen release mechanisms of various oxygen-producing biomaterials. Reproduced and adapted with permission [147]. Copyright 2021, Elsevier. B) A novel composite hydrogel scavenges ROS and prolongs oxygen production to reverse the hypoxic microenvironment in areas of bone defects. Reproduced and adapted with permission [90]. Copyright 2022, Elsevier. C) 3D-printed bionic oxygen-containing scaffolds enhance the expression of osteogenic regulatory transcription factors and accelerate osteogenesis. Reproduced and adapted with permission [91]. Copyright 2022, American Chemical Society.
Hypoxia-inducible transcription factor (HIF) is among the most well-known transcription factors that mediate oxygen-sensitive signaling pathways, stimulating the transcription of numerous genes and thereby influencing angiogenesis, precursor cell recruitment, and differentiation [146]. The development of tissue-engineered scaffolds capable of emulating local hypoxia within an environment exhibiting normal oxygen levels constitutes a rational approach, and this has emerged as a contemporary research direction to incorporate key oxygen-dependent HIF signaling pathways in scaffold design and fabrication. Quinlan et al. incorporated cobalt ions into bioactive glass/collagen-glycosaminoglycan scaffolds, which mimic hypoxia and artificially stabilize HIF-1α transcription factors [92]. The results demonstrated a significant enhancement of vascular endothelial growth factor expression, highlighting the ability to activate the HIF pathway under normoxic conditions.
However, during tissue engineering development, the material cannot be considered an adequate oxygenation mechanism due to its limited diffusion capacity and solubility in aqueous solutions. Therefore, the development of durable and homogeneous oxygen-releasing materials is critical for translation into clinical solutions.
4.2.2. pH
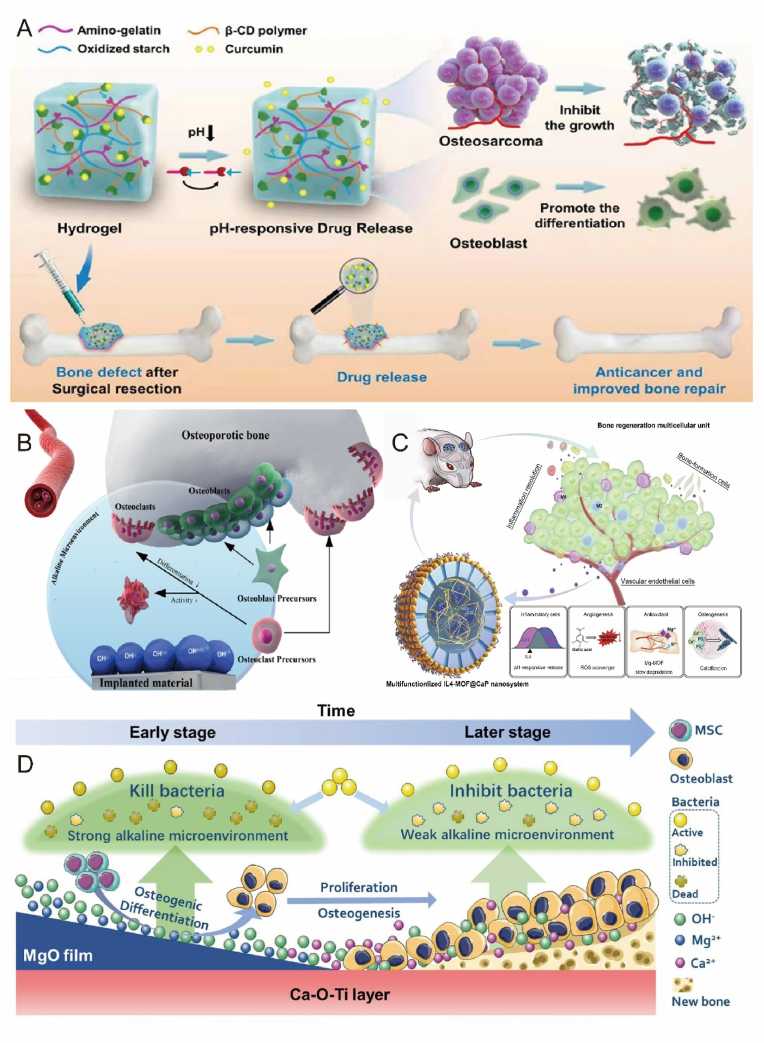
The natural human microenvironment is mildly alkaline. It is established that systemic acidosis in humans results in bone loss, potentially due to the physicochemical dissolution of bone minerals [148]. There are two common forms of acidosis, metabolic acidosis and respiratory acidosis. During metabolic acidosis, bones release buffered acids (protons) and calcium. During metabolic acidosis, bones release buffered acids (protons) and calcium. David et al. investigated the effects of metabolic acidosis on bone in mice [149]. The results show that metabolic acidosis can lead to the occurrence of bone resorption. Bone tissue pH can also be affected by inflammatory bone disease, tumor environment, and local acidic microenvironments due to immune cell enrichment. The acidic microenvironment further decreases pH, promoting increased bone resorption. Yuan et al. designed a composite hydrogel with selective toxicity to osteosarcoma tissues [150]. The loaded curcumin can be released in a pH-responsive manner at acidic osteosarcoma sites through the breakage of subamine bonds in the hydrogel, achieving selective toxicity to osteosarcoma cells. This selective toxicity and differentiation-promoting ability of pH-responsive hydrogels have been demonstrated in osteosarcoma cells and normal osteoblasts (Fig. 7A). The effective proton microenvironment boundary of degradable biomaterials was recently found to be 400 ± 50 μm, exceeding generally accepted value of 300 μm [151]. This further corroborates that biomaterials can significantly impact the cellular microenvironment (Fig. 7B). Consequently, some researchers have leveraged these microenvironment characteristics to develop smart reactive biomaterials with therapeutic and regenerative functions. Zheng et al. aimed to design multifunctional nanoplatforms capable of releasing a low pH microenvironment [93]. These nanoplatforms can continuously release encapsulated bioactive factors at low pH conditions to actively and precisely establish a reparative microenvironment for bone regeneration. Moreover, slow degradation during nanoparticle healing provides sufficient in situ magnesium and silica for angiogenesis and calcium and phosphate for osteogenesis (Fig. 7C). Alternatively, another study focused on the impact of an alkaline microenvironment on bone regeneration. The researchers constructed a weakly alkaline inner layer of Ca–O–Ti and a strongly alkaline outer membrane of MgO with Ti as the substrate [94]. This customizable alkaline microenvironment surface exhibited sustained resistance to infection and osseointegration, offering novel insights into bone implant surface design (Fig. 7D).
Fig. 7.
pH-responsive biomaterials promote bone repair. A) pH-responsive hydrogel targets selective osteosarcoma cells to release pro-bone repair drugs. Reproduced and adapted with permission [150]. Copyright 2022, American Chemical Society. B) Schematic diagram of the microenvironment of nearby cells affected by the implanted biomaterial. Reproduced and adapted with permission [151]. Copyright 2019, American Chemical Society. C) A nanoscale drug delivery system that reduces pH and can actively build a bone regenerative repair microenvironment. Reproduced and adapted with permission [93]. Copyright 2020, Elsevier. D) Customizable alkaline surface to enhance the osteogenic properties of Ti implants. Reproduced and adapted with permission [94]. Copyright 2021, Elsevier.
In the above study, different acid-base microenvironments showed different restorative effects in different pathological environments. It is still difficult to determine the optimal pH range, and further in-depth studies are needed in the future.
4.2.3. Enzymes and cytokines
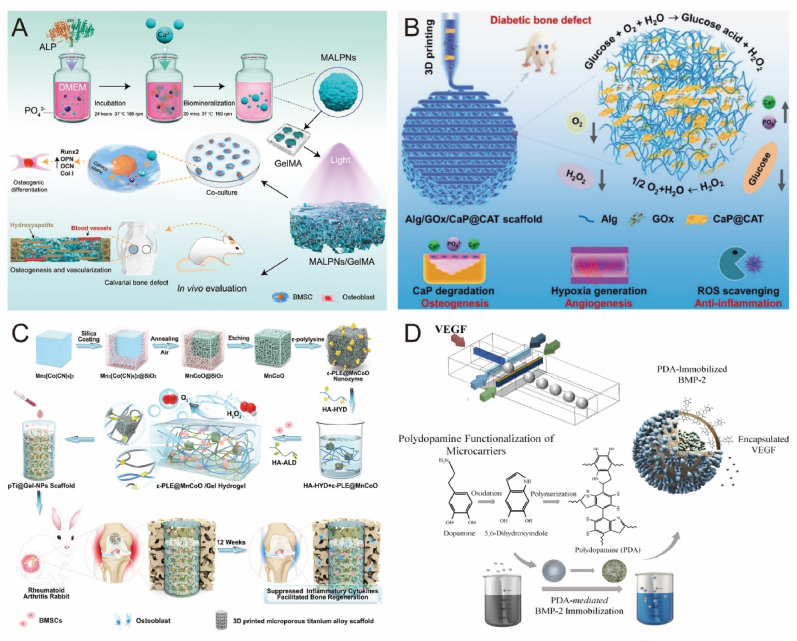
Enzymes are specific macromolecular biocatalysts, and all metabolic processes lin the body require the participation of enzymes [152]. In recent years, enzyme-based biomaterials have received a lot of attention [153]. For example, enzyme-responsive hydrogels typically utilize natural enzymes present in organisms or abnormally overexpressed at lesion sites, such as matrix metalloproteinases (MMP) [154], phosphatases [155], and tyrosinases [156]. Anjum et al. prepared a natural hydrogel based on an enzymatic reaction by grafting MMP in a hybrid system to maintain cell viability, proliferation, and migration through MMP-mediated degradation of the hydrogel to regulate cell growth factor delivery and stem cell differentiation [95]. However, MMP overexpression in bone and chondrocytes can result in pathological changes in bone, such as osteoarthritis (OA), osteoporosis, and rheumatoid arthritis (RA) [157]. In another study, mineralized ALP nanoparticles were incorporated into hydrogels to assess their bone repair effects in vivo. The osteogenic differentiation properties of BMSCs were significantly enhanced while retaining ALP enzyme activity (Fig. 8A) [96].
Fig. 8.
Biomaterials release enzymes and cytokines to promote bone repair. A) Mineralase-based hydrogels promote BMSC osteogenic differentiation to induce in situ mineralization. Reproduced and adapted with permission [96]. Copyright 2022, American Chemical Society. B) Enzymatic multifunctional scaffold loaded with GOx and catalase alleviates hyperglycemic environment and promotes bone regeneration. Reproduced and adapted with permission [158]. Copyright 2021, Wiley-VCH GmbH. C) Nanoenzyme-enhanced hydrogel improves the hypoxic environment for osseointegration at the RA prosthesis interface. Reproduced and adapted with permission [97]. Copyright 2022, Springer Nature Limited. D) Multifunctional microcarriers with sequential delivery of BMP-2 and VEGF. Reproduced and adapted with permission [168]. Copyright 2020, Springer Nature Limited.
Enzymes can not only be used as cofactors to enhance scaffold bioactivity but also be employed to create functionalized scaffolds via 3D printing. Chen et al. designed an enzyme-functionalized scaffold that releases glucose oxidase to alleviate the hyperglycemic environment and enhance bone regeneration in diabetic patients(Fig. 8B) [158]. Nanozymes, a new generation of artificial enzymes, possess unique nanomaterial properties, such as high catalytic activity, good stability, and low cost. Introducing nanozymes into hydrogels can create highly advanced bioactive platforms to address complex tissue-specific physiological challenges. A recent study demonstrated that composite hydrogels loaded with nanozymes not only scavenged endogenous overexpressed ROS but also synergistically generated dissolved oxygen. The effects of inhibiting local inflammatory cytokines and improving osseointegration were validated through in vivo and in vitro experiments (Fig. 8C) [97]. Despite these promising results, the question of how enzymes with overlapping substrates can react more precisely remains to be addressed.
Various growth factors play crucial roles in the bone repair process. Their effectiveness relies on the dose and release rate in vivo, as well as the drug delivery system, encompassing vectors, cells, and gene therapy. BMP and VEGF are the most extensively studied growth factors in bone tissue engineering. Various BMP2-loaded scaffolds have been investigated, including liposomes [159], gelatin sponges [160], hydrogels [161], exosomes [162], immune complexes [163], 3D printed scaffolds [164], etc., all of which demonstrate remarkable bone regeneration capabilities. Previous research on angiogenic factors has emphasized the role of VEGF in neovascularization and osteogenic recruitment, revealing that VEGF delivery increases vascular density and stimulates minor bone regeneration in rabbits and rats with bone defects [[165], [166], [167]]. Recent studies have indicated that the co-delivery of VEGF with osteogenic growth factors synergistically enhances osteogenesis (Fig. 8D) [168]. Bone-associated growth factors are diverse and numerous, with dynamic concentrations and distributions under varying physiological and pathological conditions, playing vital roles in promoting systemic or local bone formation. Presently, the most significant challenge in tissue engineering is delivering appropriate growth factors in a temporally and tightly regulated sequence during the repair cascade, holding immense potential for advancing bone repair medical interventions.
4.3. Physical microenvironment
Physical stimuli can significantly influence bone formation and are typically categorized into two primary classifications: internal and external bone stimuli. Internal stimuli predominantly stem from mechanical alterations, while external stimuli encompass factors such as photothermal, electrical, magnetic, and acoustic. Moreover, by integrating external stimuli with internal responses, a programmed spatiotemporally bone regeneration system can be designed and implemented.
4.3.1. Mechanical alterations
Bone, a mechanosensitive tissue, responds to mechanical signals from its environment through a process known as mechanotransduction [169]. Extensive research demonstrates that mechanical stimulation significantly impacts the development and remodeling of skeletal structures and offers a novel, drug-free approach to bone regeneration [170,171]. In bone tissue engineering, biological scaffolds are frequently required as temporary structural supports to fill bone defects, withstand early mechanical loading, and guide new bone formation [172]. Consequently, the efficacy of bone repair is strongly correlated with the mechanical properties of the scaffold, including stiffness, shear stress, and dynamic stress, which can influence osteogenic effects.
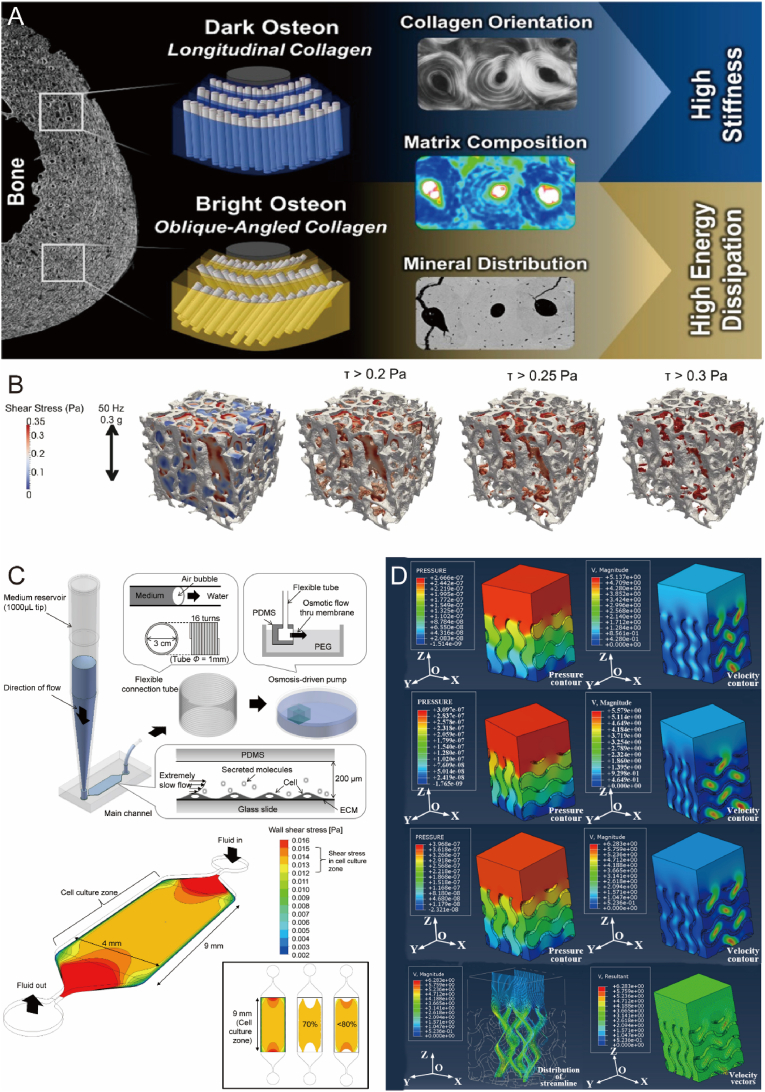
Current research has focused on investigating the impact of various materials and scaffold stiffnesses on osteogenesis. In a study by Zhang et al., polydimethylsiloxane substrates with different stiffnesses were prepared to explore the potential mechanisms of mechanotransduction [98]. The results indicated that rat primary osteoblast differentiation was more favorable on rigid substrates, with higher expression of ALP and runt-related transcription factor 2 (Runx2) observed on substrates with a stiffness of 134 kPa. In another study, researchers compared the orientation of collagen fibers in bone tissue microstructure and found that longitudinally aligned, dark-colored bone was more mineralized, containing a higher ratio of inorganic to organic matrix components and exhibiting increased stiffness and resistance to plastic deformation under compression. In contrast, brighter-colored bone, containing a higher proportion of collagen, provided enhanced ductility and energy dissipation due to lower stiffness and rigidity (Fig. 9A) [173]. These findings suggest that both intrinsic material properties and anisotropy affect surface stiffness, warranting further in-depth comparative research.
Fig. 9.
Mechanical properties of biomaterials promote bone regeneration. A) Effect of different arrangements of collagen fibers on bone stiffness. Reproduced and adapted with permission [173]. Copyright 2021, American Chemical Society. B) Comparison of bone formation under different shear stresses. Reproduced and adapted with permission [174]. Copyright 2019, Biomedical Engineering Society. C) Microfluidic chip generates constant shear stress to verify the effect of shear force on osteogenic differentiation and shear stress distribution schematic. Reproduced and adapted with permission [178]. Copyright 2014, plos.org. D) Pressure clouds and flow distribution of spiral structure brackets with the different modulus of elasticity. Reproduced and adapted with permission [183]. Copyright 2019, Elsevier.
Under physiological conditions, bone cells are constantly exposed to mechanical loads, such as shear stresses, which stimulate osteocytes and lead to changes in bone volume and structure to maintain an optimal skeletal structure (Fig. 9B) [174]. Fluid shear stresses are predicted to range from 0.8 to 3 Pa [175,176], and they have been shown to initiate a series of osteogenic signaling events, including calcium release [177], and nitric oxide [176] synthesis and release. Krekea et al. exposed planar cultures of BMSCs to shear flow [99]. The results demonstrated that expression of late phenotypic markers of osteoblast differentiation increased with the duration of exposure to shear flow, with significant enhancement of bone sialoprotein (BSP) and osteopontin (OPN) genes observed at 30 and 120 min of shear flow. Similarly, Kim et al. subjected MSCs to constant, very low shear stress generated by flow and observed increased osteogenic differentiation of MSCs (Fig. 9C) [178]. These findings suggest that immature osteoblasts are mechanosensitive and are associated with shear strength or shear patterns [179]. Therefore, shear stress is deemed an essential factor in bone scaffold development, contributing to osseointegration between the host and implant, a prerequisite for implant stability.
The dynamic stress microenvironment offers cells the capacity to influence behavior and fate through stress relaxation and remodeling [[180], [181], [182]]. Traditional bone tissue engineering strategies aim to develop a bone scaffold with an elastic modulus and yield strength comparable to human bone, employing helical structures and optimizing cell inoculation efficiency by varying porosity and pore size (Fig. 9D) [183]. In recent decades, adaptive hydrogels with reversible connections have garnered significant attention. These hydrogels are characterized by spatial dynamics of the matrix with reversible connections, providing plasticity and stress relaxation to adapt biophysical signals during the repair process [184]. Supramolecular chemistry offers numerous non-covalent interactions for obtaining reversible connections, including macrocyclic host-guest interactions [100], hydrogen bonding [185], electrostatic interactions [186], and hydrophobic interactions [187]. Additionally, dynamic covalent chemistry presents several options, such as reversible Diels-Alder reactions [188], hydrazone bonding [189], thioester exchange [190], and borate bonding [191]. Qian et al. prepared a supramolecular gelatin macromolecule [100]. The resulting hydrogels can withstand excessive compressive and tensile strains and rapidly self-repair after mechanical damage. Hydrogels with altered mechanical stress were shown to be promising carrier materials for bone tissue repair.
4.3.2. Photothermal effect
Photo and thermal reactions serve as external stimulation treatments and significantly contribute to bone regeneration promotion. Current research explores various types of light, including ultraviolet (UV), visible (Vis), near-infrared (NIR), and distinct wavelengths of laser light [192]. Notably, NIR light, with its high tissue penetration depth and photothermal effect, serves as an efficacious approach to foster osteogenesis [193,194].
UV light is frequently employed in photoresponsive biomaterial systems, as its short wavelength facilitates drug release from biomaterials [195]. In one study, a light-responsive microgel was synthesized, which, under UV irradiation, promoted the release of DEX, inducing osteogenic differentiation of hMSC [101]. AlamarBlue assay and standardized ALP activity assay results indicated that DEX released from microgels has the potential for inducing osteogenic differentiation of hMSC. By toggling the UV light source on and off, drug release can be controlled, supporting clinical drug requirements. Furthermore, zirconia surfaces treated with UV light significantly enhanced osteogenesis, potentially due to accelerated cell attachment and spreading increased cytoskeleton development, and proliferation [196]. Blue light irradiation of the photosensitive material g-C3N4/rGO generated photocurrent, rapidly inducing BMSCs into osteoblasts. The researchers co-cultured photosensitive material with BMSC and the cell culture dishes were simultaneously irradiated with blue light for 30 min per day. The spectroscopic results showed that photocurrent generation from π-π* orbitals in visible light could provide a stronger driving force for osteogenesis [197].
Temperature change also impacts bone formation. Thermally responsive systems are powerful activation mechanisms for biomedical and biomaterial applications, as body temperature typically ranges from 35 to 37°C, and temperature shifts induce functional changes [198]. Thermally responsive biomaterials play a significant role in bone regeneration, such as smart fibers and hydrogels. The researchers incorporated thermally responsive poly(N-isopropylacrylamide) fiber brushes with calcium phosphate [102]. This brush has a dual thermo-responsive transition, with the fibers dispersing in hydrophilic calcium phosphate bone cement at 21°C and transforming to a hydrophobic state at 37°C to toughen this bone cement. Thermo-responsive hydrogels are a key biomaterial in that they exhibit temperature-dependent gel-sol transition in water [199]. Many thermally responsive hydrogels based on synthetic and natural copolymers have been successfully prepared and further investigated [200,201].
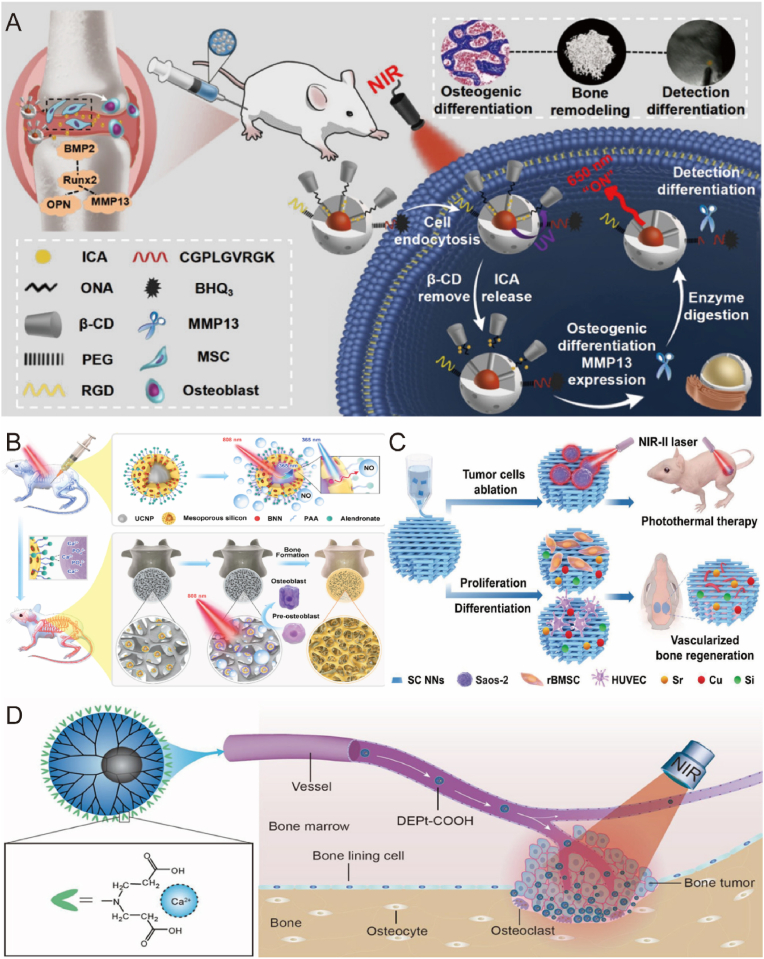
The synergistic effects of photo and thermo stimulation have proven efficacious in promoting bone regeneration. NIR light-generated photothermal effects facilitate osteogenesis, offering the advantages of non-invasiveness and high spatial and temporal accuracy [202]. Photothermal agents are capable of converting light energy into heat energy under NIR illumination, allowing for adjustments to photothermal hydrogels by modulating the concentration and ratio of the photothermal agent, irradiation time, and laser intensity [203]. Gentle local heating promotes cell proliferation, angiogenesis, wound healing, and bone regeneration [204], while moderate heat (45°C–50°C) causes minimal damage to normal tissue cells but inflicts lethal damage to tumor cells [205]. For the healing of infected wounds, heat therapy (>50°C) is effective in inhibiting bacterial proliferation. Therefore, the photothermal effect can be controlled according to different temperatures for various applications [206]. In recent studies, upconversion nanoparticles have garnered attention as efficient photo-responsive platforms. Yan [207] and Ye's [208] teams employed NIR light-mediated photothermal reactions to release Epimedium(Fig. 10A) and NO(Fig. 10B), respectively, as effective drugs against osteoporosis, with both experiments exhibiting favorable osteogenic differentiation. Photo-responsive systems can also achieve co-control of multiple targets. Qin Zhao et al. developed a dual-targeting nanoscaffold (BCP-GNC) that modulates drug release by altering the light source wavelength, thereby influencing scaffold temperature [103]. BCP-GNC releases IL-4 at 690 nm and DEX at 808 nm, modulating innate and adaptive immune responses and promoting osteoinduction. Yang et al. designed a multifunctional composite scaffold using 3D printing technology, unifying photothermal ablation of osteosarcoma, osteogenic differentiation of progenitor stem cells, and enhanced angiogenesis through bioactive ions (Fig. 10C) [209].
Fig. 10.
Biomaterials respond to photo and thermal stimulation to promote bone repair. A) Upconversion nanoparticle loaded with epimedium promotes MSC osteogenic differentiation. Reproduced and adapted with permission [207]. Copyright 2022, American Chemical Society. B) Targeted treatment of osteoporosis by upconversion nanoparticles releasing NO. Reproduced and adapted with permission [208]. Copyright 2021, American Chemical Society. C) Wesselsite nanosheet functionalized scaffold repairs tumor-induced bone defects. Reproduced and adapted with permission [209]. Copyright 2021, Wiley-VCH GmbH. D) Carboxy-capped dendrimer-mediated photothermal response inhibits bone tumor growth and tumor-associated osteolysis. Reproduced and adapted with permission [210]. Copyright 2018, American Chemical Society.
Another study reported that NIR-mediated photothermal responses inhibited osteolysis and promoted bone regeneration (Fig. 10D) [210]. Accumulating evidence supports the efficacy of NIR-mediated photothermal responses for targeted bone tumor treatment. Black phosphorus (BP), a cutting-edge two-dimensional material, exhibits exceptional photothermal properties, biocompatibility, and biodegradability [211]. Research has demonstrated that NIR-mediated photothermal heating of BP induces its oxidation in the presence of oxygen and water, effectively degrading it into phosphate ions [212]. These phosphate ions subsequently attract nearby calcium ions to form HA, thereby achieving in situ biomineralization [213]. Harnessing BP to enhance biomineralization represents an innovative approach to fostering bone formation and regeneration.
4.3.3. Electricity and magnetism
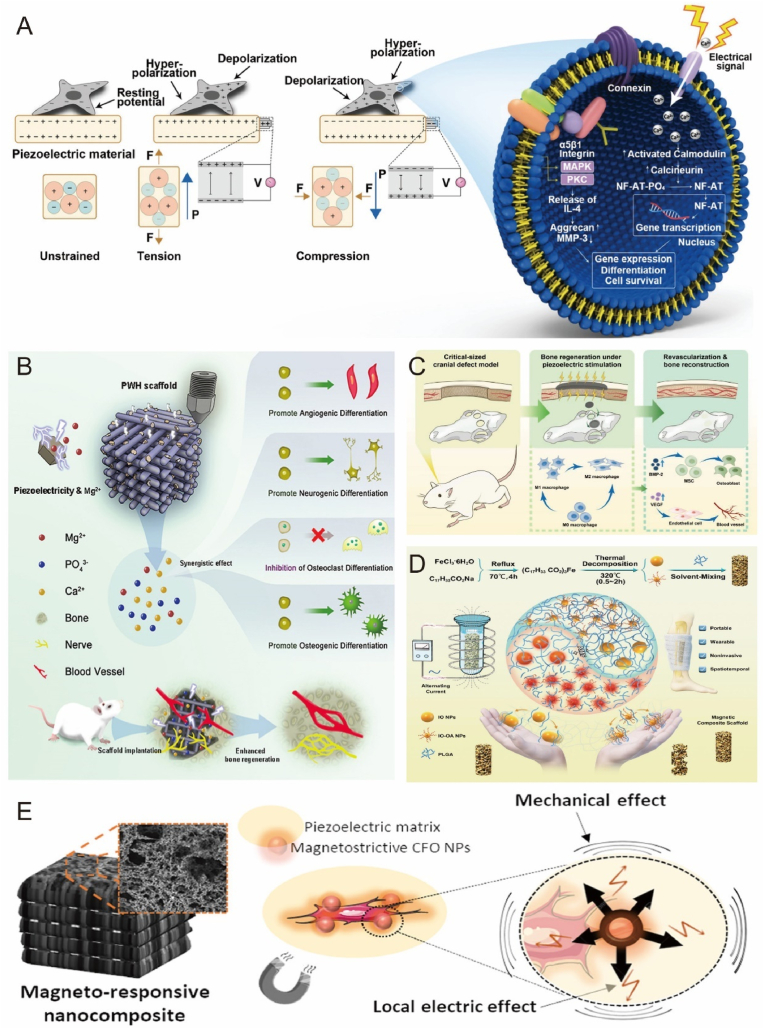
Application of electrical or magnetic stimulation in bone tissue engineering offers a promising strategy for bone regeneration [214]. Bone inherently exhibits piezoelectric properties, generating electrical and biochemical signals in response to mechanical activity for bone remodeling and repair [215]. Integrating smart materials with piezoelectric properties into bone implants can enhance bone regeneration [216]. Piezoelectric materials facilitate bone regeneration by accumulating electrical charge in response to mechanical stress, manifesting as a voltage generated by mechanical stress (the positive piezoelectric effect) or a mechanical response to an applied voltage (the converse piezoelectric effect) (Fig. 11A) [217,218]. While both effects are crucial, the positive piezoelectric effect has been predominantly investigated for bone implant applications. Common piezoelectric biomaterials include piezoelectric ceramics, piezoelectric polymers, and their composites [216,219].
Fig. 11.
Biomaterials respond to electrical and magnetic stimulation to promote bone repair. A) Schematic diagram of piezoelectric material surface mechanical strain induced charge generation triggering cell signaling pathway. Reproduced and adapted with permission [218]. Copyright 2020, WILEY-VCH Verlag GmbH. B) Composite scaffold with piezoelectric properties provides an endogenous electric field to promote bone regeneration in bone defects. Reproduced and adapted with permission [104]. Copyright 2022, Elsevier. C) Bionic piezoelectric bone membranes doped with polydopamine-modified hydroxyapatite (PHA) and barium titanate (PBT) synergistically promote osteogenesis and immunity. Reproduced and adapted with permission [220]. Copyright 2023, American Chemical Society. D) Schematic diagram of magnetron degradation in polymer implants. Reproduced and adapted with permission [106]. Copyright 2021, Wiley-VCH GmbH. E) Electromagnetic co-stimulation of bionic 3D scaffolds synergistically promotes bone repair. Reproduced and adapted with permission [226]. Copyright 2019, American Chemical Society.
Recent research has focused on designing composite scaffolds with piezoelectric properties and sustained Mg2+ release using 3D printing technology, which can restore the local endogenous electrical microenvironment and promote osteogenic differentiation (Fig. 11B) [104]. Additionally, multifunctional composite synergistic osteogenesis can be achieved with piezoelectric materials, combining piezoelectric osteogenesis with immunomodulation for rapid in situ bone regeneration (Fig. 11C) [220]. Nanogenerators can also convert environmental mechanical energy into electrical energy [221]. One study proposed a self-powered electrical system consisting of a triboelectric nanogenerator (TENG) and a flexible forked-finger electrode for in vitro osteogenesis, significantly promoting osteogenesis and demonstrating potential for clinical treatment of osteoporosis and related fractures [105]. The authors demonstrated that this electrical stimulator can clearly promote osteogenesis and has considerable potential for clinical treatment. Magnetically active biomaterials exploit external magnetic fields or direct magnetic forces to enhance bone tissue regeneration [222]. A prevailing trend in magnetically responsive biomaterials is the incorporation of iron oxide nanoparticles, as nanoparticles smaller than 100 nm exhibit superparamagnetic properties that prevent particle agglomeration [223]. One study verified that magnetic nanoparticles generate magnetothermia in alternating magnetic fields, providing crucial guidance for scaffold degradation (Fig. 11D) [106]. Magnetic fields with varying parameters may differentially affect osteogenesis in the magnetic response regime [224]. The direction and strength of the magnetic field influence bone regeneration. One study reported that cultured MC3T3-E1 cells aligned parallel to the static magnetic field (SMF) after 60 h of exposure, marking the first evidence that the growth direction of apposed cells can be regulated by the magnetic field [107]. Moreover, the biological effect of the magnetic field became more pronounced with increasing magnetic field strength within a specific range, but beyond that range, the effect diminished or even became inhibitory. Yang et al. examined the induction of osteoblasts by SMFs at three different intensities (500 nT, 0.2 T, and 16 T) and found that iron concentration and mRNA expression of transferrin receptor 1 were affected, suggesting iron involvement in the magnetic field's effect on osteoblasts [225].
Electrical and magnetic synergy can also benefit bone regeneration. Fernandes et al. demonstrated the feasibility of electromagnetic co-stimulation by assembling piezoelectric polymers and magnetostrictive nanoparticles in response to magnetic stimulation, constructing a bionic three-dimensional magnetically active scaffold for tissue recovery through co-stimulation (Fig. 11E) [226]. The applicability of pulsed electromagnetic fields (PEMF) has been evaluated using a rat cranial defect model [227]. In an 8 mm diameter rat cranial defect model, the experimental group receiving PEMF exhibited a significant effect on bone regeneration by applying a 12 μs width, a 60 Hz pulse frequency, and a 10 G magnetic field strength. However, the complex biological effects of electromagnetic fields and the underlying mechanisms of PEMF pose challenges in defining treatment options, necessitating extensive research to overcome this issue.
4.3.4. Acoustic
In vivo and in vitro studies have shown that ultrasound stimulation (e.g., low-intensity pulsed ultrasound (LIPUS), shock waves) is beneficial in promoting bone healing or reactivating failed healing processes [228]. Ultrasound-responsive biomaterials can deliver signaling molecules directly or indirectly with the help of ultrasound stimulation [229,230]. Functional fracture healing has been reported by ultrasound-mediated delivery of target genes (Fig. 12A) [108]. At present, LIPUS is the most extensively studied and researched technique in the domain of ultrasound stimulation for bone repair [231]. The biological response to LIPUS is intricate, involving numerous cell types and multiple pathways. Known mechanotransduction pathways implicated in cellular responses include MAPK [232], other kinase signaling pathways, gap junction intercellular communication [233], upregulation and aggregation of integrins, involvement of COX-2/PGE2 [234], iNOS/NO pathways [235], and activation of ATI mechanoreceptors. A recent study discovered that by altering the radiation frequency of pulsed ultrasound, not only could the release of bioactive molecules for recruiting endogenous BMSC be controlled, but also the capture of recruited BMSC into the stent could be facilitated through resonant gradient field-induced trapping forces (Fig. 12B) [109]. Similarly, several other studies have reported some effects of LIPUS on cell differentiation and protein responses. Although clinical and experimental studies have shown enhanced effects of LIPUS on bone regeneration, the physiological mechanisms involved in the complex bone healing process remain unclear and warrant further investigation (Fig. 12C–D) [[236], [237], [238], [239]].
Fig. 12.
Biomaterials respond to ultrasound stimulation to promote bone repair. A) Schematic diagram of ultrasound-mediated targeted gene delivery to the fracture site. Reproduced and adapted with permission [108]. Copyright 2017, American Association for the Advancement of Science. B) Schematic diagram of endogenous cell recruitment by pulsed ultrasound remote stimulation. Reproduced and adapted with permission [109]. Copyright 2022, Elsevier. C) Schematic diagram of LIPUS stimulation of periodontal stem cells to promote osteogenic differentiation. Reproduced and adapted with permission [238]. Copyright 2020, Springer Nature. D) Schematic diagram of LIPUS stimulated micro-arc oxidized Ti implants for bone repair. Reproduced and adapted with permission [239]. Copyright 2019, American Chemical Society.
4.3.5. Programmed spatiotemporally design
On-demand delivery of chemotactic and osteogenic biomolecules for bone regeneration is an appealing yet challenging endeavor, as it requires meeting the varying demands of distinct bone regeneration phases. Existing bone tissue engineering approaches face limitations in coordinating appropriate biomolecule concentrations and treatment time points on-demand due to insufficient spatial separation. Consequently, designing a programmable regulated delivery system remains a formidable task. In a recent study, researchers combined thermoresponsiveness with photothermal response to achieve rapid response concentrations in the initial burst release of the primary response, followed by precise modulation of therapeutic effects using photoresponsiveness [240]. The results suggest that a controlled and stable promotion of osteogenic differentiation can be achieved (Fig. 13A). Other researchers have sought to integrate immunomodulation with osteogenic process programming. By coating the programmed surface with poly(aryl ether ether ketone) (PEEK), IL-10 was released rapidly within the first week, followed by slow release of DEX for up to four weeks [110]. Suitable immunomodulatory activity sets the stage for osteogenesis, while the stable release of DEX promotes subsequent bone regeneration. Zhou et al. designed a hybrid dual growth factor delivery system with basic fibroblast growth factor (bFGF) and BMP-2 to further mimic the natural bone healing process and promote bone regeneration by synergizing osteogenic and angiogenic functions. It provides a simple and effective alternative method for bone defect treatment (Fig. 13B) [111].
Fig. 13.
Programmable design of biomaterials for bone repair. A) Thermally responsive hydrogels are combined with light-responsive nanoparticles to construct programmed delivery systems for on-demand growth factor release. Reproduced and adapted with permission [240]. Copyright 2022, Elsevier. B) Composite hydrogel that mimics the natural cascade reaction of bone healing for spatiotemporally regulated release of cytokines. Reproduced and adapted with permission [111]. Copyright 2021, Springer Nature. C) Multiple bioprinting technologies for bone repair. Reproduced and adapted with permission [244]. Copyright 2021, Elsevier. D) Constructing multi-responsive bilayers using 4D printing technology to precisely regulate stem cell fate and optimize bone repair. Reproduced and adapted with permission [258]. Copyright 2021, Wiley-VCH GmbH.
Bioprinting technology presents a promising approach to achieving programmable bio-design. By loading cells and bioactive cues directly into bioink, bioprinting can create structures that mimic natural tissue while allowing for programmability (Fig. 13C) [[241], [242], [243], [244]]. This advancement enables personalized treatment of bone-related diseases. Bioinks for bone tissue bioprinting are categorized into natural bioinks (collagen [245], chitosan [246], fibrin [247], gelatin [248], agarose [249] and alginate [250], etc.) and synthetic bioinks (Pluronic F-127 [251], methylated gelatin [252], PEG [253], etc.). Numerous challenges remain for clinical application of conventional 3D bioprinting in bone tissue engineering, such as reconstructing large and irregular bone tissue for individualized needs, achieving vascularization and nerve regeneration when repairing extensive bone defects, and addressing mechanical properties [254,255]. Four-dimensional (4D) bioprinting, which integrates the concept of time as a fourth dimension with 3D bioprinting, permits printed objects to alter their shape or function in response to external stimuli, cell fusion, or self-assembly after printing. This innovative approach offers a next-generation solution for tissue engineering, providing the potential to construct complex functional structures [256]. For instance, smart, renewable bioscaffolds synthesized using PCL and crosslinkers with predetermined amounts of castor oil demonstrated favorable shape memory effects and shape recovery at physiological temperatures [257]. Another study employed 4D printing technology to dynamically regulate stem cell fate, enabling precise switching between proliferation and differentiation phases to better promote bone regeneration (Fig. 13D) [258]. The synthesized smart polymers exhibited satisfactory surface morphology, shape memory, mechanical properties, biocompatibility, and biodegradability. In recent years, bioprinting has garnered considerable attention in the biomedical field and clinical applications due to the emergence of stimulus-responsive biomaterials and a deeper understanding of tissue regeneration. While various stimulus-responsive biomaterials and innovative strategies have been developed, 4D bioprinting remains in its infancy, necessitating further research to address numerous challenges [259].
Although responsive biomaterials have been developed for mechanical force, photothermal, electromagnetic and ultrasonic stimulation associated with the physical microenvironment, they are still in their infancy and have some common issues that need to be urgently addressed. The first is that these novel biomaterials, their immune responses and metabolic pathways have not been systematically explored. Secondly, the manufacturing process of reactive materials is complex and the specific mechanisms still need to be studied in detail. The optimal parameters of external stimuli and changes in the internal environment cannot be determined quickly in real-time and require the selection of suitable animal models for evaluation. Despite the enormous challenges, physical microenvironmental stimulation of biomaterials has far-reaching clinical applications in the future.
5. Bone microenvironment and bone organoids
Organoids, defined as in vitro 3D cell clusters derived from induced pluripotent stem cells, embryonic stem cells, or primitive tissues, rely on artificial microenvironmental matrices for self-renewal and self-organization. These formations emulate natural tissue structures and exhibit organ functions akin to native tissues [260]. Despite the variability resulting from their self-organizing nature, organoids represent the closest in vitro model system to in vivo tissue conditions and offer a promising avenue for personalized medicine. To date, organoids have been developed to simulate various human organs, including the brain [261], lung [262], kidney [263], liver [264], pancreas [265], intestine [266], and prostate [267]. However, the development of bone organoids remains in its infancy, due to limited understanding of bone-related disease mechanisms and challenges in directing stem cell differentiation [268]. Bone organoids combine stem cells with bioactive materials to form three-dimensional bone-mimicking tissue with self-renewal and self-organizing properties [260]. The successful establishment of bone organoids depends not only on the selection of stem cells but, more importantly, on the capacity of introduced biomaterials to provide the requisite microenvironmental matrix for 3D cell model growth and differentiation (Fig. 14A).
Fig. 14.
Engineered bone microenvironments assist in building bone organoids. A) Schematic diagram of the process of building bone organoids. Reproduced and adapted with permission [268]. Copyright 2022, Elsevier. B) The hydrogel-based construction of bone organoids bionically mimics the physiological microenvironment and promotes cell adhesion, proliferation and differentiation. Reproduced and adapted with permission [270]. Copyright 2022, Elsevier. C) BMSC and EC cells can form a pseudo-vascularized network within the mesenchymal compartment to generate bone marrow-like organs with in situ functional characteristics. Reproduced and adapted with permission [272]. Copyright 2022, American Institute of Physics. D) Composite hydrogel sensory electrical stimulation reconstructs the bone's physical microenvironment to promote bone regeneration. Reproduced and adapted with permission [274]. Copyright 2022, Elsevier.
As aforementioned, the bone microenvironment can be classified into physiological, chemical, and physical microenvironments, with dynamic interactions between the physiological microenvironment and bone organoids seed cells playing a critical role in regulating cellular behavior and tissue regeneration [269]. This dynamic process involves the continuous generation, degradation, and remodeling of ECM components. Matrigel, a natural physiological microenvironmental component derived from mouse tumors, is the most prevalent substrate for organoid cultures. It provides multiple physiological cues to induce stem cell differentiation, supports bone organoids growth, maintains extracellular and intercellular junctions, and facilitates self-organization into organoid tissues. Despite its widespread use in organoid cultures, Matrigel possesses certain drawbacks, such as heterogeneous origin, variable composition, and complexity, which impede its clinical advancement. Consequently, alternative biomaterials are urgently required for bone organoids development. Bone tissue engineering techniques have identified hydrogels as a viable alternative to matrix gels for bone organoids cultivation [270]. Hydrogels can provide a three-dimensional aqueous microenvironment to activate cell adhesion and proliferation, enhance cell differentiation, more closely mimic the functionality of natural tissues, and allow modulation of their properties through functional group modifications (Fig. 14B). It is anticipated that the biomimetic physiological microenvironment furnished by hydrogels will significantly advance overall regulation, thereby facilitating bone organoids culture.
The chemical microenvironment, encompassing various soluble factors, plays a crucial role in maintaining development and homeostasis during bone organoids construction. Existing in vitro skeletal models are frequently limited to basic osteogenic functions. Nevertheless, by integrating engineered microenvironmental tissue engineering strategies, organoids can present gradients of nutrients, gases, and signaling molecules, thereby achieving a comprehensive integration of immune regulation, angiogenesis, and osteogenic functions [271]. Recent research has demonstrated that bone marrow-like organoids can be formed on a hydrogel microporous platform, aided by components of the natural bone niche [272]. Morphologically, these organoids form self-organized, vascular-like networks, which strongly support the further construction of vascularized bone organoids (Fig. 14C) [273].
Constructing bone organoids with responsive physical microenvironments enables rapid detection of and response to disease environments and therapeutic effects. Various diseases exhibit distinct pathological microenvironments, such as excess reactive oxygen species and weak acidity in tumors, specific pH reductions and bacterially secreted enzymes in severe infections, and negative potential and specific ion concentrations at bone defect sites [157]. Leveraging these unique pathological characteristics, reversible or irreversible transformations in physical properties or chemical structures of biomaterials can be induced by stimulating the surrounding physical microenvironment, subsequently influencing cell fate and enhancing bone tissue healing and regeneration. Recent studies have similarly shown that re-establishing the physical microenvironment through electrical stimulation can significantly accelerate bone regeneration (Fig. 14D). [274].
In summary, the bone microenvironment is inherently linked to the development of bone organoids. Engineered bone microenvironments hold considerable potential for multifunctional co-regulation of bone organoids in simulating bone development and diseases. However, the currently reported bone organoids can only represent a single function of bone, such as bone formation, bone resorption, or hematopoiesis. Achieving multifunctional integrated bone organoids remains a great difficulty due to the different requirements of different types of stem cells for co-culture microenvironments. Future research can be conducted by constructing bone organoids with responsive microenvironments and integrating engineered microenvironmental tissue engineering strategies that can better mimic the complexity of bone tissue, create more accurate in vitro models, and ultimately facilitate advances in bone tissue regeneration and personalized medicine.
6. Summary and prospect
Bone tissue regeneration constitutes a multifaceted process that necessitates the emulation of the natural bone microenvironment, engaging various cells and factors to optimize new bone formation. This presents a critical opportunity for bone tissue engineering, which endeavors to integrate diverse physiological, chemical, and physical factors to replicate the microarchitecture and microenvironment of bone, ultimately facilitating tissue regeneration and repair. Recent advancements in the field have harnessed innovative techniques and materials to mimic the bone microenvironment, demonstrating its potential as a promising therapeutic target to promote functional bone tissue regeneration.
However, several challenges remain to be addressed in bone tissue engineering. Foremost is the attainment of multifunctional integration while emulating the bone microenvironment. The regulation of the bone healing process is stage-specific, dynamic, and coordinated, necessitating a research focus not only on the direct regulation of osteoblast cell lineage by the microenvironment but also on the interconnected systems of angiogenesis and immune regulation therein. By modulating the biological effects of these cells, a multifunctionally regulated microenvironment can be established in concordance with the natural healing process, thus enhancing bone regeneration more effectively.
Secondly, the nascent field of time-modulated four-dimensional (4D) bone microenvironments warrants further exploration. In recent years, time regulation has garnered substantial attention in biomedical research and clinical applications due to the emergence of stimuli-responsive biomaterials and enhanced comprehension of tissue regeneration. Morrison et al. showcased the successful implementation of personalized 4D printed medical devices in the treatment of pediatric tracheobronchi [275]. 4D implants possess the capacity to self-transform and self-mature over time, yielding significant benefits in the management of adolescent patients with congenital malformations. The 4D concept holds immense potential for personalized treatment and precision medicine, emerging as a leading trend within the realm of bone tissue engineering.
To fully harness the potential of bone tissue engineering, it is imperative that researchers address these challenges, focusing on the development of multifunctional integration and time-modulated 4D bone microenvironments. The successful incorporation of these advances will not only contribute to a deeper understanding of the bone healing process but also propel the field toward more effective and personalized therapeutic solutions for bone tissue regeneration.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Microenvironment-Targeted Strategy Steers Advanced Bone Regeneration”.
Acknowledgments
S.Y.H., M.K.W., and Z.F.Y. contributed equally to this work. This work was financially supported by the National Natural Science Foundation of China (82230071, 82172098), Shanghai Committee of Science and Technology (23141900600, Laboratory Animal Research Project).
Contributor Information
Yingying Jing, Email: jingy4172@shu.edu.cn.
Long Bai, Email: bailong@shu.edu.cn.
Jiacan Su, Email: drsujiacan@163.com.
Data availability
No data was used for the research described in the article.
References
- 1.Campana V., Milano G., Pagano E., Barba M., Cicione C., Salonna G., Lattanzi W., Logroscino G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014;25(10):2445–2461. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X.N., Fan H.Y., Deng X.W., Wu L.N., Yi T., Gu L.X., Zhou C.C., Fan Y.J., Zhang X.D. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials. 2018;8(11):960. doi: 10.3390/nano8110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonzo M., Primo F.A., Kumar S.A., Mudloff J.A., Dominguez E., Fregoso G., Ortiz N., Weiss W.M., Joddar B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opinion in Biomed. Eng. 2021;17 doi: 10.1016/j.cobme.2020.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian Y., Hu T., Lv Z., Xu Y., Wang Y., Wang H., Zhu W., Feng B., Liang R., Tan C., Weng X. Bone tissue engineering for treating osteonecrosis of the femoral head. Exploration. 2023;3(2) doi: 10.1002/EXP.20210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansour A., Mezour M.A., Badran Z., Tamimi F. Extracellular matrices for bone regeneration: a literature review. Tissue. Eng. Pt. A. 2017;23(23–24):1436–1451. doi: 10.1089/ten.TEA.2017.0026. [DOI] [PubMed] [Google Scholar]
- 6.Xiang G., Liu K.Y., Wang T.J., Hu X.F., Wang J., Gao Z.H., Lei W., Feng Y.F., Tao T.G.H. In situ regulation of macrophage polarization to enhance osseointegration under diabetic conditions using injectable silk/sitagliptin gel scaffolds. Adv. Sci. 2021;8(3) doi: 10.1002/advs.202002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J.Y., Han F.X., Ma J.J., Wang H., Pan J., Yang G.B., Zhao H., Zhao J.Y., Liu J.B., Liu Z., Li B. Targeting endogenous hydrogen peroxide at bone defects promotes bone repair. Adv. Funct. Mater. 2022;32(10) [Google Scholar]
- 8.Brady R.T., O’Brien F.J., Hoey D.A. Mechanically stimulated bone cells secrete paracrine factors that regulate osteoprogenitor recruitment, proliferation, and differentiation. Biochem. Bioph. Res. Co. 2015;459(1):118–123. doi: 10.1016/j.bbrc.2015.02.080. [DOI] [PubMed] [Google Scholar]
- 9.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Lin X., Patil S., Gao Y.G., Qian A. The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 2020;11:757. doi: 10.3389/fphar.2020.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loganathan R., Rongish B.J., Smith C.M., Filla M.B., Czirok A., Benazeraf B., Little C.D. Extracellular matrix motion and early morphogenesis. Development. 2016;143(12):2056–2065. doi: 10.1242/dev.127886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123(24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Y., Zheng X., Zhong X., Cui Q., Zhang S., Wen X., Heng B.C., He S., Shen Y., Zhang J., Wei Y., Deng X., Zhang X. Manipulation of heterogeneous surface electric potential promotes osteogenesis by strengthening RGD peptide binding and cellular mechanosensing. Adv. Mater. 2023;35(24) doi: 10.1002/adma.202209769. [DOI] [PubMed] [Google Scholar]
- 14.Liu J.X., Gao J.F., Liang Z.X., Gao C., Niu Q., Wu F.P., Zhang L.Y. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022;13(1):2271–2288. doi: 10.1186/s13287-022-02985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalby M.J., Gadegaard N., Tare R., Andar A., Riehle M.O., Herzyk P., Wilkinson C.D., Oreffo R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X.H., Zhang C.G., Lin Y.H., Hu P.H., Shen Y., Wang K., Meng S., Chai Y., Dai X.H., Liu X., Liu Y., Mo X.J., Cao C., Li S., Deng X.L., Chen L.L. Nanocomposite membranes enhance bone regeneration through restoring physiological electric microenvironment. ACS Nano. 2016;10(8):7279–7286. doi: 10.1021/acsnano.6b02247. [DOI] [PubMed] [Google Scholar]
- 17.de Wildt B.W.M., Ansari S., Sommerdijk N.A.J.M., Ito K., Akiva A., Hofmann S. From bone regeneration to three-dimensional in vitro models: tissue engineering of organized bone extracellular matrix. Curr. Opinion in Biomed. Eng. 2019;10:107–115. [Google Scholar]
- 18.Zhu G., Zhang T., Chen M., Yao K., Huang X., Zhang B., Li Y., Liu J., Wang Y., Zhao Z. Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021;6(11):4110–4140. doi: 10.1016/j.bioactmat.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszta M.J., Cheng X.G., Jee S.S., Kumar R., Kim Y.Y., Kaufman M.J., Douglas E.P., Gower L.B. Bone structure and formation: a new perspective. Mat. Sci. Eng. R. 2007;58(3–5):77–116. [Google Scholar]
- 20.Ferreira A.M., Gentile P., Chiono V., Ciardelli G. Collagen for bone tissue regeneration. Acta. Biomater. 2012;8(9):3191–3200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Glowacki J., Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 22.Miyata T., Taira T., Noishiki Y. Collagen engineering for biomaterial use. Clin. Mater. 1992;9(3–4):139–148. doi: 10.1016/0267-6605(92)90093-9. [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Du T., Ruan C., Niu X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021;6(5):1491–1511. doi: 10.1016/j.bioactmat.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burr D.B. The contribution of the organic matrix to bone's material properties. Bone. 2002;31(1):8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Z.Y., Wu X.D., Wang Y.F., Li M.D., Li Y., Liu X.L., Zhang X., Lan Z.Y., Wang J.L., Du Y.Y., Zhang S.M. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022;10:195–206. doi: 10.1016/j.bioactmat.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Z., Yu X., Jiang X., Brody H.D., Rowe D.W., Wei M. Fabrication and characterization of biomimetic collagen-apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013;9(7):7308–7319. doi: 10.1016/j.actbio.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu C., Deng J., Sun X., Qu Y., Man Y. Collagen membrane and immune response in guided bone regeneration: recent progress and perspectives. Tissue Eng. B Rev. 2017;23(5):421–435. doi: 10.1089/ten.TEB.2016.0463. [DOI] [PubMed] [Google Scholar]
- 28.Schwartzmann M. Use of collagen membranes for guided bone regeneration: a review. Implant Dent. 2000;9(1):63–66. doi: 10.1097/00008505-200009010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Gentile P., Chiono V., Tonda-Turo C., Ferreira A.M., Ciardelli G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011;6(10):1187–1197. doi: 10.1002/biot.201100294. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi Y., Amizuka N., Nakadate M., Ohnishi H., Fujii N., Oda K., Nomura S., Maeda T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials. 2005;26(31):6158–6166. doi: 10.1016/j.biomaterials.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Schlegel A.K., Mohler H., Busch F., Mehl A. Preclinical and clinical studies of a collagen membrane. Bio-Gide), Biomaterials. 1997;18(7):535–538. doi: 10.1016/s0142-9612(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 32.Zahedi S., Legrand R., Brunel G., Albert A., Dewe W., Coumans B., Bernard J.P. Evaluation of a diphenylphosphorylazide-crosslinked collagen membrane for guided bone regeneration in mandibular defects in rats. J. Periodontol. 1998;69(11):1238–1246. doi: 10.1902/jop.1998.69.11.1238. [DOI] [PubMed] [Google Scholar]
- 33.Chia-Lai P.J., Orlowska A., Al-Maawi S., Dias A., Zhang Y., Wang X., Zender N., Sader R., Kirkpatrick C.J., Ghanaati S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Invest. 2018;22(4):1851–1863. doi: 10.1007/s00784-017-2281-1. [DOI] [PubMed] [Google Scholar]
- 34.Hench L.L., Thompson I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface. 2010;7:S379–S391. doi: 10.1098/rsif.2010.0151.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner S., Wagner H.D. The material bone: structure mechanical function relations. Annu. Rev. Mater. Sci. 1998;28:271–298. [Google Scholar]
- 36.Kattimani V.S., Kondaka S., Lingamaneni K.P. Hydroxyapatite–-Past, present, and future in bone regeneration. Bone Tissue Regen. Insights. 2016;7:BTRI–S36138. [Google Scholar]
- 37.Guo P., Liu X.Z., Zhang P.H., He Z.Y., Li Z., Alini M., Richards R.G., Grad S., Stoddart M.J., Zhou G.Q., Zou X.N., Chan D., Tian W., Chen D.F., Gao M.M., Zhou Z.Y., Liu S.Y. A single-cell transcriptome of mesenchymal stromal cells to fabricate bioactive hydroxyapatite materials for bone regeneration. Bioact. Mater. 2022;9:281–298. doi: 10.1016/j.bioactmat.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohgushi H., Dohi Y., Tamai S., Tabata S. Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J. Biomed. Mater. Res. 1993;27(11):1401–1407. doi: 10.1002/jbm.820271107. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M., Ueno T., Tsukimura N., Ikeda T., Nakagawa K., Hori N., Suzuki T., Ogawa T. Bone integration capability of nanopolymorphic crystalline hydroxyapatite coated on titanium implants. Int. J. Nanomed. 2012;7:859–873. doi: 10.2147/IJN.S28082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao H., Wang X., Chen M., Liu Y., Cui X., Liang J., Wang Q., Fan Y., Zhang X.J.A.A.M. Interfaces, Childhood cartilage ECM enhances the chondrogenesis of endogenous cells and subchondral bone repair of the unidirectional collagen–dECM scaffolds in combination with microfracture. ACS Appl. Mater. Interfaces. 2021;13(48):57043–57057. doi: 10.1021/acsami.1c19447. [DOI] [PubMed] [Google Scholar]
- 41.Kim C., Lee J.W., Heo J.H., Park C., Kim D.H., Yi G.S., Kang H.C., Jung H.S., Shin H., Lee J.H. Natural bone-mimicking nanopore-incorporated hydroxyapatite scaffolds for enhanced bone tissue regeneration. Biomater. Res. 2022;26(1):1–13. doi: 10.1186/s40824-022-00253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto Y., Funamoto S., Kimura T., Nam K., Fujisato T., Kishida A. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials. 2011;32(29):7060–7067. doi: 10.1016/j.biomaterials.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Ozawa S., Kasugai S. Evaluation of implant materials (hydroxyapatite, glass-ceramics, titanium) in rat bone marrow stromal cell culture. Biomaterials. 1996;17(1):23–29. doi: 10.1016/0142-9612(96)80751-4. [DOI] [PubMed] [Google Scholar]
- 44.Barradas A.M., Yuan H., van Blitterswijk C.A., Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur. Cell. Mater. 2011;21:407–429. doi: 10.22203/ecm.v021a31. [DOI] [PubMed] [Google Scholar]
- 45.Grande D.A., Halberstadt C., Naughton G., Schwartz R., Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. 1997;34(2):211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 46.Li J.Y., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1(12):1–17. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C., Qazvini N.T., Sadati M., Zeng Z.Y., Huang S.F., De la Lastra A.L., Zhang L.H., Feng Y.X., Liu W., Huang B., Zhang B., Dai Z.Y., Shen Y., Wang X., Luo W.P., Liu B., Lei Y., Ye Z.Y., Zhao L., Cao D.G., Yang L.J., Chen X., Athiviraham A., Lee M.J., Wolf J.M., Reid R.R., Tirrell M., Huang W., de Pablo J.J., He T.C. A pH-triggered, self-assembled, and bioprintable hybrid hydrogel scaffold for mesenchymal stem cell based bone tissue engineering. ACS Appl. Mater. Interfaces. 2019;11(9):8749–8762. doi: 10.1021/acsami.8b19094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J., Zhang Z., Joseph J., Zhang X., Ferdows B.E., Patel D.N., Chen W., Banfi G., Molinaro R., Cosco D., Kong N., Joshi N., Farokhzad O.C., Corbo C., Tao W. Biomaterials and nanomedicine for bone regeneration: progress and future prospects. Exploration. 2021;1(2) doi: 10.1002/EXP.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chahal S., Kumar A., Hussian F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering. A review. J. Biomater. Sci. Polym. Ed. 2019;30(14):1308–1355. doi: 10.1080/09205063.2019.1630699. [DOI] [PubMed] [Google Scholar]
- 50.Lyu S., Huang C.L., Yang H., Zhang X.P. Electrospun fibers as a scaffolding platform for bone tissue repair. J. Orthop. Res. 2013;31(9):1382–1389. doi: 10.1002/jor.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Zhou G.L., Wang Y., Yang G., Ding S., Zhou S.B. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218–229. doi: 10.1016/j.biomaterials.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Badylak S.F., Weiss D.J., Caplan A., Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379(9819):943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 53.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosso F., Giordano A., Barbarisi M., Barbarisi A.J. From cell–ECM interactions to tissue engineering. J. Cell. Physiol. 2004;199(2):174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 56.Huang G., Li F., Zhao X., Ma Y., Li Y., Lin M., Jin G., Lu T.J., Genin G.M., Xu F. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017;117(20):12764–12850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsukasaki M., Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019;19(10):626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 58.Mori G., D'Amelio P., Faccio R., Brunetti G. The interplay between the bone and the immune system. Clin. Dev. Immunol. 2013;2013:16. doi: 10.1155/2013/720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirosavljevic D., Quinn J.M., Elliott J., Horwood N.J., Martin T.J., Gillespie M.T. T-cells mediate an inhibitory effect of interleukin-4 on osteoclastogenesis. J. Bone Miner. Res. 2003;18(6):984–993. doi: 10.1359/jbmr.2003.18.6.984. [DOI] [PubMed] [Google Scholar]
- 60.Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., Takaoka A., Yokochi T., Oda H., Tanaka K., Nakamura K., Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 61.Guder C., Gravius S., Burger C., Wirtz D.C., Schildberg F.A. Osteoimmunology: a current update of the interplay between bone and the immune system. Front. Immunol. 2020;11:58. doi: 10.3389/fimmu.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu S.P., Bennett S., Kuek V., Xiang C., Xu H.Z., Rosen V., Xu J.K. Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics. 2020;10(13):5957–5965. doi: 10.7150/thno.45422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin Y., Tang Q.M., Xie M.R., Hu L., Chen L.L. Insights into the mechanism of vascular endothelial cells on bone biology. Biosci. Rep. 2021;41(1) doi: 10.1042/BSR20203258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shekaran A., Garcia A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. 2011;96(1):261–272. doi: 10.1002/jbm.a.32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halloran D., Durbano H.W., Nohe A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020;8(3):19. doi: 10.3390/jdb8030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clarkin C.E., Gerstenfeld L.C. VEGF and bone cell signalling: an essential vessel for communication? Cell Biochem. Funct. 2013;31(1):1–11. doi: 10.1002/cbf.2911. [DOI] [PubMed] [Google Scholar]
- 67.Hu K., Olsen B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai J., Rabie A.B.M. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 2007;86(10):937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 69.Higuchi A., Ling Q.D., Chang Y., Hsu S.T., Umezawa A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013;113(5):3297–3328. doi: 10.1021/cr300426x. [DOI] [PubMed] [Google Scholar]
- 70.Yanagi T., Kajiya H., Kawaguchi M., Kido H., Fukushima T. Photothermal stress triggered by near infrared-irradiated carbon nanotubes promotes bone deposition in rat calvarial defects. J. Biomater. Appl. 2015;29(8):1109–1118. doi: 10.1177/0885328214556913. [DOI] [PubMed] [Google Scholar]
- 71.Lavanya K., Chandran S.V., Balagangadharan K., Selvamurugan N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C. 2020;111 doi: 10.1016/j.msec.2020.110862. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Cui H.T., Wu Z.X., Wu N.P., Wang Z.L., Chen X.S., Wei Y., Zhang P.B. Modulation of osteogenesis in MC3T3-E1 cells by different frequency electrical stimulation. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brighton C.T., Wang W., Seldes R., Zhang G.H., Pollack S.R. Signal transduction in electrically stimulated bone cells. J. Bone Joint Surg. 2001;83a(10):1514–1523. doi: 10.2106/00004623-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 2006;97(1):56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- 75.Maredziak M., Smieszek A., Tomaszewski K.A., Lewandowski D., Marycz K. The effect of low static magnetic field on osteogenic and adipogenic differentiation potential of human adipose stromal/stem cells. J. Magn. Magn Mater. 2016;398:235–245. [Google Scholar]
- 76.Wang J., An Y.X., Li F.J., Li D.M., Jing D., Guo T.W., Luo E.P., Ma C.F. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014;10(2):975–985. doi: 10.1016/j.actbio.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 77.Crane J.L., Cao X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J. Clin. Invest. 2014;124(2):466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie H., Cui Z., Wang L., Xia Z.Y., Hu Y., Xian L.L., Li C.J., Xie L., Crane J., Wan M., Zhen G.H., Bian Q., Yu B., Chang W.Z., Qiu T., Pickarski M., Duong L.T., Windle J.J., Luo X.H., Liao E.Y., Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014;20(11):1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burdick J.A., Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue. Eng. Pt. A. 2009;15(2):205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin H. Fabrication methods of an engineered microenvironment for analysis of cell-biomaterial interactions. Biomaterials. 2007;28(2):126–133. doi: 10.1016/j.biomaterials.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Hotchkiss K.M., Reddy G.B., Hyzy S.L., Schwartz Z., Boyan B.D., Olivares-Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–434. doi: 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brodbeck W.G., Nakayama Y., Matsuda T., Colton E., Ziats N.P., Anderson J.M. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18(6):311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 83.Tan S.L., Wang Y.F., Du Y.Y., Xiao Y., Zhang S.M. Injectable bone cement with magnesium-containing microspheres enhances osteogenesis via anti-inflammatory immunoregulation. Bioact. Mater. 2021;6(10):3411–3423. doi: 10.1016/j.bioactmat.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W., Yu M.Y., Chen F., Wang L.Q., Ye C., Chen Q., Zhu Q., Xie D., Shao M.Z., Yang L.L. A novel delivery nanobiotechnology: engineered miR-181b exosomes improved osteointegration by regulating macrophage polarization. J. Nanobiotechnol. 2021;19(1):1–18. doi: 10.1186/s12951-021-01015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang W., Yu Y.M., Wang J., Liu H., Pan H.B., Wang G.C., Liu C.S. Enhancement and orchestration of osteogenesis and angiogenesis by a dual-modular design of growth factors delivery scaffolds and 26SCS decoration. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120616. [DOI] [PubMed] [Google Scholar]
- 86.Ha Y.J., Ma X.J., Li S.K., Li T., Li Z.H., Qian Y.H., Shafiq M., Wang J.W., Zhou X.J., He C.L. Bone microenvironment-mimetic scaffolds with hierarchical microstructure for enhanced vascularization and bone regeneration. Adv. Funct. Mater. 2022;32(20) [Google Scholar]
- 87.Liu H.M., Du Y.Y., St-Pierre J.P., Berghlt M.S., Autefage H., Wang J.L., Cai M.L., Yang G.J., Stevens M.M., Zhang S.M. Bioenergetic-active materials enhance tissue regeneration by modulating cellular metabolic state. Sci. Adv. 2020;6(13) doi: 10.1126/sciadv.aay7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin S.H., Yin S., Shi J.F., Yang G.Z., Wen X.T., Zhang W.J., Zhou M.L., Jiang X.Q. Orchestration of energy metabolism and osteogenesis by Mg2+ facilitates low-dose BMP-2-driven regeneration. Bioact. Mater. 2022;18:116–127. doi: 10.1016/j.bioactmat.2022.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma C.Y., Tian X.G., Kim J.P., Xie D.H., Ao X., Shan D.Y., Lin Q.L., Hudock M.R., Bai X.C., Yang J. Citrate-based materials fuel human stem cells by metabonegenic regulation. P Natl Acad Sci USA. 2018;115(50) doi: 10.1073/pnas.1813000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun H., Xu J., Wang Y., Shen S., Xu X., Zhang L., Jiang Q. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 2023;24:477–496. doi: 10.1016/j.bioactmat.2022.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Xie C., Zhang Z., Liu H., Xu H., Peng Z., Liu C., Li J., Wang C., Xu T., Zhu L. 3D printed integrated bionic oxygenated scaffold for bone regeneration. ACS Appl. Mater. Interfaces. 2022;14(26):29506–29520. doi: 10.1021/acsami.2c04378. [DOI] [PubMed] [Google Scholar]
- 92.Quinlan E., Partap S., Azevedo M.M., Jell G., Stevens M.M., O'Brien F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials. 2015;52:358–366. doi: 10.1016/j.biomaterials.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Zheng Z., Chen Y., Guo B., Wang Y., Liu W., Sun J., Wang X.J.C.E.J. Magnesium-organic framework-based stimuli-responsive systems that optimize the bone microenvironment for enhanced bone regeneration. Chem. Eng. J. 2020;396 [Google Scholar]
- 94.Tan J., Wang C.F., Wang D.H., Jiang H., Qiao Y.Q., Zhang D.D., Zhang X.M., Xu R., Liu C.Y., Su J.C., Weng W.Z., Liu X.Y. Tailoring time-varying alkaline microenvironment on titanium for sequential anti-infection and osseointegration. Chem. Eng. J. 2022;431 [Google Scholar]
- 95.Anjum F., Lienemann P.S., Metzger S., Biernaskie J., Kallos M.S., Ehrbar M. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials. 2016;87:104–117. doi: 10.1016/j.biomaterials.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Z., Fan Y.S., Jiang Y.Y., Shi S., Xue C., Zhao X.Y., Tan S., Chen X., Feng C.B., Zhu Y.C., Yan J.J., Zhou Z.F., Zhao Y.F., Liu J.J., Chen F., He S.S. Mineralized enzyme-based biomaterials with superior bioactivities for bone regeneration. ACS Appl. Mater. Interfaces. 2022;14(32):36315–36330. doi: 10.1021/acsami.2c05794. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Y., Song S.L., Wang D.D., Liu H., Zhang J.M., Li Z.H., Wang J.C., Ren X.Z., Zhao Y.L. Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat. Commun. 2022;13(1):6758. doi: 10.1038/s41467-022-34481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang T., Lin S.Y., Shao X.R., Zhang Q., Xue C.Y., Zhang S., Lin Y.F., Zhu B.F., Cai X.X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017;50(3) doi: 10.1111/cpr.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kapur S., Baylink D.J., Lau K.H.W. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32(3):241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- 100.Feng Q., Wei K.C., Lin S.E., Xu Z., Sun Y.X., Shi P., Li G., Bian L.M. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials. 2016;101:217–228. doi: 10.1016/j.biomaterials.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y.N., Fang C.H., Zhang S.C., Campbell R.E., Serpe M.J. Controlled osteogenic differentiation of human mesenchymal stem cells using dexamethasone-loaded light-responsive microgels. ACS Appl. Mater. Interfaces. 2021;13(6):7051–7059. doi: 10.1021/acsami.0c17664. [DOI] [PubMed] [Google Scholar]
- 102.Petre D.G., Nadar R., Tu Y.F., Paknahad A., Wilson D.A., Leeuwenburgh S.C.G. Thermoresponsive brushes facilitate effective reinforcement of calcium phosphate cements. ACS Appl. Mater. Interfaces. 2019;11(30):26690–26703. doi: 10.1021/acsami.9b08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao Q., Shi M.S., Yin C.C., Zhao Z.F., Zhang J.L., Wang J.Y., Shen K.L., Zhang L.L., Tang H., Xiao Y., Zhang Y.F. Dual-wavelength photosensitive nano-in-micro scaffold regulates innate and adaptive immune responses for osteogenesis. Nano-Micro Lett. 2021;13(1):1–20. doi: 10.1007/s40820-020-00540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L., Pang Y., Tang Y., Wang X., Zhang D., Zhang X., Yu Y., Yang X., Cai Q. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2022:399–414. doi: 10.1016/j.bioactmat.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian J.J., Shi R., Liu Z., Ouyang H., Yu M., Zhao C.C., Zou Y., Jiang D.J., Zhang J.S., Li Z. Self-powered implantable electrical stimulator for osteoblasts' proliferation and differentiation. Nano Energy. 2019;59:705–714. [Google Scholar]
- 106.Hao L.L., Li J.X., Wang P., Wang Z.L., Wu Z.X., Wang Y., Jiao Z.X., Guo M., Shi T.F., Wang Q.G., Ito Y., Wei Y., Zhang P.B. Spatiotemporal magnetocaloric microenvironment for guiding the fate of biodegradable polymer implants. Adv. Funct. Mater. 2021;31(15) [Google Scholar]
- 107.Kotani H., Kawaguchi H., Shimoaka T., Iwasaka M., Ueno S., Ozawa H., Nakamura K., Hoshi K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 2002;17(10):1814–1821. doi: 10.1359/jbmr.2002.17.10.1814. [DOI] [PubMed] [Google Scholar]
- 108.Bez M., Sheyn D., Tawackoli W., Avalos P., Shapiro G., Giaconi J.C., Da X., David S.B., Gavrity J., Awad H.A., Bae H.W., Ley E.J., Kremen T.J., Gazit Z., Ferrara K.W., Pelled G., Gazit D. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017;9(390) doi: 10.1126/scitranslmed.aal3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He Y., Li F., Jiang P., Cai F., Lin Q., Zhou M., Liu H., Yan F. Remote control of the recruitment and capture of endogenous stem cells by ultrasound for in situ repair of bone defects. Bioact. Mater. 2023;21:223–238. doi: 10.1016/j.bioactmat.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xie L.X., Wang G.M., Wu Y.Z., Liao Q., Mo S., Ren X.X., Tong L.P., Zhang W., Guan M., Pan H.B., Chu P.K., Wang H.Y. Programmed surface on poly(aryl-ether-ether-ketone) initiating immune mediation and fulfilling bone regeneration sequentially. Innovation-Amsterdam. 2021;2(3) doi: 10.1016/j.xinn.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou X.Z., Chen J.Y., Sun H.X., Wang F.Q., Wang Y.K., Zhang Z.J., Teng W.S., Ye Y.X., Huang D.H., Zhang W., Mo X.A., Liu A., Lin P., Wu Y., Tao H.M., Yu X.H., Ye Z.M. Spatiotemporal regulation of angiogenesis/osteogenesis emulating natural bone healing cascade for vascularized bone formation. J. Nanobiotechnol. 2021;19(1):420. doi: 10.1186/s12951-021-01173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ponzetti M., Rucci N. Updates on osteoimmunology: what's new on the cross-talk between bone and immune system. Front. Endocrinol. 2019;10:236. doi: 10.3389/fendo.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steeve K.T., Marc P., Sandrine T., Dominique H., Yannick F. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Sims N.A., Jenkins B.J., Nakamura A., Quinn J.M.W., Li R.L., Gillespie M.T., Ernst M., Robb L., Martin T.J. Interleukin-11 receptor signaling is required for normal bone remodeling. J. Bone Miner. Res. 2005;20(7):1093–1102. doi: 10.1359/JBMR.050209. [DOI] [PubMed] [Google Scholar]
- 115.Gravallese E.M., Schett G. Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat. Rev. Rheumatol. 2018;14(11):631–640. doi: 10.1038/s41584-018-0091-8. [DOI] [PubMed] [Google Scholar]
- 116.Osta B., Benedetti G., Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front. Immunol. 2014;5:48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Theill L.E., Boyle W.J., Penninger J.M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 118.Kwak S.C., Lee C., Kim J.Y., Oh H.M., So H.S., Lee M.S., Rho M.C., Oh J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013;36(11):1779–1786. doi: 10.1248/bpb.b13-00430. [DOI] [PubMed] [Google Scholar]
- 119.Li J.H., Jiang X.Q., Li H.J., Gelinsky M., Gu Z. Tailoring materials for modulation of macrophage fate. Adv. Mater. 2021;33(12) doi: 10.1002/adma.202004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y.L., Zhang H., Hu Y., Jing Y.Y., Geng Z., Su J.C. Bone repair biomaterials: a perspective from immunomodulatory. Adv. Funct. Mater. 2022;32(51) [Google Scholar]
- 121.Lin Z., Chen Z., Chen Y., Yang N., Shi J., Tang Z., Zhang C., Lin H., Yin J. Wiley Online Library; Exploration: 2023. Hydrogenated Silicene Nanosheet Functionalized Scaffold Enables Immuno‐bone Remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang Z., Wei X., Li T., Wu H., Xiao X., Hao Y., Li S., Hou W., Shi L., Li X., Guo Z. Three-dimensionally printed Ti2448 with low stiffness enhanced angiogenesis and osteogenesis by regulating macrophage polarization via Piezo1/YAP signaling Axis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.750948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang S., Lyu C., Zhao P., Li W., Kong W., Huang C., Genin G.M., Du Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019;10(1):3491. doi: 10.1038/s41467-019-11397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lv L., Xie Y., Li K., Hu T., Lu X., Cao Y., Zheng X. Unveiling the mechanism of surface hydrophilicity-modulated macrophage polarization. Adv. Healthc. Mater. 2018;7(19) doi: 10.1002/adhm.201800675. [DOI] [PubMed] [Google Scholar]
- 125.Deng Y.K., Zhou Y., Liang Q.J., Ge C.L., Yang J.D., Shan B.C., Liu Y., Zhou X.Z., Yin L.C. Inflammation-instructed hierarchical delivery of IL-4/miR-21 orchestrates osteoimmune microenvironment toward the treatment of rheumatoid arthritis. Adv. Funct. Mater. 2021;31(33) [Google Scholar]
- 126.Huang X., Huang D.H., Zhu T., Yu X.H., Xu K.C., Li H.Y., Qu H., Zhou Z.Y., Cheng K., Wen W.J., Ye Z.M. Sustained zinc release in cooperation with CaP scaffold promoted bone regeneration via directing stem cell fate and triggering a pro-healing immune stimuli. J. Nanobiotechnol. 2021;19(1):1–20. doi: 10.1186/s12951-021-00956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li N., Liu L.J., Wei C.B., Ren S.C., Liu X.C., Wang X.M., Song J.Z., Li Y.H., Wang Z.R., Qiao S.W., Yan X.Y., Li S.C., Wang H., Zhou Y.M., Li D.W. Immunomodulatory blood-derived hybrid hydrogels as multichannel microenvironment modulators for augmented bone regeneration. ACS Appl. Mater. Interfaces. 2022:53523–53534. doi: 10.1021/acsami.2c16774. [DOI] [PubMed] [Google Scholar]
- 128.Deng Y.K., Zhou Y., Liang Q.J., Ge C.L., Yang J.D., Shan B.C., Liu Y., Zhou X.Z., Yin L.C. Inflammation-instructed hierarchical delivery of IL-4/miR-21 orchestrates osteoimmune microenvironment toward the treatment of rheumatoid arthritis. Adv. Funct. Mater. 2021;31(33) [Google Scholar]
- 129.Cheng S., Zhang D.D., Li M., Liu X.Y., Zhang Y., Qian S., Peng F. Osteogenesis, angiogenesis and immune response of Mg-Al layered double hydroxide coating on pure Mg. Bioact. Mater. 2021;6(1):91–105. doi: 10.1016/j.bioactmat.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang T., Bai J.X., Lu M., Huang C.L., Geng D.C., Chen G., Wang L., Qi J., Cui W.G., Deng L.F. Engineering immunomodulatory and osteoinductive implant surfaces via mussel adhesion-mediated ion coordination and molecular clicking. Nat. Commun. 2022;13(1):160. doi: 10.1038/s41467-021-27816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen L., Xiong Y., Hu Y., Yu C., Panayi A.C., Zhou W., Cao F., Sun Y., Liu M., Liu G., Xue H., Hu L., Mi B., Liu G. Regulatory T cell-exosomal miR-142-3p promotes angiogenesis and osteogenesis via TGFBR1/SMAD2 inhibition to accelerate fracture repair. Chem. Eng. J. 2022;427 [Google Scholar]
- 132.Loncar S.R., Halcrow S.E., Swales D. Osteoimmunology: the effect of autoimmunity on fracture healing and skeletal analysis. Forensic Sci Int Synerg. 2023;6 doi: 10.1016/j.fsisyn.2023.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang W., Yu Y.M., Wang J., Liv H., Pan H.B., Wang G.C., Liu C.S. Enhancement and orchestration of osteogenesis and angiogenesis by a dual-modular design of growth factors delivery scaffolds and 26SCS decoration. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119645. [DOI] [PubMed] [Google Scholar]
- 134.Bouyer M., Guillot R., Lavaud J., Plettinx C., Olivier C., Curry V., Boutonnat J., Coll J.L., Peyrin F., Josserand V., Bettega G., Picart C. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials. 2016;104:168–181. doi: 10.1016/j.biomaterials.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou X., Qian Y., Chen L., Li T., Sun X., Ma X., Wang J., He C. Flowerbed-inspired biomimetic scaffold with rapid internal tissue infiltration and vascularization capacity for bone repair. ACS Nano. 2023:5140–5156. doi: 10.1021/acsnano.3c00598. [DOI] [PubMed] [Google Scholar]
- 136.Miao Y.L., Chen Y.H., Luo J.S., Liu X., Yang Q., Shi X.T., Wang Y.J. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023;21:97–109. doi: 10.1016/j.bioactmat.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar A., Sood A., Singhmar R., Mishra Y.K., Thakur V.K.J.B.s. Manufacturing of functional hydrogels for inducing angiogenic-osteogenic coupled progressions in hard tissue repair: prospects and challenges. Biomater. Sci. 2022:5472–5497. doi: 10.1039/d2bm00894g. [DOI] [PubMed] [Google Scholar]
- 138.Ma C., Kuzma M.L., Bai X., Yang J. Biomaterial-based metabolic regulation in regenerative engineering. Adv. Sci. 2019;6(19) doi: 10.1002/advs.201900819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jr R.S., Eliseev R.A. Cell energy metabolism and bone formation. BoneKEy Rep. 2022;16 doi: 10.1016/j.bonr.2022.101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Agathocleous M., Harris W.A. Metabolism in physiological cell proliferation and differentiation. Trends Cell Biol. 2013;23(10):484–492. doi: 10.1016/j.tcb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 141.van der Knaap J.A., Verrijzer C.P. Undercover: gene control by metabolites and metabolic enzymes. Gene Dev. 2016;30(21):2345–2369. doi: 10.1101/gad.289140.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pattappa G., Heywood H.K., De Bruijn J.D., Lee D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 2011;226(10):2562–2570. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- 143.Jantsch J., Schodel J. Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology. 2015;220(2):305–314. doi: 10.1016/j.imbio.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 144.Rouwkema J., Koopman B., Blitterswijk C., Dhert W., Malda J. Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. Rev. 2010;26:163–178. doi: 10.5661/bger-26-163. [DOI] [PubMed] [Google Scholar]
- 145.Gholipourmalekabadi M., Zhao S., Harrison B.S., Mozafari M., Seifalian A.M. Oxygen-generating biomaterials: a new, viable paradigm for tissue engineering? Trends Biotechnol. 2016;34(12):1010–1021. doi: 10.1016/j.tibtech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 146.Drager J., Harvey E.J., Barralet J. Hypoxia signalling manipulation for bone regeneration. Expet Rev. Mol. Med. 2015;17:e6. doi: 10.1017/erm.2015.4. [DOI] [PubMed] [Google Scholar]
- 147.Willemen N.G.A., Hassan S., Gurian M., Li J.H., Allijn I.E., Shin S.R., Leijten J. Oxygen-releasing biomaterials: current challenges and future applications. Trends Biotechnol. 2021;39(11):1144–1159. doi: 10.1016/j.tibtech.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arnett T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 2003;62(2):511–520. doi: 10.1079/pns2003268. [DOI] [PubMed] [Google Scholar]
- 149.D.A. Bushinsky, N.S. Krieger, Acid–base Balance and Bone Health, Nutrition and bone health, Springer2015, pp. 335-357.
- 150.Yuan L., Li Z., Li X., Qiu S., Lei J., Li D., Mu C., L.J.A.A.P.M. Ge Functionalization of an injectable self-healing pH-responsive hydrogel by incorporating a curcumin/polymerized β-cyclodextrin inclusion complex for selective toxicity to. Osteosarcoma. 2022;4(2):1243–1254. [Google Scholar]
- 151.Liu W.L., Dan X.L., Lu W.W., Zhao X.L., Ruan C.S., Wang T., Cui X., Zhai X.Y., Ma Y.F., Wang D.P., Huang W.H., Pan H.B. Spatial distribution of biomaterial microenvironment pH and its modulatory effect on osteoclasts at the early stage of bone defect regeneration. ACS Appl. Mater. Interfaces. 2019;11(9):9557–9572. doi: 10.1021/acsami.8b20580. [DOI] [PubMed] [Google Scholar]
- 152.Lui Y.S., Sow W.T., Tan L.P., Wu Y., Lai Y., Li H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019;92:19–36. doi: 10.1016/j.actbio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 153.Lu Y., Aimetti A.A., Langer R., Gu Z.J.N.R.M. Bioresponsive materials. Nat. Rev. Mater. 2016;2(1):1–17. [Google Scholar]
- 154.Yu S., Duan Y.Y., Zuo X.G., Chen X.Y., Mao Z.W., Gao C.Y. Mediating the invasion of smooth muscle cells into a cell-responsive hydrogel under the existence of immune cells. Biomaterials. 2018;180:193–205. doi: 10.1016/j.biomaterials.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 155.Toda H., Yamamoto M., Uyama H., Tabata Y. Fabrication of hydrogels with elasticity changed by alkaline phosphatase for stem cell culture. Acta Biomater. 2016;29:215–227. doi: 10.1016/j.actbio.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 156.Liu H.Y., Korc M., Lin C.C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials. 2018;160:24–36. doi: 10.1016/j.biomaterials.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei H.P., Cui J.J., Lin K.L., Xie J., Wang X.D. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022;10(1):17. doi: 10.1038/s41413-021-00180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang C., Zheng Z.W., Younis M.R., Dong C.L., Chen Y.H., Lei S., Zhang D.Y., Wu J.Y.Z., Wu X.Q., Lin J., Wang X.S., Huang P. 3D printed enzyme-functionalized scaffold facilitates diabetic bone regeneration. Adv. Funct. Mater. 2021;31(20) [Google Scholar]
- 159.Dirzu N., Lucaciu O., Dirzu D.S., Soritau O., Cenariu D., Crisan B., Tefas L., Campian R.S. BMP-2 delivery through liposomes in bone regeneration. Appl Sci-Basel. 2022;12(3):1373. [Google Scholar]
- 160.Gan Q., Pan H., Zhang W.J., Yuan Y., Qian J.C., Liu C.S. Fabrication and evaluation of a BMP-2/dexamethasone co-loaded gelatin sponge scaffold for rapid bone regeneration. Regen. Biomater. 2022;9 doi: 10.1093/rb/rbac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiong A., He Y.J., Gao L., Li G.Q., Liu S., Weng J., Wang D.L., Zeng H. The fabrication of a highly efficient hydrogel based on a functionalized double network loaded with magnesium ion and BMP2 for bone defect synergistic treatment. Mater. Sci. Eng., C. 2021;128 doi: 10.1016/j.msec.2021.112347. [DOI] [PubMed] [Google Scholar]
- 162.Liang Z., Yang L., Lv Y.G. Exosome derived from mesenchymal stem cells mediates hypoxia-specific BMP2 gene delivery and enhances bone regeneration. Chem. Eng. J. 2021;422 [Google Scholar]
- 163.Xu Y.M., Yang Y., Hua Z.Y., Li S., Yang Z.Y., Liu Q.Z., Fu G., Ji P., Wu Q.Q. BMP2 immune complexes promote new bone formation by facilitating the direct contact between osteoclasts and osteoblasts. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120890. [DOI] [PubMed] [Google Scholar]
- 164.Zhuang W.D., Ye G.L., Wu J.C., Wang L.Y., Fang G.F., Ye Z.F., Lai G.H., Qiu X.Z., Sang H.X. A 3D-printed bioactive polycaprolactone scaffold assembled with core/shell microspheres as a sustained BMP2-releasing system for bone repair. Biomater. Adv. 2022;133:928–4931. doi: 10.1016/j.msec.2021.112619. [DOI] [PubMed] [Google Scholar]
- 165.Geiger F., Bertram H., Berger I., Lorenz H., Wall O., Eckhardt C., Simank H.G., Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J. Bone Miner. Res. 2005;20(11):2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 166.Kaigler D., Wang Z., Horger K., Mooney D.J., Krebsbach P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Miner. Res. 2006;21(5):735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 167.Leach J.K., Kaigler D., Wang Z., Krebsbach P.H., Mooney D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 168.Dashtimoghadam E., Fahimipour F., Tongas N., Tayebi L. Microfluidic fabrication of microcarriers with sequential delivery of VEGF and BMP-2 for bone regeneration. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-68221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 170.Ozcivici E., Luu Y.K., Adler B., Qin Y.X., Rubin J., Judex S., Rubin C.T. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 2010;6(1):50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Santos L.J., Reis R.L., Gomes M.E. Harnessing magnetic-mechano actuation in regenerative medicine and tissue engineering. Trends Biotechnol. 2015;33(8):471–479. doi: 10.1016/j.tibtech.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 172.Sanz-Herrera J.A., Garcia-Aznar J.M., Doblare M. On scaffold designing for bone regeneration: a computational multiscale approach. Acta Biomater. 2009;5(1):219–229. doi: 10.1016/j.actbio.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 173.Stockhausen K.E., Qwamizadeh M., Wölfel E.M., Hemmatian H., Fiedler I.A., Flenner S., Longo E., Amling M., Greving I., Ritchie R.O.J.A.n. Collagen fiber orientation is coupled with specific nano-compositional patterns in dark and bright osteons modulating their biomechanical properties. ACS Nano. 2021;15(1):455–467. doi: 10.1021/acsnano.0c04786. [DOI] [PubMed] [Google Scholar]
- 174.Curtis K.J., Coughlin T.R., Varsanik M.A., Niebur G.L. Shear stress in bone marrow has a dose dependent effect on cFos gene expression in in situ culture. Cell. Mol. Bioeng. 2019;12(6):559–568. doi: 10.1007/s12195-019-00594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weinbaum S., Cowin S.C., Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 176.Bacabac R.G., Smit T.H., Mullender M.G., Dijcks S.J., Van Loon J.J.W.A., Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem. Bioph. Res. Co. 2004;315(4):823–829. doi: 10.1016/j.bbrc.2004.01.138. [DOI] [PubMed] [Google Scholar]
- 177.Donahue S.W., Jacobs C.R., Donahue H.J. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am. J. Physiol. Cell Physiol. 2001;281(5):C1635–C1641. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- 178.Kim K.M., Choi Y.J., Hwang J.H., Kim A.R., Cho H.J., Hwang E.S., Park J.Y., Lee S.H., Hong J.H. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jacobs C.R., Yellowley C.E., Davis B.R., Zhou Z., Cimbala J.M., Donahue H.J. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 1998;31(11):969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lecuit T., Lenne P.F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8(8):633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 181.Burla F., Mulla Y., Vos B.E., Aufderhorst-Roberts A., Koenderink G.H. From mechanical resilience to active material properties in biopolymer networks. Nat. Rev. Phys. 2019;1(4):249–263. [Google Scholar]
- 182.Tong Z., Jin L., Oliveira J.M., Reis R.L., Zhong Q., Mao Z., Gao C. Adaptable hydrogel with reversible linkages for regenerative medicine: dynamic mechanical microenvironment for cells. Bioact. Mater. 2021;6(5):1375–1387. doi: 10.1016/j.bioactmat.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ma S., Tang Q., Feng Q.X., Song J., Han X.X., Guo F.Y. Mechanical behaviours and mass transport properties of bone-mimicking scaffolds consisted of gyroid structures manufactured using selective laser melting. J. Mech. Behav. Biomed. 2019;93:158–169. doi: 10.1016/j.jmbbm.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 184.Jongpaiboonkit L., King W.J., Lyons G.E., Paguirigan A.L., Warrick J.W., Beebe D.J., Murphy W.L. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29(23):3346–3356. doi: 10.1016/j.biomaterials.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shin M., Ryu J.H., Park J.P., Kim K., Yang J.W., Lee H. DNA/Tannic acid hybrid gel exhibiting biodegradability, extensibility, tissue adhesiveness, and hemostatic ability. Adv. Funct. Mater. 2015;25(8):1270–1278. [Google Scholar]
- 186.Zhang K.Y., Feng Q., Xu J.B., Xu X.Y., Tian F., Yeung K.W.K., Bian L.M. Self-assembled injectable nanocomposite hydrogels stabilized by bisphosphonate-magnesium (Mg2+) coordination regulates the differentiation of encapsulated stem cells via dual crosslinking. Adv. Funct. Mater. 2017;27(34) [Google Scholar]
- 187.Glassman M.J., Chan J., Olsen B.D. Reinforcement of shear thinning protein hydrogels by responsive block copolymer self-assembly. Adv. Funct. Mater. 2013;23(9):1182–1193. doi: 10.1002/adfm.201202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Delplace V., Pickering A.J., Hettiaratchi M.H., Zhao S., Kivijärvi T., Shoichet M.S.J.B. Inverse electron-demand diels–alder methylcellulose hydrogels, enable the Co-delivery of chondroitinase ABC and neural progenitor cells. Biomacromolecules. 2020;21(6):2421–2431. doi: 10.1021/acs.biomac.0c00357. [DOI] [PubMed] [Google Scholar]
- 189.Sharma P.K., Taneja S., Singh Y.J.A.a.m. interfaces, Hydrazone-linkage-based self-healing and injectable xanthan–poly (ethylene glycol) hydrogels for controlled, drug release and 3D cell culture. ACS Appl. Mater. Interfaces. 2018;10(37):30936–30945. doi: 10.1021/acsami.8b07310. [DOI] [PubMed] [Google Scholar]
- 190.Brown T.E., Carberry B.J., Worrell B.T., Dudaryeva O.Y., McBride M.K., Bowman C.N., Anseth K.S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials. 2018;178:496–503. doi: 10.1016/j.biomaterials.2018.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu J.J., Hu Q.Y., He X., Liu C.X., Kong Y.L., Cheng Y.Y., Zhang Y.D. Stimuli-responsive hydrogels with antibacterial activity assembled from guanosine, aminoglycoside, and a bifunctional anchor. Adv. Healthc. Mater. 2020;9(2) doi: 10.1002/adhm.201901329. [DOI] [PubMed] [Google Scholar]
- 192.Zhao W., Zhao Y., Wang Q., Liu T., Sun J., Zhang R. Remote light-responsive nanocarriers for controlled drug delivery: advances and perspectives. Small. 2019;15(45) doi: 10.1002/smll.201903060. [DOI] [PubMed] [Google Scholar]
- 193.Saneja A., Kumar R., Arora D., Kumar S., Panda A.K., Jaglan S. Recent advances in near-infrared light-responsive nanocarriers for cancer therapy. Drug Discov. Today. 2018;23(5):1115–1125. doi: 10.1016/j.drudis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 194.Wang S.Y., Wang F.L., Zhao X., Yang F., Xu Y.Q., Yan F.Y., Xia D.D., Liu Y.S. The effect of near-infrared light-assisted photothermal therapy combined with polymer materials on promoting bone regeneration: a systematic review. Mater. Des. 2022;217 [Google Scholar]
- 195.Wells C.M., Harris M., Choi L., Murali V.P., Guerra F.D., Jennings J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019;10(3):34. doi: 10.3390/jfb10030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Haryono A.B., Y.D.J.O.J.o.S.M. Ismiarto Zirconia surface bone graft implant with ultraviolet stimulation works toward accelerating bone healing. Orthopaedic Journal of Sports Medicine. 2019;7(11_suppl6) 2325967119S00468. [Google Scholar]
- 197.Liang J., Li W.H., Zhuang N., Wen S.N., Huang S.J., Lu W.Z., Zhou Y.Z., Liao G.Z., Zhang B., Liu C.L. Experimental study on bone defect repair by BMSCs combined with a light-sensitive material: g-C3N4/rGO. J Biomat Sci-Polym E. 2021;32(2):248–265. doi: 10.1080/09205063.2020.1827923. [DOI] [PubMed] [Google Scholar]
- 198.Kim Y.J., Matsunaga Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B. 2017;5(23):4307–4321. doi: 10.1039/c7tb00157f. [DOI] [PubMed] [Google Scholar]
- 199.Jeong B.M., Lee D.S., Shon J.I., Bae Y.H., Kim S.W. Thermoreversible gelation of poly(ethylene Oxide)Biodegradable polyester block copolymers. J. Polym. Sci., Polym. Chem. Ed. 1999;37(6):751–760. [Google Scholar]
- 200.Lv Z., Hu T., Bian Y., Wang G., Wu Z., Li H., Liu X., Yang S., Tan C., Liang R., Weng X. A MgFe-LDH nanosheet-incorporated smart thermo-responsive hydrogel with controllable growth factor releasing capability for bone regeneration. Adv. Mater. 2022 doi: 10.1002/adma.202206545. [DOI] [PubMed] [Google Scholar]
- 201.Kim J., Choi H.S., Kim Y.M., Song S.C. Thermo-responsive nanocomposite bioink with growth-factor holding and its application to bone regeneration. Small. 2022 doi: 10.1002/smll.202203464. [DOI] [PubMed] [Google Scholar]
- 202.Wang S.Y., Wang F.L., Zhao X., Yang F., Xu Y.Q., Yan F.Y., Xia D.D., Liu Y.S. The effect of near-infrared light-assisted photothermal therapy combined with polymer materials on promoting bone regeneration: a systematic review. Mater. Des. 2022;217 [Google Scholar]
- 203.Liu B., Sun J., Zhu J., Li B., Ma C., Gu X., Liu K., Zhang H., Wang F., Su J. Injectable and NIR‐responsive DNA–inorganic hybrid hydrogels with outstanding photothermal therapy. Adv. Mater. 2020;32(39) doi: 10.1002/adma.202004460. [DOI] [PubMed] [Google Scholar]
- 204.Zhang X.G., Cheng G., Xing X., Liu J.C., Cheng Y., Ye T.Y., Wang Q., Xiao X.H., Li Z.B., Deng H.B. Near-infrared light-triggered porous AuPd alloy nanoparticles to produce mild localized heat to accelerate bone regeneration. J. Phys. Chem. Lett. 2019;10(15):4185–4191. doi: 10.1021/acs.jpclett.9b01735. [DOI] [PubMed] [Google Scholar]
- 205.He J.Q., Chen G.Q., Zhao P., Ou C.W. Near-infrared light-controllable bufalin delivery from a black phosphorus-hybrid supramolecular hydrogel for synergistic photothermal-chemo tumor therapy. Nano Res. 2021;14(11):3988–3998. [Google Scholar]
- 206.Zhang X., Tan B.W., Wu Y.T., Zhang M., Liao J.F. A review on hydrogels with photothermal effect in wound healing and bone tissue engineering. Polym. Bull. 2021;13(13):2100. doi: 10.3390/polym13132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yan R., Guo Y.J., Wang X.C., Liang G.H., Yang A.L., Li J.M. Near-infrared light-controlled and real-time detection of osteogenic differentiation in mesenchymal stem cells by upconversion nanoparticles for osteoporosis therapy. ACS Nano. 2022;16(5):8399–8418. doi: 10.1021/acsnano.2c02900. [DOI] [PubMed] [Google Scholar]
- 208.Ye J., Jiang J.K., Zhou Z.R., Weng Z.Z., Xu Y.Y., Liu L.B., Zhang W., Yang Y.F., Luo J., Wang X.L. Near-infrared light and upconversion nanoparticle defined nitric oxide-based osteoporosis targeting therapy. ACS Nano. 2021;15(8):13692–13702. doi: 10.1021/acsnano.1c04974. [DOI] [PubMed] [Google Scholar]
- 209.Yang C., Ma H.S., Wang Z.Y., Younis M.R., Liu C.Y., Wu C.T., Luo Y.X., Huang P. 3D printed wesselsite nanosheets functionalized scaffold facilitates NIR-II photothermal therapy and vascularized bone regeneration. Adv. Sci. 2021;8(20) doi: 10.1002/advs.202100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yan Y., Gao X., Zhang S., Wang Y.T., Zhou Z.J., Xiao J.R., Zhang Q., Cheng Y.Y. A carboxyl-terminated dendrimer enables osteolytic lesion targeting and photothermal ablation of malignant bone tumors. ACS Appl. Mater. Interfaces. 2019;11(1):160–168. doi: 10.1021/acsami.8b15827. [DOI] [PubMed] [Google Scholar]
- 211.Tan L., Li M.H., Luo Z., Cai K.Y., Hu Y. Black phosphorus biomaterials for photo-controlled bone tissue engineering. Compos. B Eng. 2022;246 [Google Scholar]
- 212.Plutnar J., Sofer Z., Pumera M. Products of degradation of black phosphorus in protic solvents. ACS Nano. 2018;12(8):8390–8396. doi: 10.1021/acsnano.8b03740. [DOI] [PubMed] [Google Scholar]
- 213.Peng L., Abbasi N., Xiao Y., Xie Z.J. Black phosphorus: degradation mechanism, passivation method, and application for in situ tissue regeneration. Adv. Mater. Interfac. 2020;7(23) [Google Scholar]
- 214.Zhao H., Liu C., Liu Y., Ding Q., Wang T., Li H., Wu H., Ma T. Harnessing electromagnetic fields to assist bone tissue engineering. Stem Cell Res. Ther. 2023;14(1):7. doi: 10.1186/s13287-022-03217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tandon B., Blaker J.J., Cartmell S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018;73:1–20. doi: 10.1016/j.actbio.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 216.Jacob J., More N., Kalia K., Kapusetti G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018;38:2. doi: 10.1186/s41232-018-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Carter A., Popowski K., Cheng K., Greenbaum A., Ligler F.S., Moatti A. Enhancement of bone regeneration through the converse piezoelectric effect, A novel approach for applying mechanical stimulation. Bioelectricity. 2021;3(4):255–271. doi: 10.1089/bioe.2021.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kapat K., Shubhra Q.T.H., Zhou M., Leeuwenburgh S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020;30(44) [Google Scholar]
- 219.Khare D., Basu B., Dubey A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120280. [DOI] [PubMed] [Google Scholar]
- 220.Liu H.F., Shi Y.H., Zhu Y.F., Wu P., Deng Z.M., Dong Q., Wu M.H., Cai L. Bioinspired piezoelectric periosteum to augment bone regeneration via synergistic immunomodulation and osteogenesis. ACS Appl. Mater. Interfaces. 2023:12273–12293. doi: 10.1021/acsami.2c19767. [DOI] [PubMed] [Google Scholar]
- 221.Feng H., Zhao C., Tan P., Liu R., Chen X., Li Z. Nanogenerator for biomedical applications. Adv. Healthc. Mater. 2018;7(10) doi: 10.1002/adhm.201701298. [DOI] [PubMed] [Google Scholar]
- 222.Tanasa E., Zaharia C., Hudita A., Radu I.C., Costache M., Galateanu B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mat. Sci. Eng. C-Mater. 2020;110 doi: 10.1016/j.msec.2020.110714. [DOI] [PubMed] [Google Scholar]
- 223.Przekora A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019;20(2):435. doi: 10.3390/ijms20020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peng J., Zhao J., Long Y., Xie Y., Nie J., Chen L.J.F.i.M. Magnetic materials in promoting bone regeneration Front. Mater. 2019;6:268. [Google Scholar]
- 225.Yang J.C., Zhang J., Ding C., Dong D.D., Shang P. Regulation of osteoblast differentiation and iron content in MC3T3-E1 cells by static magnetic field with different intensities. Biol. Trace Elem. Res. 2018;184(1):214–225. doi: 10.1007/s12011-017-1161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fernandes M.M., Correia D.M., Ribeiro C., Castro N., Correia V., Lanceros-Mendez S. Bioinspired three-dimensional magnetoactive scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces. 2019;11(48):45265–45275. doi: 10.1021/acsami.9b14001. [DOI] [PubMed] [Google Scholar]
- 227.Yang H.J., Kim R.Y., Hwang S.J. Pulsed electromagnetic fields enhance bone morphogenetic protein-2 dependent-bone regeneration. Tissue Eng. 2015;21(19–20):2629–2637. doi: 10.1089/ten.TEA.2015.0032. [DOI] [PubMed] [Google Scholar]
- 228.Padilla F., Puts R., Vico L., Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54(5):1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 229.Kruse D.E., Mackanos M.A., O'Connell-Rodwell C.E., Contag C.H., Ferrara K.W. Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys. Med. Biol. 2008;53(13):3641–3660. doi: 10.1088/0031-9155/53/13/017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Claes L., Willie B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 2007;93(1–3):384–398. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 231.Padilla F., Puts R., Vico L., Guignandon A., Raum K. Stimulation of bone repair with ultrasound. Adv. Exp. Med. Biol. 2016;880:385–427. doi: 10.1007/978-3-319-22536-4_21. [DOI] [PubMed] [Google Scholar]
- 232.Takeuchi R., Ryo A., Komitsu N., Mikuni-Takagaki Y., Fukui A., Takagi Y., Shiraishi T., Morishita S., Yamazaki Y., Kumagai K., Aoki I., Saito T. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis Res. Ther. 2008;10(4):R77. doi: 10.1186/ar2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sena K., Angle S.R., Kanaji A., Aher C., Karwo D.G., Sumner D.R., Virdi A.S. Low-intensity pulsed ultrasound (LIPUS) and cell-to-cell communication in bone marrow stromal cells. Ultrasonics. 2011;51(5):639–644. doi: 10.1016/j.ultras.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 234.Tang C.H., Yang R.S., Huang T.H., Lu D.Y., Chuang W.J., Huang T.F., Fu W.M. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and akt pathway in osteoblasts. Mol. Pharmacol. 2006;69(6):2047–2057. doi: 10.1124/mol.105.022160. [DOI] [PubMed] [Google Scholar]
- 235.Wang F.S., Kuo Y.R., Wang C.J., Yang K.D., Chang P.R., Huang Y.T., Huang H.C., Sun Y.C., Yang Y.J., Chen Y.J. Nitric oxide mediates ultrasound-induced hypoxia-inducible factor-1alpha activation and vascular endothelial growth factor-A expression in human osteoblasts. Bone. 2004;35(1):114–123. doi: 10.1016/j.bone.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 236.Katiyar A., Duncan R.L., Sarkar K. Ultrasound stimulation increases proliferation of MC3T3-E1 preosteoblast-like cells. J. Ther. Ultrasound. 2014;2:1. doi: 10.1186/2050-5736-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang Z., Ma Y., Guo S., He Y., Bai G., Zhang W. Low-intensity pulsed ultrasound stimulation facilitates in vitro osteogenic differentiation of human adipose-derived stem cells via up-regulation of heat shock protein (HSP)70, HSP90, and bone morphogenetic protein (BMP) signaling pathway. Biosci. Rep. 2018;38(3) doi: 10.1042/BSR20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Li H., Deng Y.J., Tan M.M., Feng G., Kuang Y.C., Li J., Song J.L. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res. Ther. 2020;11(1):1–15. doi: 10.1186/s13287-020-01732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen J., Li J.J., Hu F., Zou Q., Mei Q.J., Li S.J., Hao Y.L., Hou W.T., Li J.D., Li Y.B., Zuo Y. Effect of microarc oxidation-treated Ti6Al4V scaffold following low-intensity pulsed ultrasound stimulation on osteogenic cells in vitro. ACS Biomater. Sci. Eng. 2019;5(2):572–581. doi: 10.1021/acsbiomaterials.8b01000. [DOI] [PubMed] [Google Scholar]
- 240.Wan Z.Q., Dong Q.Y., Liu Y.S., Zhang X., Zhang P., Lv L.W., Zhou Y.S. Programmed biomolecule delivery orchestrate bone tissue regeneration via MSC recruitment and epigenetic modulation. Chem. Eng. J. 2022;438 [Google Scholar]
- 241.Kang X., Zhang X.B., Gao X.D., Hao D.J., Li T., Xu Z.W. Bioprinting for bone tissue engineering. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1036375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pavek A., Nartker C., Saleh M., Kirkham M., Pour S.K., Aghazadeh-Habashi A., Barrott J.J. Tissue engineering through 3D bioprinting to recreate and study bone disease. Biomedicines. 2021;9(5):551. doi: 10.3390/biomedicines9050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ashammakhi N., Hasan A., Kaarela O., Byambaa B., Sheikhi A., Gaharwar A.K., Khademhosseini A. Advancing frontiers in bone bioprinting. Adv. Healthc. Mater. 2019;8(7) doi: 10.1002/adhm.201801048. [DOI] [PubMed] [Google Scholar]
- 244.Freeman F.E., Burdis R., Kelly D.J. Printing new bones: from print-and-implant devices to bioprinted bone organ precursors. Trends Mol. Med. 2021;27(7):700–711. doi: 10.1016/j.molmed.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 245.Rhee S., Puetzer J.L., Mason B.N., Reinhart-King C.A., Bonassar L.J. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016;2(10):1800–1805. doi: 10.1021/acsbiomaterials.6b00288. [DOI] [PubMed] [Google Scholar]
- 246.Yadav L.R., Chandran S.V., Lavanya K., Selvamurugan N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021;183:1925–1938. doi: 10.1016/j.ijbiomac.2021.05.215. [DOI] [PubMed] [Google Scholar]
- 247.Stegen S., van Gastel N., Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19–27. doi: 10.1016/j.bone.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 248.Huang J., Fu H., Wang Z.Y., Meng Q.Y., Liu S.M., Wang H.R., Zheng X.F., Dai J.W., Zhang Z.J. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016;6(110):108423–108430. [Google Scholar]
- 249.Tan Y.J., Tan X.P., Yeong W.Y., Tor S.B. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: a new biofabrication strategy. Sci. Rep. 2016;6 doi: 10.1038/srep39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Iglesias-Mejuto A., Garcia-Gonzalez C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C. 2021;131 doi: 10.1016/j.msec.2021.112525. [DOI] [PubMed] [Google Scholar]
- 251.Lee J., Kim G. Three-dimensional hierarchical nanofibrous collagen scaffold fabricated using fibrillated collagen and pluronic F-127 for regenerating bone tissue. ACS Appl. Mater. Interfaces. 2018;10(42):35801–35811. doi: 10.1021/acsami.8b14088. [DOI] [PubMed] [Google Scholar]
- 252.McBeth C., Lauer J., Ottersbach M., Campbell J., Sharon A., Sauer-Budge A.F. 3D bioprinting of GelMA scaffolds triggers mineral deposition by primary human osteoblasts. Biofabrication. 2017;9(1) doi: 10.1088/1758-5090/aa53bd. [DOI] [PubMed] [Google Scholar]
- 253.Li W.F., Dai F., Zhang S., Xu F.C., Xu Z.Y., Liao S.S., Zeng L.T., Song L., Ai F.R. Pore size of 3D-printed polycaprolactone/polyethylene glycol/hydroxyapatite scaffolds affects bone regeneration by modulating macrophage polarization and the foreign body response. ACS Appl. Mater. Interfaces. 2022;14(18):20693–20707. doi: 10.1021/acsami.2c02001. [DOI] [PubMed] [Google Scholar]
- 254.Daly A.C., Freeman F.E., Gonzalez-Fernandez T., Critchley S.E., Nulty J., Kelly D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthc. Mater. 2017;6(22) doi: 10.1002/adhm.201700298. [DOI] [PubMed] [Google Scholar]
- 255.Qasim M., Chae D.S., Lee N.Y. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int. J. Nanomed. 2019;14:4333–4351. doi: 10.2147/IJN.S209431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gao B., Yang Q.Z., Zhao X., Jin G.R., Ma Y.F., Xu F. 4D bioprinting for biomedical applications. Trends Biotechnol. 2016;34(9):746–756. doi: 10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 257.Miao S.D., Zhu W., Castro N.J., Leng J.S., Zhang L.G. Four-dimensional printing hierarchy scaffolds with highly biocompatible smart polymers for tissue engineering applications. Tissue Eng. C Methods. 2016;22(10):952–963. doi: 10.1089/ten.tec.2015.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.You D., Chen G., Liu C., Ye X., Wang S., Dong M., Sun M., He J., Yu X., Ye G. 4D printing of multi‐responsive membrane for accelerated in vivo bone healing via remote regulation of stem cell fate. Adv. Funct. Mater. 2021;31(40) [Google Scholar]
- 259.Wan Z.Q., Zhang P., Liu Y.S., Lv L.W., Zhou Y.S. Four-dimensional bioprinting: current developments and applications in bone tissue engineering. Acta Biomater. 2020;101:26–42. doi: 10.1016/j.actbio.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 260.Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 261.Qian X.Y., Song H.J., Ming G.L. Brain organoids: advances, applications and challenges. Development. 2019;146(8):dev166074. doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Miller A.J., Dye B.R., Ferrer-Torres D., Hill D.R., Overeem A.W., Shea L.D., Spence J.R. Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 2019;14(2):518–540. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Phipson B., Er P.X., Combes A.N., Forbes T.A., Howden S.E., Zappia L., Yen H.J., Lawlor K.T., Hale L.J., Sun J.E., Wolvetang E., Takasato M., Oshlack A., Little M.H. Evaluation of variability in human kidney organoids. Nat. Methods. 2019;16(1):79–+. doi: 10.1038/s41592-018-0253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Prior N., Inacio P., Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68(12):2228–2237. doi: 10.1136/gutjnl-2019-319256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Broutier L., Andersson-Rolf A., Hindley C.J., Boj S.F., Clevers H., Koo B.K., Huch M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016;11(9):1724–1743. doi: 10.1038/nprot.2016.097. [DOI] [PubMed] [Google Scholar]
- 266.Puschhof J., Pleguezuelos-Manzano C., Martinez-Silgado A., Akkerman N., Saftien A., Boot C., de Waal A., Beumer J., Dutta D., Heo I., Clevers H. Intestinal organoid cocultures with microbes. Nat. Protoc. 2021;16(10):4633–4649. doi: 10.1038/s41596-021-00589-z. [DOI] [PubMed] [Google Scholar]
- 267.Karthaus W.R., Iaquinta P.J., Drost J., Gracanin A., Van Boxtel R., Wongvipat J., Dowling C.M., Gao D., Begthel H., Sachs N., Vries R.G.J., Cuppen E., Chen Y., Sawyers C.L., Clevers H.C. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159(1):163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chen S., Chen X., Geng Z., Su J. The horizon of bone organoid: a perspective on construction and application. Bioact. Mater. 2022;18:15–25. doi: 10.1016/j.bioactmat.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kim W., Gwon Y., Park S., Kim H., Kim J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact. Mater. 2023;19:50–74. doi: 10.1016/j.bioactmat.2022.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wu S.L., Wu X.M., Wang X.H., Su J.C. Hydrogels for bone organoid construction: from a materiobiological perspective. J. Mater. Sci. Technol. 2023;136:21–31. [Google Scholar]
- 271.Cui X., Hartanto Y., Zhang H. Advances in multicellular spheroids formation. J R Soc Interface. 2017;14(127) doi: 10.1098/rsif.2016.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Giger S., Hofer M., Miljkovic-Licina M., Hoehnel S., Brandenberg N., Guiet R., Ehrbar M., Kleiner E., Gegenschatz-Schmid K., Matthes T., Lutolf M.P. Microarrayed human bone marrow organoids for modeling blood stem cell dynamics. Apl Bioeng. 2022;6(3) doi: 10.1063/5.0092860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Whelan I.T., Moeendarbary E., Hoey D.A., Kelly D.J. Biofabrication of vasculature in microphysiological models of bone. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/ac04f7. [DOI] [PubMed] [Google Scholar]
- 274.Hu Z.C., Lu J.Q., Zhang T.W., Liang H.F., Yuan H., Su D.H., Ding W., Lian R.X., Ge Y.X., Liang B., Dong J., Zhou X.G., Jiang L.B. Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment. Bioact. Mater. 2023;22:1–17. doi: 10.1016/j.bioactmat.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Morrison R.J., Hollister S.J., Niedner M.F., Mahani M.G., Park A.H., Mehta D.K., Ohye R.G., Green G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015;7(287) doi: 10.1126/scitranslmed.3010825. 285ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.