Abstract
Theranostics is the combination of two approaches—diagnostics and therapeutics—applied for decades in cancer imaging using radiopharmaceuticals or paired radiopharmaceuticals to image and selectively treat various cancers. The clinical use of theranostics has increased in recent years, with U.S. Food and Drug Administration (FDA) approval of lutetium 177 (177Lu) tetraazacyclododecane tetraacetic acid octreotate (DOTATATE) and 177Lu–prostate-specific membrane antigen vector-based radionuclide therapies. The field of theranostics has imminent potential for emerging clinical applications. This article reviews critical areas of active clinical advancement in theranostics, including forthcoming clinical trials advancing FDA-approved and emerging radiopharmaceuticals, approaches to dosimetry calculations, imaging of different radionuclide therapies, expanded indications for currently used theranostic agents to treat a broader array of cancers, and emerging ideas in the field.
Keywords: Molecular Imaging, Molecular Imaging–Cancer, Molecular Imaging–Clinical Translation, Molecular Imaging–Target Development, PET/CT, SPECT/CT, Radionuclide Therapy, Dosimetry, Oncology, Radiobiology
© RSNA, 2023
Keywords: Molecular Imaging, Molecular Imaging–Cancer, Molecular Imaging–Clinical Translation, Molecular Imaging–Target Development, PET/CT, SPECT/CT, Radionuclide Therapy, Dosimetry, Oncology, Radiobiology
Summary
Emerging approaches to theranostics, including investigational radiopharmaceuticals, expanded indications for current radionuclide therapies, and posttreatment imaging, are active areas of innovation with potential to transform clinical practice.
Essentials
■ Theranostics is a concept related to radionuclide therapy that specifically refers to the use of a pair of radiopharmaceutical agents containing radionuclides used for imaging (diagnostics) and/or therapy (therapeutics).
■ α Particles have high linear energy transfer and enable increased precision in radiation delivery, which theoretically decreases collateral damage to adjacent healthy tissues and may facilitate more focused targeting of small tumors and micrometastasis relative to the same dose of β-particle emitters required to achieve similar cytotoxic effects.
■ The imaging of theranostic agents can potentially serve multiple independent purposes, including patient selection for therapy, confirmation of delivery to tumors, dosimetry calculation, and treatment response assessment.
■ Both tetraazacyclododecane tetraacetic acid octreotate (or, DOTATATE)– and prostate-specific membrane antigen–coupled agents that now have regulatory approval for clinical use in neuroendocrine tumors and prostate cancer, respectively, are being investigated for expanded indications in other tumor classes.
Background
Radionuclide therapy refers to the application of an unstable nuclide, coupled with a targeting vector, to selectively deliver therapeutic radiation to cancer cells. The molecular targeting mechanism and the type of ionizing radiation emission vary for different radiopharmaceuticals and may have important implications for the benefits and limitations of these agents.
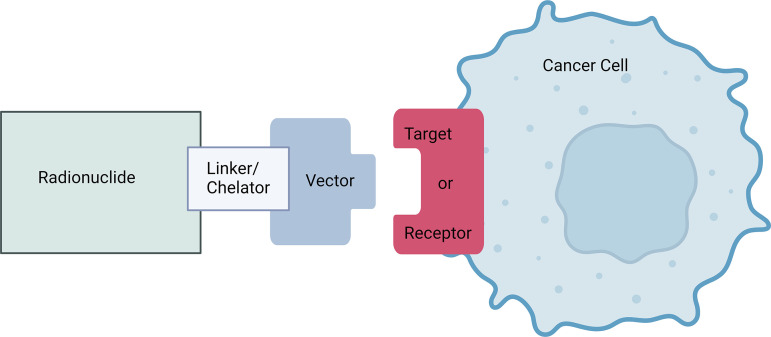
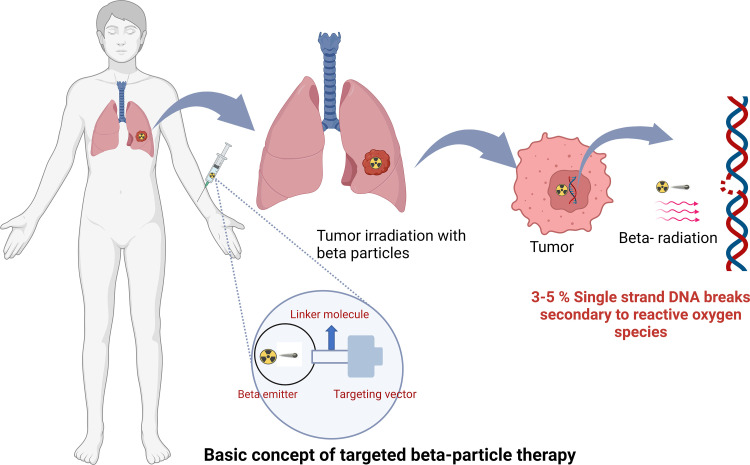
Theranostics is a concept related to radionuclide therapy, but it specifically refers to the use of a pair of radiopharmaceuticals containing radionuclides for imaging (diagnostics) and/or therapy (therapeutics) (1). Radionuclides can be attached to a vector (eg, small molecule, peptide, antibody, particle) through a linker molecule, and the vector, also referred to as a ligand, binds to a target (eg, cell surface receptor). The radiopharmaceutical location can be imaged using a SPECT or PET scanner, with the standardized uptake value used as a measure of the amount of radiopharmaceutical in each location of the body. Ideally, the radiopharmaceutical is mostly bound to the target (minimal off-target binding) or has exited the body, with trace amounts circulating in the blood. These images allow the visualization of the target for diagnosis and staging, the selection of patients whose tumors express the target, confirmation of therapeutic delivery to all intended targets in a patient, the calculation of effective radiation delivered to the target (ie, dosimetry), and follow-up treatment monitoring (Fig 1) (2). The term theragnostics (from the Greek word gnosis, meaning knowledge), which combines the words therapy and diagnosis, is sometimes used (3), but the term theranostics is now more common.
Figure 1:
Radionuclide therapy schematic. A radionuclide held in a chelator or cage or bound covalently is attached to a vector by a linker molecule. The vector binds to a molecular target to enable visualization of the target for diagnostic or treatment purposes and selective delivery of radiation therapy to the target. Alternatively, a free radionuclide ion can, in some circumstances, be used to target tumors or cancer cells, as with iodine 131, alastine 211, and radium 223. Created with BioRender.com.
This concept has been used for many decades in nuclear medicine, starting with the use of radioiodine pharmaceuticals for thyroid cancer in 1946 (4). Ideally, the diagnostic and therapeutic radiopharmaceuticals are structurally identical (a perfect theranostic pair), but this is often not the case. Most radiopharmaceuticals lack emission properties suitable for both diagnostic imaging and therapy, thus necessitating a diagnostic agent and therapeutic agent pair that bind to the same molecular target but are composed of two different molecular structures and radionuclides. Even slight differences in chemical structure from different radionuclides can lead to differences in biodistribution to target tumors, off-target binding, and clearance.
Radionuclides: Particle Emission and Relevant Types of Radiation
The types of emission from a given radionuclide are critical to understanding that radionuclide’s potential therapeutic role.
β-Particle emission occurs primarily in radionuclides with a neutron excess undergoing β-minus decay and is the most used mechanism in radionuclide therapy because of the high availability of these radionuclides (eg, radioiodine). β Particles have a negative charge, low linear energy transfer (LET, approximately 0.2 keV/µm), and a long travel distance of approximately 2–12 mm (20 to 120 cell lengths) (5,6). Clinically used radionuclides that emit a β particle include lutetium 177 (177Lu), iodine 131 (131I), and yttrium 90 (90Y), listed from lowest to highest maximum energy emission and path length (7).
An α particle is positively charged and is almost three orders of magnitude bigger than a β particle. As a result, α particles have a much higher LET (80 keV/µm) compared with that of β particles and travel a much shorter distance of 50–100 µm (one to three cell lengths) (6).
Auger electrons are low-energy electrons emitted by radionuclides when they decay by electron capture and, in doing so, eject an electron from an electron orbit around the nucleus. Auger electrons travel an extremely short distance, in the nanometer to micrometer range. They are a form of β-particle emission; however, the electron is not emitted from the nucleus.
Biologic Effects of Radiation Types
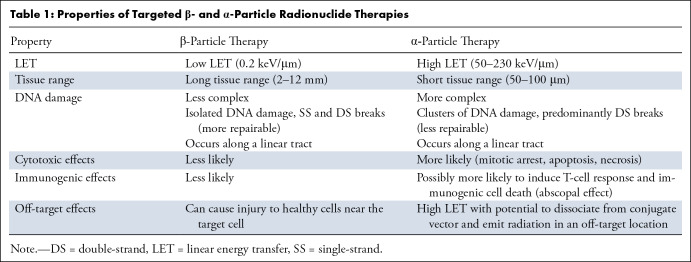
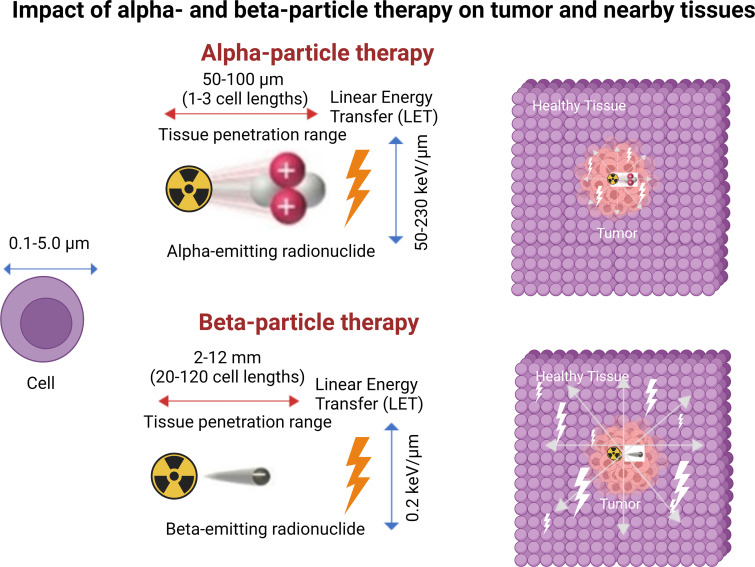
Radiopharmaceuticals can exert antitumoral biologic effects through multiple mechanisms, such as direct effects via DNA damage to a targeted cell, crossfire effects to adjacent tumor cells, and indirect effects to adjacent tumor cells through generation of reactive oxygen species and/or induction of immunogenic cell death. Relative to α particles, the longer travel distance of β particles results in a crossfire effect that is advantageous in large tumor masses with intralesional heterogeneity, allowing for irradiation of all tumor cells, even those that do not express the radiopharmaceutical target (5). β Particles exert their therapeutic effects through the generation of reactive oxygen species that cause DNA damage via single-strand DNA breaks. Such damage can elicit a cell death response or be repaired via DNA repair mechanisms. As this mechanism is dependent on the presence of oxygen, β particles potentially have lower efficacy in the treatment of ischemic tumors because of hypoxemia-induced cellular defense mechanisms (Table 1, Figs 2–4) (5). In contrast, α particles are more cytotoxic than β particles because they cause irreparable double-strand DNA breaks. Moreover, α particles incite a stronger antigen T-cell immune response (abscopal effect), are oxygen independent, and generate greater ionization events and reactive oxygen species compared with β particles (8). The high LET of α particles and increased precision of radiation delivery theoretically decrease collateral damage to adjacent healthy tissues and may facilitate more focused targeting of small tumors and micrometastasis relative to the same dose of β emitters required to achieve similar cytotoxic effects. Furthermore, the therapeutic efficacy of α-particle therapy is less dependent on the presence of oxygen, implying a theoretical advantage in hypoxic tumor microenvironments (Table 1, Figs 2–4) (6). Auger electrons travel a very short distance, on the order of the size of the cell nucleus, and could theoretically be highly lethal to tumor cells if incorporated into the cell DNA. This short range may be particularly suited for small tumors in sensitive locations, when restricting toxicity to adjacent normal tissue is especially critical (9). Despite these theoretical advantages, there has not been widespread adoption of this class of radionuclide therapy outside of limited preclinical studies, likely because of its need to reach the cell nucleus to cause DNA damage and achieve lethal cytotoxic effects.
Table 1:
Properties of Targeted β- and α-Particle Radionuclide Therapies
Figure 2:
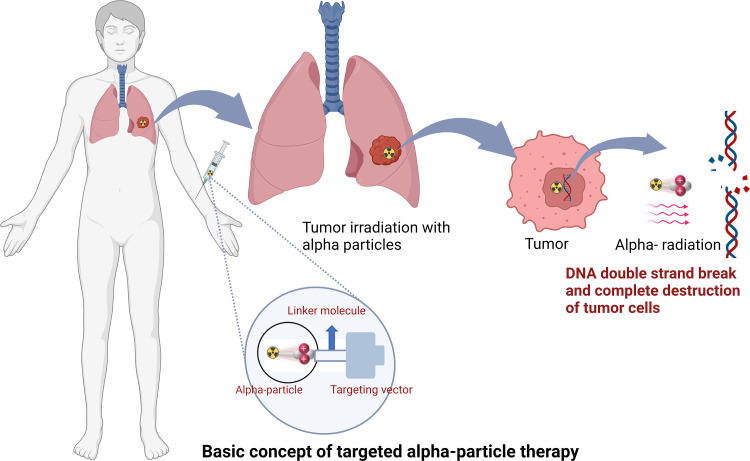
Targeted α-particle therapy schematic. The radionuclide therapy is intravenously infused and binds to the tumor through a vector linked to the radionuclide. While bound to the tumor, α-particle emission occurs, selectively delivering radiation to the tumor. α Particles are more cytotoxic than β particles because they cause irreparable double-strand DNA breaks, resulting in cell death. Created with BioRender.com.
Figure 4:
Targeted α-particle and β-particle therapy comparison. Illustration shows the characteristic features of α and β particles. α Particles are positively charged particles composed of two protons and two neutrons, essentially the nucleus of a helium atom, and β particles are negatively charged particles, essentially electrons. α Particles have much greater mass, higher linear energy transfer (LET), travel a much shorter distance in tissue, and are more cytotoxic than β particles. The illustration includes specific values of these characteristics for reference but is not to scale. Created with BioRender.com.
Figure 3:
Targeted β-particle therapy schematic. While bound to the tumor, β-particle emission occurs, selectively delivering radiation to the tumor. β Particles exert therapeutic effects through reactive oxygen species, causing DNA damage via single-strand DNA breaks that may result in cell death if not repaired via DNA repair mechanisms. Created with BioRender.com.
Patient-specific Dosimetry
Radiation dosimetry is the science by which the amount of radiation energy deposited per unit mass in tissues can be calculated. By determining the biodistribution of a radiopharmaceutical and dose delivered to healthy tissues, dose calculations can be used to establish radiopharmaceutical safety (10). Dosimetry calculations could also be applied in the posttreatment setting to verify the activity delivered to the targeted tumors and record the dose to critical organs. A critical organ is the organ most susceptible to the effects of a specific radiopharmaceutical and may be the dose-limiting factor in the amount of therapeutic radionuclide activity administered (11).
Internal dosimetry methods have been described in detail by the Medical Internal Radiation Dose (MIRD) Committee and rely on the use of a time-integrated function incorporating the source organ activity, target organ mass, cumulated activity in each source organ, and the fraction of source organ energy emission that is absorbed by target organs. Calculation of the energy absorbed by target organs can be simplified with S-factors based on computational phantoms (10,11).
A relatively simple dosimetry example is the calculation of the minimal administered activity of therapeutic sodium iodide (Na131I) for remnant ablation following thyroidectomy. This approach involves administering a trace amount of Na131I, serial imaging with a γ camera or SPECT at multiple time points to produce a time-activity curve for a region of interest including the thyroidectomy bed, deriving the effective half-life for the thyroid remnant, and determining the initial activity in the lesion. These parameters could be used in a formula to calculate the minimum activity of Na131 I needed to deliver 300 Gy to the thyroid remnant (the minimum required for adequate ablation), allowing the calculation of a personally optimized dose (12,13).
Dosimetry calculations could also be performed in the pretreatment planning phase to maximize the therapeutic radionuclide activity that can safely be administered, within the dose limits to critical organs. 131I-metaiodobenzylguanidine (131I-MIBG) therapy is another example of radionuclide therapy dosimetry adopted in clinical practice. Dosimetry is required for 131I-MIBG therapy for metastatic pheochromocytomas and paragangliomas in the U.S. Food and Drug Administration (FDA) package insert. If critical organ dose estimates are above threshold, based on MIRD calculations from images after the initial dose, reductions in the administered activity are made for the second dose (14).
The biodistribution of a therapeutic radiopharmaceutical can differ between patients because of the variable size and location of tumors, which accumulate radionuclides and emit radiation. Despite this variability, many of the radionuclide therapies discussed in this article are administered in a set quantity in clinical practice. This framework substantially differs from the treatment model of external beam radiation therapy or brachytherapy delivery, where treatment planning is the standard of care and patient-specific dosimetry is used to estimate the absorbed dose to the targeted treatment field and to organs at risk (10). Dosimetry calculation is more complex for radionuclide therapy than for external beam radiation therapy because of a heterogeneous biodistribution of the radiopharmaceuticals, varied pharmacokinetics, and different mechanisms of particle-associated cytotoxicity (10,11,15). As a result, highly accurate dosimetry for longer-lived radiopharmaceuticals may require imaging at time points over multiple days. Calculation accuracy is also strongly dependent on the calibration of equipment, including dose calibrators and imaging systems (SPECT and PET), which have quantitative capabilities that are heavily influenced by reconstruction parameters and corrections for attenuation and scatter.
Radionuclide Therapy: Current Clinical Practice and Future Directions
In current clinical practice, several theranostic pairs are widely used. Thyroid cancer is treated with Na131I and imaged with iodine 123 (123I) or technetium 99m (99mTc) pertechnetate, both relying on the Na-I symporter as the target that transports the radiopharmaceuticals into cells (16). Osteoblastic metastases in prostate cancer can be imaged with 99mTc-medronate or fluorine 18 (18F)–sodium fluoride (ie, NaF), which rely on affinity for hydroxyapatite and increased bone turnover, and treated with radium 223–dichloride (223RaCl2), strontium 89–chloride (ie, 89SrCl), or samarium 153–ethylenediaminetetramethylene phosphonate (ie, 153Sm–EDTMP), which do not target cancer cells directly but instead target the environment of the tumor in the bone (17). Norepinephrine transporter–expressing neuroendocrine neoplasms, including paraganglioma and pheochromocytoma, can be treated with 131I-MIBG and imaged with 123I-MIBG (18).
In recent years, the use of targeted radionuclide therapy in clinical practice has greatly expanded. The recent regulatory approval of multiple radionuclide therapies indicates that this is an area with momentum toward growing clinical adoption, which has found success in the setting of multiple types of malignancies.
223RaCl2 Therapy
In 2013, 223RaCl2 became the first α particle–emitting radiopharmaceutical to be granted FDA approval for clinical use and was indicated for patients with castration-resistant prostate cancer with symptomatic bone metastases and no known visceral metastatic disease (19). 223RaCl2 is a calcium analog that binds avidly to bone matrix with high bone turnover and osteoblastic activity. The naked, unstable radionuclide incorporates into the extracellular environment, and the α particle emission in the tumor microenvironment suppresses abnormal bone formation and induces cell death (20). The phase 3 ALSYMPCA (or, ΑLpharadin in SYMPtomatic Prostate CAncer) trial demonstrated that 223RaCl2 was well tolerated and improved median overall survival to 14.9 months in the treatment arm versus 11.3 months in the placebo arm (30% risk reduction; hazard ratio, 0.70 [95% CI: 0.58, 0.83]; P < .001); it also improved the median time to first symptomatic skeletal event to 15.6 months in the radium arm versus 9.8 months in the placebo arm (34% risk reduction; hazard ratio, 0.66 [95% CI: 0.52, 0.83]; P < .001) (19).
Somatostatin Receptor–targeted Radionuclide Therapy
Somatostatin receptors (SSR), particularly SSR2a, expressing neuroendocrine tumors (NETs) of the pancreas and midgut can be imaged with gallium 68 (68Ga) DOTA peptides (DOTATATE [tetraazacyclododecane tetraacetic acid octreotate], DOTANOC [tetraazacyclododecane tetraacetic acid sodium triiodide octreotide], and DOTATOC [tetraazacyclododecane tetraacetic acid D-phenylalanine 1 tyrosine 3 octreotide]), indium 111 (111In) octreotide, or 99 mTc-octreotide and treated with 177Lu-DOTA-peptides or yttrium 99 octreotate (21) as a theranostic pair. The pivotal Neuroendocrine Tumors Therapy (NETTER)–1 phase 3 trial of 177Lu-DOTATATE in patients with advanced midgut NETs demonstrated increased progression-free survival at 20 months of 65.2% versus 10.8% in the control, as well as a significantly increased tumor response rate with 177Lu-DOTATATE (22). In 2018, FDA approval of 177Lu-DOTATATE for NETs represented a major addition to the treatment options for patients with these tumors (21). Subsequently, the NETTER-1 trial did not demonstrate a statistically significant increase in overall survival at 5 years, complicated by high crossover within the randomized control group (36% of the controls subsequently received SSR-coupled radionuclide therapy) (23). Further research and innovation in radionuclide therapy are merited for patients with advanced midgut NETs.
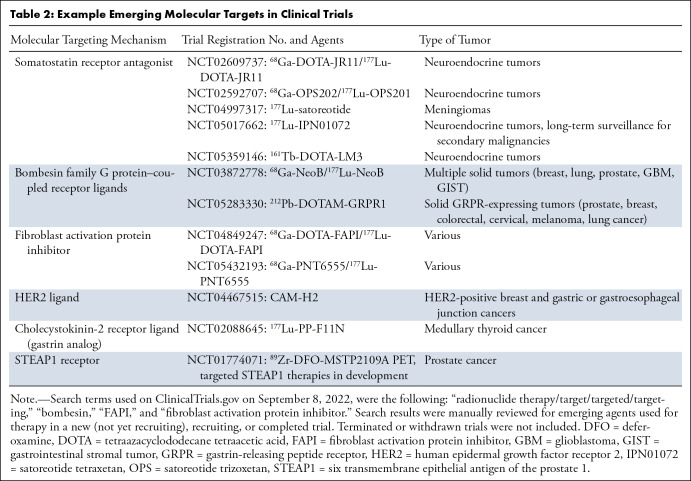
Emerging SSR-targeted radionuclide therapies are under investigation. A recent phase 2 clinical trial investigated the use of an SSR-targeted α-particle therapy using actinium 225 (225Ac) DOTATATE, demonstrating promising response and progression-free survival outcomes for gastroenteropancreatic NETs. Preclinical and early clinical studies have suggested that SSR2 antagonists may have greater receptor binding density, greater tumor-to-background ratio, and greater lesion detection compared with that of agonists (24,25). The ability of the antagonists to bind more receptors may facilitate increased sensitivity and allow treatment of tumors with lower receptor density (24). Table 2 lists five example registered trials using investigational theranostic pairs based on SSR antagonists for SSR-expressing NETs and meningiomas, an active area of innovation in radionuclide therapy. The investigational use of SSR-coupled radionuclide therapy earlier in the treatment course is also an area of interest. The NETTER-2 trial will assess 177Lu-DOTATATE as an upfront treatment by randomizing untreated patients with metastatic grade 2–3 NETs to treatment with 177Lu-DOTATATE plus long-acting octreotide or high-dose long-acting octreotide alone, and accrual goals were met in October 2022 (26).
Table 2:
Example Emerging Molecular Targets in Clinical Trials
Prostate-specific Membrane Antigen–targeted Radionuclide Therapy
Prostate-specific membrane antigen (PSMA)–coupled radiopharmaceuticals target PSMA, a transmembrane protein expressed in prostate cancer. The phase 2 TheraP trial of 177Lu-PSMA-617 demonstrated higher prostate-specific antigen (PSA) response compared with that of cabazitaxel for progressive, metastatic castration-resistant prostate cancer (mCRPC) (27). Subsequently, the landmark phase 3 VISION trial (ClinicalTrials.gov no. NCT03511664) showed that participants receiving 177Lu-PSMA-617 had increased progression-free survival (based on imaging) and overall median survival (15.3 months vs 11.3 months) compared with participants receiving standard-of-care treatment alone (28). 177Lu-PSMA-617 was recently FDA approved for mCRPC, paired with diagnostic imaging using 68Ga-PSMA-11 PET. 18F-DCFPyL PET has also been used in the clinical research setting as the PSMA-targeted diagnostic pair (29,30).
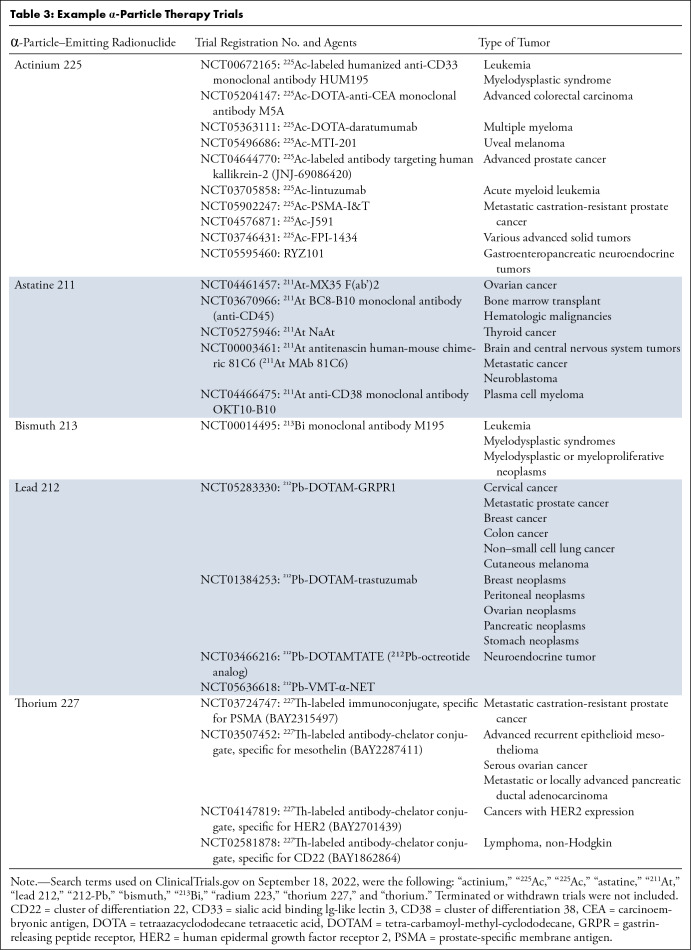
PSMA-coupled radionuclide therapy is an area of active clinical innovation. Strong interest in developing PSMA-coupled targeted α-particle therapy has emerged, supported by an initial report in 2016 demonstrating a dramatic treatment response by using 225Ac-labeled PSMA ligands (225Ac-PSMA-617) in patients with mCRPC (31). Figure 5 shows a clinical example of a patient with mCRPC responding dramatically to 225Ac-PSMA-617 following progression with multiple treatment modalities, including the α-particle therapy 223RaCl2. Since the initial report of 225Ac-PSMA-617 therapy (31), multiple studies have suggested that 225Ac-PSMA-617 is an efficacious and safe treatment option. A recent systematic review and meta-analysis comprising 256 patients treated with 225Ac-PSMA-617 showed overall biochemical response in 62.8% (95% CI: 53.4%, 71.7%) of patients, molecular response at 68Ga-PSMA-11 PET/CT in 74% (95% CI: 50.1%, 92.1%) of patients, and pooled estimates of median progression-free survival and overall survival of 9.1 months (95% CI: 3.6, 14.5) and 12.8 months (95% CI: 4.5, 21.0), respectively (32). Future randomized controlled trials are needed to further prove the therapeutic efficacy and survival benefit of 225Ac-PSMA-617. Ongoing phase 1 and 2 trials and registry data are currently active and recruiting patients (Table 3). Currently, 225Ac-PSMA-617 does not have FDA approval, has risks of adverse effects such as substantial xerostomia (31), and has not yet been investigated in a phase 3 trial. Other α-particle emitters investigated in preclinical and clinical studies, each offering different advantages and disadvantages, are summarized in Table 3 (6). While 177Lu-PSMA-617 has regulatory approval for patients with mCRPC, its investigational treatment of local-regional disease in the neoadjuvant setting has been evaluated in the recently completed LuTectomy trial (ClinicalTrials.gov registration no. NCT04430192). This trial and future clinical studies using radionuclide therapy for ablation of the primary tumor site will be informative about the potential for this targeted treatment at earlier stages of disease.
Figure 5:
Targeted α-particle therapy: radium 223 dichloride (223RaCl2) followed by actinium 225 (225Ac) prostate-specific membrane antigent (PSMA)–617. Images in a 66-year-old man with widely metastatic prostate cancer, with a Gleason score of 8 (4 + 4), that progressed following androgen deprivation, chemotherapy, and pelvic radiation. (A) Baseline gallium 68 (68Ga) PSMA-11 PET/CT image demonstrates intense PSMA uptake in numerous metastatic lesions (blue arrows indicate examples in the right scapula and both femurs). (B) 68Ga-PSMA-11 PET/CT image following three cycles of 223RaCl2 (a targeted α-particle therapy that incorporates within osteoblastic lesions but does not directly bind to cancer cells) shows progression of many osseous metastases (blue arrows), and the prostate-specific antigen (PSA) value increased. (C) Lutetium 177 (177Lu) PSMA-617 and 225Ac-PSMA-617 were both considered as treatment options. Following multidisciplinary discussion and shared decision-making with the patient, four cycles of 225Ac-PSMA-617 (a targeted α-particle therapy with affinity for PSMA) were administered 6 weeks apart, demonstrating dramatic response in the metastatic lesions, with near resolution of PSMA uptake at 68Ga-PSMA-11 PET/CT (blue arrows in the location of previous lesions) and a corresponding dramatic reduction in PSA.
Table 3:
Example α-Particle Therapy Trials
Imaging in Theranostics
Imaging theranostic agents can potentially serve multiple independent purposes, including patient selection for therapy, confirmation of delivery to tumors, dosimetry calculation, and treatment response assessment.
Patient Selection
For a theranostic pair of agents, imaging of the diagnostic agent is used to visualize the treatment target for patient selection purposes and confirm that the tumor expresses the target (33). For example, 68Ga-DOTATATE PET/CT is used to identify SSR-expressing disease in metastatic NETs. The degree of uptake is assessed with a Krenning score to select appropriate patients for 177Lu-DOTATATE radionuclide therapy (34). Similarly, 68Ga-PSMA-11 PET/CT is used to confirm PSMA-avid targets prior to initiation of 177Lu-PSMA-617 therapy. PET images are also carefully reviewed to confirm that all known or suspected live tumors express the target and show uptake.
Imaging to Monitor Therapy Response
Following treatment with radionuclide therapy, current guidelines support the use of anatomic imaging for response assessment with anatomic imaging modalities (35–37). Importantly, anatomic imaging may characterize tumors that do not express the radionuclide therapy target. For gastroenteropancreatic NETs following treatment with 177Lu-DOTATATE, anatomic imaging every 3–12 months is recommended by the National Comprehensive Cancer Network, using CT of the abdomen and pelvis with intravenous contrast media, CT of the chest with or without contrast media, and MRI of the liver for hepatic-dominant disease. Guidelines support the use of molecular imaging with SSR-targeted PET for tumor response assessment in limited scenarios, including when other evidence of progression is present, to evaluate equivocal anatomic imaging findings, and in the setting of stable disease 9–12 months after 177Lu-DOTATATE therapy completion (38). For metastatic prostate cancer treatment with 177Lu-PSMA-617 and other systemic therapies, current guidelines support the use of anatomic imaging with CT or MRI and/or 99 mTc-medronate bone scans for restaging (35). Consensus recommendations for PSMA-ligand PET/CT for response assessment have emerged recently (37). Following radionuclide therapy, scintigraphy of 177Lu-PSMA-617 performed 0–3 days after administration is recommended to confirm delivery of the therapeutic radionuclide to the targeted tumors (39). The response to therapy can also potentially be assessed with "therapy-monitoring" imaging of the therapeutic radionuclide with each cycle of therapy. For example, SPECT/CT imaging of 177Lu-PSMA-617 at approximately 24 hours after infusion can demonstrate changes in tumor uptake of the therapeutic radionuclide (Fig 6).
Figure 6:
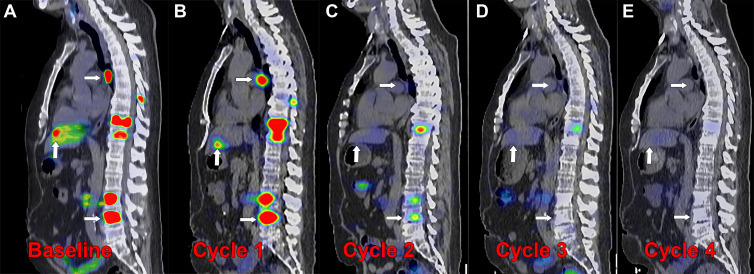
Posttherapy monitoring of index lesions with SPECT/CT imaging. Fused SPECT/CT sagittal images in a 56-year-old man with prostate-specific membrane antigen (PSMA)–avid metastatic prostate cancer undergoing lutetium 177 (177Lu) PSMA-617 therapy. (A) Baseline fluorine 18 (18F) carboxy-fluoro-pyridine-carbonyl-amino-pentyl-ureido-pentanedioic acid (DCFPyL) PET/CT image demonstrates intense PSMA uptake in nodal, osseous, and hepatic metastases (arrows). (B–E) Posttherapy SPECT/CT image with 177Lu-PSMA-617 was performed approximately 24 hours after infusion of the therapeutic radiotracer after each of four cycles administered 6 weeks apart, demonstrating localization of the therapeutic radiopharmaceutical to the metastases. Index lesions in lymph node, bone, and liver (arrows) demonstrate decreased intensity of uptake with each cycle of therapy. (C) After cycle 2, the hepatic and nodal metastases were no longer conspicuous, and (E) after cycle 4, the spine metastasis was no longer conspicuous. Imaging the therapeutic radionuclide enables confirmation of effective delivery to the sites of cancer and detection of treatment response over the course of therapy, demonstrated by the changes in the imaged index lesions over time.
A 177Lu-PSMA-617 and NOX66 combination trial (LuPIN) found that quantitative assessment of changes in radioactive total tumor volumes between baseline imaging and cycle 3 of posttherapy SPECT/CT are predictive of progression-free survival (40). This technique has the potential to generate useful quantitative biomarkers for predicting response to radionuclide therapy and may be applied to other theranostic pairs. Unanswered questions remain regarding the use of additional cycles of therapy following tumor progression or complete response as assessed with posttherapy SPECT/CT. Evidence supporting clinical benefit of 177Lu-PSMA-617 therapy is based on the VISION trial, which used a regimen of four to six cycles (28) with no SPECT/CT imaging; however, some patients may experience an early complete response (Fig 7), and at present, the benefit of completing further cycles of therapy remains uncertain.
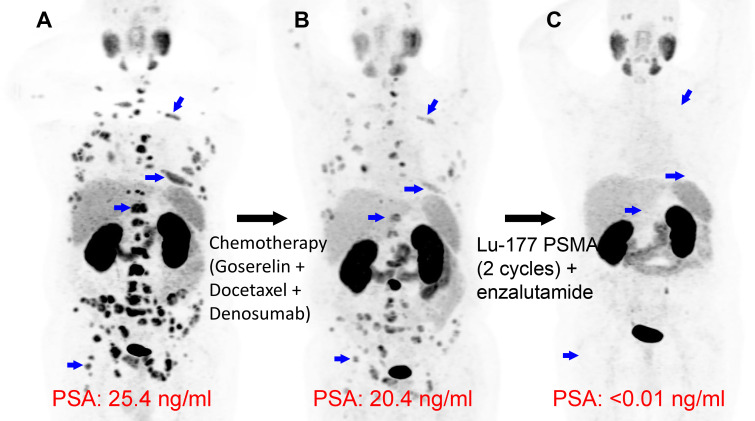
Figure 7:
Complete treatment response after two cycles of lutetium 177 (177Lu) prostate-specific membrane antigen (PSMA)–617 therapy. Images in a 63-year-old man with widely metastatic prostate cancer, with a Gleason score of 9 (4 + 5). (A) Gallium 68 (68Ga) PSMA-617 PET/CT maximum intensity projection image demonstrates widespread metastatic disease, such as numerous bone lesions (blue arrows indicate select examples). (B) Following chemotherapy and hormonal therapy, persistent PSMA-avid metastatic lesions are observed on 68Ga-PSMA-11 PET/CT image, with slight reduction in prostate-specific antigen level. (C) Following two cycles of 177Lu-PSMA-617, there is complete resolution of metastatic PSMA uptake, with undetectable PSA. Blue arrows in the expected location of previous nodal uptake show the resolved lesions on 68Ga-PSMA-11 PET/CT image.
Expanded Indications to Other Classes of Tumors for Currently Available Targeted Radioligand Therapies
While radioligand therapies binding new molecular targets or using more efficacious radionuclides will be necessary for the field of theranostics to reach its full potential, the most direct route for growth in this field is by identifying additional uses for currently available radiopharmaceuticals. Both DOTATATE- and PSMA-coupled radiopharmaceuticals that now have regulatory approval for clinical use in NETs and prostate cancer, respectively, are being investigated for expanded indications for other classes of tumors.
177Lu-DOTATATE Therapy
SSRs are expressed in a variety of neoplasms other than gastrointestinal NETs, as well as nonneoplastic processes (41). While the investigation of therapeutic intervention has lagged behind that of imaging studies, ongoing clinical trials are using 177Lu-DOTATATE for the treatment of malignancies as diverse as glioblastoma, esthesioneuroblastoma, breast cancer, small cell lung cancer, and Merkel cell carcinoma. Paraganglioma and meningioma, two areas of substantial interest for 177Lu-DOTATATE therapy, are discussed below.
Paragangliomas and pheochromocytomas.— Paragangliomas and pheochromocytomas express SSRs, are 68Ga-DOTATATE avid at PET, and may be a treatment target for DOTATATE-coupled radionuclide therapies (Fig 8) (42,43). Treatment options for both entities are limited in patients with metastatic and inoperable tumors. 123I-MIBG and 131I-MIBG have been used as a theranostic pair in combination with systemic chemotherapy for paragangliomas and pheochromocytomas, but this regimen has often failed to result in sustained remission, and bone marrow suppression is a critical disadvantage. Several relatively small clinical studies have investigated targeted radioligand therapies, including 177Lu- or 90Y-DOTATATE and -DOTATOC, in paragangliomas of the head, neck, abdomen, and urinary bladder (43–48). These early clinical studies have predominantly included inoperable paragangliomas, documenting stable disease and treatment response in many patients. SSR-targeted radionuclide therapies are reasonable therapies in such patients with limited alternative options, and 177Lu-DOTATATE is a recommended treatment in the National Comprehensive Cancer Network guidelines for metastatic paragangliomas and pheochromocytomas (49). Future studies will need to examine the efficacy of this therapy in larger cohorts, such as the forthcoming 177Lu-DOTATATE in Therapy of Inoperable Pheochromocytoma/ Paraganglioma study (ClinicalTrials.gov no. NCT03206060). Further studies are also needed to determine the long-term outcomes of 177Lu-DOTATATE, assess the impact of molecular subtypes of paragangliomas on treatment response, compare different radionuclides, and compare the therapy with other treatment modalities. For example, 177Lu-DOTATATE may be better suited for small and medium-sized tumors, whereas 90Y-peptide–receptor radionuclide therapy may be preferred for larger tumors (7,50). Recently, a high specific activity formulation of 131I-MIBG (iobenguane) has become available that is thought to have an improved adverse effect profile compared with older formulations and has received FDA approval for use in unresectable, metastatic pheochromocytomas and paragangliomas (14,51). Based on expert consensus, 177Lu-DOTATATE may be favored in patients with poor bone marrow reserve because of differences in toxicity, and high specific activity 131I-MIBG may be favored in patients at greater risk for catecholamine crisis (51). Comparative studies between these agents will be needed in the future (14). At present, a theranostic strategy may inform the choice of radionuclide therapy on a case-by-case basis by using the diagnostic agent pair to assess the degree of binding of MIBG compared with that of DOTA-based peptides.
Figure 8:
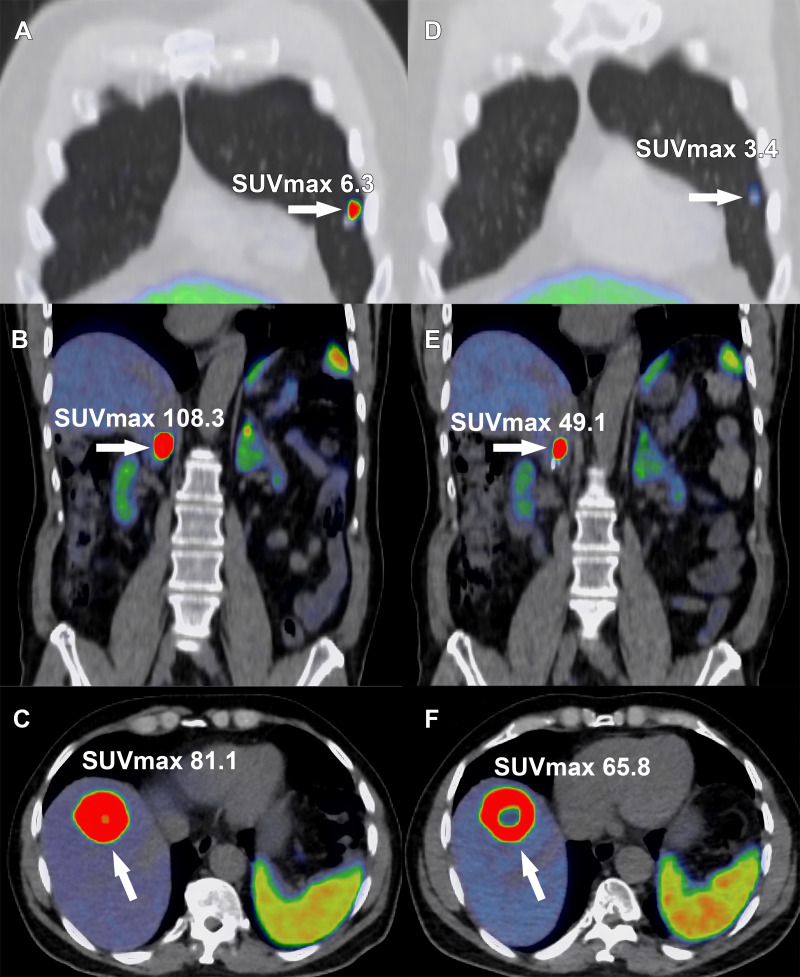
Images in a 68-year-old man with metastatic pheochromocytoma treated with four cycles of lutetium 177 (177Lu) tetraazacyclododecane tetraacetic acid octreotate (DOTATATE). A right adrenal pheochromocytoma was resected 2 years previously, with subsequent development of hepatic, osseous, and pulmonary metastasis and inferior vena cava (IVC) tumor thrombus. Selected gallium 68 (68Ga) DOTATATE PET/CT fusion images show a DOTATATE-avid (A) pulmonary metastasis (arrow), (B) IVC tumor thrombus (arrow), and (C) hepatic metastasis (arrow). Following completion of off-label 177Lu-DOTATATE therapy, (D) the pulmonary metastases (arrow), (E) IVC tumor thrombus, and (F) hepatic metastasis decreased in radiotracer uptake level. Central photopenia indicating tumor necrosis increased with the treated hepatic metastasis (E, arrow). SUVmax = maximum standardized uptake volume.
Meningiomas.— Meningiomas are classically 68Ga-DOTATATE avid and potentially serve as an ideal treatment target for radionuclides coupled to DOTATATE. Treatment of meningiomas with β-emitting SSR-targeted radiopharmaceuticals has been described in numerous reports of treatment-refractory meningiomas (52–54). These preliminary reports suggest that treatment is well tolerated and appears to have some antitumor activity. Forthcoming clinical trials (ClinicalTrials.gov nos. NCT03971461 and NCT04082520) will provide future information about the efficacy of this treatment strategy.
177Lu-PSMA-617 Therapy
Despite its name, PSMA expression is not truly prostate-specific and is observed in many other neoplasms. For many of these tumors, PSMA expression is found in the associated neovasculature rather than in the tumor cells themselves (55). However, a few other nonprostate tumors, such as adenoid cystic carcinoma, a salivary gland neoplasm, show high PSMA expression on tumor cells (56). A phase 2 clinical trial assessing 177Lu-PSMA-617 treatment of advanced salivary gland cancers (ClinicalTrials.gov no. NCT04291300) is currently enrolling patients, and a small of number of patients treated with 177Lu-PSMA-617 thought to be targeting neovascular PSMA expression in glioblastoma, thyroid cancer, and hepatocellular carcinoma have been reported (57–60).
Re-treatment
The official FDA labeling for 177Lu-DOTATATE therapy consists of a fixed four-dose series, each dose separated by 8 weeks. Similarly, the regimen for 177Lu-PSMA-617 is up to six doses separated by 6 weeks each. However, even after completion of a standard course of therapy, clinicians have re-treated patients off-label with encouraging results that have been described in multiple retrospective series for 177Lu-DOTATATE (61–64). A systematic meta-analysis of 177Lu-DOTATATE re-treatment in patients with NETs included 13 studies. The median progression-free survival time was 12.52 months, and median overall survival was 26.78 months. Only two patients with therapy-related myeloid neoplasms were reported (65). Overall, in patients with favorable responses to initial 177Lu-DOTATATE for NETs, repeat treatment appears to be a reasonable option in the absence of alternatives, albeit based on mostly retrospective data. For 177Lu-PSMA-617, a phase 2 trial re-treated 30% of the patients with subsequent progression of PSMA-positive disease, of which 73% experienced a PSA decline of 50% or greater with few grade 3 or 4 adverse events (66).
Investigational Uses of Dosimetry in Radionuclide Therapy
Dosimetry has potential investigational applications for 177Lu-coupled radiopharmaceuticals. 177Lu gives off a β-particle emission, which is used to kill cancer cells, but also gives off a γ-photon emission and thus can be imaged. As demonstrated in some reports, multiple-time-point imaging of 177Lu-DOTATATE with planar whole-body γ-camera protocols and SPECT/CT can provide quantitative assessments of the radionuclide distribution within individual patients over time, permitting personalized dosimetry calculations for tumors and critical organs (eg, kidney). In theory, knowledge of the dose delivered could be used to appropriately adjust the activity of subsequent cycles and predict outcomes.
A notable example in the literature is the use of sodium–iodine 124 (124I) PET imaging for thyroid cancer lesional dosimetry calculations to predict which patients would respond to Na131I radioablation following sensitization with selumetinib (67). Other studies of Na124I for thyroid cancer dosimetry have shown that the maximal dose could be increased using this approach (68); however, Na124I does not have FDA regulatory approval and has not been implemented for routine clinical use.
177Lu-DOTATATE Dosimetry
177Lu-DOTATATE dosimetry is not yet routinely performed in clinical practice. 177Lu-DOTATATE is typically administered as a fixed-activity regimen of 7.4 MBq (200 mCi) in four therapeutic infusions (or cycles of therapy) in patients able to tolerate the full dose, regardless of the radionuclide distribution. 177Lu-DOTATATE could be imaged during each cycle of therapy, and dosimetry can be used to extrapolate an optimal injected activity for the subsequent cycle. A 177Lu-DOTATATE dose response has been observed from the analysis of dosimetry data (69); however, no dosimetry data were reported or used to guide treatment in the landmark NETTER-1 prospective trial (22), and some smaller trials using dosimetry to guide therapy have not achieved significant correlation between tumor response outcomes and the delivered activity (70). The forthcoming COMPETE trial of 177Lu-DOTATOC (ClinicalTrials.gov no. NCT03049189) will include dosimetry data from a large cohort, which may inform the use of this technique, and the forthcoming DOBATOC trial (ClinicalTrials.gov no. NCT04917484) will evaluate the use of dosimetry for guiding treatment dose compared with standard fixed-dose regimens. As experience with SSR-targeted radionuclide therapy has grown, more complex scenarios are arising, such as the use of extended or repeated cycles. One study used dosimetry to extend cycles of 177Lu-DOTATATE until patients reached a threshold renal dose (23 Gy) or had other reasons to discontinue, such as bone marrow toxicity or progression. Response and survival outcomes were improved in those able to continue to higher doses, although selection bias was a notable limitation (71). Dosimetry may have a promising role in modulating dose regimens in the future and predicting patients likely to respond from further cycles of therapy—a gap in the existing literature that remains to be addressed in future targeted radionuclide therapy trials for NETs and other malignancies.
177Lu-PSMA-617 Dosimetry
As with 177Lu-DOTATATE, 177Lu-PSMA-617 is administered using a fixed activity regimen in current clinical practice. Dosimetry may have prognostic value for prostate cancer radionuclide therapy. 177Lu-PSMA-617 dosimetry has been performed with each cycle in the trial setting and shown to be predictive of PSA response. However, dosimetry has not been used to guide dose administration for prostate cancer radionuclide therapy or been reported in the major prospective TheraP or VISION trials (27,28).
Much-needed efforts to standardize dosimetry methods for radiopharmaceutical therapies are underway. Specifically, joint European Association of Nuclear Medicine and MIRD society guidelines on the dosimetry of 177Lu with quantitative SPECT have been issued (72) and applied in the clinical trial setting. Dosimetry is often required for clinical use of external beam radiation, and there are emerging requirements for dosimetry for radiopharmaceuticals in some countries. However, whether dosimetry calculations should be required in the clinical use of 177Lu-DOTATATE and 177Lu-PSMA-617 and the degree of benefit of using dosimetry to modify therapeutic dose in a patient-specific manner remains under debate.
An additional challenge of radionuclide therapy dosimetry is that α-particle emitters cannot be imaged successfully. An estimate of dosimetry is in fact still possible with a diagnostic surrogate radionuclide that emits γ rays, while acknowledging that the change in radionuclide may affect the biodistribution. For example, either 111In or 225Ac can be chelated in the same radiopharmaceutical, thus the version with 111In can be used to calculate dosimetry with SPECT/CT (10). These measurements may assist in tailoring treatment by maximizing the dose-response relationship within safe limits of toxicity to critical organs. Ongoing work to establish the impact of dosimetry on patient outcomes and establish standardized methods to increase the feasibility of performing dosimetry at various centers is needed to realize the potential benefits.
Auger Electron Therapy
Proof of concept of Auger electron therapy has been shown preclinically, and the therapy has been used in very few clinical studies of cancer treatment (9), including pilot studies of iodine 125 (125I) deoxyuridine and 131I-deoxyrudine for metastatic colon cancer (73), 111In-DTPA-octreotide for NETs (74,75), 111In-DTPA-hEGF for breast cancer (76), and 125I-murine anti–epidermal growth factor receptor monoclonal antibodies for glioblastoma (77).
Emerging Molecular Targets
Numerous potential theranostic targets are under investigation (78). Table 2 summarizes examples of current trials in humans with promising approaches.
Molecular agents related to bombesin are under active development for diagnostic PET and theranostic applications, with recent and ongoing early clinical studies. Bombesin, named following isolation from the Bombina bombina, or fire-bellied toad, activates G protein–coupled receptors, including the gastrin-releasing peptide receptor highly expressed in multiple human malignancies. Initially, gastrin-agonist radioligands were investigated with associated growth stimulation and adverse effects related to receptor activation (79). Subsequently, gastrin-antagonists have been developed, such as 177Lu-RM2 and 177Lu-NeoBOMB1, with improved pharmacokinetics and decreased concern of receptor activation–related adverse effects (80,81). Other challenges of the initial investigational gastrin-releasing peptide receptor ligands, including suboptimal pharmacokinetics, dose-limiting uptake in the pancreas, and metabolic instability related to proteolytic degradation, are potentially improved with the newer gastrin-antagonists and with concurrent use of peptidase inhibitors (80).
Fibroblast activation protein (FAP) inhibitors such as quinolone-based ligands are also an important class of radionuclide therapies under active development. Fibroblast proliferation is associated with many malignancies, and these tumor-associated cells become part of the tumor volume. FAP overexpression has been observed in tumor-associated cells in up to 28 different types of tumors (82). 90Y-FAP-inhibitor (FAPI)–04 was an early radiopharmaceutical in this class used in a theranostic application for metastatic breast cancer in two patients (83), demonstrating a reasonable safety profile. FAP-based radionuclides are characterized by relatively low background activity compared with common radiopharmaceuticals, an attractive feature for a theranostic candidate (84). Multiple small early clinical studies in 90Y-FAPI-46, 177Lu-FAP-2286, and 177Lu-FAPI-46 have been well tolerated in treatment-refractory sarcomas, pancreatic cancer, breast cancer, colorectal cancer, ovarian cancer, prostate cancer, and other FAP-expressing cancers (85–87). Tracer uptake in normal organs has varied with different agents of this class, with expression in the salivary glands, thyroid, and oral mucosa presenting possible disadvantages of some agents, to be explored in larger clinical studies (88).
Other potential molecular targets, such as human epidermal growth factor receptor 2, or HER2, expressed in breast cancer and gastrointestinal cancers, cholecystokinin-2 receptor ligand expressed in medullary thyroid cancer, melanocortin receptor subtype 1 expressed in melanoma, and the six transmembrane epithelial antigens of the prostate 1, or STEAP1, receptor expressed in prostate cancer are currently being investigated in clinical trials as potential candidates for theranostic pair development (Table 2).
Investigational Combination Therapies
Combined Radionuclide Therapies
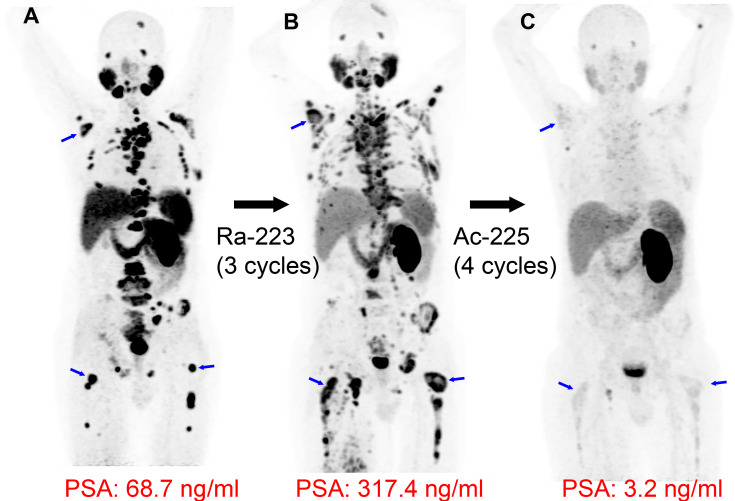
Some medical practices in countries where 225Ac-PSMA-617 is a clinical option promote the use of the α-particle emitter in patients whose cancer progressed after treatment with the β-particle emitter 177Lu-PSMA-617. One argument is that differences in the radiocytotoxic mechanism of action may facilitate benefits from α emitters in tumors that are resistant to β-particle emissions in sequence (Fig 9) (89). Similarly, 225Ac-DOTATATE α-particle therapy has been used for NETs in patients previously treated with 177Lu-DOTATATE (90). The safety and feasibility of 177Lu-PSMA-617 after 223RaCl2 (Fig 5) have been demonstrated in the recent Radium Lutetium (or, RALU) study (91). Further trials are needed comparing the effects of α- and β-particle emitters and studying the effects of using them simultaneously in combined regimens.
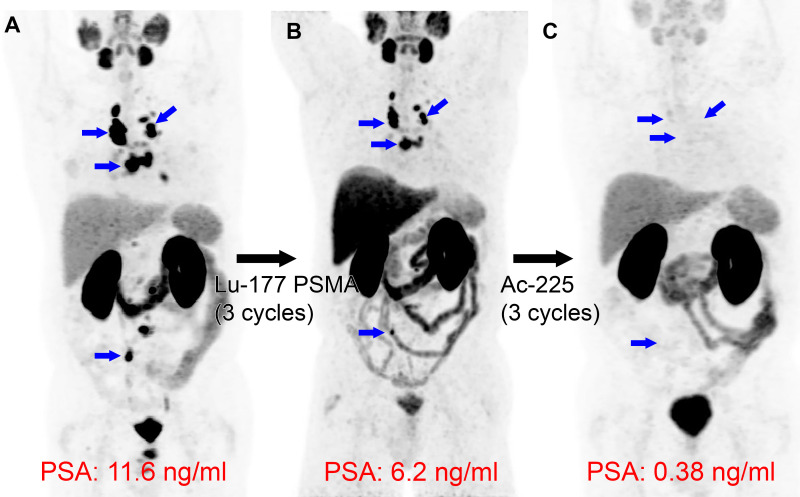
Figure 9:
Actinium 225 (225Ac) prostate-specific membrane antigen (PSMA) α-particle therapy following lutetium 177 (177Lu) PSMA-617 β-particle therapy. Images in a 71-year-old man with widely metastatic prostate cancer, with a Gleason score of 7 (3 + 4), that progressed despite androgen deprivation therapy, taxane-based chemotherapy, and thoracic spine stereotactic radiation. (A) Baseline gallium 68 (68Ga) PSMA-11 PET/CT image demonstrates intense PSMA uptake in many nodal metastases (blue arrows). (B) Following three cycles of the targeted β-particle therapy with 177Lu-PSMA-617, the 68Ga-PSMA-11 PET/CT image demonstrates persistent PSMA-avid disease (blue arrows), with a mild reduction in prostate-specific antigen (PSA). (C) Following three cycles of the targeted α-particle therapy with 225Ac-PSMA-617, nearly complete resolution of uptake in the nodal metastases is observed (blue arrows in the location of previous nodal uptake), with a substantial reduction in PSA level.
Radionuclide Therapy with Cytotoxic Chemotherapy and Other Systemic Agents
Tumor heterogeneity, in terms of varied tumor size, varied differentiation, and molecular features, especially in the setting of heavily treated metastatic disease, may ultimately benefit from a variety of combined treatment strategies relying on different mechanisms of action.
Low-dose cytotoxic chemotherapy is frequently used as a radiosensitizing agent in conjunction with external-beam radiation in a multitude of malignancies. This synergy might be expected to translate into enhancement of the antineoplastic effect of radionuclide therapy. Some phase 2 studies of 177Lu-DOTATATE in combination with cytotoxic chemotherapy (92–94) have suggested additive efficacy; however, there are concerns of increased long-term hematologic toxicity and subsequent secondary malignancies related to repairable DNA damage using this investigational approach. Improvement in survival has not yet been shown with combined radionuclide therapy and cytotoxic chemotherapy.
Likewise, combining hormonal therapies with 177Lu-PSMA-617 is an important consideration in the clinical management of metastatic prostate cancer. The landmark VISION trial of 177Lu-PSMA-617 permitted standard-of-care hormonal treatments; however, the additive impact of this combination has not yet been systematically investigated (28).
Other agents of interest in combination with radionuclide therapy are in early investigational stages. For example, poly (adenosine diphosphate–ribose) polymerase inhibitors may radiosensitize by inhibiting DNA repair and immune checkpoint inhibitors, which may augment an abscopal response to radiation-induced cell death.
Conclusion
In this review, we summarized various theranostic radiopharmaceuticals and their clinical use against a variety of cancers. Overall, theranostics for cancer imaging and treatment is rapidly evolving. Various emerging molecular targets and radiopharmaceuticals with different forms of radiation emission, such as α-emitting therapies, have a high potential to emerge as next-generation theranostics. Current and continued efforts to better estimate the dosimetry of therapeutic radiopharmaceuticals and quantify and monitor treatment response are necessary steps in the direction of individualized precision medicine for theranostics. Additional work will be needed to refine the role of radionuclide therapy in combination with other modalities of cancer treatment.
Authors declared no funding for this work.
Abbreviations:
- DOTATATE
- tetraazacyclododecane tetraacetic acid octreotate
- FAP
- fibroblast activation protein
- FAPI
- FAP inhibitor
- FDA
- U.S. Food and Drug Administration
- LET
- linear energy transfer
- mCRPC
- metastatic castration-resistant prostate cancer
- MIBG
- metaiodobenzylguanidine
- MIRD
- Medical Internal Radiation Doses
- NET
- neuroendocrine tumor
- PSA
- prostate-specific antigen
- PSMA
- prostate-specific membrane antigen
- SSR
- somatostatin receptors
Disclosures of conflicts of interest: B.J.B. Member of Radiology: Imaging Cancer trainee editorial board. D.J.B. Patent pending for a radiopharmaceutical for imaging and therapy. P.W.M. No relevant relationships. A.R.L. No relevant relationships. D.R.J. No relevant relationships. K.B. No relevant relationships. M.K.P. Multiple patents issued related to isotope production and application, as well as a patent pending for theranostics, no payments received to date. A.T.P. No relevant relationships. T.R.H. Research support to author’s institution from Ipsen and Advanced Accelerator Applications (a Novartis company); consulting fees from Ipsen and Advanced Accelerator Applications, paid to author’s institution; vice-president of the North American Neuroendocrine Tumor Society (NANETS), unpaid position; consultant to TerSera Therapeutics (personal payment). C.B.H. No relevant relationships. G.B.J. Grants or contracts from Pfizer, Novartis, MedTrace Pharma, Clarity Pharmaceuticals, Clovis Oncology, Viewpoint Molecular Targeting, and SOFIE, all paid to author’s institution; consulting fees from Pfizer, Novartis, Curium Pharma, Blue Earth Diagnostics, AstraZeneca, Siemens, and Morphimmune, paid to author’s institution; payment or honoraria from Prostate Cancer Research Institute (PCRI) for urology grand rounds; support from the Society of Nuclear Medicine and Molecular Imaging (SNMMI) for attending Gordon Research Conferences and Mayo CME courses; patents planned, issued, or pending for CRISMA PET, Alpha-PET theranostic platform, targeting meningiomas for PET imaging and therapy, cardiac PYP score; participation on a data safety monitoring board or advisory board for the SECuRE trial for Clarity Pharmaceuticals, the Targeted Imaging of Melanoma for Alpha-Particle Radiotherapy (TIMAR1) trial for Viewpoint Molecular Targeting, Pfizer, AstraZeneca, Novartis, and Siemens, all payments to author’s institution; chief scientific advisor for Nucleus RadioPharma. A.T.K. Primary investigator for Mayo Clinic Rochester for phase 3 VISION trial assessing LuPSMA therapy in patients with metastatic castration-resistant prostate cancer, sponsored by Novartis; consulting fees from Novartis for assessment of future LuPSMA therapy research; payment or honoraria for PSMA imaging presentation, an online education CME presentation sponsored by AXIS Medical Education.
References
- 1. Bannik K , Madas B , Jarzombek M , et al . Radiobiological effects of the alpha emitter Ra-223 on tumor cells . Sci Rep 2019. ; 9 ( 1 ): 18489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kendi AT , Halfdanarson TR , Packard A , Dundar A , Subramaniam RM . Therapy with 177Lu-DOTATATE: clinical implementation and impact on care of patients with neuroendocrine tumors . AJR Am J Roentgenol 2019. ; 213 ( 2 ): 309 – 317 . [DOI] [PubMed] [Google Scholar]
- 3. Modoni S , Frangos S , Iakovou I , Boero M , Mansi L . Theragnostics before we found its name . Q J Nucl Med Mol Imaging 2021. ; 65 ( 4 ): 299 – 305 . [DOI] [PubMed] [Google Scholar]
- 4. Chapman EM . History of the discovery and early use of radioactive iodine . JAMA 1983. ; 250 ( 15 ): 2042 – 2044 . [PubMed] [Google Scholar]
- 5. Kassis AI , Adelstein SJ . Radiobiologic principles in radionuclide therapy . J Nucl Med 2005. ; 46 ( Suppl 1 ): 4S – 12S . [PubMed] [Google Scholar]
- 6. Parker C , Lewington V , Shore N , et al . Targeted Alpha Therapy Working Group. Targeted alpha therapy, an emerging class of cancer agents: a review . JAMA Oncol 2018. ; 4 ( 12 ): 1765 – 1772 . [DOI] [PubMed] [Google Scholar]
- 7. Bushnell DL , Madsen MT , O’cdorisio T , et al . Feasibility and advantage of adding (131)I-MIBG to (90)Y-DOTATOC for treatment of patients with advanced stage neuroendocrine tumors . EJNMMI Res 2014. ; 4 ( 1 ): 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M , Liu D , Lee D , et al . Targeted alpha-particle radiotherapy and immune checkpoint inhibitors induces cooperative inhibition on tumor growth of malignant melanoma . Cancers (Basel) 2021. ; 13 ( 15 ): 3676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ku A , Facca VJ , Cai Z , Reilly RM . Auger electrons for cancer therapy - a review . EJNMMI Radiopharm Chem 2019. ; 4 ( 1 ): 27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawhn-Heath C , Hope TA , Martinez J , et al . Dosimetry in radionuclide therapy: the clinical role of measuring radiation dose . Lancet Oncol 2022. ; 23 ( 2 ): e75 – e87 . [DOI] [PubMed] [Google Scholar]
- 11. Wessels BW , Konijnenberg MW , Dale RG , et al . MIRD pamphlet No. 20: the effect of model assumptions on kidney dosimetry and response--implications for radionuclide therapy . J Nucl Med 2008. ; 49 ( 11 ): 1884 – 1899 . [DOI] [PubMed] [Google Scholar]
- 12. Maxon HR , Thomas SR , Hertzberg VS , et al . Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer . N Engl J Med 1983. ; 309 ( 16 ): 937 – 941 . [DOI] [PubMed] [Google Scholar]
- 13. Van Nostrand D , Atkins F , Yeganeh F , Acio E , Bursaw R , Wartofsky L . Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma . Thyroid 2002. ; 12 ( 2 ): 121 – 134 . [DOI] [PubMed] [Google Scholar]
- 14. Jungels C , Karfis I . 131I-metaiodobenzylguanidine and peptide receptor radionuclide therapy in pheochromocytoma and paraganglioma . Curr Opin Oncol 2021. ; 33 ( 1 ): 33 – 39 . [DOI] [PubMed] [Google Scholar]
- 15. Sundlöv A , Sjögreen-Gleisner K , Svensson J , et al . Individualised 177Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry . Eur J Nucl Med Mol Imaging 2017. ; 44 ( 9 ): 1480 – 1489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciarallo A , Rivera J . Radioactive iodine therapy in differentiated thyroid cancer: 2020 update . AJR Am J Roentgenol 2020. ; 215 ( 2 ): 285 – 291 . [DOI] [PubMed] [Google Scholar]
- 17. Fischer M , Kampen WU . Radionuclide therapy of bone metastases . Breast Care (Basel) 2012. ; 7 ( 2 ): 100 – 107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grünwald F , Ezziddin S . 131I-metaiodobenzylguanidine therapy of neuroblastoma and other neuroendocrine tumors . Semin Nucl Med 2010. ; 40 ( 2 ): 153 – 163 . [DOI] [PubMed] [Google Scholar]
- 19. Parker C , Nilsson S , Heinrich D , et al. ; ALSYMPCA Investigators . Alpha emitter radium-223 and survival in metastatic prostate cancer . N Engl J Med 2013. ; 369 ( 3 ): 213 – 223 . [DOI] [PubMed] [Google Scholar]
- 20. Suominen MI , Fagerlund KM , Rissanen JP , et al . Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models . Clin Cancer Res 2017. ; 23 ( 15 ): 4335 – 4346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burkett BJ , Dundar A , Young JR , et al . How we do it: a multidisciplinary approach to 177Lu DOTATATE peptide receptor radionuclide therapy . Radiology 2021. ; 298 ( 2 ): 261 – 274 . [DOI] [PubMed] [Google Scholar]
- 22. Strosberg J , El-Haddad G , Wolin E , et al. ; NETTER-1 trial investigators . phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors . N Engl J Med 2017. ; 376 ( 2 ): 125 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strosberg JR , Caplin ME , Kunz PL , et al. ; NETTER-1 investigators . 177Lu-Dotatate plus long-acting octreotide versus highdose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial . Lancet Oncol 2021. ; 22 ( 12 ): 1752 – 1763 . [Published correction appears in Lancet Oncol 2022;23(2):e59.] [DOI] [PubMed] [Google Scholar]
- 24. Fani M , Nicolas GP , Wild D . Somatostatin receptor antagonists for imaging and therapy . J Nucl Med 2017. ; 58 ( Suppl 2 ): 61S – 66S . [DOI] [PubMed] [Google Scholar]
- 25. Nicolas G , Mansi R , Vomstein S , et al . Wider safety window with radiolabeled somatostatin receptor antagonists over agonists . J Nucl Med 2015. ; 56 ( Supplement 3 ): 335 – 335 . [Google Scholar]
- 26. Study to Evaluate the Efficacy and Safety of Lutathera in Patients With Grade 2 and Grade 3 Advanced GEP-NET (NETTER-2) . National Institute of Health U.S. National Library of Medicine; . https://clinicaltrials.gov/ct2/show/NCT03972488. Posted June 3, 2019. Updated May 24, 2023. Accessed November 1, 2022. [Google Scholar]
- 27. Hofman MS , Emmett L , Violet J , et al. ; ANZUP TheraP team . TheraP: a randomized phase 2 trial of 177 Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603) . BJU Int 2019. ; 124 ( Suppl 1 ): 5 – 13 . [DOI] [PubMed] [Google Scholar]
- 28. Sartor O , de Bono J , Chi KN , et al. ; VISION Investigators . Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer . N Engl J Med 2021. ; 385 ( 12 ): 1091 – 1103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emmett L , Willowson K , Violet J , Shin J , Blanksby A , Lee J . Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy . J Med Radiat Sci 2017. ; 64 ( 1 ): 52 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mullard A . FDA approves first PSMA-targeted radiopharmaceutical . Nat Rev Drug Discov 2022. ; 21 ( 5 ): 327 . [DOI] [PubMed] [Google Scholar]
- 31. Kratochwil C , Bruchertseifer F , Giesel FL , et al . 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer . J Nucl Med 2016. ; 57 ( 12 ): 1941 – 1944 . [DOI] [PubMed] [Google Scholar]
- 32. Satapathy S , Sood A , Das CK , Mittal BR . Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer-a systematic review and meta-analysis . Prostate Cancer Prostatic Dis 2021. ; 24 ( 3 ): 880 – 890 . [DOI] [PubMed] [Google Scholar]
- 33. Sundlöv A , Sjögreen-Gleisner K . Peptide receptor radionuclide therapy - prospects for personalised treatment . Clin Oncol (R Coll Radiol) 2021. ; 33 ( 2 ): 92 – 97 . [DOI] [PubMed] [Google Scholar]
- 34. Krenning EP , Bakker WH , Breeman WA , et al . Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin . Lancet 1989. ; 1 ( 8632 ): 242 – 244 . [DOI] [PubMed] [Google Scholar]
- 35. Fendler WP , Eiber M , Beheshti M , et al . PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0 . Eur J Nucl Med Mol Imaging 2023. ; 50 ( 5 ): 1466 – 1486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hope TA , Abbott A , Colucci K , et al . NANETS/SNMMI procedure standard for somatostatin receptor-based peptide receptor radionuclide therapy with 177Lu-DOTATATE . J Nucl Med 2019. ; 60 ( 7 ): 937 – 943 . [DOI] [PubMed] [Google Scholar]
- 37. Fanti S , Briganti A , Emmett L , et al . EAU-EANM consensus statements on the role of prostate-specific membrane antigen positron emission tomography/computed tomography in patients with prostate cancer and with respect to [177Lu]Lu-PSMA radioligand therapy . Eur Urol Oncol 2022. ; 5 ( 5 ): 530 – 536 . [DOI] [PubMed] [Google Scholar]
- 38. Hope TA , Allen-Auerbach M , Bodei L , et al . SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors . J Nucl Med 2023. ; 64 ( 2 ): 204 – 210 . [DOI] [PubMed] [Google Scholar]
- 39. Kratochwil C , Fendler WP , Eiber M , et al . EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT) . Eur J Nucl Med Mol Imaging 2019. ; 46 ( 12 ): 2536 – 2544 . [DOI] [PubMed] [Google Scholar]
- 40. Pathmanandavel S , Crumbaker M , Ho B , et al . Evaluation of 177 Lu-PSMA-617 SPECT/CT quantitation as a response biomarker within a prospective 177 Lu-PSMA-617 and NOX66 combination trial (LuPIN) . J Nucl Med 2022. ; 64 ( 2 ): 221 – 226 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Helgebostad R , Revheim ME , Johnsrud K , Amlie K , Alavi A , Connelly JP . Clinical applications of somatostatin receptor (agonist) PET tracers beyond neuroendocrine tumors . Diagnostics (Basel) 2022. ; 12 ( 2 ): 528 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kong G , Grozinsky-Glasberg S , Hofman MS , et al . Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma . J Clin Endocrinol Metab 2017. ; 102 ( 9 ): 3278 – 3287 . [DOI] [PubMed] [Google Scholar]
- 43. Kolasinska-Ćwikła A , Pęczkowska M , Ćwikła JB , et al . A clinical efficacy of PRRT in patients with advanced, nonresectable, paraganglioma-pheochromocytoma, related to SDHx gene mutation . J Clin Med 2019. ; 8 ( 7 ): 952 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forrer F , Riedweg I , Maecke HR , Mueller-Brand J . Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma . Q J Nucl Med Mol Imaging 2008. ; 52 ( 4 ): 334 – 340 . [PubMed] [Google Scholar]
- 45. Pinato DJ , Black JR , Ramaswami R , Tan TM , Adjogatse D , Sharma R . Peptide receptor radionuclide therapy for metastatic paragangliomas . Med Oncol 2016. ; 33 ( 5 ): 47 . [DOI] [PubMed] [Google Scholar]
- 46. Puranik AD , Kulkarni HR , Singh A , Baum RP . Peptide receptor radionuclide therapy with (90)Y/ (177)Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours) . Eur J Nucl Med Mol Imaging 2015. ; 42 ( 8 ): 1223 – 1230 . [DOI] [PubMed] [Google Scholar]
- 47. van Essen M , Krenning EP , Kooij PP , et al . Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma . J Nucl Med 2006. ; 47 ( 10 ): 1599 – 1606 . [PubMed] [Google Scholar]
- 48. Zovato S , Kumanova A , Demattè S , et al . Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL) . Horm Metab Res 2012. ; 44 ( 5 ): 411 – 414 . [DOI] [PubMed] [Google Scholar]
- 49. Shah MH , Goldner WS , Halfdanarson TR , et al . NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018 . J Natl Compr Canc Netw 2018. ; 16 ( 6 ): 693 – 702 . [DOI] [PubMed] [Google Scholar]
- 50. Kong G , Callahan J , Hofman MS , et al . High clinical and morphologic response using 90Y-DOTA-octreotate sequenced with 177Lu-DOTA-octreotate induction peptide receptor chemoradionuclide therapy (PRCRT) for bulky neuroendocrine tumours . Eur J Nucl Med Mol Imaging 2017. ; 44 ( 3 ): 476 – 489 . [DOI] [PubMed] [Google Scholar]
- 51. Jha A , Taïeb D , Carrasquillo JA , et al . High-specific-activity-131I-MIBG versus 177Lu-DOTATATE targeted radionuclide therapy for metastatic pheochromocytoma and paraganglioma . Clin Cancer Res 2021. ; 27 ( 11 ): 2989 – 2995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bartolomei M , Bodei L , De Cicco C , et al . Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma . Eur J Nucl Med Mol Imaging 2009. ; 36 ( 9 ): 1407 – 1416 . [DOI] [PubMed] [Google Scholar]
- 53. Seystahl K , Stoecklein V , Schüller U , et al . Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake . Neuro-oncol 2016. ; 18 ( 11 ): 1538 – 1547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zahid A , Johnson DR , Kizilbash SH . Efficacy of 177Lu-Dotatate therapy in the treatment of recurrent meningioma . Mayo Clin Proc Innov Qual Outcomes 2021. ; 5 ( 1 ): 236 – 240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van de Wiele C , Sathekge M , de Spiegeleer B , et al . PSMA expression on neovasculature of solid tumors . Histol Histopathol 2020. ; 35 ( 9 ): 919 – 927 . [DOI] [PubMed] [Google Scholar]
- 56. Uijen MJM , Derks YHW , Merkx RIJ , et al . PSMA radioligand therapy for solid tumors other than prostate cancer: background, opportunities, challenges, and first clinical reports . Eur J Nucl Med Mol Imaging 2021. ; 48 ( 13 ): 4350 – 4368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kunikowska J , Charzyńska I , Kuliński R , Pawlak D , Maurin M , Królicki L . Tumor uptake in glioblastoma multiforme after IV injection of [177Lu]Lu-PSMA-617 . Eur J Nucl Med Mol Imaging 2020. ; 47 ( 6 ): 1605 – 1606 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumar A , Ballal S , Yadav MP , et al . 177Lu-/68Ga-PSMA theranostics in recurrent glioblastoma multiforme: proof of concept . Clin Nucl Med 2020. ; 45 ( 12 ): e512 – e513 . [DOI] [PubMed] [Google Scholar]
- 59. Assadi M , Ahmadzadehfar H . 177Lu-DOTATATE and 177Lu-prostate-specific membrane antigen therapy in a patient with advanced metastatic radioiodine-refractory differentiated thyroid cancer after failure of tyrosine kinase inhibitors treatment . World J Nucl Med 2019. ; 18 ( 4 ): 406 – 408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirmas N , Leyh C , Sraieb M , et al . 68Ga-PSMA-11 PET/CT improves tumor detection and impacts management in patients with hepatocellular carcinoma . J Nucl Med 2021. ; 62 ( 9 ): 1235 – 1241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Essen M , Krenning EP , Kam BL , de Herder WW , Feelders RA , Kwekkeboom DJ . Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors . J Nucl Med 2010. ; 51 ( 3 ): 383 – 390 . [DOI] [PubMed] [Google Scholar]
- 62. Sabet A , Haslerud T , Pape U-F , et al . Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours . Eur J Nucl Med Mol Imaging 2014. ; 41 ( 2 ): 205 – 210 . [DOI] [PubMed] [Google Scholar]
- 63. Hoe HJ , Wyld D . Salvage 177Lu-dotatate therapy in patients with progressive metastatic neuroendocrine tumors . J Clin Oncol 2022. ; 40 ( 16 Suppl ): e16212 . [Google Scholar]
- 64. Zemczak A , Gut P , Pawlak D , et al . The safety and efficacy of the repeated PRRT with [90Y]Y/[177Lu]Lu-DOTATATE in patients with NET . Int J Endocrinol 2021. ; 2021 : 6615511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strosberg J , Leeuwenkamp O , Siddiqui MK . Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis . Cancer Treat Rev 2021. ; 93 : 102141 . [Published correction appears in Cancer Treat Rev 2021;97:102203.] [DOI] [PubMed] [Google Scholar]
- 66. Violet J , Sandhu S , Iravani A , et al . Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase ii prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer . J Nucl Med 2020. ; 61 ( 6 ): 857 – 865 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ho AL , Grewal RK , Leboeuf R , et al . Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer . N Engl J Med 2013. ; 368 ( 7 ): 623 – 632 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Freudenberg LS , Jentzen W , Görges R , et al . 124I-PET dosimetry in advanced differentiated thyroid cancer: therapeutic impact . Nucl Med (Stuttg) 2007. ; 46 ( 4 ): 121 – 128 . [PubMed] [Google Scholar]
- 69. Ilan E , Sandström M , Wassberg C , et al . Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE . J Nucl Med 2015. ; 56 ( 2 ): 177 – 182 . [DOI] [PubMed] [Google Scholar]
- 70. Del Prete M , Buteau FA , Beauregard JM . Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study . Eur J Nucl Med Mol Imaging 2017. ; 44 ( 9 ): 1490 – 1500 . [DOI] [PubMed] [Google Scholar]
- 71. Garske-Román U , Sandström M , Fröss Baron K , et al . Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity . Eur J Nucl Med Mol Imaging 2018. ; 45 ( 6 ): 970 – 988 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ljungberg M , Celler A , Konijnenberg MW , et al . EANM Dosimetry Committee. MIRD pamphlet No. 26: joint EANM/MIRD guidelines for quantitative 177Lu SPECT applied for dosimetry of radiopharmaceutical therapy . J Nucl Med 2016. ; 57 ( 1 ): 151 – 162 . [DOI] [PubMed] [Google Scholar]
- 73. Macapinlac HA , Kemeny N , Daghighian F , et al . Pilot clinical trial of 5-[125I]iodo-2′-deoxyuridine in the treatment of colorectal cancer metastatic to the liver . J Nucl Med 1996. ; 37 ( 4 Suppl ): 25S – 29S . [PubMed] [Google Scholar]
- 74. Krenning EP , de Jong M , Kooij PP , et al . Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy . Ann Oncol 1999. ; 10 ( Suppl 2 ): S23 – S29 . [DOI] [PubMed] [Google Scholar]
- 75. Valkema R , De Jong M , Bakker WH , et al . Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience . Semin Nucl Med 2002. ; 32 ( 2 ): 110 – 122 . [DOI] [PubMed] [Google Scholar]
- 76. Vallis KA , Reilly RM , Scollard D , et al . Phase I trial to evaluate the tumor and normal tissue uptake, radiation dosimetry and safety of (111)In-DTPA-human epidermal growth factor in patients with metastatic EGFR-positive breast cancer . Am J Nucl Med Mol Imaging 2014. ; 4 ( 2 ): 181 – 192 . [PMC free article] [PubMed] [Google Scholar]
- 77. Li L , Quang TS , Gracely EJ , et al . A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme . J Neurosurg 2010. ; 113 ( 2 ): 192 – 198 . [DOI] [PubMed] [Google Scholar]
- 78. Nunes RF , Zuppani RMF , Coutinho AM , et al . General concepts in theranostics . PET Clin 2021. ; 16 ( 3 ): 313 – 326 . [DOI] [PubMed] [Google Scholar]
- 79. Mansi R , Nock BA , Dalm SU , Busstra MB , van Weerden WM , Maina T . Radiolabeled bombesin analogs . Cancers (Basel) 2021. ; 13 ( 22 ): 5766 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kurth J , Krause BJ , Schwarzenböck SM , Bergner C , Hakenberg OW , Heuschkel M . First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: a radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer . Eur J Nucl Med Mol Imaging 2020. ; 47 ( 1 ): 123 – 135 . [DOI] [PubMed] [Google Scholar]
- 81. Dalm SU , Bakker IL , de Blois E , et al . 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology . J Nucl Med 2017. ; 58 ( 2 ): 293 – 299 . [DOI] [PubMed] [Google Scholar]
- 82. Kratochwil C , Flechsig P , Lindner T , et al . 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer . J Nucl Med 2019. ; 60 ( 6 ): 801 – 805 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lindner T , Loktev A , Altmann A , et al . Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein . J Nucl Med 2018. ; 59 ( 9 ): 1415 – 1422 . [DOI] [PubMed] [Google Scholar]
- 84. Kuyumcu S , Sanli Y , Subramaniam RM . Fibroblast-activated protein inhibitor PET/CT: cancer diagnosis and management . Front Oncol 2021. ; 11 : 758958 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Assadi M , Rekabpour SJ , Jafari E , et al . Feasibility and therapeutic potential of 177Lu-fibroblast activation protein inhibitor-46 for patients with relapsed or refractory cancers: a preliminary study . Clin Nucl Med 2021. ; 46 ( 11 ): e523 – e530 . [DOI] [PubMed] [Google Scholar]
- 86. Baum RP , Schuchardt C , Singh A , et al . Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-humans results . J Nucl Med 2022. ; 63 ( 3 ): 415 – 423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ferdinandus J , Costa PF , Kessler L , et al . Initial clinical experience with 90Y-FAPI-46 Radioligand Therapy for Advanced-Stage Solid Tumors: A Case Series of 9 Patients . J Nucl Med 2022. ; 63 ( 5 ): 727 – 734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Loktev A , Lindner T , Burger EM , et al . Development of fibroblast activation protein-targeted radiotracers with improved tumor retention . J Nucl Med 2019. ; 60 ( 10 ): 1421 – 1429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ilhan H , Gosewisch A , Böning G , et al . Response to 225Ac-PSMA-I&T after failure of long-term 177Lu-PSMA RLT in mCRPC . Eur J Nucl Med Mol Imaging 2021. ; 48 ( 4 ): 1262 – 1263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bal C , Yadav M , Ballal S , Tripathi M . Safety and therapeutic efficacy of 225Ac-DOTATATE targeted alpha therapy in metastatic gastroenteropancreatic neuroendocrine tumors stable or refractory to 177Lu-DOTATATE PRRT . J Nucl Med 2020. ; 61 ( Supplement 1 ): 416 . [DOI] [PubMed] [Google Scholar]
- 91. Rahbar K , Essler M , Pabst KM , et al . Safety and survival outcomes of lutetium-177-prostate-specific membrane antigen therapy in patients with metastatic castration-resistant prostate cancer with prior radium-223 treatment: the RALU study . J Nucl Med 2023. ; 64 ( 4 ): 574 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Claringbold PG , Turner JH . Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy . Neuroendocrinology 2016. ; 103 ( 5 ): 432 – 439 . [DOI] [PubMed] [Google Scholar]
- 93. Kesavan M , Grover P , Lam WS , Claringbold PG , Turner JH . Long-term hematologic toxicity of 177Lu-octreotate-capecitabine-temozolomide therapy of GEPNET . Endocr Relat Cancer 2021. ; 28 ( 7 ): 521 – 527 . [DOI] [PubMed] [Google Scholar]
- 94. Pavlakis N , Ransom DT , Wyld D , et al . Australasian Gastrointestinal Trials Group (AGITG) CONTROL NET Study: 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) and capecitabine plus temozolomide (CAPTEM) for pancreas and midgut neuroendocrine tumours (pNETS, mNETS)—Final results . J Clin Oncol 2022. ; 40 ( 16 suppl ): 4122 . [Google Scholar]