Abstract
Background
Cardiovascular (CV) diseases are the main cause of death in maintenance hemodialysis (MHD) patients. Muscle wasting and physical function decline are common in MHD patients, and significantly impair their quality of life. These can result from abnormalities in cardiac function, which can be further worsened by physical deconditioning. Left ventricular diastolic function parameters were recently shown to be a better predictor of exercise capacity than systolic measures in patients with CV complications. But little is known about the relationship between cardiac function and physical function in MHD patients.
Methods
In 82 MHD patients, left ventricular systolic dysfunction (LVSD) was assessed by ejection fraction and fractional shortening with echocardiography, and left ventricular diastolic dysfunction (LVDD) was assessed by pulse wave and tissue Doppler indices. Physical function was assessed by gait speed, performance on a shuttle walk test, and leg muscle strength. Dual-emission X-ray absorptiometry (DXA) was used to measure whole body lean mass (WBLM).
Results
The prevalence of LVDD and LVSD was 48.8 and 12.2 %, respectively. Gait speed, shuttle walk time, leg strength, and WBLM% were significantly higher in the group without LVDD than with LVDD (p < 0.05 for all). However, there was no significant difference in any measure of physical function or body composition between patients with and without LVSD.
Conclusion
These data suggest that LVDD is more closely related to physical function and body composition than LVSD in MHD patients, and hence that LVDD may be an important therapeutic target.
Keywords: Maintenance hemodialysis patients, Cardiac abnormalities, Diastolic dysfunction, Physical function, Body composition
Introduction
The prevalence of cardiovascular (CV) disease and CV mortality are excessively high in patients undergoing maintenance hemodialysis (MHD) therapy [1]. Cardiac abnormalities such as left ventricular (LV) hypertrophy and systolic and diastolic dysfunction are present in up to 80 % of MHD patients [2]. These cardiac abnormalities independently predict adverse cardiac events, and are the strongest predictor of mortality in this population [3].
Cardiac abnormalities adversely impact physical function and also can be exacerbated by reduced physical function; however, the relationship between cardiac and physical function in MHD patients is not well established. Decrements in physical function are common in MHD patients and significantly impair their quality of life (QOL) [4]. A variety of non-cardiac factors such as decreased muscle mass [5], abnormal muscle metabolism [6], and inflammation are known to contribute to low physical function in MHD patients [7]. However, the evidence linking cardiac abnormalities and physical impairment is limited.
LV diastolic function measures have been proposed as providing better prognostic value in this population because they are less sensitive to blood volume changes than systolic function measures [8]. Furthermore, LV diastolic dysfunction (LVDD), characterized by impaired LV dilation, is strongly associated with poor exercise capacity in other clinical populations including cardiac patients [9]. While inadequate cardiac output and exertional dyspnea may contribute to poor exercise capacity during LVDD, the exact mechanism is unclear [10]. However, no studies to date have examined the relationship between LVDD and reduced physical functioning in MHD patients. Increasing our understanding of this relationship may help identify novel therapeutic approaches to improve overall health in MHD patients.
Therefore, the purpose of this study was to evaluate: (1) the prevalence of LVDD in MHD, and (2) the relationship between LVDD and physical function in patients undergoing MHD. We hypothesized that LV diastolic function would be associated with decline in physical function in MHD patients.
Research design and methods
Study population
Eighty-two patients receiving MHD therapy were recruited from hemodialysis clinics in Champaign and Oak Park, IL, USA. Patients were screened for eligibility with a health and medical history questionnaire. Inclusion criteria for participation in this study included the following: (1) >3 months of MHD treatment; (2) age 30–80 years old; (3) at least 3 days of MHD treatment per week; and (4) medical clearance from a nephrologist to determine patient eligibility for the study. Subjects were excluded if they had had chronic obstructive pulmonary disease (COPD), decompensated congestive heart failure (CHF), or cardiovascular surgery (e.g. coronary bypass or valve replacement) in the past 6 months. All participants provided written informed consent. All patients were treated using bicarbonate dialysis with blood flow rates between 400 and 600 ml/min and dialysate flow rates between 500 and 800 ml/min with treatment times between 3 and 4 h/session. Patient clinical information was available only from participants who provided a Health Insurance Portability and Accountability Act (HIPPA) release form. This study was approved by the University of Illinois at Urbana-Champaign and at Chicago Institutional Review Boards.
Echocardiography
The transthoracic echocardiographic examinations were performed using a high resolution ultrasound system (Pro-Sound SSD-α7, Aloka, Japan) by two experienced sonographers blinded to all other data, and were analyzed by a single sonographer. The measurement sessions occurred within 24 h after a MHD session on a non-dialysis day to minimize the effect of fluid overload. Two-dimensional images were obtained and analyzed according to the recommendations of the American Society of Echocardiography (ASE) [11]. At least three consecutive heartbeats in parasternal long and short axis views were acquired. LV volumes and LV mass were measured in M-mode. LV volume parameters were indexed by body surface area [BSA (m2) = 0.007184 × weight (kg)0.425 × height (cm)0.725]. LVSD was defined as left ventricular ejection fraction (LVEF) <40 % using the Teicholz method. Left ventricular mass index (LVMI) was calculated as LVM/height2.7. Left ventricular hypertrophy (LVH) was defined as LVMI > 45 (g/m2.7) for females and LVMI > 50 (g/m2.7) for males. LV diastolic function was assessed by standard Doppler echocardiographic indices [12]. LV diastolic filling patterns were assessed by placing the pulsed Doppler sample volume between the tips of the mitral valve leaflets. Based on the mitral inflow velocity curve, peak early (E) and late (A) diastolic velocities, E-wave deceleration time (DT), and E/A ratio were assessed. Peak early-diastolic mitral annulus velocity (E’) was measured using tissue Doppler imaging of mitral annulus movement. LVDD stages were graded according to ASE guidelines using an integrated ev aluation of LV filling patterns by an experienced sonographer blinded to all other data [13]: (1) mild LVDD (E/A < 0.8, E′ < 8 cm/s, E/E′ < 8 and DT > 200 ms); (2) moderate LVDD (0.8 < E/A < 2, E′ < 8, E/E′ < 9, and DT < 200); and (3) severe LVDD (E/A > 2, E′ < 8, E/E′ > 9, and DT < 200). The combination of moderate and severe LVDD was classified as ‘advanced LVDD’.
Shuttle walk test and gait speed assessment
An incremental shuttle walk test (ISWT) was conducted to estimate cardio-respiratory performance [14]. ISWT is a progressive test in which patients walk back and forth continuously over a 10 meter course. The walking speed is paced by a series of auditory signals for the termination of the 10 m walk. The test was terminated when the subject was unable to complete the 10 m course before the sub-sequent beep. Normal gait speed was measured prior to the start of the ISWT while patients walked at a self-selected speed along the 10 m walkway. Average gait speed was calculated based on three trials.
Muscle strength
Bilateral quadriceps femoris and hamstring muscle strength was evaluated using isokinetic testing modes. Following dynamometer calibration, knee extension and flexion isokinetic peak muscle torque (Nm) was evaluated at a speed of 60° per second on a dynamometer (Biodex Medical Systems, Shirley, NY, USA). Peak torque was recorded for analysis. For all tests, participants were verbally encouraged to perform as vigorously as possible.
Body composition
Whole body fat, lean and bone mass were measured by dual emission X-ray absorptiometry (DXA, Hologic QDR 4500A, Bedford, MA, USA). Whole body lean mass (WBLM) and regional mineral free lean mass (LM) were calculated by subtracting the bone mineral content from the LM quantity of the whole body or region of interest. Whole body bone mineral density (BMD) was also measured. Precision for DXA measurements of interest are 1.0–2.0 % in our laboratory.
Statistical analysis
Continuous data were compared using one-way analysis of variance testing. Categorical data were compared using χ2 tests or Fisher exact tests as appropriate. Univariate regression analysis was performed to identify correlates of physical performance and muscle strength. Significant associations at univariate regression analysis were included in the multivariable linear regression models and tested using a stepwise method with the entry and removal criteria of p < 0.05 and <0.10 respectively. Model 1 was adjusted for basic demographic (age) and anthropometric (body mass index [BMI]) measures. Model 2 included additional adjustment for other variables correlated with ISWT. Models 3 and 4 were performed for the relationship between LVDD and leg strength. The strength of the model was expressed using adjusted R-square and p-values. Standardized ß co-efficient (ß) was reported to assess its relative independent effect on the outcome variable. A p value less than 0.05 was considered statistically significant in two-sided tests using SPSS 22.0 software (IBM, Armonk, NY, USA).
Results
Subject characteristics and prevalence of LV diastolic and systolic dysfunction
Patient demographics are shown in Table 1. The mean dialysis vintage available from 59 patients was 42.9 ± 37.9 months. Races were African American (76.8 %) and Caucasian (22.0 %). The primary causes of end-stage renal disease (ESRD) were hypertension (55.7 %), diabetes (29.2 %), polycystic kidney (10.1 %) and nephritis/nephropathy (5.0 %). There was no difference in race and primary ESRD causes between groups with and without LVDD. The prevalence of LVDD was 48.7 % (34.1 % with advanced LVDD and 14.6 % with mild LVDD). LVSD was identified in 10 patients (12.2 %).
Table 1.
Subject characteristics
| Total (n = 82)a | Patients with LVDD (n = 40)a | Patients without LVDD (n = 42)a | p valueb | |
|---|---|---|---|---|
| Gender (male, %) | 48 (58.5 %) | 17 (42.5 %) | 31 (73.8 %) | 0.004* |
| Age (years) | 54.5 ± 12.0 | 53.7 ± 12.4 | 55.2 ± 11.8 | 0.559 |
| BMI (kg/m2) | 31.5 ± 7.2 | 33.5 ± 7.6 | 29.5 ± 6.3 | 0.011 |
| WBLM (%) | 66.2 ± 10.5 | 62.6 ± 9.0 | 69.6 ± 10.8 | 0.002* |
| Leg LM (%) | 68.1 ± 10.9 | 64.4 ± 10.3 | 71.4 ± 10.4 | 0.003* |
| BMD (g/cm2) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.834 |
| SBP (mmHg) | 134.0 ± 28.0 | 133.2 ± 32.1 | 134.7 ± 23.9 | 0.825 |
| DBP (mmHg) | 75.0 ± 16.8 | 74.4 ± 19 | 75.8 ± 14.6 | 0.729 |
| Smoking status (n, %) | 23 (34.2 %) | 8 (20 %) | 15 (35.7 %) | 0.113 |
| Diabetes (n, %) | 40 (48.7 %) | 21 (52.5 %) | 19 (45.2 %) | 0.511 |
BMI body mass index, WBLM whole body lean mass, LM lean mass, BMD whole body bone mineral density, SBP resting brachial systolic blood pressure, DBP resting brachial diastolic blood pressure
Statistical significance between groups
Data expressed as mean ± SD for continuous variables and numbers for countable variables
p value for group difference between patients with and without LVDD
Body composition and LVDD
WBLM% and leg LM% were significantly lower in the group with LVDD than without LVDD (Table 1).
Physical function and LVDD
Patients with LVDD had a significantly slower gait speed and poorer performance on the ISWT and leg maximal extension and flexion than the group without LVDD (Fig. 1).
Fig. 1.
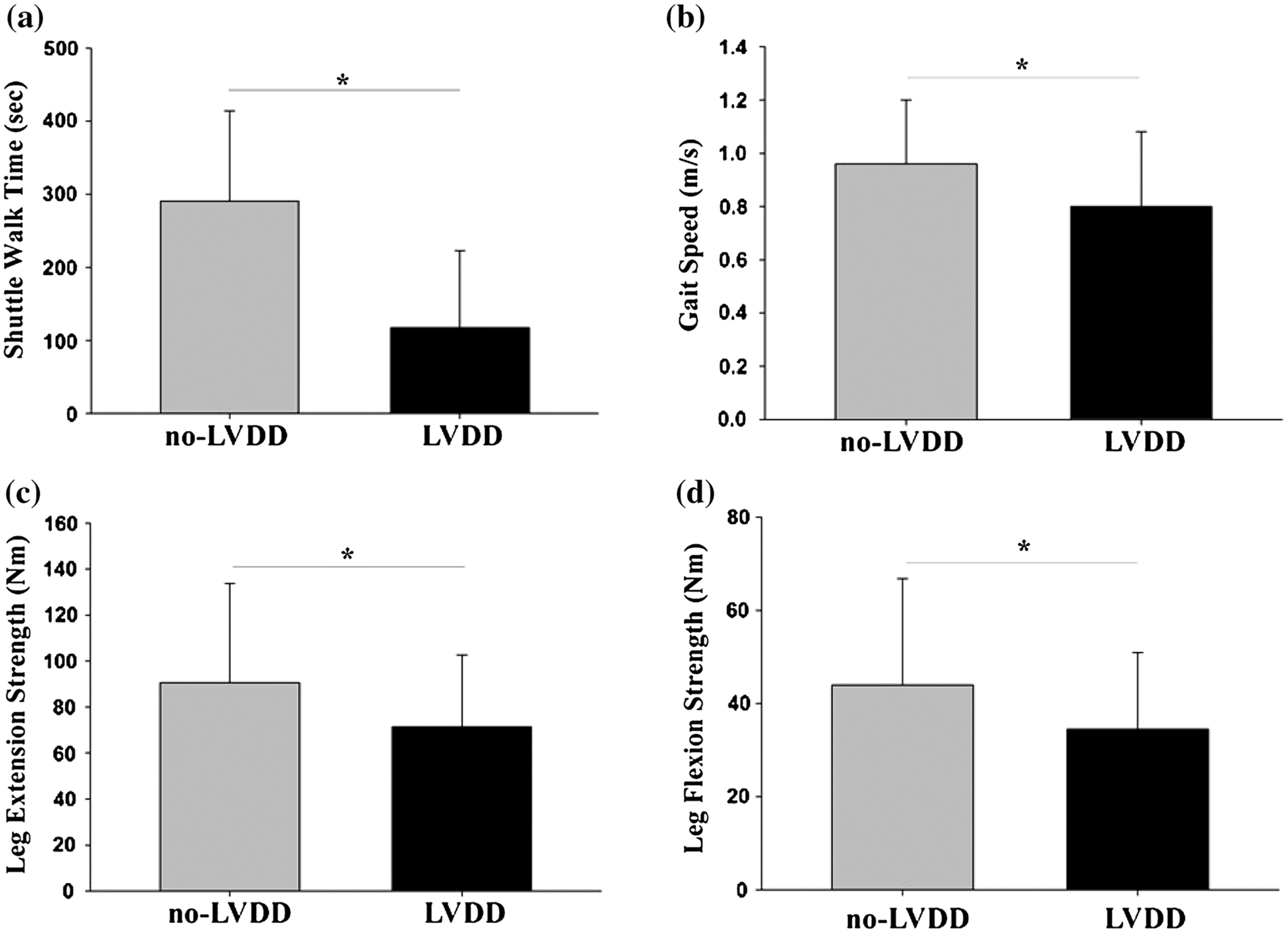
Differences in physical performance and strength measures between patients with and without LVDD. Means of a shuttle walk time, b gait speed, c leg maximal extension strength, and d leg maximal flexion strength were significantly lower in patients with LVDD than in patients without LVDD. Data expressed as mean ± SD and asterisk indicates p < 0.05
CV parameters and LVDD
There was no difference in CV parameters, with the exception of E′ and E/E′, between groups with and without LVDD (Table 2). Due to the body size difference (BMI) between groups with and without LVDD, stroke volume index (SVI) and cardiac output index (COI) were compared.
Table 2.
Cardiac function parameters in patients with and without LVDD
| Totala | Patients with LVDD (n = 40)a | Patients without LVDD (n = 42)a | p valueb | |
|---|---|---|---|---|
| SVI (ml/m2) | 28.3 ± 14.6 | 25.8 ± 13.2 | 30.7 ± 15.7 | 0.144 |
| COI (l/m2) | 2.2 ± 21.3 | 2.1 ± 1.1 | 2.2 ± 1.5 | 0.910 |
| EF (%) | 58.8 ± 18.4 | 58.4 ± 19.7 | 59.2 ± 17.3 | 0.857 |
| FS (%) | 31.3 ± 14.3 | 30.5 ± 15.2 | 32.1 ± 13.5 | 0.648 |
| LVMI (g/m2.7) | 74.9 ± 40.5 | 75.8 ± 40.8 | 73.9 ± 40.6 | 0.829 |
| LVH (n, %) | 62 (87 %) | 32 (80 %) | 30 (71.4 %) | 0.096 |
| E/A | 1.1 ± 0.4 | 1.1 ± 0.5 | 1.0 ± 0.3 | 0.336 |
| E′ (cm/s) | 11.0 ± 5.0 | 8.8 ± 3.9 | 13.0 ± 5.0 | <0.001* |
| A′ (cm/s) | 11.7 ± 4.8 | 10.5 ± 4 | 12.7 ± 5.1 | 0.039* |
| E/E′ | 7.6 ± 4.3 | 10.1 ± 4.8 | 5.5 ± 2.3 | <0.001* |
| DT of E (ms) | 164.4 ± 75.0 | 157.1 ± 63.0 | 171.4 ± 85.1 | 0.390 |
| LVSD (n) | 10 | 5 | 5 | 0.868 |
LVDD left ventricular diastolic dysfunction, SVI stroke volume indexed by BSA, COI cardiac output indexed by BSA, EF ejection fraction, FS fractional shortening, LVMI left ventricular mass index, LVM/height2.7, LVH left ventricular hypertrophy, E/A the ratio of early/late diastolic mitral valve flow velocity, E′ peak early diastolic mitral annulus velocity, A′ peak late diastolic mitral annulus velocity, DT of E deceleration time of early diastolic mitral valve flow (E), LVSD left ventricular systolic dysfunction
Statistical significance between groups
Data expressed as mean ± SD for continuous values and a number for countable values
p-value for group differences between patients with and without LVDD
Predictors of physical performance (ISWT and leg strength)
LVDD, age, BMI, WBLM% and diabetes status were each significantly correlated with ISWT at univariate analysis, and so were included in the multivariable linear regression models. In Model 1, both age and LVDD, but not BMI, significantly predicted ISWT performance. In Model 2, age, WBLM% and diabetes status, but not LVDD and BMI, significantly predicted ISWT performance (Table 3). Similar relationships were found between gait speed and LVDD (data not shown).
Table 3.
Univariate and multivariate predictors of physical performance in ISWT and leg strength
| ISWT | Univariate model | Multivariable model 1 | Multivariable model 2 | |||
|---|---|---|---|---|---|---|
| Adjusted R2 = 0.203, p<0.001 | Adjusted R2 = 0.338, p<0.001 | |||||
| β | p | β | p | β | p | |
| LVDD | −0.303 | 0.007* | −0.307 | 0.004* | −0.187 | 0.116 |
| Age | −0.359 | 0.001* | −0.361 | 0.001* | −0.335 | 0.001* |
| BMI | −0.254 | 0.025* | −0.226 | 0.051 | 0.023 | 0.850 |
| WBLM% | 0.435 | <0.001* | 0.328 | 0.001* | ||
| Diabetes | −0.369 | <0.001* | −0.290 | 0.004* | ||
| Leg strength | Univariate model | Multivariable model 3 | Multivariable model 4 | |||
| Adjusted R2 = 0.044, p = 0.039 | Adjusted R2 = 0.098, p = 0.004 | |||||
| β | p | β | p | β | p | |
| LVDD | −0.238 | 0.039* | −0.238 | 0.039* | −0.159 | 0.179 |
| Age | −0.180 | 0.119 | −0.194 | 0.087 | −0.152 | 0.174 |
| BMI | −0.108 | 0.355 | −0.047 | 0.694 | 0.240 | 0.135 |
| WBLM% | 0.332 | 0.004* | 0.332 | 0.004* | ||
| Diabetes | −0.008 | 0.944 | −0.084 | 0.469 | ||
The relationship between LVDD presence and ISWT and leg strength performance and potential confounders (age, BMI, WBLM, diabetes status) of these associations were tested in univariate regression analysis. Multivariable Models 1 and 3 tested multivariable prediction of LVDD, age and BMI on ISWT and leg strength, respectively. Multivariable Models 2 and 4 added WBLM and diabetes status into Models 1 and 3 ISWT incremental shuttle walk test, LVDD left ventricular diastolic dysfunction, BMI body mass index, WBLM whole body lean mass
Statistical significance between groups
LVDD and WBLM% were significantly correlated with leg extension strength at univariate regression. LVDD remained significant in the multivariable Model 3 when age and BMI were entered together, but was not a significant predictor when WBLM was entered into the multivariable Model 4 (Table 3).
LVDD, BMI and diabetes were each significantly correlated with WBLM at univariate regression. LVDD remained a significant predictor of WBLM when age and diabetes were entered together into a multivariable regression model (R2 = 0.10 and p = 0.002).
LVSD and physical function and body composition parameters
There was no difference in all demographic, body composition, physical function performance and cardiac parameters except systolic function measures (SVI, COI, EF and FS) between groups with and without LVSD in our study population (Table 4).
Table 4.
Demographic, physical and cardiac function parameters in patients with and without LVSD
| Patients with LVSD (n = 10)a | Patients without LVSD (n = 71)a | p valueb | |
|---|---|---|---|
| Age (years) | 54.9 ± 11.3 | 54.4 ± 12.2 | 0.905 |
| BMI (kg/m2) | 32.5 ± 8.0 | 31.3 ± 7.1 | 0.631 |
| WBLM (%) | 64.4 ± 10.3 | 66.7 ± 10.6 | 0.520 |
| Physical function | |||
| ISWT (sec) | 188.0 ± 102.1 | 261.4 ± 117.7 | 0.066 |
| Gait speed (m/sec) | 0.7 ± 0.2 | 0.9 ± 0.3 | 0.125 |
| Peak torque extension (Nm) | 87.1 ± 34.1 | 80.3 ± 39.9 | 0.627 |
| Peak torque flexion (Nm) | 41.2 ± 13.8 | 39.1 ± 21.5 | 0.780 |
| Cardiac function | |||
| SBP (mmHg) | 125.8 ± 26.2 | 135.6 ± 28.2 | 0.327 |
| DBP (mmHg) | 77.3 ± 18.1 | 74.9 ± 16.9 | 0.690 |
| SVI (ml/m2) | 11.6 ± 5.3 | 30.8 ± 13.9 | <0.001* |
| COI (l/m2) | 0.9 ± 0.5 | 2.4 ± 1.3 | <0.001* |
| FS (%) | 10.5 ± 5.0 | 34.7 ± 12.2 | <0.001* |
| LVMI (g/m2.7) | 66.4 ± 13.5 | 77.1 ± 42.1 | 0.111 |
| LVH (n, %) | 3 (30 %) | 55 (77.5 %) | 0.108 |
| E/A | 1.1 ± 0.5 | 1.0 ± 0.3 | 0.336 |
| S′ (cm/s) | 10.8 ± 4.0 | 8.9 ± 3.7 | 0.170 |
| E′ (cm/s) | 13.2 ± 6.0 | 10.7 ± 4.8 | 0.160 |
| E/E′ | 5.9 ± 3.3 | 7.9 ± 4.4 | 0.225 |
| LVDD (n) | 5 | 35 | 0.615 |
BMI body mass index, WBLM whole body lean mass, ISWT incremental shuttle walk test, SBP resting brachial systolic blood pressure, DBP resting brachial diastolic blood pressure, SVI stroke volume indexed by BSA, COI cardiac output indexed by BSA, EF ejection fraction, FS fractional shortening, LVMI left ventricular mass index, LVM/height2.7, LVH left ventricular hypertrophy, E/A the ratio of early/late diastolic mitral valve flow velocity, S′ peak systolic mitral annulus velocity, E′ peak early diastolic mitral annulus velocity
Statistical significance between groups
Data expressed as mean ± SD for continuous values and a number for countable values
p-value for group differences between patients with and without LVSD
Discussion
This study examined the relationship between cardiac function, physical function and performance, and body composition in MHD patients without overt CHF. The primary findings included the following: (1) the prevalence of LVDD was significantly higher than LVSD; (2) physical function (gait speed) and physical performance (ISWT and leg muscle strength) were reduced in those with LVDD; and (3) those with LVDD had a reduced whole body and leg LM%. By contrast, no differences in physical function and body composition were seen in MHD patients with vs. without LVSD. Our findings suggest that LVDD is associated with declines in physical performance and body composition in MHD patients. To our knowledge, this is the first study to analyze the relationship between LVDD, physical performance and body composition in MHD patients.
Our echocardiographic data showed that approximately half of the MHD patients had LVDD while the prevalence of LVSD was much lower (12 %). Other studies reported a similar incidence of LVDD (50–75 %) and LVSD (10–40 %) in MHD patients including those with CHF [15]. It should be noted that this present study excluded patients with decompensated CHF. CHF can be caused by LVSD, LVDD or both, but LVSD identified by a decreased EF is commonly used as an echocardiographic diagnostic for CHF [16]. Moreover, a lack of diagnostic knowledge in diastolic CHF has challenged early and accurate diastolic CHF diagnosis [17]. This may explain the relatively low LVSD and the high LVDD prevalence in our findings.
LVH was identified in 83.7 % of patients in our analysis, which is consistent with previous findings in MHD patients [18]. This high LVH prevalence suggests that LV structural remodeling may precede development of cardiac dysfunction regardless of the presence of decompensated CHF in MHD patients. Indeed, LVH is known to initiate a vicious cycle of cardiac maladaptation in MHD patients [19]. Together with accompanying interstitial fibrosis and myocardial ischemia, increase in LV mass contributes to impaired LV diastolic distensibility, a main feature of LVDD. As LVDD progresses, LV end-diastolic pressure increases as a consequence of inadequate LV filling in response to a given change in blood volume. Therefore, patients with LVDD may suffer from CV complications due to an inability to adjust LV volume for a given change in pressure. This results in either: (1) pulmonary congestion with an increased blood volume, or (2) hypotension with a decreased blood volume. This has significant clinical implications for MHD patients who experience frequent blood volume shifts between MHD treatments and during a MHD treatment. Therefore, identification of LVDD would provide important information for therapeutic strategies to prevent adverse CV events in MHD patients.
One interesting observation was a higher percentage of females in the LVDD group than in the non-LVDD group (57.5 vs. 26.2 % respectively, p = 0.004). Although not accounted for in this study, hormonal factors may have contributed to the high prevalence of LVDD in female dialysis patients. Female MHD patients have female-hormone related symptoms and accelerated rates of CVD and mortality compared to the general female population [20]. Further investigation is needed to confirm the relationship between female hormonal abnormalities and cardiac dysfunction in this population.
Growing evidence suggests that MHD patients experience reduced physical functioning, which is associated with a poor prognosis and impaired QOL [21]. Exercise capacity has been shown to be approximately 50 % of the level of healthy sedentary controls [22]. Physiologically, exercise capacity is affected by the efficiency of oxygen delivery (central factors) and oxygen utilization (peripheral factors). The peripheral contributors such as decreased muscle mass [5] and muscle metabolism [6] have been reported in MHD patients. However, few studies have examined cardiac mechanisms underlying decline in physical function, and the data that exist mostly used LV systolic function measures that are volume dependent in MHD patients [23].
In our study, patients with LVDD had significantly slower gait speed, poorer performance on the shuttle walk test, and reduced hamstring and quadriceps strength. Regression analysis revealed that LVDD was an independent predictor of walking performance and muscle strength even after adjusting for age, but not when additionally adjusting for WBLM and diabetes in our study population.
A possible pathophysiological explanation for this association is that LVDD leads to limited LV filling and decreased cardiac output even with preserved systolic function. Especially, during exercise, the failure to increase cardiac output in response to the increased oxygen demand may significantly limit exercise performance [24]. Additionally, an increased LV filling pressure, a hallmark of LVDD, frequently coincides with an augmented left atrial pressure and consequently leads to ventilation-perfusion abnormalities. This can limit exercise capacity as well [10]. Respiratory muscle weakness, a cause for dyspnea and tachypnea, has also been shown to be closely related to LVDD [25]. Regarding strength, abnormal skeletal muscle metabolism, including impaired mitochondrial energy transfer and ATP production, have been found in heart failure models, and may also partially explain the strength decline in patients with LVDD [26].
In MHD patients, it has been suggested that LV diastolic performance may reflect CV fitness more than systolic function due to the volume dependence of LV systolic function measures [15]. Although the contribution of LV systolic function to physical performance has been studied widely, recent studies have reported that echocardiographic LV systolic function parameters are poor predictors of exercise capacity in patients with mild and severe cardiac disorders [24]. Studies have demonstrated that LV diastolic function surrogates such as E′, E/E′ and left atrial volume are strongly associated with exercise capacity in cardiac patients [27]. This present study found that only LVDD, not LVSD, was significantly related to physical function and body composition in MHD patients.
We also found correlations between body composition (LM %) and body size (BMI) and cardiac function in MHD patients. Previous studies demonstrated an unfavorable effect of high BMI on mortality when body sizes were assessed separately as LM and fat mass (FM) to further stratify wasting symptoms. For example, a high BMI with a low ratio of LM to FM, called sarcopenic obesity, was associated with increased systemic inflammation and high mortality rates in MHD patients [28]. Apart from mortality data, little is known about the contribution of increased body size, and even less about FM or LM, on cardiac function in MHD patients. In the present study, patients with LVDD had a higher BMI and lower WBLM% than the group without LVDD. Also, decreasing WBLM% was significantly correlated with impaired walking capacity (p < 0.001, data not shown), but this trend was not significant after controlling for body weight in our analysis. Indeed, LM predicts exercise capacity better than total body weight in the general population [29]. Furthermore, whole body FM was associated with unfavorable CV adaptations such as increased blood pressure, impaired LV contractility and LVH, whereas increased WBLM was primarily related to preload determinants such as cardiac output (CO) and stroke volume (SV), perhaps due to the increased metabolic needs of skeletal muscle [30]. Taken together, our findings suggest that body FM and LM should be used to further refine stratification of CV risk in relation to cardiac dysfunction in clinical settings in MHD patients.
Strengths and limitations
To our knowledge, this is the first study to assess the correlation between LV diastolic function and functional capacity in MHD patients. We excluded patients with CV complications that are known to limit physical function. Therefore, the impact of LVDD on physical performance was not confounded by other common CV complications in our analysis. However, these exclusions resulted in our study population being younger and fitter than the general dialysis population; thus our results may not be valid for older and less fit MHD patients. The low prevalence of LVSD may limit the statistical power to detect significant difference between groups with and without LVSD. It is possible that use of other criteria to identify LVSD such as tissue Doppler S-wave velocity and global strain by speckle tracking could have added more precision to our determination of LVSD. However, because there are no clinically accepted cut points for defining LVSD using these methodologies, we did not include them in this analysis. The most validated technique to estimate LV filling pressures—invasive catheter—was not used, and other possible contributors that affect LV diastolic function such as left atrial volume and filling profiles and arterial stiffness parameters were not available in this study. However, integrated indices using echocardiographic pulsed and tissue Doppler assessments that our study used are widely validated to estimate LV filling pressure for LVDD classification, and their subclinical prognostic values have been well confirmed in patients with ESRD [15]. Body composition measures by DXA are fluid dependent, but the measurement sessions in our study occurred 24 h after a dialysis session on a non-dialysis day to minimize the effect of fluid overload. Finally, the design was cross-sectional, making a causal relationship impossible.
Conclusions
The prevalence of LVDD was higher than LVSD in MHD patients without major CV complications such as CHF. The severity of LVDD was related to physical functional capacity and body composition in this population. Furthermore, our data suggest that distinguishing between body fat and lean mass may improve CV risk stratification in relation to cardiac dysfunction. Further investigation is needed to confirm these findings, including in MHD patients with diagnosed CV comorbidities.
Acknowledgments
This study was funded the National Institute of Health, NIH (RO1 DK084016-01 to KR. Wilund).
Abbreviations
- BMD
Bone mineral density
- BMI
Body mass index
- BSA
Body surface area
- CHF
Congestive heart failure
- COI
Cardiac output index
- COPD
Chronic obstructive pulmonary disease
- CV
Cardiovascular
- DBP
Diastolic blood pressure
- DT of E
Deceleration time of E’
- DXA
Dual emission x-ray absorptiometry
- E/A
Diastolic early to late mitral flow velocity ratio
- E′, A′
Peak early/late diastolic mitral annulus velocity
- EF
Ejection fraction ESRD End-stage renal disease FM Fat mass
- FS
Fractional shortening
- ISWT
Incremental shuttle walk test
- LM
Lean mass
- LV
Left ventricular
- LVDD
Left ventricular diastolic dysfunction
- LVH
Left ventricular hypertrophy
- LVMI
Left ventricular mass index
- LVSD
Left ventricular systolic dysfunction
- MHD
Maintenance hemodialysis patients
- SBP
Systolic blood pressure
- SVI
Stroke volume index
- WBLM
Whole body lean mass
Footnotes
Conflict of interest The authors of this study have no conflict of interests to report.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Contributor Information
Jin Hee Jeong, University of Illinois at Urbana-Champaign, Kinesiology and Community Health, Urbana, IL, USA.
Pei-Tzu Wu, School of Nursing, University of California, Los Angeles, Los-Angeles, CA, USA.
Brandon Michael Kistler, University of Illinois at Urbana-Champaign, Kinesiology and Community Health, Urbana, IL, USA.
Peter John Fitschen, Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, USA.
Annabel Guzman Biruete, Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, USA.
Shane Aaron Phillips, University of Illinois at Chicago, Kinesiology, Nutrition, and Rehabilitation, Chicago, IL, USA.
Mohamed M. Ali, University of Illinois at Chicago, Kinesiology, Nutrition, and Rehabilitation, Chicago, IL, USA
Bo Fernhall, University of Illinois at Chicago, Kinesiology, Nutrition, and Rehabilitation, Chicago, IL, USA.
Kenneth Robert Wilund, University of Illinois at Urbana-Champaign, Kinesiology and Community Health, Urbana, IL, USA.
References
- 1.Go AS et al. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305 [DOI] [PubMed] [Google Scholar]
- 2.London GM, Parfrey PS (1997) Cardiac disease in chronic uremia: pathogenesis. Adv Ren Replace Ther 4(3):194–211 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J et al. (1999) Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99(18):2434–2439 [DOI] [PubMed] [Google Scholar]
- 4.Moore GE et al. (1993) Determinants of VO2peak in patients with end-stage renal disease: on and off dialysis. Med Sci Sports Exerc 25(1):18–23 [DOI] [PubMed] [Google Scholar]
- 5.Kemp GJ et al. (1995) ATP production and mechanical work in exercising skeletal muscle: a theoretical analysis applied to 31P magnetic resonance spectroscopic studies of dialyzed uremic patients. Magn Reson Med 33(5):601–609 [DOI] [PubMed] [Google Scholar]
- 6.Thompson CH et al. (1997) The effect of propionyl L-carnitine on skeletal muscle metabolism in renal failure. Clin Nephrol 47(6):372–378 [PubMed] [Google Scholar]
- 7.Moore GE et al. (1993) Uremic myopathy limits aerobic capacity in hemodialysis patients. Am J Kidney Dis 22(2):277–287 [DOI] [PubMed] [Google Scholar]
- 8.Dogan U et al. (2012) Evaluation of echocardiographic indices for the prediction of major adverse events during long-term follow-up in chronic hemodialysis patients with normal left ventricular ejection fraction. Eur Rev Med Pharmacol Sci 16(3):316–324 [PubMed] [Google Scholar]
- 9.Aljaroudi WA et al. (2012) Relationship between baseline resting diastolic function and exercise capacity in patients with hypertrophic cardiomyopathy undergoing treadmill stress echocardiography: a cohort study. BMJ Open 2(6). doi: 10.1136/bmjopen-2012-002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skaluba SJ, Litwin SE (2004) Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation 109(8): 972–977 [DOI] [PubMed] [Google Scholar]
- 11.Cheitlin MD et al. (2003) ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr 16(10):1091–1110 [DOI] [PubMed] [Google Scholar]
- 12.Teske AJ et al. (2007) Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound 5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagueh SF et al. (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133 [DOI] [PubMed] [Google Scholar]
- 14.Singh SJ et al. (1992) Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 47(12):1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barberato SH et al. (2010) Prevalence and prognostic impact of diastolic dysfunction in patients with chronic kidney disease on hemodialysis. Arq Bras Cardiol 94(4):457–462 [DOI] [PubMed] [Google Scholar]
- 16.Marantz PR et al. (1988) The relationship between left ventricular systolic function and congestive heart failure diagnosed by clinical criteria. Circulation 77(3):607–612 [DOI] [PubMed] [Google Scholar]
- 17.Hancock HC et al. (2014) Barriers to accurate diagnosis and effective management of heart failure have not changed in the past 10 years: a qualitative study and national survey. BMJ Open 4(3):e003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parfrey PS et al. (1996) Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11(7):1277–1285 [PubMed] [Google Scholar]
- 19.London GM et al. (1987) Uremic cardiomyopathy: an inadequate left ventricular hypertrophy. Kidney Int 31(4):973–980 [DOI] [PubMed] [Google Scholar]
- 20.Guglielmi KE (2013) Women and ESRD: modalities, survival, unique considerations. Adv Chronic Kidney Dis 20(5):411–418 [DOI] [PubMed] [Google Scholar]
- 21.Sietsema KE et al. (2004) Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 65(2):719–724 [DOI] [PubMed] [Google Scholar]
- 22.Deligiannis A, Kouidi E, Tourkantonis A (1999) Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol 84(2):197–202 [DOI] [PubMed] [Google Scholar]
- 23.Deligiannis A et al. (1999) Cardiac effects of exercise rehabilitation in hemodialysis patients. Int J Cardiol 70(3):253–266 [DOI] [PubMed] [Google Scholar]
- 24.Grewal J et al. (2009) Left ventricular function and exercise capacity. JAMA 301(3):286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavietes MH et al. (2004) Inspiratory muscle weakness in diastolic dysfunction. Chest 126(3):838–844 [DOI] [PubMed] [Google Scholar]
- 26.Ventura-Clapier R, De Sousa E, Veksler V (2002) Metabolic myopathy in heart failure. News Physiol Sci 17:191–196 [DOI] [PubMed] [Google Scholar]
- 27.Matsumura Y et al. (2002) Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart 87(3):247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segall L et al. (2014) Protein-energy wasting, as well as over-weight and obesity, is a long-term risk factor for mortality in chronic hemodialysis patients. Int Urol Nephrol 46(3):615–621 [DOI] [PubMed] [Google Scholar]
- 29.Batterham AM et al. (1999) Modeling the influence of body size on V(O2) peak: effects of model choice and body composition. J Appl Physiol 87(4):1317–1325 [DOI] [PubMed] [Google Scholar]
- 30.Kardassis D et al. (2012) The influence of body composition, fat distribution, and sustained weight loss on left ventricular mass and geometry in obesity. Obesity (Silver Spring) 20(3):605–611 [DOI] [PubMed] [Google Scholar]


