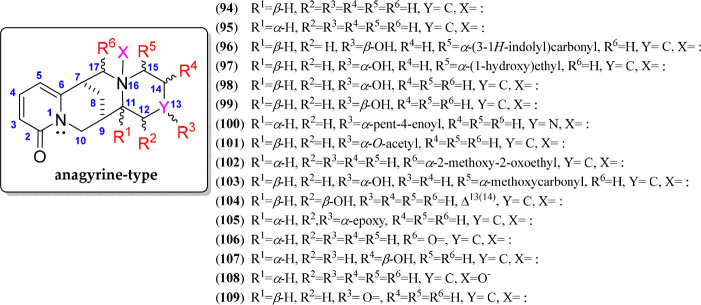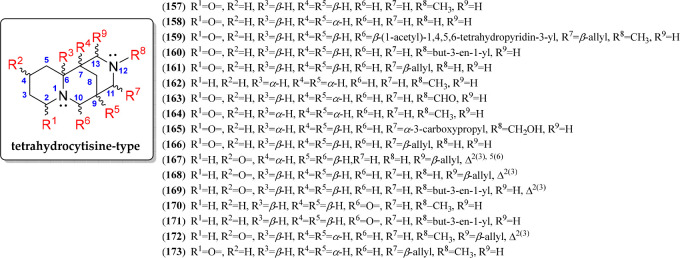Abstract
Quinolizidine alkaloids (QAs) are nitrogen-containing compounds produced naturally as specialized metabolites distributed in plants and animals (e.g., frogs, sponges). The present review compiles the available information on the chemical diversity and biological activity of QAs reported during the last three decades. So far, 397 QAs have been isolated, gathering 20 different representative classes, including the most common such as matrine (13.6%), lupanine (9.8%), anagyrine (4.0%), sparteine (5.3%), cytisine (6.5%), tetrahydrocytisine (4.3%), lupinine (12.1%), macrocyclic bisquinolizidine (9.3%), biphenylquinolizidine lactone (7.1%), dimeric (7.1%), and other less known QAs (20.9%), which include several structural patterns of QAs. A detailed survey of the reported information about the bioactivities of these compounds indicated their potential as cytotoxic, antiviral, antimicrobial, insecticidal, anti-inflammatory, antimalarial, and antiacetylcholinesterase compounds, involving favorable putative drug-likeness scores. In this regard, research progress on the structural and biological/pharmacological diversity of QAs requires further studies oriented on expanding the chemical space to find bioactive scaffolds based on QAs for pharmacological and agrochemical applications.
Introduction
The quinolizidine alkaloids (QAs) are nitrogenous heterocycles with a 1-azabicyclo[4.4.0]decane moiety obtained from natural sources.1 QAs are specialized metabolites biosynthesized from the amino acid l-lysine.2 Their core structure can be built from one or two quinolizidines, differentiating them from other alkaloids derived from the l-lysine pathway, such as piperidine, indolizidine, and lycopodium alkaloids.3 QAs have been reported to possess various pharmacological effects such as sedative,4 anticonvulsant,5 anti-inflammatory,6 antiviral,7 antitumor,8 antipyretic,9,10 antihepatitis B,11 antifibrotic, antiallergic, antidiarrheal, analgesic,6 and antimicrobial.12,13 Sparteine and lupinine were the first QAs to be isolated from Lupinus luteus leaves and stems at the onset of the 20th century.14 With the development of chromatographic and spectroscopic techniques, many naturally occurring QAs have been isolated and identified, and they still attract widespread attention for their plausible applications. In this sense, there are different natural sources for which QAs have been reported, whose structural variability is highlighted. Consequently, they can be classified according to the different QA moieties depending on the number of cycles and cyclic arrangement. The seven most-known structural QA types can be globally gathered (Figure 1), characterized by one quinolizidine moiety, as in the case of the substituted, fused bicycle quinolizidines (i.e., lupinine-type). Bridged tricycles, such as cytisine and tetrahydrocytisine, are also characteristic quinolizidine classes. Finally, the tetracyclic quinolizidines are divided into two groups, such as fused (i.e., matrine-type) and bridged (i.e., lupanine, anagyrine, and sparteine) heterocycles.
Figure 1.
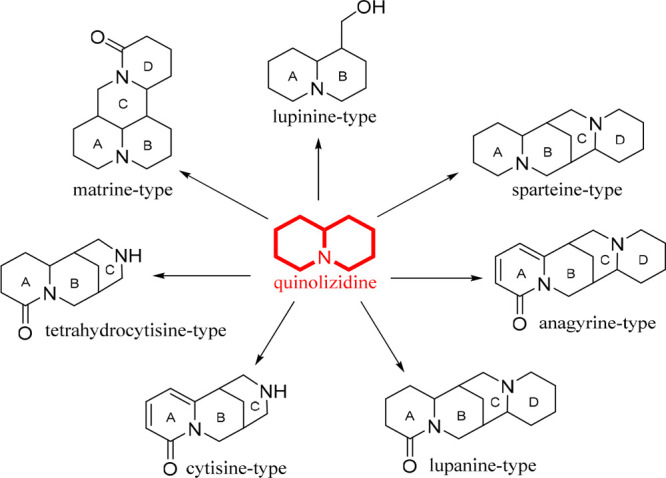
Structural types of the most common quinolizidine alkaloids.
Considering that these most-known QA types are mainly associated with Fabaceae-related taxa, the present compilation was organized on the basis of three motivations: (1) lack of exclusive and comprehensive information on QAs reported to date from diversified sources, even incorporating animal sources such as sponges and frogs,15 since the available reviews on QAs are emphasized on occurrence in particular taxa, e.g., Sophora,8,16,17 their bioactivities,18 biosynthesis,19−21 and diversity;22−24 (2) classification of QA structures beyond the plant-derived QAs; and (3) recognition of structural diversity and substituent variations within common QA skeletons to be described in light of their chemodiversity. Hence, this review provides comprehensive background information on the chemodiversity of QAs, focusing on their structural variants, substitutions, occurrences, and reported biological activities, harmonizing previous reviews.
Roles for Natural Producers
One of the leading chemical functions of alkaloids in plants is the defense against predators and herbivores.25 QAs have antimicrobial properties but have also been reported to be teratogenic to some ruminants.26 However, the importance of quinolizidine function in plants is essential. For instance, Lupinus plants use compounds such as QAs in periods of biotic stress as a repellent strategy against insects.27 Likewise, humans have also used these QAs in mixtures with carotenoids and tannins for crop bioremediation purposes, taking advantage of the direct influence and allelopathic properties of the tannins and the toxicity of the alkaloids.28,29
Another relevant function of these QAs is the chemical similarity to some molecules participating in signal transmission from the nervous system. Hence, they can block neuroreceptors, intermediaries of neuronal signal transduction, and ion channels in vertebrates and insects.1 Additionally, they can serve as plant growth regulators since, in some cases, cadaverine- and putrescine-derived alkaloids increase significantly during germination.30 Finally, the alkaloids are associated with fatty acids facilitating translocation within the plant since they can serve as storage products or transportation of nonmetabolized nitrogen. Indeed, QAs in Fabaceae can serve as nitrogen storage, especially the atmospherically fixed N2.31−33
General Distribution
Although QAs have predominantly been isolated from the Fabaceae family,25 it is worth noting that other plant families, such as Saururaceae,23 Acanthaceae,34 Phyllanthaceae,35 Rubiaceae,36 Lycopodiaceae,37 Lycopodiaceae,38−41 Urticaceae,42 Ericaceae,43 Euphorbiaceae,44 and Connaraceae,23 have also been investigated for the presence of relevant QAs. Additionally, QAs have been reported in families of terrestrial and marine animal species such as Dendrobatidae,45,46 Mantellidae,47,48 Formicidae,49 Clavelinidae,50 and Petrosiidae.15 Particularly, a series of petrosins, xestospongins, and araguspongines have also been identified from marine sponges belonging to the genera Petrosia, Xestospongia, and Oceanapia(15,51−54) but have also been identified in frog skins, specifically in the families Dendrobatidae and Mantellidae, highlighting species such as Phyllobates aurotaenia, Melanophryniscus moreirae, Melanophryniscus toads,45Epipedobates tricolor,46Mantella baroni,48 and Mantella basileo.47 These alkaloids constitute a unique type of macrocyclic QAs, formed by the union of two quinolizidines (precisely two 1-oxaquinolizidine fragments), called bisquinolizidines.15 They have exhibited biological activities such as cytotoxicity,55 anti-inflammatory,56 selective inhibition of the IP3 receptor, and HIV-1 RT inhibitory activity.7
The Fabaceae family, one of the world’s largest flowering plants (Angiosperms), is the primary source of QAs. It has cosmopolitan distribution but is also well-represented in the flora of the Andes.57 It is the third largest family worldwide among the Angiosperms, surpassed by the Asteraceae and Orchidaceae families.58 Remarkably, the QAs are biosynthesized and accumulated in the so-called primitive legumes of the tribes Genisteae, Lupinus, Sophoreae, Dalbergieae, Euchresteae, Thermopsidae, Bossiaeae, Brongniartieae, Podalyrieae, Liparieae, and Crotalarieae.1 The previous tribes include the six most relevant genera due to the highest occurrence of QAs, such as Lupinus, Ulex, Cytisus, Sophora, Genista, and Orphanodendron.1,19,59
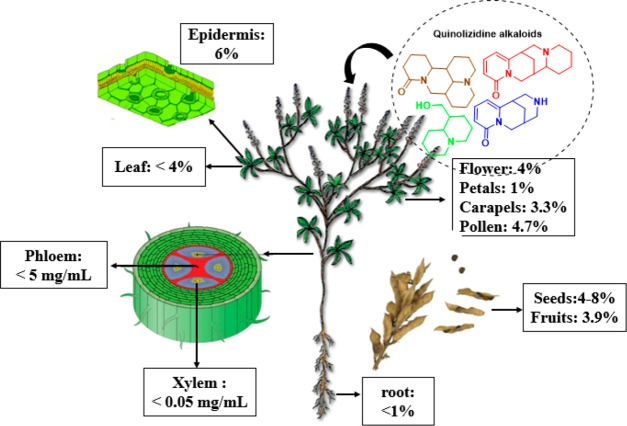
The peripheral part of the seeds, bark, root, fruit, and leaf epidermis can majorly accumulate QAs.60 Previous studies suggest that QAs play an essential role in plant defense against insects due to the bitter taste and toxicity conferred by QAs, especially in the seeds, where their most significant accumulation occurs.1 The biosynthesis of most Fabaceae alkaloids (quinolizidines) is carried out in the green aerial parts of the plant, specifically in the chloroplast. These alkaloids are transported by the phloem to other plant organs and tissues and are predominantly accumulated in subepidermal cellular structures.61,62 The organs necessary for survival and reproduction, such as flowers and seeds, store exceptionally high amounts of defensive alkaloids.1 The seeds of Fabaceae plants are rich in alkaloids and can reach up to 3–4%, as they are moved from the senescent leaves during the growing season. In general, QAs are widely distributed in different plant parts/organs of QA-producing legumes, so there is an estimate of the alkaloid percentage gathered in each plant part, as depicted in Figure 2.
Figure 2.
General distribution of quinolizidines in different plant parts of QA-producing legumes.
Relevant Biosynthetic Remarks
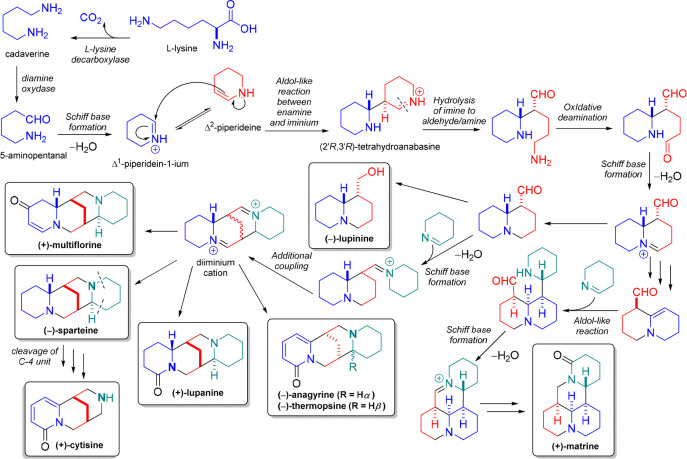
QAs are biosynthesized from the amino acid l-lysine (Figure 3). This amino acid undergoes oxidative decarboxylation due to the action of the lysine decarboxylase (LDC), a pyridoxal phosphate (PLP)-dependent enzyme.3 This enzymatic process results in cadaverine, a precursor and intermediary between l-lysine and QAs. Thus, the nitrogen atoms of the quinolizidine skeleton (C15N2) are derived from l-lysine-derived cadaverine.19 However, despite the relevant abundance and distribution of QAs, there is a lack of knowledge of QA biosynthesis, although several hypotheses and proposals have been raised in recent decades. For more information, a recent review carefully discussed the mechanistic insights into the QA biosynthesis, particularly the pathway related to the sparteine formation based on the often-ignored precursor feeding studies.20
Figure 3.
Biosynthesis of the most common quinolizidine alkaloids produced by legume plants.
The preferred hypothesis of the QA biosynthetic pathway starts with the copper amine oxidase (CAO)-catalyzed oxidative deamination of the precursor cadaverine and its subsequent cyclization to form the next important intermediary (i.e, Δ1-piperidein-1-ium cation), whose step involves an aldol-like coupling between the two piperideine-related tautomers.20 This dimerization occurs under plant physiological pH (pH = 6.5–7.0), forming two stereocenters having four potential stereochemical variants but being stereoselective to the product (2′R,3′R)-tetrahydroanabasine (THA) and, in turn, to the bicyclic (−)-lupinine.63,64 In this transformation, the imine-containing ring of THA is hydrolyzed, and another oxidative deamination proceeds (Figure 3), forming the basic quinolizidine core, achieved through a Schiff base formation.19 Although the experimental evidence remains to be generated, it has been proposed that incorporating another Δ1-piperideine molecule could produce the additional diazatetra(tri)cyclic moieties.65,66 In addition, the cleavage of the fourth ring and the oxidation to a 2-pyridone system offer a potential route to cytisine, considering that any of the outermost rings could be cleaved to produce the same product.3,67 Once the bicyclic, tricyclic, and tetracyclic structures are assembled, they can be modified by dehydrogenation, oxygenation, hydroxylation, glycosylation, or esterification (Figure 3). These transformations can afford a wide variety of structurally related quinolizidines.68 For instance, acetylated products of 13α-hydroxylupanine/13α-hydroxymultiflorine and lupinine/epilupinine are produced by acyltransferases (e.g., HMT/HLT and ECT/EFT-LCT/LFT).69 So far, only two enzymes have been identified in the QA pathway. Therefore, the discovery of biosynthetic genes remains as an opportunity for understanding the attractive chemistry and biology involved in QA biosynthesis.19,21
Structural Variations and Occurrence of Quinolizidine Alkaloids
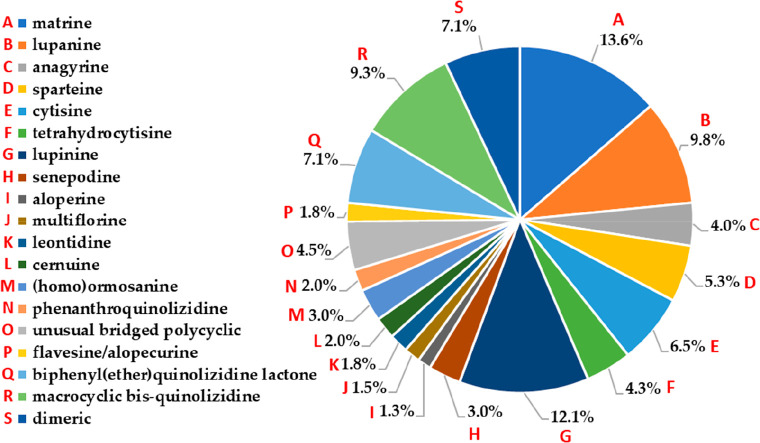
This review aimed to gather the chemical structures of naturally occurring QAs, reported between 1990 and 2023, to disclose the available QA-related chemical space. The presence of intriguing QA diversity and complexity has broadened our understanding of their structural characteristics, including their varied natural sources (plants and animals). Thus, the compiled compounds (n = 397) were mainly related to bridged or fused polycyclic QAs, including the most known QA types such as matrine, lupanine, anagyrine, sparteine, cytisine, tetrahydrocytisine, and lupinine-type compounds,18,20,66 as depicted in Figure 1. In addition, other QAs with a more complex structure were also found, such as macrocyclic bisquinolizidine and biphenyl quinolizidine lactones, and widely reported as marine natural products51 and from frog skins.46 In this context, Figure 4 shows the percentage of alkaloids subdivided into classified QA types. Matrine-type QAs are the most frequently isolated (13.6%), followed by lupinine (12.1%), lupanine-type (9.8%), macrocyclic bisquinolizidines (9.3%), and biphenyl(ether)quinolizidine lactones (7.1%). The remaining QA types encompass 48.1% of the total representatives and comprise 15 distinct structural variants (Figure 4). To disclose the QAs’ chemical diversity, the reported structural variants for each QA type and the global features of the compiled chemical space are expanded on below. To support such an expansion, the names and codified structural information on QAs (1–397) in the simplified molecular input line entry specification (SMILES) are presented in Table S1, and their structures are depicted in Figures S1–S22 (Supporting Information).
Figure 4.
Distribution of the compiled quinolizidines (n = 397) according to the structural type.
Matrine-Type QAs
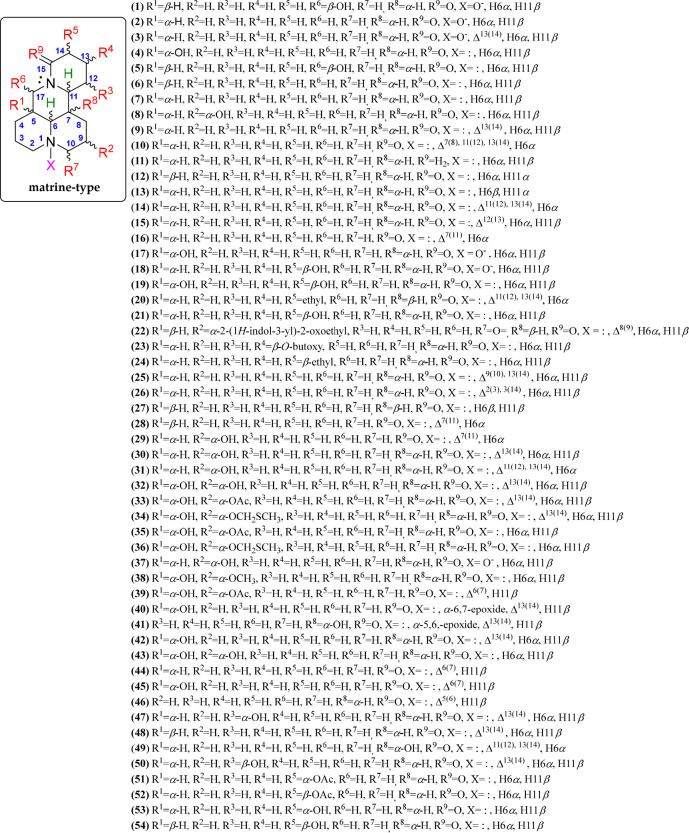
The matrine-type QAs are significant representatives and abundant in species of the Sophora genus.16 This QA type and its sources have been used in traditional Chinese medicine for many years. Matrine-type QAs contain two condensed quinolizidine units, having a 6/6/6/6 diazatetracyclic building block and forming a fused, nonlinear bisquinolizidine. More than 50 matrine alkaloids have been isolated and described thus far, and they are disclosed in compounds 1–54 (Figure 5, Figure S1). The basic structure of this type of QA is the matrine alkaloid (7), having the mentioned tetracyclic moiety formed by two quinolizidine moieties and four contiguous stereogenic centers. The relative configurations of these chiral centers in matrine-related QAs have α- (at C-5, C-6, and C-7) and β-oriented (at C-11) hydrogens. Other basic structures have β-oriented hydrogens at C-6 (14) and C-7 (e.g., 15).
Figure 5.
Matrine-type quinolizidine alkaloids 1–54.
In addition, the matrine-type QAs are sometimes found in the N-oxide form (e.g., 1, 2, 17, 18, and 37), with N-1 being the most common point for such oxidation. The matrine-type QAs 1–54 differ mainly in their substitution patterns and stereochemistry.70 They contain structural variations associated with double bonds (specifically in the D-ring) and α- or β-oriented substitutions around each tetracycle, such as hydroxyl (e.g., 1, 3, 4, 8, 18, 19, 21, 29, 30–43, 45, 47, 49, 50, 53, and 54), acetyl (e.g., 33, 35, 39, 51, and 52), epoxy (e.g., 40 and 41), (methylthio)methoxy (e.g., 34 and 36), methoxy (e.g., 38), and indolyl (e.g., 22) groups.71 Thus, nine different positions for matrine substitutions (R1 to R9) were evidenced, the structural distribution of which is outlined in Figure 5.
The matrine-type compounds (1–54) have been extensively obtained from Sophora plants (96% of records) and mostly isolated from seeds and aerial parts from S. flavescens,16S. alopecuroides,72S. tonkinensis,73S. leachiana,74S. velutina,75 and Oxytropis plants, e.g., O. ochrocephala,76,77 and even widely reported from seeds and leaves of Genista plants.27 Additionally, according to Table 1, these compounds have been mainly isolated from the aerial parts, roots, and seeds, which comprise the plant parts where the QAs accumulate the most.1
Table 1. Sources of Isolated Matrine-Type (1–54) and Lupanine-Type (55–93) Quinolizidine Alkaloids (QAs).
| QAs | Species | Plant part | Ref |
|---|---|---|---|
| 1–11 | Sophora flavescens | roots | (16) |
| 12–16 | Sophora alopecuroides | roots | (17) |
| 17–21 | Sophoratonkinensis | roots | (73) |
| 22 | Sophora alopecuroides | seed | (78) |
| 23–24 | Oxytropis ochrocephala Bunge | whole plants | (76,77) |
| 25–26 | Sophora flavescens Ait., Subprostrate sophora | roots | (79) |
| 27–28 | Sophora flavescens | roots | (9) |
| 29–31 | Sophora flavescens Ait. | chipped roots | (80) |
| 32–46 | Sophora tonkinensis Gagnep | seeds | (71) |
| 47–48 | Sophora flavescens | roots | (11) |
| 49–50 | Sophora alopecuroides | aerial parts | (4) |
| 51–54 | Sophora alopecuroides, S. tonkinensis, S. viciifolia, Thermopsis lanceolata | fresh leaves | (81) |
| 55–56 | Sophora flavescens Ait. | roots | (16) |
| 57–59 | Sophora flavescens | roots | (78) |
| 60 | Lupinus albus L. | seeds | (67) |
| 61 | Cytisus purgans | aerial parts | (82) |
| 62–64 | Lupinus angustifolius | aerial parts | (83) |
| 65 | Sophora velutina subsp. zimbabweensis | fruits and pods | (75) |
| 66–67 | Lupinus lanatus | aerial parts | (84) |
| 68 | Lupinus albus, L. angustifolius | seeds | (85) |
| 69 | Lupinus sp. | leaves | (23) |
| 70 | Gonocytisus pterocladus | whole plant | (86) |
| 71–73 | Acosmium panamense | bark | (87) |
| 74–75 | Cytisus scoparius | seeeds | (88) |
| 76 | Lupinus lanatus | seeds | (89) |
| 77–82 | Genus pearsonia | aerial parts | (88) |
| 83 | Ormosia krugii | seeds | (90) |
| 84 | Lupinus polyphyllus | leaves | (91) |
| 85 | Lygos raetam | aerial parts | (92) |
| 86–87 | Lupinus sp. | aerial parts | (93) |
| 88–93 | Personia cajanifolia subsp. Cryptantha, P. sessilifolia subsp. marginata | aerial parts | (94) |
Lupanine-Type QAs
Two condensed quinolizidine units constitute lupanine-type QAs. However, they differ from other QA types, e.g., sparteine-type, by the presence of a carbonyl group at C-2, forming a lactam moiety with N-1 as ring A. Lupanines also differ from the matrine-type compounds because they involve a 6/6/6/6 diazatetracyclic building block to form a bridged bisquinolizidine.95 So far, almost 40 lupanine-like QAs have been reported, comprising a chemical diversification illustrated by compounds 55–93 (Figure 6, Figure S2). The central structural core agrees with the alkaloid lupanine (levo or dextrorotatory, i.e., 56 or 61, respectively), which is mostly substituted in rings A and D.85 Compounds 55–93 exhibited C-3, C-4, and C-5 as preferred substitution positions in ring A, while the most substituted positions in ring D are associated with C-12, C-13, and C-15. In general, the most common substitutions are hydroxyl groups, affording alkaloids such as 5α-hydroxylupanine (57) or 13α-hydroxylupanine (63) but also ester moieties such as angeloyl (91), tigloyl (75), cinnamoyl (70), or pyrroyl (90). Common unsaturations are also present at positions Δ5(6) (55) or Δ7(17) (77), forming dehydrolupanines. Furthermore, the N-oxide derivatives at N-16 are also very common in these QA types. Ten distinct positions for lupanine substitutions (R1 to R10) were then revealed, the structural distribution of which is depicted in Figure 6.
Figure 6.
Lupanine-type quinolizidine alkaloids 55–93.
On the other hand, compounds 55–93 (lupanine-type) show their most significant occurrence in the genus Lupinus, followed by Sophora and other genera such as Genista, Acosmium, Ormosine, Thermopsis, and Cytisus (Table 1). Thus, lupanine-type QAs are abundant mainly in the Lupinus genus and distributed in the aerial parts, leaves, seeds, bark, and root,25 according to the gathered information in Table 1. More than 170 QAs have been identified in different species of the genus Lupinus.95 Some species, such as L. argenteus,96L. exaltatus,97L. angustifolius,98L. albus,67L. mexicanus,99 and L. lanatus,89 are important representatives, where the QA diversity related to lupanine-type compounds has been widely investigated.
Anagyrine-Type QAs
Anagyrine-type alkaloids are structurally very similar to lupanine-type alkaloids since they have the 6/6/6/6 diazatetracyclic building block, forming a bridged bisquinolizidine, but differ in the 2-pyridone moiety in ring A as the typical feature of these QAs. There are currently 19 anagyrines reported as natural QAs, as represented by compounds 94–109 (Figure 7, Figure S3). The alkaloids anagyrine (94) and thermopsine (95) are the representatives of this QA type, which are epimerically related and differentiated by the relative configuration of C-11 (i.e., β-H for 94 and α-H for 95). Compounds 94–109 exhibit various substitutions on ring D at C-15, C-14, and C-12, while ring C appeared to be substituted at C-17 with a methoxyoxoethyl moiety (102). In addition, the C-7–C-8–C-9 bridge is mostly α-oriented. Like lupanines, an N-oxide thermopsine derivative at N-16 was also isolated (108), and other common substitutions, such as hydroxyl, epoxy, acetyl, alkanoyl, and indolyl, are also found in these QAs. An isolated alkaloid exhibited a double bond in Δ13(14) (104), whereas other QA molecules exhibited an additional nitrogen instead of C-13 (100). Six substitution points in anagyrine-type QAs (R1 to R6) are evidenced, as illustrated in Figure 7. These 2-pyridone-containing QAs (94–109) are typical alkaloids of many Papilionoideae subfamily-belonging genera,95 such as Anagyris, Thermopsis, Genista, Clathrotropis, and Sophora (Table 2), and generally absent in Lupinus, excepting L. arboreus and L. argenteus.96
Figure 7.
Anagyrine-type quinolizidine alkaloids 94–109.
Table 2. Sources of Isolated Anagyrine-Type (94–109) and Sparteine-Type (110–130) Quinolizidine Alkaloids (QAs).
| QAs | Species | Plant Part | Ref |
|---|---|---|---|
| 94 | Anagyris fetida | aerial parts | (100) |
| 95 | Thermopsis rhombifolia, Genista sessilifolia, G. tinctoria L. | leaves | (101) |
| 96 | Sophora alopecuroides | seed | (78) |
| 97–99 | Clathrotropis glaucophylla | bark | (102) |
| 100 | Sophora grzfithii. | leafy shoots | (88) |
| 101 | Thermopsis chinensis | seeds | (93) |
| 102–109 | Thermopsis lanceolata | seeds | (103) |
| 110 | Lupinus sp. | aerial parts | (104) |
| 111–113 | Thermopsis chinensis, Laburnum watereri | leaves | (93) |
| 114 | Lupinus angustifolius | aerial parts | (83) |
| 115–119 | Lupinus sp. | aerial parts | (105) |
| 120 | Acosmium dasycarpum (Vog.) | root bark | (106) |
| 121–124 | Cytisus monspessulanus | leaves | (107) |
| 125 | Lupinus sericeus Purshl | aerial parts | (108) |
| 126 | Lygos ruetam var. surcocurpa | aerial part | (109) |
| 127 | Genista sesslifolia DC | aerial parts | (110) |
| 128 | Laburnum watereri | leaves | (93) |
| 129–130 | Lupinus varius | seeds | (111) |
Sparteine-Type QAs
(+)- or (−)-Sparteine (110 or 111, respectively) constitutes the basic structure of this type of QA. They are also constituted by a bridged tetracyclic formed by two quinolizidine units, having a 6/6/6/6 building block similar to the lupanine moiety,95 but differentiated by the absence of the carbonyl group at C-2 in ring A, mentioned above. Sparteine-type QAs appeared to have fewer reported chemical variants than lupanine-type QAs. Over 20 sparteine alkaloids have been currently reported, and their structural variations are represented by compounds 110–130 (Figure 8, Figure S4). Most of these sparteine-like compounds are characterized by an α-H at C-6 and a β-H at C-11. Generally, an α-orientation for C-7–C-8–C-9 is usually presented, with some β-oriented exceptions (121–124). Furthermore, some sparteines have substitutions at C-10 and C-17, specifically oxygenated groups (carbonyl and hydroxyl), and the presence of bulkier substitutions, such as piperidine derivatives. The substitution pattern (R1 to R11) of these QAs is depicted in Figure 8. Finally, unsaturations have also been observed, specifically at Δ2(3) and Δ5(6) in ring A and at Δ11(12), affording dehydrosparteine-like QAs (e.g., 114–116, 121, 123, 124, and 130).
Figure 8.
Sparteine-type quinolizidine alkaloids 110–130.
Regarding sparteine-type alkaloids (110–130), Lupinus plants produce and accumulate this QA type in seeds and leaves112 and other genera such as Acosmium, Lygos, and Houttuynia (Saururaceae) (Table 2). Additionally, this QA type has also been isolated from seeds, leaves, flowers, and aerial parts of species belonging to the genera Cytisus,113Ormosia,114Ulex,115Genista,116Lupinus,25 and Sophora,117 which contain structurally related sparteine-type compounds.
Cytisine-Type QAs
The cytisine-type QA is a class of bridged tricycle alkaloids containing a 2-pyridone moiety in ring A and mainly isolated from plants of the Faboideae subfamily.118 These QAs are characterized by having a 6/6/6 diazatricyclic building block, forming the base structure of 1,5-methanopyrido[1,2-a][1,5]diazocine, whose C-7–C-8–C-9 bridge has an α-orientation. Twenty-five cytisine-like alkaloids have been currently described, whose structural variations are represented by compounds 131–155 (Figure 9, Figure S5). Such variations comprise a particular substitution pattern on ring C, as shown as R1 to R3 in Figure 9. In general, the different derivatives of cytisine (132) are mostly substituted at N-12, specifically with oxide, carbonyl, hydroxyl, alkanoyl, and alk(en)yl groups. Additionally, cytisine-like QAs can be hydroxylated at C-9 (140), while other cytisines have a carbonyl group at C-11 (e.g., 148–150) or an allyl group (137), which is generated if a sparteine-like tetracycle undergoes a ring D cleavage.119 The alkaloid 3-hydroxy-11-norcytisine (156) is a cytisine-like QA isolated from Laburnum anagyroides green pods,120 having an unusual 7,11-diazatricyclo[7,2,1,02,7]dodeca-2,4-dien-6-one (6/6/5) moiety formed by the C-13 loss (Figure 9).
Figure 9.
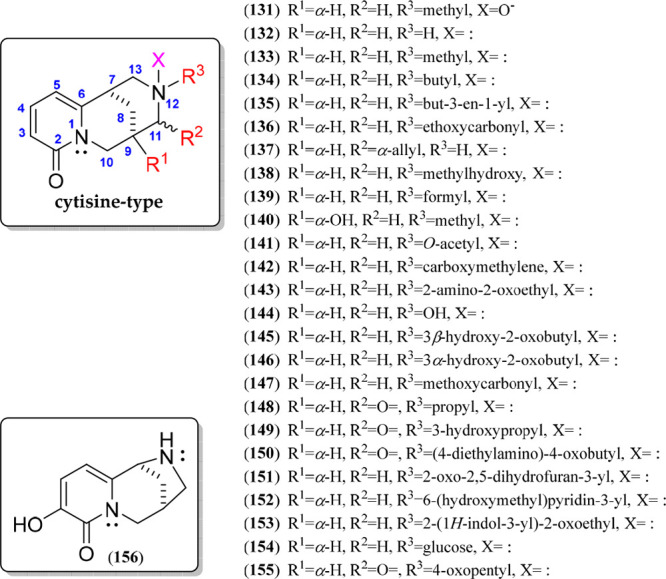
Cytisine-type quinolizidine alkaloids 131–156.
Cytisine-type QAs (131–156) are also characteristic of the Fabaceae family plant species, and their distribution is widespread in various Fabaceae genera, widely distributed in the genera Sophora, Genista, Cytisus, Osyris, Spartium, Petteria, Euchresta, Dermatophyllum, and Styphnolobium and isolated from leaves, aerial parts, roots, seeds, and flowers (Table 3). Notably, these compounds accumulate mainly in the seeds and leaves and are obtained on a commercial scale from Laburnum anagyroides (=Cytisus laburnum),121Sophora alopecuroides,4Thermopsis alterniflora,122Thermopsis lanceolata,123 and Caragana sinica.124
Table 3. Sources of Isolated Cytisine-Type (131–156) and Tetrahydrocytisine-Type (157–173) Quinolizidine Alkaloids (QAs).
| QAs | Species | Part | Ref |
|---|---|---|---|
| 131–133 | Sophora flavescens | roots | (16) |
| 134 | Sophora flavescens | roots | (125) |
| 135 | Genista quadriflora Munby | roots and aerial parts | (126) |
| 136–137 | Dermatophyllum arizonicum, Dermatophyllum gypsophilum, Dermatophyllum secundiflorum, Styphnolobium affine, Styphnolobium japonicum | leaf tissue | (119) |
| 138 | Sophora velutina subsp. zimbabweensis | fruits and pods | (75) |
| 139 | Spartium junceum | fresh flowers | (127) |
| 140 | Osyris alba L. | aerial parts | (128) |
| 141 | Euchresta tubulosa Dunn | stem | (129) |
| 142–144 | Sophora exigua | aerial parts | (88) |
| 145–146 | Sophora griffithii | leaves | (130) |
| 147 | Petteria ramentacea | buds, leaves, and flowers | (131) |
| 148–155 | Thermopsis lanceolata | seeds | (103,132) |
| 156 | Laburnum anagyroides | green pods | (120) |
| 157 | Sophora flavescens | roots | (125) |
| 158 | Genista quadriflora | roots and aerial parts | (126) |
| 159 | Guianodendron praeclarum | leaves | (133) |
| 160–161 | Lupinus angustifolius | aerial parts | (83) |
| 162 | Lupinus sp. | leaves | (23) |
| 163–164 | Templetonia biloba | leaves | (134) |
| 165 | Lupinus termis | seeds | (135) |
| 166 | Thermopsis mongolica | aerial parts | (136) |
| 167 | Lupinus termis | seeds | (137) |
| 168 | Lupinus angustifolius | seeds | (85) |
| 169 | Lupinus angustifolius, L. campestris | aerial parts | (23) |
| 170–171 | Virgilia diuaricata, V. oroboides | left | (138) |
| 172 | Lupinus albus | aerial parts | (139) |
| 173 | Lupirnus polyphyllus | stems, leaves, and pods | (140) |
Tetrahydrocytisine-Type QAs
The tetrahydrocytisine-type QAs are also characterized to be tricycles and differ from those of the cytisine type by the absence of a 2-pyridone moiety in the A ring. They also have a 6/6/6 building block, forming the base structure of a 1,5-methanopyrido[1,2-a][1,5]diazocine. The C-7–C-8–C-9 bridge can be α- or β-oriented (e.g., 158 and 157, respectively). Despite the fact that fewer chemical variants are reported for tetrahydrocytisine-type than for cytisine-type QAs, more substitutions were evidenced for the 17 alkaloids belonging to this QA class, illustrated by the structures of compounds 157–173 (Figure 10, Figure S6). Seven positions were observed to be substituted in tetrahydrocytisine-type QAs (R1 to R9, Figure 10). Tetrahydrocytisine (158) has chemical variants commonly substituted at N-12, such as cytisine-like QAs. In addition, C-13 is also substituted with an allyl group, affording angustifoline (161) derivatives. On the carbonyl group, the position can occur at C-2 in this QA type as cytisine derivatives but also at C-4 in ring A, forming a cyclohexenone moiety, typical for albine (168) derivatives. Particularly, compound 167, a Δ5-dehydroalbine, exhibited a 4-pyridone moiety.
Figure 10.
Tetrahydrocytisine-type quinolizidine alkaloids 157–173.
These tetrahydrocytisine-type alkaloids (157–173) appear as the main QA in some lupin species such as L. angustifolius.19,28 Very often, these alkaloids (such as 169) are considered minor components in Old World species (L. micranthus, L. albus), in South American species (L. gibertianus, L. mutabilis), and in North American plants (L. perennis, L. elegans, L. leucophyllus).141 The most significant accumulation of this QA type has been reported in leaves and flowers of Templetonia(134) and Lupinus(142) plants (Table 3).
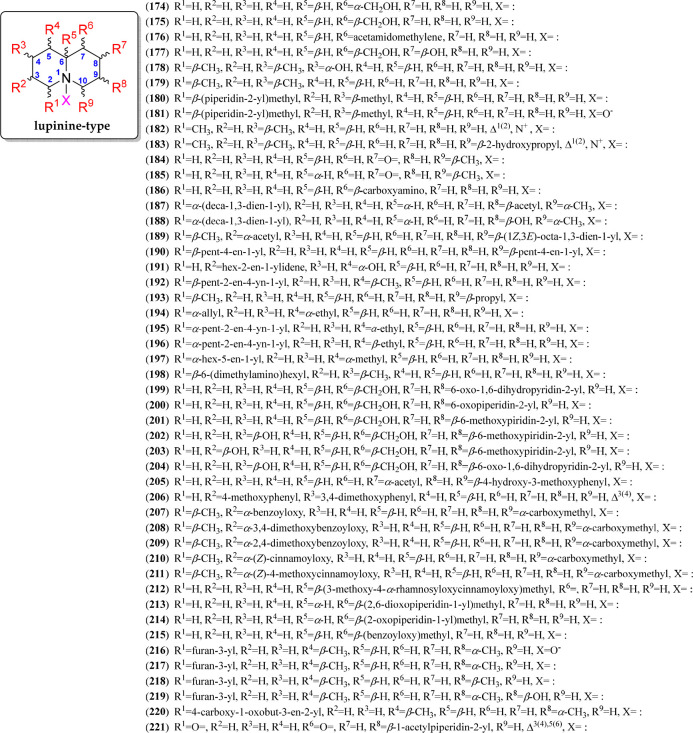
Lupinine-Type QAs
Lupinine-type QAs represent the most basic quinolizidine unit, whose building block is a 6/6, forming the 1-azabicyclo[4.4.0]decane moiety, to comprise the quinolizidine core.143 From this basic structure, based on (−)-lupinine and (+)-epilupinine (174 and 175, respectively), many substituted homologues have been identified in different plant and animal sources.22 Hence, more than 40 lupinine-like compounds have been reported, with structural variations illustrated with the reported compounds 174–220 (Figure 11, Figure S7). They are characterized by having oxygenated substitutions such as hydroxyl (177–178), hydroxyalkyl (183), acetyl (187 and 189), (substituted) benzoyl (207–209, 215), (substituted) cinnamoyl (210–212), furan-3-yl (216–220), and other substitutions associated with alk(en/in)yl (187–197, typically found in frog skins), phenyl (205–206), pyridyl (201–204), and (substituted) piperidin-1-yl (199–200, 213–214). These substitutions can be found in the different positions of the quinolizidine ring, comprising a substitution pattern represented by nine different positions (R1 to R9, Figure 11). However, substitutions at C-6 have not yet been reported but involve chemical variants with α- or β-oriented hydrogens. In addition, two iminium salts between C-2 and N-1 have also been reported (182–183) as structural variations of this type of QAs. Finally, thermlanseedline A (221) is an alkaloid containing the quinolizidine moiety coupled with an acetylpiperidyl fragment, which was proposed to be derived from thermopsine after oxidative ring D cleavage/demethylation/acetylation biosynthetic steps.132
Figure 11.
Lupinine-type quinolizidine alkaloids 174–221.
This type of alkaloid (174–221) is the most abundant in the Fabaceae family, and they generally occur in the genera Lupinus, Baptisia, Thermopsis, Maackia, Genista, Lycopodium, Ulex, Prosopis, Cytisus, and Sophora.21 The first reported structure of lupinine was carried out in 1938, isolated from the leaves of the Lupinus luteus,144 and different Lupinus genotypes have shown the presence of lupinine-type compounds.145 In addition, furan-3-yl-containing lupinine-type QAs were isolated from Nuphar plants. Additionally, 26% of records correspond to other genera of other families that can also biosynthesize QAs, such as Hypoestes (Acanthaceae), Flueggea (Phyllanthaceae), Myrioneuron (Rubiaceae), Huperzia (Lycopodiaceae), Heimia (Lythraceae), Boehmeria (Urticales), Vaccinium (Ericaceae), Croton (Euphorbiaceae), and Clavelina (Clavelinidae), whereas the other 16% of records correspond to animal species such as Solenopsis picea(49) and frog skin of the Dendrobates,146Mantella,48 and Epipedobates(46) genera (Table 4).
Table 4. Sources of Isolated Lupinine- (174-221) and Senepodine-Type (222–233) Quinolizidine Alkaloids (QAs).
| QAs | Species | Part | Ref |
|---|---|---|---|
| 174–175 | Lupinus sp. | aerial parts | (147) |
| 176 | Maackia amurensis var. Buergeri, M. tashiroi | fresh stems | (148) |
| 177 | Virgilia divaricata, V. oroboides | leaves | (93) |
| 178 | Lycopodium cernuum var. sikkimense | whole plants | (149) |
| 179–183 | Lycopodium cernuum, L. chinense | club moss | (150) |
| 184–185 | Vaccinium myrtillus | aerial parts | (43) |
| 186 | Epipedobates tricolor | skin | (151) |
| 187–189 | Clavelina picta | leaves and aerial part | (93,152) |
| 190–191 | Melanophryniscus klappenbachi, M. cupreuscapularis | skin of poison frogs | (45) |
| 192–193 | Mantella basileo | skin | (47) |
| 194–197 | Mantella baroni | skin | (48) |
| 198 | Huperzia phlegmaria | club moss | (37) |
| 199–200 | Sophora chrysophylla | bark | (153) |
| 201–203 | Ulex jussiaei | aerial parts | (154) |
| 204 | Maackia amurensis var. buergeri | leaves | (155) |
| 205 | Heimia salicifolia | leaves | (41) |
| 206 | Pilea aff. martinii | aerial parts | (156) |
| 207–211 | Cylicomorpha solmsii | leaves | (157) |
| 212 | Lupinus varius ssp. orientalis | leaves | (81) |
| 213 | Sophora nuttalliana, S. stenophylla | leaf and stem tissue | (158) |
| 214 | Bongardia Chrysogonum | tubers | (159) |
| 215 | Lupinus varius ssp. orientalis | aerial parts | (81) |
| 216–219 | Nuphar pumilum | rhizomes | (160) |
| 220 | Nuphar japonicum | rhizomes | (161) |
| 221 | Thermopsis lanceolata | seeds | (132) |
| 222–233 | Lycopodium chinense | club moss | (23,38,150,162−164) |
Senepodine-Type QAs
A group of alkaloids, also known for being part of theLycopodium QAs, comprise the C22N2 adduct formed between a lupinine-like moiety with an α- or β-oriented (1,7-dimethyldecahydroquinolin-5-yl)methyl moiety at C-10.38 (+)- and (−)-Senepodine (222 and 223, respectively) can be considered the basic structure of this QA type.165 Eleven senepodine-related compounds have been reported, having structural variations at six different positions (R1 to R6, Figure 12), involving three variations for the lupinine moiety and the other three for the decahydroquinoline substitution. The four substituting positions involved α- or β-oriented methyl groups at C-2, C-4, N-1′, and C-8′ (224–225), formyl and acetyl groups at N-1′ (226–227, 229), and piperidin-2-yl at C-2 (230–232). Iminium salts between C-2 and N-1 have also been reported (224). In addition, α- or β-oriented hydrogens can be found at C-6 and C-10′ in the lupinine and decahydroquinoline moieties, respectively (Figure 12, Figure S8). Finally, a particular senepodine-like alkaloid, consisting of a fastigiatine-quinolizidine adduct (C27N3), himeradine A (233), was isolated from Lycopodium chinense (Table 4), having the fastigiatine moiety attached to C-2 through a methylene bridge.164
Figure 12.
Senepodine-type quinolizidine alkaloids 222–233.
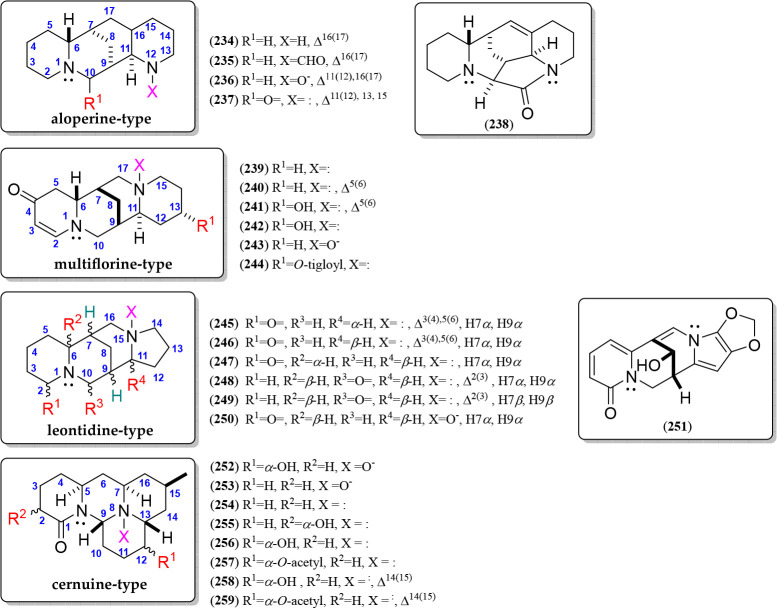
Uncommon Diazatetracyclic QAs (Aloperine, Multiflorine, Leontidine, and Cernuine Types)
These QA types (234–259) have structural similarities to sparteine or lupanine since they contain a diazatetracyclic moiety (Figure 13). However, they did not fall into the above-described QA types, but they can be gathered into four subclasses due to their structural similarities, such as aloperine, multiflorine, leontidine, and cernuine types. The first uncommon diazatetracyclic QA, i.e., aloperine type, have been isolated from Sophora and Oxytropis plants (Table 5). Thus, aloperine (234) is the base structure of this QA variant (234–237), sharing a sparteine-like structure but differing by the nitrogen position, i.e., N-12 in ring D. Aloperines have exclusively been reported with an α-oriented C-7–C-8–C-9 bridge. The other two related alkaloids have been isolated with formyl and oxide substitutions at N-12 (235 and 236, respectively)166 and have a carbonyl at C-10 and an aromatic D ring (237) (Figure 13). Ochrocephalamine D (238) is an aloperine-like QA, isolated from Oxytropis ochrocephala,166 having an additional pyrrolidin-2-one moiety formed by a further carbonyl group linked to N-12 and C-10 (Figure 13, Figure S9).
Figure 13.
Aloperine, multiflorine, leontidine, and cernuine-type quinolizidine alkaloids 234–259.
Table 5. Sources of Isolated Aloperine- (234–238), Multiflorine- (239–244), Leontidine- (245–251), and Cernuine-Type (252–259) Quinolizidine Alkaloids (QAs).
| QAs | Species | Part | Ref |
|---|---|---|---|
| 234 | Sophora alopecuroides | seeds and leaves | (169) |
| 235–238 | Oxytropis ochrocephala Bunge | whole plant | (166,170) |
| 239 | Lupinus lanatus | aerial parts | (84) |
| 240–242 | Lupinus albus | seeds | (171) |
| 243 | Lupinus hirsutus Linn | seedlings | (172) |
| 244 | L. albus, L. varius, L. orientalis, L. hartwegii, L. densiflorus | whole plant | (173) |
| 245 | Leontice ewersmannii | leaves | (174) |
| 246–248 | Orphanodendron bernalii, O. grandiflorum | leaves | (133) |
| 249 | Guianodendron praeclarum | leaves | (133) |
| 250 | Maackia tashiroi | stems | (175) |
| 251 | Sophora velutina subsp. zimbabweensis | fruits and pods | (75) |
| 252–253 | Lycopodium cernuum, L. chinense | club moss | (150) |
| 254–259 | Lycopodium cernuum var. Sikkimense | club moss | (149) |
On the other hand, (−)-multiflorine (239) comprises the basic structure of another related QA type, with a particularly high occurrence in the genus Lupinus(167) (Table 5), differing from lupanine by the C-4 carbonyl group, a nitrogen position at the D-ring (i.e., N-16), and a characteristic double bond at C-2. The β-oriented C-7–C-8–C-9 bridge has exclusively been reported for these QAs. Five additional multiflorine-like alkaloids have also been isolated (239–244), representing the structural variations of this QA type, which involve a further double bond at C-5 (240), a hydroxyl group at C-13 (241–242), an oxide at N-16 (243) or O-tigloyl ester (244) (Figure 13, Figure S10).
Another type of lupanine-like diazatetracyclic QA is related to leontidine (245), widely distributed in the genera Camoensia, Guianodendron, and Orphanodendron (Table 5), which differs from lupanine by the presence of a five-membered D-ring instead of a six-membered one, forming a quinolizidine/indolizidine adduct.59 This type of QA exhibits an α- or β-oriented C-7–C-8–C-9 bridge and a carbonyl group at C-2 (e.g., 245–247, 250) but also at C-10 (e.g., 248–249). In addition, unsaturations at C-2 (248–249), C-3 and C-5 (245–246), and the oxide group at N-15 (250) can also be found. In addition, velutinine (251) is a highly unsaturated leontidine-like alkaloid isolated from Sophora velutina subsp. Zimbabweensis stem bark,75 having a hydroxyl at C-8, an α-pyridone moiety at ring A, and a methylenedioxy group at C-13/C-14 (Figure 13, Figure S11).
Finally, cernuine (254) is the representative alkaloid of a QA type constituted by a tetradecahydro-2H-pyrido[1′,2′:3,4]pyrimido[2,1,6-de]quinolizine moiety, which is also known to be part of the Lycopodium alkaloids168 (Table 5). Eight cernuine-type QAs (252–259) have been reported, characterized by having a carbonyl group at C-1 and can involve an α-oriented hydroxyl or acetyl group at C-12 (252, 256–259) or an α-hydroxyl at C-2 (255). They also have a ΔC14(15) unsaturation (258–259) and an N-oxide at N-8 (252–253) (Figure 13, Figure S12).
Triazapolycyclic QAs (Ormosanine- and Homoormosanine-Type)
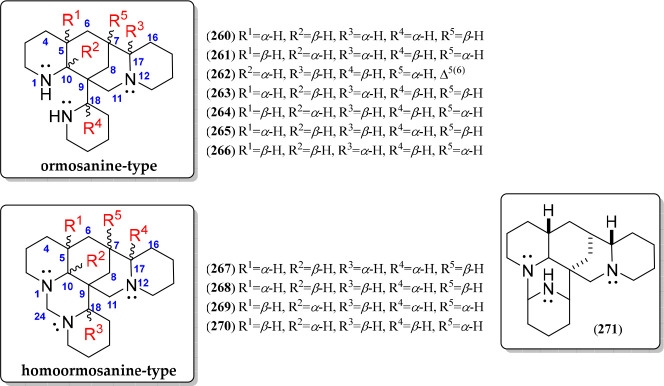
An interesting QA type involves those compounds having triazapolycyclic moieties (260–271) being related to (+)- or (−)-ormosanine (260 or 264, respectively) and homoormosanine (267) as the basic structures (Figure 14). The ormosanine-type alkaloids (260–266) have a diazatetracycle moiety bonded with a piperidine unit at C-9, commonly distributed in the Podopetalum(176) and Bowdichia(177) genera (Table 6). The other four ormosanine-like diastereomers have been reported due to the configuration of six chiral carbons (i.e., C-5, C-7, C-9, C-10, C-17, and C-18) (261, 263, 265, and 266). Finally, a Δ5(6)-containing structural variant of 264 was also reported ((−)-podopetaline, 262).24 On the other hand, the homoormosanine-type QAs are based on a singular triazahexacyclic structure (267–271) involving an aloperine moiety fused with an additional azabicyclic fragment bonded at N-1 and C-9 of the aloperine moiety (Figure 14, Figure S13). The additional azabicyclic moiety can be formed through a methylenediaza bridge (267–270) or an N-1–C-20 linking (271). Homoormosanine (267) has these three epimeric variants (268–270) differentiated by the α/β-oriented hydrogen patterns at five chiral carbons, i.e., C-5, C-6, C-8, C-21, and C-22 (R1 to R5, Figure 14, Figure S14).
Figure 14.
Ormosanine- and homoormosanine-type quinolizidine alkaloids 260–271.
Table 6. Sources of Isolated Ormosanine- (260–266) and Homoormosanine-Type (267–271) Quinolizidine Alkaloids (QAs).
Phenanthroquinolizidine QAs
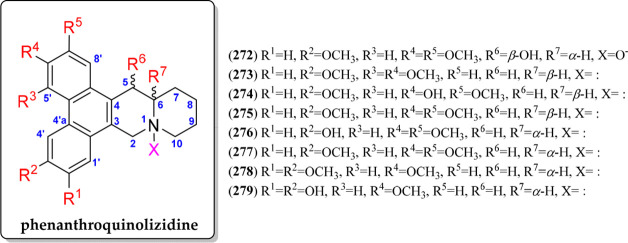
This QA type has a phenanthrene moiety fused with a quinolizidine, sharing the carbons C-3–C-4 of the quionolizidine fragments (272–279). The alkaloids (+)- or (−)-cryptopleurine (275 or 277, respectively) can be considered to be the basic structure of this QA type (Figure 15, Figure S15). Apart from 275/277, six phenanthroquinolizidine QAs have been additionally reported (272–274, 276, 278–279), varying by six different substitutions (R1 to R6, Figure 15) involving hydroxyl or methoxyl groups and the α- or β-orientation of H-6. A representative of this QA type was first isolated in 1935 from the species Tylophora indica. In addition, the genus Tylophora, Pilea, Boehmeria, and Hypoestes are the reported plant sources of these particular QAs (Table 7).
Figure 15.
Phenanthroquinolizidine alkaloids 272–279.
Table 7. Sources of Isolated Phenanthroquinolizidines (272–279), Unusual Bridged Polycycles (280–297), Flavesine (298–301), and Alopecurine-Type (302–304) Quinolizidine Alkaloids (QAs).
| QAs | Species | Part | Ref |
|---|---|---|---|
| 272 | Hypoestes forskaolii | aerial part | (34) |
| 273–275 | Pilea aff. martinii | leaves | (179) |
| 276 | Tylophora indica | aerial parts | (180) |
| 277 | Tylophora indica | leaves | (181) |
| 278–279 | Boehmeria siamensis | whole plants | (42) |
| 280 | Acosmium dasycarpum (Vog.) Yakovlev | root bark | (106) |
| 281–282 | Acosmium panamense | seed | (182) |
| 283 | Acosmium dasycarpum (Vog.) Yakovlev | root bark | (106) |
| 284 | Guianodendron praeclarum | leaves | (133) |
| 285–293 | Flueggea virosa | twigs and leaves | (35) |
| 294–297 | Myrioneuron faberi | aerial parts | (36) |
| 298–303 | Sophora flavescens | roots | (183) |
| 304 | Oxytropis ochrocephala Bunge | whole plant | (170) |
Unusual Bridged Polycyclic QAs
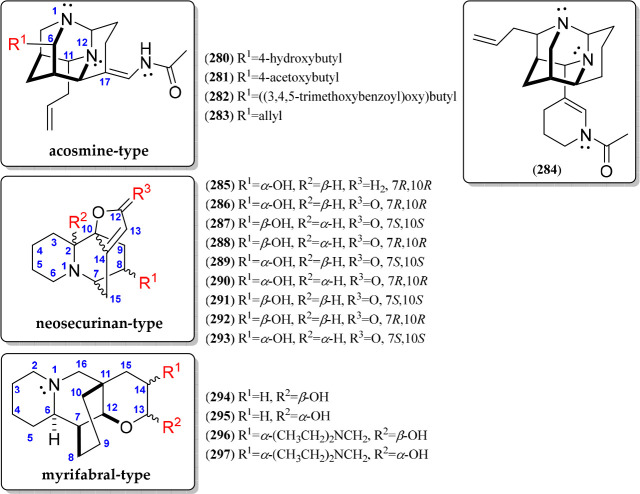
Other QA types can be gathered into unique alkaloids containing unusually bridged polycycles (280–297). In this group, the N1,N12-diaza-adamantane alkaloids are included (280–283), highly isolated from the genus Acosmium; therefore, acosmine (280) is the basic structure for this kind of alkaloid106 (Figure 16, Figure S17). Few structural variants have been reported for the acosmine-type QAs, involving a substitution at C-6 (R1), which comprises esterified 4-hydroxybutyl chains (282–282) or an allyl group (283). In addition, panacosmine (284) is a special diaza-adamantane alkaloid since it involves a 1-acetyl-1,4,5,6-tetrahydropyridin-3-yl substitution (284) instead of an acetamidomethylene (280) in the absence of the 4-hydroxybutyl substitution (Figure 16). On the other hand, neosecurinan (285) is an interesting hexahydro-2H,7H-5,10b-ethanofuro[2,3-a]quinolizine-containing QA, which was isolated for the first time in 1956 from the genus Securinegaen (Phyllanthaceae).35 Eight securinol-type stereoisomers have been isolated (286–293) (Figure 16, Figure S17) from twigs and leaves of Flueggea virosa (Phyllanthaceae).35 These stereoisomers differed from 285 by the presence of a carbonyl group at C-12, forming a furan-2(5H)-one moiety, whose differences between them are related to the absolute configuration of C-2, the C-7–C-15–C-14–C-10 bridge, and the carbinol carbon at C-835 (Figure 16). Finally, myrifabral-type QAs (294–297) are an uncommon group of QAs possessing a particular cyclohexane-bridged, tetrahydro-2H-pyran-fused quinolizidine skeleton (Figure 16, Figure S18), involving two pairs of epimers at C-13 (α- or β-OH as R2) and an α-oriented (diethylamino)methyl group at C-14 (R1) (296–297). These QAs represent the first quinolizidine alkaloids of the genus Myrioneuron.36
Figure 16.
Unusual bridged polycyclic quinolizidine alkaloids 280–297.
Modified Matrine-Related QAs (Flavesine- And Alopecurine-Type)
Flavesine-type QAs (298–301) represent a particular group of modified alkaloids isolated from Sophora and Oxytropis plants (Table 7). They have a matrine-like structure with an open-loop ring D, forming a 3-carboxypropyl moiety and having structural variations related to the unsaturation pattern in ring C (298–300) and a piperidine amide (301) (Figure 17, Figure S19).183 Other modified matrine-related QAs involve alopecurine A (302) or B (303), which constitutes the first reported example of a matrine-type alkaloid with C-5–C-6 and C-6–C-7 bond fragmentations,183 respectively, and the ochrocephalamine E (304), which was identified as a 14-nor methylene matrine with a unique 6/6/6/5 ring system170 (Figure 17, Figure S19).
Figure 17.
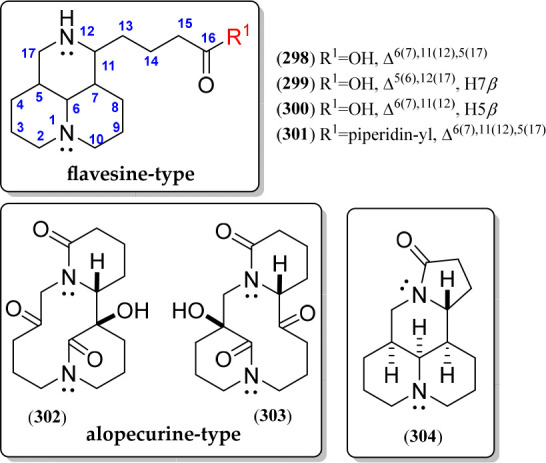
Flavesine and alopecurine quinolizidine alkaloids 298–304.
Biphenyl and Phenyl Ether Quinolizidine Lactones
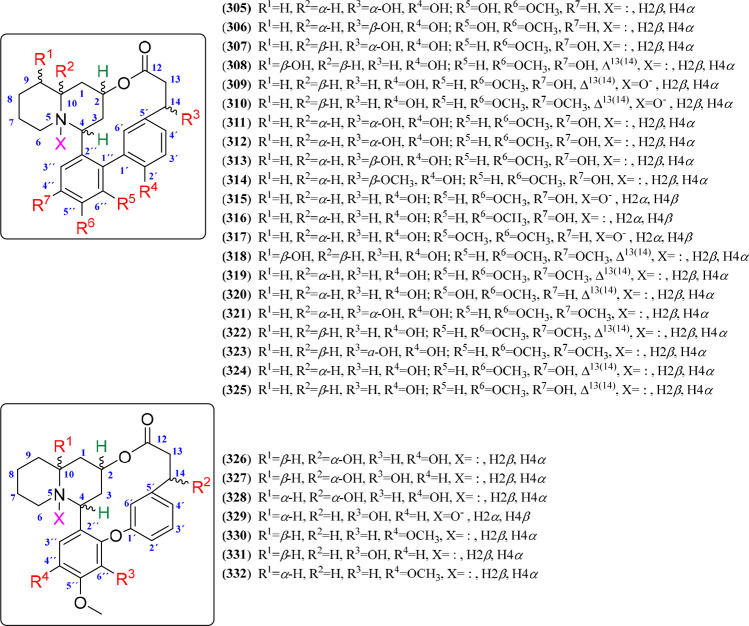
This QA type corresponds to a complex alkaloid class particularly occurring in Lythraceae plants,41 typified by having biphenyl or phenyl ether quinolizidine lactone skeletons. The structure contains quinolizidine (rings A and B) and biphenyl or phenyl ether (rings C and D), connected by C-4–C-2″ and C-2–O–C-12 bonds for both skeletons and the C1′–C1″ or C1′–O–C1″ bond for biphenyl or phenyl ether, respectively, forming the typical dodecano-12-lactone or 7-oxatridecane-13-lactone moiety, respectively. The basic structural core is represented by the alkaloid lythrine (319) or lagerine (331), although some of them are characterized by a hydroxyl group at C-14, involving those QAs related to lythridine (321).39,40 In this regard, various phytochemical studies have led to the isolation of 21 biphenyl-containing (305–325) and seven phenyl-ether-bearing (326–332) QAs, whose structural variations are depicted in Figures 18 and S20, implying seven substituting positions (R1 to R7) for biphenyl and four substituting positions (R1 to R4) for phenyl ether quinolizidine lactones. Such variations comprise hydroxyl or methoxyl groups at C-2′, C-4″, C-5″, and C-6″ as the distinctive substitution pattern on biphenyl and phenyl ether substructures. In addition, hydroxyl substitutions at C-9 (e.g., 308) or C-10 (e.g., 326), having α- and β-orientation, respectively, also occurred. Some variants include an N-oxide group at N-5 (309–310, 315, 317, 329), which was reported for the first time from Lagerstroemia indica (Lyrthraceae).184 Finally, reported quinolizidine lactones (90%) include α-hydrogen at C-10. In addition, these lactones have also been isolated from Heimia (Lythraceae) plants (Table 8).
Figure 18.
Biphenyl (305–325) and phenyl ether (326–332) quinolizidine lactones.
Table 8. Sources of Isolated Biphenyl (305–325), Phenyl Ether (326–332), and Macrocyclic (305–332) QAs.
| QAs | Species | Part | Ref |
|---|---|---|---|
| 305–314 | Heimia salicifolia | leaves | (39,40) |
| 315–317 | Lagerstroemia indica | aerial parts | (184) |
| 318–328 | Heimia salicifolia | leaves | (41) |
| 329 | Lagerstroemia indica | aerial parts | (184) |
| 330–332 | Heimia salicifolia | leaves | (40) |
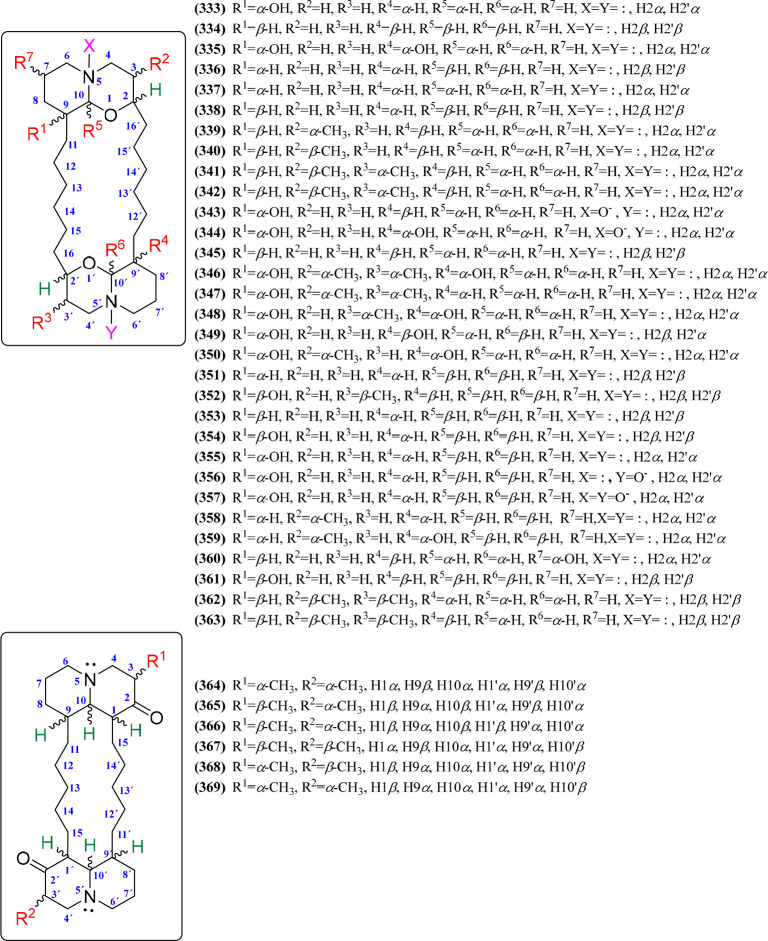
| 333 | Xestospongia muta | sponges | (15) |
| 334 | Neopetrosia chaliniformis | sponges | (185) |
| 335 | Xestospongia muta | sponges | (15) |
| 336 | Neopetrosia chaliniformis | sponges | (185) |
| 337 | Xestospongia muta | sponges | (15) |
| 338 | Neopetrosia chaliniformis | sponges | (185) |
| 339–342 | Xestospongia sp. | sponges | (186) |
| 343–344 | Xestospongia exigua | sponges | (187) |
| 345 | Neopetrosia exigua | sponges | (55) |
| 346–349 | Xestospongia muta | sponges | (15) |
| 350 | Haliclona exigua | sponges | (188) |
| 351–354 | Xestospongia exigua | sponges | (189) |
| 355–359 | Oceanapia sp. | sponges | (190) |
| 360–362 | Xestospongia sp. | sponges | (191,192) |
| 363 | Neopetrosia exigua | sponges | (193) |
| 364–365 | Xestospongia muta | sponges | (15) |
| 366 | Petrosia seriata | sponges | (51) |
| 367 | Xestospongia exigua | sponges | (194) |
| 368–369 | Neopetrosia chaliniformis | sponges | (185) |
Macrocyclic Bisquinolizidines
Macrocycle-containing QAs are a class of marine natural products found mainly in sponges, which are considered to be biogenetically derived from bis-3-alkylpyridine units.185 Araguspongins/xestospongins and petrosins are the two distinct macrocyclic QA subtypes, chemically characterized by possessing bis-1-oxaquinolizidine (6/6) and bisquinolizidine-2-one (6/6) moieties, respectively.55 Thus, the bis-1-oxaquinolizidine units are connected by two six-carbon chains at C-2 and C-9 of each quinolizidine unit (i.e., C-2–(CH2)6–C-9′ and C-9–(CH2)6–C-2′), while the two bisquinolizidine-2-one units have the C-1–(CH2)5–C-9′ and C-9–(CH2)5–C-1′ connectivity, which comprise the respective macrocyclic substructure.54 Thirty-seven chemical variants are reported for macrocyclic QAs, and the bis-1-oxaquinolizidine-containing QAs (n = 31) are more abundant than bis-quinolizidine-2-one-containing QAs (n = 6), the structures of which are depicted in Figures 19 and S21. In the case of araguspongins/xestospongins (333–363), the chemical diversity is mainly represented by stereochemical variations in six positions (R1 to R6, Figure 19). Hence, hydroxyl groups at C-9(9′) (R1 and R4), methyl groups at C-3(3′) (R2 and R3), hydrogens at C-10(10′) (R5 and R6), and the connecting six-carbon chains can exhibit α- or β-orientation. A similar case is found for petrosins (364–369), having stereochemical variations at C-1(1′), C-9(9′), C-10(10′) (having α- or β-oriented hydrogens), and C-3(3′) (having α- or β-oriented methyl groups; Figure 19, Figure S21). These marine-origin macrocycles are mainly found in the genera Xestospongia, Neopetrosia, and Petrosia, belonging to the Petrosiidae family, but also from Haliclona (Chalinidae) and Oceanapia (Phloeodictyidae) (Table 8).
Figure 19.
Macrocyclic bisquinolizidines 333–369.
Dimeric QAs
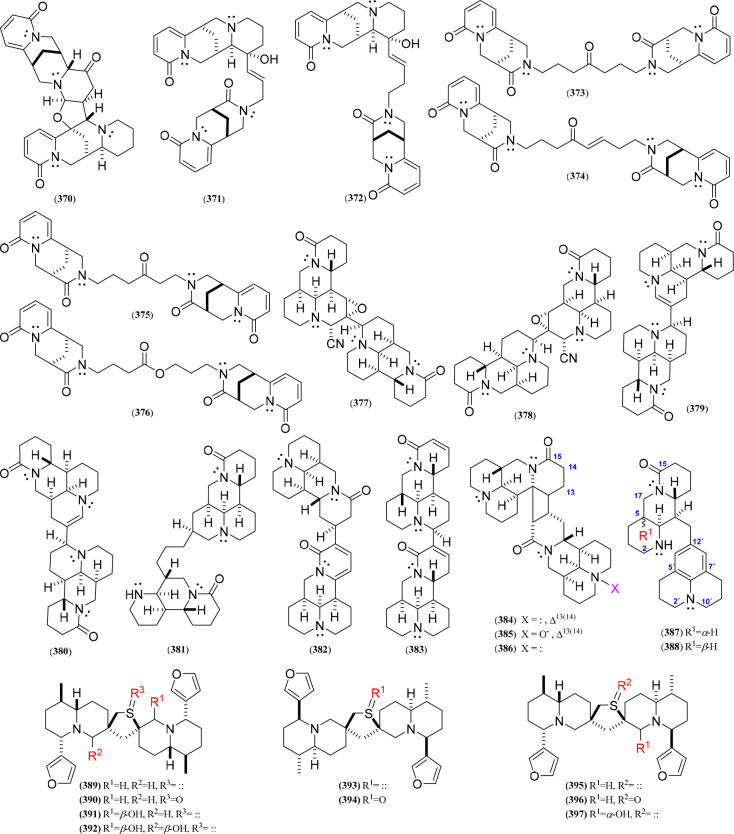
Within this group are gathered those QAs that have unusual patterns to afford dimers or form interesting two-pair quinolizidine adducts (Figure 20, Figure S22), isolated from plants of the genera Thermopsis, Sophora, Oxytropis, and Nuphar (Table 9). In this sense, a series of QA adducts (370–376), namely, thermlanseedlines B–F and thermseedlines F–G, were isolated from Thermopsis lanceolata,103,132 involving thermopsine or cytisine dimers or thermopsine/cytisine adducts. In this regard, a thermopsine dimer involved linking through an additional tetrahydrofuran ring (370), whereas the thermopsine/cytisine adducts (371–372) comprised an alkenyl chain between C-12 and N-12 of the 12-hydroxythermopsine and 11-oxocytisine moieties, respectively. In addition, the cytisine dimers contained an N12,N12′-oxoalkyl (373–375) or N12,N12′-alkoxyoxoalkyl (376) bridge between two 11-oxocytisine units. On the other hand, matrine-type dimers have also been discovered, mostly isolated from Sophora alopecuroides, which include different dimerization patterns, e.g., C-9–C-2′ (377, 379), C-10–C-3′ (378, 380), C-13–C-14′ (382), C-10–C-14′ (383), and C-11/C-12–C-13′/C-14′ (384–386), involving various substituted matrine units. Furthermore, other matrine adducts also involved nor-matrine derivatives, which contain loops formed by ring cleavage, such as ring A of the second matrine unit attached at C-3 of the first matrine unit (381) or ring B in the first unit linked to C-9 of a julolidine unit, representing rare epimeric nor-matrine/julolidine alkaloids (387–388) isolated from seeds.78 Finally, the dimeric thiospirane quinolizidines (389–397), particularly isolated from Nuphar plants and known consequently as Nuphar alkaloids,160 are composed of two lupinine-type units (usually substituted by α/β-methyl, α/β-furan-3-yl, and α/β-hydroxyl groups at C-6, C-3, and C-10, respectively) and connected by a spirocyclic tetrahydrothiophene ring. In addition, the sulfur can be oxygenated, forming a sulfoxide group (390, 394, and 396), or unfunctionalized (389, 391–393, 395, and 397).
Figure 20.
Dimeric quinolizidine alkaloids 370–397.
Table 9. Sources of Isolated Dimeric (370–397) Quinolizidine Alkaloids (QAs).
| QAs | Species | Part | Ref |
|---|---|---|---|
| 370–376 | Thermopsis lanceolata | seeds | (103,132) |
| 377–381 | Sophora alopecuroides L. | aerial parts | (195) |
| 382 | Oxytropis ochrocephala Bunge | whole plant | (196) |
| 383 | Sophora alopecuroides L. | seeds | (78) |
| 384–386 | Sophora alopecuroides | leaves | (197) |
| 387–388 | Sophora alopecuroides L. | seeds | (78) |
| 389–394 | Nuphar pumilum | rhizomes | (160) |
| 395 | Nuphar Alkaloids | rhizomes | (198) |
| 396–397 | Nuphar pumilum | rhizomes | (160) |
Bioactivity of Quinolizidine Alkaloids: An Overview
There is relevant chemical QA variability and a wide distribution in animal and plant sources, as described above (Figures 1–20, Tables 1–9). Apart from this generous chemodiversity and origin, these QA groups have great importance due to their biological activity18 but are also recognized as toxic agents.199 For instance, the QAs present in seeds, pods, leaves, aerial parts, and roots of some genistoid plants have been broadly studied, and the alkaloids causing the lupin bittering contain mostly lupanine, lupinine, and hydroxylupanine.53 Such bitter-related QAs have an excitatory effect on the CNS, depressing the respiratory and vasomotor centers, mainly observed in sheep,200 and exhibiting acute anticholinergic toxicity. These facts promoted lupin seed debittering or the research on low QA-containing lupin varieties since lupin seeds are good food options due to their protein content and quality.201 Likewise, “twisted calf disease” cases have been reported in cattle due to the anagyrine presence in some herbaceous plants, which is responsible for teratogenic effects.202,203 However, QAs have other relevant biological activities that can be exploited for several applications. In general, matrine-type QAs are mostly cytotoxic and anticancer bioactive (e.g., 7), whereas lupanine-type (e.g., 57–59) and sparteine-type (e.g., 110–113) QAs have potential against insects and microorganisms. Likewise, the cytisine and tetrahydrocytisine-type QAs have effects as cytotoxic and antiviral agents (e.g., 132–133, and 157), and in the case of the lupinine and macrocycle types, they exhibited relevant antiviral and anticancer properties. In this context, the bioactivities of the most abundant QAs, such as matrine, lupanine, sparteine, cytisine, lupinine, and other QA types, are described below and summarized in Table 10, focusing specifically on the most promising results of the alkaloid types.
Table 10. Overview of Biological Activities of Quinolizidine Alkaloids (QAs).
| QAs | Activity tested | Outcome | Ref |
|---|---|---|---|
| 4, 131–133 | NO production in LPS-stimulated RAW 264.7 cells | IC50 = 22.1 μM | (16) |
| 4–5, 27–28 | Effect against HL-60, A-549, and SW480 cell lines | IC50 < 50 μM | (9) |
| 7 | Inhibition of Botryosphaeria dothidea mycelial growth | MIC = 1.682 mg/mL | (218) |
| 7 | Acaricidal (Tetranychus cinnabarinus) and aphicidal (Aphis citricola) activities | LC50 < 2 mg/mL | (217) |
| 12–13 | Cytotoxic activity (endothelial cells) | IC50 = 15.2 μM | (16,214) |
| 12–16 | Cytotoxic activity | IC50 = 57.8 μM (HepG-2) and 83.1 μM (CNE-2) for 12 | (242) |
| 17–21 | In vivo anti-inflammatory activity | Significant inflammation reduction of 17 and 19 | (73) |
| 22 | Antiviral activity against the hepatitis B virus | 53.8% inhibition under the noncytotoxic concentration of 0.035 mM | (78) |
| 23 | Cytotoxic activity | IC50 = 20 μM (A-549) | (76) |
| 32–46 | Insecticidal activity | LC50 < 50 mg/mL | (71) |
| 47–48 | Antiviral activity against the hepatitis B virus | 48.3–79.3% inhibition | (11) |
| 47–48 | Antiviral activity against the hepatitis B virus | 41.3% inhibition | (11) |
| 55–56 | Cytotoxic activity | 56 inhibit the growth of GSC-3# at 20 μg/mL | (16) |
| 56 | Glucose homeostasis | Improved glycemic control at 1 mM | (222) |
| 57 | Antiviral activity against the hepatitis B virus | 53.8% inhibition | (125) |
| 57–59, 134, 157 | Antibacterial activities against Staphylococcus aureus and Escherichia coli | 8 μg/mL < MIC < 32 μg/mL | (125) |
| 65 | Antibacterial activity against Pseudomonas aeruginosa and Enterococcus faecalis | 10.9 μg/mL < MIC < 20.8 μg/mL | (75) |
| 66–67, 239 | Antibacterial activity against: Staphylococcus aureus, S. epidermides, S. saprophyticum, and Streptococcus pyogenes | 25 μg/mL < MIC < 100 μg/mL | (84) |
| 110–113 | Insecticidal activity against Spodoptera frugiperda | Mortality = 83% at day 7 for 110 | (82,223,243,244) |
| 132 | Cytotoxic activity | IC50 = 5.36 μM, inducing apoptosis. | (229,231) |
| 132 | Antiviral activity against human influenza virus A | IC50 < 200 μM | (235) |
| 132 | Cytotoxic activity | Cell percentage in the G2/M phase at 24 h increased from 21.1 to 50.0% (HEK293, Hep-G2, and Jurkat cell lines) | (229) |
| 138 | Antibacterial activity against E. faecalis | MIC = 208.3 μg/mL | (75) |
| 148–155 | Insecticidal activity against Aphis fabae | LC50 < 50 μg/mL | (103,132) |
| 148–155 | Inhibition against tomato spotted wilt virus | Protective effect >70% | (103,132) |
| 161 | Cytotoxic activity | IC50 = 10 μM (COLO-205) | (245) |
| 179–183 | Cytotoxic activity and acetylcholinesterase inhibition | IC50 < 330 μM (AChE) | (150) |
| IC50 < 5.5 μg/mL (L1210) | |||
| 189 | Neuronal nicotinic acetylcholine receptors | IC50 = 1.5 μM (α4β2-nAChRs) and 1.3 μM (α7-nAChRs) | (152) |
| 198 | Cytotoxic activity HL-60 cells | IC50 = 39 μM (HL-60) | (37) |
| 205, 311–314 | Antimalarial activity | IC50 < 5 μg/mL (P. falciparum) | (40) |
| 333–337 | Cytotoxic activity | IC50 < 1.02 μM against all tested cancer cell lines | (15) |
| 234 | Cell growth and in vitro tumorigenesis of human thyroid cancer cells | IC50 < 161.7 μM (IHH-4) | (246) |
| 234 | Inhibition of human immunodeficiency virus 1 | EC50 = 1.2 and 1.6 μM (NL4-3 and YU2) | (240) |
| 234–237 | Anticonvulsant effect | 234 exhibited a better anticonvulsant effect | (17,240) |
| 272 | Antimalarial activity | IC50 < 6.11 nM (P. falciparum) | (34) |
| 276 | Cytotoxic activity | IC50 = 1.6 nM (A375), 2.5 nM (A549), 1.4 nM (HCT116) and 4.1 nM (Namalwa–Burkitt’s lymphoma) | (180) |
| 278–279 | Cytotoxic activity | 0.2 ng/mL < IC50 < 100 ng/mL | (42) |
| 294–297 | Inhibition of hepatitis C virus replication | 0.9 μM < IC50 < 4.7 μM | (36) |
| 303 | Antiviral activity against the hepatitis B virus | 46.0–14.1% inhibition | (183) |
| 311–314 | Antimalarial activity | IC50 = 4.76 μg/mL (P. falciparum) | (40) |
| 333, 335, 337 | Cytotoxic activity | IC50 < 1.02 μM against all tested cancer cell lines | (15) |
| 337–339 | Cytotoxic activity | ED50 > 20 μM | (166) |
The matrine-type alkaloids have been reported as the most biologically active QAs,6 exhibiting a wide spectrum of biological properties, including antitumor,204 antiviral,205 and anti-inflammatory10 activities. In addition, they have attracted attention due to their capacity to reduce hand and foot diseases caused by enterovirus (EV-71), which does not have an available vaccine. Hence, therapeutics based on derivatives of 1 have shown relevant results that could lead to the management and control of EV-71 (e.g., 34–35) in the future.205 Moreover, matrine derivatives (e.g., 3) have reduced disease symptoms by compensating for the decreased T-cell levels.206 It has also been suggested that 12 can inhibit and suppress the expression of TLR4, a pattern recognition receptor whose activation produces pro-inflammatory cytokines.207 Compounds 1–17 have been reported to inhibit the growth of malignant cells and tumors with promising results (IC50 < 20 μM against different cancer cell lines) through proliferation inhibition and apoptosis induction, whose advances were compiled in a comprehensive review recently published on the anticancer properties of 1 and its derivatives.208 However, matrine and some naturally occurring derivatives are limited by various factors (i.e., toxicity, bioavailability, and low water solubility), and different matrine-inspired compounds have been synthesized to improve the inhibitory action against cancer cells.208 Compound 15 can improve the clinical signs of experimental autoimmune encephalomyelitis (EAE).209,210 These studies have reported that 15 delays the disease progress, attenuates the clinical severity of EAE when tested in rats, decreases inflammation and demyelination generated in the brain, and suppresses apoptosis of oligodendrocytes (OLG) in the rat central nervous system.211
Compounds 1–11 exhibited cytotoxic, anti-inflammatory, and antianaphylactic activities. Recent studies have examined the expression of the hypoxia-inducible 1-alpha factor (HIF-1a) and endothelial vascular growth factor in different phases of human hemangioma (HA). At different concentrations (0–2 μg/μL) of 2, it was demonstrated that the HIF-1a expression increases significantly in the proliferation phase of HA but decreases in the involuntary phase of HA.212 On the other hand, alkaloid 9 has been widely studied for its anti-inflammatory properties. Recent studies have shown its excellent effects against lupus nephritis (LN) and lupus erythematosus (SLE) since it reduces the inflammatory response and inhibits the activation of the inflammatory NLRP3.213,214 Additionally, compound 2 has also been reported to inhibit epidermal growth factor receptor (EGFR) related signaling pathways from suppressing the proliferation and invasion of malignant cells responsible for gastric cancer.215 Alkaloid 2 significantly inhibited migration and invasion of human gastric cancer cells by decreasing phospho-cofilin (Ser3) and phospho-LIMK1 (Thr508) without changing the total expression of cofilin and LIMK1.216 In addition, alkaloid 7 showed acaricidal and aphicidal effects on Tetranychus cinnabarinus and Aphis citricola,217 respectively, and antifungal activity against Botryosphaeria dothidea(218) and Fusarium oxysporum.219
It has been reported that the consumption of seeds from Lupinus plants containing alkaloid 62 has led to intoxication events in humans due to the acute anticholinergic toxicity of some lupanine-type QAs. The most common symptoms are blurred vision, dry mouth, easy flushing, and confusion.201 Such symptoms are reported in a human who consumed 0.5 L of bitter water from Lupinus seeds. The immediate symptoms were weakness, accelerated palpitations, extrasystoles, and different anticholinergic symptoms.220 According to the antecedents, the lethal dose in rats for alkaloids 62 and 63 was investigated, determining a DL50 = 1664 mg/kg.221 However, other lupanines, e.g., 56, positively influenced pancreatic cells in an animal model of type-2 diabetes mellitus.222 In the presence of glucose at 15 mM, insulin secretion was significantly elevated by compound 56 (0.5 mM). At the same time, the alkaloid did not stimulate insulin release with lower glucose concentrations, suggesting that 56 improved glycemic control in response to an oral glucose tolerance test in streptozotocin-diabetic rats.222 In this context, the effect on insulin secretion of three alkaloids isolated from Lupinus has recently been studied, such as compounds 56, 59, and 70, along with a synthetic derivative involving in vitro evidence of an increase in glucose-induced insulin release, whose effect intensity depended on glucose concentration and ATP-sensitive K channel blocking.32 Also, various lupanine-type QAs have shown cytotoxic activities, such as 56 and 61 against human glioma stem cells GSC-3#,16 human breast cancer (MDA-MB-231), and human lung cancer (A549).82 Furthermore, esterified lupanines, such as 64 and 67, exhibited high deterrent effects against coleopteran and lepidopteran insects such as Spodoptera frugiperda(223) and Choristoneura fumiferana,224 as well as antibacterial activity by 65 and 66 against Pseudomonas aeruginosa, Enterococcus faecalis,75Staphylococcus aureus, S. epidermides, S. saprophyticum, and Streptococcus pyogenes.84
Sparteine-type alkaloids are relevant QAs since studies report their neuroprotective effects against cellular diseases associated with Alzheimer’s.225 Sparteine-type compounds, such as 110, may inhibit protein synthesis and acetylcholine receptors,226 while 125 presented nematicidal activity against Hemonchus contortus and Teladorsagia circumcincta.227 Similarly, 110 has toxic effects by inhibiting K+ channels and the tDNA synthesis and formation.95 In addition, compound 110 and analogs have shown that subcutaneous administration of 25 mg/kg in neonatal rats decreases the mRNA levels of muscarinic acetylcholine receptors, specifically of M1–M3 subtypes, and generates an increase of M7 mRNA between 7 and 14 days after administration.228 Furthermore, the anticonvulsant effects of 110 on the behavior and electroencephalic activity were studied in three states of epilepsy (SE) models.5
Some studies have investigated the effect of some cytisine-type QAs (e.g., 131 and 132) on human lung and breast cancer. Results showed that 132 (i.e., cytisine) inhibited the growth of lung cancer cell lines, including A549 (IC50 = 26.83 μM), NCI-H23 (IC50 = 49.79 μM), and NCI-H460 (IC50 = 32.45 μM) cells using the CCK-8 assay.229 Alkaloid 132 was influential in suppressing lung cancer cells through cell cycle arrest and the induction of mitochondrial-mediated apoptosis, suggesting that compound 132 may be a promising candidate for developing lung cancer treatments.229 In addition, compound 132 and homologues induce apoptosis of tumor cells via the endoplasmic reticulum (ER) pathway.230 The information suggested that calcium overload promotes ER stress-induced apoptosis in cytisine-induced HepG2 cells, modulating the CHOP/GADD153, JNK, and caspase-4 pathways.231 Finally, the caspase cascade is activated to induce apoptosis of HepG2 cells, through the mitochondrial pathway, according to the reduction in the mitochondrial membrane potential.231 Following cytisine treatment, mitochondrial permeability may increase, leading to mitochondrial matrix expansion, outer membrane rupture, and a cytochrome C release.231
Alkaloid 132 and structurally related compounds (131–155) have been shown to have a high affinity for the neuronal nicotinic acetylcholine receptors (nAChR) and are essential probes in the investigation of central nervous system disorders.232 Particularly, cytisine showed affinity to nAChRs and can activate α7-nAChR expression.230 Moreover, some synthetic derivatives (e.g., cytisine-12-carbamide and N-allylcytisine-12-carbamide) are acetylcholinesterase inhibitors and are toxic against Artemia salina at concentrations below 1000 ppm.233 Additionally, the antiviral activity of 132 was also evaluated against the human influenza A (H1N1) virus, the human parainfluenza virus type-3 (HPIV-3), and SARS-CoV-2 virus.234132–136 showed remarkable activity against HPIV-3 with a selectivity index (SI) of 58, calculated as the ratio of CC50/IC50.235 Compounds 148–155 isolated from seeds of Thermopsis lanceolata had moderate insecticidal activity against Aphis fabae (LC50 = 43.15 and 46.47 mg/L, respectively),123 and compound 132 showed antifungal activity against Fusarium oxysporum.219 On the other hand, compounds 150–151 isolated from the rhizomes of the Chinese plant known as “Shan-Dou-Gen” (Sophora tonkinensis) were evaluated against the cancer lines T24 (human bladder cancer cell line), SPC-A2 (human lung adenocarcinoma), and A549 (human lung adenocarcinoma). The best results were obtained for 150 against the A549 cancer line, with an IC50 = 10.36 μM.236
Alkaloid 174 is one of the most representative alkaloids of the genus Lupinus and can be considered the basic form of QAs, i.e., the 6/6 azabicycle quinolizidine moiety.95 This QA type has had several biological activity records in recent years.8 In this regard, 179–180 inhibited acetylcholinesterase at IC50 = 330 and 220 μM and cytotoxicity against murine lymphoma L1210 cells (IC50 = 4.9 and 5.5 μg/mL),150 while QA 189 was a potent blocker of α4β2- and α7-nAChRs (IC50 = 1.5 and 1.3 μM, respectively). Alkaloid 199 was tested against HL-60 (human myeloid leukemia cell line), SMMC-7721 (human hepatocarcinoma cell line), and SW480 (human colon carcinoma), and the results were found to be promising (IC50 < 10 μM).9 Compounds 206 and 207 displayed lower cytotoxicity compared with the commercial standard, being potent antiangiogenic agents.156 Senepodines 222 and 227 showed moderate cytotoxicity against human blood promyelocytic leukemia (HL-60, 46% inhibition at 100 μM), whereas 224 and 225 did not show activity.38
Those compounds structurally related to 234 (aloperine-type) show excellent anticancer, anti-inflammatory, antifibrotic, antiviral, and antiarrhythmic activities.17 In this regard, alkaloid 234 has been explored as an anti-inflammatory and antitumor agent.237 Recent studies have demonstrated that 234 generates protection against acute renal injury induced by ischemia-reperfusion.238 Additionally, studies have shown that 234 and its derivatives selectively repress IL-1β and IFN-α expression, regulating PI3K/Akt/mTOR signaling and NF-ob transcriptional activity.8,239 In addition, 234 has also been one of the most important compounds because it inhibits HIV infection by blocking HIV-1 entry.240 This compound responded well by inhibiting cell–cell fusion mediated by the HIV envelope at low concentrations. This study demonstrated that the naturally occurring 234 and synthetic derivatives are key bioactives for inhibiting this globally problematic infection.240 Additionally, alkaloids 235–237 demonstrated potent antihepatitis B virus activities (HBV) and are more potent against the hepatitis B e-antigen (HBeAg) secretion than the hepatitis B surface antigen (HBsAg).166 On the other hand, the antimicrobial activity of 239 against four Gram-positive bacteria (i.e., Staphylococcus aureus, S. epidermides, S. saprophyticum, and S. pyogenes), three Gram-negative bacteria (i.e., Escherichia coli, Klebsiela pneumonia, and Shigella sonei), and three yeasts (i.e., Candida albicans, Sacharomyes cerevisae, and Criptococcus neoformans) was evaluated, demonstrating moderate to good results (MIC < 50.0 μg/mL).84 Regarding (homo)ormosanine-type QAs, compounds 260 and 267 showed good in vitro activity against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum (IC50 = 0.5 μg/mL).241 Alkaloid 276 (a phenanthroquinolizidine QA) was evaluated against a panel of 30 cancer cell lines and found to inhibit the proliferation of all tested cell lines, including three multidrug-resistant cell lines (average IC50 value of 2.1 nM), which is much lower than that of those previously reported for commercial standards.180 Phenanthroquinolizidines 278 and 279 show cytotoxic activity against six cancer lines, including colon, lung, breast, prostate, kidney, and leukemia, with IC50 between 0.2 and 100 ng/mL,42 whereas 272 exhibited high activity against malaria.34 The myrifabrals 294–297 inhibited hepatitis C virus replication (HCV, IC50 0.9–4.7 μM) with cytotoxicity lower than reference standards.36
Compendium of Biological Activities Reported for Quinolizidine Alkaloids
The studies conducted have primarily centered on investigating the biological properties of QAs, which can be categorized into eight classes, including cytotoxic, antiviral, antimicrobial, insecticidal, anti-inflammatory, antimalarial, antiacetylcholinesterase, and miscellaneous activities. A comprehensive compilation of each QA (1–397) can be found in Table S2 (Supporting Information), and the subsequent description highlights the most pertinent findings.
Cytotoxic Activity
Tumor cells are characterized by uncontrolled growth and unlimited proliferation. The primary function of many anticancer drugs is to damage these tumor cells directly. In the case of quinolizidine treatments, they have demonstrated the ability to inhibit the proliferation of tumor cells in various cancer types, including HL-60 (human promyelocytic leukemia cells), SMMC-7721 (hepatocellular carcinoma), human glioma stem cells (GSC), A-549 (adenocarcinomic human alveolar basal epithelial), MCF-7, and SW-480 (human colon adenocarcinoma). Considering the above, alkaloids have shown bioactivity against these specific cell lines depending on their respective types. For instance, matrine-type compounds (5,96,2477,248,24923,7727,9 and 55(250)) have demonstrated cytotoxic activity with average IC50 values greater than 50 μM against SMMC-7721, A549, HepG2, HL-60, MCF-7, and SW480 lines. Furthermore, lupanine- and cytisine-type compounds have also exhibited promising results in terms of cytotoxicity. Specifically, compounds 56,1694,251135, and 139(236) have shown cytotoxic activity against GSC-3#, GSC-12#, GSC-18#, MCF-7, and HEPG-2 lines, with IC50 values ranging between 117 and 20 μM.
In addition, an important group of lupinine-type quinolizidines, specifically compounds 179–184 (IC50 > 8.2 μg/mL), demonstrated activity against murine lymphoma cells L1210.150 Compound 186 exhibited activity against TE-671, SH-SY5Y, IMR-32, and K-177 with IC50 values greater than 55 μM,46 while 187–188 showed activity against P-388, A-549, U-251, and SNl2KI with IC50 values above 24.7 μg/mL.252 QA 198 was evaluated against HL-60 (IC50 = 39 μM),37 while 298 was evaluated against five cancer lines with IC50 values below 100 μM.9 Compounds 206–211 were assessed against the HCT-116 line, demonstrating IC50 > 80.2 μM.157 On the other hand, compounds 216–219 were evaluated against B16 melanoma cells, resulting in inhibitions of less than or equal to 50%.160 Regarding compounds 222–236, they were tested against L1210 lymphoma cells, exhibiting IC50 values below 7.5 μg/mL.163 Furthermore, QAs 230–236 were tested against various cancer lines, including MG-63, U2OS-OS, HepG2 2.2.15, papillary thyroid carcinoma (IHH-4), and anaplastic thyroid carcinoma (8505c and KMH-2), showing IC50 values above 100 μM (inactive),38,246,253 except for the L1210 line, which demonstrated IC50 values below 10 μg/mL.164 Compounds 244 and 245 displayed moderate activity (IC50 > 50 μM) against U-87 (glioblastoma), 518-A2 (melanoma), and HCT-116 (colon cancer).174 Finally, compounds 272–276 were successfully evaluated against KB (mouth epidermal carcinoma cells, CCL-17), HepG-2 (human liver hepatocellular carcinoma cells, HB-8065), LU-1 (human lung adenocarcinoma cells, HTB-57), and MCF-7 (human breast cancer cells, HTB-22), with IC50 values above 1 μM, demonstrating promising potential.179 QA 277 was evaluated against human gastric cancer AGS (hypoxia-inducible factor-1) with an IC50 of 8.7 nM,254,255 while 285 and 288 were evaluated against the P388 cell line; however, no promising activity was obtained.256 These results indicate that matrine- and lupinine-type quinolizidines show the most promising activity against the investigated cancer cell lines. Therefore, it is crucial to continue expanding the experimental knowledge regarding the biological activity of these QA types.
Antiviral Activity
Viral infections significantly threaten humans, animals, and economically important crops worldwide, leading to substantial mortality and disease-related losses. In order to mitigate the detrimental effects caused by various viruses, natural targets have been investigated, yielding noteworthy outcomes in both in vitro and in vivo studies. Several QAs have been examined for their ability to affect specific viruses, including the hepatitis B virus, hepatitis C, nonhuman influenza virus (H3N2), enterovirus EV-71, coxsackie B virus, human herpesvirus-6 (HHV-6), tobacco mosaic virus (TMV), tomato spotted wilt virus (TSWV), and others. In this regard, QAs 2,2577,7813,18315,1121,25822,7828,18348,1196,78132,258235,166237,170238,166298,183304,170383,78387,78 and 388(78) showed promising results against the hepatitis B virus, with inhibition percentages between 10 and 60% for the serologic marker (HBsAg) and between 10 and 40.5% for the antigen (HBeAg). On the other hand, compounds 294–297 were active against the hepatitis C virus with CC50 values between 119 and 170 μM and EC50 between 2 and 5 μM.36 The importance of the effect of QAs 3, 14, 47, and 50 against the nonhuman influenza virus (H3N2) has also been reported, with mean inhibitory concentrations between 60 and 400 μM.259,260 In addition, the matrine-type compounds 32–46 showed valuable results against the tobacco mosaic virus (TMV) with a protective effect above 50% and a curative effect between 20 and 65%. In another study, the effect of quinolizidines 4, 8, 21, and 202 against the Coxsackie B virus (pathogenic enterovirus) was evaluated, and the best result was obtained for compound 202 (IC50 = 4.66 μM).261 Finally, the inhibitory effect of 9 against the human herpes virus 6 (HHV-6) exhibited an IC50 = 3.9 μM,262 while compounds 150–155, 221, and 370–376 were evaluated against the tomato spotted wilt virus (TSWV) in Nicotiana tabacum cv.K326, whose results showed protective and curative effects between 16 and 60% and 18–50%, respectively.132
Antimicrobial Activity
The antimicrobial effect of QAs has been evaluated against several microorganisms such as bacteria and fungi. Recent studies have evaluated the antifungal activity of compounds 2, 7, 15, 56, 59, 62, 63, 92, 94, 110, 132, 133, 139, 160, 161, 174, and 239 against the phytopathogen Fusarium oxysporum, involving IC50 values between 10 and 400 μM.219 On the other hand, some studies were performed on the effect of quinolizidines 351–354 against Candida albicans ATCC 14503, C. albicans UCD-FR1, C. glabrata, and C. krusei, with MIC values between 30 and 100 μg/mL.191 Other studies have evaluated the antibacterial activity of compounds 65, 133, 138, and 251 against Enterococcus faecalis, with MIC values between 20 and 200 μg/mL.75 In addition, alkaloids 57–59, 66–67, 134, 157, and 202 were evaluated against two Gram-positive bacteria, i.e., Staphylococcus aureus and Escherichia coli, including a broad MIC range between 25 and 200 μg/mL,84,125,261 whose best outcome was obtained for compound 202 (MIC = 8 μg/mL for S. aureus and MIC = 0.8 μg/mL for E. coli).261 Finally, the antibacterial activity of 214 was evaluated against Proteus mirabilis, P. vulgaris, Klebsiella pneumoniae, Escherichia coli, and Shigellu dysenteriue, with MIC values between 100 and 150 μg/mL.159
Insecticidal Activity
Insecticidal studies of QAs have also been investigated to counteract the problems caused by some insects on economically important crops, such as the black aphid (Aphis fabae), the common house mosquito (Culex pipiens), the red spider mite (Tetranychus urticae), and the brown leafhopper (Nilaparvata lugens). According to these studies, compounds 37, 103, 105–109, 148–155, 221, and 370–376 showed insecticidal activity with LC50 between 25 and 32 ppm and inhibitions between 35 and 80%, demonstrating their promising protective capacity for Vicia faba crops.71,103,132 Another study examined quinolizidines 94 and 133 against Culex pipiens, involving LC50 ranging between 3.42 and 8.26 ppm and LC90 between 43.83 and 154.18 ppm.251 Insecticidal activity studies have also been conducted with the QAs 102–109, 103–108, and 375–376, involving an LC50 between 49 and 65 ppm and inhibitions greater than 50% against Nilaparvata lugens and Tetranychus urticae.103,132
Anti-inflammatory Activity
The anti-inflammatory activity of QAs on the tumor necrosis factor (TNF-α), associated with inflammation, apoptosis, and joint destruction, and the interleukin-6 factor (IL-6), associated with endothelial cells and fibroblasts, has also been evaluated. These studies have shown that compounds 1, 7, 17, 94, 133, and 377–381 could positively inhibit the TNF-α with values greater than 50%. The best result was obtained with compound 377, which showed an inhibition of 96.64%. In the case of the IL-6 factor, the studies reported 40–68% inhibitions.78,195,263−265
Antimalarial Activity
Significant research efforts have been focused on exploring active compounds against tropical diseases with malaria being one of the primary targets. In this regard, the investigation of QAs against the parasitic protozoan Plasmodium falciparum has been conducted. Thus, QAs have shown promising results comparable to commercial standards, highlighting the promising activity of 260 (IC50 = 5 μg/mL),241267 (IC50 < 20 μg/mL),241 and 268 (IC50 = 6.11 nM for KI strain and 5.13 nM for FCR3 strain of P. falciparum).34318–325 were also active against D6 and W2 P. falciparum clones, and the best results were obtained with 322 and 325 with IC50s between 2.80 and 4.76 μg/mL.41 Finally, compound 335 was also tested against the African clone D6 (IC50 = 670 ng/mL) and Indochinese clone W2 (IC50 = 280 ng/mL) of P. falciparum.187
Antiacetylcholinesterase Activity
The antiacetylcholinesterase activity is a significant and medically relevant biological effect. This activity is particularly important because it has the potential to inhibit the degradation of acetylcholine, a neurotransmitter released in the synaptic clefts. Natural substances, including QAs, that possess the ability to inhibit the enzyme responsible for acetylcholine breakdown can enhance cholinergic neurotransmission by slowing acetylcholine degradation. With the aforementioned benefits in mind, studies were conducted to assess the inhibitory effects of compounds 110, 111, 126, 189, and 253–259 on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). These compounds exhibited inhibitions ranging from 15% to 73.9%, and their mean inhibitory concentrations ranged from 21 to 331 μM.5,150,266 Finally, compound 189 blocked neuronal nicotinic acetylcholine receptors (α4β2 and α7) with IC50 between 1.3 and 1.5 μM.152
Miscellaneous Activities
Finally, some of the compiled QAs have undergone testing for specific types of biological activity that are less commonly observed. Despite their particularity, these reports provide valuable information regarding the bioactivity of these diverse and structurally intriguing compounds. Among these activities, the antiarrhythmic effect of QA 7 was tested on mice, whose lethal dose (LD50) was 72.1 mg/kg,267 and the same compound was effective as a biopesticide against Diaphorina citri (LC50 = 1247 ppm and LC90 = 5712 ppm), Panonychus citri (LC50 = 42 ppm and LC90 = 73.7 ppm), Sitophilus zeamais (LC50= 463.9 ppm and LC90 = 1121 ppm), and Spodoptera frugiperda (LC50 = 384.3 and LC90 = 1034 ppm).195 In the case of compound 174, dietary activity was investigated in rainbow trout, and no toxic effects were observed at a recommended dietary dose of 500 mg/kg.268 In addition, the inhibitory activity against hPTP1B (human protein tyrosine phosphatase 1B) of compounds 286–287 and the vasodilatory activity of compounds 339–342 have been documented. However, in both cases, these compounds were found to be inactive.186,269 On the other hand, compound 352 was evaluated as a possible inhibitor of somatostatin and vasoactive intestinal peptide, and the results were very promising with IC50 = 12 μM.270 Furthermore, a compound of the same QA type, i.e., 353, was a potent inhibitor of the inositol 1,4,5-trisphosphate receptor and endoplasmic reticulum Ca2+ pumps with an inhibition of 78%.271 Finally, the RLAR (rat lens aldose reductase) activity of compounds 315–317 and 329 was evaluated with inhibitions between 25 and 32%184 and the antimetastatic activity of compounds 389–397, whose best result was obtained for QAs 391–392 with inhibitions of 86.6% and 86.8%, respectively, classifying them as potent antimetastatic agents.160
Structural and Drug-Likeness Comparison of Compiled Quinolizidine Alkaloids
The compiled information shows that QAs, a category of natural compounds, have garnered considerable attention in drug discovery research due to their wide range of pharmacological activities (Table 10, Table S2). QAs possess intricate structural complexity and distinctive properties, which position them as potential candidates for creating innovative therapeutics. However, an essential aspect to consider for this purpose, beyond biological properties, pertains to the physicochemical characteristics required for a compound to be suitable for drug development.272 This aspect can be rationalized under the drug-likeness concept, which refers to a set of physicochemical properties that a compound should possess to effectively access body cells and perform physiological or pharmacological functions while maintaining safety within the host organism.273 Therefore, it can be considered a qualitative measure that aims to strike a balance between molecular and structural features, indicating how closely a substance resembles a potential drug regarding bioavailability.274 In this regard, structural factors come into consideration when evaluating the drug-likeness of QAs, considering that they exhibit a complexity with multiple stereocenters, which provides numerous variations and expands the range of potential drug-like properties that can be attributed to them. To explore such factors, the custom-made QA-based library (n = 397) was structurally compared using the similarity analysis module included in the DataWarrior ver. 5.5.0 program275 to visualize plausible structural relationships and patterns to define interesting QA-based scaffolds. Thus, the similarity plot (Figure 21a) on associating the FragFP descriptor (i.e., a substructure fragment dictionary-based binary fingerprint similar to the MDL keys) and the DataWarrior-based drug-likeness approach275 led to finding eight main clusters having similar fingerprints and positive drug-likeness score (d) (calculated values in Table S3). In this regard, the cluster with the best drug-likeness profile (d > 3, clusters 2 and 5) involved matrine and lupanine-type compounds having a 3-hydroxypiperidin-2-one moiety (related to 53 and 78, respectively) and the (homo)ormosanine-type QAs (e.g., 260–272), which seem to exhibit more drug-like properties. Compounds 53 and 78 have no reported activity, but they are related to 18 and 21, which exhibited promising anti-HBV activity.258 Other important clusters (i.e., 1, 3, and 5–8) involved bismacrocyclic (e.g., related to 355 and 336), piperidin-2-one-containing (e.g., related to darvasamine (12), camoensidine (247), N-formyltetrahydrocytisine (163), alopecuroide E (381)), cytisine-type (e.g., 132 and 133), and leontidine-type (e.g., 247) QAs with positive drug-likeness scores (d > 0).
Figure 21.
(a) Similarity plot combining the FragFP descriptor and drug-likeness. Colors were determined according to the drug-likeness score (d). Numbers in small boxes are related to QA numbering 1–397. Ball size is related to the structure–activity landscape index (SALI) of d/FragFp. Numbers in yellow rectangles are related to the relevant clusters 1–8. (b) Box plots of calculated values of c Log P, c Log S, and drug-likeness (d) values of QAs 1–397.
The d/FragFp ratio, which serves as the structure–activity landscape index (SALI), demonstrated values exceeding 200 (represented by the size of the balls in Figure 21a) for approximately 26% of the compiled QAs (SALI > 200). This enabled the prediction of the drug-like potential of these QAs based on their chemical structure, utilizing the principle of molecular similarity to known drugs. In essence, drug-likeness refers to the inherent characteristics of a chemical compound that are necessary to attain the desired optimal pharmacological properties.272 Generally, QAs exhibited a favorable drug-likeness profile, with a positive d score observed for 62.7% of QAs (Figure 21a,b, Table S3). These facts suggested that most QAs contained fragments that are commonly found in commercially available drugs. However, some QAs were part of the exception (d < −5) with bad drug-like profiles, such as alkenyl-substituted lupinines (e.g., 188 and 189), biphenylquinolizidine lactones (e.g., 315–317), acosmine-type (e.g., 280), and dimeric quinolizidines (e.g., 377). In addition, most QAs also exhibited reasonable hydrophilicity, involving medians within the range of commercial drugs (c Log P > 0; 0 > c Log S > −4) (Figure 21b), rationalized by the presence of various H-acceptors (ca. four on average) (Table S3).
These findings indicated that the biological activities of QAs might be of paramount importance in drug discovery since their drug-like properties place QAs as attractive starting points for the development of drug candidates targeting various diseases and conditions. Although QAs present challenges and advantages due to their structural complexity, several offer immense potential as drug candidates. Continued research efforts focused on improving their drug-likeness through expanding the chemical space by isolation of more variants or synthetic modifications and computational modeling, and optimization strategies will pave the way for the development of novel therapeutics based on QAs.276
Perspectives
QAs have emerged as a fascinating class of naturally occurring compounds with diverse chemical structures and significant biological activities. The chemistry and biological activities of QAs have been the subject of extensive research, and their perspectives hold great promise for various scientific disciplines. From a chemical perspective, QAs exhibit remarkable structural complexity and diversity. Multiple substituted variants and stereocenters within the quinolizidine scaffold add to their structural intricacy. This complexity provides a fertile ground for studying stereochemistry, synthetic methodologies, and structure–activity relationships (SARs) in the context of drug discovery and natural product chemistry.18 In addition, the biological activity of QAs is another intriguing aspect that has attracted significant attention. These alkaloids have demonstrated a wide range of pharmacological properties, making them promising candidates for the development of therapeutics (Table 2). QAs have exhibited antimicrobial activity against various bacterial and fungal strains, including multidrug-resistant pathogens. Their cytotoxicity against cancer cells has also been investigated, showing potential as anticancer agents. Moreover, QAs have shown antiviral activity against several viral infections, such as hepatitis B and C viruses, and have been explored for their insecticidal and insect-repellent properties.
Understanding the mechanisms underlying the biological activities of QAs is crucial for their further development and utilization.17 Further studies can reveal that their bioactivities are often mediated through the modulation of specific molecular targets and cellular pathways, interacting with enzymes, receptors, ion channels, and signaling pathways, leading to their diverse pharmacological effects.277 However, several of them remain to be examined, and consequently, elucidating the molecular mechanisms of action can provide valuable insights into the design and optimization of QA-based therapeutics.
In recent years, advancements in analytical techniques, synthetic methodologies, and medicinal chemistry techniques have facilitated the obtention of diverse QA derivatives and analogs. These efforts to expand QA-related chemical space have contributed to SAR studies and structure-based drug design, enabling the development of potent and selective QA-based compounds.278,279 Moreover, the discovery of natural sources of QAs, including plants, marine organisms, and animals, continues to expand the chemical space and offers new prospects for exploring QA chemistry and biological activity. In this context, the research perspectives on the chemistry, occurrence, and biological activity of QAs are multifaceted and hold immense potential. They provide opportunities for the discovery of novel drugs, exploration of natural product chemistry, and development of sustainable insecticides and antimicrobial agents. Furthermore, the unique structural features and diverse biological activities of QAs make them interesting subjects for interdisciplinary research, encompassing fields such as synthetic chemistry, pharmacology, biochemistry, and molecular biology.
Concluding Remarks
The present review encompasses a compilation of 397 quinolizidine alkaloids (QAs) representing the chemical diversity of these specialized metabolites isolated and reported over the past three decades. This compilation was organized into various QA types; as such, categorization had not been previously undertaken but was necessary. These QA types exhibit a high degree of structural complexity, characterized by intricate stereoisomerism, which renders them attractive as leads and scaffolds for various purposes. Most of these compounds have been isolated from the seeds, leaves, and aerial parts of Fabaceae plants, although other families, such as Lythraceae, also contain an intriguing group of macrocycle-type QAs. Additionally, macrocycle-type QAs have been identified in marine sponges (Petrosia, Xestospongia, and Oceanapia), frogs (Dendrobatidae), and ants (Formicidae). Despite initially being recognized for their natural defensive properties, various types of QAs have attracted considerable attention for research and utilization due to their wide range of biological activities. These activities include cytotoxic, antiviral, antimicrobial, insecticidal, anti-inflammatory, antimalarial, and antiacetylcholinesterase effects. Importantly, these QAs also exhibit a favorable putative drug-likeness profile, making them promising candidates to be considered in drug discovery endeavors. Accordingly, the chemistry, occurrence, and biological activity perspectives of QAs offer a rich landscape for scientific exploration and innovation. Continued research efforts, including synthetic studies, structure–activity relationship investigations, and mechanistic studies, will undoubtedly contribute to unlocking the full potential of QAs as valuable chemical entities with significant therapeutic applications.
Thus, the information gathered in this review underscores the need for further research to expand the chemodiversity and identify more potent bioactive compounds based on QAs as valuable scaffolds for pharmacological and agrochemical applications.
Acknowledgments
The authors thank UMNG and USP for the financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c02179.
Names and SMILES of 397 compiled quinolizidine alkaloids (QAs) (Table S1), individual structures of quinolizidine alkaloids 1–397 (Figures S1 to S22), compendium of reported biological activity of the 397 quinolizidine alkaloids (Table S2), and calculated properties of compiled QAs 1–397 (Table S3) (PDF)
This study was funded by the Vicerrectoría de Investigaciones at the Universidad Militar Nueva Granada (UMNG) through the project IMP-CIAS-2924, validity 2020.
The authors declare no competing financial interest.
Supplementary Material
References
- Wink M.Introduction: Biochemistry, Physiology and Ecological Functions of Secondary Metabolites. In Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Oxford, UK, 2010; Vol. 40, pp 1–19. 10.1002/9781444320503.ch1. [DOI] [Google Scholar]
- Yang T.; Nagy I.; Mancinotti D.; Otterbach S. L.; Andersen T. B.; Motawia M. S.; Asp T.; Geu-Flores F. Transcript Profiling of a Bitter Variety of Narrow-Leafed Lupin to Discover Alkaloid Biosynthetic Genes. J. Exp. Bot. 2017, 68 (20), 5527–5537. 10.1093/jxb/erx362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsupa S.; Katayama K.; Ikeura E.; Oikawa A.; Toyooka K.; Saito K.; Yamazaki M. Lysine Decarboxylase Catalyzes the First Step of Quinolizidine Alkaloid Biosynthesis and Coevolved with Alkaloid Production in Leguminosae. Plant Cell 2012, 24 (3), 1202–1216. 10.1105/tpc.112.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta-ur-Rahman; Choudhary M. I.; Parvez K.; Ahmed A.; Akhtar F.; Nur-e-Alam M.; Hassan N. M. Quinolizidine Alkaloids from Sophora alopecuroides. J. Nat. Prod. 2000, 63 (2), 190–192. 10.1021/np990351v. [DOI] [PubMed] [Google Scholar]
- Villalpando-Vargas F.; Medina-Ceja L. Sparteine as an Anticonvulsant Drug: Evidence and Possible Mechanism of Action. Seizure 2016, 39 (1), 49–55. 10.1016/j.seizure.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Wang R.; Deng X.; Gao Q.; Wu X.; Han L.; Gao X.; Zhao S.; Chen W.; Zhou R.; Li Z.; Bai C. Sophora alopecuroides L.: An Ethnopharmacological, Phytochemical, and Pharmacological Review. J. Ethnopharmacol. 2020, 248, 112172 10.1016/j.jep.2019.112172. [DOI] [PubMed] [Google Scholar]
- Venkateshwar Goud T.; Srinivasa Reddy N.; Raghavendra Swamy N.; Siva Ram T.; Venkateswarlu Y. Anti-HIV Active Petrosins from the Marine Sponge Petrosia similis. Biol. Pharm. Bull. 2003, 26 (10), 1498–1501. 10.1248/bpb.26.1498. [DOI] [PubMed] [Google Scholar]
- Wang H.; Xia C.; Chen L.; Zhao J.; Tao W.; Zhang X.; Wang J.; Gao X.; Yong J.; Duan J. Phytochemical Information and Biological Activities of Quinolizidine Alkaloids in Sophora: A Comprehensive Review. Curr. Drug Targets 2019, 20 (15), 1572–1586. 10.2174/1389450120666190618125816. [DOI] [PubMed] [Google Scholar]
- Lei W.; Xing-De W.; Juan H.; Gen-Tao L.; Li-Yan P.; Yan L.; Liu-Dong S.; Qin-Shi Z. A New Quinolizidine Alkaloid from Sophora flavescens. Chem. Nat. Compd. 2014, 50 (5), 876–879. 10.1007/s10600-014-1104-8. [DOI] [Google Scholar]
- Ao C.; Araki N.; Tawata S. Cyclooxygenase Inhibitory Compounds with Antioxidant Activities from Sophora subprostrata. Asian J. Chem. 2009, 21 (1), 745–754. [Google Scholar]
- Ding P. L.; Liao Z. X.; Huang H.; Zhou P.; Chen D. F. (+)-12α-Hydroxysophocarpine, a New Quinolizidine Alkaloid and Related Anti-HBV Alkaloids from Sophora flavescens. Bioorg. Med. Chem. Lett. 2006, 16 (5), 1231–1235. 10.1016/j.bmcl.2005.11.073. [DOI] [PubMed] [Google Scholar]
- Adig N.; Tosun F. Alkaloid Profiles and Biological Activities of Different Sophora jaubertii Extracts. Turk J. Pharm. Sci. 2010, 7 (1), 1–7. [Google Scholar]
- Erdemoglu N.; Ozkan S.; Tosun F. Alkaloid Profile and Antimicrobial Activity of Lupinus angustifolius L. Alkaloid Extract. Phytochem. Rev. 2007, 6 (1), 197–201. 10.1007/s11101-006-9055-8. [DOI] [Google Scholar]
- Kinder D. H.; Knecht K. T.. Lupine (Lupinus caudatus L., Lupinus albus L.) Seeds: History of Use, Use as an Antihyperglycemic Medicinal, and Use as a Food. In Nuts and Seeds in Health and Disease Prevention; Preedy V. R., Watson R. R., Patel V. B. B. T.-N., Eds.; Academic Press: San Diego, 2011; pp 711–716. 10.1016/B978-0-12-375688-6.10084-2. [DOI] [Google Scholar]
- Dung D. T.; Hang D. T. T.; Yen P. H.; Quang T. H.; Nhiem N. X.; Tai B. H.; Minh C. V.; Kim Y.-C.; Kim D. C.; Oh H.; Kiem P. V. Macrocyclic Bis-Quinolizidine Alkaloids from Xestospongia muta. Nat. Prod. Res. 2019, 33 (3), 400–406. 10.1080/14786419.2018.1455043. [DOI] [PubMed] [Google Scholar]
- Li J. C. C.; Zhang Z. J. J.; Liu D.; Jiang M. Y. Y.; Li R. T. T.; Li H. M. M. Quinolizidine Alkaloids from the Roots of Sophora flavescens. Nat. Prod. Res. 2022, 36 (7), 1781–1788. 10.1080/14786419.2020.1817011. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang G.; Liu J.; Ouyang L. Quinolizidine Alkaloids Derivatives from Sophora alopecuroides Linn: Bioactivities, Structure-Activity Relationships and Preliminary Molecular Mechanisms. Eur. J. Med. Chem. 2020, 188, 111972 10.1016/j.ejmech.2019.111972. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Liu Y.-Q.; Fang J.. The Biological Activities of Quinolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Knölker H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2023; Vol. 89, pp 1–37. 10.1016/bs.alkal.2022.06.001. [DOI] [PubMed] [Google Scholar]
- Frick K. M.; Kamphuis L. G.; Siddique K. H. M.; Singh K. B.; Foley R. C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 8, 1–12. 10.3389/fpls.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinotti D.; Frick K. M.; Geu-Flores F. Biosynthesis of Quinolizidine Alkaloids in Lupins: Mechanistic Considerations and Prospects for Pathway Elucidation. Nat. Prod. Rep. 2022, 39 (7), 1423–1437. 10.1039/D1NP00069A. [DOI] [PubMed] [Google Scholar]
- Bunsupa S.; Yamazaki M.; Saito K. Quinolizidine Alkaloid Biosynthesis : Recent Advances and Future Prospects. Front. Plant Sci. 2012, 3, 1–7. 10.3389/fpls.2012.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J. P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2008, 25 (1), 139–165. 10.1039/B612166G. [DOI] [PubMed] [Google Scholar]
- Michael J. P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2007, 24 (1), 191–222. 10.1039/b509525p. [DOI] [PubMed] [Google Scholar]
- Michael J. P. Indolizidine and Quinolizidine Alkaloids. Natural Product Reports 1991, 8, 553. 10.1039/np9910800553. [DOI] [PubMed] [Google Scholar]
- Wink M. Evolution of Secondary Metabolites in Legumes (Fabaceae). South African J. Bot. 2013, 89 (1), 164–175. 10.1016/j.sajb.2013.06.006. [DOI] [Google Scholar]
- Thawabteh A.; Juma S.; Bader M.; Karaman D.; Scrano L.; Bufo S.; Karaman R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins (Basel) 2019, 11 (11), 656. 10.3390/toxins11110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornoy B.; Atlan A.; Tarayre M.; Dugravot S.; Wink M. Alkaloid Concentration of the Invasive Plant Species Ulex europaeus in Relation to Geographic Origin and Herbivory. Naturwissenschaften 2012, 99 (11), 883–892. 10.1007/s00114-012-0970-9. [DOI] [PubMed] [Google Scholar]
- Resta D.; Boschin G.; D’Agostina A.; Arnoldi A. Evaluation of Total Quinolizidine Alkaloids Content in Lupin Flours, Lupin-Based Ingredients, and Foods. Mol. Nutr. Food Res. 2008, 52 (4), 490–495. 10.1002/mnfr.200700206. [DOI] [PubMed] [Google Scholar]
- Guemes-Ver N.; Martinez-H J.; Hernand J.; Yanez-Fern J.; Totosaus A. Comparison of Chemical Composition and Protein Digestibility, Carotenoids, Tanins and Alkaloids Content of Wild Lupinus Varieties Flour. Pakistan J. Nutr. 2012, 11 (8), 774. 10.3923/pjn.2012.774.780. [DOI] [Google Scholar]
- Dalton H. L.; Blomstedt C. K.; Neale A. D.; Gleadow R.; DeBoer K. D.; Hamill J. D. Effects of Down-Regulating Ornithine Decarboxylase upon Putrescine-Associated Metabolism and Growth in Nicotiana tabacum L. J. Exp. Bot. 2016, 67 (11), 3367–3381. 10.1093/jxb/erw166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda J. G.; Porta D. H.; Y S. R. The Participation of Secondary Metabolites in the Plant Defense. Rev. Mex. Fitopatol. 2003, 21, 355–363. [Google Scholar]
- Wink M.Evolution of the Angiosperms and Co-evolution of Secondary Metabolites, Especially of Alkaloids. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon J. M., Ramawat K., Eds.; Springer: Cham, 2022; pp 151–174. 10.1007/978-3-319-96397-6_22. [DOI] [Google Scholar]
- Baptista M. S. P.; Assunção V. A.; Bueno M. L.; Casagrande J. C.; Sartori Â. L. B. Species Representativeness of Fabaceae in Restrictive Soils Explains the Difference in Structure of Two Types of Chaco Vegetation. Acta Bot. Brasilica 2020, 34 (3), 559–569. 10.1590/0102-33062020abb0064. [DOI] [Google Scholar]
- Abdel-Sattar E.; Abdallah H. M.; El-Mekkawy S.; Ichino C.; Kiyohara H.; Yamada H. Antimalarial Alkaloid from Hypoestes forskaolii. Exp. Parasitol. 2020, 211, 107851. 10.1016/j.exppara.2020.107851. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhu K. K.; Gao X. H.; Yue J. M. Natural Occurrence of All Eight Stereoisomers of a Neosecurinane Structure from Flueggea virosa. Tetrahedron 2017, 73 (31), 4692–4697. 10.1016/j.tet.2017.06.035. [DOI] [Google Scholar]
- Cao M. M.; Zhang Y.; Li X. H.; Peng Z. G.; Jiang J. D.; Gu Y. C.; Di Y. T.; Li X. N.; Chen D. Z.; Xia C. F.; He H. P.; Li S. L.; Hao X. J. Cyclohexane-Fused Octahydroquinolizine Alkaloids from Myrioneuron faberi with Activity against Hepatitis C Virus. J. Org. Chem. 2014, 79 (17), 7945–7950. 10.1021/jo501076x. [DOI] [PubMed] [Google Scholar]
- Hirasawa Y.; Kato Y.; Wong C. P.; Uchiyama N.; Goda Y.; Hadi A. H. A.; Ali H. M.; Morita H. Hupermine A, a Novel C16N2-Type Lycopodium Alkaloid from Huperzia phlegmaria. Tetrahedron Lett. 2014, 55 (11), 1902–1904. 10.1016/j.tetlet.2014.01.141. [DOI] [Google Scholar]
- Hirasawa Y.; Tanaka T.; Koyama K.; Morita H. Lycochinines A-C, Novel C27N3 Alkaloids from Lycopodium chinense. Tetrahedron Lett. 2009, 50 (34), 4816–4819. 10.1016/j.tetlet.2009.05.072. [DOI] [Google Scholar]
- Kitajima M.; Yamaguchi Y.; Yanagisawa T.; Kogure N.; Ogata J.; Kikura-Hanajiri R.; Takayama H. Biphenyl Quinolizidine Lactone Alkaloids from “Sinicuichi” (Heimia salicifolia). Tetrahedron 2019, 75 (27), 3733–3739. 10.1016/j.tet.2019.05.045. [DOI] [Google Scholar]
- Kitajima M.; Yanagisawa T.; Tsukahara M.; Yamaguchi Y.; Kogure N.; Kikura-Hanajiri R.; Goda Y.; Iida O.; Sugimura Y.; Kawahara N.; Takayama H. Biphenyl Ether and Biphenyl Quinolizidine Lactone Alkaloids from Heimia salicifolia. Tetrahedron 2018, 74 (4), 441–452. 10.1016/j.tet.2017.12.012. [DOI] [Google Scholar]
- Rumalla C. S.; Jadhav A. N.; Smillie T.; Fronczek F. R.; Khan I. A. Alkaloids from Heimia salicifolia. Phytochemistry 2008, 69 (8), 1756–1762. 10.1016/j.phytochem.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Liu Y.; Luo D.; Gao X.; Li B.; Zhang G. Cytotoxic Alkaloids from Boehmeria siamensis. Planta Med. 2003, 69 (9), 842–845. 10.1055/s-2003-43215. [DOI] [PubMed] [Google Scholar]
- Gardette D.; Gelas-Mialhe Y.; Gramain J. C.; Perrin B.; Remuson R. Enantioselective Synthesis of the Quinolizidine Alkaloids (+)-Myrtine and (−)-Epimyrtine. Tetrahedron Asymmetry 1998, 9 (10), 1823–1828. 10.1016/S0957-4166(98)00170-0. [DOI] [Google Scholar]
- Amaral A. C. F.; Barnes R. A. A Tetrahydroprotoberberine Alkaloid from Croton hemiargyreus. Phytochemistry 1998, 47 (7), 1445–1447. 10.1016/S0031-9422(97)00764-4. [DOI] [Google Scholar]
- Daly J. W.; Garraffo H. M.; Spande T. F.; Yeh H. J. C.; Peltzer P. M.; Cacivio P. M.; Baldo J. D.; Faivovich J. Indolizidine 239Q and Quinolizidine 275I. Major Alkaloids in Two Argentinian Bufonid Toads (Melanophryniscus). Toxicon 2008, 52 (8), 858–870. 10.1016/j.toxicon.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch R. W.; Garraffo H. M.; Spande T. F.; Yeh H. J. C.; Daly J. W. Bioassay-Guided Isolation of Epiquinamide, a Novel Quinolizidine Alkaloid and Nicotinic Agonist from an Ecuadoran Poison Frog, Epipedobates tricolor. J. Nat. Prod. 2003, 66 (10), 1345–1350. 10.1021/np030306u. [DOI] [PubMed] [Google Scholar]
- Lourenço A. M.; Máximo P.; Ferreira L. M.; Pereira M. M. A.. Indolizidine and Quinolizidine Alkaloids Structure and Bioactivity. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Vol. 27, pp 233–298. 10.1016/S1572-5995(02)80038-2. [DOI] [Google Scholar]
- Jain P.; Garraffo H. M.; Yeh H. J. C.; Spande T. F.; Daly J. W.; Andriamaharavo N. R.; Andriantsiferana M. A 1,4-Disubstituted Quinolizidine from a Madagascan Mantelline Frog (Mantella). J. Nat. Prod. 1996, 59 (12), 1174–1178. 10.1021/np9605100. [DOI] [PubMed] [Google Scholar]
- Jones T. H.; et al. Further Alkaloids Common to Ants and Frogs: Decahydroquinolines and a Quinolizidine. J. Chem. Ecol. 1999, 25 (5), 1179–1193. 10.1023/A:1020898229304. [DOI] [Google Scholar]
- Kong F.; Faulkner D. J. Pictamine, a Quinolizidine Alkaloid from the Tunicate Clavelina picta. Tetrahedron Lett. 1991, 32 (30), 3667–3668. 10.1016/S0040-4039(00)79761-9. [DOI] [Google Scholar]
- Braekman J. C.; Daloze D.; Defay N.; Zimmermann D. Petrosin-A and -B, Two New Bis-Quinolizidine Alkaloids from the Sponge Petrosia seriata. Bull. des Sociétés Chim. Belges 1984, 93 (11), 941–944. 10.1002/bscb.19840931102. [DOI] [Google Scholar]
- Ibrahim S. R. M.; Mohamed G. A.; Elkhayat E. S.; Fouad M. A.; Proksch P. Sagitol C, a New Cytotoxic Pyridoacridine Alkaloid from the Sponge Oceanapia Sp. Bull. Fac. Pharmacy, Cairo Univ. 2013, 51 (2), 229–232. 10.1016/j.bfopcu.2013.05.004. [DOI] [Google Scholar]
- Sbihi H. M.; Nehdi I. A.; Tan C. P.; Al-Resayes S. I. Bitter and Sweet Lupin (Lupinus albus L.) Seeds and Seed Oils: A Comparison Study of Their Compositions and Physicochemical Properties. Ind. Crops Prod. 2013, 49 (1), 573–579. 10.1016/j.indcrop.2013.05.020. [DOI] [Google Scholar]
- Kobayashi M.; Miyamoto Y.; Aoki S.; Murakami N.; Kitagawa I.; In Y.; Ishida T. Isomerization of Dimeric 2,9-Disubstituted 1-Oxa-Quinolizidine Alkaloids and Structural Revision of Araguspongines B and E, Isolated from a Marine Sponge of Xestospongia Sp. Heterocycles 1998, 47 (1), 195–203. 10.3987/COM-97-S(N)4. [DOI] [Google Scholar]
- Liu H.; Mishima Y.; Fujiwara T.; Nagai H.; Kitazawa A.; Mine Y.; Kobayashi H.; Yao X.; Yamada J.; Oda T.; Namikoshi M. Isolation of Araguspongine M, a New Stereoisomer of an Araguspongine/Xestospongin Alkaloid, and Dopamine from the Marine Sponge Neopetrosia exigua Collected in Palau. Mar. Drugs 2004, 2 (4), 154–163. 10.3390/md204154. [DOI] [Google Scholar]
- El-Shitany N. A.; Shaala L. A.; Abbas A. T.; Abdel-dayem U. A.; Azhar E. I.; Ali S. S.; van Soest R. W. M.; Youssef D. T. A. Evaluation of the Anti-Inflammatory, Antioxidant and Immunomodulatory Effects of the Organic Extract of the Red Sea Marine Sponge Xestospongia testudinaria against Carrageenan Induced Rat Paw Inflammation. PLoS One 2015, 10 (9), e0138917 10.1371/journal.pone.0138917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Ortiz A. J.-M.; et al. The Acaulescent Rosette Species of Lupinus L. (Fabaceae) of Colombia and Ecuador Including a New Species from Colombia. Phytotaxa 2018, 364 (1), 61. 10.11646/phytotaxa.364.1.3. [DOI] [Google Scholar]
- Forero E.; Romero C. Studies of Legumes in Colombia. J. Colomb. Acad. Exact, Phys. Nat. Sci. 2005, 25, 12–15. [Google Scholar]
- Kite G. C. Leontidine-Type Quinolizidine Alkaloids in Orphanodendron (Leguminosae). Biochem. Syst. Ecol. 2017, 73 (2017), 47–49. 10.1016/j.bse.2017.06.002. [DOI] [Google Scholar]
- Ganzera M.; Krüger A.; Wink M. Determination of Quinolizidine Alkaloids in Different Lupinus Species by NACE Using UV and MS Detection. J. Pharm. Biomed. Anal. 2010, 53 (5), 1231–1235. 10.1016/j.jpba.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Wink M.; Botschen F.; Gosmann C.; Schfer H.; Waterman P. G.. Chemotaxonomy Seen from a Phylogenetic Perspective and Evolution of Secondary Metabolism. In Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Oxford, UK, 2010; pp 364–433. 10.1002/9781444320503.ch7. [DOI] [Google Scholar]
- Lee M. J.; Pate J. S.; Harris D. J.; Atkins C. A. Synthesis, Transport and Accumulation of Quinolizidine Alkaloids in Lupinus albus L. and L. angustifolius L. J. Exp. Bot. 2007, 58 (5), 935–946. 10.1093/jxb/erl254. [DOI] [PubMed] [Google Scholar]
- Golebiewski W. M.; Spenser I. D. Biosynthesis of the Lupine Alkaloids. II. Sparteine and Lupanine. Can. J. Chem. 1988, 66 (7), 1734–1748. 10.1139/v88-280. [DOI] [Google Scholar]
- Robins D. J.; Sheldrake G. N. Stereochemistry of Quinolizidine Alkaloid Biosynthesis: Incorporation of the Enantiomeric [2–2H]Cadaverines into Lupinine. J. Chem. Soc.{,} Chem. Commun. 1994, 11, 1331–1332. 10.1039/c39940001331. [DOI] [Google Scholar]
- Ramírez-Betancourt A.; Hernández-Sánchez A. M.; Salcedo-Morales G.; Ventura-Zapata E.; Robledo N.; Wink M.; Bermúdez-Torres K. Unraveling the Biosynthesis of Quinolizidine Alkaloids Using the Genetic and Chemical Diversity of Mexican Lupins. Diversity 2021, 13 (8), 375. 10.3390/d13080375. [DOI] [Google Scholar]
- Dewick P. M.Medicinal Natural Products: A Biosynthetic Approach; Wiley-Blackwell: Oxford, UK, 2009; Vol. 1. [Google Scholar]
- Kurek J.; Jasiewicz B.; Katrusiak A. Unexpected Dimeric Form of 17-Hydroxylupanine - MS and Crystallographic Studies. ChemRxiv 2017, 3 (1), 1–21. 10.26434/chemrxiv.5480422. [DOI] [Google Scholar]
- Wink M.; Hartmann T. Enzymatic Synthesis of Quinolizidine Alkaloid Esters: A Tigloyl-CoA: 13-Hydroxylupanine O-Tigloyltransferase from Lupinus albus L. Planta 1982, 156 (6), 560–565. 10.1007/BF00392781. [DOI] [PubMed] [Google Scholar]
- Okada T.; Hirai M. Y.; Suzuki H.; Yamazaki M.; Saito K. Molecular Characterization of a Novel Quinolizidine Alkaloid O-Tigloyltransferase: CDNA Cloning, Catalytic Activity of Recombinant Protein and Expression Analysis in Lupinus Plants. Plant Cell Physiol. 2005, 46 (1), 233–244. 10.1093/pcp/pci021. [DOI] [PubMed] [Google Scholar]
- Tsukano C.; Oimura A.; Enkhtaivan I.; Takemoto Y. Synthetic Study of Matrine-Type Alkaloids: Stereoselective Construction of the AB Rings of the Quinolizidine Skeleton. Synlett 2014, 25 (05), 653–656. 10.1055/s-0033-1340179. [DOI] [Google Scholar]
- Zou J.; Zhao L.; Yi P.; An Q.; He L.; Li Y.; Lou H.; Yuan C.; Gu W.; Huang L.; Hu Z.; Hao X. Quinolizidine Alkaloids with Antiviral and Insecticidal Activities from the Seeds of Sophora tonkinensis Gagnep. J. Agric. Food Chem. 2020, 68 (50), 15015–15026. 10.1021/acs.jafc.0c06032. [DOI] [PubMed] [Google Scholar]
- Rashid H.; Rasool S.; Ali Y.; Khan K.; Martines M. A. U. Anti-Cancer Potential of Sophoridine and Its Derivatives: Recent Progress and Future Perspectives. Bioorg. Chem. 2020, 99, 103863 10.1016/j.bioorg.2020.103863. [DOI] [PubMed] [Google Scholar]
- He L. J.; Liu J. S.; Luo D.; Zheng Y. R.; Zhang Y. B.; Wang G. C.; Li Y. L. Quinolizidine Alkaloids from Sophora tonkinensis and Their Anti-Inflammatory Activities. Fitoterapia 2019, 139, 104391 10.1016/j.fitote.2019.104391. [DOI] [PubMed] [Google Scholar]
- Lee S. T.; Cook D.; Molyneux R. J. Identification of the Quinolizidine Alkaloids in Sophora leachiana. Biochem. Syst. Ecol. 2014, 54 (1), 1–4. 10.1016/j.bse.2013.12.020. [DOI] [Google Scholar]
- Korir E.; Kiplimo J. J.; Crouch N. R.; Moodley N.; Koorbanally N. A. Quinolizidine Alkaloids from Sophora velutina Subsp. Zimbabweensis (Fabaceae: Sophoreae). Nat. Prod. Commun. 2012, 7 (8), 1934578 10.1177/1934578X1200700810. [DOI] [PubMed] [Google Scholar]
- Tan C. J.; Yi P.; Goto M.; Morris-Natschke S. L.; Liu L. N.; Lee K. H.; Zhao B. Y. (+)-(14β)-14-Ethylmatridin-15-One, a New Quinolizidine Alkaloid from the Poisonous Plant Oxytropis ochrocephala Bunge. Helv. Chim. Acta 2016, 99 (3), 225–227. 10.1002/hlca.201500239. [DOI] [Google Scholar]
- Tan C. J. J.; Liu L. N. N.; Zhao B. Y. Y. A New Quinolizidine Alkaloid from Oxytropis ochrocephala. Chem. Nat. Compd. 2017, 53 (2), 322–324. 10.1007/s10600-017-1979-2. [DOI] [Google Scholar]
- Zhang Y. B.; Yang L.; Luo D.; Chen N. H.; Wu Z. N.; Ye W. C.; Li Y. L.; Wang G. C. Sophalines E-I, Five Quinolizidine-Based Alkaloids with Antiviral Activities against the Hepatitis B Virus from the Seeds of Sophora alopecuroides. Org. Lett. 2018, 20 (18), 5942–5946. 10.1021/acs.orglett.8b02637. [DOI] [PubMed] [Google Scholar]
- Wu L.; Zhong W.; Liu J.; Han W.; Zhong S.; Wei Q.; Liu S.; Tang L. Human Microsomal Cyttrochrome P450-Mediated Reduction of Oxysophocarpine, an Active and Highly Toxic Constituent Derived from Sophora flavescens Species, and Its Intestinal Absorption and Metabolism in Rat. Fitoterapia 2015, 105 (1), 26–36. 10.1016/j.fitote.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Liu X. J.; Cao M. A.; Li W. H.; Shen C. S.; Yan S. Q.; Yuan C. S. Alkaloids from Sophora flavescens Aition. Fitoterapia 2010, 81 (6), 524–527. 10.1016/j.fitote.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Michael J. P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2000, 17 (6), 579–602. 10.1039/a904849i. [DOI] [PubMed] [Google Scholar]
- Benaiche G.; Belattar N.; Trifunnovic S.; Vukovic N.; Todorovic D.; Todorovic M.; Baskic D.; Vukic M. Isolation of Alkaloids and Anti-Tumor Activity of the Crude Methanolic Extract of Algerian Cytisus purgans. Orient. J. Chem. 2015, 31 (4), 1943–1948. 10.13005/ojc/310411. [DOI] [Google Scholar]
- Chludil H. D.; Leicach S. R.; Corbino G. B.; Barriga L. G.; Vilariño M. D. P. Genistin and Quinolizidine Alkaloid Induction in L. angustifolius Aerial Parts in Response to Mechanical Damage. J. Plant Interact. 2013, 8 (2), 117–124. 10.1080/17429145.2012.672660. [DOI] [Google Scholar]
- Neto A. T.; Oliveira C. Q.; Ilha V.; Pedroso M.; Burrow R. A.; Dalcol I. I.; Morel A. F. Quinolizidine Alkaloids from Lupinus lanatus. J. Mol. Struct. 2011, 1004 (1–3), 174–177. 10.1016/j.molstruc.2011.07.061. [DOI] [Google Scholar]
- Boschin G.; Annicchiarico P.; Resta D.; D’Agostina A.; Arnoldi A. Quinolizidine Alkaloids in Seeds of Lupin Genotypes of Different Origins. J. Agric. Food Chem. 2008, 56 (10), 3657–3663. 10.1021/jf7037218. [DOI] [PubMed] [Google Scholar]
- Tosun F.; Akyüz Ç. Alkaloids from Gonocytisus pterocladus. Pharm. Biol. 1999, 37 (5), 343–345. 10.1076/phbi.37.5.343.6060. [DOI] [Google Scholar]
- Veitch N. C.; Goodwin B. L.; Kite G. C.; Simmonds M. S. J. Methoxylated Quinolizidine Alkaloids from Acosmium panamense. Phytochemistry 1997, 45 (4), 847–850. 10.1016/S0031-9422(97)00072-1. [DOI] [Google Scholar]
- Michael J. P. Lndolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 1994, 11 (1), 17–39. 10.1039/np9941100017. [DOI] [PubMed] [Google Scholar]
- Mohamed M. H.; Ibraheim Z. Z.; Abdallah O. M.; Murakoshi I. (+)-15β-Hydroxy-17-Oxolupanine a Lupin Alkaloid from the Seeds of Lupinus albus. Phytochemistry 1994, 37 (6), 1751–1753. 10.1016/S0031-9422(00)89604-1. [DOI] [Google Scholar]
- Nasution M. P.; Kinghorn A.D. (+)-13a-(4′-Hydroxytigloyloxy)Lupanine from Ormosia krugii. Phytochemistry 1993, 32 (6), 1603–1605. 10.1016/0031-9422(93)85188-W. [DOI] [Google Scholar]
- Veen G.; Schmidt C.; Witte L.; Wray V.; Czygan F. C. Lupin Alkaloids from Lupinus polyphyllus. Phytochemistry 1992, 31 (12), 4343–4345. 10.1016/0031-9422(92)80471-P. [DOI] [Google Scholar]
- Abdel-Halim O. B.; Sekine T.; Saito K.; Halim A. F.; Abdel-Fattah H.; Murakoshi I. (+)-12α-Hydroxylupanine, a Lupin Alkaloid from Lygos raetam. Phytochemistry 1992, 31 (9), 3251–3253. 10.1016/0031-9422(92)83486-I. [DOI] [Google Scholar]
- Michael J. P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 1990, 7 (46), 485–513. 10.1039/np9900700485. [DOI] [PubMed] [Google Scholar]
- Verdoorn G. H.; van Wyk B.-E. Esters of Quinolizidine Alkaloids from the Genus Pearsonia. Phytochemistry 1990, 29 (4), 1297–1302. 10.1016/0031-9422(90)85446-M. [DOI] [Google Scholar]
- Boschin G.; Resta D.. Alkaloids Derived from Lysine: Quinolizidine (a Focus on Lupin Alkaloids). In Natural Products; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 381–403. 10.1007/978-3-642-22144-6_11. [DOI] [Google Scholar]
- Wink M.; Carey D. B. Variability of Quinolizidine Alkaloid Profiles of Lupinus argentous (Fabaceae) from North America. Biochem. Syst. Ecol. 1994, 22 (7), 663–669. 10.1016/0305-1978(94)90052-3. [DOI] [Google Scholar]
- Zamora-Natera J. F.; Bernal-alcocer A.; Ruiz-lópez M.; Soto-hernández M.; Vibrans-lindemann H. Seed alkaloid profile Lupinus exaltatus ZUCC (Fabaceae) and the antifungal evaluation of the alkaloid extract and lupanine against phytopathogens. Rev. Mex. Fitopatol. 2005, 23 (2), 124–129. [Google Scholar]
- Chludil H. D.; Leicach S. R.; Corbino G. B.; Barriga L. G.; Vilarino M. d. P. Genistin and Quinolizidine Alkaloid Induction in L. angustifolius Aerial Parts in Response to Mechanical Damage. J. Plant Interact. 2013, 8 (2), 117–124. 10.1080/17429145.2012.672660. [DOI] [Google Scholar]
- Zamora-Natera F.; García-López P.; Ruiz Lopez M. A. A.; Salcedo-Perez E. Composition of alkaloids in seeds of Lupinus mexicanus (Fabaceae) and antifungal and allelopathic evaluation of the alkaloid extract. Agrociencia 2008, 1, 185–192. [Google Scholar]
- Rycroft D. S.; Robins D. J.; Sadler I. H. Assignment of the 1H and 13C NMR Spectra of the Quinolizidine Alkaloid Anagyrine and Determination of Its Conformation. Magn. Reson. Chem. 1991, 29 (1), 936–940. 10.1002/mrc.1260290913. [DOI] [Google Scholar]
- Tsypysheva I. P.; Petrova P. R.; Koval’skaya A. V.; Lobov A. N.; Maksimova M. A.; Zainullina L. F.; Vinogradova V. I.; Vakhitov V. A.; Vakhitova Y. V.; Galin F. Z. Synthesis and Cytotoxic Activity of Conjugates of (−)-Cytisine and Thermopsin Amine Derivatives with 1,3-Dimethyl-5-Formyluracil. Chem. Nat. Compd. 2018, 54 (5), 938–946. 10.1007/s10600-018-2517-6. [DOI] [Google Scholar]
- Sagen A.-L.; Gertsch J.; Becker R.; Heilmann J.; Sticher O. Quinolizidine Alkaloids from the Curare Adjuvant Clathrotropis glaucophylla. Phytochemical 2002, 61 (1), 975–978. 10.1016/S0031-9422(02)00394-1. [DOI] [PubMed] [Google Scholar]
- Hu Z.-X.; Zhang P.; Zou J.-B.; An Q.; Yi P.; Yuan C.-M.; Zhang Z.-K.; Zhao L.-H.; Hao X.-J. Quinolizidine Alkaloids with Antitomato Spotted Wilt Virus and Insecticidal Activities from the Seeds of Thermopsis lanceolata R. Br. J. Agric. Food Chem. 2022, 70 (29), 9214–9226. 10.1021/acs.jafc.2c02546. [DOI] [PubMed] [Google Scholar]
- Tomioka K.; Yamamoto Y.; Yamada K. I. 3.20 Stoichiometric Auxiliary Ligands for Metals and Main Group Elements: Ligands for Lithium. Compr. Chirality 2012, 3, 626–654. 10.1016/B978-0-08-095167-6.00320-7. [DOI] [Google Scholar]
- Jasiewicz B.; Wyrzykiewicz E. Mass Spectrometry of Bis-Quinolizidine Alkaloids: Differentiation of Stereoisomers and Metamers Using ESI and FAB Mass Spectrometry. Spectrosc. Lett. 2009, 42 (1), 49–57. 10.1080/00387010802428435. [DOI] [Google Scholar]
- Trevisan T. C.; Silva E. A.; Dall’Oglio E. L.; da Silva L. E.; Silva Velozo E. da; Vieira P. C.; de Sousa P. T. New Quinolizidine and Diaza-Adamantane Alkaloids from Acosmium dasycarpum (Vog.) Yakovlev—Fabaceae. Tetrahedron Lett. 2008, 49 (44), 6289–6292. 10.1016/j.tetlet.2008.06.124. [DOI] [Google Scholar]
- Nihei K.-i.; Shibata K.; Kubo I. (+)-2,3-Dehydro-10-Oxo-α-Isosparteine in Uresiphita reversalis Larvae Fed on Cytisus monspessulanus Leaves. Phytochemistry 2002, 61 (8), 987–990. 10.1016/S0031-9422(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Carmack M.; Goldberg S. I.; Martin E. W. (−)-7-Hydroxy-.Beta.-Isosparteine, an Alkaloid Accompanying. Beta.-Isosparteine in Lupinus sericeus. J. Org. Chem. 1967, 32 (10), 3045–3049. 10.1021/jo01285a024. [DOI] [PubMed] [Google Scholar]
- Abdel-Halim O. B.; Sekine T.; Halim A. F.; Abdel-Fattah H.; Saito K.; Ogata K.; Murakoshi I. Reconfirmation of the Structure of (+)-retamine from Lygos raetam Var. Sarcocarpa by X-ray Analysis. Phytochem. Anal. 1995, 6 (6), 302–305. 10.1002/pca.2800060606. [DOI] [Google Scholar]
- Nasution M. P.; Hussain R. A.; Kinghorn A. D.; Tosun A.; Tosun F.; Tanker M.; Özden T. 10α-Hydroxymethylsparteine, a New Type of Quinolizidine Alkaloid from Genista sessilifolia. Tetrahedron Lett. 1991, 32 (42), 5915–5918. 10.1016/S0040-4039(00)79425-1. [DOI] [Google Scholar]
- Mohamed M. H.; Hassanean H. A. Alkaloids from Seeds of Lupinus varius and L. hartwegii. Phytochemistry 1997, 46 (2), 365–369. 10.1016/S0031-9422(97)00275-6. [DOI] [Google Scholar]
- Ruiz López M.; García López P.; Rodríguez Macías R.; Zamora Natera J.; Isaac Virgen M.; Múzquiz M. Mexican Wild Lupines as a Source of Quinolizidine Alkaloids of Economic Potential. Polibotánica 2010, 29 (1), 159–164. [Google Scholar]
- Gavilan J.; Mennickent D.; Ramirez-Molina O.; Triviño S.; Perez C.; Silva-Grecchi T.; Godoy P. A.; Becerra J.; Aguayo L. G.; Moraga-Cid G.; Martin V. S.; Yévenes G. E.; Castro P. A.; Guzman L.; Fuentealba J. 17 Oxo Sparteine and Lupanine, Obtained from Cytisus scoparius, Exert a Neuroprotection against Soluble Oligomers of Amyloid-β Toxicity by Nicotinic Acetylcholine Receptors. J. Alzheimer’s Dis. 2019, 67 (1), 343–356. 10.3233/JAD-180945. [DOI] [PubMed] [Google Scholar]
- Ricker M.; Daly D. C.; Veen G.; Robbins E. F.; Sinta M. V.; Chota J. I.; Czygan F. C.; Kinghorn A. D. Distribution of Quinolizidine Alkaloid Types in Nine Ormosia Species (Leguminosae-Papilionoideae). Brittonia 1999, 51 (1), 34–43. 10.2307/2666554. [DOI] [Google Scholar]
- Máximo P.; Lourenço A.; Tei A.; Wink M. Chemotaxonomy of Portuguese Ulex: Quinolizidine Alkaloids as Taxonomical Markers. Phytochemistry 2006, 67 (17), 1943–1949. 10.1016/j.phytochem.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Wink M.; Witte L. Quinolizidine Alkaloids in Genista acanthoclada and Its Holoparasite, Cuscuta palaestina. J. Chem. Ecol. 1993, 19 (3), 441–448. 10.1007/BF00994317. [DOI] [PubMed] [Google Scholar]
- Abdel-Baky A.; Makboul M. Sparteine and 13-Hydroxy Sparteine from Leaves of Sophora secundiflora (Orteg.) Lag. Bull. Pharm. Sci. Assiut 1985, 8 (1), 99–108. 10.21608/bfsa.1985.75885. [DOI] [Google Scholar]
- Gotti C.; Clementi F. Cytisine and Cytisine Derivatives. More than Smoking Cessation Aids. Pharmacol. Res. 2021, 170, 105700 10.1016/j.phrs.2021.105700. [DOI] [PubMed] [Google Scholar]
- Lee S. T.; Cook D.; Molyneux R. J.; Davis T. Z.; Gardner D. R. Alkaloid Profiles of Dermatophyllum arizonicum, Dermatophyllum gypsophilum, Dermatophyllum secundiflorum, Styphnolobium affine, and Styphnolobium japonicum Previously Classified as Sophora Species. Biochem. Syst. Ecol. 2013, 49, 87–93. 10.1016/j.bse.2013.03.018. [DOI] [Google Scholar]
- Hayman A. R.; Gray D. O. Hydroxynorcytisine, a Quinolizidone Alkaloid from Laburnum anagyroides. Phytochemistry 1989, 28 (2), 673–675. 10.1016/0031-9422(89)80087-1. [DOI] [Google Scholar]
- Petruczynik A.; Wróblewski K.; Misiurek J.; Plech T.; Szalast K.; Wojtanowski K.; Mroczek T.; Szymczak G.; Waksmundzka-Hajnos M.; Tutka P. Determination of Cytisine and N-Methylcytisine from Selected Plant Extracts by High-Performance Liquid Chromatography and Comparison of Their Cytotoxic Activity. Toxins (Basel). 2020, 12 (9), 557. 10.3390/toxins12090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko L. D.; Mamatkhanov A. U.; Turakhozhaev M. T.; Yuldashev M. P. Quantitative Analysis of Flavonoids in the Total Preparation of Thermopsis alterniflora. Chem. Nat. Compd. 2001, 37 (2), 137–139. 10.1023/A:1012374702242. [DOI] [Google Scholar]
- Zhang P.; Zou J.-B.; An Q.; Yi P.; Yuan C.-M.; Huang L.-J.; Gu W.; Hu Z.-X.; Hao X.-J. Two New Cytisine-Type Alkaloids from the Seeds of Thermopsis lanceolata. J. Asian Nat. Prod. Res. 2022, 24 (12), 1141. 10.1080/10286020.2021.2020759. [DOI] [PubMed] [Google Scholar]
- Pérez E. G.; Méndez-Gálvez C.; Cassels B. K. Cytisine: A Natural Product Lead for the Development of Drugs Acting at Nicotinic Acetylcholine Receptors. Nat. Prod. Rep. 2012, 29 (5), 555–567. 10.1039/c2np00100d. [DOI] [PubMed] [Google Scholar]
- Zhang S.-Y.; Li W.; Nie H.; Liao M.; Qiu B.; Yang Y.-L.; Chen Y.-F. Five New Alkaloids from the Roots of Sophora flavescens. Chem. Biodivers. 2018, 15 (3), e1700577 10.1002/cbdv.201700577. [DOI] [PubMed] [Google Scholar]
- Kacem N.; Goossens J.-F.; Duhal N.; Roumy V.; Hennebelle T.; Christen P.; Hostettmann K.; Rhouati S. Determination of Alkaloids in Endemic Genista quadriflora Munby (Fabaceae). Biochem. Syst. Ecol. 2014, 56 (1), 83–87. 10.1016/j.bse.2014.05.005. [DOI] [Google Scholar]
- Belsito E. L.; Chidichimo G.; Di Gioia M. L.; Leggio A.; Liguori A.; Perri F.; Siciliano C. Extraction of Quinolizidine Alkaloids in Non Aqueous Basic Conditions: The Case of Spartium junceum Flowers. Chromatographia 2008, 68 (5–6), 345–349. 10.1365/s10337-008-0706-3. [DOI] [Google Scholar]
- Woldemichael G. M.; Wink M. Concomitant Occurrence of Pyrrolizidine and Quinolizidine Alkaloids in the Hemiparasite Osyris alba L. (Santalaceae). Biochem. Syst. Ecol. 2002, 30 (2), 139–149. 10.1016/S0305-1978(01)00065-5. [DOI] [Google Scholar]
- Li W.; Wang H.; Dong A. Preparative Separation of Alkaloids from Stem of Euchresta tubulosa Dunn. by High-Speed Counter-Current Chromatography Using Stepwise Elution. Molecules 2019, 24 (24), 4602. 10.3390/molecules24244602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta-ur-Rahman; Pervin A.; Perveen S.; Nasir H.; Hasan N. Two Lupin Alkaloids from Sophora griffithii. Phytochemistry 1991, 30 (3), 1001–1003. 10.1016/0031-9422(91)85295-B. [DOI] [Google Scholar]
- Veen G.; Günther S.; Greinwald R.; Bachmann P.; Witte L.; Kustrak D.; Czygan F. C. Quinolizidine Alkaloids in Petteria ramentacea. Phytochemistry 1992, 31 (10), 3487–3490. 10.1016/0031-9422(92)83712-8. [DOI] [Google Scholar]
- Zhang P.; An Q.; Yi P.; Cui Y.; Zou J. B.; Yuan C. M.; Zhang Y.; Gu W.; Huang L. J.; Zhao L. H.; Hu Z. X.; Hao X. J. Thermlanseedlines A–G, Seven Thermopsine-Based Alkaloids with Antiviral and Insecticidal Activities from the Seeds of Thermopsis lanceolata R. Br. Fitoterapia 2022, 158, 105140 10.1016/j.fitote.2022.105140. [DOI] [PubMed] [Google Scholar]
- Kite G. C.; Cardoso D.; Veitch N. C.; Lewis G. P. Quinolizidine Alkaloid Status of Acosmium, Guianodendron and Leptolobium, the Segregate Genera of Acosmium L. South African J. Bot. 2013, 89 (2013), 176–180. 10.1016/j.sajb.2013.05.009. [DOI] [Google Scholar]
- Greinwald R.; Henrichs C.; Veen G.; Ross J. H.; Witte L.; Czygan F. C. A Survey of Alkaloids in Templetonia biloba (Benth.) Polhill (Fabaceae: Brongniartieae). Biochem. Syst. Ecol. 1995, 23 (6), 649–654. 10.1016/0305-1978(95)00035-6. [DOI] [Google Scholar]
- Mohamed M. H.; El-Shorbagi A.-N. A. (±)-Termisine, a Novel Lupine Alkaloid from the Seeds of Lupinus termis. J. Nat. Prod. 1993, 56 (11), 1999–2002. 10.1021/np50101a023. [DOI] [Google Scholar]
- Christov V.; Dutschewska H.; Selenghe D.; Zhavsan S.; Zhamyansan Y. 13-Epi-Hydroxysparteine and Desoxyangustifoline, New Alkaloids from Thermopsis mongolica. J. Nat. Prod. 1991, 54 (5), 1413–1415. 10.1021/np50077a030. [DOI] [Google Scholar]
- Mohamed M. H.; Saito K.; Kadry H. A.; Khalifa T. I.; Ammar H.; Murakoshi I. Lupin Alkaloids from the Seeds of Lupinus termis. Phytochemistry 1991, 30 (9), 3111–3115. 10.1016/S0031-9422(00)98264-5. [DOI] [Google Scholar]
- Veen G.; Greinwald R.; Witte L.; Wray V.; Czygan F.-C. Alkaloids of Virgilia divaricata and V. oroboides. Phytochemistry 1991, 30 (6), 1891–1895. 10.1016/0031-9422(91)85034-W. [DOI] [Google Scholar]
- Brukwicki T.; Wysocka W. Some Stereochemical Aspects of Multiflorine and Seco(11,12)-12-Dehydromultiflorine Previously Named Nmethylalbine. J. Mol. Struct. 1989, 196 (1), 343–352. 10.1016/0022-2860(89)85030-6. [DOI] [Google Scholar]
- Wink M.; Witte L.; Hartmann T. Quinolizidine Alkaloid Composition of Plants and of Photomixotrophic Cell Suspension Cultures of Sarothamnus scoparius and Orobanche rapum-Genistae. Planta Med. 1981, 43 (12), 342–352. 10.1055/s-2007-971522. [DOI] [PubMed] [Google Scholar]
- Brooke P.; Harris D. J.; Longmore R. B. Isolation of Minor Lupin Alkaloids. 1. A Simple Procedure for the Isolation of Angustifoline from Lupinus angustifolius (Cv. Fest) Seeds, with Application to Other Lupin Alkaloids. J. Agric. Food Chem. 1996, 44 (8), 2129–2133. 10.1021/jf950020y. [DOI] [Google Scholar]
- Bermúdez Torres K.; Robledo Quintos N.; Barrera Necha L. L.; Wink M. Alkaloid Profile of Leaves and Seeds of Lupinus hintonii C. P. Smith. Zeitschrift fur Naturforsch. - Sect. C J. Biosci. 2002, 57 (3–4), 243–247. 10.1515/znc-2002-3-408. [DOI] [PubMed] [Google Scholar]
- Aniszewski T.Alkaloids - Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role; Elsevier: New York, NY, USA, 2007. 10.1016/B978-0-444-52736-3.X5000-4. [DOI] [Google Scholar]
- Kozioł A.; Kosturkiewicz Z.; Podkowińska H. Structure of the Alkaloid Lupinine. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34 (11), 3491–3494. 10.1107/S0567740878011437. [DOI] [Google Scholar]
- Romeo F.; Fabroni S.; Ballistreri G.; Muccilli S.; Spina A.; Rapisarda P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus Spp. Sustainability 2018, 10 (3), 788. 10.3390/su10030788. [DOI] [Google Scholar]
- Tokuyama T.; Nishimori N.; Shimada A.; Edwards M. W.; Daly J. W. New Classes of Amidine, Indolizidine and Quinolizidine Alkaloids from a Poison-Frog, (Dendrobatidae). Tetrahedron 1987, 43 (3), 643–652. 10.1016/S0040-4020(01)89998-1. [DOI] [Google Scholar]
- Nurkenov O.A.; Abulyaissova L.K.; Zhaksybayeva G.S. Structural and Spectral Properties of Quinolizidine Alkaloids: Quantum Chemical Calculations. Chem. Bull. Kazakh Natl. Univ. 2019, 3, 28–36. 10.15328/cb1089. [DOI] [Google Scholar]
- Saito K.; Yoshino T.; Tsai S.; Ohmiya S.; Kubo H.; Otomasu H.; Murakoshi I. Absolute Configuration of (−)-Lusitanine, a New Lupin Alkaloid in Maackia Species. Chem. Pharm. Bull. (Tokyo). 1987, 35 (3), 1308–1310. 10.1248/cpb.35.1308. [DOI] [Google Scholar]
- Tang Y.; Li N.; Zou Y.; Ai Y.; Ma G. L.; Osman E. E. A.; Xiong J.; Li J.; Jin Z. X.; Hu J. F. LC-MS Guided Isolation and Dereplication of Lycopodium Alkaloids from Lycopodium cernuum Var. Sikkimense of Different Geographical Origins. Phytochemistry 2019, 160, 25–30. 10.1016/j.phytochem.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Morita H.; Hirasawa Y.; Shinzato T.; Kobayashi J. New Phlegmarane-Type, Cernuane-Type, and Quinolizidine Alkaloids from Two Species of Lycopodium. Tetrahedron 2004, 60 (33), 7015–7023. 10.1016/j.tet.2003.09.106. [DOI] [Google Scholar]
- Sangsuwan W.; Kongkathip B.; Chuawong P.; Kongkathip N. Total Synthesis of (+)-Epiquinamide and (−)-Epiepiquinamide from D-Mannose. Tetrahedron 2017, 73 (52), 7274–7281. 10.1016/j.tet.2017.11.016. [DOI] [Google Scholar]
- Tsuneki H.; You Y.; Toyooka N.; Sasaoka T.; Nemoto H.; Dani J. A.; Kimura I. Marine Alkaloids (−)-Pictamine and (−)-Lepadin B Block Neuronal Nicotinic Acetylcholine Receptors. Biol. Pharm. Bull. 2005, 28 (4), 611–614. 10.1248/bpb.28.611. [DOI] [PubMed] [Google Scholar]
- Kadooka M. M.; Chang M. Y.; Fukami H.; Scheuer P. J.; Clardy J.; Solheim B. A.; Springer J. P. Mamanine and Pohakuline, Two Unprecedented Quinolizidine Alkaloids from Sophora chrysophylla. Tetrahedron 1976, 32 (8), 919–924. 10.1016/0040-4020(76)85049-1. [DOI] [Google Scholar]
- Máximo P.; Lourenço A. New Quinolizidine Alkaloids from Ulex jussiaei. J. Nat. Prod. 2000, 63 (2), 201–204. 10.1021/np9903604. [DOI] [PubMed] [Google Scholar]
- Grundon M. F. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 1989, 6 (5), 523–536. 10.1039/np9890600523. [DOI] [PubMed] [Google Scholar]
- Banwell M. G.; Bezos A.; Burns C.; Kruszelnicki I.; Parish C. R.; Su S.; Sydnes M. O. C8c–C15 Monoseco-Analogues of the Phenanthroquinolizidine Alkaloids Julandine and Cryptopleurine Exhibiting Potent Anti-Angiogenic Properties. Bioorg. Med. Chem. Lett. 2006, 16 (1), 181–185. 10.1016/j.bmcl.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Pouny I.; Long C.; Batut M.; Aussagues Y.; Jean Valère N.; Achoundong G.; David B.; Lavaud C.; Massiot G. Quinolizidine Alkaloids from Cylicomorpha solmsii. J. Nat. Prod. 2021, 84 (4), 1198–1202. 10.1021/acs.jnatprod.0c01261. [DOI] [PubMed] [Google Scholar]
- Lee S. T.; Cook D.; Molyneux R. J.; Marcolongo-Pereira C.; Stonecipher C. a.; Gardner D. R. The Alkaloid Profiles of Sophora nuttalliana and Sophora stenophylla. Biochem. Syst. Ecol. 2013, 48 (1), 58–64. 10.1016/j.bse.2012.11.022. [DOI] [Google Scholar]
- Rahman A. U.; Shahwar D.; Parween Z.; Choudhary M. I.; Sener B.; Toker G.; Can Baser K. H. New Alkaloids from Bongardia chrysogonum. Nat. Prod. Lett. 1998, 12 (3), 161–173. 10.1080/10575639808048287. [DOI] [PubMed] [Google Scholar]
- Matsuda H.; Morikawa T.; Oda M.; Asao Y.; Yoshikawa M. Potent Anti-Metastatic Activity of Dimeric Sesquiterpene Thioalkaloids from the Rhizome of Nuphar pumilum. Bioorg. Med. Chem. Lett. 2003, 13 (24), 4445–4449. 10.1016/j.bmcl.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Takayama H.; Kogure N.; Nozoe A.; Kitajima M. Nupharic Acid, a New Sesquiterpene Alkaloid from Nuphar japonicum. Heterocycles 2009, 79 (1), 1101. 10.3987/COM-08-S(D)62. [DOI] [Google Scholar]
- Hirasawa Y.; Morita H.; Kobayashi J. Senepodine F, a New C22N2 Alkaloid from Lycopodium chinense. Heterocycles 2004, 64 (1), 515–521. 10.3987/COM-04-S(P)44. [DOI] [Google Scholar]
- Hirasawa Y.; Morita H.; Kobayashi J. Senepodines B–E, New C22N2 Alkaloids from Lycopodium chinense. Tetrahedron 2003, 59 (20), 3567–3573. 10.1016/S0040-4020(03)00545-3. [DOI] [Google Scholar]
- Morita H.; Hirasawa Y.; Kobayashi J. I. Himeradine A, a Novel C27N3-Type Alkaloid from Lycopodium chinense. J. Org. Chem. 2003, 68 (11), 4563–4566. 10.1021/jo034294t. [DOI] [PubMed] [Google Scholar]
- Morita H.; Hirasawa Y.; Yoshida N.; Kobayashi J. Senepodine A, a Novel C22N2 Alkaloid from Lycopodium chinense. Tetrahedron Lett. 2001, 42 (25), 4199–4201. 10.1016/S0040-4039(01)00688-8. [DOI] [Google Scholar]
- Zhou K. S.; Yi P.; Yang T.; Tian W.; Yang F. M.; Lee K. H.; Zhao B. Y.; Wang Y. H.; Tan C. J. Ochrocephalamines B-D, Three Alkaloids from Oxytropis ochrocephala Bunge. Org. Lett. 2019, 21 (13), 5051–5054. 10.1021/acs.orglett.9b01643. [DOI] [PubMed] [Google Scholar]
- Hernández E. M.; Rangel M. L. C.; Corona A. E.; Angel J. A. C.; López J. A. S.; Sporer F.; Wink M.; Torres K. B. Quinolizidine Alkaloid Composition in Different Organs of Lupinus aschenbornii. Rev. Bras. Farmacogn. 2011, 21 (5), 824–828. 10.1590/S0102-695X2011005000149. [DOI] [Google Scholar]
- Veerasamy N.; Carter R. G. Synthesis of Quinolizidine-Containing Lycopodium Alkaloids and Related Natural Products. Tetrahedron 2016, 72 (33), 4989–5001. 10.1016/j.tet.2016.06.043. [DOI] [Google Scholar]
- Zhou H.; Li J.; Sun F.; Wang F.; Li M.; Dong Y.; Fan H.; Hu D. A Review on Recent Advances in Aloperine Research: Pharmacological Activities and Underlying Biological Mechanisms. Front. Pharmacol. 2020, 11 (1), 1–12. 10.3389/fphar.2020.538137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z.; Zhang Y. K.; Yi P.; Yang F. M.; Huo X. M.; Wang T. T.; Zhang H. Y.; Zhao B. Y.; Zeng Y. R.; Wang Y. H.; Tan C. J. Ochrocephalamines E and F, Two New Alkaloids from Oxytropis ochrocephala Bung. Tetrahedron Lett. 2022, 102, 153943. 10.1016/j.tetlet.2022.153943. [DOI] [Google Scholar]
- Wysocka W.; Brukwicki T. Conformational Equilibria in Quinolizidine Alkaloids. J. Mol. Struct. 1996, 385 (1), 23–33. 10.1016/S0022-2860(96)09393-3. [DOI] [Google Scholar]
- Takamatsu S.; Saito K.; Ohmiya S.; Murakoshi I. Stereostructure of (−)-Multiflorine N-Oxide: A New Lupin Alkaloid from Lupinus hirsutus. Heterocycles 1991, 32 (6), 1167–1171. 10.3987/COM-91-5716. [DOI] [Google Scholar]
- El-Shazly A.; Ateya A.-M. M.; Wink M. Quinolizidine Alkaloid Profiles of Lupinus varius orientalis, L. albus albus, L. hartwegii, and L. densiflorus. Zeitschrift für Naturforsch. 2001, 56 (1–2), 21. 10.1515/znc-2001-1-204. [DOI] [PubMed] [Google Scholar]
- Wagner S.; Sigl S.; Schenkl M.; Breuning M. Diastereodivergent Synthesis of the Quinolizidine-Indolizidine Alkaloids of the Leontidine/Camoensine Family. Eur. J. Org. Chem. 2021, 2021 (17), 2498–2505. 10.1002/ejoc.202100270. [DOI] [Google Scholar]
- Ohmiya S.; Kubo H.; Nakaaze Y.; Otomasu H.; Saito K.; Murakoshi I. (—)-Camoensidine N-Oxide; A New Alkaloid from Maackia tashiroi. Chem. Pharm. Bull. 1991, 39 (5), 1123–1125. 10.1248/cpb.39.1123. [DOI] [Google Scholar]
- Mackay M. F.; Satzke L.; Mathieson A. M. The Absolute Molecular Structure of (−)-Podopetaline. Tetrahedron 1975, 31 (10), 1295–1298. 10.1016/0040-4020(75)80171-2. [DOI] [Google Scholar]
- Torrenegra R.; Bauereiß P.; Achenbach H. Homoormosanine-Type Alkaloids from Bowdichia virgiloides. Phytochemistry 1989, 28 (8), 2219–2221. 10.1016/S0031-9422(00)97953-6. [DOI] [Google Scholar]
- Guimarães P. R.; José J.; Galetti M.; Trigo J. R. Quinolizidine Alkaloids in Ormosia arborea Seeds Inhibit Predation but Not Hoarding by Agoutis (Dasyprocta leporina). J. Chem. Ecol. 2003, 29 (5), 1065–1072. 10.1023/A:1023817203748. [DOI] [PubMed] [Google Scholar]
- Doan Thi Thuy A.; Trinh Thi Thanh V.; Doan Thi Mai H.; Le H. T.; Litaudon M.; Nguyen V. H.; Chau V. M.; Pham V. C. Cytotoxic Alkaloids from Leaves of Pilea aff. martinii. Planta Med. 2019, 85 (6), 496–502. 10.1055/a-0826-0483. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Qing L.; Meng C.; Shi J.; Yang Y.; Wang Z.; Han G.; Wang Y.; Ding J.; Meng L.; Wang Q. 6-OH-Phenanthroquinolizidine Alkaloid and Its Derivatives Exert Potent Anticancer Activity by Delaying S Phase Progression. J. Med. Chem. 2017, 60 (7), 2764–2779. 10.1021/acs.jmedchem.6b01502. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Kennedy J. W. J. Total Syntheses of the Tylophora Alkaloids Cryptopleurine, (−)-Antofine, (−)-Tylophorine, and (−)-Ficuseptine C. Chem. - A Eur. J. 2006, 12 (28), 7398–7410. 10.1002/chem.200600592. [DOI] [PubMed] [Google Scholar]
- Suslov E. V.; Ponomarev K. Y.; Volcho K. P.; Salakhutdinov N. F. Azaadamantanes, a New Promising Scaffold for Medical Chemistry. Russ. J. Bioorganic Chem. 2021, 47 (6), 1133–1154. 10.1134/S1068162021060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. B.; Luo D.; Yang L.; Cheng W.; He L. J.; Kuang G. K.; Li M. M.; Li Y. L.; Wang G. C. Matrine-Type Alkaloids from the Roots of Sophora flavescens and Their Antiviral Activities against the Hepatitis B Virus. J. Nat. Prod. 2018, 81 (10), 2259–2265. 10.1021/acs.jnatprod.8b00576. [DOI] [PubMed] [Google Scholar]
- Lee I.; Youn U.; Kim H.; Min B.; Kim J. S.; Bae K. Biphenyl and Biphenyl Ether Quinolizidine N -Oxide Alkaloids from Lagerstroemia indica L. Planta Med. 2011, 77 (18), 2037–2041. 10.1055/s-0031-1280064. [DOI] [PubMed] [Google Scholar]
- Chen B.; Huan X.-J.; Miao Z.-H.; Voogd N. J.; Gu Y.-C.; Wang C.-Y.; Guo Y.-W.; Li X.-W. Uncommon Bis-Quinolizidine Alkaloids from the Hainan Sponge Neopetrosia chaliniformis. Chin. J. Chem. 2021, 39 (7), 1838–1842. 10.1002/cjoc.202100091. [DOI] [Google Scholar]
- Kobayashi M.; Kawazoe K.; Kitagawa I. Araguspongines B, C, D, E, F, G, H, and J, New Vasodilative Bis-1-Oxaquinolizidine Alkaloids from an Okinawan Marine Sponge, Xestospongia sp. Chem. Pharm. Bull. (Tokyo). 1989, 37 (6), 1676–1678. 10.1248/cpb.37.1676. [DOI] [PubMed] [Google Scholar]
- Orabi K. Y.; El Sayed K. A.; Hamann M. T.; Chuck Dunbar D.; Al-Said M. S.; Higa T.; Kelly M. Araguspongines K and L, New Bioactive Bis-1-Oxaquinolizidine N-Oxide Alkaloids from Red Sea Specimens of Xestospongia exigua. J. Nat. Prod. 2002, 65 (12), 1782–1785. 10.1021/np0202226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswarlu Y.; Reddy M. V. R.; Rao J. V. Bis-1-Oxaquinolizidines from the Sponge Haliclona exigua. J. Nat. Prod. 1994, 57 (9), 1283–1285. 10.1021/np50111a017. [DOI] [Google Scholar]
- Nakagawa M.; Endo M.; Tanaka N.; Gen-Pei L. Structures of Xestospongin A,B,C and D, Novel Vasodilativecompounds from Marine Sponge. Xestospongia exigua. Tetrahedron Lett. 1984, 25 (30), 3227–3230. 10.1016/S0040-4039(01)91016-0. [DOI] [Google Scholar]
- Singh K. S.; Das B.; Naik C. G. Quinolizidines Alkaloids: Petrosin and Xestospongins from the Sponge Oceanapia sp. J. Chem. Sci. 2011, 123 (5), 601–607. 10.1007/s12039-011-0124-1. [DOI] [Google Scholar]
- Moon S.-S.; MacMillan J. B.; Olmstead M. M.; Ta T. A.; Pessah I. N.; Molinski T. F. (+)-7S-Hydroxyxestospongin A from the Marine Sponge Xestospongia sp. and Absolute Configuration of (+)-Xestospongin D. J. Nat. Prod. 2002, 65 (3), 249–254. 10.1021/np010427z. [DOI] [PubMed] [Google Scholar]
- Moon S.-S.; MacMillan J. B.; Olmstead M. M.; Ta T. A.; Pessah I. N.; Molinski T. F. (+)-7S-Hydroxyxestospongin A from the Marine Sponge Xestospongia sp. and Absolute Configuration of (+)-Xestospongin D. J. Nat. Prod. 2002, 65 (3), 249–254. 10.1021/np010427z. [DOI] [PubMed] [Google Scholar]
- Li Y.; Qin S.; Guo Y.-W.; Gu Y.-C.; van Soest R. W. M. 9′-Epi-3β,3′β–Dimethylxestospongin C, a New Macrocyclic Diamine Alkaloid from the Hainan Sponge Neopetrosia exigua. Planta Med. 2011, 77 (02), 179–181. 10.1055/s-0030-1250164. [DOI] [PubMed] [Google Scholar]
- Iwagawa T.; Kaneko M.; Okamura H.; Nakatani M.; van Soest R. W. M.; Shiro M. A New Quinolizidine Alkaloid from the Papua New Guinean Sponge Xestospongia exigua. J. Nat. Prod. 2000, 63 (9), 1310–1311. 10.1021/np000111b. [DOI] [PubMed] [Google Scholar]
- Fan C. L.; Zhang Y. B.; Chen Y.; Xie P.; Wang G. C.; Tian H. Y.; Li Y. L.; Huang X. J.; Zhang X. Q.; Li Z. Y.; Liu J. S.; Ye W. C.; Chen W. M. Alopecuroides A-E, Matrine-Type Alkaloid Dimers from the Aerial Parts of Sophora alopecuroides. J. Nat. Prod. 2019, 82 (12), 3227–3232. 10.1021/acs.jnatprod.8b01081. [DOI] [PubMed] [Google Scholar]
- Liu L. N.; Ran J. Q.; Li L. J.; Zhao Y.; Goto M.; Morris-Natschke S. L.; Lee K. H.; Zhao B. Y.; Tan C. J. Ochrocephalamine A, a New Quinolizidine Alkaloid from Oxytropis ochrocephala Bunge. Tetrahedron Lett. 2016, 57 (46), 5047–5049. 10.1016/j.tetlet.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.; Jiang J.; Yang Y.; Zhang X.; Feng Z.; Zhang P. Three Quinolizidine Dimers from the Seeds of Sophora alopecuroides and Their Hepatoprotective Activities. Chin. Chem. Lett. 2022, 33 (6), 2923–2927. 10.1016/j.cclet.2021.10.085. [DOI] [Google Scholar]
- LaLonde R. T.; Donvito T. N.; Tsai A. I. M. 13C Chemical Shifts of Quinolizidines. 2. 13C Spectra of Some Nuphar Alkaloids. Can. J. Chem. 1975, 53 (12), 1714–1725. 10.1139/v75-243. [DOI] [Google Scholar]
- Gunthardt B. F.; Hollender J.; Hungerbuhler K.; Scheringer M.; Bucheli T. D. Comprehensive Toxic Plants–Phytotoxins Database and Its Application in Assessing Aquatic Micropollution Potential. J. Agric. Food Chem. 2018, 66 (29), 7577–7588. 10.1021/acs.jafc.8b01639. [DOI] [PubMed] [Google Scholar]
- Panter K. E.; Welch K. D.; Gardner D. R.. Poisonous Plants. In Biomarkers in Toxicology; Elsevier, 2014; pp 563–589. 10.1016/B978-0-12-404630-6.00033-6. [DOI] [Google Scholar]
- Schrenk D.; Bodin L.; Chipman J. K.; del Mazo J.; Grasl-Kraupp B.; Hogstrand C.; Hoogenboom L.; Leblanc J.; Nebbia C. S.; Nielsen E.; Ntzani E.; Petersen A.; Sand S.; Schwerdtle T.; Vleminckx C.; Wallace H.; Alexander J.; Cottrill B.; Dusemund B.; Mulder P.; Arcella D.; Baert K.; Cascio C.; Steinkellner H.; Bignami M. Scientific Opinion on the Risks for Animal and Human Health Related to the Presence of Quinolizidine Alkaloids in Feed and Food, in Particular in Lupins and Lupin-derived Products. EFSA J. 2019, 17 (11), 15–16. 10.2903/j.efsa.2019.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. T.; Lee S. T.; Welch K. D.; Cook D. Anagyrine Desensitization of Peripheral Nicotinic Acetylcholine Receptors. A Potential Biomarker of Quinolizidine Alkaloid Teratogenesis in Cattle. Res. Vet. Sci. 2017, 115, 195–200. 10.1016/j.rvsc.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Ringuelet J.; Viña S.. Plant Natural Products; Universidad de la Plata: Buenos Aires, 2013; pp 18–60. [Google Scholar]
- Jiang J.; Wang G. Matrine Protects PC12 Cells from Lipopolysaccharide-Evoked Inflammatory Injury via Upregulation of MiR-9. Pharm. Biol. 2020, 58 (1), 314–320. 10.1080/13880209.2020.1719165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Xiu J.; Zhang X.; Zhang L.; Yan K.; Qin C.; Liu J. Antiviral Effect of Matrine against Human Enterovirus 71. Molecules 2012, 17 (9), 10370–10376. 10.3390/molecules170910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J.; Zhang Y.; Wang X.; Xiang J.; Deng S.; Wu D.; Chen J.; Yu L.; Zhou Y.; Wang Y.; Shen J. Matrine Inhibits the Growth of Natural Killer/T-Cell Lymphoma Cells by Modulating CaMKIIγ-c-Myc Signaling Pathway. BMC Complement. Med. Ther. 2020, 20 (1), 214. 10.1186/s12906-020-03006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. Y.; Zeng X.; Wang Y.; Shi J.; Qian H.; Zhang Y.; Fang J. Q.; Sheng X.; Zheng J. M.; Chen Y. X. Sophocarpine Attenuates Toll-like Receptor 4 in Steatotic Hepatocytes to Suppress pro-Inflammatory Cytokines Synthesis. J. Gastroenterol. Hepatol. 2015, 30 (2), 405–412. 10.1111/jgh.12691. [DOI] [PubMed] [Google Scholar]
- Rashid H. ur; Xu Y.; Muhammad Y.; Wang L.; Jiang J. Research Advances on Anticancer Activities of Matrine and Its Derivatives: An Updated Overview. Eur. J. Med. Chem. 2019, 161 (1), 205–238. 10.1016/j.ejmech.2018.10.037. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Kan Q.-C.; Xu Y.; Zhang G.-X.; Zhu L. Inhibitory Effect of Matrine on Blood-Brain Barrier Disruption for the Treatment of Experimental Autoimmune Encephalomyelitis. Mediators Inflamm. 2013, 2013, 1–10. 10.1155/2013/736085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao X.; Zhang X.; Lv Y.; Xu Y.; Li M.; Pan Q.; Chu Y.; Liu N.; Zhang G.-X.; Zhu L. Matrine Downregulates IL-33/ST2 Expression in the Central Nervous System of Rats with Experimental Autoimmune Encephalomyelitis. Immunol. Lett. 2016, 178, 97–104. 10.1016/j.imlet.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Pan Q.; Zhang X. J.; Xu Y. M.; Chu Y.; Liu N.; Lv P.; Zhang G. X.; Kan Q. C. Protective Effects of Matrine on Experimental Autoimmune Encephalomyelitis via Regulation of ProNGF and NGF Signaling. Exp. Mol. Pathol. 2016, 100 (2), 337–343. 10.1016/j.yexmp.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Fei Z. W.; Qiu M. K.; Qi X. Q.; Dai Y. X.; Wang S. Q.; Quan Z. W.; Liu Y. B.; Ou J. M. Oxymatrine Suppresses Proliferation and Induces Apoptosis of Hemangioma Cells through Inhibition of HIF-1α Signaling. Int. J. Immunopathol. Pharmacol. 2015, 28 (2), 201–208. 10.1177/0394632015578342. [DOI] [PubMed] [Google Scholar]
- Zheng H.; Chen G.; Shi L.; Lou Z.; Chen F.; Hu J. Determination of Oxymatrine and Its Metabolite Matrine in Rat Blood and Dermal Microdialysates by High Throughput Liquid Chromatography/Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2009, 49 (2), 427–433. 10.1016/j.jpba.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang Y.; Tang J.; Zhao S.; Li C.; Huang Y. P.; Yi M. Oxymatrine Inhibits Homocysteine-Mediated Autophagy via MIF/MTOR Signaling in Human Umbilical Vein Endothelial Cells. Cell. Physiol. Biochem. 2018, 45 (5), 1893–1903. 10.1159/000487912. [DOI] [PubMed] [Google Scholar]
- Chen S.-F.; Zhang Z.-Y.; Zhang J.-L. Matrine Increases the Inhibitory Effects of Afatinib on H1975 Cells via the IL-6/JAK1/STAT3 Signaling Pathway. Mol. Med. Rep. 2017, 16 (3), 2733–2739. 10.3892/mmr.2017.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B.; Zhang T.; Su J.; Wang K.; Li X. Oxymatrine Targets EGFRp-Tyr845and Inhibits EGFR-Related Signaling Pathways to Suppress the Proliferation and Invasion of Gastric Cancer Cells. Cancer Chemother. Pharmacol. 2015, 75 (2), 353–363. 10.1007/s00280-014-2651-1. [DOI] [PubMed] [Google Scholar]
- Kong W.; Bao Y.; Ma Q.; Xu H. Synthesis and Biological Activities of Novel Pyrazolomatrine Derivatives. Bioorg. Med. Chem. Lett. 2018, 28 (20), 3338–3341. 10.1016/j.bmcl.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Pan J.; Hao X.; Yao H.; Ge K.; Ma L.; Ma W. Matrine Inhibits Mycelia Growth of Botryosphaeria dothidea by Affecting Membrane Permeability. J. For. Res. 2019, 30 (3), 1105–1113. 10.1007/s11676-019-00883-3. [DOI] [Google Scholar]
- Cely-Veloza W.; Yamaguchi L.; Quiroga D.; Kato M. J.; Coy-Barrera E. Antifungal Activity against Fusarium oxysporum of Quinolizidines Isolated from Three Controlled-Growth Genisteae Plants: Structure–Activity Relationship Implications. Nat. Products Bioprospect. 2023, 13 (1), 9. 10.1007/s13659-023-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daverio M.; Cavicchiolo M. E.; Grotto P.; Lonati D.; Cananzi M.; Da Dalt L. Bitter Lupine Beans Ingestion in a Child: A Disregarded Cause of Acute Anticholinergic Toxicity. Eur. J. Pediatr. 2014, 173 (12), 1549–1551. 10.1007/s00431-013-2088-2. [DOI] [PubMed] [Google Scholar]
- Koleva I. I.; van Beek T. A.; Soffers A. E. M. F.; Dusemund B.; Rietjens I. M. C. M. Alkaloids in the Human Food Chain - Natural Occurrence and Possible Adverse Effects. Mol. Nutr. Food Res. 2012, 56 (1), 30–52. 10.1002/mnfr.201100165. [DOI] [PubMed] [Google Scholar]
- Wiedemann M.; Gurrola-Díaz C. M.; Vargas-Guerrero B.; Wink M.; García-López P. M.; Düfer M. Lupanine Improves Glucose Homeostasis by Influencing KATP Channels and Insulin Gene Expression. Molecules 2015, 20 (10), 19085–19100. 10.3390/molecules201019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Torres K.; Martinez Herrera J.; Figueroa Brito R.; Wink M.; Legal L. Activity of Quinolizidine Alkaloids from Three Mexican Lupinus against the Lepidopteran Crop Pest Spodoptera frugiperda. Biocontrol 2009, 54, 459–466. 10.1007/s10526-008-9180-y. [DOI] [Google Scholar]
- Bentley M. D.; Leonard D. E.; Reynolds E. K.; Leach S.; Beck A. B.; Murakoshi I. Lupine Alkaloids as Larval Feeding Deterrents for Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 1984, 77 (4), 398–400. 10.1093/aesa/77.4.398. [DOI] [Google Scholar]
- Zhao J.; Zhang G.; Li M.; Luo Q.; Leng Y.; Liu X. Neuro-Protective Effects of Aloperine in an Alzheimer’s Disease Cellular Model. Biomed. Pharmacother. 2018, 108, 137–143. 10.1016/j.biopha.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Voitenko S.; Purnyn S.; Omeltchenko I.; Dyadyusha G. G.; Zhorov B.; Brovtsina N.; Skok V. Effect of (+)-Sparteine on Nicotinic Acetylcholine Receptors in the Neurons of Rat Superior Cervical Ganglion. Mol. Pharmacol. 1991, 40 (2), 180–185. [PubMed] [Google Scholar]
- Dubois O.; Allanic C.; Charvet C. L.; Guégnard F.; Février H.; Théry-Koné I.; Cortet J.; Koch C.; Bouvier F.; Fassier T.; Marcon D.; Magnin-Robert J. B.; Peineau N.; Courtot E.; Huau C.; Meynadier A.; Enguehard-Gueiffier C.; Neveu C.; Boudesocque-Delaye L.; Sallé G. Lupin (Lupinus spp.) Seeds Exert Anthelmintic Activity Associated with Their Alkaloid Content. Sci. Rep. 2019, 9 (1), 9070. 10.1038/s41598-019-45654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Soto; et al. Neuronal Damage and Changes in the Expression of Muscarinic Acetylcholine Receptor Subtypes in the Neonatal Rat Cerebral Cortical upon Exposure to Sparteine, a Quinolizidine Alkaloid. Int. J. Dev. Neurosci. 2006, 24 (6), 401–410. 10.1016/j.ijdevneu.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Xu W. T.; Li T. Z.; Li S. M.; Wang C.; Wang H.; Luo Y. H.; Piao X. J.; Wang J. R.; Zhang Y.; Zhang T.; Xue H.; Cao L. K.; Jin C. H. Cytisine Exerts Anti-Tumour Effects on Lung Cancer Cells by Modulating Reactive Oxygen Species-Mediated Signalling Pathways. Artif. Cells, Nanomedicine Biotechnol. 2020, 48 (1), 84–95. 10.1080/21691401.2019.1699813. [DOI] [PubMed] [Google Scholar]
- Yu L.; Jiang B.; Chen Z.; Wang X.; Shang D.; Zhang X.; Sun Y.; Yang J.; Ji Y. Cytisine Induces Endoplasmic Reticulum Stress Caused by Calcium Overload in HepG2 Cells. Oncol. Rep. 2018, 39 (3), 1475–1484. 10.3892/or.2018.6200. [DOI] [PubMed] [Google Scholar]
- Yu L.; Wang X.; Chen Z.-F.; Jiang B.; Shang D.-Y.; Sun Y.-X.; Yang J.-H.; Zhang L.-F.; Ji Y.-B. Cytisine Induces Apoptosis of HepG2 Cells. Mol. Med. Rep. 2017, 16 (3), 3363–3370. 10.3892/mmr.2017.6991. [DOI] [PubMed] [Google Scholar]
- Mineur Y. S.; Somenzi O.; Picciotto M. R. Cytisine, a Partial Agonist of High-Affinity Nicotinic Acetylcholine Receptors, Has Antidepressant-like Properties in Male C57BL/6J Mice. Neuropharmacology 2007, 52 (5), 1256–1262. 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybył A. K.; Maj E.; Wietrzyk J.; Kubicki M. Spectroscopic, Structural and Anticancer Activity Studies of (−)-Cytisine Halogenated N-Benzyl Derivatives. J. Mol. Struct. 2019, 1176, 871–880. 10.1016/j.molstruc.2018.09.029. [DOI] [Google Scholar]
- Majnooni M. B.; Fakhri S.; Bahrami G.; Naseri M.; Farzaei M. H.; Echeverría J. Alkaloids as Potential Phytochemicals against SARS-CoV-2: Approaches to the Associated Pivotal Mechanisms. Evidence-Based Complement. Altern. Med. 2021, 2021 (1), 1–21. 10.1155/2021/6632623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova V. A.; Kadyrova R. A.; Slita A. V.; Muryleva A. A.; Petrova P. R.; Kovalskaya A. V.; Lobov A. N.; Zileeva Z. R.; Tsypyshev D. O.; Borisevich S. S.; Tsypysheva I. P.; Vakhitova J. V.; Zarubaev V. V. Antiviral Activity of Amides and Carboxamides of Quinolizidine Alkaloid (−)-Cytisine against Human Influenza Virus A (H1N1) and Parainfluenza Virus Type 3. Nat. Prod. Res. 2021, 35 (22), 4256–4264. 10.1080/14786419.2019.1696791. [DOI] [PubMed] [Google Scholar]
- Pan Q. M.; Zhang G. J.; Huang R. Z.; Pan Y. M.; Wang H. S.; Liang D. Cytisine-Type Alkaloids and Flavonoids from the Rhizomes of Sophora tonkinensis. J. Asian Nat. Prod. Res. 2016, 18 (5), 429–435. 10.1080/10286020.2015.1131680. [DOI] [PubMed] [Google Scholar]
- Kim H.; Lee M. R.; Lee G. S.; An W. G.; Cho S. I. Effect of Sophora Flavescens Aiton Extract on Degranulation of Mast Cells and Contact Dermatitis Induced by Dinitrofluorobenzene in Mice. J. Ethnopharmacol. 2012, 142 (1), 253–258. 10.1016/j.jep.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Hu S.; Zhang Y.; Zhang M.; Guo Y.; Yang P.; Zhang S.; Simsekyilmaz S.; Xu J. F.; Li J.; Xiang X.; Yu Q.; Wang C. Y. Aloperine Protects Mice against Ischemia-Reperfusion (IR)-Induced Renal Injury by Regulating PI3K/AKT/MToR Signaling and AP-1 Activity. Mol. Med. 2015, 21, 912–923. 10.2119/molmed.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Shi J.; Zhu L.; Dong L.; Luo F.; Zhao M.; Wang Y.; Hu M.; Lu L.; Liu Z. Reductive Metabolism of Oxymatrine Is Catalyzed by Microsomal CYP3A4. Drug Des. Devel. Ther. 2015, 9, 5771–5783. 10.2147/DDDT.S92276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Z.; Zhu L.; Lai W.; Bogerd H.; Lee K. H.; Huang L.; Chen C. H. Aloperine and Its Derivatives as a New Class of HIV-1 Entry Inhibitors. ACS Med. Chem. Lett. 2016, 7 (3), 240–244. 10.1021/acsmedchemlett.5b00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J. A.; Lavaud C.; Bourdy G.; Deharo E.; Gimenez A.; Sauvain M. Antimalarial Activity of Ormosanine and Hormoormosanine from Bowdichia virgiloides. Rev. Boliv. Quim. 2002, 19 (1), 12–17. [Google Scholar]
- Cai X.; Zhang H.; Xie B. Matrine-Family Alkaloids: Versatile Precursors for Bioactive Modifications. Med. Chem. 2020, 16 (4), 431–453. 10.2174/1573406415666190507121744. [DOI] [PubMed] [Google Scholar]
- Cuadra C. De; Muzquiz M. Bactericide-like Effect of Lupinus Alkaloids. Ind. Crop. Prod. Int. J. 1996, 6690 (96), 141–148. 10.1016/0926-6690(96)88414-7. [DOI] [Google Scholar]
- Villalpando-Vargas F.; Medina-Ceja L.; Santerre A.; Enciso-Madero E. A. The Anticonvulsant Effect of Sparteine on Pentylenetetrazole-Induced Seizures in Rats: A Behavioral, Electroencephalographic, Morphological and Molecular Study. J. Mol. Histol. 2020, 51 (5), 503–518. 10.1007/s10735-020-09899-0. [DOI] [PubMed] [Google Scholar]
- Ding Z.; Chen Q.; Xiong B.; Cun Y.; Wang H.; Xu M. Angustifoline Inhibits Human Colon Cancer Cell Growth by Inducing Autophagy along with Mitochondrial-Mediated Apoptosis, Suppression of Cell Invasion and Migration and Stimulating G2/M Cell Cycle Arrest. J. B.U.ON. 2019, 24 (1), 236–241. [PubMed] [Google Scholar]
- Lee Y. R.; Chen S. H.; Lin C. Y.; Chao W. Y.; Lim Y. P.; Yu H. I.; Lu C. H. In Vitro Antitumor Activity of Aloperine on Human Thyroid Cancer Cells through Caspase-Dependent Apoptosis. Int. J. Mol. Sci. 2018, 19 (1), 312. 10.3390/ijms19010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Zhang F.; Bai C.; Yao C.; Zhong H.; Zou C.; Chen X. Sophoridine Induces Apoptosis and S Phase Arrest via ROS-Dependent JNK and ERK Activation in Human Pancreatic Cancer Cells. J. Exp. Clin. Cancer Res. 2017, 36 (1), 1–10. 10.1186/s13046-017-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhang H.; Yu P.; Liu Q.; Liu K.; Duan H.; Luan G.; Yagasaki K.; Zhang G. Effects of Matrine against the Growth of Human Lung Cancer and Hepatoma Cells as Well as Lung Cancer Cell Migration. Cytotechnology 2009, 59 (3), 191–200. 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Xu Y.; Ji W.; Li X.; Sun B.; Gao Q.; Su C. Anti-Tumor Activities of Matrine and Oxymatrine: Literature Review. Tumor Biol. 2014, 35 (6), 5111–5119. 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- Rong Z. J.; Gao X. X.; Hu G. S.; Yan T.; Jia J. M.; Wang A. H. A Novel Alkaloid from the Seeds of Sophora alopecuroides L. Nat. Prod. Res. 2022, 36 (7), 1864–1869. 10.1080/14786419.2020.1824226. [DOI] [PubMed] [Google Scholar]
- Aly S. H.; Elissawy A. M.; Allam A. E.; Farag S. M.; Eldahshan O. A.; Elshanawany M. A.; Singab A. N. B. New Quinolizidine Alkaloid and Insecticidal Activity of Sophora secundiflora and Sophora tomentosa against Culex pipiens (Diptera: Culicidae). Nat. Prod. Res. 2022, 36 (11), 2722. 10.1080/14786419.2021.1919108. [DOI] [PubMed] [Google Scholar]
- Raub M. F.; Cardellina J. H.; Choudhary M. I.; Ni C. Z.; Clardy J.; Alley M. C. Clavepictines A and B: Cytotoxic Quinolizidines from the Tunicate Clavelina picta. J. Am. Chem. Soc. 1991, 113 (8), 3178–3180. 10.1021/ja00008a060. [DOI] [Google Scholar]
- Wang H.; Yang S.; Zhou H.; Sun M.; Du L.; Wei M.; Luo M.; Huang J.; Deng H.; Feng Y.; Huang J.; Zhou Y. Aloperine Executes Antitumor Effects against Multiple Myeloma through Dual Apoptotic Mechanisms. J. Hematol. Oncol. 2015, 8 (1), 1–13. 10.1186/s13045-015-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotic E.; Farnsworth N. R.; Messmer W. M. Cryptopleurine, an Active Antiviral Alkaloid from Boehmeria cylindrica (L.) Sw. (Urticaceae). J. Pharm. Sci. 1972, 61 (9), 1508–1509. 10.1002/jps.2600610945. [DOI] [PubMed] [Google Scholar]
- Cai X. F.; Jin X.; Lee D.; Yang Y. T.; Lee K.; Hong Y. S.; Lee J. H.; Lee J. J. Phenanthroquinolizidine Alkaloids from the Roots of Boehmeria pannosa Potently Inhibit Hypoxia-Inducible Factor-1 in AGS Human Gastric Cancer Cells. J. Nat. Prod. 2006, 69 (7), 1095–1097. 10.1021/np060081y. [DOI] [PubMed] [Google Scholar]
- Ohsaki A.; Nagaoka T.; Yoneda K.; Kishida A. Secu’amamines E-G, New Alkaloids from Securinega suffruticosa Var. Amamiensis. Tetrahedron Lett. 2009, 50 (50), 6965–6967. 10.1016/j.tetlet.2009.09.138. [DOI] [Google Scholar]
- Wang Y. P.; Zhao W.; Xue R.; Zhou Z. X.; Liu F.; Han Y. X.; Ren G.; Peng Z. G.; Cen S.; Chen H. S.; Li Y. H.; Jiang J. D. Oxymatrine Inhibits Hepatitis B Infection with an Advantage of Overcoming Drug-Resistance. Antiviral Res. 2011, 89 (3), 227–231. 10.1016/j.antiviral.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Ding P. L.; Huang H.; Zhou P.; Chen D. F. Quinolizidine Alkaloids with Anti-HBV Activity from Sophora tonkinensis. Planta Med. 2006, 72 (9), 854–856. 10.1055/s-2006-946639. [DOI] [PubMed] [Google Scholar]
- Liu G.; Wang J.; Deng X. H.; Ma P. S.; Li F. M.; Peng X. D.; Niu Y.; Sun T.; Li Y. X.; Yu J. Q. The Anticonvulsant and Neuroprotective Effects of Oxysophocarpine on Pilocarpine-Induced Convulsions in Adult Male Mice. Cell. Mol. Neurobiol. 2017, 37 (2), 339–349. 10.1007/s10571-016-0411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q. M.; Li Y. H.; Hua J.; Huang F. P.; Wang H. S.; Liang D. Antiviral Matrine-Type Alkaloids from the Rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015, 78 (7), 1683–1688. 10.1021/acs.jnatprod.5b00325. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Hu W.-Z.; Che X.-Z.; Peng Y.-B.; Song L.-Y.; Shi Y.-S. Bioactive Quinolizidine Alkaloids from Sophora tonkinensis. China J. Chinese Mater. Medica 2016, 41 (12), 2261–2266. 10.4268/cjcmm20161215. [DOI] [PubMed] [Google Scholar]
- Qavi H. B.; Wyde P. R.; Khan M. A. In Vitro Inhibition of HHV-6 Replication by Sophocarpines. Phyther. Res. 2002, 16 (2), 154–156. 10.1002/ptr.949. [DOI] [PubMed] [Google Scholar]
- Luo D.; Chen N. H.; Wang W. Z.; Zhang J. H.; Li C. J.; Zhuo X. F.; Tu Z. C.; Wu Z. N.; Fan C. L.; Zhang H. P.; Li Y. L.; Wang G. C.; Zhang Y. B. Structurally Diverse Matrine-Based Alkaloids with Anti-Inflammatory Effects from Sophora alopecuroides. Chin. J. Chem. 2021, 39 (12), 3339–3346. 10.1002/cjoc.202100526. [DOI] [Google Scholar]
- Roufosse F.; Kahn J.; Rothenberg M. E.; Wardlaw A. J.; Klion A. D.; Kirby S. Y.; Martyn J.; Bentley J. H.; Bradford E. S.; Yancey S. W.; Steinfeld J.; Gleich G. J. Quinolizidine Alkaloids from Sophora tonkinensis and Their Anti-Inflammatory Activities. J. Allergy Clin. Immunol. 2020, 2 (1), 102592. [Google Scholar]
- Li J. C.; Dai W. F.; Liu D.; Zhang Z. J.; Jiang M. Y.; Rao K. R.; Li R. T.; Li H. M. Quinolizidine Alkaloids from Sophora alopecuroides with Anti-Inflammatory and Anti-Tumor Properties. Bioorg. Chem. 2021, 110, 104781 10.1016/j.bioorg.2021.104781. [DOI] [PubMed] [Google Scholar]
- Orhan I.; Naz Q.; Kartal M.; Tosun F.; Şener B.; Choudhary M. I. In Vitro Anticholinesterase Activity of Various Alkaloids. Zeitschrift fur Naturforsch. - Sect. C J. Biosci. 2007, 62 (9–10), 684–688. 10.1515/znc-2007-9-1010. [DOI] [PubMed] [Google Scholar]
- Zhang B. H.; Wang N. S.; Li X. J.; Kong X. J.; Cai Y. L. Anti-Arrhythmic Effects of Matrine. Acta Pharmacol. Sin. 1990, 11 (3), 253–257. [PubMed] [Google Scholar]
- Serrano E.; Storebakken T.; Penn M.; Øverland M.; Hansen J. Øv.; Mydland L. T. Responses in Rainbow Trout (Oncorhynchus mykiss) to Increasing Dietary Doses of Lupinine, the Main Quinolizidine Alkaloid Found in Yellow Lupins (Lupinus luteus). Aquaculture 2011, 318 (1–2), 122–127. 10.1016/j.aquaculture.2011.05.004. [DOI] [Google Scholar]
- Qin S.; Liang J. Y.; Guo Y. W. Two New Securinega Alkaloids from Securinega suffruticosa. Helv. Chim. Acta 2009, 92 (2), 399–403. 10.1002/hlca.200800263. [DOI] [Google Scholar]
- Vassas A.; Bourdy G.; Paillard J. J.; Lavayre J.; Païs M.; Quirion J. C.; Debitus C. Naturally Occurring Somatostatin and Vasoactive Intestinal Peptide Inhibitors. Isolation of Alkaloids from Two Marine Sponges. Planta Med. 1996, 62 (1), 28–30. 10.1055/s-2006-957790. [DOI] [PubMed] [Google Scholar]
- De Smet P.; Parys J. B.; Callewaert G.; Weidema A. F.; Hill E.; De Smedt H.; Erneux C.; Sorrentino V.; Missiaen L. Xestospongin C Is an Equally Potent Inhibitor of the Inositol 1,4,5-Trisphosphate Receptor and the Endoplasmic-Reticulum Ca2+ Pumps. Cell Calcium 1999, 26 (1–2), 9–13. 10.1054/ceca.1999.0047. [DOI] [PubMed] [Google Scholar]
- Agarwal P.; Huckle J.; Newman J.; Reid D. L. Trends in Small Molecule Drug Properties: A Developability Molecule Assessment Perspective. Drug Discovery Today 2022, 27 (12), 103366 10.1016/j.drudis.2022.103366. [DOI] [PubMed] [Google Scholar]
- Ntie-Kang F.; Nyongbela K. D.; Ayimele G. A.; Shekfeh S. Drug-Likeness” Properties of Natural Compounds.. Physical Sciences Reviews 2019, 10.1515/psr-2018-0169. [DOI] [Google Scholar]
- Ahire E. D.; Sonawane V. N.; Surana K. R.; Talele G. S.. Drug Discovery, Drug-Likeness Screening, and Bioavailability: Development of Drug-Likeness Rule for Natural Products. In Applied Pharmaceutical Practice and Nutraceuticals; Mahapatra D. K., Aguilar C. N., Haghi A. K., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2021; pp 1–18. 10.1201/9781003054894-13. [DOI] [Google Scholar]
- Sander T.; Freyss J.; von Korff M.; Rufener C. DataWarrior: An Open-Source Program For Chemistry Aware Data Visualization And Analysis. J. Chem. Inf. Model. 2015, 55 (2), 460–473. 10.1021/ci500588j. [DOI] [PubMed] [Google Scholar]
- Li G.; Lou M.; Qi X. A Brief Overview of Classical Natural Product Drug Synthesis and Bioactivity. Org. Chem. Front. 2022, 9 (2), 517–571. 10.1039/D1QO01341F. [DOI] [Google Scholar]
- Xu J.; Lv M.; Xu H. The Advances in Bioactivities, Mechanisms of Action and Structural Optimizations of Matrine and Its Derivatives. Mini-Reviews in Medicinal Chemistry. 2022, 22, 1716–1734. 10.2174/1389557522666220113124717. [DOI] [PubMed] [Google Scholar]
- Konrath E. L.; Passos C. S.; Klein-Júnior L. C.; Henriques A. T. Alkaloids as a Source of Potential Anticholinesterase Inhibitors for the Treatment of Alzheimer’s Disease. J. Pharm. Pharmacol. 2013, 65 (12), 1701–1725. 10.1111/jphp.12090. [DOI] [PubMed] [Google Scholar]
- Turabekova M. A.; Vinogradova V. I.; Werbovetz K. A.; Capers J.; Rasulev B. F.; Levkovich M. G.; Rakhimov S. B.; Abdullaev N. D. Structure–Activity Relationship Investigations Of Leishmanicidal N-Benzylcytisine Derivatives. Chem. Biol. Drug Des. 2011, 78 (1), 183–189. 10.1111/j.1747-0285.2011.01092.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.