Abstract
Given the central role of the androgen receptor (AR) in prostate cancer cell biology, AR-targeted therapies have been the backbone of prostate cancer treatment for over 50 years. New data indicate that AR is expressed in additional cell types within the tumor microenvironment. Moreover, targeting AR for the treatment of prostate cancer has established side effects such as bone complications and an increased risk of developing cardiometabolic disease, indicating broader roles for AR. With the advent of novel technologies, such as single-cell approaches and advances in preclinical modeling, AR has been identified to have clinically significant functions in other cell types. In this mini-review, we describe new cancer cell–extrinsic roles for AR within the tumor microenvironment as well as systemic effects that collectively impact prostate cancer progression and patient outcomes.
Keywords: androgen receptor, prostate cancer, tumor microenvironment, bone, muscle, adipose, metabolic syndrome, cardiovascular
The androgen receptor (AR) has been the major therapeutic target in prostate cancer since even before the discovery of the receptor in the late 1960s (1‐3). Two decades earlier, Charles Huggins performed his seminal work demonstrating that depletion of androgens via surgical castration or chemical castration with estrogen benefitted men with metastatic prostate cancer through a mechanism that was unknown at the time (4). Following the discovery of AR and antibodies that could recognize the protein, it was quickly deduced that AR expression and transcriptional activity promoted prostate cancer cell biology and was, thus, the central driver of the disease. Correspondingly, prostate cancer cells became the predominant models to study AR pharmacology and biology. Within prostate cancer cells, AR regulates multiple processes including cell proliferation, survival, migration, invasion, metabolism, differentiation, and DNA repair (5‐8). The broad array of genetic and enzymatic alterations that occur within prostate cancer cells to help maintain AR activity following hormone therapy, such as AR gene and enhancer amplifications, expression of constitutively active AR splice variants, increased intratumoral androgens, and somatic AR ligand-binding domain mutations, further underscores the sustained importance of AR even in the late stages of the disease (9, 10). Yet we know from multiple clinical trials testing AR-targeting therapies, and preclinical studies disrupting AR in specific cell types, that AR signaling modulators clearly have on-target side effects (ie, disruption of physiological AR functions in other tissues) (11). Defining how cancer cell–extrinsic AR activity impacts prostate cancer stands to improve our understanding of how AR-directed therapies work and why they fail. This knowledge will aid the development of new rational, biologically based combination therapies, and inform the intensity of AR-directed therapy necessary to treat the disease more effectively. In this mini-review, we discuss emerging roles for AR in prostate cancer. More specifically, recently described cancer cell–extrinsic functions for AR in prostate cancer are described to help provide a broader perspective of how targeting this receptor impacts both tumor growth and patient quality of life.
Immune System
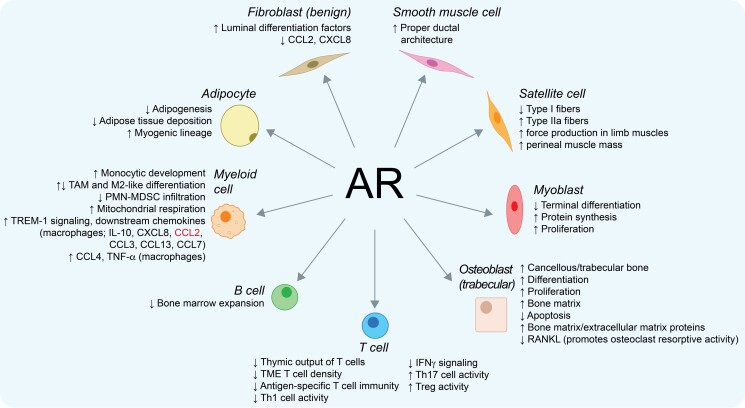
Epidemiological data reveals sex differences with regards to both autoimmune disease and cancer incidence (12). On average, females have lower rates of cancer incidence and mortality, but increased risk of developing autoimmune diseases (13, 14). These sex differences have in part been attributed to sex hormones such as estrogen and testosterone (15, 16). These observations raised the possibility that AR signaling, which predominates in men compared with women, may have immunosuppressive functions and partly explain these sex differences. With regards to androgens, administration of the potent androgen dihydrotestosterone leads to involution of the thymus, the primary lymphoid organ in which T cells mature (17). AR is expressed across cell types of both the adaptive (T cells and B cells) and innate (eg, macrophages and monocytes) immune system (15). Consistent with this immunosuppressive role for AR signaling, androgen deprivation therapy (ADT, the backbone of treatment for advanced prostate cancer) increases thymic output of T cells (18), interferon-γ signaling (which is necessary for T cell–mediated antitumor immune responses) (19), antigen-specific T cell immunity (20), density of T cells within the prostate tumor microenvironment (21), and expansion of bone marrow B cells (22) (Fig. 1). It has recently been shown that AR bound directly to Ifng gene enhancers in CD8+ T cells, and that suppression of T cell–intrinsic AR function promoted interferon-γ signaling (23).
Figure 1.
Cancer-cell extrinsic roles for AR in prostate cancer. Shown are direct AR functions in indicated cell types that can impact prostate cancer patients. Text in red indicates that contradictory data exists (CCL2 has been reported to be both positively and negatively regulated by AR in macrophages). AR, androgen receptor; CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; IL, interleukin; PMN-MDSC, polymorphonuclear-myeloid–derived suppressor cell; RANKL, receptor activator of nuclear factor kappa-Β ligand; TAM, tumor-associated macrophage; Th, T helper; TME, tumor microenvironment; TNF-α, tumor necrosis factor-alpha; Treg, regulatory T cell; TREM-1, triggering receptor expressed on myeloid cells-1.
The AR-mediated immunological effects led to the hypothesis that combining AR inhibition with immune checkpoint therapies (ICTs), which block T cell inhibitory molecules, would promote antitumor responses. Unlike tumor types such as melanoma and lung cancer that are characterized by high tumor mutational burden and neoantigen load associated with a high density of intratumoral T cells and have demonstrated improved long-term survival in many patients with ICTs, prostate cancer has relatively few intratumoral T cells and low response rates to ICTs (24‐26). Although, preclinical data in murine models supported the combination of AR inhibition plus ICTs (27), clinical trials of ADT plus anticytotoxic T lymphocyte associated protein-4 (28‐30) and enzalutamide (a second-generation AR inhibitor) plus anti-programmed death-ligand 1 (31) showed no benefit.
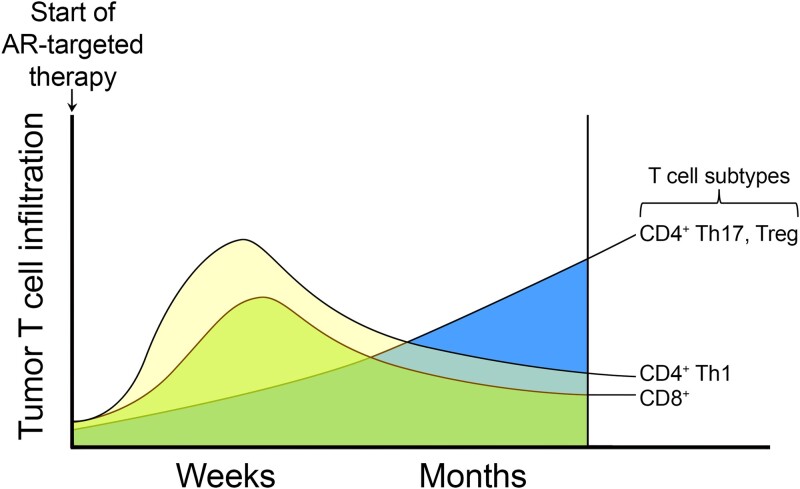
The lack of demonstrated benefit with combined anti-AR and ICT is likely due to the complex and dynamic interactions of AR signaling within the tumor immune microenvironment that remain incompletely understood. For example, maximal T cell infiltration occurs within weeks after AR inhibition, followed by a subsequent decline, suggesting that the timing of ICT relative to AR inhibition may influence the response (21) (Fig. 2). Additionally, the initial T cell infiltrate following AR inhibition is biased toward Th1 cells, which promote antitumor immunity, but is subsequently replaced by Th17 and regulatory T cells, which suppress antitumor immunity (32, 33). Furthermore, in addition to lymphoid cells such as T cells, subsets of myeloid cells, specifically myeloid-derived suppressor cells, have been demonstrated to drive castration resistance and promote tumor survival via secretion of interleukin-23 (34) and interleukin-8 (35). Interestingly, myeloid-specific genetic ablation of Ar in syngeneic mouse models increases prostate cancer growth, suggesting that AR may play an antitumor role in certain innate myeloid cell populations (36, 37). Given the breadth of myeloid cell types, additional studies are needed to delineate the specific myeloid subtypes directly regulated by AR, their individual contributions to tumorigenesis, and how their roles evolve over the course of the disease. Critically, given the known differences between human and murine immunity, detailed characterization (eg, utilizing single-cell and spatial technologies) of biopsies from patients both prior to and while receiving treatment will be essential. This will allow prioritization of relevant biological mechanisms that can subsequently be tested in coclinical animal models and to increase the probability of successful translation to patients.
Figure 2.
Effects of AR inhibition over time on T cell infiltration into the prostate and prostate tumor. Inhibition of AR leads to an initial increase in the infiltration of anticancer T cells such as CD8+ T cells and CD4+ Th1 T cells. However, this initial influx of anticancer T cells is eventually followed by the emergence of procancer CD4+ Th17 and regulatory T cells (Tregs).
Fibroblasts and Smooth Muscle Cells
AR is prominently expressed (∼25-75% by immunohistochemistry (IHC)) in the stromal compartment (38‐42), in both fibroblasts and smooth muscle cells (SMCs) of the prostate gland (43). AR's functional roles in these different compartments were determined using genetically engineered mouse models in which the Ar gene was specifically deleted in either the epithelium, fibroblasts, or SMCs using Probasin-Cre, FSP-Cre, or (transgelin) Tgln-CRE, respectively (43‐47). Strikingly, loss of AR in the epithelium did not significantly disrupt glandular structure, but it did inhibit the expression of AR target genes involved in glandular secretion and led to hyperproliferation of epithelium, suggesting a normal growth suppressive role for epithelial AR (46, 47). Loss of AR in fibroblasts severely inhibited luminal cell differentiation, a phenotype also seen upon chemical castration (44). Loss of AR in SMCs led to altered ductal invaginations (45). Combined loss of AR in both fibroblasts and SMCs generated a more profound phenotype, including epithelial cell apoptosis, suggesting both are contributing unique signals to epithelial homeostasis (48). These findings corroborated prior tissue recombination and implantation models using urogenital mesenchyme from mice deficient in AR (49). This stromal AR dependency was observed in a human microfluidic-based bioengineered epithelia/stroma coculture model, where stimulation of stromal cells with androgens was sufficient to induce luminal cell differentiation of the neighboring basal epithelial population, and stromal cells with inactive AR did not (50). In the same model system, intrinsic AR function in the stroma is also not required for luminal cell survival or proliferation (51). In fact, in normal epithelium, AR overexpression suppresses growth (38). This is in stark contrast to prostate cancer, where AR drives both survival and proliferation. The basis for this difference remains unresolved. However, these developmental studies suggest that AR in the stromal compartment is critical for prostate gland organogenesis and normal glandular homeostasis.
One of the major outstanding questions is how stromal AR exerts its effects on the epithelia. It was originally postulated that AR must induce the expression of secreted factors that act on the epithelium (52). Attempts to unambiguously identify the nature of these factors have been complicated by the lack of accurate models. Ex vivo studies have identified 2 morphogens, FGF10 and FGF7 (aka KGF), which are sufficient to induce luminal cell differentiation of basal cells and, in some models, are induced by androgen (44, 52‐56). However, other reports provide evidence that neither is robustly controlled by androgens in the stroma (57‐59). While much work has focused on the canonical nuclear functions of AR in stroma cells, it is possible that non-nuclear AR actions are important as has been reported in prostate cancer cells (60). The recent identification of putative membrane-localized ARs including membrane-localized AR and unrelated androgen-interacting receptors may add to the complexity (61). Resolution of all these questions will require additional studies and improved models.
Human tissue studies demonstrate that stromal AR expression is lost with increasing Gleason grade and is associated with poor outcomes (40, 62, 63). In 1 study, AR in patient-derived fibroblasts was found to suppress the migration of cocultured human prostate cancer cells by blocking chemokine production (64). Since stromal AR maintains tissue homeostasis, loss of stromal AR is further expected to disrupt normal glandular structure through decreased secretion of androgen-dependent morphogens. The mechanism by which AR is lost in the stroma is not known. One possibility is that simple cancer-associated fibroblast (CAF) conversion could alter the mesenchymal lineage such that AR is no longer expressed (65). Alternatively, there may be more direct mechanisms mediated by other tumor-secreted factors. In fact, the mechanisms that control AR gene expression in fibroblasts have not been identified. Studies in different prostate cancer lines and other cell types suggest fundamental differences in how AR gene transcription is controlled depending on the context and species (66‐68). Understanding these mechanisms will be critical for efforts attempting to restore stromal AR function in prostate cancer to help renormalize the tissue and impair disease progression. Therefore, AR's stromal functions are fundamentally important in patients receiving ADT, where AR function is not only suppressed in the tumor but also in the stroma. One study suggests that in some cells, NF-kB signaling suppresses AR expression (67), whereas in tumor cells, it enhances AR expression (66, 69). Hence, 1 possibility is to therapeutically suppress NF-kB, which would lead to the correct repression of AR in tumor cells and enhance its activity in stroma.
While human tissue studies have repeatedly linked AR loss in the stroma as a mechanism of progression, preclinical studies have suggested that AR expression in the stroma is critical for tumor initiation in prostate cancer genetically engineered mouse models (48, 70, 71). However, in another study in which Ar was genetically ablated in SMCs, loss of AR promoted oncogenesis through a mechanism that leads to disruption of luminal cells. This result is consistent with a loss in glandular homeostasis (72). It is not clear what the source of discrepancy is in these studies. Of note in the mouse, oncogenic disruption often occurs in the context of gland development, whereas, in humans, oncogenic disruption occurs postdevelopment and during aging. Another possibility is that there are fundamental differences in AR actions in fibroblasts vs SMCs. Signaling in the stroma may also be needed early in tumor initiation to drive the proliferation or maintenance of the “cell-of-origin” until the tumor is established and then is no longer dependent on stromal AR. It is established that normal stroma is repressive to tumor initiation, while the subsequent disruption of the stromal compartment is required to switch from noninvasive prostatic intraepithelial neoplasia (PIN) to high-grade invasive carcinoma. Invasive carcinoma is accompanied by a CAF phenotype and loss of stromal AR. The relative contribution of AR loss vs CAF conversion and how they each contribute to tumor progression needs to be further established.
Systemic Metabolism, Adipocytes, and the Cardiovascular System
Contemporary ADT is primarily via gonadotropin hormone-releasing hormone (GnRH) agonists, such as leuprolide or goserelin, or antagonists, such as degarelix or relugolix, that decrease production of luteinizing hormone and follicle-stimulating hormone. Greater androgen signaling inhibition (ASI) has also improved survival for men with advanced prostate cancer. ASI may be AR antagonists to target paracrine and autocrine signaling or CYP17A1 inhibition to target production of androgen precursors from the adrenal gland and adipose tissue. While ADT and ASI are effective at stopping prostate cancer cell growth, these therapies also have profound effects on the host given the central role of androgen signaling in male physiology, including impacts on the musculoskeletal system (described in the next section), adipocytes, insulin and lipid metabolism, and ultimately cardiovascular health.
Clinically, body composition significantly changes in men receiving ADT and ASI. ADT increases total fat mass, with subcutaneous adipose tissue increasing more than visceral adipose tissue (73). ADT also causes a loss of skeletal muscle mass and quality, which puts men treated with ADT at risk for sarcopenia and sarcopenic obesity (74). Adverse body composition changes occur in ∼70% of men treated with ADT and the risk increases with age, but the individual vulnerabilities to body composition toxicity are not well understood (75). Human preadipocytes and adipocytes express AR, and murine studies demonstrated that androgens shift mesenchymal pluripotent cells towards a myogenic lineage and an AR-mediated pathway inhibits differentiation into adipocytes, which is mitigated with ASI treatment (76, 77). In human subcutaneous adipose tissue, androgens, via AR, inhibit adipogenesis where adipose stem cells normally would differentiate into preadipocytes and then into adipocytes, ultimately limiting adipocyte number and storage (78). Androgens may also modulate lipolysis in a depot-specific manner (79). Adipose tissue regulates exposure to androgens in an autocrine manner by modulating levels of androgens via AKR1C, 17-βHSD, and SRD5A activity (79).
ADT and ASI impact endocrine and paracrine levels of insulin and adipokines, which worsens hemoglobin A1C control and exacerbates body composition changes (80). In murine models, germline Ar knockout reduces insulin sensitivity, increases leptin levels (a satiety hormone with other functions), and produces leptin resistance (81). In these same models, AR in the hypothalamus regulates leptin-mediated STAT3 signaling, and loss of AR impairs STAT3 nuclear localization in the arcuate nucleus potentially explaining leptin resistance (82). To date, the mechanism(s) underlying insulin resistance with ADT is unclear.
Ultimately, ADT increases total cholesterol, low-density lipoprotein, high-density lipoprotein, and the risk for cardiovascular disease (83). Clinical studies suggest that treatment with GnRH agonists is associated with an increased risk of cardiovascular disease compared with GnRH antagonists or orchiectomy (84). It is worth noting that the degree of cardiovascular risk with ADT varies between population-based studies and clinical trial cohorts where risk is actively managed by cardiologists (85‐87). The aforementioned changes with ADT indirectly increase the risk for cardiovascular events, and preclinical studies also suggest a direct role of the androgen–AR axis in atherosclerotic disease. In murine models, a high-fat diet increases the size of atherosclerotic lesions in castrated mice compared with sham-operated mice, and subsequent testosterone supplementation in mice reduces the atherosclerotic lesions in castrated mice, if aromatase is active, suggesting in this case a protective role for the estrogen receptor (88). These findings were supported by other murine models of cardiovascular disease such as ApoE-deficient mice (89).
Bone and Muscle
AR also plays essential functions in the musculoskeletal system (90). Androgens stimulate longitudinal and radial bone growth, with an accelerated bone apposition in men during puberty, and are essential to maintain bone mass in adults (90). Conditional ablation of Ar in prepubertal and postpubertal mice results in reduced total body bone mineral density, bone volume fraction, and increased bone turnover, confirming that AR is required to support bone health in both young and adult mice (91). Bone homeostasis depends on the well-controlled balance between bone-forming (osteoblasts) and resorbing (osteoclasts) cells. Osteoblasts express AR at higher levels in cortical bone than trabecular bone, with no sex-related differences (92). Male mice with targeted deletion of Ar in mature osteoblasts or osteocytes have lower cancellous bone mass, but no cortical bone phenotype (91). AR activation stimulates osteoblast differentiation and proliferation, improves the production and organization of bone matrix, the biosynthesis of extracellular matrix proteins, and suppresses apoptosis (93, 94). AR is also expressed by osteoclasts; however, experimental evidence suggests that it plays an indirect role in modulating the biology of these cells, most likely by regulating their interactions with osteoblasts (95). As an example, low testosterone levels lead to increased secretion of receptor activator of nuclear factor kappa-B ligand by osteoblasts, which stimulates osteoclast resorptive activity (96). Interestingly, targeted deletion of Ar in the mesenchymal cell lineage, besides decreasing bone volume and trabecular number, shows an increased osteoclast number in the cancellous compartment, while targeted deletion of Ar in myeloid cells does not show any bone phenotype (97). These results suggest that the effects of androgens on cancellous bone result from AR signaling in osteoblasts—not osteoclasts. Accordingly, osteoclast-specific knockout of Ar driven by cathepsin K-Cre does not alter the bone microarchitecture or osteoclast surface (98).
AR also critically regulates muscle mass and function. AR is expressed in several muscle cell types, including muscle fibers, fibroblasts, smooth muscle cells, and endothelial cells, with predominant expression in satellite cells (99). Conditional knockout of Ar in satellite cells affects fiber-type distribution, decreases force production of the limb muscles, and perineal muscle mass (100). Genomic Ar knockout in male mice shows decreased muscle mass, reduced force production, and increased fatigue resistance due to altered expression of molecular regulators that maintain myoblasts in a proliferative state and delay their terminal differentiation (101). Recent studies in myofiber-specific Ar knockout mice identified a fast-type, muscle-specific novel splicing variant of myosin light-chain kinase 4 as a target of AR in skeletal muscles (102). In addition, AR increases the reutilization of intracellular amino acids that favors protein synthesis and muscle fibers generation, including both type I and type II (103).
ADT side effects translate to bone fragility in patients (93, 104). A prospective study on ∼50 000 prostate cancer patients identified a significantly higher fracture rate in men who underwent ADT compared with non-ADT (105), with a 21% to 37% increase in fracture risk in the absence of antiresorptive therapy (105). Other analyses on additional patient cohorts treated with ADT confirmed a higher risk of bone fractures (106, 107) and showed a significant decline of bone mineral density, with a mean decline of 1.6% to 3.7%, depending on the bone type, which is faster than the normal yearly bone loss rate for what is already an aging population (108). As noted above, a further contribution to androgen deprivation–dependent bone fragility is related to sarcopenia, the loss of muscle mass that is replaced by fat deposition (109). Sarcopenia has been shown to be a predictor of fracture risk (110). Taken together, these data indicate that targeting AR has detrimental side effects on body composition and bone quality. Whether targeting AR in bone cells directly impacts the ability of metastatic prostate cancer cells to form skeletal lesions is less clear.
Conclusions and Future Perspectives
AR is the central driver of prostate cancer and, thus, remains the primary therapeutic target for this disease. Research over the past decade has highlighted important roles for AR not only in prostate cancer cells, but also in other cell types. AR cancer cell–extrinsic functions within the tumor microenvironment as well as peripheral to the tumor impact the efficacy of anti-AR treatments and mediate on-target side effects (prostate cancer cell nonautonomous activity) of this class of drugs. As new technologies are deployed to help map AR functions across different tissue and cell types throughout disease progression, it is anticipated that we will be able to develop improved treatment combinations that optimize the therapeutic index for individual patients. For instance, while great strides have been made toward delineating AR's roles in T cells, AR's function in myeloid cells has remained more enigmatic, suggesting a need to better define AR functions in explicit myeloid subtypes while accounting for potential species-specific differences (eg, rodent vs human). Given the immunosuppressive roles of AR in T cells, but the potential immune-activating roles of AR in some myeloid cells, the ratio of certain myeloid cells to T cells could ultimately determine the effectiveness of AR signaling inhibitors in the tumor immune microenvironment and, thus, sensitivity to immunotherapies. A more in-depth understanding of the myeloid subsets that promote disease progression, when they appear, and how they respond to AR-targeted therapy could aid in the development of novel treatment regimens capable of overcoming the immunosuppressive prostate cancer milieu.
Our understanding of how systemic AR inhibition impacts patient quality of life, and, conversely, how the patient's composition influences antitumor drug efficacy continues to evolve. It is anticipated that an improved understanding of AR's regulation and role throughout the body will allow us to move beyond the current “1 size fits all” approach for treating men with prostate cancer. With the advent of new therapies with better antitumor effects, patients are living longer. As such, greater consideration for how these therapies also impact quality of life and linked comorbidities is required to improve patient care in this growing population.
Acknowledgments
We thank Kelly Kage for assistance with preparing the figures. This work was supported by a grant from the National Institutes of Health (NIH P50CA140388 [D.E.F.]) and support from the Mike Slive Foundation for Prostate Cancer Research (D.E.F.). A.W.H is supported by a Rob Hayvaert and Paul Heynen Prostate Cancer Foundation Young Investigator Award, a Department of Defense Early Investigator Research Award (W81XWH-22-1-0117), and philanthropic donations from Michael and Patricia Berns.
Abbreviations
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ASI
androgen signaling inhibition
- CAF
cancer-associated fibroblast
- GnRH
gonadotropin-releasing hormone
- ICT
immune checkpoint therapy
- SMC
smooth muscle cell
Contributor Information
Andrew W Hahn, Department of Genitourinary Medical Oncology and the David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Bilal A Siddiqui, Department of Genitourinary Medical Oncology and the David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Javier Leo, Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA; The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Houston, TX 77030, USA.
Eleonora Dondossola, Department of Genitourinary Medical Oncology and the David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Kaitlin J Basham, Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA.
Cindy K Miranti, Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, University of Arizona, Tucson, AZ 85721, USA.
Daniel E Frigo, Department of Genitourinary Medical Oncology and the David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA; Center for Nuclear Receptors and Cell Signaling, University of Houston, Houston, TX 77204, USA; Department of Biology and Biochemistry, University of Houston, Houston, TX 77204, USA.
Disclosures
D.E.F. has received research funding from GTx, Inc. and has familial relationships with Hummingbird Bioscience, Maia Biotechnology, Alms Therapeutics, Hinova Pharmaceuticals, and Barricade Therapeutics. A.W.H reports advisory board consultation for Janssen and travel support from Dava Oncology. The other authors report no potential conflicts of interest. The funders had no role in the conceptualization of the study or writing of the manuscript, or in the decision to publish this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Mainwaring WI. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969;45(4):531‐541. [DOI] [PubMed] [Google Scholar]
- 2. Anderson KM, Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968;219(5151):277‐279. [DOI] [PubMed] [Google Scholar]
- 3. Bruchovsky N, Wilson JD. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. J Biol Chem. 1968;243(22):5953‐5960. [PubMed] [Google Scholar]
- 4. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on Serum phosphatases in metastatic carcinoma of the prostate*. Cancer Res. 1941;1(4):293‐297. [DOI] [PubMed] [Google Scholar]
- 5. Copeland BT, Pal SK, Bolton EC, Jones JO. The androgen receptor malignancy shift in prostate cancer. Prostate. 2018;78(7):521‐531. [DOI] [PubMed] [Google Scholar]
- 6. Frigo DE, Howe MK, Wittmann BM, et al. Cam kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 2011;71(2):528‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10(480):eaam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin C, Salzillo TC, Bader DA, et al. Prostate cancer energetics and biosynthesis. Adv Exp Med Biol. 2019;1210:185‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7(9):276‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol. 2014;11(6):365‐376. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825‐836. [DOI] [PubMed] [Google Scholar]
- 12. Losada-Garcia A, Cortes-Ramirez SA, Cruz-Burgos M, et al. Hormone-Related cancer and autoimmune diseases: A Complex interplay to be discovered. Front Genet. 2021;12:673180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Cancer Institute . Data from: Surveillance, Epidemiology, and End Results (SEER) Program. 2022. Accessed January 1, 2023. www.seer.cancer.gov
- 14. Wilkinson NM, Chen HC, Lechner MG, Su MA. Sex differences in immunity. Annu Rev Immunol. 2022;40:75‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buskiewicz IA, Huber SA, Fairweather D. Sex hormone receptor expression in the immune system. In: Neigh GN and Mitzelfelt MM, eds. Sex Differences in Physiology. Academic Press; 2016:45‐60. [Google Scholar]
- 16. Chakraborty B, Byemerwa J, Krebs T, Lim F, Chang CY, McDonnell DP. Estrogen receptor signaling in the immune system. Endocr Rev. 2023;44(1):117‐141. [DOI] [PubMed] [Google Scholar]
- 17. Olsen NJ, Kovacs WJ. Effects of androgens on T and B lymphocyte development. Immunol Res. 2001;23(2-3):281‐288. [DOI] [PubMed] [Google Scholar]
- 18. Roden AC, Moser MT, Tri SD, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098‐6108. [DOI] [PubMed] [Google Scholar]
- 19. Kissick HT, Sanda MG, Dunn LK, et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A. 2014;111(27):9887‐9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565‐14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsen NJ, Gu X, Kovacs WJ. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J Clin Invest. 2001;108(11):1697‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guan X, Polesso F, Wang C, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. 2022;606(7915):791‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38(5):395‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrylak DP, Loriot Y, Shaffer DR, et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: A phase I study. Clin Cancer Res. 2021;27(12):3360‐3369. [DOI] [PubMed] [Google Scholar]
- 26. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen YC, Ghasemzadeh A, Kochel CM, et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the myc-CaP model. Prostate Cancer Prostatic Dis. 2018;21(1):113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao J, Ward JF, Pettaway CA, et al. VISTA Is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Graff JN, Stein MN, Surana R, et al. Phase II study of ipilimumab in men with metastatic prostate cancer with an incomplete response to androgen deprivation therapy. Front Oncol. 2020;10:1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subudhi SK, Aparicio A, Gao J, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113(42):11919‐11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Powles T, Yuen KC, Gillessen S, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med. 2022;28(1):144‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morse MD, McNeel DG. T cells localized to the androgen-deprived prostate are TH1 and TH17 biased. Prostate. 2012;72(11):1239‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang S, Moore ML, Grayson JM, Dubey P. Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72(8):1975‐1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calcinotto A, Spataro C, Zagato E, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez-Bujanda ZA, Haffner MC, Chaimowitz MG, et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat Cancer. 2021;2(8):803‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izumi K, Fang LY, Mizokami A, et al. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med. 2013;5(9):1383‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Consiglio CR, Udartseva O, Ramsey KD, Bush C, Gollnick SO. Enzalutamide, an androgen receptor antagonist, enhances myeloid cell-mediated immune suppression and tumor progression. Cancer Immunol Res. 2020;8(9):1215‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Antony L, van der Schoor F, Dalrymple SL, Isaacs JT. Androgen receptor (AR) suppresses normal human prostate epithelial cell proliferation via AR/beta-catenin/TCF-4 complex inhibition of c-MYC transcription. Prostate. 2014;74(11):1118‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh M, Jha R, Melamed J, Shapiro E, Hayward SW, Lee P. Stromal androgen receptor in prostate development and cancer. Am J Pathol. 2014;184(10):2598‐2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wikstrom P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69(8):799‐809. [DOI] [PubMed] [Google Scholar]
- 41. Henshall SM, Quinn DI, Lee CS, et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61(2):423‐427. [PubMed] [Google Scholar]
- 42. Li Y, Li CX, Ye H, et al. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12(6B):2790‐2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498‐13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu S, Yeh CR, Niu Y, et al. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate. 2012;72(4):437‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu S, Zhang C, Lin CC, et al. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate. 2011;71(5):517‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu CT, Altuwaijri S, Ricke WA, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104(31):12679‐12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simanainen U, Allan CM, Lim P, et al. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148(5):2264‐2272. [DOI] [PubMed] [Google Scholar]
- 48. Lai KP, Yamashita S, Huang CK, Yeh S, Chang C. Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines. EMBO Mol Med. 2012;4(8):791‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76(6):578‐586. [DOI] [PubMed] [Google Scholar]
- 50. Jiang L, Ivich F, Tahsin S, et al. Human stroma and epithelium co-culture in a microfluidic model of a human prostate gland. Biomicrofluidics. 2019;13(6):064116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lamb LE, Knudsen BS, Miranti CK. E-cadherin-mediated survival of androgen-receptor-expressing secretory prostate epithelial cells derived from a stratified in vitro differentiation model. J Cell Sci. 2010;123(Pt 2):266‐276. [DOI] [PubMed] [Google Scholar]
- 52. Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6(12):2123‐2128. [DOI] [PubMed] [Google Scholar]
- 53. Planz B, Wang Q, Kirley SD, Lin CW, McDougal WS. Androgen responsiveness of stromal cells of the human prostate: regulation of cell proliferation and keratinocyte growth factor by androgen. J Urol. 1998;160(5):1850‐1855. [DOI] [PubMed] [Google Scholar]
- 54. Nakano K, Fukabori Y, Itoh N, et al. Androgen-stimulated human prostate epithelial growth mediated by stromal-derived fibroblast growth factor-10. Endocr J. 1999;46(3):405‐413. [DOI] [PubMed] [Google Scholar]
- 55. Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev Biol. 2005;278(2):396‐414. [DOI] [PubMed] [Google Scholar]
- 56. Fasciana C, van der Made AC, Faber PW, Trapman J. Androgen regulation of the rat keratinocyte growth factor (KGF/FGF7) promoter. Biochem Biophys Res Commun. 1996;220(3):858‐863. [DOI] [PubMed] [Google Scholar]
- 57. Nemeth JA, Zelner DJ, Lang S, Lee C. Keratinocyte growth factor in the rat ventral prostate: androgen-independent expression. J Endocrinol. 1998;156(1):115‐125. [DOI] [PubMed] [Google Scholar]
- 58. Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126(16):3693‐3701. [DOI] [PubMed] [Google Scholar]
- 59. Ropiquet F, Giri D, Kwabi-Addo B, Schmidt K, Ittmann M. FGF-10 is expressed at low levels in the human prostate. Prostate. 2000;44(4):334‐338. [DOI] [PubMed] [Google Scholar]
- 60. Zamagni A, Cortesi M, Zanoni M, Tesei A. Non-nuclear AR signaling in prostate cancer. Front Chem. 2019;7:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mauvais-Jarvis F, Lange CA, Levin ER. Membrane-Initiated estrogen, androgen, and progesterone receptor signaling in health and disease. Endocr Rev. 2022;43(4):720‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leach DA, Need EF, Toivanen R, et al. Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome. Oncotarget. 2015;6(18):16135‐16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Palethorpe HM, Leach DA, Need EF, Drew PA, Smith E. Myofibroblast androgen receptor expression determines cell survival in co-cultures of myofibroblasts and prostate cancer cells in vitro. Oncotarget. 2018;9(27):19100‐19114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cioni B, Nevedomskaya E, Melis MHM, et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol Oncol. 2018;12(8):1308‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barcellos-de-Souza P, Comito G, Pons-Segura C, et al. Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-beta1. Stem Cells. 2016;34(10):2536‐2547. [DOI] [PubMed] [Google Scholar]
- 66. Nadiminty N, Lou W, Sun M, et al. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010;70(8):3309‐3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Supakar PC, Jung MH, Song CS, Chatterjee B, Roy AK. Nuclear factor kappa B functions as a negative regulator for the rat androgen receptor gene and NF-kappa B activity increases during the age-dependent desensitization of the liver. J Biol Chem. 1995;270(2):837‐842. [DOI] [PubMed] [Google Scholar]
- 68. Ko S, Shi L, Kim S, Song CS, Chatterjee B. Interplay of nuclear factor-kappaB and B-myb in the negative regulation of androgen receptor expression by tumor necrosis factor alpha. Mol Endocrinol. 2008;22(2):273‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang L, Altuwaijri S, Deng F, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hiroto A, Kim WK, Pineda A, et al. Stromal androgen signaling acts as tumor niches to drive prostatic basal epithelial progenitor-initiated oncogenesis. Nat Commun. 2022;13(1):6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yu S, Xia S, Yang D, et al. Androgen receptor in human prostate cancer-associated fibroblasts promotes prostate cancer epithelial cell growth and invasion. Med Oncol. 2013;30(3):674. [DOI] [PubMed] [Google Scholar]
- 72. Liu Y, Wang J, Horton C, et al. Stromal AR inhibits prostate tumor progression by restraining secretory luminal epithelial cells. Cell Rep. 2022;39(8):110848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599‐603. [DOI] [PubMed] [Google Scholar]
- 74. Smith MR, Saad F, Egerdie B, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30(26):3271‐3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gupta D, Salmane C, Slovin S, Steingart RM. Cardiovascular complications of androgen deprivation therapy for prostate cancer. Curr Treat Options Cardiovasc Med. 2017;19(8):61. [DOI] [PubMed] [Google Scholar]
- 76. Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 1998;274(6):C1645‐C1652. [DOI] [PubMed] [Google Scholar]
- 77. Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144(11):5081‐5088. [DOI] [PubMed] [Google Scholar]
- 78. Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78(9):920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O'Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277‐284. [DOI] [PubMed] [Google Scholar]
- 80. Keating NL, Liu PH, O'Malley AJ, Freedland SJ, Smith MR. Androgen-deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol. 2014;65(4):816‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54(6):1717‐1725. [DOI] [PubMed] [Google Scholar]
- 82. Fan W, Yanase T, Nishi Y, et al. Functional potentiation of leptin-signal transducer and activator of transcription 3 signaling by the androgen receptor. Endocrinology. 2008;149(12):6028‐6036. [DOI] [PubMed] [Google Scholar]
- 83. Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24(24):3979‐3983. [DOI] [PubMed] [Google Scholar]
- 84. O'Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243‐1251. [DOI] [PubMed] [Google Scholar]
- 85. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448‐4456. [DOI] [PubMed] [Google Scholar]
- 86. Zhao J, Zhu S, Sun L, et al. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS One. 2014;9(9):e107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lopes RD, Higano CS, Slovin SF, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144(16):1295‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98(6):3589‐3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bourghardt J, Wilhelmson AS, Alexanderson C, et al. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010;151(11):5428‐5437. [DOI] [PubMed] [Google Scholar]
- 90. Chen JF, Lin PW, Tsai YR, Yang YC, Kang HY. Androgens and androgen receptor actions on bone health and disease: from androgen deficiency to androgen therapy. Cells. 2019;8(11):1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu J, Henning P, Sjogren K, et al. The androgen receptor is required for maintenance of bone mass in adult male mice. Mol Cell Endocrinol. 2019;479:159‐169. [DOI] [PubMed] [Google Scholar]
- 92. Kasperk C, Helmboldt A, Borcsok I, et al. Skeletal site-dependent expression of the androgen receptor in human osteoblastic cell populations. Calcif Tissue Int. 1997;61(6):464‐473. [DOI] [PubMed] [Google Scholar]
- 93. Hussain A, Tripathi A, Pieczonka C, et al. Bone health effects of androgen-deprivation therapy and androgen receptor inhibitors in patients with nonmetastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021;24(2):290‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kasperk CH, Wergedal JE, Farley JR, Linkhart TA, Turner RT, Baylink DJ. Androgens directly stimulate proliferation of bone cells in vitro. Endocrinology. 1989;124(3):1576‐1578. [DOI] [PubMed] [Google Scholar]
- 95. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. The effects of androgen deficiency on murine bone remodeling and bone mineral density are mediated via cells of the osteoblastic lineage. Endocrinology. 1997;138(9):4013‐4021. [DOI] [PubMed] [Google Scholar]
- 96. Huber DM, Bendixen AC, Pathrose P, et al. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology. 2001;142(9):3800‐3808. [DOI] [PubMed] [Google Scholar]
- 97. Ucer S, Iyer S, Bartell SM, et al. The effects of androgens on murine cortical bone do not require AR or ERalpha signaling in osteoblasts and osteoclasts. J Bone Miner Res. 2015;30(7):1138‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sinnesael M, Jardi F, Deboel L, et al. The androgen receptor has no direct antiresorptive actions in mouse osteoclasts. Mol Cell Endocrinol. 2015;411:198‐206. [DOI] [PubMed] [Google Scholar]
- 99. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89(10):5245‐5255. [DOI] [PubMed] [Google Scholar]
- 100. Dubois V, Laurent MR, Sinnesael M, et al. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 2014;28(7):2979‐2994. [DOI] [PubMed] [Google Scholar]
- 101. MacLean HE, Chiu WS, Notini AJ, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22(8):2676‐2689. [DOI] [PubMed] [Google Scholar]
- 102. Sakakibara I, Yanagihara Y, Himori K, et al. Myofiber androgen receptor increases muscle strength mediated by a skeletal muscle splicing variant of Mylk4. iScience. 2021;24(4):102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275(5):E864‐E871. [DOI] [PubMed] [Google Scholar]
- 104. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 Suppl 1):79‐85. [PubMed] [Google Scholar]
- 105. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154‐164. [DOI] [PubMed] [Google Scholar]
- 106. Wallander M, Axelsson KF, Lundh D, Lorentzon M. Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures-a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos Int. 2019;30(1):115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang A, Obertova Z, Brown C, et al. Risk of fracture in men with prostate cancer on androgen deprivation therapy: a population-based cohort study in New Zealand. BMC Cancer. 2015;15:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim DK, Lee JY, Kim KJ, et al. Effect of androgen-deprivation therapy on bone mineral density in patients with prostate cancer: a systematic review and meta-analysis. J Clin Med. 2019;8(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Berruti A, Dogliotti L, Terrone C, et al. Gruppo Onco Urologico Piemontese, Rete Oncologica Piemontese. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361‐2367. [PubMed] [Google Scholar]
- 110. Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) study. J Am Med Dir Assoc. 2014;15(8):551‐558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.