Abstract
Introduction
Smoking cessation is more than 50% heritable. Genetic studies of smoking cessation have been limited by short-term follow-up or cross-sectional design.
Aims and Methods
This study tests single nucleotide polymorphism (SNP) associations with cessation during long-term follow-up throughout adulthood in women. The secondary aim tests whether genetic associations differ by smoking intensity. Associations between 10 SNPs in CHRNA5, CHRNA3, CHRNB2, CHRNB4, DRD2, and COMT and the probability of smoking cessation over time were evaluated in two longitudinal cohort studies of female nurses, the Nurses’ Health Study (NHS) (n = 10 017) and NHS-2 (n = 2793). Participant follow-up ranged from 2 to 38 years with data collected every 2 years.
Results
Women with the minor allele of either CHRNA5 SNP rs16969968 or CHRNA3 SNP rs1051730 had lower odds of cessation throughout adulthood [OR = 0.93, p-value = .003]. Women had increased odds of cessation if they had the minor allele of CHRNA3 SNP rs578776 [OR = 1.17, p-value = .002]. The minor allele of DRD2 SNP rs1800497 was associated with lower odds of cessation in moderate-to-heavy smokers [OR = 0.92, p-value = .0183] but increased odds in light smokers [OR = 1.24, p-value = .096].
Conclusions
Some SNP associations with short-term smoking abstinence observed in prior studies were shown in the present study to persist throughout adulthood over decades of follow-up. Other SNP associations with short-term abstinence did not persist long-term. The secondary aim findings suggest genetic associations may differ by smoking intensity.
Implications
The results of the present study expand on previous studies of SNP associations in relation to short-term smoking cessation to demonstrate some of these SNPs were associated with smoking cessation throughout decades of follow-up, whereas other SNP associations with short-term abstinence did not persist long-term. The rate of relapse to smoking remains high for several years after quitting smoking, and many smokers experience multiple quit attempts and relapse episodes throughout adulthood. Understanding genetic associations with long-term cessation has potential importance for precision medicine approaches to long-term cessation management.
Introduction
Most US adult smokers attempt to quit smoking each year, but only a small proportion achieve sustained cessation1 as 50% of those who quit relapse within 12 months.2 Understanding factors associated with smoking cessation can lead to improved intervention approaches, increasing long-term cessation rates. Smoking cessation is more than 50% heritable,3 emphasizing the importance of research to identify genetic variants associated with cessation because of their value for refining precision medicine approaches to smoking cessation.
Nicotinic acetylcholine receptor (nAChR) and dopaminergic genes are strong candidates for genetic studies of smoking cessation because of their influence on nicotine response. Nicotine binds to nAChRs,4 a family of neurotransmitter receptors,4 stimulating dopamine release producing neurological reward and reinforcing smoking behavior.5 Continued nicotine intake causes neuroadaptations within nAChRs producing craving, tolerance, and dependence.6 Furthermore, nAChRs are implicated in memory and associative learning7 which are reinforced by increased dopamine levels and signaling.8 This leads to cue-primed behavior, drug-seeking in the absence of contextual biological need.9 Dopamine-driven inhibitory control mechanisms also contribute to addictive behaviors.10 Cue-primed behavior and inhibitory control are behavioral factors that contribute to relapse risk long after nicotine withdrawal.2
The nAChR and dopaminergic pathways may influence addiction differentially via biochemical and behavioral mechanisms depending on smoking intensity. Light smokers (≤5 cigarettes/day [CPD]) experience low or no nicotine dependence yet still smoke for an average of 19 years.11 Light smokers report smoking for the “boost” in mood or concentration,12 thus, for light smokers, persistent smoking may be driven by positive reinforcement and corresponding behavioral conditioning.12 Conversely, nicotine dependence predominates in heavy smokers via a negative reinforcement mechanism of addiction (e.g. withdrawal-avoidance).1
Addiction mechanisms may also differ by biological sex. Women are more likely to respond to sensory stimuli of cigarette smoking, smoke to regulate mood, and have increased cue-reactivity.13,14 Conversely, men are more sensitive to nicotine content and craving.13
Despite the plausible differences in factors associated with smoking intensity and differences by biological sex, most genetic studies of smoking cessation have excluded light smokers and few studies of women focused on pregnancy.15,16 Thus, studies examining differences in genetic risk by smoking intensity and studies focused on women are needed. Furthermore, most evidence of genetic variants in relation to smoking cessation is from cross-sectional studies or studies measuring abstinence 2–6 months post-cessation. Studies are needed to understand genetic markers that are associated with long-term smoking outcomes. Using data from two all-female cohort studies with up to 38 years of repeated smoking status measures, the primary aim of this study was to examine associations between single nucleotide polymorphisms (SNPs) within nAChR and dopaminergic genes and the likelihood of smoking cessation throughout adulthood in women.
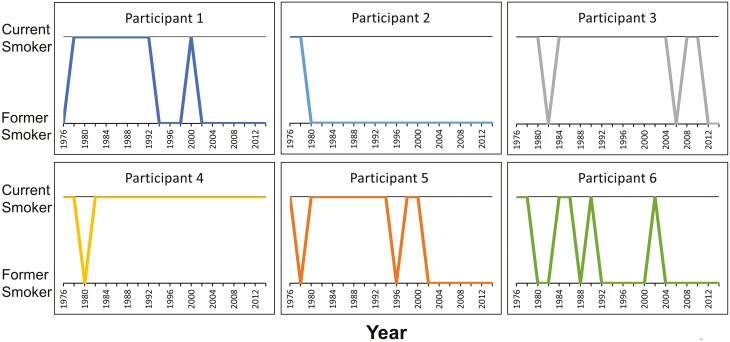
The novelty of this research is not the SNPs studied, as the SNPs were selected based on a systematic review of prior studies.17,18 Rather, the novelty of this research is the investigation of genetic associations with smoking cessation over long-term follow-up. To illustrate the innovation and importance of this research, Figure 1 depicts the smoking status over time between 1976 and 2014 for six participants of one of the cohort studies. Examination of genetic associations with participant smoking behavior throughout this period provides insight not gained by single timepoint smoking status comparisons or shorter follow-up. For example, all six participants in Figure 1 would be considered former smokers in 2012 despite the variability in smoking status at previous timepoints.
Figure 1.
Smoking status over time of six participants from the Nurses’ Health Study from 1976 to 2014. Smoking status (former versus current smoker) was collected every 2 years.
In addition to the importance of examining genetic associations with long-term smoking status, this research also stratifies SNP associations by <65 and ≥65 years of age. Because prior genetic studies of older smokers are limited, this provides an important addition to the body of evidence on genetic associations with smoking cessation. The secondary study's aim was to investigate the potential heterogeneity of genetic associations between light versus moderate-to-heavy smokers based on the differential impact of smoking intensity on behavioral versus biochemical addiction pathways.
Methods
Study Population
The study population included a subset of participants from the Nurses’ Health Study (NHS) (N = 121 700) and NHS-2 (N = 116 429), two prospective cohort studies of female registered nurses that have been previously described.19 Data were collected every 2 years beginning in 1976 for NHS and 1989 for NHS-2 and were available up to 2014 for NHS and 2015 for NHS-2. Genotype data were available in 18 527 and 8276 participants in the NHS and NHS-2 cohorts, respectively,20 of which 10 049 and 2804 were ever-smokers. Quality control of this whole-genome data were previously conducted and documented and only SNPs that met quality control criteria were included in these data.20 Of those women who were ever-smokers with genotype data, only 41 reported non-European ancestry and because of this small sample size combined with the potential for ethnic differences in allele frequencies, they were excluded. This resulted in a final study population of 10 017 participants from the NHS and 2793 participants from the NHS-2.
SNP Selection
A literature review identified 10 SNPs within nAChR or dopaminergic genes with findings suggesting an association with short-term smoking cessation or cross-sectional smoking status.17,18 Six SNPs were located within the CHRNA5-A3-B4 gene complex on chromosome 15, encoding for the α-5, α-3, and β-4 nAChR subunits: CHRNA5 SNPs rs16969968 G>A,21,22 rs680244 T>C,16,23 rs588765 T>C24; CHRNA3 SNPs rs1051730 G>A15,25 and rs578776 G>A24; and CHRNB4 SNP rs12914008 G>A.23 An additional nAChR variant, CHRNB2 SNP rs2072661 G>A,26 was also identified; CHRNB2 encodes for the β-2 nAChR subunit. Three dopaminergic variants were identified. Two SNPs of DRD2, encoding for D2 dopamine receptors: SNPs rs1800497 G>A27 and rs6277 G>A28; and one SNP of COMT, encoding for an enzyme regulating brain dopamine levels: SNP rs4680 G>A.29
Variables
The dependent variable was smoking status dichotomized for each subject at each follow-up time as former or current. Follow-up time was treated as continuous with the baseline designated as year 0. At each follow-up, participants reported former or current ordinal CPD data with the following categories: 1–4, 5–14, 15–24, 25–34, 35–44, ≥45, and unknown. Smoking intensity was classified so that participants who reported smoking 1–4 CPD throughout the follow-up were categorized as light smokers and all others were classified as moderate-to-heavy smokers. This variable definition captures lifetime light smokers and prevents misclassifying moderate-to-heavy smokers who reported reducing smoking to <5 CPD as light smokers.30
Additional relevant variables included baseline age, early age of smoking initiation, menopausal status, hormone replacement therapy (HRT) use, and body mass index (BMI).31,32 Because of differences between cohorts in data collection, early age of smoking initiation was defined as participants who reported smoking prior to age 16 years for NHS and those who reported smoking prior to age 15 years for NHS-2. To account for changes during follow-up, time-varying dichotomized variables were used for menopausal status and HRT use and a continuous time-varying variable was used for BMI.
Missing Data
Less than 0.5% of observations were missing smoking status data (510, 374, and 1 observation were missing smoking status among women in NHS <65 years of age, women in NHS ≥65 years of age, and women in NHS-2 <65 years of age, respectively); these observations were not included in the analysis. Among time-varying covariates, 7.4% and 3.5% of observations within NHS and NHS-2, respectively, were missing BMI, and 2.8% and 3% of observations within NHS and NHS-2, respectively, were missing menopausal status. Most participants missing either menopausal status or BMI were missing these data for only 1 or 2 observations, thus missing data were imputed utilizing the values obtained from observations prior to or subsequent to those with missing data. We also used age to determine missing menopausal status, assuming observations in which the participant was over 55 years of age were post-menopause. Those missing data for early initiation of smoking (<1%) were considered smokers who did not initiate smoking early.
Statistical Analysis
Generalized estimating equation models were used to evaluate the association between the 10 SNPs and the probability of smoking cessation over time using repeated smoking status measurements. Instead of focusing on smoking status at a single timepoint, these models account for the proportion of observations each participant was a former smoker and thus characterize if SNP associations with cessation persist throughout the follow-up. An autoregressive covariance structure was assumed to account for the correlation between repeated measures for each participant. Codominant, dominant, recessive, and additive genetic models were considered for SNPs with minor allele frequencies >0.05, whereas the dominant model was assumed if minor allele frequencies ≤0.05. The association between each SNP and the probability of smoking cessation over time was modeled with fixed effects for potential confounders (smoking intensity, early initiation of smoking, menopausal status, HRT use, and BMI). A SNP genotype by year interaction was considered but was not retained in the models because of the lack of statistical significance. Thus, the SNP genotype associations with the probability of smoking cessation were consistent over time. A minimally adjusted model including only baseline age, year, and genotype was also examined as a sensitivity analysis to assess if missingness impacted the SNP genotype effect. Analyses were stratified on age <65 years at the time of follow-up assessments to account for documented differences in smoking prevalence and cessation attempts between adults aged <65 years and those aged ≥65 years.33 Age stratification also enabled a more valid comparison between NHS and the more recent NHS-2 which had an insufficient sample size of participants aged ≥65 years for longitudinal analysis.
Statistical analyses were conducted using SAS 9.4 statistical software and model assumptions were checked graphically. The best genetic model for each SNP was selected based on quasi-likelihood under the independence model criterion (QIC) score and consistency with inheritance patterns.34 To accomplish the secondary goal of the study of evaluating heterogeneity of genetic associations between moderate-to-heavy and light smokers, a genotype by smoking intensity interaction was then considered.
Each cohort was analyzed separately. Results were combined via meta-analysis using the %METAANAL SAS macro to produce fixed effects estimators and test for between-study heterogeneity.35 Random effects pooling was assumed. A false discovery rate <0.05 was used to control for testing multiple SNPs and genetic models. In the individual analyses of the two cohorts, we controlled for the testing of 9 SNPs using 4 different genetic models and the testing of the 10th SNP (rs12914008) using only the dominant genetic model, thus, we controlled for 39 tests using false discovery rate <0.05. In the summary measures from the combined meta-analysis of the results of NHS and NHS-2, we controlled for the testing of the 10 SNPs across the selected genetic model, thus controlling for 10 tests using a false discovery rate <0.05.
Results
Participant characteristics are summarized in Table 1. At baseline, the average NHS ever-smoker was 43 years old (SD 6.7 years) with a BMI of 23.7 kg/m2 (SD 4.0) and the average NHS-2 ever-smoker was 36.3 years old (SD 4.3 years) with a BMI of 24.0 kg/m2 (SD 4.7). At the beginning of follow-up, 32.0% of NHS participants were post-menopause and 9.2% used HRT whereas only 6.1% of NHS-2 participants were post-menopause and 5.3% used HRT. The prevalence of early initiation of smoking was similar in both cohorts (12.1% NHS, 14.1% NHS-2), whereas the prevalence of light smokers was higher (p-value <.001) in NHS-2 (16.8%) compared to NHS (5.7%). Genotype frequencies are reported in Supplementary Table 1.
Table 1.
Descriptive Statistics of Nurses’ Health Study (NHS) and Nurses’ Health Study 2 (NHS-2) Cohorts. Baseline Status Data Were Collected in 1976 for NHS and 1989 for NHS-2
| NHS 1976–2014 |
NHS-2 1989–2015 |
||||
|---|---|---|---|---|---|
| (N = 10 017) | (N = 2793) | ||||
| Age (years), baseline | mean, (SD) | 43.0 (6.7) | 36.3 (4.3) | ||
| Range | 29–55 | 24–43 | |||
| BMI (kg/m2), baseline | mean, (SD) | 23.7 (4.0) | 24.0 (4.7) | ||
| Female | n (%) | 10 017 (100) | 2793 (100) | ||
| Race, White | n (%) | 10 017 (100) | 2793 (100) | ||
| Postmenopausal, baseline | Yes, n (%) | 3208 (32.0) | 171 (6.1) | ||
| HRT use, baseline | Yes, n (%) | 923 (9.2) | 148 (5.3) | ||
| Early initiation of smoking (<16 years old in NHS, <15 years old in NHS-2) | Yes, n (%) | 1214 (12.1) | 393 (14.1) | ||
| Lifetime light smoker (<5 cigarettes/day) | n (%) | 574 (5.7) | 471 (16.8) | ||
| Participants <65 years of age | Participants ≥65 years of age | Participants <65 years of age | Participants ≥65 years of age | ||
| # of participants, N | 10 017 | 9788 | 2793 | N/A | |
| Total # of observations | 111 493 | 75 169 | 36 387 | N/A | |
| # of repeated measures per participant | 3–18 | 1–15 | 5–14 | N/A | |
BMI = body mass index, HRT = hormone replacement therapy.
Associations in Participants Aged <65 Years
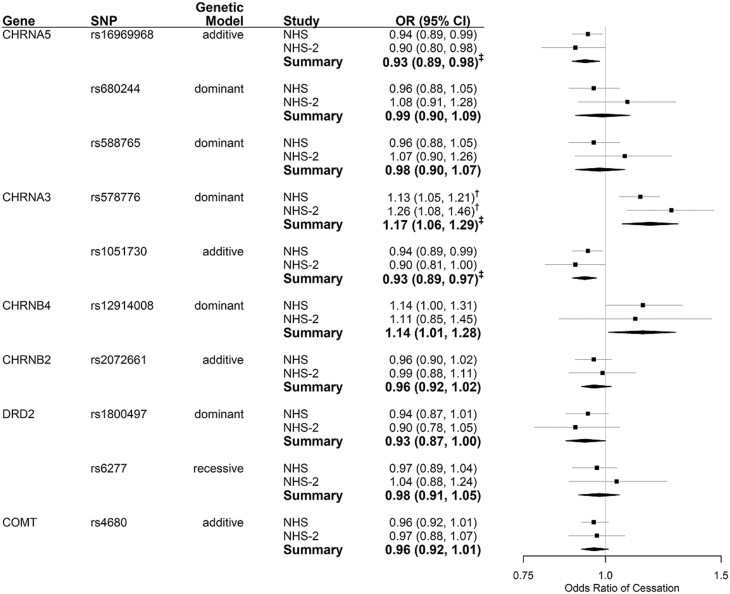
Among NHS and NHS-2 participants aged <65 years, associations with cessation were not statistically significant for five of the ten SNPs: CHRNA5 SNPs rs588765 and rs680244, CHRNB2 SNP rs2072661, DRD2 SNP rs6277, and COMT SNP rs4680. Of the remaining five SNPs, the association with smoking cessation was nominally significant but not significant after correction for multiple testing for two SNPs (CHRNB4 rs12914008 and DRD2 rs1800497) and three of the SNP associations remained significant after correction for multiple testing (CHRNA5 rs16969968, CHRNA3 rs1051730, and CHRNA3 rs578776). The direction of the associations among the five SNPs that were nominally significant varied, with three of the SNPs, two nAChR, and one dopaminergic, associated with reduced odds of cessation and two nAChR SNPs associated with increased odds of cessation. The odds ratios (ORs) and 95% confidence intervals (CIs) under the best genetic model for each SNP are presented in Figure 2. Associations (ORs, 95% CIs) for all SNPs and genetic models (including minimally adjusted and fully adjusted models) are shown in Supplementary Table 2. Results were similar between the minimally adjusted and fully adjusted models. Hereafter, we present the fully adjusted results.
Figure 2.
SNP associations with odds of smoking cessation, timepoints where participants are <65 years old. Controlling for year, age, smoking intensity (<5 or ≥5 cigarettes per day), early initiation of smoking (NHS = <16 years of age, NHS-2 = <15 years of age), menopausal status, use of hormone replacement therapy, body mass index. † FDR < 0.05, accounting for multiple comparisons for all genetic models and SNPs analyzed. ‡ FDR < 0.05, accounting for multiple comparisons of the selected genetic model for all 10 SNPs. SNP = single nucleotide polymorphism, NHS = Nurses' Health Study, NHS-2 = Nurses' Health Study 2.
The nAChR SNPs associated with reduced odds of cessation, CHRNA5 rs16969968 and CHRNA3 rs1051730, are nonrandomly associated loci or in linkage disequilibrium (r2 = 1.00, Dʹ = 1.00) in populations with European ancestry. Thus, findings are nearly identical, with participants having 7% lower odds of quitting smoking with each additional copy of the minor allele [rs16969968: Summary OR = 0.93, 95% CI 0.89–0.98, rs1051730: Summary OR = 0.93, 95% CI 0.89–0.97]. Among dopaminergic SNPs, those with at least one copy of the minor allele DRD2 rs1800497 were less likely to quit smoking than those homozygous for the common allele [Summary OR = 0.93, 95% CI 0.87–1.00]. This association was not statistically significant after adjusting for multiple testing.
The nAChR SNPs associated with increased odds of cessation were CHRNA3 rs578776 and CHRNB4 rs12914008. Within European ancestry populations, these SNPs are in high linkage disequilibrium (Dʹ = 0.999) but low correlation (r2 = 0.12). Participants with at least one copy of the minor allele of rs578776 had increased odds of smoking cessation compared to homozygous common allele carriers [Summary OR = 1.17, 95% CI 1.06–1.29]. A weaker association that was not statistically significant after adjusting for multiple testing was observed for rs12914008 [Summary OR = 1.14, 95% CI 1.01–1.28].
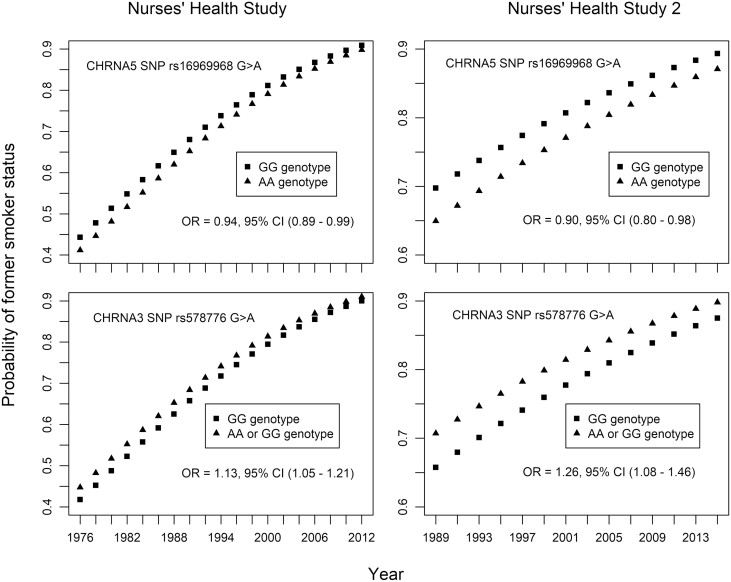
Though SNP genotype by time interactions were not observed, time was significantly associated with the odds of being a former smoker. Figure 3 depicts the probability of being a former smoker over time in both the NHS and NHS-2 cohort studies by genotype for SNPs rs16969968 and 578776. The ORs of smoking cessation between genotypes are consistent over time; however, participants of all genotypes have a higher probability of being a former smoker at later timepoints. Supplementary Figure 1 depicts the same relationship between the probability of being a former smoker over time by genotype for the remaining eight SNPs.
Figure 3.
The probability of former smoker status over time by SNP genotype for CHRNA5 SNP rs16969968 and CHRNA3 SNP rs578776.
Controlling for year, age, smoking intensity (<5 or ≥5 cigarettes per day), early initiation of smoking (NHS = <16 years of age, NHS-2 = <15 years of age), menopausal status, use of hormone replacement therapy, body mass index. The odds ratios of smoking cessation between genotypes are consistent over time; however, participants of all genotypes have higher probability of being a former smoker at later timepoints.
Associations by Smoking Intensity in Participants Aged <65 Years
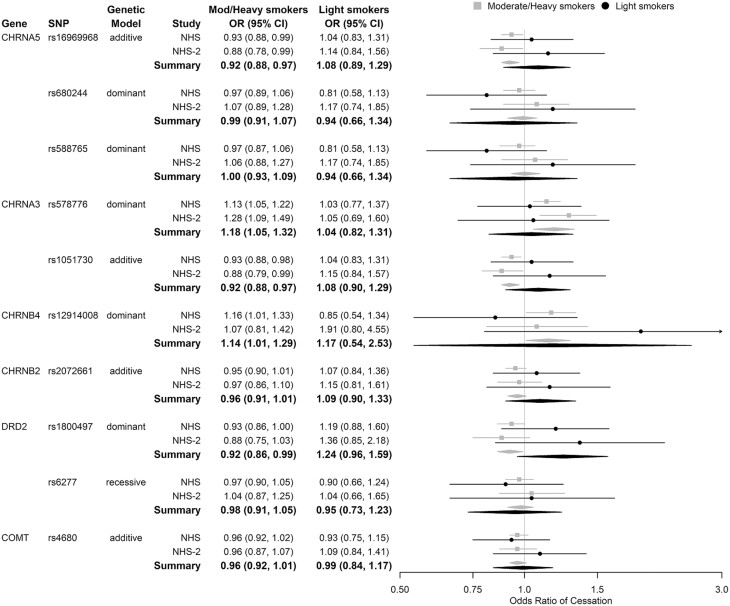
Examining SNP associations under the best genetic model by smoking intensity, the genotype by smoking intensity interactions were not statistically significant. However, in both cohorts, there was heterogeneity for some SNPs in the direction of the genetic association between light and moderate-to-heavy smokers. (Figure 4)
Figure 4.
Genetic associations with odds of smoking cessation by smoking intensity.
SNP = single nucleotide polymorphism, NHS = Nurses’ Health Study, NHS-2 = Nurses’ Health Study 2.
Light smoking <5 cigarettes per day (CPD), Moderate/Heavy smoking ≥5 CPD.
Adjusted for year, age, smoking intensity, genotype × smoking intensity interaction, early initiation of smoking (NHS = <16 years of age, NHS -2 = <15 years of age), menopausal status, use of hormone replacement therapy, and body mass index. Assessing timepoints in which participants are <65 years of age.
The strongest heterogeneity was for DRD2 rs1800497. Among moderate-to-heavy smokers, those with at least one copy of the minor allele were less likely to quit smoking than those homozygous for the common allele [Summary OR = 0.92, 95% CI 0.86–0.99]. Conversely, among light smokers, minor allele carriers had increased odds of quitting [Summary OR = 1.24, 95% CI 0.96–1.59].
Similar heterogeneity in the direction of the association was observed for SNPs rs16969968 and rs1051730. In moderate-to-heavy smokers, the odds of smoking cessation were reduced by 8% per copy of the minor allele of either SNP [Summary OR = 0.92, 95% CI 0.88–0.97], whereas in light smokers, the odds of smoking cessation increased 8% per copy of the minor allele of either SNP [Summary OR = 1.08, 95% CI 0.89–1.29].
Associations in NHS Participants, ≥65 Years of Age
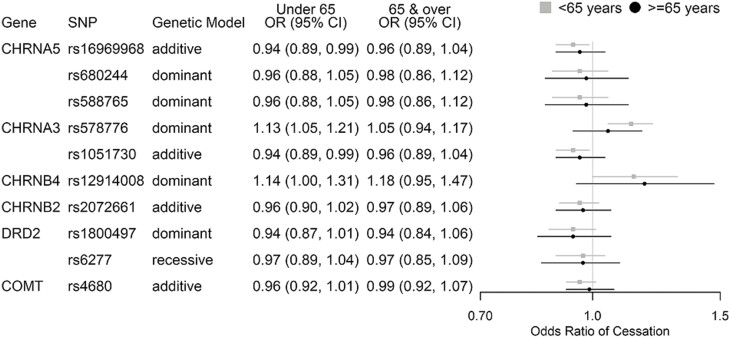
The associations were not statistically significant in NHS participants aged ≥65 years; however, the point estimates were similar or slightly weaker compared to those observed in NHS participants aged <65 years (Figure 5).
Figure 5.
SNP associations with odds of smoking cessation, NHS participants <65 and ≥65 years old. Controlling for baseline age, year, smoking intensity (<5 or ≥5 cigarettes per day), early initiation of smoking (<16 years of age), menopausal status (for <65 years of age), and hormone replacement therapy use. SNP = Single Nucleotide Polymorphism, NHS = Nurses’ Health Study.
Post Hoc Analysis
We conducted post hoc analyses testing associations between rs578776/rs16969968 haplotypes and smoking cessation (Supplementary Figure 2). Compared to participants with GG/AA haplotypes, participants with (AG + AA)/GG haplotypes [Summary OR = 1.33, 95% CI 1.10–1.61] or (AG + AA)/AG haplotypes [Summary OR = 1.19, 95% CI 1.06–1.33] had higher odds of smoking cessation throughout adulthood. The (AG + AA)/AA haplotypes were rare (NHS: n = 3; NHS-2: n = 0), and thus were not examined.
Discussion
This study focused on 10 selected SNPs in relation to smoking cessation, with methods and results that expand upon prior evidence. Though these 10 SNPs were previously studied in cross-sectional and/or short-term prospective studies, prior studies have not examined long-term smoking cessation outcomes. Thus, the novelty of this study is the longitudinal design with long-term repeated smoking behavior measures to investigate the association between these 10 SNPs and smoking cessation throughout adulthood. In addition to the long-term longitudinal design, innovative study characteristics include a focus on smoking cessation in women, a large enough sample size to explore the genetic associations by smoking intensity, and data on elderly individuals for whom evidence is sparse. Each of these novel aspects of the study is discussed below.
This study provides important proof of principle that some SNPs associated with short-term smoking cessation remain associated with cessation during long-term follow-up, whereas other SNP associations may not persist long-term. These findings suggest this long-term longitudinal research approach to study genetic associations with smoking cessation could be used to identify high-risk smokers who may benefit from long-term smoking cessation management. In this study, five of the ten SNPs tested were nominally associated with smoking cessation throughout adulthood and three of these SNPs remained significant after correction for multiple testing, documenting that these genetic associations persist during long-term follow-up in white women.
The minor alleles of CHRNA5 rs16969968 and CHRNA3 rs1051730 were associated with a lower likelihood of quitting smoking throughout adulthood among women aged <65 years, and these associations remained significant after correction for multiple testing. Though some null findings for either SNP have been reported,23,36–38 the present findings are consistent with several prior studies with much shorter follow-up.15,16,22,25,39,40
The present study also found a robust association, which remained significant after correction for multiple testing, between the minor allele of CHRNA3 rs578776 and increased odds of smoking cessation throughout adulthood. These results were consistent with those previously observed among Han Chinese males.24 However, to the best of our knowledge this is the first study to observe this association with cessation in a population of European ancestry as prior studies reported null associations.25,38 The minor allele was previously shown to be protective against relapse during the first 90-day post-cessation41 and may influence smoking cessation through cognitive-behavioral mechanisms as smokers with the minor allele are less likely to have a heightened intrinsic reward response to visual smoking cues.42 This may partially explain the strong association observed in the present all-female study as women are more likely than men to respond to sensory stimuli of cigarette smoking.43
An association was also observed between the minor allele of CHRNB4 SNP rs12914008 and increased odds of smoking cessation that was no longer statistically significant after correcting for multiple testing. The findings for rs12914008 are consistent with those previously observed in a short-term smoking cessation trial,23 but the present association was weak and could be an artifact of the linkage disequilibrium with rs578776.
Among the dopaminergic SNPs studied, the minor allele of DRD2 rs1800497 was weakly associated with a lower likelihood of smoking cessation throughout adulthood in women aged <65 years. The direction of this association is consistent with previous studies.27,44 The weaker association we observed in the present study could be due to the all-female study population because the minor allele is associated with reductions in D2 dopamine receptor density and binding capacity45 which are more pronounced in males.46
The present study also extends prior research by examining differences in genetic associations by smoking intensity. Smoking intensity is an important tobacco control issue because the proportion of US adult smokers who are light smokers is growing.47 This increase was evident in the present study as the proportion of light smokers increased from 5.7% in NHS to 16.8% in NHS-2. This study’s considerable sample size and long-term follow-up are notable strengths for investigating whether SNP associations with smoking cessation differ by smoking intensity. Consistent heterogeneity in the direction of the associations with smoking cessation by light versus moderate-to-heavy smokers were observed for rs1800497, rs16969968, and rs1051730. The minor alleles of these SNPs were inversely associated with persistent smoking among light smokers but associated with an increased risk of persistent smoking among moderate-to-heavy smokers. Despite the heterogeneity in ORs, inferences are limited because the tests for statistical interaction were not statistically significant, possibly because of the lack of statistical power resulting from the low prevalence of light smokers. Nevertheless, these findings are biologically plausible. The reduced D2 receptor density and binding capacity associated with the minor allele of rs1800497 could lead to decreased positive reinforcement, and thereby protect against persistent light smoking. The rs16969968 and rs1051730 SNPs are associated with nicotine dependence,48–50 and thus could lead to persistent heavier smoking because of the key role of withdrawal avoidance in heavier smoking.
Another strength of the present study was the data to examine genetic associations over time in women aged ≥65 years. Genetic studies of smoking cessation in elderly populations are rare as the mean age of study populations have typically been 37.7–57.3 years.16,25,36 Compared to women aged <65 years, observed associations were weaker and were not statistically significant among women aged ≥65 years, but the point estimates of the ORs were consistent across age strata suggesting the associations are relevant among older women. These results provide insights into smoking cessation in elderly populations, but also highlight challenges to this research area. The lower prevalence of smoking and quit attempts among older women smokers increases the difficulty of detecting genetic associations in this population.
The present investigation is innovative because of the longitudinal analysis of long-term repeated smoking measures to determine if the associations between the 10 selected SNPs and smoking cessation persist throughout adulthood. Nonetheless, some important study limitations are acknowledged. Though the all-female study population was important for identifying genetic factors among women, it was not possible to examine these genetic associations with long-term smoking cessation among men. Furthermore, it was not feasible to evaluate differences in genetic associations by ancestry because there was insufficient genotype data for participants of non-European ancestry. Thus, similar studies of men and of non-European ancestral populations are necessary to understand where associations differ by biological sex and ancestry.
In addition, even though the present study expanded on previous studies by examining associations among light and older smokers, the necessary stratification of the study population to do so may have limited the statistical power to determine genetic factors associated with cessation among these smaller subsets of smokers. Despite the challenges present when conducting research to determine genetic associations among light and older smokers, this study provided evidence that some genetic associations may differ by smoking intensity and that associations observed among younger smokers may be relevant for the elderly. These findings are important because identifying how genetic factors predictive of long-term cessation differ by smoking intensity and their relevance to elderly smokers holds promise for high-precision tailoring of smoking cessation interventions.
Furthermore, this study revealed some of the CHRNA5-A3-B4 and DRD2 SNPs previously associated with short-term smoking cessation were also associated with long-term cessation. The clinical implications of these results include the potential use of genetic markers to identify smokers who may benefit from long-term cessation/relapse management. Improving rates of long-term smoking abstinence has important public health implications. Though quitting smoking has health benefits in the short-term, long-term cessation is necessary to realize significant reductions in smoking-attributable morbidity and mortality. In addition, comparison of the results from this all-female study to findings from studies including both sexes suggests the strength of some associations may differ by biological sex. Understanding genetic differences by biological sex could fine-tune these precision medicine cessations and relapse prevention approaches. Our findings also raise the possibility that genetic associations with smoking cessation may vary by smoking intensity, and if these results are corroborated then smoking intensity may be another factor used to fine-tune precision medicine approaches to smoking cessation.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Stephanie K Jones, Department of Public Health, Baylor University, Waco, TX, USA; Department of Public Health Sciences, Medical University of South Carolina, Charleston, SC, USA.
Anthony J Alberg, Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA.
Kristin Wallace, Department of Public Health Sciences, Medical University of South Carolina, Charleston, SC, USA; Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, USA.
Brett Froeliger, Department of Psychological Sciences, University of Missouri, Columbia, MO, USA.
Matthew J Carpenter, Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, USA; Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
Bethany J Wolf, Department of Public Health Sciences, Medical University of South Carolina, Charleston, SC, USA.
Funding
This study was supported by UM1 CA186107 (NHS infrastructure grant), U01 CA176726 (NHS II Infrastructure grant), TL1 grant (TL1TR001451), Hollings Cancer Center Abney Graduate Fellowship, and NIH/NIAMS P30AR072582 (CCCR Improving Minority Health in Rheumatic Diseases).
Declaration of Interests
The authors declare that there is no conflict of interest. Dr. Brett Froeliger is a consultant for Promentis Pharmaceuticals, Inc. (BF) for work unrelated to the content of the manuscript. Dr. Matthew Carpenter has received consulting honoraria from both Pfizer and Frutarom for work unrelated to the content of the manuscript.
Data Availability
Harvard University and Brigham and Women’s Hospital have set up an external Advisory Committee to review outside requests for Nurses’ Health Study (NHS) and NHS II data in accordance with the 2003 NIH Data Sharing Policy (NOT-OD-03-032), and with the NIH Genomic Data Sharing Policy (NOT-OD-14-124). Dissemination of data used for this manuscript will be shared per the NHS/NHS II Data Availability Policy which states, “Because of participant confidentiality and privacy concerns, we do NOT send out cohort analytic datasets to journals or anyone else where there is uncontrolled access. Programs that generated the results, but without the UNIX paths to datasets, can be shared. To ensure appropriate adherence to human subjects/ethical requirements for sharing the cohort data, individuals wanting to access the data must request access to log onto our Channing computer system. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (contact E-mail: nhsaccess@channing.harvard.edu).”
References
- 1. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia-Rodriguez O, Secades-Villa R, Florez-Salamanca L, Okuda M, Liu SM, Blanco C.et al. Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2013;132(3):479–485. doi:10.1016/j.drugalcdep.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munafò MR, Johnstone EC.. Genes and cigarette smoking. Addiction. 2008;103(6):893–904. [DOI] [PubMed] [Google Scholar]
- 4. Wu J. Understanding of nicotinic acetylcholine receptors. Acta Pharmacol Sin. 2009;30(6):653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trifilieff P, Martinez D.. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacol. 2014;76(Pt B):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DiFranza JR, Wellman RJ.. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: integrating the clinical and basic science literature. Nicotine Tob Res. 2005;7(1):9–26. [DOI] [PubMed] [Google Scholar]
- 7. Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53(4):633–640. [DOI] [PubMed] [Google Scholar]
- 8. De Biasi M, Salas R.. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med. 2008;233(8):917–929. [DOI] [PubMed] [Google Scholar]
- 9. Garland EL, Bryan CJ, Kreighbaum L, Nakamura Y, Howard MO, Froeliger Bet al. Prescription opioid misusing chronic pain patients exhibit dysregulated context-dependent associations: investigating associative learning in addiction with the cue-primed reactivity task. Drug Alcohol Depend. 2018;187:13–21. doi:10.1016/j.drugalcdep.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 10. Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang FA.. Beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiffman S, Paty JA, Kassel JD, et al. Smoking behavior and smoking history of tobacco chippers. Exp Clin Psychopharmacol. 1994;2(2):126–142. [Google Scholar]
- 12. Shiffman S, Terhorst L.. Intermittent and daily smokers’ subjective responses to smoking. Psychopharmacol. 2017;234(19):2911–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkins KA, Karelitz JL.. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carpenter MJ, Saladin ME, Larowe SD, et al. Craving, cue reactivity, and stimulus control among early-stage young smokers: effects of smoking intensity and gender. Nicotine Tob Res. 2014;16(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freathy RM, Ring SM, Shields B, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18(15):2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012;169(7):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones SK. Genetic Variation Within Nicotine Addiction Pathways in Relation to Smoking Cessation and Smoking Relapse Throughout Adulthood: A Longitudinal Study of Female Registered Nurses. Medical University of South Carolina; 2022. https://medica-musc.researchcommons.org/theses/669. Accessed April 22, 2022. [Google Scholar]
- 18. Jones SK, Wolf BJ, Froeliger B, Wallace K, Carpenter MJ, Alberg AJet al. A systematic review of genetic variation within nicotinic acetylcholine receptor genes and cigarette smoking cessation. Drug Alcohol Depend. 2022;239:109596. Epub 2022 Aug 5. PMID: 35981468. doi:10.1016/j.drugalcdep.2022.109596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health. 2016;106(9):1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindstrom S, Loomis S, Turman C, et al. A comprehensive survey of genetic variation in 20,691 subjects from four large cohorts. PLoS One. 2017;12(3):e0173997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen LS, Hung RJ, Baker T, et al. CHRNA5 risk variant predicts delayed smoking cessation and earlier lung cancer diagnosis – a meta-analysis. J Natl Cancer Inst. 2015;107(5):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevens VL, Jacobs EJ, Gapstur SM, et al. Evaluation of a novel difficulty of smoking cessation phenotype based on number of quit attempts. Nicotine Tob Res. 2017;19(4):435–441. [DOI] [PubMed] [Google Scholar]
- 23. Sarginson JE, Killen JD, Lazzeroni LC, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B: Neuropsych Genet. 2011;156b(3):275–84. [DOI] [PubMed] [Google Scholar]
- 24. Wang Q, Li S, Pan L, et al. Association between variants in nicotinic acetylcholine receptor genes and smoking cessation in a Chinese rural population. Am J Addict. 2016;25(4):297–300. [DOI] [PubMed] [Google Scholar]
- 25. Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23(2):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conti DV, Lee W, Li D, et al. ; Pharmacogenetics of Nicotine Addiction and Treatment Consortium. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17(18):2834–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma Y, Wang M, Yuan W, Su K, Li MD.. The significant association of Taq1A genotypes in DRD2/ANKK1 with smoking cessation in a large-scale meta-analysis of Caucasian populations. Transl Psychiatry. 2015;5:e686. doi:10.1038/tp.2015.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lerman C, Jepson C, Wileyto EP, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacol. 2006;31(1):231–242. [DOI] [PubMed] [Google Scholar]
- 29. Omidvar M, Stolk L, Uitterlinden AG, Hofman A, Van Duijn CM, Tiemeier Het al. The effect of catechol-O-methyltransferase Met/Val functional polymorphism on smoking cessation: retrospective and prospective analyses in a cohort study. Pharmacogenet Genomics. 2009;19(1):45–51. doi:10.1097/FPC.0b013e328317f3f8 [DOI] [PubMed] [Google Scholar]
- 30. Husten CG. How should we define light or intermittent smoking? Does it matter? Nicotine Tob Res. 2009;11(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Viron S, Malats N, Van der Heyden J, Van Oyen H, Brand A.. Environmental and genomic factors as well as interventions influencing smoking cessation: a systematic review of reviews and a proposed working model. Public Health Genomics. 2013;16(4):159–173. [DOI] [PubMed] [Google Scholar]
- 32. Perkins KA. Smoking cessation in women: special considerations. CNS Drugs. 2001;15(5):391–411. [DOI] [PubMed] [Google Scholar]
- 33. Messer K, Trinidad DR, Al-Delaimy WK, Pierce JP.. Smoking cessation rates in the United States: a comparison of young adult and older smokers. Am J Public Health. 2008;98(2):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J.. A method for meta-analysis of molecular association studies. Stat Med. 2005;24(9):1291–1306. [DOI] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N.. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen LS, Baker TB, Jorenby D, Piper M, Saccone N, Johnson E, et al. Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation. Drug Alcohol Depend. 2015;154:278–282. doi:10.1016/j.drugalcdep.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bousman CA, Rivard C, Haese JD, Ambrosone C, Hyland A.. Alpha-5 and -3 nicotinic receptor gene variants predict nicotine dependence but not cessation: findings from the COMMIT cohort. Am J Med Genet B: Neuropsych Genet. 2012;159b(2):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tyndale RF, Zhu AZX, George TP, et al. ; PGRN-PNAT Research Group. Lack of associations of CHRNA5-A3-B4 genetic variants with smoking cessation treatment outcomes in Caucasian smokers despite associations with baseline smoking. PLoS One. 2015;10(5):e0128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard Pet al. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res. 2011;13(10):982–988. doi:10.1093/ntr/ntr106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen LS, Baker TB, Piper ME, Smith SS, Gu C, Grucza RA, et al. Interplay of genetic risk (CHRNA5) and environmental risk (partner smoking) on cigarette smoking reduction. Drug Alcohol Depend. 2014;143:36–43. doi:10.1016/j.drugalcdep.2014.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baker TB, Weiss RB, Bolt D, et al. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11(7):785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robinson JD, Versace F, Lam CY, Minnix JA, Engelmann JM, Cui Y, et al. The CHRNA3 rs578776 variant is associated with an intrinsic reward sensitivity deficit in smokers. Front Psychiatry. 2013;4:114. PMID: 24065931; PMCID: PMC3779859. doi: 10.3389/fpsyt.2013.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchinson Set al. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3(2):141–150. doi:10.1080/14622200110043059 [DOI] [PubMed] [Google Scholar]
- 44. Styn MA, Nukui T, Romkes M, Perkins K, Land SR, Weissfeld JL.. The impact of genetic variation in DRD2 and SLC6A3 on smoking cessation in a cohort of participants 1 year after enrollment in a lung cancer screening study. Am J Med Genet B: Neuropsych Genet. 2009;150b(2):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ.. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48(7):648–654. [DOI] [PubMed] [Google Scholar]
- 46. Thompson J, Thomas N, Singleton A, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenet. 1997;7(6):479–484. [DOI] [PubMed] [Google Scholar]
- 47. Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mort Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 48. Berrettini W, Yuan X, Tozzi F, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13(4):368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berrettini WH, Doyle GA.. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Mol Psychiatry. 2012;17(9):856–866. [DOI] [PubMed] [Google Scholar]
- 50. Weiss RB, Baker TB, Cannon DS, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4(7):e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Harvard University and Brigham and Women’s Hospital have set up an external Advisory Committee to review outside requests for Nurses’ Health Study (NHS) and NHS II data in accordance with the 2003 NIH Data Sharing Policy (NOT-OD-03-032), and with the NIH Genomic Data Sharing Policy (NOT-OD-14-124). Dissemination of data used for this manuscript will be shared per the NHS/NHS II Data Availability Policy which states, “Because of participant confidentiality and privacy concerns, we do NOT send out cohort analytic datasets to journals or anyone else where there is uncontrolled access. Programs that generated the results, but without the UNIX paths to datasets, can be shared. To ensure appropriate adherence to human subjects/ethical requirements for sharing the cohort data, individuals wanting to access the data must request access to log onto our Channing computer system. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (contact E-mail: nhsaccess@channing.harvard.edu).”