Abstract
Contrast-enhanced ultrasound (CEUS) uses an intravascular contrast agent to enhance blood flow signals and assess microcirculation in different parts of the human body. Over the past decade, CEUS has become more widely applied in musculoskeletal (MSK) medicine, and the current review aims to systematically summarize current research on the application of CEUS in the MSK field, focusing on 67 articles published between January 2001 and June 2021 in online databases including PubMed, Scopus, and Embase. CEUS has been widely used for the clinical assessment of muscle microcirculation, tendinopathy, fracture nonunions, sports-related injuries, arthritis, peripheral nerves, and tumors, and can serve as an objective and quantitative evaluation tool for prognosis and outcome prediction. Optimal CEUS parameters and diagnostic cut off values for each disease category remain to be confirmed.
Keywords: Bone, contrast agent, contrast-enhanced ultrasound, joint, muscle, musculoskeletal medicine, nerve, sonography, tendon, tumor
INTRODUCTION
Contrast-enhanced ultrasound (CEUS) entails the use a contrast medium in ultrasonography. With sound waves reflecting differences in the two media, the interface between the two substances creates a clear-cut border due to differences in acoustic impedance, leading to contrast enhancement under ultrasonography. The most commonly used contrast agent is gas-filled microbubbles with phospholipid shells. The microbubbles are injected into the vein and spread systematically throughout the bloodstream. They can also be designed to target specific ligands in certain parts of the body. Microbubble oscillation under ultrasound waves and backscattering intensity creates a contrasted image on CEUS.[1,2] The temporal and spatial spread of these microbubbles in the blood circulation also indicates the dynamics of regional perfusion and microcirculation in the human body.[3]
Over the past decade, CEUS has been increasingly applied in the field of musculoskeletal (MSK) medicine, mostly to detect muscle microcirculation, tendinopathies, inflammatory arthritis, fracture nonunions, and the nature of tumors.[3,4] A recent position paper by the World Federation for Ultrasound in Medicine and Biology also detailly described the contrast agent dynamics, CEUS parameters, and the suggested standardized administration of CEUS in various MSK territories.[5] Our study will not only focus on the application and capability of CEUS in the aforementioned MSK realms but also expand the scope to a broader field of MSK medicine, including the assessment of peripheral nerve pathologies, sport injuries, and medical diseases that alter muscle microcirculation. There has also been emerging evidence in the application of CEUS in the children, especially in the evaluation of inflammatory arthropathies, postoperative hip perfusion, and soft-tissue masses, which was described in a recent review article.[6] It is recommended to refer to the review for further discussion of the pediatric application of CEUS. This article provides an updated review of the current applications of CEUS in the MSK field, focusing on the adult population, as a reference for the future clinical implementation of CEUS.
LITERATURE SEARCH
This literature research focuses on articles published between January 2001 and June 2021 in online databases including PubMed, Scopus, and Embase, using a keyword search for “contrast-enhanced ultrasound,” combined with terms including muscle, tendon, ligament, or bone (e.g., [“contrast-enhanced ultrasound”] AND [muscle OR tendon OR ligament OR bone OR MSK]). Abstracts of the searched articles were reviewed for relevance. Search results were limited to human studies published in English. Original articles, meta-analyses, and review articles were reviewed.
RESULTS AND DISCUSSIONS
A total of 1699 studies were initially identified. Removing duplications, articles with irrelevant topics, animal studies, or case reports left a total of 67 studies for review and analysis [Figure 1].
Figure 1.
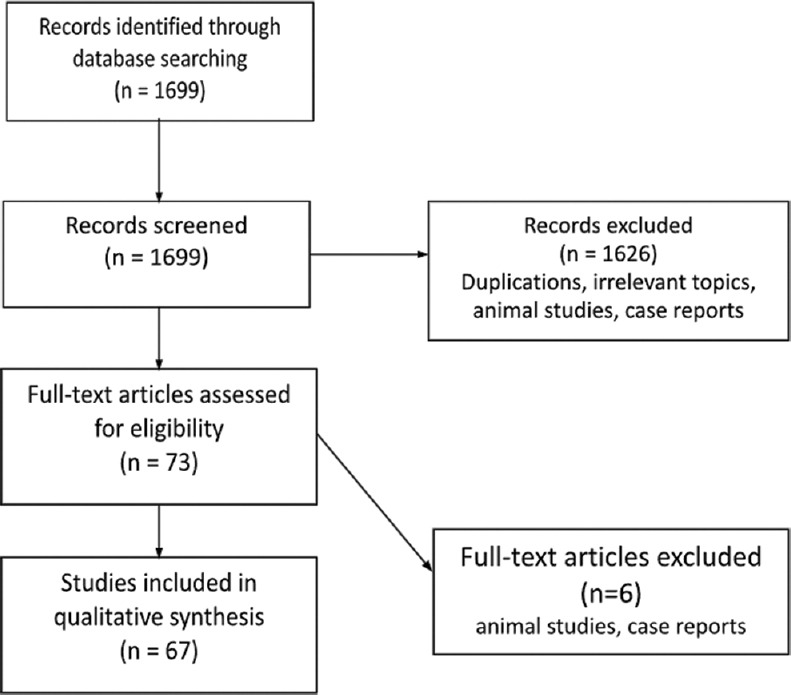
Flowchart illustrating our search strategy. Exclusions were mostly due to duplications, non-English publications or animal studies
Table 1 summarizes the main findings of CEUS applications in different MSK fields. CEUS was most commonly used in the clinical assessment of muscle microcirculation and diagnosis of tendinopathy. Other applications include the evaluation of fracture nonunions, sports-related injuries, arthritis, peripheral nerves, tumors, and the flap transplantation condition.
Table 1.
A summarization of the application of contrast enhanced ultrasound in different musculoskeletal fields
| Application (evaluation/diagnosis) | Main findings | Reference |
|---|---|---|
| Muscle microcirculation | DM | [6-11] |
| Impaired muscular microcirculation | ||
| DM patients with MC: Reduced capillary recruitment and arterial blood reserve after exercise | ||
| Uncomplicated Type-1 DM: Diminished MBV in muscular capillaries | ||
| PAOD | [12-19] | |
| Impaired muscular microcirculation correlated to the severity of ABI reduction | ||
| Prolonged TTP that increases with disease severity. TTP reduces after revascularization | ||
| Elevated MBV after exercise | [20-22] | |
| Aging: Conflicting results | ||
| Middle-aged versus young-aged: Lower muscle microcirculation response to isometric exercise | ||
| Old-aged versus young-aged: Similar microvascular perfusion after cuff occlusion or exercise | ||
| Smokers: Impaired muscular microcirculation | [23] | |
| Compartment syndrome: Higher time to arrival, TTP, MBV, MTT | [25] | |
| Myositis: Higher blood flow velocity and MBV | [26] | |
| Systemic sclerosis: Decreased MTT and AUC of TIC | [27] | |
| Tendinopathy and tendon repair | Achilles tendinopathy | [29-32] |
| Increased MBV | ||
| Increased vascularity in chronic Achilles tendinopathy | ||
| Increased vascularity in the uninjured Achilles tendon in patients with previous Achilles tendinopathy | ||
| Adhesive capsulitis | [34-35] | |
| Contrast enhancement at the rotator interval | ||
| Detects capsular inflammation | ||
| Differentiates RCT subtypes better than traditional US and MRI | ||
| RCT repair surgical outcome | [36-43] | |
| Postoperative measurements | ||
| Higher PE and WiR at the peribursa and suture anchor | ||
| Increased AUC in the distal bursal and articular areas | ||
| Diminished perfusion of the rotator cuff correlated with poorer functional outcomes | ||
| Preoperative measurements | ||
| Deltoid perfusion and caliber correlated with shoulder function after RSA | ||
| Impaired deltoid perfusion correlated with limited ROM after RSA | ||
| Supraspinatus vascularization correlated with early postoperative function and the risks of tendon retear | ||
| Fracture nonunions | Higher perfusion, PE, WiAUC, WiR, TTP in patients with better prognosis (i.e., future consolidation) Increased PE, WiR and TTP in septic nonunions | [44-50] |
| Sports-related injuries | Contrast enhancement pattern in muscle strains aid in determining the time of return to play Hypoenhancement of CEUS in Grade I muscle lesions No perfusion changes after cryotherapy in cases with DOMS Reduced PE and WiAUC after PRICE in healthy athletes after cycling | [51-54] |
| Arthritis | Psoriatic arthritis: Amplifies changes in bone outline and synovium detected by US; aids in diagnosis RA: Discriminates RA from other arthritis, sensitive in detecting synovitis | [55-58] |
| Peripheral nerves | A potential tool to visualize the vasculature of the median nerves CTS Greater blood flow in the sub-synovial connective tissue Increased blood flow in sub-synovial connective tissue and median nerve in the early phase after carpal tunnel release surgery |
[60-61] |
| Characterization of tumors | May aid in differentiating benign or malignant soft tissue tumor Improves the successful rate of soft tissue tumors biopsy | [62-64] |
| Flap transplantation | Ability to detect flap necrosis, hematoma or insufficient tissue perfusion was similar to contrast-enhanced MRI Flaps with microvascular complications requiring revision: Decreased TTP, MTT, regional blood volume and flow Buried-flap transplantation with wound healing disturbance: Longer TTP and smaller AUC of TIC | [65-67] |
CEUS: Contrast-enhanced ultrasound, DM: Diabetes mellitus, MC: Microvascular complication, MBV: Microvascular blood volume, PAOD: Peripheral arterial occlusion disease, ABI: Ankle-brachial index, TIC: Time-intensity curve, TTP: Time to peak intensity, MTT: Mean transit time, WiAUC: Wash-in AUC, AUC: Area under curve, RCT: Rotator cuff tear, US: Ultrasonography, MRI: Magnetic resonance imaging, PE: Peak enhancement, WiR: Wash-in rate, RSA: Reverse shoulder arthroplasty, ROM: Range of motion, DOMS: Delayed-onset muscle soreness, PRICE: Protection, rest, ice, compression, and elevation, RA: Rheumatoid arthritis, CTS: Carpal tunnel syndrome
Parameters of contrast-enhanced ultrasound
The quantitative parameters of CEUS are measured based on the dynamic changes of the contrast agent over a period of time at a fixed region of interest (ROI). The time-intensity curve (TIC) of contrast agent perfusion shows an initial exponential rise, peaks, and then slowly decays. The following are some common temporal and amplitude parameters of CEUS.[4,7]
Contrast agent arrival time: The time interval from contrast injection to its arrival at the ROI and detection by US
Peak enhancement (PE) or peak intensity (PI): The value of maximum contrast agent intensity
Time to peak intensity (TTP): The time required to reach PE
Area under curve (AUC): The area under the TIC, which is proportional to the regional blood volume
Wash-in rate (WiR): The maximum slope of the signal enhancement curve
Wash-in AUC (WiAUC): The AUC from contrast agent injection until PE
Mean transit time (MTT): The period when intensity exceeds the mean value
Microvascular blood volume (MBV): The total perfusion volume in the ROI within a defined period
Blood flow velocity: The total blood volume divided by total time.
Safety profile of contrast-enhanced ultrasound
One of the extensively used, ultrasound contrast agent (UCA), SonoVue (Bracco, Milan, Italy), that contains sulfur hexafluoride had been proven to have a very low risk of complication, which was comparable or lower than the gadolinium-based or iodinated contrast agent.[8] The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guideline in 2017 had given the recommendation of UCA in nonhepatic use.[9] As the guideline described, it is not necessary to have a blood test before a CEUS examination, and it is considered to be safe to administer UCA to renal insufficiency patients. A more recent retrospective study focusing on the MSK application of CEUS compiled a total of 2268 examinations.[10] Only 2 cases experienced mild adverse effects (AE) and 1 case experienced severe AE (<0.04%). Symptoms of mild AE included sensation of warmth, dizziness, discomfort, nausea, itchiness, and headache. These symptoms were self-limited and the patients required no medical support. The severe AE was an anaphylactic response to the contrast agent which induced increased blood pressure, tachycardia, and dyspnea in the patient. The study concluded that sulfur hexafluoride contrast agent had a lower rate of AEs than the other agents even in elderlies with concomitant diseases. An earlier study including 30,222 patients undergoing abdominal CEUS also demonstrated a high safety profile with only 6 patients (0.020%) experiencing AEs.[11]
Analysis of muscle microcirculation
Muscular CEUS has been used to quantify changes in muscle microcirculation in various diseases, including type-2 diabetes mellitus (DM), and peripheral arterial occlusion disease (PAOD). One study demonstrated impaired microcirculation in advanced DM patients when compared with PAOD and control groups.[12] Another showed that DM patients with microvascular complications (MC) had impaired muscle microcirculation compared with uncomplicated DM and control subjects.[13] Moreover, Womack et al. discovered that patients with DM and MC experienced reduced capillary recruitment and blood flow during exercise compared to patients with uncomplicated DM and normal subjects.[14] This was later echoed by Xu et al.’s finding that patients with DM and MC have a significantly prolonged arrival time difference of contrast agent in the arteries, muscles and veins, implying a lower arterial blood reserve compared to those without MC.[15] Irace et al. induced temporary ischemia in the forearm of type-1 DM patients without known vascular complications. Diminished MBV was found in muscle capillaries both at rest and after induced ischemia, indicating CEUS may be a reliable tool to detect early vascular abnormalities in DM patients.[16] A recent meta-analysis included 15 studies comparing the muscle microcirculation in terms of CEUS parameters between healthy individuals and patients with DM.[17] The study revealed significantly impaired perfusion in the DM group with no improvement in muscle perfusion indices on CEUS even after insulin administration.
Different groups of researchers have demonstrated the detection of impaired muscle microcirculation and flow reserve by CEUS in PAOD patients compared to healthy individuals.[18-20] A more recent study further established a correlation between symptom severity and the ankle-brachial index (ABI) with CEUS parameters in PAOD patients,[21] indicating that impaired muscle microcirculation is correlated to the severity of ABI reduction. When comparing limbs with normal ABI in PAOD patients to the healthy population, the muscle microcirculation detected by CEUS was still significantly reduced. However, in a study comparing PAOD patients with age-matched healthy controls, MBV was found to be elevated in the PAOD group after submaximal leg exercise. This may represent a compensatory strategy in the bodies of these patients to maintain sufficient muscle perfusion after exercise.[22] One parameter commonly used to evaluate microvascular dysfunction in PAOD is TTP after bolus injection of contrast medium.[23] Compared to the control group, PAOD patients had a longer TTP despite being asymptomatic, and TTP also tends to increase with disease severity.[24] After revascularization, TTP significantly shortened and those with a shorter TTP had more significant symptom relief.[25]
A few studies used CEUS to investigate changes to muscle microcirculation with aging.[26,27] Hildebrandt et al. found a lower microcirculation response to isometric exercise in the middle aged compared to the young aged.[27] However, a more recent study discovered no differences in microvascular perfusion (defined as MBV × flow velocity) after cuff occlusion or submaximal leg exercise in healthy elders compared to the young population.[28] Mancini et al. found no difference between smokers and nonsmokers in terms of color Doppler ultrasound and ABI, while the CEUS succeeded in detecting impairment in the smokers’ muscle microcirculation compared to the nonsmokers.[29] A review article outlining ultrasound biomarkers for sarcopenia also suggested CEUS as a potential technique to evaluate muscle quality.[30]
CEUS could also be utilized to detect ongoing compartment syndrome by assessing lower limb muscle perfusion pressures. The hypoperfusion group, induced by tourniquet in healthy volunteers, showed significantly higher values in several CEUS parameters, including the time to arrival of the bolus contrast, TTP, MBV, and MTT, with significantly lower regional blood flow.[31]
CEUS may also play a role in patients with myositis. Significantly higher blood flow velocity and MBV were found in patients with dermatomyositis or polymyositis.[32] However, sensitivity, specificity, and predicted values were lower compared to the MRI. In patients with systemic sclerosis, MMT and AUC were also decreased in the gastrocnemius muscles compared to that of healthy counterparts, suggesting impaired muscular microcirculation in these patients.[33]
While CEUS is increasingly used to assess muscle microcirculation, there is still no standard CEUS protocol. Young et al. used CEUS to measure the microvascular blood flow within the calf muscle 1, 3, and 5 min after cuff-occlusion, finding that the 5-min cuff-occlusion protocol had the best reliability and largest magnitude. Therefore, a standardized 5-min cuff-occlusion period was proposed to be used in future studies and clinical practice.[34]
Assessment of tendinopathy and tendon repair
Several studies used CEUS to detect tendinopathy. Pingel et al. discovered an increase in MBV before and after treadmill exercise at all times in patients with Achilles tendinopathy compared to healthy controls.[35] The result implied that microvascular changes may be involved in the pathogenesis of tendinopathy. Other research also showed increased vascularity of the uninjured Achilles tendon in patients who had previous contralateral Achilles tendon rupture under CEUS, while the power Doppler failed to identify the same alternation.[36] This may suggest that vascularization increased long before actual injuries took place in those without clinical signs or traditional US abnormalities of tendinopathy. Further studies using CEUS revealed a greater sensitivity to detect vascularization in chronic Achilles tendinopathy than power Doppler. However, the research failed to demonstrate the correlation of pain or disability to such neovascularization of the tendon.[37] In contrast, another cross-sectional study found a significant association between the MBV and clinical severity and symptoms duration of Achilles tendinopathy.[38]
A pilot study used CEUS to follow-up on patients with lateral epicondylitis who had undergone platelet-rich plasma (PRP) injection, concluding that CEUS was a sensitive tool to display improved vascularity at the myotendinous junction of the common extensor tendon after PRP treatment.[39]
CEUS was also used to diagnose adhesive capsulitis (AC).[40,41] One study discovered that CEUS enhancement at the rotator interval of affected shoulders was seen in all AC patients, while no enhancement was noted in asymptomatic shoulders.[41] The study also compared CEUS to MRI and concluded that CEUS has similar sensitivity for detecting capsular inflammation. A study by Tang et al. demonstrated a higher detection rate of rotator cuff tear (RCT) subtypes, later proved by arthroscopy, using percutaneous US-guided subacromial bursography and tendon lesionography under CEUS when compared to the traditional US and MRI (96.9%, 74.2%, and 76.3%, respectively.) This held true, especially for small full-thickness tears combined with partial-thickness tears.[42]
CEUS is not only a diagnostic tool for tendinopathy but also for the assessment of surgical intervention outcomes. Gamradt et al. assessed shoulders after arthroscopic rotator cuff repair using CEUS.[43] At 3 months after repair, the researchers found that the PE and WiR were significantly higher in the peribursal soft tissue and anchor site compared to the supraspinatus tendon itself. A later study measuring AUC of the TIC in patients with rotator cuff repair surgeries also showed that AUC peaked at 1 and 2 months in the distal bursal and distal articular areas and decreased at 3 months after surgery.[44] Adler et al., retrospectively evaluated the topographic map of rotator cuff vascularity after surgical repair. Among the peribursal, articular medial, articular lateral, and suture anchor, they found significantly higher enhancement signals in the peribursal and suture anchor regions.[45] Cadet et al., followed up with patients who had undergone arthroscopic rotator cuff repair with CEUS for at least 10 months. CEUS enhancement was found to be consistent with previous findings, and they further discovered that the increase in blood flow gradually fell over time.[46] These patterns of postoperative vascularization in different areas of the repaired rotator cuff may aid in the decision for appropriate repair methods or postoperative rehabilitation.
Later studies focused on specific ROI based on the topographic map of rotator cuff vascularity. Fischer et al. found a strong correlation between diminished perfusion under CEUS at the supraspinatus fossa and functional impairment in operated shoulders after supraspinatus tendon repair.[47] They further discovered that preoperative perfusion and caliber of the deltoid muscles were significantly correlated with shoulder function after reverse shoulder arthroplasty (RSA).[48] Kunz et al. assessed CEUS parameters for the supraspinatus tendon preoperatively (with the probe positioned medial to the scapular notch) and discovered that preoperative supraspinatus vascularization was correlated with early postoperative function and risk of tendon retear.[49]
Most studies evaluating surgical outcomes focused on microvascularization of the supraspinatus before or after tendon repair surgeries. Fischer et al. used dynamic CEUS to assess surgical outcomes after RSA.[50] In their work, deltoid muscles on both operated and nonoperated sides were selected as the target of CEUS. Perfusion deficit in the operated shoulder, indicating an impaired deltoid integrity, was associated with a limited range of motion (ROM). Their most recent work also demonstrated that dynamic CEUS could be used to visualize the microperfusion of healing tendons using parameters such as PE, WiAUC, and TTP with a strong correlation with dynamic contrast-enhanced MRI.[51]
Evaluation of fracture nonunions
Other studies have focused on using CEUS to assess the microperfusion of bones, especially its ability to evaluate fracture union. Pozza et al. demonstrated the capability of CEUS to monitor the healing process in noninfected long bone fractures as it could show early progressive neovascularization.[52] Krammer et al. performed CEUS in patients with tibial nonunions 12 weeks after revision surgeries. CEUS revealed significantly higher osseous perfusion in patients with future consolidation versus those with persistent nonunions in all quantification parameters (e.g., PE, WiAUC, TTP, and WiR).[53] The ability to predict eventual nonunions in the early postoperative stage provided crucial information for decision-making for early re-revision surgeries.
One study compared the capability of CEUS and contrast-enhanced MRI in differentiating septic and aseptic long bone nonunions.[54] CEUS found significant differences between septic and aseptic nonunions both qualitatively and quantitatively using parameters such as WiR, TTP, and PE. While no qualitative change was observed in contrast-enhanced MRI, a relation with microbiological results from the nonunion tissue was seen following data quantification. Higher perfusion values, contrast enhancement, and larger initial AUC were observed on the quantified MRI images in septic nonunions. Doll et al. recently published the results of the Advanced Microperfusion Assessed Non-Union Diagnostics with CEUS (AMANDUS) project, focusing on the efficacy of CEUS to differentiate septic and aseptic nonunions in upper and lower limb fractures.[55,56] Both studies showed a significant perfusion difference in CEUS parameters, such as TICs and PE, between septic and nonseptic nonunions. The authors thus recommended that CEUS should be included in the diagnostic algorithm before a revision surgery due to the different treatment strategies between septic and nonseptic nonunions. Another recent study further revealed that PE yielded the most coherent results with tissue culture in long bone nonunions.[57]
CEUS has been used to compare the outcome between different operative methods in fractured cases. A study compared the deltoid perfusion under CEUS after traditional open reduction and internal fixation with locked plates and the novel minimally invasive plate osteosynthesis (MIPO) methods in proximal humeral fractures.[58] The MIPO method was hypothesized to reduce soft tissue injury in such cases. Nevertheless, the CEUS, power Doppler of the deltoid perfusion, and the shoulder functional score all failed to show a significant difference between the two surgical methods.
Application in assessing sports-related injuries
Sports-related muscle injury and its healing process could be assessed by CEUS. Early research prospectively and longitudinally evaluated muscle strains at different locations of lower extremity with CEUS in professional athletes.[59] The contrast-enhanced area, including the scars of the injured site, became larger and then shrank with completely resolved hemorrhagic collection at 40 and 60 days, respectively. The clinician may thus be able to schedule the athlete’s return to play based on the use of CEUS imaging as the contrast enhancement may imply the extent of repair in these strained muscles. A recent pilot study including 15 patients with acute muscle injury demonstrated that CEUS was superior to traditional ultrasound modalities in the early detection of grade I muscle lesions, showing focal hypoenhancement.[60]
Kellermann et al. used CEUS to detect delayed-onset muscle soreness (DOMS) induced by eccentric exercise on healthy volunteers. CEUS seems to be able to detect increased intramuscular perfusion, corresponding to intramuscular edema shown in MRI.[61] A randomized-controlled trial (RCT) further applied CEUS to observe laboratory-controlled eccentric muscle damage in the lower extremities in healthy participants who were later treated with cryotherapy.[62] Although the VAS pain score decreased at 34 and 48 h after cryotherapy, there was no interaction between cryotherapy and CEUS perfusion change. The authors postulated that such analgesic effect by cryotherapy in DOMS might not necessarily be caused by suppressing muscle inflammation. Another study focusing on the effect of wearing compression garments in DOMS concluded that compression garments reduce muscle stiffness, but CEUS showed no significant changes in intramuscular perfusion.[63]
Hotfiel et al. evaluated the effect of PRICE (protection, rest, ice, compression, and elevation) on muscular perfusion using quantifiable CEUS parameters, including PE and WiAUC in a RCT.[64] Healthy athletes underwent either the PRICE protocol or control after cycling exercise. In the PRICE group, PE and WiAUC in the rectus femoris and vastus intermedius reduced significantly right after intervention and continued to decrease in rectus femoris at 60 minutes follow-up, while the control group showed a continuous rise at the time of intervention and no change at 60 min follow-up. The mechanism of intramuscular perfusion constriction after PRICE remains unclear, but the change detected by CEUS may help develop a more delicate care plan in athletes after intense exercise and play.
Diagnosis of arthritis
Traditional B-mode and power Doppler ultrasound had been used to detect morphological changes in arthritis such as synovial hypertrophy and increased local blood flow. CEUS has been compared to these traditional ultrasound methods in terms of efficacy in detecting inflammatory arthritis.
Solivetti et al. compared the use of traditional ultrasound, CEUS, and contrast-MRI in patients with suspicion of psoriatic arthritis.[65] CEUS was found to potentially amplify changes in bone outline and synovium detected by ultrasound, thereby raising confidence in cases with suspicious symptoms but without clinical diagnosis.
Ohrndorf et al. applied grayscale, power Doppler sonography, and CEUS in studying the wrist and finger joints in patients with rheumatoid arthritis before and after anti-inflammatory treatment, finding that CEUS showed the greatest sensitivity in detecting synovitis and follow-up treatment effect when compared to MRI.[66]
More recently, Rizzo et al. developed a novel, pixelwise quantification-based method to investigate the kinetics of contrast agent perfusion patterns in differentiating rheumatoid arthritis from other different forms of inflammatory arthritis.[67] Their proposed method demonstrated the accuracy of up to 97% in discriminating rheumatoid arthritis from psoriatic arthritis, spondyloarthritis, and arthritis in connective tissue disease.
CEUS was also used to investigate degenerative joint diseases, specifically osteoarthritis (OA). Song et al. demonstrated that in patients with painful knee OA, CEUS had even better sensitivity than contrast MRI to detect synovitis activity using time/intensity analysis when focusing on the superior recess synovium of the affected knee.[68]
Application in assessing peripheral nerves
CEUS had also attracted researchers in the field of peripheral nerve pathology, particularly carpal tunnel syndrome (CTS). Detecting microvascularization around the injured median nerve by power Doppler is challenging. Various sensitivity and specificity levels have been reported by previous studies due to equipment limitations and low accuracy in detecting blood flow direction.[69] CEUS, with an increasing signal-to-noise ratio, was seen as a potential solution for improving sensitivity and specificity. Volz et al. conducted a pilot study using CEUS to evaluate the median nerves in patients who were simultaneously undergoing contrast-enhanced transthoracic echocardiography.[70] The result showed that CEUS could be used to visualize the vasculature of the median nerve that remains inaccessible by traditional methods.
Motomiya et al. used CEUS to investigate the microcirculation of the subsynovial connective tissue (SSCT) as well as the median nerve at the carpal tunnel segment.[71] Patients with CTS were found to have greater blood flow in the SSCT than healthy volunteers. Furthermore, the blood flow significantly increased in both SSCT and median nerve three months after carpal tunnel release surgery. The author proposed that such a microcirculation increase in the SSCT in the early postoperative phase played a role in neural recovery in CTS.
Although limited literatures were available regarding the application of CEUS in assessing peripheral nerves, it has shown the potential to detect microvascularity in peripheral nerve abnormalities with better sensitivity compared to traditional ultrasound alone or power Doppler.
Characterization of tumors
CEUS has been widely used for differential diagnosis between benign and malignant soft-tissue mass. A recent meta-analysis covered 5 studies including 746 patients with soft tissue mass who underwent CEUS to identify the nature of tumor that was later confirmed by pathological findings or clinical image appearance.[72] Three of the studies differentiate benign and malignant tumors by the perfusion pattern of CEUS of the mass, while the remaining studies considered the contrast filling rate in addition to morphology. The pooled sensitivity and specificity were, respectively, 76% and 67%. The author proposed using CEUS alongside traditional US to help differentiate benign and malignant tumors.
An earlier pilot study showed that CEUS increased the successful rate of MSK mass biopsy compared to using traditional US.[73] Daniels et al. used CEUS to help localize the target area and the traditional B-mode image to trace the needle tract in MSK mass biopsy.[74] They concluded that CEUS-guided mass biopsy was effective and safe, especially when the targets were heterogeneous lesions or suspected necrosis.
Evaluation of flap transplantation
CEUS can also be used for flap perfusion evaluation and prognosis. CEUS allows for the dynamic flow detection of the transplanted flap as deep as 3 cm with a quantitative TIC of its microcirculation.[75] Lamby et al. used CEUS to assess postoperative perfusion of cutaneous, subcutaneous, fatty tissue, and muscle tissues in flap transplantation, with flap necrosis, hematoma or insufficient tissue perfusion detection results similar to those of contrast-enhanced MRI.[73] In patients undergoing osteocutaneous flap transplantation, significantly lower CEUS perfusion parameter values were detected in patients with postoperative complications that require revision, including TTP, MTT, and regional blood volume and flow.[76] The MC of buried-flap transplantation was previously difficult to detect due to its deep location. Using the better tissue penetration provided by CEUS, Geis et al. found longer TTP and smaller AUC in patients with wound healing complications after buried-flap transplantation.[77]
PERSPECTIVES AND CONCLUSIONS
The development of CEUS has led to many innovative and promising application in the MSK field. CEUS serves as an objective and quantitative evaluation tool not only for the diagnosis of various MSK pathologies but also for treatment outcome prediction. A growing number of studies has demonstrated its use in quantifying microperfusion and evaluating several vascular-related or muscular diseases, tendinopathy, nerve injuries, sports-related injuries, and flap transplant condition. It is also shown to be effective in differentiating etiologies of fracture nonunions and the nature of tumors. Moreover, it can be used to predict treatment outcomes, including tendon surgeries and flap transplantation and also provides valuable information for earlier decision-making of whether to perform further surgeries in patients with persistent nonunions. Novel applications of CEUS are continuing to emerge, including the evaluation of peripheral nerves, a potential topic for future endeavors. Finally, appropriate CEUS parameters and optimal cut off values for the diagnosis of different categories of diseases warrant additional research.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Wen-Shiang Chen, an editorial board member at Journal of Medical Ultrasound, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
REFERENCES
- 1.Chung YE, Kim KW. Contrast-enhanced ultrasonography: Advance and current status in abdominal imaging. Ultrasonography. 2015;34:3–18. doi: 10.14366/usg.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Averkiou MA, Bruce MF, Powers JE, Sheeran PS, Burns PN. Imaging methods for ultrasound contrast agents. Ultrasound Med Biol. 2020;46:498–517. doi: 10.1016/j.ultrasmedbio.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Chang KV, Lew HL, Wang TG, Chen WS. Use of contrast-enhanced ultrasonography in musculoskeletal medicine. Am J Phys Med Rehabil. 2012;91:449–57. doi: 10.1097/PHM.0b013e31823caaa3. [DOI] [PubMed] [Google Scholar]
- 4.Gitto S, Messina C, Vitale N, Albano D, Sconfienza LM. Quantitative musculoskeletal ultrasound. Semin Musculoskelet Radiol. 2020;24:367–74. doi: 10.1055/s-0040-1709720. [DOI] [PubMed] [Google Scholar]
- 5.Fischer C, Krix M, Weber MA, Loizides A, Gruber H, Jung EM, et al. Contrast-enhanced ultrasound for musculoskeletal applications: A world federation for ultrasound in medicine and biology position paper. Ultrasound Med Biol. 2020;46:1279–95. doi: 10.1016/j.ultrasmedbio.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Ntoulia A, Barnewolt CE, Doria AS, Ho-Fung VM, Lorenz N, Mentzel HJ, et al. Contrast-enhanced ultrasound for musculoskeletal indications in children. Pediatr Radiol. 2021;51:2303–23. doi: 10.1007/s00247-021-04964-6. [DOI] [PubMed] [Google Scholar]
- 7.Tang MX, Mulvana H, Gauthier T, Lim AK, Cosgrove DO, Eckersley RJ, et al. Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus. 2011;1:520–39. doi: 10.1098/rsfs.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piscaglia F, Bolondi L Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of sonovue in abdominal applications: Retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–75. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: Update 2017 (long version) Ultraschall Med. 2018;39:e2–44. doi: 10.1055/a-0586-1107. [DOI] [PubMed] [Google Scholar]
- 10.Fischer C, Kunz P, Strauch M, Weber MA, Doll J. Safety profile of musculoskeletal contrast-enhanced ultrasound with sulfur hexafluoride contrast agent. Ther Clin Risk Manag. 2020;16:269–80. doi: 10.2147/TCRM.S235235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang C, Fang K, Guo Y, Li R, Fan X, Chen P, et al. Safety of sulfur hexafluoride microbubbles in sonography of abdominal and superficial organs: Retrospective analysis of 30,222 Cases. J Ultrasound Med. 2017;36:531–8. doi: 10.7863/ultra.15.11075. [DOI] [PubMed] [Google Scholar]
- 12.Duerschmied D, Maletzki P, Freund G, Olschewski M, Seufert J, Bode C, et al. Analysis of muscle microcirculation in advanced diabetes mellitus by contrast enhanced ultrasound. Diabetes Res Clin Pract. 2008;81:88–92. doi: 10.1016/j.diabres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Li Y, Wang PJ, Gao Y. Contrast-enhanced ultrasonography of skeletal muscles for type 2 diabetes mellitus patients with microvascular complications. Int J Clin Exp Med. 2014;7:573–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175–83. doi: 10.1016/j.jacc.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu ZH, Chen JH, Huang FB, Lv GR. Evaluation of skeletal muscle microcirculation and reserve function of the type 2 diabetes with contrast-enhanced ultrasonography. Ultrasound Q. 2020;36:38–42. doi: 10.1097/RUQ.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 16.Irace C, Messiniti V, Tassone B, Cortese C, Barrett EJ, Gnasso A. Evidence for congruent impairment in micro and macrovascular function in type 1 diabetes. PLoS One. 2017;12:e0187525. doi: 10.1371/journal.pone.0187525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LL, Zhai JX, Kang J, Li YS. Utility of contrast-enhanced ultrasound for the assessment of skeletal muscle perfusion in diabetes mellitus: A meta-analysis. Med Sci Monit. 2019;25:4535–43. doi: 10.12659/MSM.915252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas KN, Cotter JD, Lucas SJ, Hill BG, van Rij AM. Reliability of contrast-enhanced ultrasound for the assessment of muscle perfusion in health and peripheral arterial disease. Ultrasound Med Biol. 2015;41:26–34. doi: 10.1016/j.ultrasmedbio.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Amarteifio E, Weber MA, Wormsbecher S, Demirel S, Krakowski-Roosen H, Jöres A, et al. Dynamic contrast-enhanced ultrasound for assessment of skeletal muscle microcirculation in peripheral arterial disease. Invest Radiol. 2011;46:504–8. doi: 10.1097/RLI.0b013e3182183a77. [DOI] [PubMed] [Google Scholar]
- 20.Lindner JR, Womack L, Barrett EJ, Weltman J, Price W, Harthun NL, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1:343–50. doi: 10.1016/j.jcmg.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson BP, Hodovan J, Mason OR, Moccetti F, Gupta A, Muller M, et al. Limb perfusion during exercise assessed by contrast ultrasound varies according to symptom severity in patients with peripheral artery disease. J Am Soc Echocardiogr. 2019;32:1086–94. doi: 10.1016/j.echo.2019.05.001. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meneses AL, Nam MC, Bailey TG, Magee R, Golledge J, Hellsten Y, et al. Leg blood flow and skeletal muscle microvascular perfusion responses to submaximal exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol. 2018;315:H1425–33. doi: 10.1152/ajpheart.00232.2018. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen T, Davidson BP. Contrast enhanced ultrasound perfusion imaging in skeletal muscle. J Cardiovasc Imaging. 2019;27:163–77. doi: 10.4250/jcvi.2019.27.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duerschmied D, Maletzki P, Freund G, Olschewski M, Bode C, Hehrlein C. Success of arterial revascularization determined by contrast ultrasound muscle perfusion imaging. J Vasc Surg. 2010;52:1531–6. doi: 10.1016/j.jvs.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Amarteifio E, Krix M, Wormsbecher S, Demirel S, Braun S, Delorme S, et al. Dynamic contrast-enhanced ultrasound for assessment of therapy effects on skeletal muscle microcirculation in peripheral arterial disease: Pilot study. Eur J Radiol. 2013;82:640–6. doi: 10.1016/j.ejrad.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Dunford EC, Au JS, Devries MC, Phillips SM, MacDonald MJ. Cardiovascular aging and the microcirculation of skeletal muscle: Using contrast-enhanced ultrasound. Am J Physiol Heart Circ Physiol. 2018;315:H1194–9. doi: 10.1152/ajpheart.00737.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildebrandt W, Schwarzbach H, Pardun A, Hannemann L, Bogs B, König AM, et al. Age-related differences in skeletal muscle microvascular response to exercise as detected by contrast-enhanced ultrasound (CEUS) PLoS One. 2017;12:e0172771. doi: 10.1371/journal.pone.0172771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meneses AL, Nam MC, Bailey TG, Anstey C, Golledge J, Keske MA, et al. Skeletal muscle microvascular perfusion responses to cuff occlusion and submaximal exercise assessed by contrast-enhanced ultrasound: The effect of age. Physiol Rep. 2020;8:e14580. doi: 10.14814/phy2.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini M, Di Donato O, Saldalamacchia G, Liuzzi R, Rivellese A, Salvatore M. Contrast-enhanced ultrasound evaluation of peripheral microcirculation in diabetic patients: Effects of cigarette smoking. Radiol Med. 2013;118:206–14. doi: 10.1007/s11547-012-0830-x. [DOI] [PubMed] [Google Scholar]
- 30.Ivanoski S, Vasilevska Nikodinovska V. Future ultrasound biomarkers for sarcopenia: Elastography, contrast-enhanced ultrasound, and speed of sound ultrasound imaging. Semin Musculoskelet Radiol. 2020;24:194–200. doi: 10.1055/s-0040-1701630. [DOI] [PubMed] [Google Scholar]
- 31.Sellei RM, Waehling A, Weber CD, Jeromin S, Zimmermann F, McCann PA, et al. Contrast enhanced ultrasound (CEUS) reliably detects critical perfusion changes in compartmental muscle: A model in healthy volunteers. Eur J Trauma Emerg Surg. 2014;40:535–9. doi: 10.1007/s00068-014-0443-2. [DOI] [PubMed] [Google Scholar]
- 32.Weber MA, Jappe U, Essig M, Krix M, Ittrich C, Huttner HB, et al. Contrast-enhanced ultrasound in dermatomyositis- and polymyositis. J Neurol. 2006;253:1625–32. doi: 10.1007/s00415-006-0318-5. [DOI] [PubMed] [Google Scholar]
- 33.Partovi S, Kaspar M, Aschwanden M, Robbin MR, Bilecen D, Walker UA, et al. Quantitative dynamic contrast-enhanced ultrasound for the functional evaluation of the skeletal muscle microcirculation in systemic sclerosis. Clin Hemorheol Microcirc. 2016;62:35–44. doi: 10.3233/CH-151929. [DOI] [PubMed] [Google Scholar]
- 34.Young GM, Krastins D, Chang D, Lam J, Quah J, Stanton T, et al. Influence of cuff-occlusion duration on contrast-enhanced ultrasound assessments of calf muscle microvascular blood flow responsiveness in older adults. Exp Physiol. 2020;105:2238–45. doi: 10.1113/EP089065. [DOI] [PubMed] [Google Scholar]
- 35.Pingel J, Harrison A, Simonsen L, Suetta C, Bülow J, Langberg H. The microvascular volume of the Achilles tendon is increased in patients with tendinopathy at rest and after a 1-hour treadmill run. Am J Sports Med. 2013;41:2400–8. doi: 10.1177/0363546513498988. [DOI] [PubMed] [Google Scholar]
- 36.Genovese E, Ronga M, Recaldini C, Fontana F, Callegari L, Maffulli N, et al. Analysis of achilles tendon vascularity with second-generation contrast-enhanced ultrasound. J Clin Ultrasound. 2011;39:141–5. doi: 10.1002/jcu.20789. [DOI] [PubMed] [Google Scholar]
- 37.De Marchi A, Pozza S, Cenna E, Cavallo F, Gays G, Simbula L, et al. In Achilles tendinopathy, the neovascularization, detected by contrast-enhanced ultrasound (CEUS), is abundant but not related to symptoms. Knee Surg Sports Traumatol Arthrosc. 2018;26:2051–8. doi: 10.1007/s00167-017-4710-8. [DOI] [PubMed] [Google Scholar]
- 38.Praet SF, Ong JH, Purdam C, Welvaert M, Lovell G, Dixon L, et al. Microvascular volume in symptomatic Achilles tendons is associated with VISA-A score. J Sci Med Sport. 2018;21:1185–91. doi: 10.1016/j.jsams.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhury S, de La Lama M, Adler RS, Gulotta LV, Skonieczki B, Chang A, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: Sonographic assessment of tendon morphology and vascularity (pilot study) Skeletal Radiol. 2013;42:91–7. doi: 10.1007/s00256-012-1518-y. [DOI] [PubMed] [Google Scholar]
- 40.Mezian K, Chang KV. Contrast-enhanced ultrasonography for the diagnosis of frozen shoulder. J Med Ultrasound. 2019;27:146–7. doi: 10.4103/JMU.JMU_103_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn K, Kang CH, Jeong W. Contrast-Enhanced ultrasonography in patients with adhesive capsulitis: Preliminary experience. Iranian Journal of Radiology. 2017;14:e33069. [Google Scholar]
- 42.Tang YQ, Zeng C, Su XT, Li SS, Yi WH, Xu JJ, et al. The value of percutaneous shoulder puncture with contrast-enhanced ultrasound in differentiation of rotator cuff tear subtypes: A preliminary prospective study. Ultrasound Med Biol. 2019;45:660–71. doi: 10.1016/j.ultrasmedbio.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Gamradt SC, Gallo RA, Adler RS, Maderazo A, Altchek DW, Warren RF, et al. Vascularity of the supraspinatus tendon three months after repair: Characterization using contrast-enhanced ultrasound. J Shoulder Elbow Surg. 2010;19:73–80. doi: 10.1016/j.jse.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Funakoshi T, Iwasaki N, Kamishima T, Nishida M, Ito Y, Nishida K, et al. In vivo vascularity alterations in repaired rotator cuffs determined by contrast-enhanced ultrasound. Am J Sports Med. 2011;39:2640–6. doi: 10.1177/0363546511420077. [DOI] [PubMed] [Google Scholar]
- 45.Adler RS, Johnson KM, Fealy S, Maderazo A, Gallo RA, Gamradt SC, et al. Contrast-enhanced sonographic characterization of the vascularity of the repaired rotator cuff: Utility of maximum intensity projection imaging. J Ultrasound Med. 2011;30:1103–9. doi: 10.7863/jum.2011.30.8.1103. [DOI] [PubMed] [Google Scholar]
- 46.Cadet ER, Adler RS, Gallo RA, Gamradt SC, Warren RF, Cordasco FA, et al. Contrast-enhanced ultrasound characterization of the vascularity of the repaired rotator cuff tendon: Short-term and intermediate-term follow-up. J Shoulder Elbow Surg. 2012;21:597–603. doi: 10.1016/j.jse.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Fischer C, Gross S, Zeifang F, Schmidmaier G, Weber MA, Kunz P. Contrast-Enhanced ultrasound determines supraspinatus muscle atrophy after cuff repair and correlates to functional shoulder outcome. Am J Sports Med. 2018;46:2735–42. doi: 10.1177/0363546518787266. [DOI] [PubMed] [Google Scholar]
- 48.Fischer C, Flammer S, Kauczor HU, Zeifang F, Schmidmaier G, Kunz P. Preoperative deltoid assessment by contrast-enhanced ultrasound (CEUS) as predictor for shoulder function after reverse shoulder arthroplasty: A prospective pilot study. Arch Orthop Trauma Surg. 2020;140:1001–12. doi: 10.1007/s00402-019-03281-w. [DOI] [PubMed] [Google Scholar]
- 49.Kunz P, Mick P, Gross S, Schmidmaier G, Zeifang F, Weber MA, et al. Contrast-enhanced ultrasound (CEUS) as predictor for early retear and functional outcome after supraspinatus tendon repair. J Orthop Res. 2020;38:1150–8. doi: 10.1002/jor.24535. [DOI] [PubMed] [Google Scholar]
- 50.Fischer C, Krammer D, Hug A, Weber MA, Kauczor HU, Krix M, et al. Dynamic contrast-enhanced ultrasound and elastography assess deltoid muscle integrity after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:108–17. doi: 10.1016/j.jse.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Fischer C, Miska M, Jung A, Weber MA, Saure D, Schmidmaier G, et al. Posttraumatic perfusion analysis of quadriceps, patellar, and achilles tendon regeneration with dynamic contrast-enhanced ultrasound and dynamic contrast-enhanced magnetic resonance imaging: Preliminary results. J Ultrasound Med. 2021;40:491–501. doi: 10.1002/jum.15424. [DOI] [PubMed] [Google Scholar]
- 52.Pozza S, De Marchi A, Albertin C, Albano D, Biino G, Aloj D, et al. Technical and clinical feasibility of contrast-enhanced ultrasound evaluation of long bone non-infected nonunion healing. Radiol Med. 2018;123:703–9. doi: 10.1007/s11547-018-0902-7. [DOI] [PubMed] [Google Scholar]
- 53.Krammer D, Schmidmaier G, Weber MA, Doll J, Rehnitz C, Fischer C. Contrast-Enhanced ultrasound quantifies the perfusion within tibial non-unions and predicts the outcome of revision surgery. Ultrasound Med Biol. 2018;44:1853–9. doi: 10.1016/j.ultrasmedbio.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Fischer C, Preuss EM, Tanner M, Bruckner T, Krix M, Amarteifio E, et al. Dynamic contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging for preoperative diagnosis of infected nonunions. J Ultrasound Med. 2016;35:933–42. doi: 10.7863/ultra.15.06107. [DOI] [PubMed] [Google Scholar]
- 55.Doll J, Gross S, Weber MA, Schmidmaier G, Fischer C. The AMANDUS project-advanced microperfusion assessed non-union diagnostics with contrast-enhanced ultrasound (CEUS) for the detection of infected lower extremity non-unions. Ultrasound Med Biol. 2019;45:2281–8. doi: 10.1016/j.ultrasmedbio.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Doll J, Streblow J, Weber MA, Schmidmaier G, Fischer C. The AMANDUS project PART II-Advanced microperfusion assessed non-union diagnostics with contrast-enhanced ultrasound (CEUS): A reliable diagnostic tool for the management and pre-operative detection of infected upper-limb non-unions. Ultrasound Med Biol. 2021;47:478–87. doi: 10.1016/j.ultrasmedbio.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 57.Dapunt U, Zhao Y, Schmidmaier G, Fischer C. Preoperative contrast-enhanced ultrasound (CEUS) of long bone nonunions reliably predicts microbiology of tissue culture samples but not of implant-sonication. Orthop Traumatol Surg Res. 2022;108:102862. doi: 10.1016/j.otsr.2021.102862. [DOI] [PubMed] [Google Scholar]
- 58.Fischer C, Frank M, Kunz P, Tanner M, Weber MA, Moghaddam A, et al. Dynamic contrast-enhanced ultrasound (CEUS) after open and minimally invasive locked plating of proximal humerus fractures. Injury. 2016;47:1725–31. doi: 10.1016/j.injury.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Genovese EA, Callegari L, Combi F, Leonardi A, Angeretti MG, Benazzo F, et al. Contrast enhanced ultrasound with second generation contrast agent for the follow-up of lower-extremity muscle-strain-repairing processes in professional athletes. Radiol Med. 2007;112:740–50. doi: 10.1007/s11547-007-0177-x. [DOI] [PubMed] [Google Scholar]
- 60.Hotfiel T, Heiss R, Swoboda B, Kellermann M, Gelse K, Grim C, et al. Contrast-Enhanced ultrasound as a new investigative tool in diagnostic imaging of muscle injuries-a pilot study evaluating conventional ultrasound, CEUS, and findings in MRI. Clin J Sport Med. 2018;28:332–8. doi: 10.1097/JSM.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 61.Kellermann M, Heiss R, Swoboda B, Gelse K, Freiwald J, Grim C, et al. Intramuscular perfusion response in delayed onset muscle soreness (DOMS): A quantitative analysis with contrast-enhanced ultrasound (CEUS) Int J Sports Med. 2017;38:833–41. doi: 10.1055/s-0043-112501. [DOI] [PubMed] [Google Scholar]
- 62.Selkow NM, Herman DC, Liu Z, Hertel J, Hart JM, Saliba SA. Blood flow after exercise-induced muscle damage. J Athl Train. 2015;50:400–6. doi: 10.4085/1062-6050-49.6.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heiss R, Kellermann M, Swoboda B, Grim C, Lutter C, May MS, et al. Effect of compression garments on the development of delayed-onset muscle soreness: A multimodal approach using contrast-enhanced ultrasound and acoustic radiation force impulse elastography. J Orthop Sports Phys Ther. 2018;48:887–94. doi: 10.2519/jospt.2018.8038. [DOI] [PubMed] [Google Scholar]
- 64.Hotfiel T, Hoppe MW, Heiss R, Lutter C, Tischer T, Forst R, et al. Quantifiable contrast-enhanced ultrasound explores the role of protection, rest, ice (Cryotherapy), compression and elevation (PRICE) therapy on microvascular blood flow. Ultrasound Med Biol. 2021;47:1269–78. doi: 10.1016/j.ultrasmedbio.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Solivetti FM, Elia F, Teoli M, De Mutiis C, Chimenti S, Berardesca E, et al. Role of contrast-enhanced ultrasound in early diagnosis of psoriatic arthritis. Dermatology. 2010;220:25–31. doi: 10.1159/000258049. [DOI] [PubMed] [Google Scholar]
- 66.Ohrndorf S, Hensch A, Naumann L, Hermann KG, Scheurig-Münkler C, Meier S, et al. Contrast-enhanced ultrasonography is more sensitive than grayscale and power Doppler ultrasonography compared to MRI in therapy monitoring of rheumatoid arthritis patients. Ultraschall Med. 2011;32(Suppl 2):E38–44. doi: 10.1055/s-0031-1281770. [DOI] [PubMed] [Google Scholar]
- 67.Rizzo G, Raffeiner B, Coran A, Ciprian L, Fiocco U, Botsios C, et al. Pixel-based approach to assess contrast-enhanced ultrasound kinetics parameters for differential diagnosis of rheumatoid arthritis. J Med Imaging (Bellingham) 2015;2:034503. doi: 10.1117/1.JMI.2.3.034503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song IH, Althoff CE, Hermann KG, Scheel AK, Knetsch T, Schoenharting M, et al. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis. 2008;67:19–25. doi: 10.1136/ard.2006.067462. [DOI] [PubMed] [Google Scholar]
- 69.Vanderschueren GA, Meys VE, Beekman R. Doppler sonography for the diagnosis of carpal tunnel syndrome: A critical review. Muscle Nerve. 2014;50:159–63. doi: 10.1002/mus.24241. [DOI] [PubMed] [Google Scholar]
- 70.Volz KR, Evans KD, Kanner CD, Dickerson JA. Detection of intraneural median nerve microvascularity using contrast-enhanced sonography: A pilot study. J Ultrasound Med. 2016;35:1309–16. doi: 10.7863/ultra.15.07012. [DOI] [PubMed] [Google Scholar]
- 71.Motomiya M, Funakoshi T, Ishizaka K, Nishida M, Matsui Y, Iwasaki N. Blood flow changes in subsynovial connective tissue on contrast-enhanced ultrasonography in patients with carpal tunnel syndrome before and after surgical decompression. J Ultrasound Med. 2018;37:1597–604. doi: 10.1002/jum.14500. [DOI] [PubMed] [Google Scholar]
- 72.Wang P, Wu M, Li A, Ye X, Li C, Xu D. Diagnostic value of contrast-enhanced ultrasound for differential diagnosis of malignant and benign soft tissue masses: A Meta-analysis. Ultrasound Med Biol. 2020;46:3179–87. doi: 10.1016/j.ultrasmedbio.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Lamby P, Prantl L, Fellner C, Geis S, Jung EM. Post-operative monitoring of tissue transfers: Advantages using contrast enhanced ultrasound (CEUS) and contrast enhanced MRI (ceMRI) with dynamic perfusion analysis?Clin Hemorheol Microcirc. 2011;48:105–17. doi: 10.3233/CH-2011-1405. [DOI] [PubMed] [Google Scholar]
- 74.Daniels SP, Mankowski Gettle L, Blankenbaker DG, Lee KS, Ross AB. Contrast-enhanced ultrasound-guided musculoskeletal biopsies: Our experience and technique. Skeletal Radiol. 2021;50:673–81. doi: 10.1007/s00256-020-03604-8. [DOI] [PubMed] [Google Scholar]
- 75.Prantl L, Pfister K, Kubale R, Schmitt S, Stockhammer V, Jung W, et al. Value of high resolution ultrasound and contrast enhanced US pulse inversion imaging for the evaluation of the vascular integrity of free-flap grafts. Clin Hemorheol Microcirc. 2007;36:203–16. [PubMed] [Google Scholar]
- 76.Geis S, Prantl L, Mueller S, Gosau M, Lamby P, Jung EM. Quantitative assessment of bone microvascularization after osteocutaneous flap transplantation using contrast-enhanced ultrasound (CEUS) Ultraschall Med. 2013;34:272–9. doi: 10.1055/s-0033-1335133. [DOI] [PubMed] [Google Scholar]
- 77.Geis S, Prantl L, Schoeneich M, Lamby P, Klein S, Dolderer J, et al. Contrast enhanced ultrasound (CEUS) –An unique monitoring technique to assess microvascularization after buried flap transplantation. Clin Hemorheol Microcirc. 2016;62:205–14. doi: 10.3233/CH-151964. [DOI] [PubMed] [Google Scholar]


