Abstract
Adaptive radiation therapy is a feedback process by which imaging information acquired over the course of treatment, such as changes in patient anatomy, can be used to reoptimize the treatment plan, with the end goal of improving target coverage and reducing treatment toxicity. This review describes different types of adaptive radiation therapy and their clinical implementation with a focus on CT-guided online adaptive radiation therapy. Depending on local anatomic changes and clinical context, different anatomic sites and/or disease stages and presentations benefit from different adaptation strategies. Online adaptive radiation therapy, where images acquired in-room before each fraction are used to adjust the treatment plan while the patient remains on the treatment table, has emerged to address unpredictable anatomic changes between treatment fractions. Online treatment adaptation places unique pressures on the radiation therapy workflow, requiring high-quality daily imaging and rapid recontouring, replanning, plan review, and quality assurance. Generating a new plan with every fraction is resource intensive and time sensitive, emphasizing the need for workflow efficiency and clinical resource allocation. Cone-beam CT is widely used for image-guided radiation therapy, so implementing cone-beam CT–guided online adaptive radiation therapy can be easily integrated into the radiation therapy workflow and potentially allow for rapid imaging and replanning. The major challenge of this approach is the reduced image quality due to poor resolution, scatter, and artifacts.
Keywords: Adaptive Radiation Therapy, Cone-Beam CT, Organs at Risk, Oncology
© RSNA, 2023
Keywords: Adaptive Radiation Therapy, Cone-Beam CT, Organs at Risk, Oncology
Summary
Online adaptive radiation therapy aims to address unpredictable anatomic changes occurring over the course of treatment; this approach requires high-quality daily imaging, as well as rapid recontouring, replanning, plan review, and quality assurance.
Essentials
■ Online adaptive radiation therapy aims to decrease toxicity to local organs at risk and increase target coverage by accounting for random, unpredictable anatomic changes that occur between treatment fractions.
■ Online adaptive radiation therapy enables daily treatment plan adaptation to anatomy visualized at in-room imaging and requires high-quality daily imaging and rapid recontouring, replanning, plan review, and quality assurance.
■ Online adaptive radiation therapy based on cone-beam CT allows relative ease for integration into the radiation therapy workflow and the potential for rapid imaging and replanning, with the major challenge of reduced image quality.
Introduction
The concept of adaptive radiation therapy (ART) was introduced in 1997 by Yan et al (1) as a feedback process by which imaging information acquired over the course of treatment (such as changes in a patient's anatomy) can be used to reoptimize the treatment plan. The end goals of such replanning include improving target coverage and reducing treatment toxicity. Between initial planning and the first treatment fraction and over the course of treatment, changes in anatomy, including weight loss and tumor progression; reduction in tumor volume; and physiologic motion of organs at risk (OARs) may occur (2–4). Traditional methods of radiation therapy (RT) do not account for these changes because OAR and target segmentation are defined only in the initial CT data set acquired as the patient first begins their RT treatment. Physician-defined margins around target volumes are expanded to account for positional uncertainty, at the cost of larger irradiated volumes and increased toxicity (5). The process of adapting treatment to account for changes in patient anatomy introduces new technical challenges, and several ART platforms have emerged in recent years. This review will provide an overview of the implementation of ART, both off- and online, with a focus on approaches using medical linear accelerators equipped with onboard CT-based imaging systems as well as basic considerations when evaluating current, commercially available platforms.
Adaptive RT: Concepts
Traditional RT Process
Traditional RT treatment planning involves acquiring an initial three-dimensional data set (CT simulation scan), delineating normal and target structures in the initial data set (contouring), and creating an optimized RT plan that maximizes dose to targeted volumes while maintaining acceptable levels of dose to normal tissues. Once the plan is created, the patient receives the prescribed radiation dose over the course of numerous treatment sessions, or fractions, often delivered daily. Traditionally, the structures and plan as defined on the initial scan are used for the duration of treatment.
Anatomic Changes
Certain anatomic changes during RT, such as tumor regression or progression and weight loss, can occur over the course of weeks, while others, such as bladder or rectal filling, peristalsis, and uterus motion, occur over minutes to hours (4,6). These changes, which vary by treatment site, impact the coverage of target volumes and dose delivered to normal tissues (2,4). Historically, the ART paradigm has been investigated in treating rapidly growing cancers (eg, head and neck disease). For example, Kishan et al (7) reported a median increase of 16% in the gross tumor volume between treatment planning and initiation for head and neck cancer. The parotid glands, local OARs, may move medially due to patient weight loss and tumor regression during treatment (2).
ART has also been investigated for treatment of pelvic tumors. Pelvic anatomy can shift in response to changes in bladder, bowel, and rectum filling; thus, consistency in organ filling is desirable. While laxatives, dietary plans, and drinking protocols help achieve consistent rectal and bladder filling, volume variability persists and is influenced by patient compliance, chemotherapy timing, and baseline hydration (8).
In cervical cancer treatment, the cervix-uterus complex can undergo complex inter- and intrafraction motion, most heavily influenced by bladder and rectal volumes (9). Bladder volume predominantly affects the position of the tip of the uterus while rectal filling primarily impacts the cervix and upper vagina (9). Moreover, tumor regression over the course of treatment has been observed on both MR and cone-beam CT (CBCT) images, the latter being the most common onboard volumetric imaging modality used by current-generation medical linear accelerators (3). These changes in local anatomy can impact the dose to OARs and coverage of the clinical target (10,11).
ART Approaches
Three general approaches to ART exist: (a) offline ART (treatment replanning between treatment fractions), (b) online ART (treatment replanning immediately before each treatment fraction), and (c) real-time ART (real-time motion monitoring and/or gating [eg, to compensate for respiratory motion]) (6). The most suitable timing, frequency, and strategy of treatment adaptation depend on the potential clinical benefit, which in turn is dictated by local anatomic changes and the clinical context (6). For example, sites with little interfraction change, such as the lungs, may not benefit from frequent adaptation or online ART; the head and neck is another site where progressive anatomic changes predominate (6,12). Conversely, abdominal or pelvic sites where OARs are present and target motion occurs between fractions may benefit from more frequent adaptation, particularly online ART. Although additional degrees of change in organ position may occur over the course of treatment planning itself (see below for average treatment planning duration), the incremental magnitude of these changes may be minimal compared with the overall day-to-day changes accommodated through online ART. Further, a second CBCT acquisition taken just before treatment delivery can help verify the final position of the organs and treatment plan accuracy. Real-time ART, a promising approach to compensate for intrafraction motion, may be helpful for abdominal and thoracic targets where respiratory and cardiac motion predominantly exert their effect.
Offline ART
Of the ART approaches currently under investigation, offline ART has been the most extensively explored to date, as it can be implemented within the existing RT workflow. One offline strategy that has been used in cervical and prostate cancer is to create an average anatomic model by quantifying and averaging anatomic changes over the first few treatment fractions (4). Another strategy is to create several plans during the initial treatment planning session. For example, plans can be created representing anatomy with a full and empty bladder, with the most appropriate plan selected on each day of treatment (ie, a plan-of-the-day approach) (4,10). Alternatively, plan adaptation may be triggered ad hoc, such as when the treating physician observes changes that necessitate the creation of a new plan or when the current plan violates predetermined dosimetric criteria (4). Adaptation can also be scheduled for specific time points throughout the treatment course, and there has been some investigation as to which schedule may be most suitable (2,13,14). For example, weekly offline replanning may reduce the margin from the clinical target volume (CTV) to planning target volume (PTV) in cervical and head and neck cancer without losing target coverage (13).
Offline ART may be an excellent strategy to account for slow and progressive changes such as weight loss. It allows for more selective treatment adaptation than daily online adaptation and can be implemented without substantial infrastructural change because it relies on traditional RT workflows. However, offline adaptation is unsuitable for some anatomic changes (especially random interfraction changes), and better-defined thresholds and schedules for treatment adaptation are needed (4). Additionally, dosimetric changes due to changes in anatomy are difficult to assess, being both time-consuming and imprecise to estimate.
Online ART
Online ART has emerged to address unpredictable anatomic changes between treatment fractions, such as uterocervical complex motion. For online ART, images acquired in-room before each fraction are used to adjust the treatment plan while the patient remains on the treatment table. As such, online ART places unique pressures on the RT workflow, requiring high-quality daily imaging, rapid recontouring, replanning, plan review, and quality assurance (6). Generating a new plan with every fraction is resource intensive and time sensitive; therefore, workflow efficiency and clinical resource allocation are key (6,15).
Image Quality Requirements
Current online ART modalities rely on either in-room CBCT or hybrid MRI-guided linear accelerator treatment systems. While both systems may not demonstrate equivalent utility for segmentation compared with planning CT or diagnostic MRI, images must meet quality and accuracy specifications particular to radiation oncology, such as those from the International Commission on Radiation Units and Measurements. Such specifications aim to ensure overall dosimetric uncertainty of ±5% and overall spatial uncertainty of ±5 mm (16–18).
Image quality and soft-tissue contrast are essential for accurate organ delineation and replanning during online ART. Additionally, a high-resolution matrix of spatially accurate electronic density information is required for accurate dose calculations. Furthermore, overall image quality is critical for trustworthy deformable image registration (DIR), which is used at every fraction to deform OARs and target contours (6). DIR is the process of matching two images to maximize spatial correspondence, allowing for the propagation of contours or electron density mapping from image to image. However, DIR can introduce error and uncertainty, and a wide range of reported DIR accuracy exists (6). DIR accuracy can be compromised by poor quality of the input images (the planning image and the daily in-room image), as well as presence of artifacts and distortion (6,19).
One must consider geometric alignment and distortion when evaluating potential online ART systems. For CT-based image-guided RT, imaging and treatment isocenter coincidence, scaling and distance accuracy, low-contrast resolution, spatial resolution, uniformity, and noise are essential for quality assurance. Scanner-related artifacts that impact clinical image interpretation are detected and corrected through the quality assurance process. For ART, Hounsfield unit accuracy is also important for consistency and accuracy of dose calculations (20). Currently, the Hounsfield units for CBCT can be restored by deforming the planning CT to CBCT for online dose calculation. The recommended tolerance of isocenter coincidence and geometric fidelity is 1 mm for stereotactic body RT–based applications and 2 mm for non–stereotactic body RT applications. High-contrast spatial resolution is recommended to be within less than or equal to 2 mm or less than or equal to 5 line pairs per centimeter; however, in clinical practice, systems may operate at lower spatial resolution due to the large size of volumetric data sets at full resolution, with most authors reporting spatial resolution of 6–9 line pairs per centimeter (20). Furthermore, low-contrast resolution and detectability are important for localization of preidentified or presegmented structures, and requirements may vary based on clinical site (eg, to accommodate a contrast difference between prostate and rectum of 2%) (20). Imaging quality assurance baseline values for in-room imaging including CBCT are established during the acceptance and commissioning process of linear accelerator equipment, with quality assurance conducted at regular intervals (further described in the American Association of Physicists in Medicine task group reports TG-142 and TG-179) (18,20). MR images are subject to geometric distortion due to machine-based magnetic field errors and a patient's chemical makeup (which affects the local magnetic field) (16).
Workflow Considerations
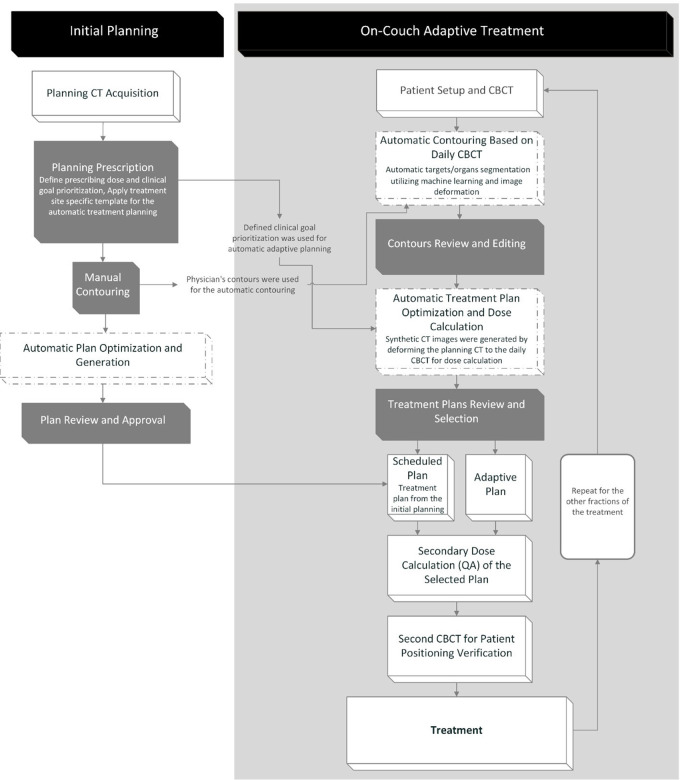
Two technological considerations that render online ART feasible are speed of replanning and workflow efficiency (Fig 1). Automated recontouring, replanning, and Hounsfield unit mapping (as necessary) allow for maximal efficiency in the use of clinical resources and a feasible timeline on the order of minutes. These processes depend heavily on image quality, spatial image accuracy, and the accuracy of DIR algorithms. Overall times for the image acquisition, recontouring, replanning, and quality analysis process have been reported in several MRI-guided online ART studies to be between 45 and 79 minutes, with the time to recontour, reoptimize the plan, and perform quality assurance taking 26 minutes (21–23). For CBCT-based online ART in rectal cancer, the average time at the treatment machine was 34 minutes in one study (24), while another study reported the average time from patient setup to posttreatment CBCT verification to be 28 minutes (25). Median replanning duration (not including setup, imaging, or treatment delivery) was 17.6 minutes in one study (26) and 19.6 minutes in another (27).
Figure 1:
Workflow for Ethos CT-based online adaptive radiation therapy. CBCT = cone-beam CT.
Rapid recontouring approaches require manual editing but are hastened with DIR to propagate previously used contours (21,28). Manual editing of contours for ART replanning has been shown to take a median of 9 minutes, with a range of 2–24 minutes (22). Other approaches for rapid contouring, including deep learning and atlas-based segmentation, have been proposed (29). When evaluating auto-contouring or rapid contouring strategies, the accuracy needs to be assessed such that it is similar to the uncertainty of a human expert contouring the structure manually; the tolerance value between any two contours has been suggested to be within a Dice similarity coefficient value of approximately 0.8–0.9 (6,19).
Approaches to propagating the target volumes differ, with some suggesting rigid propagation and others proposing that, in certain cases, the contours can be adjusted as changes occur. For example, as a gross tumor volume shrinks, an OAR may move into an area formerly occupied by the gross tumor volume; it is not yet clear whether it is best to prioritize reducing the dose to OARs or maintaining coverage of the region formerly occupied by the gross tumor volume. Sonke et al (4) recommend against altering the gross tumor volume, CTV, and PTV since in-room imaging used for online ART does not provide the necessary information to update contours, and complementary diagnostic information (such as pathology reports and other imaging modalities) is not available during daily replanning. Furthermore, the area encapsulated initially by the gross tumor volume may contain traces of microscopic disease, so prospective clinical trials may be needed before the field size can be safely reduced during ART (4). In a phase 1 study of stereotactic MRI-guided ART, conservative PTV margins of 5 mm were used to remain in line with the standard of care (22). On the other hand, the Adaptive Radiation Therapy in Locally Advanced Non-Small Cell Lung Cancer (LARTIA) trial in non–small cell lung cancer evaluated adaptive reduction of PTV with tumor shrinkage over the course of treatment (30).
To generate a new treatment plan that considers the dosimetric influence of material inhomogeneities, a new electron density (ie, Hounsfield unit) map of the patient must be created with each fraction. In the traditional RT workflow, the Hounsfield unit from the planning CT can be mapped directly to the electron density values used in dose calculation and treatment planning. When using in-room MRI for treatment planning, images must first be converted to Hounsfield units via the generation of synthetic CT scans from MRI data (this process has been shown to have a clinically acceptable accuracy) (6,31). For CBCT scans, the accuracy of electron density maps is compromised by image artifacts and x-ray scatter and requires correction or calibration (20). However, Hounsfield unit accuracy for CBCT may be improved with the iterative CBCT reconstruction algorithm, and several approaches to improve dose calculation accuracy from CBCT images have been explored (32,33).
Rapid online generation of a new treatment plan needs to be largely automated. One approach is knowledge-based planning which uses prior knowledge (either a model or an atlas) to predict the dose distribution. Other approaches involve protocol-based automatic iterative optimizations that make clinically desirable trade-offs to better adhere to predetermined prioritized constraints (34). Additional approaches to reduce planning time include combining OARs into a single optimization structure, relying on artificial neural networks, and using the final objectives of the initial plan to reoptimize the new plan on the anatomy of the day (4,28). Online plan reoptimization has been successfully achieved on the order of minutes (22,26).
Quality assurance of the new plan must be performed prior to initiating treatment while the patient is on the treatment table (6). The lack of a daily planning-quality CT scan necessitates novel technological quality assurance solutions unique to online ART. While commercial options are limited, the MRIdian Linac (ViewRay) and Ethos (Varian Medical Systems) systems come with a vendor-provided online adaptive quality assurance tool.
Clinical Benefit of ART
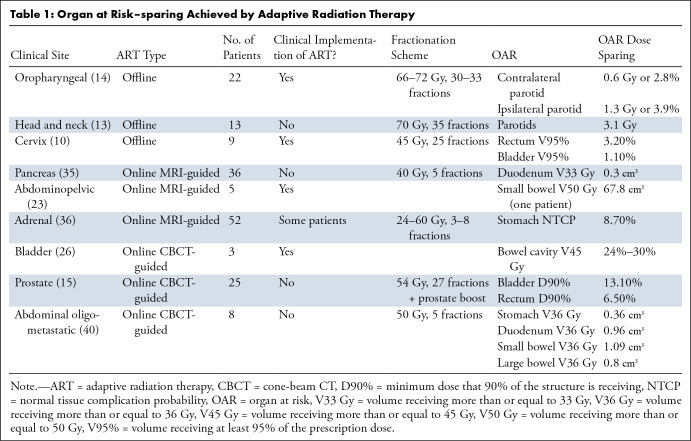
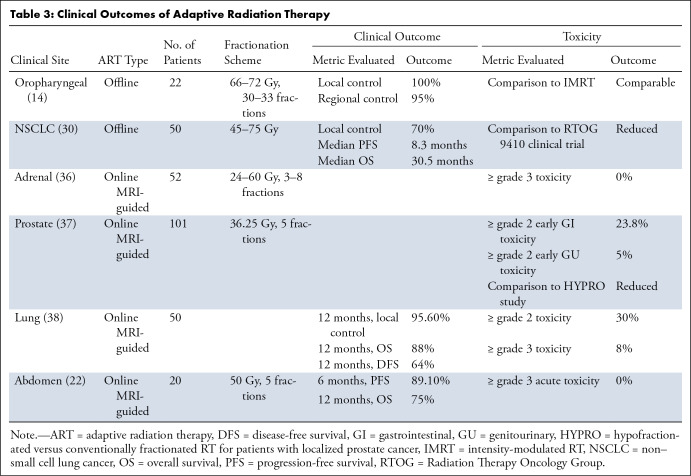
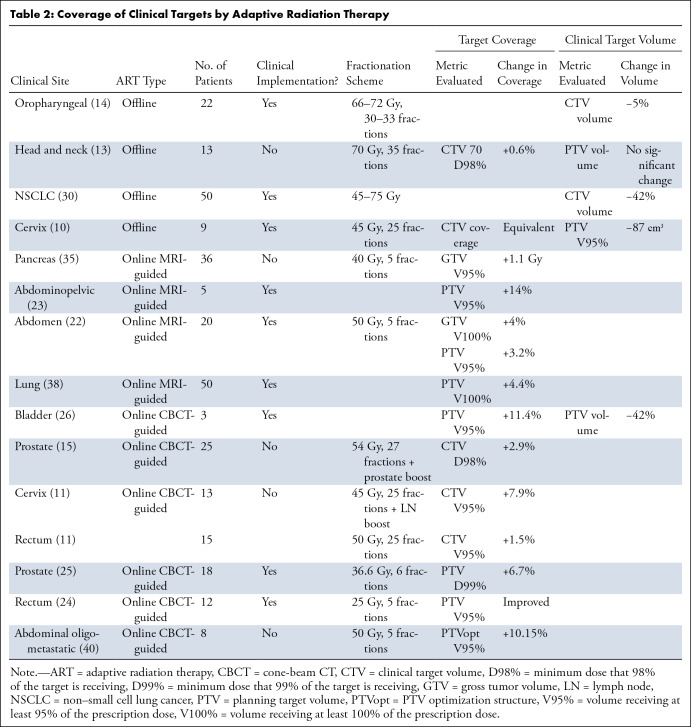
The toxicities of RT are associated with the dose delivered to local OARs, and guidelines for OAR constraints aim to reduce this risk. However, as anatomy shifts over the course of treatment, OARs often receive doses higher than predicted by the initial RT plan. Adaptive planning minimizes dose to normal tissues and increases coverage of the clinical target, translating clinically to decreased toxicity and/or improved local control. Dosimetric and nonrandomized clinical benefits have been investigated for offline (10,13,14,30) and online (11,15,22,23,26,35–38) ART strategies (Tables 1–3).
Table 1:
Organ at Risk–sparing Achieved by Adaptive Radiation Therapy
Table 3:
Clinical Outcomes of Adaptive Radiation Therapy
Table 2:
Coverage of Clinical Targets by Adaptive Radiation Therapy
Offline ART Dosimetric and Clinical Benefits
Offline ART has been applied in multiple tumor sites, including rectal, cervical, bladder, head and neck, and lung cancer (Tables 1–3). For example, weekly adaptation for locally advanced non–small cell lung cancer to accommodate tumor shrinkage led to reduced toxicity, with acute and late grade 3 or higher pulmonary toxicities of 2% and 4%, respectively, compared with grade 3 or higher lung toxicities of 13%–17% reported in Radiation Therapy Oncology Group 9410 clinical trial and a rate of local failure of 30% (30).
Online ART Dosimetric Benefits
Much investigation of online ART benefit has occurred for stereotactic body RT of abdominopelvic malignancies, a treatment modality where high doses of radiation are delivered over just a few treatment fractions. For one series of 137 patients with abdominal cancer (of which 98 had pancreatic cancer) treated with MRI-guided ART, 961 of 1185 (81.1%) fractions were adapted. Of these, 870 (90.5%) fractions adhered to critical OAR constraints that were previously violated by unadapted fractions. Target coverages were improved for 888 (92.4%) cases of plan adaptation (39). For additional studies investigating dosimetric benefits of MRI-guided ART, see Tables 1–3.
In an in-silico model of daily online CBCT-based treatment adaptation for eight patients receiving stereotactic RT for abdominal oligometastatic disease, plan adaptation resulted in only two of 40 (5%) fractions with OAR constraint violations, whereas the initial plan applied to daily anatomy violated OAR constraints in 30 of 40 (75%) fractions (40). For CBCT-guided online ART of three patients with bladder cancer, a 42% median primary PTV reduction was achieved which led to a 24%–30% reduction in volume receiving more than or equal to 45 Gy (V45 Gy) to the bowel cavity (26). At the same time, coverage of the PTV receiving at least 95% of the scheduled dose (PTV V95%) was restored from 88.2% to 99.6% through adaptive replanning (26). Retrospective simulation of CBCT-based online ART in anal cancer showed that a reduction in CTV to PTV margins could reduce bowel cavity V45 Gy by an average of 11.4%, while reoptimizing the plan to match the anatomy of the day could reduce bowel cavity V45 Gy by 13.1% (41). In a study of simulated CBCT-guided online ART in cervical cancer, replanning changed the CTV V95% by 7.9% and modestly reduced the D2cc (the minimum dose to the maximally exposed 2 cm3) to each pelvic OAR by 0.02–0.08 Gy for each fraction (11). For patients with rectal cancer, online replanning led to an increase in CTV V95% by 1.5%, while the D2cc for the bladder and bowel changed by 0.02 and -0.02 Gy, respectively, for each fraction (11). For a series of 18 patients with prostate cancer undergoing ultrahypofractionated RT with manual adaptation of organ contours to match anatomy of the day, PTV D99% (the minimum dose that 99% of the PTV is receiving) increased from 90.7% to 97.1% (42). In a different study using a CBCT-based online ART platform, 24 of 25 patients with prostate cancer experienced an average improvement in CTV D98% (the minimum dose that 98% of the CTV is receiving) of 2.9% ± 5.3 (SD) (15).
Online ART Clinical Benefits
Most clinical outcome results for online ART to date have been published for MRI-based systems. Early toxicity results from a prospective single-arm phase 2 study of MRI-guided ART for stereotactic RT of prostate cancer showed no grade 3 gastrointestinal toxicity with maximum cumulative grade 2 or higher early genitourinary and gastrointestinal toxicities of 23.8% and 5.0%, respectively, which is markedly lower than toxicities reported for the moderately hypofractionated RT arm in a separate study (61% and 42%, respectively) (37,43).
For patients with high-risk lung tumors, daily online MRI-guided plan adaptation resulted in early local control of 95.6% and disease-free survival of 63.6% at 12 months, as well as low rates of 30% and 8% for grade 2 and 3 or higher toxicities, respectively (38). For stereotactic MRI-guided online ART in five patients with ultracentral thorax malignancies, 10 of 25 total fractions were adapted to either improve PTV coverage or address OAR violations, with no grade 3 or higher acute toxicities reported and local control of 100% at 6 months (44).
In a study of stereotactic MRI-guided RT in renal cancer, only 16.1% of 180 fractions required online reoptimization, with local control and overall survival rates of 95.2% and 91.2% at 1 year and no grade 3 or higher toxicities reported (45). For a set of patients with abdominal malignancies undergoing online adaptive stereotactic MRI-guided RT, plans were adapted for 81 of 97 fractions with most fractions requiring adaptation due to violation of OAR constraints. In 64 of 97 fractions, adaptation increased PTV coverage; no acute grade 3 or higher toxicities were observed (22).
Review of Online CBCT-based ART
Implementation of CBCT-based Online ART
CBCT is widely used for image-guided RT, so implementing CBCT-guided online ART requires little infrastructural change or specialized training (24,46). Other advantages include a short image acquisition time (approximately 2 minutes for CBCT acquisition) and ease of delivery (no caveats for metal implants, large patient size, or claustrophobia) (25,26,46).
One study evaluated the feasibility of daily online CBCT-based adaptation for prostate cancer. Contour propagation and manual contour adaptation took an average of 8 minutes with a total time from patient setup to posttreatment CBCT verification of 28 minutes. Adaptation increased target coverage (D99%) from 90.4% to 97.1% and restored optimal prostate coverage in 35% of fractions (25).
The Ethos system has been introduced for CBCT-guided online ART (29). This platform uses the iterative CBCT reconstruction process to reduce noise and improve image quality, and therefore features improved soft-tissue delineation and dose calculation (32,47,48). Iterative CBCT is prone to motion artifacts and thus has limited use for sites such as the pancreas but is optimal for sites such as the prostate, rectum, anus, and bladder.
Clinical implementation of the Ethos platform is an area of active interest. For five patients with bladder or rectal cancer, the median replanning duration was 17.6 minutes between CBCT acceptance and treatment initiation (26). For retrospectively simulated online ART in various pelvic sites, 76% of automatically segmented structures required little to no manual editing with the adaptive plan selected in 88% of simulated treatment sections (26). This study suggests that disadvantages of the current treatment planning software include a fixed set of beam configurations and inferior volumetric modulated arc therapy plan quality (26). These disadvantages can be overcome by creating a novel beam arrangement in a separate treatment planning system, Eclipse (Varian Medical Systems), and importing the plan back into the Ethos platform, which is a cumbersome process.
Twelve patients with rectal cancer were treated with neoadjuvant RT to 25 Gy with the Ethos linear accelerator. The average treatment time slot was 34 minutes, with an average on-table time of 26 minutes. Evaluation of target volumes took 4 minutes with an additional 5 minutes required for adjustments. Plan adaptation increased V95% of the PTV for 52 of 55 fractions (24). Complications encountered during adaptive plan treatment delivery included workflow interruption in individual fractions due to full bladder, intrafraction motion of a large gas pocket, error in bony anatomy registration, and synthetic CT error due to body contour definition (24).
Online ART for patients with head and neck cancer was retrospectively simulated using the Ethos software emulator, with a median online ART planning duration (not including patient setup, CBCT image acquisition, or treatment delivery) of 19 minutes 34 seconds and overall satisfactory quality of OAR delineation. Adapted plans resulted in persistent OAR sparing, even without human revision of automated contours (27). In this study, automation (including contour delineation and treatment plan generation) required a median of 6.5 minutes, with human review of key OARs taking a median of 5 minutes 38 seconds (27).
Alternatively, in a simulation of online ART using the Ethos emulator in 25 patients with prostate cancer, 96% of fractions required additional editing after contour autosegmentation, with generally minor corrections of largely less than 10% of the CTV. Even without manual editing, these patients would have experienced dosimetric benefits. However, one patient required substantial modification (15).
For a series of 13 patients with cervical cancer and 15 patients with rectal cancer, the average workflow duration was 24.4 minutes for patients with cervical cancer and 9.2 minutes for patients with rectal cancer, with 13.1 minutes and 2.7 minutes, respectively, devoted to editing target contours. This adaptation increased PTV and CTV coverage and decreased the dose to the maximum 2 cm3 of local OARs (11).
Challenges Facing CBCT-based Online ART
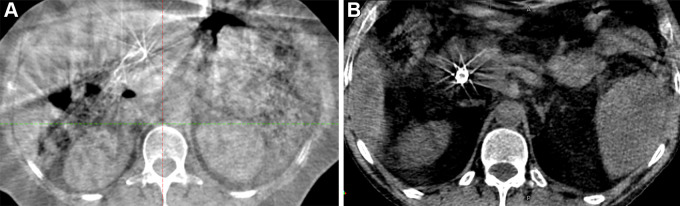
One of the critical limitations of online ART using CBCT is inferior soft-tissue definition (46). Image quality is reduced due to reasons such as patient movement (due to long acquisition time), radiation scattering, and image artifacts, including gas, ring, noise, and beam-hardening artifacts (20,33). Scatter, of particular concern with CBCT, occurs due to cone-beam geometry, with the amount of scatter dependent on acquisition and reconstruction parameters, beam angle, and object size (49). Increased scatter reduces CBCT image quality by reducing image contrast and increasing noise and artifacts (20,32). The iterative CBCT algorithm can help reduce the effects of scatter by using a scatter estimation algorithm such as Acuros (Varian Medical Systems). Several other approaches to mitigate the impact of scatter have also been investigated (47,50). See Figure 2 for representative images comparing conventional CBCT and iterative CBCT imaging.
Figure 2:
(A) Representative axial cone-beam CT (CBCT) image acquired with a conventional linear accelerator. (B) Representative axial image acquired using iterative CBCT reconstruction.
Inferior CBCT image quality reduces soft-tissue contrast, leading to potential inaccuracy in segmenting structures based on CBCT images (4). Both manual and automatic segmentation are subject to this inaccuracy. However, contour propagation from planning CT and artificial intelligence–guided segmentation has been developed to aid CBCT segmentation. The latter is now commercially available as part of the Ethos linear accelerator system (29,51).
Artifacts and poorer image quality also lead to reduced accuracy in determining electron density for dose calculation purposes, though postprocessing algorithms can mitigate the impact (33,50,52). Approaches to dose calculation based on CBCT include CBCT calibration curves derived from phantoms or patient images, DIR with planning CT, overriding of CBCT Hounsfield unit values with either Hounsfield unit density or Hounsfield units from CT images, and dose deformation (33). Another method proposes corrections based on prior image information to restore Hounsfield units and improve uniformity (52). A wide range of dose calculation accuracies has been reported for various CBCT-based dose calculation methods at different tumor sites, with some methods yielding good accuracy. CBCT calibration methods, for example, yield dose differences of less than 2%, and DIR algorithms produced calculated doses with differences in accuracy of 2%–3% (33,52).
Image acquisition and reconstruction techniques to address challenges in image quality are currently under investigation with the potential to improve dose calculation accuracy and facilitate segmentation (20,48,50,51,53). A ring-shaped medical linear accelerator system with fast kilovolt CBCT has been introduced for faster CBCT image acquisition and may reduce the effects of motion (54).
One additional concern with CBCT images is the limited field of view of images, which with a length of 16 cm, is particularly limited in the longitudinal direction (55). Therefore, certain anatomic regions, including portions of OARs, clinical targets, or elective lymph nodes, may not be captured on CBCT images during treatment. This limitation is addressed by acquiring and stitching together two CBCT images or compensating for the missing field of view with information from the planning CT (32,55).
Radiation exposure during the acquisition of the CBCT scan, while minimal compared with the dose delivered during RT treatment, nevertheless prevents the use of CBCT for repeated images throughout the fraction; therefore, CBCT cannot be used for real-time ART or intrafraction motion assessment (46,56). The imaging dose for kilovoltage-CBCT typically ranges from 0.2 to 2 cGy per image (20,29). Skin dose has been measured to be a fraction of a centigray for low-dose head and neck imaging and 7 cGy for high-dose pelvic imaging (56).
In summary, the key advantages of CBCT-based ART include its ease of integration into the RT workflow and potential for rapid imaging and replanning, leading to feasible time frames for online adaptation. The major drawback is the inferior image quality due to poor resolution, scatter, and artifacts, which leads to reduced soft-tissue contrast and increased dosimetric uncertainty.
Additional Approaches to ART
As online ART relies heavily on the quality of onboard imaging for treatment adaptation, there has been much enthusiasm about MRI platforms for online ART. Hybrid systems that combine linear accelerators with onboard MRI (MRI-linear accelerator systems) are commercially available and have been used in most clinical implementations of online ART to date (46), including in patients with lung tumors (38), liver and other abdominal malignancies (22), pancreas cancer (28), colorectal cancer (23), prostate cancer (21,37), and adrenal metastases (36).
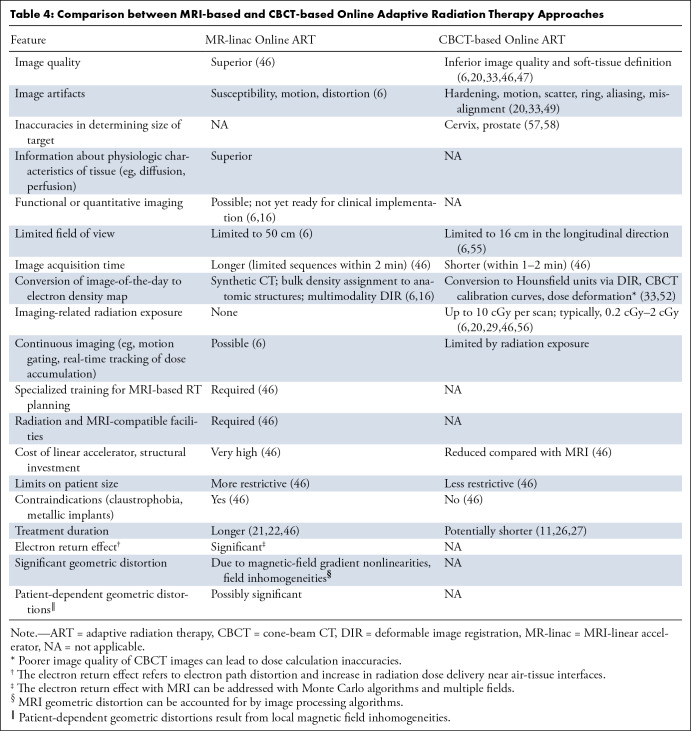
While implementing MRI-guided ART can require a substantial investment of resources and pose many technical challenges, the increased soft-tissue resolution over CBCT and the potential for real-time and functional imaging offer important advantages to MRI-linear accelerator implementation for ART (Table 4). CT-based imaging can lead to inaccuracies in determining the target size and overestimates the size of anatomic boundaries, such as cervical cancer CTV width and prostate size, compared with MRI (57,58). Therefore, contour definition based on CT alone without MRI input may not be optimal for specific treatment sites. The possibility of continuous imaging with MRI enables real-time positional monitoring for sites affected by constant motion, such as in lung cancer. In addition, functional imaging, where quantitative or physiologic information is obtained from the image to help guide treatment, is feasible with MRI but not CBCT. For example, information obtained from diffusion-weighted imaging or dynamic contrast-enhanced MRI sequences may help guide treatment adaptation or dose escalation to specific regions of the tumor. A PET scanner coupled to a linear accelerator has been introduced with the promise of PET-guided ART which may enable assessment of the biologic response to treatment and subsequent treatment adaptation (6). So-called biology-guided RT has the potential for dynamic guidance of radiation delivery to PET-avid targets, with adaptive planning based on kilovoltage CT scans acquired prior to each fraction, though the logistics and feasibility of fluorodeoxyglucose or other PET tracers used for each fraction of radiation are potential barriers to wide implementation (59,60).
Table 4:
Comparison between MRI-based and CBCT-based Online Adaptive Radiation Therapy Approaches
Conclusion
Emerging technologies offer exciting possibilities for online RT treatment adaptation. However, many technological and practical challenges to online ART implementation remain. CBCT and MRI-based ART technologies offer trade-offs between practical considerations such as cost, speed of adaptation (and thereby patient throughput), and image quality. Speed of adaptive replanning is critical in improving patient experience and compliance with treatment. Depending on local anatomic changes and clinical context, different anatomic sites and/or disease stages and presentations will likely benefit from different ART strategies. Assessment of the clinical benefit from online ART and comparison of various ART strategies in the setting of clinical trials are still needed, as well as an objective way to determine the clinical scenarios in which treatment adaptation is most beneficial.
Authors declared no funding for this work.
Disclosures of conflicts of interest: E.L. No relevant relationships. M.D.G. No relevant relationships. Y.F.W. No relevant relationships. C.C. No relevant relationships. C.E. No relevant relationships. M.S. No relevant relationships. M.P. No relevant relationships. L.A.K. Grants from Varian Medical Systems for a trial assessing barriers to clinical trial enrollment in Latinx patients receiving breast or prostate radiation therapy and a trial for adaptive radiation therapy for anal cancer; payment as an editor for an UpToDate chapter on bone metastases; member of the formal data safety monitoring committee to assess phase 1 drug development for New Beta Innovation and an unpaid board member for the Radiation Therapy Oncology Group; unpaid leadership role at NRG Oncology. D.P.H. Grants from the National Institute of Allergy and Infectious Diseases and the Radiological Society of North America; support from the Southwest Oncology Group.
Abbreviations:
- ART
- adaptive RT
- CBCT
- cone-beam CT
- CTV
- clinical target volume
- DIR
- deformable image registration
- OAR
- organ at risk
- PTV
- planning target volume
- RT
- radiation therapy
References
- 1. Yan D , Vicini F , Wong J , Martinez A . Adaptive radiation therapy . Phys Med Biol 1997. ; 42 ( 1 ): 123 – 132 . [DOI] [PubMed] [Google Scholar]
- 2. Barker JL Jr , Garden AS , Ang KK , et al . Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system . Int J Radiat Oncol Biol Phys 2004. ; 59 ( 4 ): 960 – 970 . [DOI] [PubMed] [Google Scholar]
- 3. Chen W , Bai P , Pan J , Xu Y , Chen K . Changes in Tumor Volumes and Spatial Locations Relative to Normal Tissues During Cervical Cancer Radiotherapy Assessed by Cone Beam Computed Tomography . Technol Cancer Res Treat 2017. ; 16 ( 2 ): 246 – 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sonke JJ , Aznar M , Rasch C . Adaptive Radiotherapy for Anatomical Changes . Semin Radiat Oncol 2019. ; 29 ( 3 ): 245 – 257 . [DOI] [PubMed] [Google Scholar]
- 5. van Herk M . Errors and margins in radiotherapy . Semin Radiat Oncol 2004. ; 14 ( 1 ): 52 – 64 . [DOI] [PubMed] [Google Scholar]
- 6. Glide-Hurst CK , Lee P , Yock AD , et al . Adaptive Radiation Therapy (ART) Strategies and Technical Considerations: A State of the ART Review From NRG Oncology . Int J Radiat Oncol Biol Phys 2021. ; 109 ( 4 ): 1054 – 1075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kishan AU , Cui J , Wang P-C , Daly ME , Purdy JA , Chen AM . Quantification of gross tumour volume changes between simulation and first day of radiotherapy for patients with locally advanced malignancies of the lung and head/neck . J Med Imaging Radiat Oncol 2014. ; 58 ( 5 ): 618 – 624 . [DOI] [PubMed] [Google Scholar]
- 8. Eminowicz G , Motlib J , Khan S , Perna C , McCormack M . Pelvic Organ Motion during Radiotherapy for Cervical Cancer: Understanding Patterns and Recommended Patient Preparation . Clin Oncol (R Coll Radiol) 2016. ; 28 ( 9 ): e85 – e91 . [DOI] [PubMed] [Google Scholar]
- 9. Jadon R , Pembroke CA , Hanna CL , et al . A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer . Clin Oncol (R Coll Radiol) 2014. ; 26 ( 4 ): 185 – 196 . [DOI] [PubMed] [Google Scholar]
- 10. Buschmann M , Majercakova K , Sturdza A , et al . Image guided adaptive external beam radiation therapy for cervix cancer: Evaluation of a clinically implemented plan-of-the-day technique . Z Med Phys 2018. ; 28 ( 3 ): 184 – 195 . [DOI] [PubMed] [Google Scholar]
- 11. Yock AD , Ahmed M , Ayala-Peacock D , Chakravarthy AB , Price M . Initial analysis of the dosimetric benefit and clinical resource cost of CBCT-based online adaptive radiotherapy for patients with cancers of the cervix or rectum . J Appl Clin Med Phys 2021. ; 22 ( 10 ): 210 – 221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Q , Chi Y , Chen PY , Krauss DJ , Yan D , Martinez A . Adaptive replanning strategies accounting for shrinkage in head and neck IMRT . Int J Radiat Oncol Biol Phys 2009. ; 75 ( 3 ): 924 – 932 . [DOI] [PubMed] [Google Scholar]
- 13. Zhang P , Simon A , Rigaud B , et al . Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer . Radiother Oncol 2016. ; 120 ( 1 ): 41 – 47 . [DOI] [PubMed] [Google Scholar]
- 14. Schwartz DL , Garden AS , Thomas J , et al . Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial . Int J Radiat Oncol Biol Phys 2012. ; 83 ( 3 ): 986 – 993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moazzezi M , Rose B , Kisling K , Moore KL , Ray X . Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation . J Appl Clin Med Phys 2021. ; 22 ( 10 ): 82 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thorwarth D , Low DA . Technical Challenges of Real-Time Adaptive MR-Guided Radiotherapy . Front Oncol 2021. ; 11 ( 332 ): 634507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landberg T , Chavaudra J , Dobbs J , et al . Report 50: Prescribing, Recording and Reporting Photon Beam Therapy . J ICRU 1993. ; os26 ( 1 ). [Google Scholar]
- 18. Klein EE , Hanley J , Bayouth J , et al. Task Group 142, American Association of Physicists in Medicine . Task Group 142 report: quality assurance of medical accelerators . Med Phys 2009. ; 36 ( 9 ): 4197 – 4212 . [DOI] [PubMed] [Google Scholar]
- 19. Brock KK , Mutic S , McNutt TR , Li H , Kessler ML . Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132 . Med Phys 2017. ; 44 ( 7 ): e43 – e76 . [DOI] [PubMed] [Google Scholar]
- 20. Bissonnette J-P , Balter PA , Dong L , et al . Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179 . Med Phys 2012. ; 39 ( 4 ): 1946 – 1963 . [DOI] [PubMed] [Google Scholar]
- 21. Tetar SU , Bruynzeel AME , Lagerwaard FJ , Slotman BJ , Bohoudi O , Palacios MA . Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer . Phys Imaging Radiat Oncol 2019. ; 9 : 69 – 7 6 [Published correction appears in Phys Imaging Radiat Oncol 2020;15:98.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henke L , Kashani R , Robinson C , et al . Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen . Radiother Oncol 2018. ; 126 ( 3 ): 519 – 526 . [DOI] [PubMed] [Google Scholar]
- 23. Acharya S , Fischer-Valuck BW , Kashani R , et al . Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications . Int J Radiat Oncol Biol Phys 2016. ; 94 ( 2 ): 394 – 40 3 [Published correction appears in Int J Radiat Oncol Biol Phys 2016;96(1):243.]. [DOI] [PubMed] [Google Scholar]
- 24. de Jong R , Visser J , van Wieringen N , Wiersma J , Geijsen D , Bel A . Feasibility of Conebeam CT-based online adaptive radiotherapy for neoadjuvant treatment of rectal cancer . Radiat Oncol 2021. ; 16 ( 1 ): 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calvo-Ortega JF , Moragues-Femenía S , Laosa-Bello C , Torices-Caballero J , Hermida-López M , Casals-Farran J . Clinical Experience in Prostate Ultrahypofractionated Radiation Therapy With an Online Adaptive Method . Pract Radiat Oncol 2022. ; 12 ( 2 ): e144 – e152 . [DOI] [PubMed] [Google Scholar]
- 26. Sibolt P , Andersson LM , Calmels L , et al . Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region . Phys Imaging Radiat Oncol 2020. ; 17 : 1 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoon SW , Lin H , Alonso-Basanta M , et al . Initial Evaluation of a Novel Cone-Beam CT-Based Semi-Automated Online Adaptive Radiotherapy System for Head and Neck Cancer Treatment - A Timing and Automation Quality Study . Cureus 2020. ; 12 ( 8 ): e9660 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bohoudi O , Bruynzeel AME , Senan S , et al . Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer . Radiother Oncol 2017. ; 125 ( 3 ): 439 – 444 . [DOI] [PubMed] [Google Scholar]
- 29. Archambault Y , Boylan C , Bullock D , et al . THompson S . Making on-line adaptive radiotherapy possible using artificial intelligence and machine learning for efficient daily re-planning . Med Phys Int J 2020. ; 8 ( 2 ): 77 – 86 . [Google Scholar]
- 30. Ramella S , Fiore M , Silipigni S , et al . Local Control and Toxicity of Adaptive Radiotherapy Using Weekly CT Imaging: Results from the LARTIA Trial in Stage III NSCLC . J Thorac Oncol 2017. ; 12 ( 7 ): 1122 – 1130 . [DOI] [PubMed] [Google Scholar]
- 31. Persson E , Gustafsson C , Nordström F , et al . MR-OPERA: A Multicenter/Multivendor Validation of Magnetic Resonance Imaging-Only Prostate Treatment Planning Using Synthetic Computed Tomography Images . Int J Radiat Oncol Biol Phys 2017. ; 99 ( 3 ): 692 – 700 . [DOI] [PubMed] [Google Scholar]
- 32. Washio H , Ohira S , Funama Y , et al . Accuracy of dose calculation on iterative CBCT for head and neck radiotherapy . Phys Med 2021. ; 86 : 106 – 112 . [DOI] [PubMed] [Google Scholar]
- 33. Giacometti V , Hounsell AR , McGarry CK . A review of dose calculation approaches with cone beam CT in photon and proton therapy . Phys Med 2020. ; 76 : 243 – 276 . [DOI] [PubMed] [Google Scholar]
- 34. Hussein M , Heijmen BJM , Verellen D , Nisbet A . Automation in intensity modulated radiotherapy treatment planning-a review of recent innovations . Br J Radiol 2018. ; 91 ( 1092 ): 20180270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bohoudi O , Bruynzeel AME , Meijerink MR , et al . Identification of patients with locally advanced pancreatic cancer benefitting from plan adaptation in MR-guided radiation therapy . Radiother Oncol 2019. ; 132 : 16 – 22 . [DOI] [PubMed] [Google Scholar]
- 36. Chen H , Schneiders FL , Bruynzeel AME , et al . Impact of daily plan adaptation on organ-at-risk normal tissue complication probability for adrenal lesions undergoing stereotactic ablative radiation therapy . Radiother Oncol 2021. ; 163 : 14 – 20 . [DOI] [PubMed] [Google Scholar]
- 37. Bruynzeel AME , Tetar SU , Oei SS , et al . A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results . Int J Radiat Oncol Biol Phys 2019. ; 105 ( 5 ): 1086 – 1094 . [DOI] [PubMed] [Google Scholar]
- 38. Finazzi T , Haasbeek CJA , Spoelstra FOB , et al . Clinical Outcomes of Stereotactic MR-Guided Adaptive Radiation Therapy for High-Risk Lung Tumors . Int J Radiat Oncol Biol Phys 2020. ; 107 ( 2 ): 270 – 278 . [DOI] [PubMed] [Google Scholar]
- 39. Yang D , Kim H , Green OL , et al . Co-60 MR Guided Adaptive Radiation Treatment Improves Target Coverage and Organs-At-Risk Sparing: Dosimetric Analysis of 1185 Adaptive Fractions and 5 Years’ Experience . Int J Radiat Oncol Biol Phys 2020. ; 108 ( 3 Supplement ): e300 – e301 . [Google Scholar]
- 40. Schiff JP , Stowe HB , Price A , et al . In Silico Trial of Computed Tomography-Guided Stereotactic Adaptive Radiation Therapy (CT-STAR) for the Treatment of Abdominal Oligometastases . Int J Radiat Oncol Biol Phys 2022. ; 114 ( 5 ): 1022 – 1031 . [DOI] [PubMed] [Google Scholar]
- 41. Andersson L , Behrens CF , Serup-Hansen E , Sibolt P . Simulated Cone-Beam Computed Tomography Based Online Adaptive Radiotherapy of Anal Cancer — Potential Dosimetric Benefit . Int J Radiat Oncol Biol Phys 2021. ; 111 ( 3 ): e509 – e510 . [Google Scholar]
- 42. Calvo-Ortega JF , Moragues-Femenía S , Laosa-Bello C , Torices-Caballero J , Hermida-López M , Casals-Farran J . Clinical Experience in Prostate Ultrahypofractionated Radiation Therapy With an Online Adaptive Method . Pract Radiat Oncol 2022. ; 12 ( 2 ): e144 – e152 . [DOI] [PubMed] [Google Scholar]
- 43. Aluwini S , Pos F , Schimmel E , et al . Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial . Lancet Oncol 2015. ; 16 ( 3 ): 274 – 283 . [DOI] [PubMed] [Google Scholar]
- 44. Henke LE , Olsen JR , Contreras JA , et al . Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial . Adv Radiat Oncol 2018. ; 4 ( 1 ): 201 – 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tetar SU , Bohoudi O , Senan S , et al . The Role of Daily Adaptive Stereotactic MR-Guided Radiotherapy for Renal Cell Cancer . Cancers (Basel) 2020. ; 12 ( 10 ): 2763 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shelley CE , Barraclough LH , Nelder CL , Otter SJ , Stewart AJ . Adaptive Radiotherapy in the Management of Cervical Cancer: Review of Strategies and Clinical Implementation . Clin Oncol (R Coll Radiol) 2021. ; 33 ( 9 ): 579 – 590 . [DOI] [PubMed] [Google Scholar]
- 47. Gardner SJ , Mao W , Liu C , et al . Improvements in CBCT Image Quality Using a Novel Iterative Reconstruction Algorithm: A Clinical Evaluation . Adv Radiat Oncol 2019. ; 4 ( 2 ): 390 – 400 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Studenski MT , Delgadillo R , Xu Y , et al . Margin verification for hypofractionated prostate radiotherapy using a novel dose accumulation workflow and iterative CBCT . Phys Med 2020. ; 77 : 154 – 159 . [DOI] [PubMed] [Google Scholar]
- 49. Bootsma GJ , Verhaegen F , Jaffray DA . The effects of compensator and imaging geometry on the distribution of x-ray scatter in CBCT . Med Phys 2011. ; 38 ( 2 ): 897 – 914 . [DOI] [PubMed] [Google Scholar]
- 50. Stankovic U , Ploeger LS , van Herk M , Sonke J-J . Optimal combination of anti-scatter grids and software correction for CBCT imaging . Med Phys 2017. ; 44 ( 9 ): 4437 – 4451 . [DOI] [PubMed] [Google Scholar]
- 51. Zhao J , Chen Z , Wang J , et al . MV CBCT-Based Synthetic CT Generation Using a Deep Learning Method for Rectal Cancer Adaptive Radiotherapy . Front Oncol 2021. ; 11 : 655325 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marchant TE , Joshi KD , Moore CJ . Accuracy of radiotherapy dose calculations based on cone-beam CT: comparison of deformable registration and image correction based methods . Phys Med Biol 2018. ; 63 ( 6 ): 065003 . [DOI] [PubMed] [Google Scholar]
- 53. Liu Y , Lei Y , Wang T , et al . CBCT-based synthetic CT generation using deep-attention cycleGAN for pancreatic adaptive radiotherapy . Med Phys 2020. ; 47 ( 6 ): 2472 – 2483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai B , Laugeman E , Mazur TR , et al . Characterization of a prototype rapid kilovoltage x-ray image guidance system designed for a ring shape radiation therapy unit . Med Phys 2019. ; 46 ( 3 ): 1355 – 1370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rafic KM , Timothy Peace SB , Manu M , Arvind S , Ravindran BP . A rationale for cone beam CT with extended longitudinal field-of-view in image guided adaptive radiotherapy . Phys Med 2019. ; 62 : 129 – 139 . [DOI] [PubMed] [Google Scholar]
- 56. Alaei P , Spezi E . Imaging dose from cone beam computed tomography in radiation therapy . Phys Med 2015. ; 31 ( 7 ): 647 – 658 . [DOI] [PubMed] [Google Scholar]
- 57. Viswanathan AN , Dimopoulos J , Kirisits C , Berger D , Pötter R . Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours . Int J Radiat Oncol Biol Phys 2007. ; 68 ( 2 ): 491 – 498 . [DOI] [PubMed] [Google Scholar]
- 58. Rasch C , Barillot I , Remeijer P , Touw A , van Herk M , Lebesque JV . Definition of the prostate in CT and MRI: a multi-observer study . Int J Radiat Oncol Biol Phys 1999. ; 43 ( 1 ): 57 – 66 . [DOI] [PubMed] [Google Scholar]
- 59. Oderinde OM , Han C , Sun Z , et al . Feasibility and Dosimetric Benefits of Adaptive Planning in Prostate Cancer Radiotherapy Using a Novel Treatment Planning Machine with Integrated Dual kVCT/PET Imaging Systems . Int J Radiat Oncol Biol Phys 2022. ; 114 ( 3 Supplement ): e592 . [Google Scholar]
- 60. Olcott P , Khan S , Bal G , et al . PhD GK . BgRT Motion Management Maintains Target Dose Coverage for Respiratory and Non-Respiratory Motion . Int J Radiat Oncol Biol Phys 2022. ; 114 ( 3 Supplement ): S116 – S117 . [Google Scholar]