Abstract
Background
Maintaining adequate nutrition is critical for people with amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND). Enteral tube feeding is offered to people experiencing difficulty swallowing (dysphagia) to prevent weight loss and aspiration pneumonia. Among the types of enteral tube feeding, percutaneous endoscopic gastrostomy (PEG) is the typical procedure offered to people with ALS and will be mainly discussed here.
Objectives
To examine the effectiveness of percutaneous endoscopic gastrostomy or other enteral tube feeding in people with ALS, compared to oral feeds without enteral tube feeding on:
1. survival;
2. nutritional status;
3. quality of life.
To examine the incidence of minor and major complications of percutaneous endoscopic gastrostomy (PEG) and other enteral tube feeding procedures in people with ALS.
Search methods
On 3 January 2020 and 6 February 2021, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE. Embase, ClinicalTrials.gov and WHO ICTRP. We screened the results to identify randomized controlled studies on enteral tube feeding in ALS. We reviewed all references from the search in published articles to identify any additional references.
Selection criteria
We included randomized controlled trials (RCTs), quasi‐RCTs, and cross‐over trials evaluating the effectiveness and complications of PEG or other enteral tube feeding placement in ALS.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We found no RCTs or quasi‐RCTs comparing the effectiveness of enteral tube feeding versus oral feeds without enteral tube feeding.
Authors' conclusions
There are no RCTs or quasi‐RCTs to indicate whether enteral tube feeding is effective compared to continuation of oral feeding for any of the outcome measures. Such RCTs are very unlikely to be performed for ethical reasons. RCTs evaluating the effect of different enteral tube insertion techniques and timings of tube placement on survival and quality of life of people with ALS dysphagia are feasible and warranted.
Keywords: Humans; Amyotrophic Lateral Sclerosis; Amyotrophic Lateral Sclerosis/complications; Amyotrophic Lateral Sclerosis/therapy; Deglutition Disorders; Deglutition Disorders/complications; Deglutition Disorders/therapy; Enteral Nutrition; Enteral Nutrition/methods; Intubation, Gastrointestinal; Motor Neuron Disease; Motor Neuron Disease/complications
Plain language summary
Enteral tube feeding in people with amyotrophic lateral sclerosis, also known as motor neuron disease
Key messages
We found no randomized or quasi‐randomized (partially randomized) controlled trials that compare the use of a feeding tube to continued feeding by mouth for people with amyotrophic lateral sclerosis (ALS), or that investigate the safety and timing of different types or methods of feeding tube placement in people with ALS. ALS is the most common form of motor neuron disease (MND) and the two terms are often used interchangeably.
Randomized and quasi‐randomized trials are studies that aim to ensure that groups of participants are similar. Such studies could inform clinicians and people with ALS on whether insertion of a feeding tube prolongs survival and improves quality of life compared to continued feeding by mouth. However, there are ethical challenges in conducting such a trial, as international expert consensus and guidelines support tube feeding for people with ALS despite the lack of high‐quality evidence.
What is ALS?
ALS is a condition where the nerves that control movement cease to work. ALS causes weakness that worsens over time until the person becomes paralyzed. It is almost always fatal.
Most people with ALS develop swallowing difficulties. These can cause serious weight loss and people affected are at risk of inhaling food or drink, since effective swallowing normally protects the airway from foods and liquids. If food or drink enters the lower airways or lung, these may become inflamed or infected (a condition called aspiration pneumonia).
What is enteral tube feeding?
Enteral tube feeding is feeding through a tube. One of the ways the feeding tube is inserted is through the stomach wall into the stomach. This type of tube feeding is often recommended for people with ALS who develop swallowing difficulties, so they can maintain adequate nutrition and to lower the risk of aspiration pneumonia.
What did we want to do?
We wanted to review the evidence for using a feeding tube compared to not using a feeding tube on survival, nutritional status, and quality of life in people with ALS. We also wanted to collect evidence on complications of feeding tube placement.
What did we do?
We searched for randomized controlled trials that looked at enteral feeding compared with feeding by mouth in people with ALS, or that studied the safety and timing of different types or methods of feeding tube placement in people with ALS. We planned to summarize their results and evaluate the evidence.
What did we find?
We found no randomized controlled trials to indicate whether enteral tube feeding has benefits compared to continuation of feeding by mouth. We summarized the results of non‐randomized studies in the discussion. However, these provide poorer quality evidence and can produce misleading results.
What are the limitations of the evidence?
We found no evidence that met our requirements for inclusion in the review.
How up to date is this review?
This review updates the previous review. The evidence is up to date to February 2021.
Background
Description of the condition
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a fatal, progressive, neurodegenerative disease, characterized by the death of both upper and lower motor neurons (Karitzky 2001). ALS is the most common form of motor neuron disease (MND) and the two terms are often used interchangeably; in this review we use ALS. Its etiology is unknown. The initial symptom of ALS is usually progressive weakness of the arms and legs. However, in one‐third of all patients, bulbar symptoms (speech and voice changes, difficulty chewing and swallowing, and sialorrhea (excessive drooling)) are the presenting complaint (Van Es 2017). Respiratory insufficiency usually presents in advanced disease states and is the primary cause of death in patients with ALS. Aspiration pneumonia, malnutrition, and dehydration are complications that result from difficulty swallowing, and can also be fatal. In patients with ALS, the median survival from symptom onset ranges from 1.98 to 4.32 years (Abdul Aziz 2020; Chhetri 2016; Chiò 2002; Czaplinski 2006; Murphy 2008).
Description of the intervention
Maintaining adequate nutrition is of great importance in ALS, as weight loss, malnutrition, and dehydration may hasten muscle weakness, contribute to respiratory impairment, and shorten life span. Dysphagia (difficulty swallowing) may contribute to weight loss, but poor appetite and reduced ability to feed oneself may also lead to reduced oral intake and subsequent malnutrition, dehydration, or both. When chewing and swallowing difficulties develop, dietary modification is indicated in order to maintain appropriate caloric intake and prevent aspiration. When dysphagia becomes severe and a supplemental or alternative nutritional route is required, enteral tube feeding may provide a safer and more reliable route for nutrition. Methods include nasogastric (NG) tubes, percutaneous endoscopic gastrostomy (PEG), PEG with jejunal extension (PEG‐J), surgical gastrostomy, percutaneous endoscopic jejunostomy (PEJ), and surgical jejunostomy. Except for the NG tube, all other feeding tubes require surgical insertion directly into the patient's stomach or intestine. These procedures are performed either as an outpatient or inpatient procedure in the endoscopy suite, the radiology suite, or the operating room.
Patients with ALS typically undergo PEG under conscious sedation. The stomach is accessed endoscopically and the tube is inserted across the abdominal wall. A recent development is the placement of feeding tubes by a less invasive technique called percutaneous radiological gastrostomy (PRG), which is also known as radiologically inserted gastrostomy (RIG). During PRG/RIG, air is insufflated into the stomach via a temporary NG tube and the percutaneous tube is placed into the stomach under local anesthesia. Per‐oral image‐guided gastrostomy (PIG) is a hybrid enteral tube feeding technique that involves fluoroscopic‐guided stomach access and per‐oral tube insertion.
In general, the overall use of PEG has increased over time. The proportion of people undergoing PEG tube insertion varies geographically and has been reported as follows:
Australia: 19% to 36% (Talman 2016; Zhang 2012);
Canada: 16% to 53% (Hodgkinson 2018; Jackson‐Tarlton 2016; Strong 1999);
France: 49% (Limousin 2010);
Germany: 11% to 27% (Andersen 2018; Silani 1998);
Great Britain: 14% to 38% (Allison 2000; Chhetri 2016; Neudert 2001; Rio 2010);
Italy: 11% to 51% (Boitano 2001; Chiò 2002; Fasano 2017; Fini 2014; Mandrioli 2018; Mazzini 1995; Spataro 2011);
Malaysia: 25.4% (Abdul Aziz 2020);
Mexico: 68.8% (Sánchez‐Martínez 2019);
Japan: 21% to 60% (Seki 2000; Yanagisawa 1996);
Norway: 22% (Bak 1994);
Poland: 16% (Andersen 2018);
Portugal: 7.4% (Braga 2020);
South Africa: 9.4% (Braga 2020);
Spain: 28% (Paipa 2019);
Sweden: 35% to 50% (Andersen 2018; Longinetti 2018);
Switzerland: 65% (Burkhardt 2017);
Taiwan: 3.8% (Lee 2013);
USA: 13% to 40% (Atassi 2011; Bond 2019; Bradley 2004; Lechtzin 2001).
How the intervention might work
After tube placement, food and liquid can be safely delivered enterally, bypassing normal oral entry. While oral feeding is not contraindicated after PEG placement, the majority of the caloric intake and medications can be reliably administered through the tube. This intervention maintains caloric intake, thereby reducing the risk of weight loss and aspiration.
Why it is important to do this review
ALS practice guidelines from the American Academy of Neurology (AAN) were disseminated in 1999 and again in 2009 (Miller 1999; Miller 2009), and reaffirmed three times, in 2014, 2017, and 2020; the European Federation of Neurological Societies (EFNS) task force published in 2005 and revised in 2012 (EFNS 2012); and the Canadian Best Practice Recommendations were recently published in 2020 (Shoesmith 2020). They recommend that PEG be offered to patients with ALS when there is symptomatic dysphagia and accelerated weight loss. In 2004, data gathered from over 1000 ALS patients in the USA who were cared for prior to the publication of the American Practice Parameters in 1999 (Miller 1999), were compared to an equivalent number of patients cared for after the publication (Bradley 2004). Data showed that the proportion of people with ALS receiving PEG had risen after 1999, especially in those with forced vital capacity (FVC) less than 50% of the predicted value. Of those people studied after 1999, 48% of patients met the Practice Parameter criteria for enteral support. PEG was recommended for 46% of patients, but less than half of them (43%) underwent the procedure. It was therefore determined that only 20% of people who met the AAN practice parameter criteria for enteral support, actually received a feeding tube. This finding suggests that a significant proportion of people with ALS and caregivers remain unconvinced of the benefit of gastrostomy. The benefits of PEG in maintaining adequate nutritional intake and weight stabilization have been shown in a number of observational studies, but have not been tested rigorously (Kasarskis 1999; Klor 1999). Improvement in survival time has been reported by some investigators (Mazzini 1995), but there are limited data from controlled studies.
In addition, the optimal timing of PEG tube insertion in the course of ALS progression is of central importance, but there are limited data from clinical studies. The 2009 AAN Practice Parameter stated that there was insufficient evidence to support or refute the specific timing of PEG insertion in people with ALS (Miller 2009). Recently published best practice guidelines in Canada recommended that PEG should be considered in people with ALS who have an increased risk of aspiration despite diet modification, weight loss of more than 5% to 10% of baseline, more than 1‐point BMI reduction, who are underweight (BMI < 18.5), when total daily energy expenditure surpass daily energy intake, or with FVC decline approaching 50%, even without signs of dysphagia (Shoesmith 2020). It is also advised that when the decision to proceed with a feeding tube has been made, it should be inserted within four weeks (Shoesmith 2020). The potential rationale explaining the connection between enteral tube feeding placement and worse nutritional status includes the potential association between weight loss and increased adjusted hazard ratio of death in ALS (Marin 2011), as well as shorter survival in men with low subcutaneous adipose tissue measured by magnetic resonance imaging (MRI) (Lindauer 2013).
Previous versions of the review did not find any RCTs that addressed this topic (Katzberg 2011; Langmore 2006). Therefore, it is important to conduct an update of this review, which was last updated in 2011. This review searched for randomized clinical trials to determine the best available evidence for the effectiveness of enteral tube feeding in patients with ALS.
Objectives
To examine the effectiveness of percutaneous endoscopic gastrostomy or other enteral tube feeding in people with ALS, compared to oral feeds without enteral tube feeding on:
1. survival;
2. nutritional status;
3. quality of life.
To examine the incidence of minor and major complications of percutaneous endoscopic gastrostomy (PEG) and other enteral tube feeding procedures in people with ALS.
Methods
Criteria for considering studies for this review
Types of studies
We planned to select randomized controlled trials (RCTs), quasi‐RCTs, and cross‐over trials, irrespective of whether allocation was concealed (e.g. alternate allocation). We would have included studies reported as full text, those published as abstract only, and unpublished data. There were no language restrictions. Quasi‐RCTs are studies that allocate participants to groups using methods that are partly systematic, for example by alternation, use of a case record number, or date of attendance. Cross‐over trials are studies that allocate each participant to a sequence of interventions, in which participants are randomized initially to intervention A or intervention B, and then ‘cross over’ to intervention B or intervention A, respectively.
Types of participants
We included studies with participants diagnosed with definite, possible, probable, or probable‐laboratory supported ALS, according to the El Escorial (Brooks 1994), or revised El Escorial criteria (Brooks 2000).
Types of interventions
The primary intervention of interest was the placement of enteral tube feeding (NG tube, PEG, PEG‐J, surgical gastrostomy, PEJ, surgical jejunostomy, PRG/RIG, and PIG) at any time during the course of ALS. The comparator was no enteral tube feeding and continued oral intake. People with ALS who received enteral tube feeding could still continue to consume some food or liquid orally. Other studies of interest were those that evaluated the safety and timing of different types or methods of feeding tubes placed in people with ALS, in particular, use of a less invasive radiographic‐guided gastrostomy tube insertion, i.e. PRG/RIG.
Types of outcome measures
The outcomes listed here were not eligibility criteria for this review but were outcomes of interest within included studies.
Primary outcomes
Survival time in months either from the onset of ALS, time of diagnosis, or placement of the feeding tube.
Secondary outcomes
Quantitative index of change in nutritional status, such as weight change, change in body mass index (BMI), or other nutritional markers such as pre‐albumin level or insulin‐like growth factor I (IGF‐I) level. If different measures of nutritional status were used in studies, we would compare if the scores could be normalized.
Self‐perceived quality of life (QOL), rated using an ordinal or interval scale that addressed quality of life issues.
Safety of PEG was also of interest in this review. We looked for frequency of adverse events related to the PEG procedure or use of a feeding tube, as indicated by minor and major complications. Examples of minor complications include transient laryngeal spasm, localized infections, or failure to place PEG due to technical difficulties. Examples of major complications include gastric hemorrhage, death due to respiratory arrest during the procedure, or aspiration pneumonia.
Search methods for identification of studies
Electronic searches
On 3 January 2020 and 6 February 2021, the Cochrane Neuromuscular Information Specialist searched the following sources for this version of the review:
Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web (Issue 2, 2021; Appendix 2);
MEDLINE (1946 to 4 February 2021; Appendix 3);
Embase (1974 to 2021 Week 4; Appendix 4);
ClinicalTrials.gov via https://clinicaltrials.gov/ (until search date; Appendix 1);
WHO International Clinical Trials Registry Portal via https://trialsearch.who.int/ (ICTRP; Appendix 2).
We searched all resources from their inception date and we did not exclude any document because of language, type, publication date or publication status.
Searching other resources
We also checked references in published articles to identify any additional references.
Data collection and analysis
In the absence of RCTs, we identified non‐randomized studies (cohort and case‐control studies) and summarized their results in the Discussion and Appendix 3.
Selection of studies
Four review authors (AS, AA, BR, and EF) screened the search results to identify RCTs to be included in the review and non‐randomized studies that may be worthy of review and discussion. All RCTs, quasi‐RCTs, or cross‐over trials that met the inclusion criteria would have been selected for inclusion. We resolved any disagreements about studies meeting the inclusion criteria between all four review authors (AS, AA, BR, and EF).
In Appendix 4, we document methods for use in future updates if RCTs for inclusion are identified and meta‐analysis is possible.
Data extraction and management
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Assessment of risk of bias in included studies
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Measures of treatment effect
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Unit of analysis issues
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Dealing with missing data
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Assessment of heterogeneity
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Assessment of reporting biases
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Data synthesis
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Subgroup analysis and investigation of heterogeneity
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Sensitivity analysis
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Summary of findings and assessment of the certainty of the evidence
No studies were eligible for this review. We report methods for use in future updates in Appendix 4.
Results
Description of studies
Results of the search
The literature search for the first version of this review identified a total of 242 references, from which the review authors identified no RCTs or quasi‐RCTs. In the Discussion we comment on seven observational, non‐randomized studies comparing ALS participants with and without enteral tube feeding.
The second version of this review, using the same searches, identified 413 references. None of these were RCTs or quasi‐RCTs. In the Discussion, we comment on an additional four controlled studies comparing people with ALS with and without enteral tube feeding.
For this update, we re‐ran the searches and identified a total of 1276 new references, 970 after duplicates were removed (see Figure 1). We found no RCTs or quasi‐RCTs but comment on 12 non‐randomized studies in the Discussion.
1.
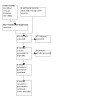
Study flow diagram.
Included studies
No studies fulfilled the search criteria.
Excluded studies
We listed no excluded studies.
Risk of bias in included studies
We did not identify any RCTs.
Effects of interventions
We did not identify any RCTs, quasi‐RCTs, or cross‐over trials evaluating the effectiveness of enteral tube feeding versus continued oral feeds in people with ALS.
Discussion
RCTs or quasi‐RCTs of enteral tube feeding versus continued oral intake would provide high‐quality evidence on survival, quality of life, and other clinically meaningful outcomes in people with ALS. However, conducting these studies poses significant ethical challenges, as multiple international guidelines endorse enteral tube feeding for people with ALS (EFNS 2012; Miller 1999; Miller 2009; Shoesmith 2020). Our updated search did not identify any RCTs or quasi‐RCTs and none were included in the previous versions of the review.
Alternative RCT designs could be considered to address other relevant clinical questions, such as optimal enteral tube feeding timing (i.e. early versus late in the disease course) and the safest technique (e.g. less‐invasive RIG versus traditional PEG). It is possible that enteral tube feeding would only be superior to continued oral intake in improving survival and quality of life in people with bulbar‐onset ALS with relatively preserved limb and respiratory function, rather than those with diffuse disease. Ultimately, to determine optimal modes of enteral tube insertion, single‐blind randomized trials can be performed to assess procedure safety, insertion success rate, complication risk, survival, quality of life, and cost‐effectiveness of the different methods.
In prior versions of this review, authors summarized non‐randomized studies (NRS) assessing survival, nutritional status, quality of life, safety, and procedure timing of enteral tube feeding compared to continued oral intake in people with ALS in the discussion (Katzberg 2011; Langmore 2006). However, they evaluated these studies without a systematic quality and risk of bias appraisal. In this version of the review, the literature search retrieved 12 additional observational studies, and we have updated the summary of non‐randomized studies accordingly. Due to the nature of non‐randomized studies, the certainty of the evidence is poor. These studies are also associated with a high risk of bias, including reporting, selection, confounding, and information bias.
We summarized the results of 23 non‐randomized studies comparing enteral tube feeding to oral feeding in people with ALS in Table 1 and Appendix 3. We identified non‐randomized studies by screening the search results for all prospective and retrospective observational studies that included a control group. Most of these studies utilized PEG.
1. Controlled non‐randomized studies assessing survival in amyotrophic lateral sclerosis (ALS) participants using percutaneous enteral gastrostomy (PEG) versus oral feeding alone.
| Author and date of publication | Number of participants in each group | Survival | Nutrition | Quality of life (QOL) | Study feature | Control for confounders | Funding source | ||
| Direction of effecta | Result | 95% CI/P value | |||||||
| Mazzini 1995 | 31 PEG versus 35 PO | Better with PEG | 38 months versus 30 months | P = 0.03 | Benefit with PEG. 0.5‐point increase in BMI after PEG vs 4.5‐point decrease in non‐PEG group | Only anecdotal benefit from PEG | Prospective cohort | No matching or regression analysis performed | Not stated |
| Chiò 1999 | 50 PEG versus 100 PO | Better with PEG | Protective OR 1.55 | P = 0.02 | Benefit with PEG, observation only | Not reported | Case‐control | Matched on age, FVC, site of onset, severity of disease (Norris Score) | Not stated |
| Strong 1999 | 73 PEG versus 293 PO | Worse with PEG | Bulbar‐onset versus control: 22 months versus 30 months Limb‐onset versus control 24 months versus 35.5 months |
Bulbar P < 0.001; Limb P value not reported |
Not reported | Not reported | Case‐control | No matching or regression performed | Not stated |
| Desport 2000 | 30 PEG versus 30 PO | No effect | Not reported | Not reported | Not reported | Not reported | Case‐control | Multivariate regression used. Confounders not reported | Not stated |
| Chiò 2002 / Chiò 2006 | 52 PEG versus 169 PO | Better with PEG | Protective HR 3.38 | P = 0.0006 | Not reported | Not reported | Prospective cohort | Multivariate regression performed to match on riluzole and NIV | Piemonte and Valle d’Aosta Register for ALS, Regione Piemonte, Ricerca Sanitaria Finalizzata, and Compagnia San Paolo |
| Mitsumoto 2003 | 137 PEG versus 187 PO | Worse with PEG | 47 months versus 58 months | P = 0.33 | Benefit with PEG; observation only | 17% with improved psychological well‐being and 28% with less fatigue and less time spent on meals/medications with PEG | Case‐control | Patients with bulbar scores of five or less selected and adjustment for bulbar subscore performed in analysis | Not stated |
| Forbes 2004 | 142 PEG versus 1084 PO | Little or no effect | 25.0 months versus 24.7 months | P = 0.52 | Not reported | Not reported | Case‐control | No matching or regression performed | Not stated |
| Czaplinski 2006 | 275 PEG versus 766 PO | Better with PEG | HR 0.75 | 0.63 ‐ 0.90; P = 0.003 | Not reported | Not reported | Retrospective cohort | No matching or regression performed | Muscular Dystrophy Association, the Houston Endowment, the Swiss National Science Foundation, and Fonds zur Foerderung des Akademischen Nachwuchses, University of Basel |
| Mitchell 2006 | 127 PEG versus 348 PO | Better with PEG | HR 0.59 | 0.22 ‐ 1.61; P = 0.30 | Not reported | Not reported | Case‐control | Multivariate regression performed controlling for age, sex, onset site, riluzole use | The author accepted speaker's honoraria from Aventis. |
| Sorenson 2007 | 12 PEG versus 28 PO | No effect | Not reported | Not reported | Not reported | Not reported | Case‐control | No matching or regression performed | Not stated |
| Murphy 2008 | 57 PEG versus 187 PO | Little or no effect | 26 months versus 27 months | Not reported | Not reported | Not reported | Prospective cohort | No matching or multivariate regression performed | Not stated |
| Atassi 2011 | 38 PEG versus 262 PO | Worse with PEG | HR for death increased by 36% and 28% using GEE and marginal structural model respectively. | P < 0.001; P = 0.02 | Not reported | Not reported | Post hoc analysis | Marginal structural models were used to account for confounding by indication. | Muscular Dystrophy Association clinical research training grant and NIH training grant |
| Spataro 2011 | 76 PEG versus 74 PO | Better with PEG | 38 months versus 32 months | P = 0.05 | Not reported | Anecdotal advantage for PEG only | Retrospective cohort | No matching or regression analysis performed | Not stated |
| Zhang 2012 | 31 PEG versus 35 PO | Better with PEG | 21.6 months versus 16.8 months | P = 0.089 | Benefit with PEG. Stabilization of weight after PEG (BMI at 3 months (22.6 ± 2.2 kg/m2) and 6 months (22.5 ± 2.0 kg/m2) versus weight at the time of the procedure (22.5 ± 3.0 kg/m2)); people without PEG had ongoing weight loss. | Not reported | Retrospective cohort | No matching or regression analysis performed | Not stated |
| Chhetri 2016 | 91 PEG versus 249 PO | Little or no effect between PEG users and non‐PEG users. Better if PEG inserted after 500 days after onset | PEG inserted 500 days after onset 1046 days versus PEG inserted within 500 days after onset 454 days versus non PEG 715 days | P < 0.001 | Not reported | Not reported | Retrospective cohort | Multivariate regression performed to adjust the effects of sex, onset age, onset site, time from onset to diagnosis, mechanical ventilation, and riluzole treatment | Not stated |
| Burkhardt 2017 | 46 PEG versus 25 PO | Better with PEG | 40.1 months versus 29.8 months; HR for death 0.24 | 0.09 ‐ 0.62; P < 0.01 | No benefit with PEG. Little or no difference in BMI at death and BMI loss during the disease course in people with PEG (BMI 23.8, ‐16.8%) and without PEG (BMI 23.8, ‐17.4%) | Not reported | Retrospective cohort | Multivariate regression performed controlling for diagnostic delay, region of onset, predicted ALSFRS‐R, gender and age at diagnosis, and BMI loss | The authors received honoraria from Biogen Idec, USA and Merz Pharma Schweiz AG. And supported by Swiss ALS Foundation and the EU Joint Programme‐Neurodegenerative Disease Research |
| Fasano 2017 | 152 PEG versus 41 PO | Worse with PEG | 25 months versus 32 months; HR 1.28 | 0.87 ‐ 1.90; P = 0.21 | Not reported | Not reported | Prospective cohort | Cox multivariate regression performed to control for age at onset, ALSFRS‐R score at diagnosis, diagnostic delay, and sex | Not stated |
| Kak 2017 | 36 PEG + 5 PRG/RIG versus 61 PO | Little or no effect | HR 0.85 | P = 0.53 | Not reported | Not reported | Retrospective cohort | Multivariate regression performed to control FVC subgroup | Not stated |
| McDonnell 2017 | 224 PEG versus 257 PO | Worse with PEG | HR 2.1 | P < 0.001 | Not reported | No benefit with PEG. G‐tube worsened the ALS‐Specific Quality of Life (ALSSQOL) inventory average total score by 0.23 points, though CI allow for no effect (95% CI −0.50 to 0.03, P = 0.078) | Post hoc analysis | Structural nested accelerated failure time model was used to adjust treatment and confounder histories. Marginal structural models for QOL | National Institutes of Health (NIH)/National Institute of Neurologic Disorders and Stroke |
| Bond 2019 | 278 PEG versus 649 PO | Better with PEG | 20.8 months versus 14.8 months | P < 0.001 | No weight difference after PEG (P > 0.05) | PEG did not affect clinical impression of mood (CIM) (P > 0.05) | Retrospective cohort | No matching or regression analysis performed | National Institute of Health |
| Abdul Aziz 2020 | 34 PEG versus 25 PO | No effect in entire group. May improve in slow progressors | HR = 0.80 (slow progressors) | 0.76 ‐ 0.90; P < 0.000 | Not reported | Not reported | Prospective cohort | No matching or regression analysis performed for the survival analysis | Malaysian Ministry of Higher Education, ALS Association, and the Sydney Southeast Asia Center. |
| Klavžar 2020 | 42 PEG versus 82 PO | Worse with PEG | HR 2.23 | 1.36 ‐ 3.67; P = 0.002 | Not reported | Not reported | Retrospective cohort | Multiple regression performed to control for age, site of onset, diagnostic delay, riluzole treatment, use of NIV, and interaction between observation time and NIV use | Slovenian Research Agency |
| Vergonjeanne 2020 | 116 PEG versus 66 PO | Worse with PEG | HR 1.25 | 0.88 ‐ 1.79; P = 0.216 | Not reported | Not reported | Prospective cohort | Multivariate regression performed to adjust for site of onset, Norris bulbar score, weight loss, and BMI loss | Association ALAIR |
a Note that this column shows the direction of effect, without consideration of statistical significance. When study authors report no effect without numerical data we entered 'no effect'.
ALSFRS‐R: ALS functional rating score‐revised; BMI: body mass index; FVC: forced vital capacity; GEE: generalized estimating equation; HR: hazard ratio; NIV: non‐invasive ventilation; OR: odds ratio; PEG: percutaneous enteral gastrostomy; PO: per os (oral); PRG/RIG: percutaneous radiologic gastrostomy/radiologically inserted gastrostomy; QOL: quality of life
In summary, these observational studies aimed to determine if enteral tube feeding insertion extends survival in ALS. Two prospective and five retrospective studies reported a longer survival in people with ALS after PEG compared to those without PEG, even when controlling for possible confounders in multivariate analysis. Four prospective, 10 retrospective, and two post hoc analysis studies did not report a survival advantage with PEG. However, some of these studies had multiple design flaws, including not adjusting for survival confounders in ALS. Survival advantage does not appear to be limited to people with either limb or bulbar onset ALS.
Nutrition was not studied as rigorously, with only five of 23 studies reporting relevant nutritional metrics. Overall, results suggested that enteral tube feeding facilitates weight maintenance or increase in people with ALS.
QOL‐associated with gastrostomy is understudied in this population. Only two studies measured QOL using standardized tools, while four other studies addressed QOL narratively or by using non‐standard QOL measures. Most of these studies reported no change in QOL after PEG insertion in people with ALS.
The frequency of minor complications of PEG tube insertion in people with ALS ranged from 1.3% to 60%, with post‐procedural pain and site‐related issues the most common. Major complications were less common, occurring in 2% to 16% of participants. Death within 30 days of PEG placement was the most extreme outcome. People with more advanced disease were more likely to experience major complications. There is no consensus regarding the optimal timing for PEG tube insertion. Studies reported that the procedure can generally be performed safely even in people with respiratory impairment, as NIV can be used during the procedure to support breathing. There is also evidence that suggests PEG insertion as early as possible, while respiratory and nutritional status is considerably better, could be associated with longer survival. Alternative insertion techniques such as PRG/RIG and PIG, appear to be feasible and have similar complication rates to traditional PEG.
The optimal time to undergo PEG placement in the ALS disease course has not been definitely determined from the non‐randomized studies.
Limited evidence from non‐randomized studies suggests that PEG offers a nutritional advantage. However, the evidence is weak and mostly demonstrated by survival surrogates, such as body weight or body mass index. Prospective cohort studies are needed to compare outcomes in patients who have a PEG placed at different levels of nutrition or malnutrition. In addition to weight, future studies should include other nutritional biomarkers to assess nutritional status of patients with ALS receiving PEG.
The effects of PEG placement on patient and caregivers' quality of life need to be evaluated in controlled studies with standardized ALS‐specific QOL instruments. Ultimately, in an almost universally terminal disease like ALS, improvement in QOL is paramount in the assessment and application of an invasive measure such as gastrostomy.
Summary of main results
The review identified no RCTs or quasi‐RCTs for inclusion.
Overall completeness and applicability of evidence
The review identified no RCTs or quasi‐RCTs for inclusion.
Quality of the evidence
The review identified no RCTs or quasi‐RCTs for inclusion.
Potential biases in the review process
This review was conducted using Cochrane methodology. A comprehensive search of multiple registries was done without restriction on language, type, publication date, or publication status. Thus, it was unlikely that trials were overlooked. As this review did not identify RCTs that fulfilled the inclusion criteria, we cannot completely rule out the possibility of publication bias.
Agreements and disagreements with other studies or reviews
We identified two systematic reviews that compared the outcomes of enteral tube insertion via PEG and RIG. Stavroulakis 2013 demonstrated a significantly higher 30‐day mortality rate in people with MND after PEG insertion compared to those who received an enteral tube via RIG. More recently in a larger systematic review, Yuan 2020 showed no difference in the mortality rate between PEG and RIG but noted a higher technical success rate of RIG versus PEG.
Authors' conclusions
Implications for practice.
There is no evidence from randomized controlled trials (RCTs) or quasi‐RCTs to indicate whether enteral tube feeding improves survival, nutritional status, or quality of life in people with amyotrophic lateral sclerosis (ALS) compared to continuation of only oral feeding. Nor is there evidence from RCTs on the optimal timing and safety of enteral tube feeding tube insertion, or on radiologically‐guided versus standard insertion.
Implications for research.
RCTs would provide less biased estimates of survival and quality of life after enteral tube feeding compared to continued oral feeding in people with ALS. However, such RCTs are very unlikely to be performed for ethical reasons, as international expert consensus and guidelines support enteral tube feeding for patients with progressive ALS‐related dysphagia. Therefore, the best evidence will come from well‐conducted non‐randomized studies.
A structured, systematic review of non‐randomized studies on enteral tube feeding in ALS could be performed with careful assessment of the risk of bias to synthesize the evidence qualitatively and quantitively. However, this was not the scope of this review and the non‐RCT data summarized in the discussion was not systematically appraised.
RCTs evaluating the effect of different enteral tube insertion techniques and timings of tube placement on the survival and quality of life of people with ALS dysphagia are ethically feasible and warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 10 August 2023 | New citation required but conclusions have not changed | New authors updated the review. Previous authors Hans Dieter Katzberg and Michael Benatar withdrew. We updated the methods section to current standards. We identified no RCTs. We included new non‐randomized evidence from an updated search in the Discussion. |
| 10 August 2023 | New search has been performed | We updated searches to February 2021. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 4 August 2010 | New citation required but conclusions have not changed | New team of authors. |
| 4 June 2010 | New search has been performed | Searches updated to September 2009. No studies identified for inclusion. Additional studies considered in the Discussion. |
| 9 July 2008 | Amended | Converted to new review format. |
| 1 June 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Ruth Brassington (Managing Editor, Cochrane Neuromuscular) for her guidance throughout this project. Farhad Shokraneh (Information Specialist, Cochrane Neuromuscular) ran searches.
We thank the following peer reviewers:
Brian Dickie, MND Association, UK
Professor David Oliver, University of Kent, UK
Catrin Tudur Smith, University of Liverpool, UK
Dr Shea Morrison MBBS (Hons) FAFRM (RACP) Rehabilitation Physician Medical Director, Medical Rehabilitation Unit, St Joseph's Hospital, Normanby Rd, Auburn NSW 2144, St Vincent's Health Australia, Sydney, Australia.
Appendices
Appendix 1. ClinicalTrials.gov search strategy
Advanced Search
Condition or disease: Motor Neuron Disease OR MND OR Charcot OR Amyotrophic Lateral Sclerosis OR ALS
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Gastrostomy OR Enteral Nutrition OR Enteric Feeding OR PEG OR Tube OR Intubation
8 Studies found
Appendix 2. WHO ICTRP search strategy
We searched this source but the system did not return any results. We suggest the following search strategy for the next updates:
Advanced Search
Motor Neuron Disease OR MND OR Charcot OR Amyotrophic Lateral Sclerosis OR ALS in the Condition
Gastrostomy OR Enteral Nutrition OR Enteric Feeding OR PEG OR Tube OR Intubation in the Intervention
Recruitment status is ALL
Search results were not shown.
Appendix 3. Summary of non‐randomized studies
This appendix reports findings from non‐randomised studies as reported by the study authors. The studies report no consistent effect measure or data. The review authors have not formally evaluated the risk of bias in these studies. The evidence is likely to be of very low certainty and findings may not be reliable.
Survival outcomes of enteral nutrition
Prospective non‐randomised studies
Six prospective studies measured the effect of percutaneous endoscopic gastrostomy (PEG) insertion in amyotrophic lateral sclerosis (ALS) on survival. Two studies showed a survival benefit, while the other four did not.
Two prospective cohorts in a total of 287 participants with ALS suggested that PEG improved survival. In Mazzini 1995, mean survival in 31 participants who underwent PEG insertion was 38 months (standard deviation (SD) 17) compared to 30 months (SD 13) in 35 participants who refused the procedure (P < 0.03). However, multivariate regression was not performed to assess for possible confounders. Chiò and colleagues prospectively followed 221 people with ALS in 26 neurology departments in northwestern Italy (Chiò 2004; Chiò 2006). Multivariate regression analysis using PEG as a time‐dependent variable, reported that the 169 participants not using a PEG tube had a probability of death at any point in time more than three times higher than the 52 participants using PEG (HR 3.38; P = 0.0006).
Four prospective studies in a total of 678 participants with ALS, showed no improvement in survival outcomes with PEG tube placement. Murphy 2008 identified 244 people in North Canterbury, New Zealand diagnosed with ALS between 1985 and 2006. No survival benefit was observed between those who underwent PEG insertion and those who continued oral feeding. Median survival time was 26 months (range 5 months to 88 months) from onset of symptoms in the PEG group, which was similar to the entire cohort (27.6 months) (P value not reported). Fasano 2017 included 193 participants with ALS in Emilia Romagna, Italy, diagnosed between 2009 and 2013, who required supplemental tube feeding. The trial authors reported that overall median survival was 25 months in 152 PEG users and 32 months in 41 participants who continued oral feeding (P = 0.21). Survival was also measured from the time of PEG recommendation. After adjusting for known confounders, median survival was six months in PEG users and two months in non‐PEG users (HR 1.72, 95% confidence interval (CI) 1.15 to 2.57; P = 0.008). Another prospective study included 182 participants with ALS in whom PEG was indicated, but only 116 received the intervention (Vergonjeanne 2020). The result of the multivariate survival analysis was imprecise (HR 1.25, 95% CI 0.88 to 1.79; P = 0.216). Finally, a study by Abdul Aziz 2020 conducted in Malaysia compared 34 PEG users to 25 non‐PEG users and found no difference in survival between the two groups (P = 0.12). However, univariate Cox analysis demonstrated better survival with PEG among people who had a longer time between disease onset and PEG usage (i.e. slower progressors) (HR = 0.80, 95% CI 0.76 to 0.90; P < 0.000 (P value as given)). It also showed that PEG (HR = 0.823, 95% CI 0.755 to 0.897, P < 0.000 (P value as given)) was associated with better survival.
Retrospective studies
Fifteen retrospective studies measured the effect of PEG insertion on survival outcomes in ALS.
Seven retrospective studies reported better survival with PEG (in two studies the difference was not statistically significant). Czaplinski 2006 was an American cohort study of 1041 participants with ALS, followed from 1984 to 2004. In a multivariate model accounting for possible confounders, those who underwent PEG tube insertion showed a significantly improved survival (HR 0.75, CI 0.63 to 0.90; P = 0.003). Another American cohort compared 278 ALS PEG users to 649 ALS non‐PEG users (Bond 2019). Median survival, measured from the first ALS clinic visit until death, was significantly longer in PEG users (20.8 months versus 14.8 months, respectively; P < 0.001), irrespective of the type of ALS onset. Two smaller studies were conducted in Italy. Chiò 1999 studied 50 cases with ALS undergoing PEG and 100 historical controls without PEG. Cases and controls were matched for age, forced vital capacity (FVC), severity of disease, and site of onset. Multivariate analyses showed that PEG was associated with improved survival (odds ratio (OR) 1.55, 95% CI 1.28 to 1.88; P = 0.02). Spataro 2011 studied ALS participants who developed dysphagia and required PEG. Survival was significantly longer in PEG users (median 38 months (interquartile range (IQR) 24 to 56)) compared to non‐PEG users (32 months (IQR 21 to 49)) (P = 0.05). Interestingly, these two Italian studies show conflicting results when survival was analyzed by site of ALS onset. Chiò 1999 found that PEG was associated with an increase in survival in bulbar‐onset PEG users (HR 1.83; P = 0.02) but not in spinal‐onset PEG users. Spataro 2011 found the opposite; the benefit of using PEG was observed in spinal‐onset PEG users (median 44 months, IQR 33 to 58) compared to non‐PEG users (median 36 months, IQR 23 to 54) (P = 0.046), but the study found no benefit of PEG in bulbar‐onset ALS (PEG users: median 28 months, IQR 22 to 45) versus non‐PEG users: median 25 months, IQR 16 to 34; P = 0.14). Burkhardt 2017 compared 46 participants PEG users to 20 non‐PEG users. Median survival was 40.1 months and 29.8 months in the two groups, respectively. However, the difference was not significant (statistical comparison not reported). After adjusting for confounders (gender, age at diagnosis, onset region, diagnostic delay, predicted Revised ALS Functional Rating Scale (ALSFRS‐R), and body mass index (BMI) loss), PEG usage appeared to improve survival (HR 0.24, 95% CI 0.09 to 0.62; P < 0.01).
Mitchell 2006 conducted a retrospective analysis of the effects of riluzole on outcomes in 475 people diagnosed with ALS from 1980 to 2003. Cox regression analysis including PEG tube usage as a variable, found a protective effect of PEG tube use, but the data were imprecise and allowed for effects in either direction (HR 0.59, 95% CI 0.22 to 1.61; P = 0.30). Another study assessed 66 people with ALS who developed dysphagia in a tertiary care center in Australia: 31 of them received PEG and 35 did not (Zhang 2012). The group as a whole who received PEG survived longer than those who did not receive PEG, although the difference was not statistically significant (mean (± SD) 21.6 ± 15.6 months versus 16.8 ± 11 months; P = 0.089). Within this cohort, the subgroup with limb‐onset ALS survived longer with PEG (26.4 ± 20.4 months versus 14.4 ± 10.8 months; P = 0.063), but there was little or no difference with PEG among people with bulbar‐onset ALS (19.2 ± 12 months versus 18.0 ± 10.8 months, P = 0.656).
Eight other retrospective studies, did not demonstrate important effects of PEG on survival or indicted that survival may be worse with PEG.
Two large ALS patient registries, the Scottish Motor Neuron Disease Register (Forbes 2004), and the ALS Patient Care Database (ALS‐CARE) database in the USA (Mitsumoto 2003), evaluated survival with and without PEG across multiple ALS clinics. Unmatched analysis of all 1226 people in the Scottish Motor Neuron Disease Register and 324 people with ALSFRS ‐ Bulbar subscale scores of five or less in the ALS‐CARE database, showed little or no survival difference with PEG tube feeding. In the Scottish register, median survival in PEG users was 2.08 years, IQR 1.44 to 2.99 versus 2.06 years, IQR 1.29 to 3.49 in non‐PEG users (P = 0.52). The ALS‐CARE database reported mean (range) survival of 47 months (8 to 146) for PEG users, 43 months (IQR 13 to 163) for people undergoing later PEG insertion, and 58 months (IQR 6 to 303) for non‐PEG users (P = 0.33 comparing the three groups). Furthermore, PEG did not improve survival in people with bulbar symptoms in either cohort. Kak 2017 evaluated whether gastrostomy tube (G‐tube) insertion was safe and could prolong survival when placed in the later stages of respiratory involvement. Little or no difference in survival was observed between 41 people who had a G‐tube (36 were inserted via PEG and 5 via radiologically inserted gastrostomy (RIG)) and 61 people who received no supplemental feeding tube, regardless of respiratory involvement (HR 0.85; P = 0.53). A medium‐sized Slovenian study demonstrated that enteral tube feeding (42 people with PEG compared to 82 people without PEG) was associated with increased HR for death of 2.23 (95% CI 1.36 to 3.67; P = 0.002), even after controlling for multiple factors (Klavžar 2020). Strong 1999 studied 73 participants with ALS undergoing PEG feeding and 293 people fed orally. The authors found shorter survival with PEG in both subgroups of people with bulbar onset ALS and limb onset ALS, each compared to a control group (bulbar onset median 22 months versus 30 months, P < 0.001; limb onset median 24 months versus 35.5 months, P value not reported). In an English cohort, Chhetri 2016 found little or no difference in survival between 91 PEG users and 249 non‐PEG users. The investigators also performed an analysis by dividing the PEG group into two, according to timing of PEG insertion (less than or more than 500 days from symptom onset), and compared median survival measured in days from symptom onset. They found that survival in 43 people who received PEG more than 500 days after symptom onset was longer (median 1046 days, 95% CI 895.67 to 1196.34, P < 0.001), than survival in 43 people who received PEG at less than 500 days of disease onset (median survival 454 days, 95% CI 396.29 to 511.71) and 249 people who did not undergo PEG (median survival 715 days, 95% CI 625.93 to 804.1). Two additional studies evaluating outcomes in small ALS cohorts (< 60 people) found no difference in survival between those undergoing PEG and those fed orally (Desport 2000; Sorenson 2007).
Post hoc analyses
Two post hoc studies used data from clinical trials originally designed to test the efficacy of drugs in people with ALS (Atassi 2011; McDonnell 2017). Both found that PEG decreased survival. Atassi 2011 performed a post hoc analysis of results from two prospective clinical trials on a total of 38 participants treated with PEG and 262 participants who did not receive PEG. An increase in the hazard ratio (HR) for mortality was observed with PEG. The study analysed results using both a standard statistical approach (generalized estimating equation) and an advanced model that adjusted for confounding by indications (marginal structural model), which revealed increases of 36% (P < 0.001) and 28% (P = 0.02) in the hazard for death, respectively. McDonnell 2017 used data from a placebo‐controlled randomized controlled trial (RCT) assessing the effects of ceftriaxone on survival and functional decline in people with ALS. McDonnell 2017 compared 224 people who received PEG with 257 people who did not. The use of advanced statistical models to adjust for confounding that varies over time (structural nested accelerated failure time models) allowed the study authors to estimate the effects of PEG on survival. PEG decreased survival time by 46% and was associated with HR for death of 2.1 (P < 0.001).
Nutritional outcomes of enteral tube feeding
Seven controlled studies used anthropometric measures, such as weight and body mass index (BMI), to assess nutritional benefit following PEG insertion. These measures were used as the primary indicators and surrogate markers of effectiveness. Weight loss, as a consequence of dysphagia, was the major reason for PEG insertion in these studies.
Nutritional benefit is interpreted differently across studies. Two studies reported stabilization of weight (measured as BMI, BMI loss, or both) before and after PEG insertion as nutritional benefit (Bond 2019; Zhang 2012). Burkhardt 2017 found little to no difference in BMI loss between people who underwent PEG insertion (BMI 23.8, ‐16.8%) with a group who did not (BMI 23.8, ‐17.4%). Zhang 2012 also observed stabilization of weight, with a mean (SD) percentage weight gain of 0.1 ± 6.0 % at three months post‐PEG insertion, whereas weight loss was ongoing in people who did not receive PEG (no CI or P values given for the comparison). Furthermore, weight loss reoccurred at the terminal stage of the disease, despite PEG use.
A prospective cohort study by Mazzini 1995 found that, over a period of 12 months, BMI significantly increased by 0.5 points after PEG but decreased by 4.5 points in the non‐PEG group. Two studies used body weight and BMI as survival predictors (Fasano 2017; McDonnell 2017). Fasano 2017 examined whether two nutritional outcomes led to better survival in people with ALS undergoing PEG. They found that the HR for weight loss of more than 10% from diagnosis to PEG insertion was 2.21 (95% CI 1.25 to 3.92; P = 0.007) and HR for the presence of any weight loss at six months post‐PEG insertion was 2.51 (95% CI 1.09 to 5.81; P = 0.03). With this approach, it is suggested that even with PEG insertion, weight loss is still inevitable in the majority of people with ALS who have dysphagia. One study that used data from the ceftriaxone clinical trial measured BMI from baseline to PEG placement (McDonnell 2017). People with ALS with a BMI loss of 15% or more at the start of PEG usage had shorter median survival compared to those with less BMI loss before PEG insertion (6.1 months versus 9.5 months), but the difference was not significant (P = 0.464). This finding suggests that although not used as an outcome measure, weight loss before PEG is an indicator of disease severity and may also determine the success of PEG usage.
Quality of life outcomes of enteral nutrition
Several quality of life (QOL) questionnaires have been specifically designed for or validated in people with ALS, including the ALS‐specific quality of life (ALSSQOL) instrument (Simmons 2006), the Schedule for the Evaluation of Individual QOL (SEIQoL) (O'Boyle 1994), and the Sickness Impact Profile (SIP) (Bergner 1981). However, only two studies measured QOL quantitatively using validated questionnaires. Mitsumoto 2003 used a shortened form of the SIP questionnaire. In this study, most people with ALS (79%) reported that stabilized nutritional and hydration status was the most positive effect of PEG. Seventeen per cent of PEG users reported improved psychological well‐being with regard to nutrition and 28% listed less fatigue or less time spent consuming meals and medications. McDonnell 2017 looked at the causal effect of PEG insertion on the total score of ALSSQOL, while controlling confounders such as FVC, ALSFRS‐R total, and bulbar scores. In PEG users, the ALSSQOL average total score decreased by 0.23 points (95% CI ‐0.5 to 0.03; P = 0.078).
Other studies discussed quality of life anecdotally without concrete data, or used instruments that are not generally considered a measure of QOL. Mazzini 1995 relayed anecdotal impressions from people with ALS that their quality of life improved after PEG. In contrast, in a study conducted in southern Italy, where eating with other people is an important cultural factor related to sense of well‐being, PEG was seen to eliminate this experience and therefore have a negative impact on quality of life (Spataro 2011). Zhang 2012 used the Revised ALS Functional Rating Scale (ALSFRS‐R) (Cedarbaum 1999), as a measure for quality of life and reported stability of ALSFRS‐R scores after PEG insertion. Another instrument used in two quality of life studies was the clinical impression of mood (CIM) (Oliver 2010), which is a binomial scoring system based on clinic notes denoted by the clinician's perspective to classify a person's mood during the day of visit. Based on the CIM, PEG did not increase or decrease quality of life (Bond 2019; Bond 2020).
Safety and timing of enteral tube feeding insertion
Safety and timing of enteral feeding tube placement (typically PEG) in people with ALS was another outcome of interest in this review. In the PEG studies discussed above, complication rates were usually noted. Minor complications included tube site‐related issues (tube dislodgement, infection, bleeding, and leak occurred in 2.2% to 13%), mild peritonitis (1.3%), pneumoperitoneum (2.4%), hoarseness due to arytenoid edema (2.4%), respiratory distress requiring peri‐procedural ventilatory support (2.4% to 13.3%), bloating (3%), constipation (6.4%), diarrhea (6.4%), vomiting (6.4%), nausea (9.6%), poor appetite (13%), and postoperative pain (9.4% to 60%) (Chiò 1999; Desport 2000; Kak 2017; McDonnell 2017; Sorenson 2007; Spataro 2011; Strong 1999; Zhang 2012). Major complications included gastric hemorrhage (3%) (Mazzini 1995; Zhang 2012), spleen rupture and peritonitis (2%) (Burkhardt 2017), and death within 30 days (4% to 16%) (Desport 2000; Fasano 2017; McDonnell 2017; Sorenson 2007; Strong 1999; Zhang 2012). The majority of people with ALS who developed significant complications of PEG insertion were in a more advanced clinical state (Fasano 2017; McDonnell 2017).
Bond 2019 demonstrated that time elapsed since disease onset or diagnosis is not a significant predictor for PEG placement. The American Academy of Neurology (AAN) Practice parameter also quoted a study which found that the risk of PEG insertion increased when forced vital capacity (FVC) declined to less than 50% in an uncontrolled series of ALS patients (Kasarskis 1999). Chiò 2006 showed that rate of respiratory decline was the most important prognostic factor for longer survival time. Five studies reported that longer survival was associated with higher FVC at the time of PEG (Bond 2019; Chiò 1999: Desport 2000; Mazzini 1995; McDonnell 2017). However, these observations did not take into account that people with higher FVC may have less severe disease at the time of PEG insertion, which would confound the survival analysis. Conversely, other studies reported no association between FVC and survival in ALS patients with PEG (Fasano 2017; Kak 2017; Spataro 2011; Vergonjeanne 2020).
Three studies investigated the safety of the PEG procedure in participants with FVC less than 50%. Two uncontrolled retrospective studies reported on the safety of PEG tube placement in ALS participants with low vital capacity (Boitano 2001; Gregory 2002). Both studies reported few complications when they used non‐invasive ventilation (NIV) to support respiration during PEG placement. In Boitano 2001, five participants with an FVC ranging from 21% to 44% reported no major complications. In Gregory 2002, 29 out of 33 (88%) PEG tubes were successfully placed with NIV support in participants whose FVCs ranged from 7% to 52%. Additionally, a controlled study that included 12 participants with ALS and a low FVC (mean 42.4%), reported that three participants experienced minor complications, but there were no procedure‐related deaths (Kak 2017). This was also true for those with higher FVCs. The only significant difference between the low and high FVC groups was the 6‐month mortality rate (50% versus 17% respectively; P = 0.03).
Method of enteral tube insertion
We found seven studies that specifically compared the safety and outcome of traditional versus radiologically placed gastrostomy (percutaneous radiological gastrostomy (PRG), also known as radiologically inserted gastrostomy (RIG)), in participants with ALS. Comparisons with other insertion techniques were also found and described.
Five studies attempted to control for potential confounders in comparing PEG and PRG/RIG groups by using multivariable regression to evaluate efficacy and hazards. Two studies compared survival between participants who underwent PEG, PRG/RIG, and nasogastric (NG) tube feeding. In a cohort of 98 participants with ALS receiving gastrostomy (PEG = 18; RIG = 72; NG = 8), Shaw 2006 found that median survival was not significantly different in the PEG (7.1 months) versus RIG groups (6.8 months) (P = 0.50). In Rio 2010, there was no significant difference in survival between those who had gastronomy inserted via PEG (median 200 days (IQR 106 to 546)) or RIG (median 216 days (IQR 83 to 383)) (P = 0.902). Blondet 2010 compared PEG and PRG/RIG techniques in 18 and 22 ALS participants, respectively. Multivariate analysis revealed no significant difference in survival (median survival PEG 302 days versus PRG 191 days; P = 0.05). Of note, three participants in the PRG/RIG group had PEG insertions attempted before finally undergoing RIG. Complications experienced by both groups were similar, but more participants in the PRG/RIG group experienced post‐procedural pain (81.8% versus 52.4%; P = 0.05). Desport 2005 assessed survival and complications in 30 participants undergoing PEG tube insertion and 20 participants undergoing RIG tube insertion. Multivariate analysis did not show a significant difference in survival or overall complications between the two groups. Tube blockages/migration occurred less in the RIG group (35% versus 10.4%, P = 0.003); however, the RIG group experienced more post‐procedure pain (10% versus 39.4%, P = 0.003). Similarly, Allen 2013 reported no difference in survival post‐feeding tube insertion between 57 PEG (mean 10.5 (SD 7.5) months) and 51 RIG insertions (mean 8.3 (SD 7.9) months) (P = 0.12), though failure rate was significantly higher in the PEG procedure (15.7% PEG versus 1.9% RIG; P = 0.02).
Two additional studies assessed outcomes based on the method of feeding tube insertion without accounting for potential confounders. Thornton 2002 compared 20 PEG to 16 PRG procedures in participants with ALS and reported no survival difference between the two groups. However, many of the PRG procedures were performed in participants in whom traditional PEG insertion had failed. Thornton 2002 reported one death with each procedure and one case of peritonitis with PEG. In a retrospective case‐control study, Chiò 2004 reported improved survival in 25 PRG users compared to 25 similar but unmatched, PEG users (mean survival after the procedure 204 days versus 85 days; P = 0.004). However, in this cohort, all those treated with PEG underwent the procedure prior to October 2000 when the standard of care and timing of gastrostomy placement in ALS would have been different. The rates of minor and major complications in both groups were similar to previous studies.
One case‐control study compared cases with PRG/RIG to controls without a feeding tube. Without adjusting for confounders, Jiménez 2015 found that the 48 cases who underwent PRG/RIG had shorter mean survival than the 92 controls (32 versus 33.9 months; P = 0.39); the difference was not statistically significant.
Other techniques that have been compared to traditional PEG are per‐oral image‐guided gastrostomy (PIG) and PEG with jejunal extension (PEG‐J). PIG is a hybrid technique of PRG and PEG. Chavada 2010 compared 16 PEG‐users to 19 participants who received per‐oral image‐guided gastrostomy (PIG); four out of the 19 from the PIG group were failed attempts at the PEG procedure. The median survival between the groups was similar (PIG 192 days versus PEG 183 days; P = 0.960), as were the complication rates; PIG infection (n = 1), local tenderness (n = 1), and minor bleeding (n = 1). PEG with jejunal extension (PEG‐J) placement is thought to cause fewer complications related to feeding and aspiration, as nutrition is delivered directly to the small intestines. Kirstein 2018 evaluated the outcome of 43 PEG users and 39 PEG‐J users and reported no difference in overall survival between the groups. (Median overall survival in PEG 14 months versus PEG‐J was not reached, P = 0.392 (written as reported)). However, complication‐free survival, calculated from tube placement until the first complication or death, was shorter in those with PEG‐J (median survival 14 months versus 5 months, P = 0.007). The PEG‐J group also faced more complications than PEG group (36 versus 4, respectively; P = 0.001). The most frequent complication following PEG‐J placement was dislodgement or dysfunction of the tube, or both (65%). Kirstein 2018 concluded that PEG is a more tolerable option due to its lower complications.
To conclude, the traditional PEG procedure displays similar survival and complication outcomes to alternative tube insertion methods (RIG or PIG) in people with ALS. In the case of PEG insertion failure, alternative methods such as RIG or PIG are available. Compared to PEG, post‐procedural pain may be higher with PRG/RIG placement.
Appendix 4. Methods from Cochrane Neuromuscular protocol template
Data extraction and management
We will use a data extraction form for study characteristics and outcome data which has been piloted on at least one study in the review. One review author will extract study characteristics from included studies. We will extract the following study characteristics: study design and setting, characteristics of participants (e.g. disease severity and age), eligibility criteria, intervention details, the outcomes assessed, source(s) of study funding, and any conflicts of interest among investigators.
Two review authors will independently extract outcome data from included studies. We will note in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We will resolve disagreements by consensus or by involving a third author. One review author will transfer data into RevMan Web 2022. A second review author will check the outcome data entries. A second review author will spot‐check study characteristics for accuracy against the study report.
When reports require translation, the translator will extract data directly using a data extraction form, or review authors will extract data from the translation provided. Where possible a review author will check numerical data in the translation against the study report.
Assessment of risk of bias in included studies
Two review authors will independently assess the risk of material bias for each result (as a minimum those included in summary of findings tables) using the Cochrane RoB 2 criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a). We will resolve any disagreements by discussion or by involving another review author. We will assess the risk of bias according to the following domains:
bias arising from the randomization process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
We will grade each potential source of bias as high, low, or some concerns and provide a justification for our judgment. We will report the source(s) of information for each decision using, for example, a quote from the study report. Where information on the risk of bias relates to unpublished data or correspondence with a trialist, we will note this in the risk of bias table. We will summarize the risk of bias judgments across different studies for each of the domains listed. We will summarize the risk of bias for each key outcome (across domains) for each study and address the risk of bias when synthesizing results (see Data synthesis) (Higgins 2021a).
When considering bias due to deviations from intended interventions, we will consider assignment to the intervention.
Conflicts of interest and risk of bias
We will assess whether there is a reason for ‘notable concern’ about conflicts of interest for each study. We will include a table indicating our judgment for each study and provide a rationale for each assessment. We will define a notable concern as described in Boutron 2021, "important conflicts of interest expected to have a potential impact on study design, risk of bias in study results or risk of bias in a synthesis due to missing results".
Assessment of bias in conducting the systematic review
We will conduct the review according to these methods and report any deviations in Differences between protocol and review.
Measures of treatment effect
We will analyze dichotomous data (i.e. PEG usage) as risk ratios (RR) with 95% confidence intervals (CSs). We will analyze continuous data (i.e. survival) as mean differences, or standardized mean differences for results across studies with outcomes that are conceptually the same but measured in different ways. We will report corresponding 95% confidence intervals (CIs). We will enter data presented as a scale with a consistent direction of effect.
Unit of analysis issues
Where multiple arms are reported in a single study, we will include only the treatment arms relevant to the review topic. If two comparisons from a single study (e.g. drug A versus placebo and drug B versus placebo) are combined in the same meta‐analysis, we will follow guidance in Section 23.3.4 in the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting (Higgins 2021b). Our preferred approach will be to combine intervention groups if clinically appropriate.
Dealing with missing data
We will contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is available as an abstract only).
Assessment of heterogeneity
We will use the I² statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). If we identify substantial unexplained heterogeneity we will report it and explore possible causes by prespecified subgroup analysis. We will use the rough guide to interpretation as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions, as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We will avoid the use of absolute cut‐off values, but interpret I²in relation to the size and direction of effects and strength of evidence for heterogeneity (e.g. P value from the Chi‐squared test, or CI for I²) (Deeks 2021).
Assessment of reporting biases
If we are able to pool more than 10 studies, we will create and examine a funnel plot to explore possible small study biases. If searches identify study protocols, clinical trial registrations or abstracts indicating the existence of unpublished studies; we will attempt to determine the status of any unpublished studies by contact with the investigators.
Data synthesis
As a general rule, we will use a random‐effects model in RevMan Web 2022, as this is usually a more conservative approach. Where analyses include both small and large studies, we will perform a sensitivity analysis to determine whether their results are systematically different, since in these circumstances, the use of a random‐effects meta‐analysis will exacerbate the effects of the bias. If they are systematically different, we will report both analyses.
We will incorporate risk of bias assessments in our analyses by presenting all studies and providing a narrative description of the risk of bias.
If meta‐analysis is not possible, we will define contingencies in the analysis plan to group one or more of the research question elements at a broader level.
Summary of findings and assessment of the certainty of the evidence
We will create a summary of findings table using GRADEpro GDT software and present the following outcomes:
survival;
nutritional outcomes;
quality of life;
major and minor complications.
Two review authors will use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to independently assess the certainty of a body of evidence (studies that contribute data for the prespecified outcomes). We will use methods and recommendations described in Chapters 14 and 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021a; Schünemann 2021b). We will resolve any disagreements by discussion or by involving another review author. We will assess study quality according to the GRADE criteria. We will consider RCTs as high‐quality evidence if the five factors above are not present to any serious degree, but may downgrade the quality to moderate, low, or very low. We will downgrade evidence once if a certainty consideration is serious and twice if very serious. We will justify all decisions to downgrade or upgrade the certainty of the evidence using footnotes and make comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We will analyze the following outcomes in subgroup analyses, if the sample sizes are sufficient in one or more studies. These predefined subgroups have been chosen because of their prognostic importance in previous prospective studies:
younger adults (under 60 years) and older adults (> 60 years);
forced vital capacity (FVC) < 50% and FVC ≥ 50%;
bulbar‐onset and limb‐onset;
time from onset of symptoms to placement of the feeding tube (< 3 years and > 3 years duration from onset of first symptom).
We will use the formal test for subgroup interactions in RevMan Web 2022.
Sensitivity analysis
We plan to conduct the following sensitivity analyses:
repeat the analysis excluding any unpublished studies;
repeat the analysis excluding studies at high risk of bias;
repeat the analysis excluding other types of studies.
Reaching conclusions
We will base our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We will avoid making recommendations for practice and our implications for research will suggest priorities for future research and outline what the remaining uncertainties are in the area.
Appendix 5. Cochrane Neuromuscular Specialised Register (CRS Web) search strategy
1 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND INREGISTER 342
2 "motor neuron disease*" or "motor neurone disease*" AND INREGISTER 162
3 "motoneuron disease*" or "motoneurone disease*" AND INREGISTER 4
4 "motorneuron disease*" or "motorneurone disease*" AND INREGISTER 2
5 "charcot disease" AND INREGISTER 1
6 "amyotrophic lateral sclerosis" AND INREGISTER 582
7 als:ti or als:ab or mnd:ti or mnd:ab AND INREGISTER 537
8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 717
9 gastrostomy AND INREGISTER 16
10 "enteral nutrition" or "enteric feeding" AND INREGISTER 20
11 peg or "feeding tube" or "nasogastric tube" or "digestive tract intubation" AND INREGISTER 57
12 gastrointestinal NEAR2 intubation AND INREGISTER 5
13 #9 OR #10 OR #11 OR #12 75
14 #8 AND #13 18
Appendix 6. CENTRAL search strategy
1 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND CENTRAL:TARGET 713
2 "motor neuron disease*" or "motor neurone disease*" AND CENTRAL:TARGET 445
3 "motoneuron disease*" or "motoneurone disease*" AND CENTRAL:TARGET 6
4 "motorneuron disease*" or "motorneurone disease*" AND CENTRAL:TARGET 3
5 "charcot disease" AND CENTRAL:TARGET 2
6 "amyotrophic lateral sclerosis" AND CENTRAL:TARGET 1355
7 als:ti or als:ab or mnd:ti or mnd:ab AND CENTRAL:TARGET 1800
8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 2290
9 gastrostomy AND CENTRAL:TARGET 672
10 "enteral nutrition" or "enteric feeding" AND CENTRAL:TARGET 5714
11 peg or "feeding tube" or "nasogastric tube" or "digestive tract intubation" AND CENTRAL:TARGET 6789
12 gastrointestinal NEAR2 intubation AND CENTRAL:TARGET 755
13 #9 OR #10 OR #11 OR #12 12610
14 #8 AND #13 62
Appendix 7. Embase (Ovid SP) search strategy
Database: Embase <1974 to 2021 Week 04>
1 motor neuron disease/ or amyotrophic lateral sclerosis/ (46725)
2 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (14849)
3 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (238)
4 charcot disease.tw. (34)
5 amyotrophic lateral sclerosis.tw. (32377)
6 or/1‐5 (52099)
7 gastrostomy/ (11520)
8 percutaneous endoscopic gastrostomy/ (6255)
9 enteric feeding/ (33431)
10 nasogastric tube/ (11443)
11 digestive tract intubation/ (3128)
12 (gastrostomy or enteral nutrition or enteral feeding or enteric feeding or enteral nutrition or enteral feeding or enteric feeding or nasogastric tube or gastrointestinal intubation or digestive tract intubation or peg).tw. (97587)
13 or/7‐12 (127153)
14 6 and 13 (1107)
15 limit 14 to (conference abstracts or embase) (1032)
16 limit 15 to dc=20200103‐20210204 (111)
Appendix 8. MEDLINE (Ovid SP) search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to February 04, 2021>
1 exp Motor Neuron Disease/ (28647)
2 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (9450)
3 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (232)
4 charcot disease.tw. (28)
5 Amyotrophic Lateral Sclerosis.mp. (28844)
6 or/1‐5 (39406)
7 Gastrostomy/ (8198)
8 Enteral Nutrition/ (20033)
9 Intubation, Gastrointestinal/ (9776)
10 (gastrostomy or enteral nutrition or enteral feeding or enteric feeding or peg or feeding tube or nasogastric tube or digestive tract intubation).tw. (68189)
11 or/7‐10 (85841)
12 6 and 11 (479)
13 limit 12 to ed=20200103‐20210204 (23)
14 limit 12 to dt=20200103‐20210204 (42)
15 13 or 14 (56)
Differences between protocol and review
In the 2010 update, the review authors revised the Methods to incorporate updated risk of bias methodology, and partially rewrote the Background section.
For this update we added Appendix 4, which documents methods to be used if RCTs are identified and meta‐analysis becomes possible. The text was developed from a standard template provided by Cochrane Neuromuscular. Changes to the original protocol by Langmore 2003, include the following;
inclusion of cross‐over RCTs;
the number of types of feeding tubes eligible for inclusion has increased;
the number of databases that were searched has increased to include several not mentioned in the original review;
the authors specified that reporting of outcomes was not a selection criteria;
the original model for meta‐analysis was given as fixed‐effect.
Contributions of authors
AS, AA, BR, and EF performed screening of title and abstracts. AS wrote the first draft of the current update, while all other authors reviewed and edited the manuscript. All authors agreed on the final text.
Sources of support
Internal sources
-
Student Stipend, Canada
AS worked on the review as part of her master's degree.
External sources
-
National Institute for Health Research and Motor Neurone Disease Association, UK
This project was supported by the National Institute for Health and Care Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the Motor Neurone Disease Association and the Queen Square Centre for Neuromuscular Diseases.
Declarations of interest
AS: none known.
AA: none known. AA is a specialist in neurology and manages patients with neuromuscular disorders
EF: none known.
BR: none known. BR is a specialist in Physical Medicine and Rehabilitation and manages patients with neurological disorders, including motor neuron disease.
LZ: declares consultancy fees from Amylyx, Biogen Idec, and Mitsubishi Tanabe Pharma America, Inc.
These authors should be considered joint first author
New search for studies and content updated (no change to conclusions)
References
Additional references
Abdul Aziz 2020
- Abdul Aziz NA, Toh TH, Goh KJ, Loh EC, Capelle DP, Abdul Latif L, et al. Natural history and clinical features of ALS in Malaysia. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2020;22(1-2):108-16. [DOI] [PubMed] [Google Scholar]
Allen 2013
- Allen JA, Chen R, Ajroud-Driss S, Sufit RL, Heller S, Siddique T, et al. Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: A retrospective study of complications and outcome. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2013;14(4):308-14. [DOI] [PubMed] [Google Scholar]
Allison 2000
- Allison SP, Rawlings J, Field J, Bean N, Stephen AD. Nutrition in the elderly hospital patient - Nottingham studies. Journal of Nutrition, Health & Aging 2000;4(1):54-7. [PMID: ] [PubMed] [Google Scholar]
Andersen 2018
- Andersen PM, Kuzma-Kozakiewicz M, Keller J, Aho-Oezhan HEA, Ciecwierska K, Szejko N, et al. Therapeutic decisions in ALS patients: cross-cultural differences and clinical implications. Journal of Neurology 2018;265(7):1600-6. [DOI] [PubMed] [Google Scholar]
Atassi 2011
- Atassi N, Cudkowicz ME, Schoenfeld DA. Advanced statistical methods to study the effects of gastric tube and non-invasive ventilation on functional decline and survival in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2011;12(4):272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bak 1994
- Bak S, Bak L. Symptomatic treatment of amyotrophic lateral sclerosis [Symptomatisk behandling af amyotrofisk lateralsklerose]. Ugeskrift for Laeger 1994;156(28):4138-40. [PMID: ] [PubMed] [Google Scholar]
Bergner 1981
- Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Medical Care 1981;19(8):787-805. [DOI] [PubMed] [Google Scholar]
Blondet 2010
- Blondet A, Lebigot J, Nicolas G, Boursier J, Person B, Laccoureye L, et al. Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. Journal of Vascular and Interventional Radiology 2010;21(4):527-33. [DOI] [PubMed] [Google Scholar]
Boitano 2001
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001;56(3):413-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bond 2019
- Bond L, Ganguly P, Khamankar N, Mallet N, Bowen G, Green B, et al. A comprehensive examination of percutaneous endoscopic gastrostomy and its association with amyotrophic lateral sclerosis patient outcomes. Brain Sciences 2019;9(9):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 2020
- Bond L, Bowen G, Mertens B, Denson K, Jordan K, Vidakovic B, et al. Associations of patient mood, modulators of quality of life, and pharmaceuticals with amyotrophic lateral sclerosis survival duration. Behavioral Sciences 2020;10(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2021
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Bradley 2004
- Bradley WG, Anderson F, Gowda N, Miller RG, ALS CARE Study Group. Changes in the management of ALS since the publication of the AAN ALS Practice Parameter 1999. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2004;5(4):240-4. [PMID: ] [PubMed] [Google Scholar]
Braga 2020
- Braga AC, Gromicho M, Pinto S, Carvalho M, Henning F. A comparative study of South African and Portuguese amyotrophic lateral sclerosis cohorts. Journal of the Neurological Sciences 2020;414:116857. [DOI] [PubMed] [Google Scholar]
Brooks 1994
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial 'Clinical limits of ALS' Workshop Contributors. Journal of the Neurological Sciences 1994 Suppl;124:96-107. [DOI] [PubMed] [Google Scholar]
Brooks 2000
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Disease. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2000;1:293-9. [DOI] [PubMed] [Google Scholar]
Burkhardt 2017
- Burkhardt C, Neuwirth C, Sommacal A, Andersen PM, Weber M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLOS ONE 2017;12(5):e0177555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cedarbaum 1999
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of the Neurological Sciences 1999;169(1):13-21. [DOI] [PubMed] [Google Scholar]
Chavada 2010
- Chavada G, El-Nayal A, Lee F, Webber SJ, McAlindon M, Walsh T, et al. Evaluation of two different methods for per-oral gastrostomy tube placement in patients with motor neuron disease (MND): PIG versus PEG procedures. Amyotrophic Lateral Sclerosis 2010;11(6):531-6. [DOI] [PubMed] [Google Scholar]
Chhetri 2016
- Chhetri SK, Bradley BF, Majeed T, Lea RW. Motor neurone disease in Lancashire and South Cumbria in North West England and an 8-year experience with enteral nutrition. Journal of Clinical Neuroscience 2016;24:47-51. [DOI] [PubMed] [Google Scholar]
Chiò 1999
- Chiò A, Finocchiaro E, Meineri P, Bottacchi E, Schiffer D. Safety and factors related to survival after percutaneous endoscopic gastrostomy in ALS. ALS Percutaneous Endoscopic Gastrostomy Study Group. Neurology 1999;53(5):1123-5. [DOI] [PubMed] [Google Scholar]
Chiò 2002
- Chiò A, Mora G, Leone M, Mazzini L, Cocito D, Giordana MT, et al. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology 2002;59(1):99-103. [DOI] [PubMed] [Google Scholar]
Chiò 2004
- Chiò A, Galletti R, Finocchiaro C, Righi D, Ruffino MA, Calvo A, et al. Percutaneous radiological gastrostomy: a safe and effective method of nutritional tube placement in advanced ALS. Journal of Neurology, Neurosurgery and Psychiatry 2004;75(4):645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiò 2006
- Chiò A, Bottacchi E, Buffa C, Mutani R, Mora G, PARALS. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. Journal of Neurology, Neurosurgery and Psychiatry 2006;77(8):948-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czaplinski 2006
- Czaplinski A, Yen AA, Simpson EP, Appel SH. Slower disease progression and prolonged survival in contemporary patients with amyotrophic lateral sclerosis: is the natural history of amyotrophic lateral sclerosis changing? Archives of Neurology 2006;63(8):1139-43. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Desport 2000
- Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2000;1(2):91-6. [DOI] [PubMed] [Google Scholar]
Desport 2005
- Desport JC, Mabrouk T, Bouillet P, Perna A, Preux PM, Couratier P. Complications and survival following radiologically and endoscopically-guided gastrostomy in patients with amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2005;6(2):88-93. [DOI] [PubMed] [Google Scholar]
EFNS 2012
- The EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis: Andersen PM, Abrahams S, Borasio GD, Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS) – revised report of an EFNS task force. European Journal of Neurology 2012;19:360-75. [DOI] [PubMed] [Google Scholar]
Fasano 2017
- Fasano A, Fini N, Ferraro D, Ferri L, Vinceti M, Errals, et al. Percutaneous endoscopic gastrostomy, body weight loss and survival in amyotrophic lateral sclerosis: a population-based registry study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2017;18(3-4):233-42. [DOI] [PubMed] [Google Scholar]
Fini 2014
- Fini N, Georgoulopoulou E, Vinceti M, Monelli M, Pinelli G, Vacondio P, et al. Noninvasive and invasive ventilation and enteral nutrition for ALS in Italy: management of ALS in Italy. Muscle & Nerve 2014;50(4):508-16. [DOI] [PubMed] [Google Scholar]
Forbes 2004
- Forbes RB, Colville S, Swingler RJ, Scottish Motor Neurone Disease Research Group. Frequency, timing and outcome of gastrostomy tubes for amyotrophic lateral sclerosis/motor neurone disease - a record linkage study from the Scottish Motor Neurone Disease Register. Journal of Neurology 2004;251(7):813-7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 August 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gregory 2002
- Gregory S, Siderowf A, Golaszewski AL, McCluskey L. Gastrostomy insertion in ALS patients with low vital capacity: respiratory support and survival. Neurology 2002;58(3):485-7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021a
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021b
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hodgkinson 2018
- Hodgkinson VL, Lounsberry J, Mirian A, Genge A, Benstead T, Briemberg H, et al. Provincial Differences in the Diagnosis and Care of Amyotrophic Lateral Sclerosis. Canadian Journal of Neurological Sciences/Journal Canadien des Sciences Neurologiques 2018;45(6):652-9. [DOI] [PubMed] [Google Scholar]
Jackson‐Tarlton 2016
- Jackson-Tarlton CS, Benstead TJ, Doucette S, CNDR INVESTIGATOR NETWORK. Correlating factors in the recommendation of feeding tubes in the nutritional management of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2016;17(7-8):515-21. [DOI] [PubMed] [Google Scholar]
Jiménez 2015
- Jiménez García I, Sala Moya N, Riera Munt M, Herrera Rodríguez MV, Povedano Panadés M, Virgili Casas MN. The Patient's Opinion Matters: Exeprience in the Nutritional Care in ALS Multidisciplinary Team [La opinión del paciente cuenta: Experiencia en la atención nutricional en un equipo multidisciplinar de ELA]. Nutrición Hospitalaria 2015;31 Suppl 5:56-66. [DOI] [PubMed] [Google Scholar]
Kak 2017
- Kak M, Issa NP, Roos RP, Sweitzer BJ, Gottlieb O, Guralnick A, et al. Gastrostomy tube placement is safe in advanced amyotrophic lateral sclerosis. Neurological Research 2017;39(1):16-22. [DOI] [PubMed] [Google Scholar]
Karitzky 2001
- Karitzky J, Ludolph AC. Imaging and neurochemical markers for diagnosis and disease progression in ALS. Journal of the Neurological Sciences 2001;191(1-2):35-41. [DOI] [PubMed] [Google Scholar]
Kasarskis 1999
- Kasarskis EJ, Scarlata D, Hill R, Fuller C, Stambler N, Cedarbaum JM. A retrospective study of percutaneous endoscopic gastrostomy in ALS patients during the BDNF and CNTF trials. Journal of the Neurological Sciences 1999;169(1-2):118-25. [DOI] [PubMed] [Google Scholar]
Kirstein 2018
- Kirstein MM, Körner S, Schneider A, Manns MP, Petri S, Voigtländer T. Percutaneous endoscopic gastrostomy with and without jejunal extension in patients with amyotrophic lateral sclerosis. European Journal of Gastroenterology & Hepatology 2018;30(3):257-62. [DOI] [PubMed] [Google Scholar]
Klavžar 2020
- Klavžar P, Koritnik B, Leonardis L, Dolenc Grošelj L, Kirbiš M, et al. Improvements in the multidisciplinary care are beneficial for survival in amyotrophic lateral sclerosis (ALS): experience from a tertiary ALS center. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2020;21(3-4):203-8. [DOI] [PubMed] [Google Scholar]
Klor 1999
- Klor BM, Milianti FJ. Rehabilitation of neurogenic dysphagia with percutaneous endoscopic gastrostomy. Dysphagia 1999;14(3):162-4. [DOI] [PubMed] [Google Scholar]
Lechtzin 2001
- Lechtzin N, Wiener CM, Clawson L, Chaudhry V, Diette GB. Hospitalization in amyotrophic lateral sclerosis: causes, costs and outcomes. Neurology 2001;56(6):753-7. [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee CTC, Chiu YW, Wang KC, Hwang CS, Lin KH, Lee IT, et al. Riluzole and prognostic factors in amyotrophic lateral sclerosis long-term and short-term survival: a population-based study of 1149 cases in Taiwan. Journal of Epidemiology 2013;23(1):35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Limousin 2010
- Limousin N, Blasco H, Corcia P, Gordon PH, De Toffol B, Andres C, et al. Malnutrition at the time of diagnosis is associated with a shorter disease duration in ALS. Journal of the Neurological Sciences 2010;297(1-2):36-9. [DOI] [PubMed] [Google Scholar]
Lindauer 2013
- Lindauer E, Dupuis L, Muller HP, Neumann H, Ludolph AC, Kassubek J. Adipose Tissue Distribution Predicts Survival in Amyotrophic Lateral Sclerosis. PLoS One 2013;8(6):e67783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longinetti 2018
- Longinetti E, Regodón Wallin A, Samuelsson K, Press R, Zachau A, Ronnevi LO, et al. The Swedish motor neuron disease quality registry. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2018;19(7-8):528-37. [DOI] [PubMed] [Google Scholar]
Mandrioli 2018
- Mandrioli J, Malerba SA, Beghi E, Fini N, Fasano A, Zucchi E, et al. Riluzole and other prognostic factors in ALS: a population-based registry study in Italy. Journal of Neurology 2018;265(4):817-27. [DOI] [PubMed] [Google Scholar]
Marin 2011
- Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, Preux PM, Couratier P. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. Journal of Neurology, Neurosurgery and Psychiatry 2011;82(6):628-34. [DOI] [PubMed] [Google Scholar]
Mazzini 1995
- Mazzini L, Corra T, Zaccala M, Mora G, Del Piano M, Galante M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. Journal of Neurology 1995;242(10):695-8. [DOI] [PubMed] [Google Scholar]
McDonnell 2017
- McDonnell E, Schoenfeld D, Paganoni S, Atassi N. Causal inference methods to study gastric tube use in amyotrophic lateral sclerosis. Neurology 2017-10-03;89(14):1483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1999
- Miller RG, Rosenberg JA, Gelinas DF, Mitsumoto H, Newman D, Sufit R, et al. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology: ALS Practice Parameters Task Force. Neurology 1999;52(7):1311-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2009
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice Parameter update: The care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73(15):1218-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2006
- Mitchell JD, O'Brien MR, Joshi M. Audit of outcomes in motor neuron disease (MND) patients treated with riluzole. Amyotrophic Lateral Sclerosis 2006;7(2):67-71. [DOI] [PubMed] [Google Scholar]
Mitsumoto 2003
- Mitsumoto H, Davidson M, Moore D, Gad N, Brandis M, Ringel S, et al. Percutaneous endoscopic gastrostomy (PEG) in patients with ALS and bulbar dysfunction. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2003;4(3):177-85. [DOI] [PubMed] [Google Scholar]
Murphy 2008
- Murphy M, Quinn S, Young J, Parkin P, Taylor B. Increasing incidence of ALS in Canterbury, New Zealand: a 22-year study. Neurology 2008;71(23):1889-95. [DOI] [PubMed] [Google Scholar]
Neudert 2001
- Neudert C, Oliver D, Wasner M, Borasio GD. The course of the terminal phase in patients with amyotrophic lateral sclerosis. Journal of Neurology 2001;248(7):612-6. [DOI] [PubMed] [Google Scholar]
O'Boyle 1994
- O'Boyle CA. The Schedule for the Evaluation of Individual Quality of Life (SEIQoL): the concept of quality of life in clinical research. International Journal of Mental Health 1994;23(3):3-23. [Google Scholar]
Oliver 2010
- Oliver DJ, Turner MR. Some difficult decisions in ALS/MND. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 2010;11(4):339-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paipa 2019
- Paipa AJ, Povedano M, Barcelo A, Domínguez R, Saez M, Turon J, et al. Survival benefit of multidisciplinary care in amyotrophic lateral sclerosis in Spain: association with noninvasive mechanical ventilation. Journal of Multidisciplinary Healthcare 2019;12:465-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rio 2010
- Rio A, Ellis C, Shaw C, Willey E, Ampong M-A, Wijesekera L, et al. Nutritional factors associated with survival following enteral tube feeding in patients with motor neurone disease: complications following gastrostomy insertion. Journal of Human Nutrition and Dietetics 2010;23(4):408-15. [DOI] [PubMed] [Google Scholar]
Schünemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Schünemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Seki 2000
- Seki H, Kameya T, Kimura I. A survey of current nutrition therapy for the ALS patients in Japanese national sanatoriums. Rinsho Shinkeigaku - Clinical Neurology 2000;40(11):1083-9. [PubMed] [Google Scholar]
Shaw 2006
- Shaw AS, Ampong MA, Rio A, Al-Chalabi A, Sellars ME, Ellis C, et al. Survival of patients with ALS following institution of enteral feeding is related to pre‐procedure oximetry: a retrospective review of 98 patients in a single centre. Amyotrophic Lateral Sclerosis 2006;7(1):16-21. [DOI] [PubMed] [Google Scholar]
Shoesmith 2020
- Shoesmith C, Abrahao A, Benstead T, Chum M, Dupre N, Izenberg A, et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. Canadian Medical Association Journal 2020;192(46):E1453-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silani 1998
- Silani V, Kasarskis EJ, Yanagisawa N. Nutritional management in amyotrophic lateral sclerosis: a worldwide perspective. Journal of Neurology 1998;245(Suppl 2):S13-9. [DOI] [PubMed] [Google Scholar]
Simmons 2006
- Simmons Z, Felgoise SH, Bremer BA, Walsh SM, Hufford DJ, Bromberg MB, et al. The ALSSQOL: balancing physical and nonphysical factors in assessing quality of life in ALS. Neurology 2006;67(9):1659-64. [DOI] [PubMed] [Google Scholar]
Sorenson 2007
- Sorenson EJ, Crum B, Stevens JC. Incidence of aspiration pneumonia in ALS in Olmsted County, MN. Amyotrophic Lateral Sclerosis 2007;8(2):87-9. [DOI] [PubMed] [Google Scholar]
Spataro 2011
- Spataro R, Ficano L, Piccoli F, La Bella V. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: Effect on survival. Journal of the Neurological Sciences 2011;304(1-2):44-8. [DOI] [PubMed] [Google Scholar]
Stavroulakis 2013
- Stavroulakis T, Walsh T, Shaw PJ, McDermott CJ. Gastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practice. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2013;14(2):96-104. [DOI] [PubMed] [Google Scholar]
Strong 1999
- Strong MJ, Rowe A, Rankin RN. Percutaneous gastrojejunostomy in amyotrophic lateral sclerosis. Journal of the Neurological Sciences 1999;169(1-2):128-32. [DOI] [PubMed] [Google Scholar]
Sánchez‐Martínez 2019
- Sánchez-Martínez CM, Choreño-Parra JA, Nuñez-Orozco L, Placencia-Álvarez N, Alvis-Castaño LM, Guadarrama-Ortiz P. A retrospective study of the clinical phenotype and predictors of survival in non-Caucasian Hispanic patients with amyotrophic lateral sclerosis. BMC Neurology 2019;19(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talman 2016
- Talman P, Duong T, Vucic S, Mathers S, Venkatesh S, Henderson R, et al. Identification and outcomes of clinical phenotypes in amyotrophic lateral sclerosis/motor neuron disease: Australian National Motor Neuron Disease observational cohort. BMJ Open 2016;6(9):e012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornton 2002
- Thornton FJ, Fotheringham T, Alexander M, Hardiman O, McGrath FP, Lee MJ. Amyotrophic lateral sclerosis: enteral nutrition provision - endoscopic or radiologic gastrostomy? Radiology 2002;224(3):713-7. [DOI] [PubMed] [Google Scholar]
Van Es 2017
- Van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, et al. Amyotrophic Lateral Sclerosis. Lancet 2017;390(10107):2084-98. [DOI] [PubMed] [Google Scholar]
Vergonjeanne 2020
- Vergonjeanne M, Fayemendy P, Marin B, Penoty M, Lautrette G, Sourisseau H, et al. Predictive factors for gastrostomy at time of diagnosis and impact on survival in patients with amyotrophic lateral sclerosis. Clinical Nutrition 2020;39(10):3112-8. [DOI] [PubMed] [Google Scholar]
Yanagisawa 1996
- Yanagisawa N, Shindo M. Nationwide study on the natural course of amyotrophic lateral sclerosis in Japan. Annual report of the Research Committee of CNS Degenerative Diseases, Ministry of Health and Welfare in Japan for the year 1995. Matsumoto 1996:253-5.
Yuan 2020
- Yuan TW, He Y, Wang SB, Kong P, Cao J. Technical success rate and safety of radiologically inserted gastrostomy versus percutaneous endoscopic gastrostomy in motor neuron disease patients undergoing: a systematic review and meta-analysis. Journal of the Neurological Sciences 2020;410:116622. [DOI] [PubMed] [Google Scholar]
Zhang 2012
- Zhang L, Sanders L, Fraser RJ. Nutritional support teams increase percutaneous endoscopic gastrostomy uptake in motor neuron disease. World Journal of Gastroenterology : WJG 2012;18(44):6461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Katzberg 2011
- Katzberg HD, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD004030. [DOI: 10.1002/14651858.CD004030.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langmore 2003
- Langmore S, Kasarskis E, Manca ML, Olney R. Enteral feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD004030. [DOI: 10.1002/14651858.CD004030] [DOI] [PubMed] [Google Scholar]
Langmore 2006
- Langmore SE, Kasarskis EJ, Manca ML, Olney RK. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD004030. [DOI: 10.1002/14651858.CD004030.pub2] [DOI] [PubMed] [Google Scholar]


