Abstract
It has been reported that human immunodeficiency virus type 1 (HIV-1) bound to follicular dendritic cells (FDCs) remains highly infectious to CD4+ T cells even when it forms immune complexes with neutralizing antibody (HIV-1/IC). To elucidate the role of FDCs in HIV-1 transmission to CD4+ T cells in lymph nodes, we have isolated and purified FDCs from human tonsils and examined whether the HIV-1/IC trapped on their surface is infectious to CD4+ T cells. To our surprise, not the HIV-1/IC but the antibody-free HIV-1 on FDCs could be transmitted to CD4+ T cells. Furthermore, in contrast to previous studies showing that FDCs are productively infected with HIV-1, the present study clearly demonstrated that FDCs were not the target cells for HIV-1 infection. FDCs could capture the viral particles on their surface; however, the binding of HIV-1 to FDCs was strongly inhibited by the presence of anti-CD54 (ICAM-1) monoclonal antibody (MAb) and anti-CD11a (LFA-1) MAb, suggesting that the adhesion molecules play an important role in the interaction between HIV-1 and FDCs.
The germinal centers play a key role in producing memory B cells and plasma cells. The main constituents of germinal center microenvironment are activated germinal-center B cells (centroblasts and centrocytes), follicular dendritic cells (FDCs), macrophages, CD4+ and CD8+ T cells, and dendritic cells (6, 10, 12, 13, 16, 17, 26). FDCs belong to so-called B-cell-associated dendritic cells with desmosomal junctions (29), and they have two notable characteristics. First, FDCs have very intimate relationships with the germinal-center B cells such as thymic nurse cells in vitro and in vivo (13, 15, 34, 36). Second, they are long-term antigen-presenting cells for the germinal-center B cells (18, 19, 28). The latter function seems important in pathological conditions such as with infectious diseases.
In human immunodeficiency virus type 1 (HIV-1) infection, FDCs not only handle the viral antigens but also interact with infectious virions. This is particularly relevant to the pathogenesis of this disease, since the CD4+ T cells that migrated into the light zone of the germinal centers can be infected with HIV-1 trapped on the surface of the FDCs. The first evidence that the germinal centers were infected with HIV-1 was provided by ultrastructural analysis for lymph nodes obtained from infected patients (1). The virions were located in the interdendritic extracellular spaces and embedded in a moderately electron-dense material covering the surface of the FDCs. This localization indicated that the capture of HIV-1 with immunoglobulins by FDCs was responsible for the infection of the germinal centers. Stahmer et al. reported that normal human tonsil FDCs were highly susceptible to HIV-1 infection in a CD4-independent fashion in vitro (25). Furthermore, the HIV-1-infected FDCs not only contain proviral DNA but also produce viral particles and transmit them to CD4+ T cells (24). When HIV-1 formed immune complexes with its neutralizing antibody (HIV-1/IC) and was trapped by FDCs, the virus remained highly infectious to CD4+ T cells (11). However, our previous study demonstrated that FDCs themselves were not susceptible to HIV-1 infection (33). We also found that more HIV-1 particles were trapped by fresh FDCs than by dedifferentiated FDCs or control fibroblasts, yet the surface molecules mediating this binding could not be determined. In the present study, we have isolated and purified FDCs from human tonsils and examined the infectivity of HIV-1 particles trapped on their surface. We have shown that not the HIV-1/IC but the antibody-free HIV-1 on FDCs can be transmitted to CD4+ T cells. Furthermore, the adhesion molecules CD54 (ICAM-1) and CD11a (LFA-1) play an important role in the interaction between HIV-1 particles and FDCs.
MATERIALS AND METHODS
Isolation and cultivation of FDCs.
FDCs were isolated and purified from surgically resected fresh palatine tonsils, which were kindly provided by M. Hattori (Fujita General Hospital, Fukushima, Japan). The methods for enucleation and subsequent digestion of lymph follicles have been previously described (31–33). Briefly, more than 200 lymph follicles were enucleated from ca. 1-mm-thick slices of tonsil under an anatomical microscope. The follicles were then digested with an enzyme cocktail of collagenase A (0.5 mg/ml; Boehringer Mannheim, Mannheim, Germany), dispase II (0.5 mg/ml; Boehringer Mannheim) and DNase I (0.04 mg/ml; Boehringer Mannheim). FDC clusters (FDCs engulfing several germinal-center lymphocytes) (14) were then separated from the free cell suspension by four repeated sedimentations. After final purification, the ratio of FDC clusters to mixed free cells was approximately 97% (vol/vol). To remove contaminating macrophages, the FDC cluster-enriched fraction was cultured in plastic culture dishes with RPMI 1640 supplemented with 10% fetal calf serum (BioWhittaker, Walkersville, Md.) at 37°C for 60 min. The nonadherent population was transferred to other plastic culture dishes (Corning Glass Works, Corning, N.Y.) and maintained under the same conditions. After a 6-h incubation, the plastic wells were washed with culture medium, and weakly adherent cells (FDC clusters) were collected with a cell scraper. To eliminate possible contamination with CD4+ T cells, the final FDC clusters were further purified by negative selection with CD4 antibody-labeled magnetic beads (Dynabeads M-450; Dynal A.S., Oslo, Norway).
Purification and stimulation of human CD4+ T cells.
Autologous tonsillar lymphocytes were separated with Lymphprep (Nyomed Pharma, Oslo, Norway) from fresh tonsillar cells. The CD4+ T-cell population was further purified by positive selection with the above-mentioned CD4 antibody-labeled magnetic beads and detached antibody. CD4+ T cells were activated by the incubation with 100 pg of staphylococcal enterotoxin E (Toxin Technology, Sarasota, Fla.) per ml.
HIV-1 exposure of FDCs and coculture with CD4+ T cells.
HIV-1/IC were prepared by incubating 5,000 50% cell culture infective doses per 100 μl of HIV-1 (IIIB strain) at 37°C for 2 h with 100 μl of fresh serum obtained from HIV-1-infected patients. FDCs were exposed to either HIV-1/IC or free HIV-1 for 12 h at 4°C. The cells were then extensively washed to remove untrapped virions and cocultured with CD4+ T cells in fresh culture medium. After a 5-day incubation at 37°C, DNA was isolated from the cells and stored at −20°C until use. The p24 antigen levels in culture supernatants were measured by a p24 antigen capture enzyme-linked immunosorbent assay (ELISA) kit (Cellular Products, Inc., Buffalo, N.Y.), according to the manufacturer’s instructions. In some experiments, the HIV-1-exposed FDCs were cocultured with activated CD4+ T cells by using a cell culture insert (0.4-μm diameter; Becton Dickinson, Franklin Lakes, N.J.) to avoid cell-to-cell contact between FDCs and CD4+ T cells. In the experiments with anti-adhesion molecule monoclonal antibodies (MAbs), FDCs were exposed to HIV-1 together with 10 μg of anti-CD54, anti-CD11a, or anti-CD106 MAb per ml at 4°C for 12 h, extensively washed, and incubated at 37°C for 5 days. The antibodies were purchased from Becton Dickinson. The cells were cocultured with activated CD4+ T cells in fresh culture medium for 4 days, and the p24 antigen production and the proviral DNA synthesis were determined.
Detection of HIV-1 proviral DNA.
DNA was isolated from the cells (106 cells) with a nucleic acid extraction kit (IsoQuick; Orch Research, Bothell, Wash.). PCR analysis for HIV-1 gag and β-globin DNA was performed with the primer pairs SK38/39 (20) and GH20/PC04 (3), respectively. The PCR products were analyzed by electrophoresis (1.5% agarose), stained with ethidium bromide for detection of β-globin DNA, and transferred to Hybond-N+ membranes (Amersham, Buckinghamshire, United Kingdom) for detection of HIV-1 gag DNA. The blot was hybridized with the 32P-labeled oligonucleotide probe SK-19 (20). OM-10.1 cells (7), a chronically infected clone harboring a single HIV-1 provirus, were used as a positive control.
TEM analysis.
FDCs were exposed to HIV-1/IC or HIV-1 at 4°C for 12 h in the absence or presence of anti-CD54 and anti-CD11a MAbs. For transmission electron microscopy (TEM) analysis, the FDCs were treated in a routine manner, including fixation in ice-cold 2.5% glutaraldehyde buffered with 0.1 M phosphate solution (pH 7.4) for 2 h, postfixation in 1% osmium tetroxides for 1 h, dehydration, embedding in Epon, ultrathin sectioning, counterstaining, and observation by TEM (Jeol, Tokyo, Japan).
RESULTS
FDCs are not the target cells for HIV-1 but transmit the virus to CD4+ T cells.
Heath et al. reported that FDCs could convert the neutralized HIV-1 into an infectious form even in the presence of a vast excess of neutralizing antibody (11). Therefore, we first examined whether CD4+ T cells could be infected with HIV-1/IC, which might be trapped by FDCs. Establishment of infection was monitored by p24 antigen production in culture supernatants and by proviral DNA synthesis in the cells. When purified FDCs were exposed to HIV-1/IC for 12 h at 4°C, extensively washed, and cocultured with activated CD4+ T cells for 5 days at 37°C, the levels of p24 antigen in the culture supernatants were 0.16 and 0.10 ng/ml in experiments 1 and 2, respectively (Table 1). These values were only four- to fivefold higher than those in the FDC culture without CD4+ T cells (0.04 and 0.02 ng/ml, respectively).
TABLE 1.
Infectivity and replication of HIV-1 trapped on the surface of FDCs
| Virus | Cell combination | p24 antigen (ng/ml)
|
Presence of proviral DNAa | ||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |||
| HIV-1/ICb | FDC/TCc | 0.16 | 0.10 | ND | |
| HIV-1/IC | FDC/− | 0.04 | 0.02 | ND | |
| HIV-1/IC | −/TC | 0.05 | 0.20 | ND | |
| HIV-1 | FDC/TC | 6.0 | 26.0 | 12.9 | + |
| HIV-1 | FDC/− | 0.07 | 0.01 | ND | ND |
| HIV-1 | −/TC | 0.34 | 6.0 | 12.5 | + |
| Control | FDC/TC | ND | ND | ND | ND |
Determined by PCR and Southern blot analysis. ND, not detectable (below the threshold of ELISA or PCR-Southern blot analysis).
HIV-1 in the form of immune complexes. Sera from different patients were used for HIV-1/IC formation in experiments 1 and 2.
Activated CD4+ T cells.
The patient sera used in this assay seemed to efficiently neutralize the infectivity of HIV-1 because a high level of HIV-1 production (>1 ng/ml) was not observed in CD4+ T cells simply exposed to HIV-1/IC without FDCs (Table 1). In order to confirm their neutralizing activity and specificity, the patients’ sera, as well as HIV-1-negative human sera, were examined for the effects on HIV-1 infectivity in MT-4 cells (a T-lymphoblastoid cell line). The patients’ sera but not the HIV-1-negative sera could reduce the viral infectivity in a dose-dependent fashion (data not shown). For instance, a patient serum achieved >3.6, 2.5, and 1.4 log10 reductions of the infectivity of the viral suspension when mixed at ratios (vol/vol) of 1:1, 1:9, and 1:99, respectively. Furthermore, HIV-1 particles could be identified on the surface of FDCs, as determined by TEM analysis (Fig. 1A). Twenty-eight viral particles were observed on the surface of 54 FDCs (51.9%). These results suggest that the reduced infectivity of HIV-1/IC is not due to the lack of HIV-1/IC capture by FDCs. Thus, we could not confirm the previous finding that FDCs converted the neutralized HIV-1 into an infectious form in the presence of neutralizing antibody (11). PCR and Southern blot analysis revealed that specific amplified products (proviral DNA) were not detectable for all samples extracted from the culture cells (Table 1), indicating that the transmission of HIV-1/IC from FDCs to CD4+ T cells does not commonly occur.
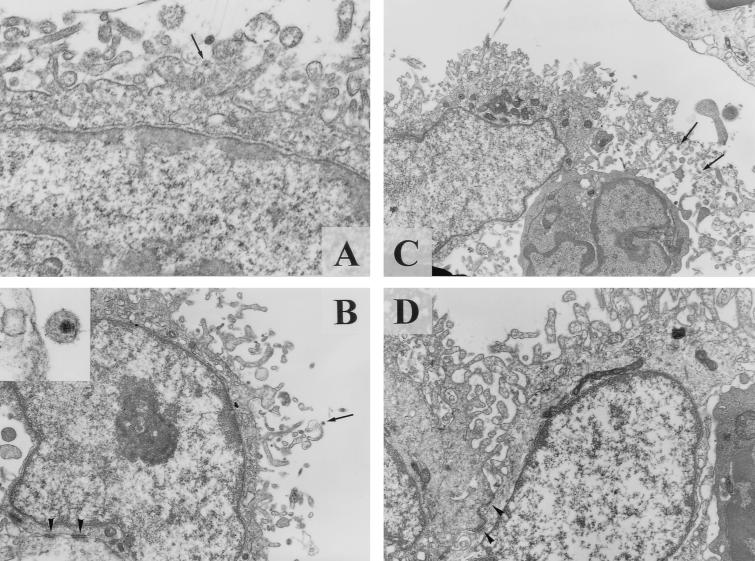
FIG. 1.
TEM for HIV-1/IC or HIV-1-exposed FDCs. An HIV-1/IC particle (arrow in panel A) was observed on the surface of FDC (×25,000). Twenty-eight viral particles were observed on the surface of 54 FDCs (51.9%). Similarly, an antibody-free HIV-1 particle (arrow in panel B) was captured on the top of a delicate cytoplasmic process of FDCs (×10,000; inset, ×75,000). Twenty-seven particles were observed on the surface of 55 FDCs (49.1%). Pleural virions (arrows in panel C) were often observed among the extracellular spaces of fine cytoplasmic processes of FDCs in isolated FDC-lymphocytes complex (FDC cluster) (×8,000). In contrast, such virions were scarcely identified on the surfaces of FDCs treated with a combination of anti-CD54 MAb and anti-CD11a MAb (panel D). Only one particle was identified on the 64 FDCs (1.6%). Desmosomal junctions, which are a reliable morphological marker of FDCs, were observed in panels B and D (arrowheads). These pictures are representative electron micrographs of some FDCs analyzed in this study.
We next examined the same experiments with antibody-free virions. In contrast to the experiments with HIV-1/IC, the free HIV-1 retained a high degree of infectivity and, except for experiment 1, infection of the CD4+ T cells with the virus brought about a high level of p24 antigen production (6.0 and 12.5 ng/ml in experiments 2 and 3, respectively) (Table 1). When FDCs were exposed to the free virus and extensively washed, the trapped HIV-1 was found to be highly infectious. After cocultivation with CD4+ T cells, the p24 levels in the culture supernatants ranged between 6.0 ng/ml (experiment 1) and 26.0 ng/ml (experiment 2). However, it is unlikely that FDCs produced such amounts of viral antigens, and almost all of the viral particles and antigens were considered to be produced by CD4+ T cells after viral transmission from FDCs because the p24 antigen levels in the culture supernatant of FDCs alone were extremely low (undetectable levels to 0.07 ng/ml). Furthermore, the PCR analysis showed that the proviral DNA was undetectable for the samples extracted from the FDCs alone after viral exposure (Table 1), confirming that FDCs themselves are not the target cells for HIV-1 replication. In addition, it is also clear that the virions trapped on the surface of FDCs (Fig. 1B and C) were highly transmissible to the susceptible cells.
Cell-to-cell contact is necessary for HIV-1 transmission from FDCs to CD4+ T cells.
To examine whether the transmission of HIV-1 from FDCs to CD4+ T cells requires cell-to-cell contact, HIV-1-exposed FDCs were separated from CD4+ T cells in the same wells by using a filter with a 0.4-μm diameter (cell culture insert). Again, high levels of p24 antigen production were observed in two separate experiments when HIV-1-exposed FDCs and CD4+ T cells were in contact with each other (Table 2). In contrast, much less amounts (undetectable levels and 0.7 ng/ml) of the antigens were produced in the culture supernatants when HIV-1-exposed FDCs and CD4+ T cells were incubated without this contact. In these experiments, proviral DNA was detected for the cocultured cells but not for the cells separately cultured (Table 2). These results indicate that cell-to-cell contact is required for HIV-1 transmission from FDCs to CD4+ T cells.
TABLE 2.
Infectivity and replication of HIV-1 trapped on the surface of FDCs under different culture conditions
| Virus | Cell combination | p24 antigen (ng/ml)
|
Presence of proviral DNAa | |
|---|---|---|---|---|
| Expt 1 | Expt 2 | |||
| HIV-1 | FDC/TCb | 26.3 | 7.1 | + |
| HIV-1 | FDC/TC (filterc) | ND | 0.7 | ND |
| HIV-1 | FDC/− | 0.01 | 0.8 | ND |
| HIV-1 | −/TC | 5.95 | 1.9 | + |
| Control | FDC/TC | ND | ND | ND |
Determined by PCR and Southern blot analysis. ND, not detectable (below the thresholds of ELISA or PCR-Southern blot analysis).
Activated CD4+ T cells.
HIV-1-exposed FDCs were separated from CD4+ T cells by a filter (0.4-μm diameter).
Adhesion molecules play a crucial role in HIV-1 binding to FDCs.
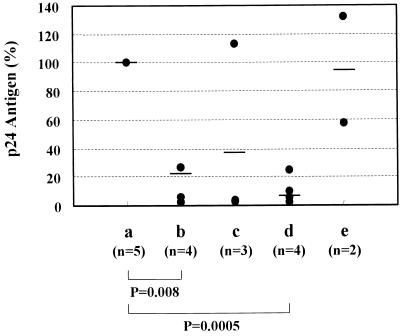
Although it is clear that FDCs capture HIV-1 on their surface, the molecular mechanism of their interaction is not yet fully understood. Therefore, we examined the influence of various anti-adhesion molecule MAbs on the HIV-1 capture activity of FDCs. To this end, FDCs were isolated and purified from the tonsils of several donors and exposed to HIV-1 in the absence or presence of the MAbs. After a 12-h incubation at 4°C, the cells were extensively washed to remove untrapped virions and cultured at 37°C for 5 days. Then the cells were cocultured with CD4+ T cells. As shown in Fig. 2, the treatment with anti-CD54 MAb or the combination of anti-CD54 MAb with anti-CD11a MAb significantly reduced the production of p24 antigens from cocultured CD4+ T cells. However, the treatment with anti-CD106 (VCAM-1) MAb did not affect the p24 antigen production. These results suggest that anti-CD54 and anti-CD11a MAbs either block the entrapment of virions on the surface of FDCs or interfere with the transmission of trapped virions to cocultured CD4+ T cells. To distinguish between these two possibilities, HIV-1-exposed FDCs in the absence or presence of the MAbs were analyzed by TEM. HIV-1 particles were observed on the surface of FDCs frequently (49.1% of total FDCs observed) when the cells were not treated with the anti-CD54 and anti-CD11a MAbs (Fig. 1B and C). In contrast, such particles were hardly (1.6%) identified on the surface of FDCs that had been treated with the MAbs (Fig. 1D).
FIG. 2.
Inhibitory effects of anti-adhesion molecule MAbs on the entrapment and infectivity of HIV-1 in FDC cultures. FDCs were isolated and purified from human tonsils and exposed to HIV-1 at 4°C for 12 h in the absence (a) or in the presence of anti-CD54 (ICAM-1) MAb (b), anti-CD11a (LFA-1) MAb (c), anti-CD54 MAb plus anti-CD11a MAb (d), or anti-CD106 (VCAM-1) MAb (e). The cells were extensively washed and incubated at 37°C. After a 5-day incubation, FDCs were cocultured with activated CD4+ T cells in fresh culture medium for 4 days. The p24 antigen levels were determined by antigen capture ELISA and expressed as a percentage of the control culture (a). The mean values in each group are indicated by horizontal bars, and the statistical significance was analyzed by the t test.
DISCUSSION
Initial studies on HIV-1 pathogenesis in lymph nodes have revealed a novel and surprising finding that FDCs are responsible for the infection of germinal centers. FDCs are peculiar dendritic cells that carry immune complexes on their surfaces (18, 35). Health et al. reported that, once HIV-1 was trapped by FDCs, the virus became highly infectious to CD4+ T cells even in the presence of antibodies that would otherwise neutralize it (11). However, we demonstrated that FDCs could not transmit HIV-1/IC to CD4+ T cells, though FDCs could capture HIV-1/IC on their surface (Table 1 and Fig. 1A). The reason for this discrepancy may be due to the differences in neutralizing antibodies used in our experiments. We speculate that, in the earlier experiments, the formation of HIV-1/IC might have been incomplete and therefore the antibody-free particles were also trapped on the surface of FDCs together with HIV-1/IC. In our study, we repeated the experiment with FDCs from different donors and with sera from different patients, yet a reproducible result was obtained (Table 1). Furthermore, HIV-1/IC was found to be much less infectious to CD4+ T cells than was the free HIV-1 and, even when trapped, FDCs could not convert the virus into its infectious form.
Another important finding in this study is that FDCs themselves were not susceptible to HIV-1 infection, irrespective of the viral forms (immune complex or free) and did not produce the viral antigens during at least a 5-day culture period (Table 1). Previous studies in immunohistochemistry and electron microscopy demonstrated that FDCs play an important role in the pathogenesis of AIDS, serving as a major reservoir of HIV-1 (1, 23, 27). Our electron microscopic observations also indicated that the trapped virions were morphologically intact. Thus, FDCs appear to be sequentially supplied with infectious virions and to function as a viral reservoir, where the virus remains infectious to CD4+ T cells for several days.
Although the molecular mechanism involved in the entrapment of HIV-1 by FDCs is of particular importance, it has not been elucidated yet. Blauvelt et al. reported that the productive infection of T-cell-associated dendritic cells with HIV-1 and their ability to capture the virus were mediated through separate machineries. The former was dependent on the CD4 molecule and HIV-1 coreceptors, while the latter was not (4). FDCs do not express CD4 and the coreceptors on their surface (5, 22, 37), suggesting that FDCs use other mechanisms for the capture HIV-1 particles. We previously reported that the infectivity of FDC-trapped HIV-1 was lost during a 25-day incubation period (33). The expression of adhesion and costimulatory molecules on FDCs were rapidly downregulated in vitro, and the amount of expressed CD54 after a 35-day incubation was less than one-hundredth of that after a 7-day incubation (32). Furthermore, the interaction between the virus-incorporated and the host cellular adhesion molecules CD54 and CD11a is known to markedly enhance the capacity of HIV-1 for its binding and entry into CD4+ T cells (2, 8, 9, 21, 30). These findings and our present observations strongly suggest the use of CD54 and CD11a in the FDC capture of HIV-1 particles.
In conclusion, we have demonstrated that (i) FDCs are able to capture HIV-1 on their surfaces in vitro, (ii) that the FDC-trapped virions remain infectious and are transmitted to cocultured CD4+ T cells but (iii) that the FDCs themselves are not susceptible to these virions, and (iv) that the adhesion molecules expressed on FDCs and the viral envelope play an important role in the interaction between FDCs and HIV-1. Further studies are required to elucidate the complete functions of the FDCs in the pathogenesis of HIV-1 infection.
ACKNOWLEDGMENTS
M.F. and R.T. contributed equally to this work.
OM-10.1 cells were obtained through the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, Bethesda, Md. (the contributor was S. Butera).
REFERENCES
- 1.Armstrong J A, Horne R. Follicular dendritic cells and virus-like particles in AIDS-related lymphadenopathy. Lancet. 1984;ii:370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- 2.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer H M, Ting Y, Greer C E, Chambers J C, Tashiro C J, Chimera J, Reingold A, Manos M M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–477. [PubMed] [Google Scholar]
- 4.Blauvelt A, Asada H, Saville N W, Klaus-Kovtun V, Altman D J, Yarchoan R, Katz S I. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Invest. 1997;100:2043–2053. doi: 10.1172/JCI119737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butch A W, Chung G-H, Hoffmann J W, Nahm M H. Cytokine expression by germinal center cells. J Immunol. 1993;150:39–47. [PubMed] [Google Scholar]
- 6.Butcher E C, Rouse R V, Coffman R L, Nottenburg C N, Hardy R R, Weissman I L. Surface phenotype of Peyer’s patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982;129:2698–2707. [PubMed] [Google Scholar]
- 7.Butera S T, Perez P L, Wu B-Y, Nabel G J, Folks T M. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J Virol. 1991;65:4645–4653. doi: 10.1128/jvi.65.9.4645-4653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortin J-F, Cantin R, Lamontagne G, Tremblay M J. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin J-F, Cantin R, Tremblay M J. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grouard G, Durand I, Filgueira L, Banchereau J, Liu Y J. Dendritic cells capable of stimulating T cells in germinal centres. Nature. 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 11.Heath S L, Tew J G, Szakal A K, Burton G F. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 12.Heinen E, Cormann N, Kinet-Denoël C. The lymph follicle: a hard nut to crack. Immunol Today. 1988;9:240–243. doi: 10.1016/0167-5699(88)91223-6. [DOI] [PubMed] [Google Scholar]
- 13.Imai Y, Yamakawa M. Morphology, function and pathology of follicular dendritic cells. Pathol Int. 1996;46:807–833. doi: 10.1111/j.1440-1827.1996.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 14.Lilet-Leclercq C, Radoux D, Heinen E, Kinet-Denoel C, Defraigne J O, Houben-Defresne M P, Simar L J. Isolation of follicular dendritic cells from human tonsils and adenoids. I. Procedure and morphological characterization. J Immunol Methods. 1984;66:235–244. doi: 10.1016/0022-1759(84)90334-x. [DOI] [PubMed] [Google Scholar]
- 15.Lindhout E, Lakeman A, de Groot C. Follicular dendritic cells inhibit apoptosis in human B lymphocytes by a rapid and irreversible blockade of preexisting endonuclease. J Exp Med. 1995;181:1985–1995. doi: 10.1084/jem.181.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y J, Johnson G D, Gordon J, MacLennan I C. Germinal centers in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 17.MacLennan I C, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 18.Nossal G J V. Differentiation of the secondary B-lymphocyte repertoire: The germinal center reaction. Immunol Rev. 1994;137:173–183. doi: 10.1111/j.1600-065x.1994.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 19.Nossal G J V, Abbott A, Mitchell J, Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968;127:277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou C-Y, Kwok S, Mitchell S W, Mack D H, Sninsky J J, Krebs J W, Feorino P, Warfield D, Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988;239:295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schriever F, Freedman A S, Freeman G, Messner E, Lee G, Daley J, Nadler L M. Isolated human follicular dendritic cells display a unique antigenic phenotype. J Exp Med. 1989;169:2043–2058. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel H, Herbst H, Niedobitek G, Foss H-D, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992;140:15–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Sprenger R, Toellner K-M, Schmetz C, Lüke W, Stahl-Henning C, Ernst M, Hunsmann G, Schmitz H, Flad H-D, Gerdes J, Zimmer J P. Follicular dendritic cells productively infected with immunodeficiency viruses transmit infection to T cells. Med Microbiol Immunol. 1995;184:129–134. doi: 10.1007/BF00224349. [DOI] [PubMed] [Google Scholar]
- 25.Stahmer I, Zimmer J P, Ernst M, Fenner T, Finnern R, Schmitz H, Flad H-D, Gerdes J. Isolation of normal human follicular dendritic cells and CD4-independent in vitro infection by human immunodeficiency virus (HIV-1) Eur J Immunol. 1991;21:1873–1878. doi: 10.1002/eji.1830210814. [DOI] [PubMed] [Google Scholar]
- 26.Stein H, Gerdes J, Mason D Y. The normal and malignant germinal centre. Clin Haematol. 1982;11:531–559. [PubMed] [Google Scholar]
- 27.Tenner-Rácz K, Rácz P, Dietrich M, Kern P. Altered follicular dendritic cells and virus-like particles in AIDS and AIDS-related lymphadenopathy. Lancet. 1985;i:105–106. doi: 10.1016/s0140-6736(85)91994-4. [DOI] [PubMed] [Google Scholar]
- 28.Tew J G, Mandel T E, Burgess A W. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell Immunol. 1979;45:207–212. doi: 10.1016/0008-8749(79)90378-2. [DOI] [PubMed] [Google Scholar]
- 29.Tew J G, Thorbecke G J, Steinman R M. Dendritic cells in the immune response: characteristic and recommended nomenclature (a report from the Reticuloendothelial Society Committee on nomenclature) J Reticuloendothel Soc. 1982;31:371–380. [PubMed] [Google Scholar]
- 30.Tremblay M J, Fortin J F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 31.Tsunoda R. Long-term cultivation of human follicular dendritic cells. In: Heinen E, editor. Follicular dendritic cells in normal and pathological conditions. R. G. Austin, Tex: Landes Co.; 1995. pp. 111–124. [Google Scholar]
- 32.Tsunoda R, Bosseloir A, Onozaki K, Heinen E, Miyake K, Okamura H, Suzuki K, Fujita T, Simar L J, Sugai N. Human follicular dendritic cells in vitro and follicular dendritic-cell-like cells. Cell Tissue Res. 1997;288:381–389. doi: 10.1007/s004410050824. [DOI] [PubMed] [Google Scholar]
- 33.Tsunoda R, Hashimoto K, Baba M, Shigeta S, Sugai N. Follicular dendritic cells in vitro are not susceptible to infection by HIV-1. AIDS. 1996;10:595–602. doi: 10.1097/00002030-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Tsunoda R, Nakayama M, Heinen E, Miyake K, Suzuki K, Sugai N, Kojima M. Emperipolesis of lymphoid cells by human follicular dendritic cells in vitro. Virchows Arch B Cell Pathol. 1992;62:69–78. doi: 10.1007/BF02899667. [DOI] [PubMed] [Google Scholar]
- 35.Van Rooijen N. The role of the FDC-retained immune complex network and its dynamics in the activity of germinal centres. Res Immunol. 1993;144:545–552. doi: 10.1016/s0923-2494(05)80001-x. [DOI] [PubMed] [Google Scholar]
- 36.Wekerle H, Ketelsen U-P. Thymic nurse cells. Ia-bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980;283:402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. In vitro distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]