Abstract
Problem:
Preeclampsia (PE) is associated with an increased risk of maternal cardiovascular disease (CVD), however, it is unclear whether this is due to shared underlying physiology or changes which occur during the disease process. Fetal microchimerism (FMc) within the maternal circulation can durably persist decades after pregnancy, is known to occur at greater frequency in PE, and can potentially affect local and systemic immune programming, thus changes in cellular FMc may provide a mechanism for long-term health outcomes associated with PE.
Method of Study:
We investigated whether PE is associated with alterations in FMc immune and stem cell populations. We analyzed maternal peripheral blood mononuclear cells (PBMC) from PE cases (n=16) and matched controls from normal pregnancies (n=16), from which immune and stem cell subsets were isolated by flow cytometry. Genomic DNA was extracted from total PMBC and individual cell subsets, and FMc frequency was quantified by quantitative polymerase chain reaction assays targeting a fetal-specific non-shared polymorphism identified from family genotyping.
Results:
There was a significant increase in FMc concentration in immune cell subsets in PE cases compared to controls, predominantly in B cell, and NK cell lymphocyte populations. There was no significant difference in FMc frequency or concentration within the stem cell population between PE and controls.
Conclusions:
The altered concentrations of immune cells within FMc in the maternal blood provides a potential mechanism for the inflammation which occurs during PE to induce long-lasting changes to the maternal immune system and may potentially promote chronic maternal disease.
Keywords: Microchimerism, preeclampsia, NK cell, T cell, Regulatory T cell, B cell, Monocytes, Pluripotent stem cell
Introduction
PE complicates 2–8% of pregnancies and is among the leading causes of maternal mortality worldwide1–3. In addition to the immediate risks, individuals who develop PE are at risk for later-life complications such as cardiovascular, autoimmune, and metabolic disorders4–9. It is currently unclear if PE is simply an early manifestation of underlying high-risk physiology or whether events that occur during the pathogenic process of PE also contribute to these long-term health risks10,11.
The pathophysiology of PE is associated with disruption of typically tolerogenic immune cells at the maternal-fetal interface, triggering maternal systemic inflammation, oxidative stress, dysregulation of angiogenic factors, and endothelial dysfunction12,13. As such, maternal-fetal immune interactions are an important component of PE, especially given that intimate interactions and immune crosstalk between mother and fetus occur throughout gestation. A variety of innate and adaptive immune cells perform complementary and interdependent functions at the maternal-fetal interface during pregnancy to support an uncomplicated pregnancy14–17. PE is associated with disruption of these normal immunologic mechanisms. Patients with PE have altered NK cell and Treg function18–20, and this loss of immune tolerance at the maternal-fetal interface leads to the production of inflammatory cytokines and activation of monocytes, T cells, and B cells which have been identified both within the placenta and the maternal circulation and are thought to contribute to the maternal pathology of PE12,13. There is also evidence of decreased maternal stem cell frequency and function in PE21–23. Inflammation is considered an important contributing factor for cardiovascular, autoimmune, and metabolic disorders,24–27 and immune and stem cells can be long-lived. As such, it is possible that the inflammation associated with PE may contribute to the increased incidence of the later-life complications observed in patients with a history of PE. An important question remains as to whether the inflammation during PE can have long lasting effects on maternal immune programming.
The invasive hemochorial human placenta results in the close proximity of the maternal and fetal circulatory systems28, facilitating the bidirectional transfer of cellular and non-cellular material (cell-free DNA, microRNA, exosomes, etc.)29–31. Fetal microchimerism (FMc), the presence of fetal cells and material that enters the maternal compartment and can persist for decades, is a normally occurring physiologic process32,33. FMc acquisition increases throughout gestation34 and is affected by obstetric factors35,36. Fetal origin microchimerism (Mc) persistence is implicated in later-life maternal autoimmune diseases32, protection from some malignancies37, and preliminary analysis supports a potential association with the development of CVD38,39. Importantly, in PE pregnancies, there is increased FMc transfer compared to normal pregnancy40. FMc is detected in immunologically varied cell subsets, including pluripotent CD34+ cells, T cells, B cells, monocytes, NK cells, and CD66+b granulocytes34,41–43, however, the functional role of these cells and whether there is selective transfer of certain cell types during PE pregnancy is incompletely understood. Because fetal origin Mc can persist long beyond the pregnancy, it provides a potential mechanism for transmission of inflammatory signals during PE pregnancies indicating a possible role in the observed long-term health risks. In this study, we investigated whether the immune cellular phenotype of FMc is altered in PE compared to healthy controls. We hypothesized that individuals with PE will have significantly increased detection of FMc in adaptive immune and stem cell populations in the maternal blood compared to controls.
Methods
Patient population
Samples were obtained from prospectively collected specimens from two clinical studies. These studies were approved by the Institutional Review Boards of the participating institutions University of Washington (IRB STUDY00001636) and the Fred Hutchinson Cancer Research Center (Ref 9569). All individual participants gave written informed consent prior to participation. We included pregnant individuals ≥18 years old carrying a non-anomalous singleton fetus. Individuals with the following conditions were excluded as these conditions may affect FMc detection or transfer: multiple gestation, conception via in vitro fertilization, cephalic version in the current pregnancy, threatened abortion, placental abnormalities (invasive placentation, previa, abruption), preterm labor, and maternal transplant.
Participants with PE were recruited at the time of clinical diagnosis. PE was defined as hypertension (systolic blood pressure > 140 or diastolic blood pressure > 90) persistent for at least four hours with proteinuria (defined as a timed urine collection with ≥300mg of protein in 24 hours, a random urine sample with a protein to creatinine ratio of ≥0.3, or a urine dipstick assessment of ≥3+). PE with severe features was defined by the presence of any of the following: severe hypertension (systolic blood pressure >160 or diastolic blood pressure >110) persistent for at least four hours, seizures (eclampsia), hemolysis, elevated liver enzymes, thrombocytopenia, pulmonary edema, or renal dysfunction44. Subjects with chronic hypertension were included if a timed urine collection from early in pregnancy was available and a twofold elevation in proteinuria was demonstrated in conjunction with worsening hypertension. Medical records were reviewed for clinical and demographic information. As PE with severe features is most strongly associated with maternal systemic and fetal-placental inflammation,13,45 we only included PE cases with severe features in this study.
Participants with uncomplicated pregnancy outcomes were derived from a population of healthy individuals with a singleton pregnancy. Delivery and medical records were reviewed to confirm inclusion in the study for all participants. Among the controls, we excluded subjects with any of the following obstetric complications: PE, gestational hypertension, preterm birth (spontaneous or indicated), pregestational diabetes, placenta previa, placental abruption/antenatal bleeding, trauma, fetal growth restriction, and other complications listed above.
Sample collection and processing
For participants with PE, maternal peripheral blood was drawn at the time of diagnosis, before the onset of labor or delivery. Control participants underwent peripheral blood draws each trimester, and samples drawn during the latter half of pregnancy (20–42 weeks gestation, the same gestational age range at which women could be diagnosed with PE) were matched by gestational age and parity to PE cases and included in this study. For both PE cases and controls, an umbilical cord blood sample was also collected after delivery. Samples from all participants were processed and stored in an identical manner. All blood samples were collected in acid citrate dextrose solution A-vacutainer tubes and underwent processing (unfrozen) within 24 hours. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Histopaque (Pharmacia Biotech) gradient centrifugation at a density of 1.077 g/mL and cryopreserved in dimethylsulfoxide (DMSO). Genomic DNA was extracted from whole blood and PBMC using PureLink® Genomic DNA Mini Kit (Invitrogen).
Immune and stem cell subset isolation
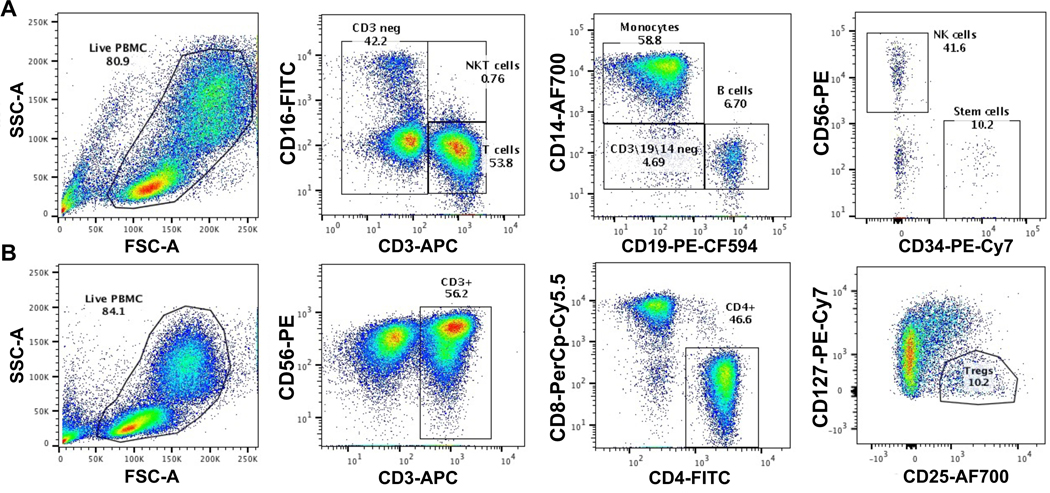
PBMC from PE cases and controls (n=16 for each) were thawed and stained with a fluorescent marker for viability (LIVE/DEAD Violet, Invitrogen) and antibodies specific for immune and stem cells populations in two separate panels: Panel 1: CD3 (APC conjugated, BD Biosciences cat#555342), CD14 (AlexaFluor700 conjugated, BD Biosciences cat#557923), CD16 (FITC conjugated, BD Biosciences cat#561308), CD19 (PE-CF594 conjugated, BD Biosciences cat#562321), CD34 (PE-Cy7 conjugated, BD Biosciences cat#560710), and CD56 (PE conjugated, BD Biosciences cat#340363) or Panel 2: CD3 (APC conjugated), CD4 (FITC conjugated, BD Biosciences cat#340422), CD8 (PerCp-Cy5.5 conjugated, BD Biosciences cat#341051), CD25 (AlexaFluor700 conjugated, BD Biosciences cat#561398), CD56 (PE conjugated), and CD127 (PE-Cy7 conjugated, BD Biosciences cat#560822). The cells were fixed (Fix/Perm Buffer, BD Biosciences) and sorted by flow cytometry using a FACS Aria cell sorter to isolate B cells, Monocytes, NK cells, stem cells, T cells, and Treg cells by their characteristic cell surface markers (Figure 1). The frequency of each subset population was determined by percentage of total live cells (FlowJo). An aliquot of sorted cell subsets from each sample were tested for purity by flow cytometry and confirmed >95% specific for target cell subset. Genomic DNA was extracted from sorted cell subsets as above.
Figure 1. Flow Cytometry Gating Strategy for Cell Sorting.
Maternal PBMCs from PE cases and gestational-age matched (range between 26–37 weeks) controls were stained with a viability marker and antibodies for immune and stem cell subsets from Panel 1 (A) CD3-APC, CD14-AlexaFluor700, CD16-FITC, CD19-PE-CF594, CD34-PE-Cy7, and CD56-PE or Panel 2 (B) CD3-APC, CD4-FITC, CD8-PerCp-Cy5.5, CD25-AlexaFluor700, CD56-PE, and CD127-PE-Cy7. Cells were fixed and cell sorting performed on FACS Aria with isolation of stem and immune cells subsets by serial gating on the live (viability negative, not shown) lymphocyte/monocyte population by size and granularity (FSC-A and SSC-A) and the following markers to isolate B cells (CD3/CD14 negative, CD19+), Monocytes (CD3 negative, CD14+), NK cells (CD3/CD14/CD19 negative/CD56+), stem cells (CD3/CD19/CD14/CD56 negative, CD34+) (A) and T cells (CD56 negative, CD3+) and Treg cells (CD3+/CD4+/ CD25high/ CD127low) (B)
Genotyping of mother-baby pairs
Because of extensive polymorphism in human leukocyte antigen (HLA) genes, HLA genotyping of pregnant individuals and their fetuses usually results in identification of a polymorphism unique to the fetus that can be targeted by quantitative polymerase chain reaction (quantitative PCR, or QPCR) to identify and quantify FMc in the maternal blood. HLA genotyping was conducted using a Luminex-based (One Lambda, Thermo Fisher Scientific) PCR-sequence-specific oligonucleotide probe-based technique or via commercial HLA genotyping (Sisco Genetics, Inc, Seattle, WA). Maternal and cord blood samples were HLA genotyped for the class II loci DRB1, DQA1, and DQB1. HLA relationships were then examined to identify non-shared HLA polymorphisms targetable to identify FMc. Because an HLA polymorphism unique to the fetus may not always be available for all mother-baby pairs, genotyping for several other polymorphic non-HLA genes was also performed as necessary using a conventional PCR system. These genes included antithrombin III, thyroglobulin, and glutathione S-transferase theta 1.
FMc detection and quantification
After identifying a polymorphism unique to the neonate, we used the appropriate assay from a panel of qPCR assays previously developed to test DNA extracted from maternal PBMC for FMc46. Sensitivity of utilized primers was previously established via testing of each HLA-specific qPCR primer with an extended panel of well-characterized HLA cell lines. Of note, primers are designed to amplify specific HLA allele groups (i.e. amplification of only the DRB1*01 allele group and no other groups such as DRB1*03, 04, etc.). Testing is performed on a cell line known to be positive for the HLA target of interest at increasing concentrations (lowest of 0.5 cell equivalents, highest of 500 cell equivalents) amongst a background of cells known to be negative for that HLA polymorphism. The maximum amount of DNA tested per well was 25,000 genome equivalents (gEq), as higher concentrations of DNA may inhibit the PCR reaction. Six replicates of DNA from PBMC or subsets were tested from each sample with total reaction volumes of 50 μL. A calibration curve for the polymorphism-specific assay using commercially available cell lines known to be positive for the HLA target of interest was included to quantify the amount of FMc and to validate the assay for each experiment (positive control). Every sample was also tested for a nonpolymorphic gene, beta-globin (BGLOB). A BGLOB calibration curve (prepared from commercially available human genomic DNA (Promega) was concurrently evaluated on each plate to quantify the total number of gEq of DNA tested in each reaction. DNA quantities were reported as the DNA gEq number of FMc cells per 100,000 total gEq tested, using a conversion factor of 6.6 pg of DNA per cell47.
Multiple precautions were taken to minimize potential for contamination in qPCR experiments. DNA extractions and qPCR preparations were performed under an ultraviolet light-equipped safety hood cleaned with bleach and filtered tips were used during pipetting. Each experiment included multiple negative control wells to ensure the absence of contamination.
Statistical Analysis
Clinical and demographic characteristics were compared using student’s t-test, Kruskal-Wallis rank test, χ2 test, or Fisher’s exact test as appropriate. Statistical analysis of subset distribution was performed using Wilcoxon rank sum test. Linear regression analysis was also performed to assess for effects of gestational age on subset distribution. Overall FMc detection and FMc detection in each subset was compared using χ2 or Fisher’s exact test as appropriate. Negative binomial regression was used to compare FMc concentration by cases status within each subset. The use of negative binomial models for analysis of Mc concentrations has previously been tested and described48.
Results
Demographic and Clinical Data
There was no significant difference in terms of maternal age, race, or neonatal sex between PE cases and controls (Table 1). PE cases delivered at significantly earlier gestational ages (30.3 (±3.2) vs. 39.2 (±1.4) weeks, p<0.01) with lower infant birth weights (1580 (±1447) vs. 3439 (±486) grams, p<0.01) and had a higher rate of Cesarean delivery (81% vs. 18%, p<0.01) compared to controls. These were iatrogenic deliveries due to severe PE and preterm labor was not observed in either population.
Table 1.
Demographic and Clinical Data.
| Normal Subjects (n=16) | PE Subjects (n=16) | p-value | |
|---|---|---|---|
| Maternal age (years), mean (s.d.) | 31.5 (±3.9) | 28.9 (±5.8) | 0.14 |
| Maternal white race, n (%) | 12 (75) | 13 (81.3) | 0.10 |
| Gravidity, median (range) | 1 (1–3) | 1 (1–4) | 0.47 |
| Parity, median (range) | 0 (0–1) | 0 (0–3) | 0.44 |
| Delivery gestational age (wks), mean (s.d.) | 39.2 (±1.4) | 30.3 (±3.2) | <0.01 |
| Cesarean delivery, n (%) | 3 (18%) | 13 (81%) | <0.01 |
| Neonatal birth weight (g), mean (s.d.) | 3439 (±486) | 1580 (±1447) | <0.01 |
| Female neonatal sex, n (%) | 5 (31%) | 10 (62%) | 0.16 |
Immune and Stem Cell Subsets in PE Cases and Controls
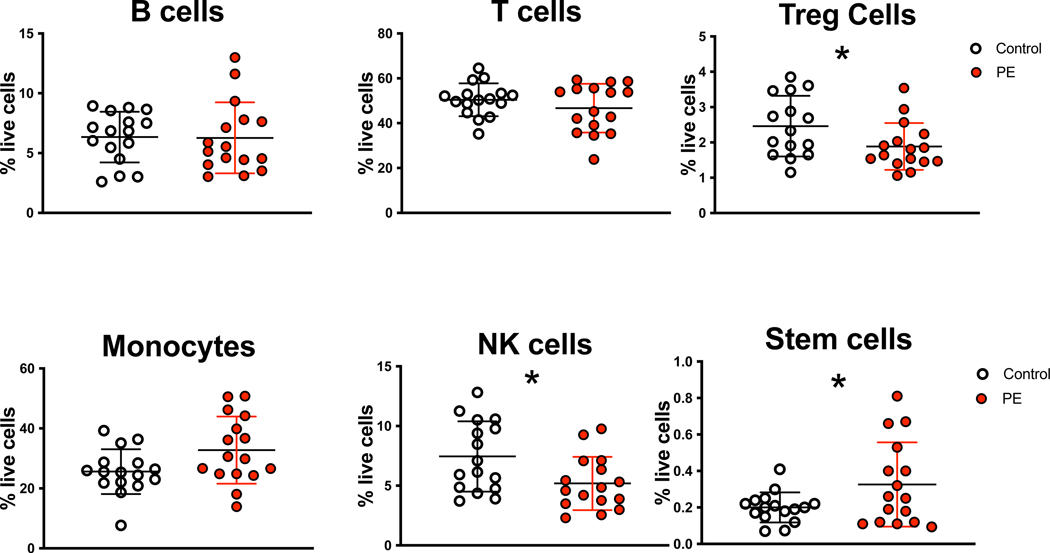
Maternal PBMC from PE cases and controls were stained with antibodies specific for B cells, monocytes, NK cells, T cells, Tregs, and stem cells as these populations have been associated with altered frequency or function in PE. The frequency of these subsets was determined in each PBMC sample as percentage of total live cells (Figure 2). There was no significant difference in B cells (6.3 ± 3.0% cases vs 6.3 ± 2.1% controls p=0.93), T cells (46.7 ± 10.8% cases vs 50.4 ± 7.3% controls p=0.26), or monocytes (32.9 ± 11.2% cases vs 26.5 ± 7.5% controls p=0.07) in the maternal blood in PE cases and controls. There was a significant decrease in maternal NK cell (5.2 ± 2.3% in cases vs. 7.2 ± 3.1% in controls p=0.04) and Treg (1.9 ± 0.6% in cases vs. 2.5 ± 0.9% in controls p=0.03) frequency in PE cases compared to controls. There was a significant increase in maternal stem cells (0.3 ± 0.2% in cases vs. 0.2 ± 0.1% in controls p=0.04) in PE cases compared to controls.
Figure 2. Immune and Stem Cell Frequency in PE Cases and Controls.
Maternal PBMC from PE cases and gestational-age matched (range between 26–37 weeks) controls were stained with immune and stem cell specific antibodies as described in Figure 1. The frequency of each subset was determined as a fraction of the total live PBMC cell population. Statistical significant was determined by Wilcoxon rank sum test (*=p<0.05).
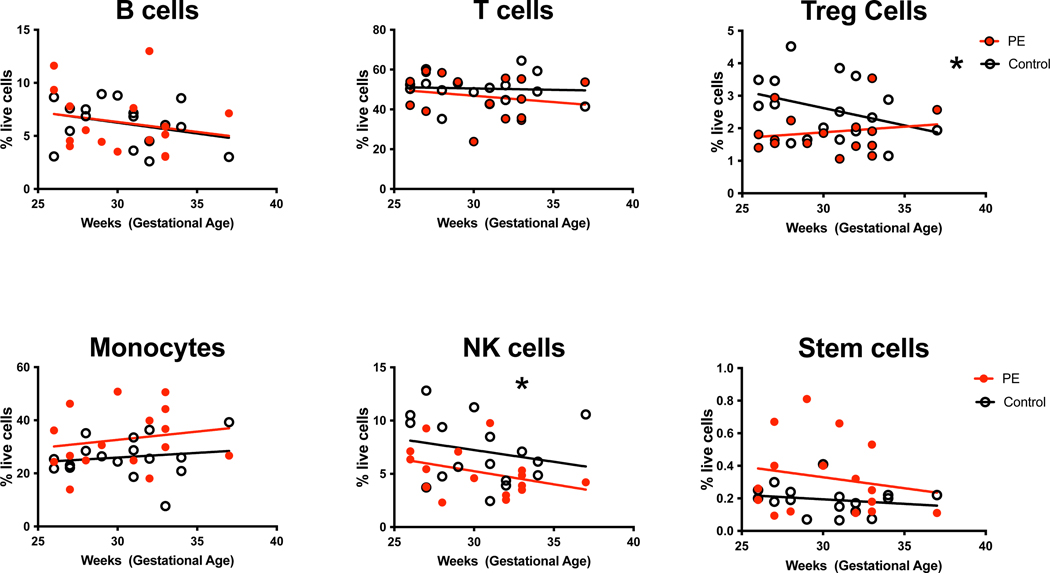
Maternal immune and stem cell frequency is known to vary by gestational age in the peripheral circulation, so we performed linear regression analysis of subset frequency by gestational age (Figure 3). There was no significant difference in peripheral blood B cell (p=0.19), T cell (p=0.48), monocyte (p=0.37), or stem cell (p=0.38) frequency by gestational age and there was no significant difference in these subsets between PE cases compared to controls by gestational age (B cells p=0.99, T cells p=0.53, monocytes p=0.18, stem cells p=0.12). There was significantly decreased NK cell frequency seen with increased gestational age (p=0.04), but no difference in NK cell frequency in PE cases compared to controls (p=0.16) by gestational age. There was a significant decrease in Treg frequency in controls with increased gestational age (p=0.03). In contrast, there was no difference in Treg frequency by gestational age in PE cases (p=0.43), however there was not a significant difference between Tregs in PE cases and controls by gestational age (p=0.10).
Figure 3. Immune and Stem Cell Frequency by Gestational Age.
Maternal PBMC from PE cases and gestational-age matched controls were stained with immune and stem cell specific antibodies as described in Figure 1. The frequency of each subset among the total live cell population was plotted by gestational age. Statistical significant was determined by linear regression (*=p<0.05).
FMc Detection in PE Cases and Controls
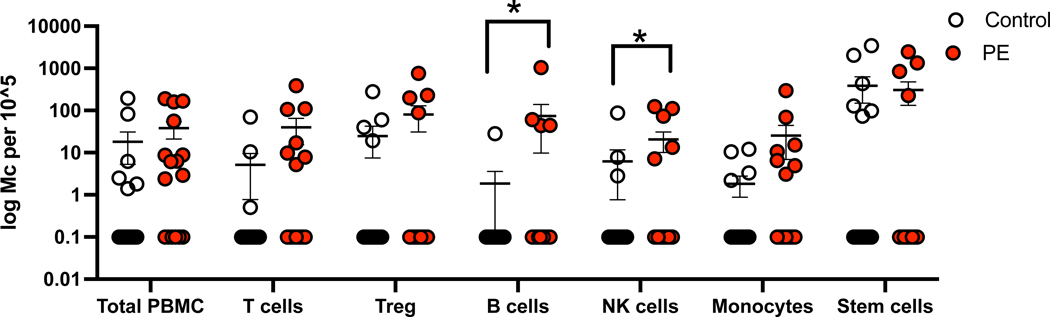
A higher proportion of PE cases (75%) were positive for FMc detection compared to controls (43.8%), however this was not statistically significant (p=0.15) (Table 2). FMc were detected at varying frequencies (6–44%) within all types of sorted immune and stem cell lineages. There was no significant difference in proportions of FMc frequency in individual immune or stem cell subsets between PE cases and controls. Although there was no significant difference in overall FMc concentration from total PBMCs between PE cases (median 38 genomic equivalents (gEq), 95%ile (2–189) and controls (18 gEq (1–194) (p=0.40), there was a significant increase in FMc concentration in the total sorted immune subsets in PE cases (545 gEq (5–2598)) compared to controls (428 gEq (22–3823)) (p=0.02) (Figure 4). There was a statistically significant increase in FMc concentration in B cell (p<0.01) and NK cell (p=0.01) subsets from PE cases versus controls and a trend towards increased FMc concentration in T cell (p=0.05), Treg (p=0.10), and monocyte (p=0.08) subsets in PE cases compared to controls. There was no difference in FMc concentrations in the stem cell subset (p=0.93).
Table 2.
FMc Detection and Concentration
| Normal Subjects (n=16) | PE Subjects (n=16) | p-value | |
|---|---|---|---|
| FMc detection overall | 7 (43.8%) | 12 (75%) | 0.15 |
| FMc detection in subsets | |||
| Innate/stem cell subsets positive | 7 (43.8%) | 8 (50%) | 1.0 |
| Adaptive cell subsets positive | 5 (31.2%) | 10 (62.5%) | 0.16 |
| T cell subsets positive | 3 (18.8%) | 7 (43.8%) | 0.25 |
| Treg subsets positive | 4 (25%) | 4 (25%) | 1.0 |
| B cell subsets positive | 1 (6.3%) | 4 (25%) | 0.33 |
| NK cell subsets positive | 3 (18.8%) | 5 (31.2%) | 0.69 |
| Monocyte subsets positive | 4 (25%) | 7 (43.8%) | 0.46 |
| Stem cell subsets positive | 6 (37.5%) | 5 (31.2%) | 1.0 |
| FMc concentration in PBMCs (gEq/105 gEq tested), mean, (range) |
18 (1–194) | 38 (2–189) | 0.40 |
| FMc concentration in subsets1 (gEq/105 gEq tested), mean, (range) |
428 (22–3823) | 545 (5–2598) | 0.02 |
| FMc concentration in T cells (gEq/105 gEq tested), mean, (range) |
5 (1–70) | 40 (5–385) | 0.05 |
| FMc concentration in Treg (gEq/105 gEq tested), mean, (range) |
25 (19–278) | 80 (88–764) | 0.10 |
| FMc concentration in B cells (gEq/105 gEq tested), mean, (range) |
2 (28) | 74 (44–1038) | <0.01 |
| FMc concentration in NK cells (gEq/105 gEq tested), mean, (range) |
6 (3–87) | 20 (7–123) | 0.01 |
| FMc concentration in Monocytes (gEq/105 gEq tested), mean, (range) |
2 (2–12) | 25 (3–297) | 0.08 |
| FMc concentration in Stem cells (gEq/105 gEq tested), | 389 (73–3437) | 305 (226–2464) | 0.933 |
| Normal Subjects (n=16) | PE Subjects (n=16) | 95% CI | |
| Likelihood of FMc detection (OR) | Ref | 3.9 | 0.84–17.7 |
Sum total of FMc among sorted immune and stem cell subsets
Figure 4. FMc Detection in Immune and Stem Cell Subsets.
FMc concentration was calculated by qPCR targeting of a fetal HLA-specific polymorphism from genomic DNA from total PBMC or sorted cell subsets in gestational-age matched (range between 26–37 weeks) PE cases and controls. FMc concentration is displayed as number of FMc per 100,000 total gEq tested.
Conclusions
Principal Findings
We found that FMc was present among multiple immune and stem cell subsets in maternal PBMC. There was no difference in the frequency of FMc detection between innate and adaptive immune subsets. While there was no statistical difference in FMc positivity between groups, there was a significant increase in FMc concentration in total immune cell subsets as well as individual B and NK cell subsets in PE cases compared to controls.
Results
We found no statistical difference in overall FMc frequency between PE cases and controls. This is in contrast to previous findings of increased FMc detection and concentration in PE cases compared to normal pregnancies40, likely related to a smaller sample size in this study. We did find, however, a trend towards increased FMc frequency in PE cases compared to controls, which is in line with the prior study40. Preeclampsia results in necrosis and apoptosis of syncytiotrophoblasts which is associated with disruption and increased permeability of the maternal-fetal interface49–52. The increased disruption of the maternal-fetal interface observed during the inflammatory process of PE is a plausible explanation for the increase in FMc concentration in certain immune cell subsets in PE cases compared to controls. This is supported from data demonstrating decreased maternal Mc from the woman’s own mother during PE, which would not be subject to alterations of the maternal-fetal interface53.
In this study of maternal blood samples, we found a similar frequency of FMc in innate (43%) and adaptive (31%) immune cell subsets in normal pregnancies. In contrast, in prior study of cord blood, maternal Mc is represented at higher frequency in adaptive immune cells, and particularly in memory T cells46. We did not test for FMc in memory T cells as these cells are infrequent in the fetus compared to adults and we would be unlikely to detect FMc within this population. The levels of T cells is similar and Tregs increased in the fetus compared to adults54,55, so the specific increase in T cells in maternal microchimerism (MMc) but not FMc compared to other immune populations may suggest the potential for selective transfer of T cells to the fetus, which may play a role in early neonatal immune protection. This study confirms prior findings demonstrating the presence of CD34+ pluripotent stem cells among the FMc population33,56,57. Stem cells were the least abundant cell type in maternal PBMC (~0.2% of total, ~200-fold less total stem cells sorted compared to T cells), but represented the majority of FMc positive subsets. This may reflect the increased frequency of circulating stem cells in the fetus compared to adults58. Human and animal studies have demonstrated that pluripotent FMc participate in the process of inflammation and wound healing59–62. The presence of substantial FMc in stem cells suggests a mechanism for long term maintenance of FMc which may provide a mechanism for the maintenance of pro- or anti-inflammatory programing.
Previous studies have demonstrated decreased NK cell and Treg frequency in the peripheral blood and decidua, which may be associated with the pathophysiology of PE63–70. We similarly found decreased maternal peripheral NK cells and Tregs in PE cases compared to controls. We also found decreased NK cell and Treg frequency with advancing gestational age in controls, similar to prior findings71,72. Interestingly, a decrease in Treg frequency with gestational age was not observed in the PE cases, potentially due to the overall decreased Tregs in the PE subjects.
Clinical Implications
PE is associated with long term maternal risks of CVD and metabolic disorders through largely unknown mechanisms. Here we demonstrate that FMc is present among the stem cell population in the maternal circulation during pregnancy. These long-lived pluripotent cells are acquired during pregnancy and have the potential to influence long-term maternal health as persistence of fetal origin Mc well beyond the reproductive years is well documented33,73–75. Our findings also suggest that PE may be associated with an altered distribution of FMc with increased concentration of B and NK cells from PE pregnancies. Further studies are needed to determine whether this altered FMc distribution influences gene expression, which may ultimately affect maternal inflammatory programing, with the possibility for long-lasting effects given the known persistence of FMc beyond pregnancy. Although PE is a heterogeneous disease with multiple potential inciting factors and varied pathophysiology, further investigations of the functional role of FMc during pregnancy and beyond may provide insights into therapeutic interventions to the reduce later-life maternal health complications associated with a history of PE.
Research Implications
This study suggests that PE may be associated with a different cellular phenotype of FMc in the maternal circulation, however whether gene expression of FMc is also altered remains unknown. PE is associated with inflammation and exposure to inflammation is known to alter cellular programing via epigenetic mechanisms, which can have long term health effects76,77. Given the rarity of Mc, detailed assessment of gene expression on the population level is technically challenging. The recent development of single cell techniques has enhanced the ability to study rare cells type and application of these techniques to Mc will allow more detailed characterization of FMc cellular phenotype as well as gene expression.
Strengths and Limitations
The strengths of this study include the use of well-defined PE cases, all of which have severe features, and comparison with controls confirmed to have uncomplicated pregnancies and matched by parity and gestational age of sample collection. The methodology of FMc detection is well established and validated. This is the first study to use flow cytometry to comprehensively investigate the phenotype of FMc in maternal peripheral immune and stem cell subsets.
A limitation of Mc studies is that Mc is present in only a fraction of samples. We found that FMc was present in 75% and 44% of PE cases and controls respectively which is similar to prior studies36,40. Despite this, differences are inherently present between cases and controls. Given the high rates of iatrogenic preterm delivery in the setting of PE with severe features, the vast majority of participants in this cohort received at least one dose of betamethasone, which was not received by the controls. Systemic steroids are known to affect immune cell mobilization and may influence our observed findings. The PE cases also had a higher frequency of Cesarean delivery, and mode of delivery is associated with differences in FMc detection, which may influence the findings in this study36.
Conclusions
Our study suggests that PE is associated with altered concentrations of immune cells within FMc in the maternal blood. Animal studies have demonstrated that transfer of pluripotent FMc can lead to development of functional immune cells78,79. It is currently unclear whether pluripotent FMCs are biased to distinct lineages prior to passage across the placenta, or if they acquire alternative fates in response to maternal environment80,81. This altered immune phenotype, as well as the presence of FMc in the stem cell population provides a potential mechanism for the inflammation which occurs during PE to have a long-lasting changes to the maternal immune system and may potentially play a role in promotion of chronic maternal disease81–84. Studies of larger populations and of the genetic programing of FMc are necessary to reveal the nuances in FMc phenotypes in normal and PE pregnancies.
Funding
This work was funded by a Preeclampsia Vision Grant to SM and RS and NIH R03 AI-159288 to SK and JLN.
Footnotes
Ethics approval
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The study conformed to the US Federal Policy for the Protection of Human Subjects.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Duley L.The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. The Lancet. 2010;376(9741):631–644. doi: 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- 3.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–333. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 4.Drost JT, Arpaci G, Ottervanger JP, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM). Eur J Prev Cardiol. 2012;19(5):1138–1144. doi: 10.1177/1741826711421079 [DOI] [PubMed] [Google Scholar]
- 5.Clifton VL, Stark MJ, Osei-Kumah A, Hodyl NA. Review: The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta. 2012;33 Suppl:S37–41. doi: 10.1016/j.placenta.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Veerbeek JHW, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65(3):600–606. doi: 10.1161/HYPERTENSIONAHA.114.04850 [DOI] [PubMed] [Google Scholar]
- 7.Bokslag A, Teunissen PW, Franssen C, et al. Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Obstet Gynecol. 2017;216(5):523.e1–523.e7. doi: 10.1016/j.ajog.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 8.Haug EB, Horn J, Markovitz AR, et al. Life Course Trajectories of Cardiovascular Risk Factors in Women With and Without Hypertensive Disorders in First Pregnancy: The HUNT Study in Norway. J Am Heart Assoc. 2018;7(15):e009250. doi: 10.1161/JAHA.118.009250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rana S, Lemoine E, Granger J, Karumanchi SA. Preeclampsia. Circ Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276 [DOI] [PubMed] [Google Scholar]
- 10.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122(6):579–584. doi: 10.1161/CIRCULATIONAHA.110.943407 [DOI] [PubMed] [Google Scholar]
- 11.de Havenon A, Delic A, Stulberg E, et al. Association of Preeclampsia With Incident Stroke in Later Life Among Women in the Framingham Heart Study. JAMA Netw Open. 2021;4(4):e215077. doi: 10.1001/jamanetworkopen.2021.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redman CWG, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta. 2003;24 Suppl A:S21–27. [DOI] [PubMed] [Google Scholar]
- 13.Kalagiri RR, Carder T, Choudhury S, et al. Inflammation in Complicated Pregnancy and Its Outcome. Am J Perinatol. 2016;33(14):1337–1356. doi: 10.1055/s-0036-1582397 [DOI] [PubMed] [Google Scholar]
- 14.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5(10):971–974. doi: 10.1038/ni1004-971 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol. 2017;124:44–53. doi: 10.1016/j.jri.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 16.Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol. 2016;38(6):635–649. doi: 10.1007/s00281-016-0574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141(6):715–724. doi: 10.1530/REP-10-0360 [DOI] [PubMed] [Google Scholar]
- 18.Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018;128(10):4224–4235. doi: 10.1172/JCI122182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui A, Yokota M, Funamizu A, et al. Changes of NK cells in preeclampsia. Am J Reprod Immunol. 2012;67(4):278–286. doi: 10.1111/j.1600-0897.2012.01120.x [DOI] [PubMed] [Google Scholar]
- 20.Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. 2016;12(2):209–227. doi: 10.1586/1744666X.2016.1105740 [DOI] [PubMed] [Google Scholar]
- 21.Luppi P, Powers RW, Verma V, Edmunds L, Plymire D, Hubel CA. Maternal circulating CD34+VEGFR-2+ and CD133+VEGFR-2+ progenitor cells increase during normal pregnancy but are reduced in women with preeclampsia. Reprod Sci. 2010;17(7):643–652. doi: 10.1177/1933719110366164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsanezhad ME, Attar A, Namavar-Jahromi B, et al. Changes in endothelial progenitor cell subsets in normal pregnancy compared with preeclampsia. J Chin Med Assoc. 2015;78(6):345–352. doi: 10.1016/j.jcma.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 23.Gammill HS, Lin C, Hubel CA. Endothelial progenitor cells and preeclampsia. Front Biosci. 2007;12:2383–2394. [DOI] [PubMed] [Google Scholar]
- 24.Lange LG, Schreiner GF. Immune mechanisms of cardiac disease. N Engl J Med. 1994;330(16):1129–1135. doi: 10.1056/NEJM199404213301607 [DOI] [PubMed] [Google Scholar]
- 25.Sjöholm A, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005;365(9459):610–612. doi: 10.1016/S0140-6736(05)17912-4 [DOI] [PubMed] [Google Scholar]
- 26.Panico C, Condorelli G. Unmet Needs in the Pathogenesis and Treatment of Cardiovascular Comorbidities in Chronic Inflammatory Diseases. Clin Rev Allergy Immunol. 2018;55(3):254–270. doi: 10.1007/s12016-017-8624-5 [DOI] [PubMed] [Google Scholar]
- 27.Medina G, Vera-Lastra O, Peralta-Amaro AL, et al. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol Res. 2018;133:277–288. doi: 10.1016/j.phrs.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 28.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6(8):584–594. doi: 10.1038/nri1897 [DOI] [PubMed] [Google Scholar]
- 29.Manokhina I, Wilson SL, Robinson WP. Noninvasive nucleic acid-based approaches to monitor placental health and predict pregnancy-related complications. Am J Obstet Gynecol. 2015;213(4 Suppl):S197–206. doi: 10.1016/j.ajog.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 30.Mitchell MD, Peiris HN, Kobayashi M, et al. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol. 2015;213(4 Suppl):S173–181. doi: 10.1016/j.ajog.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Mouillet JF, Ouyang Y, Coyne CB, Sadovsky Y. MicroRNAs in placental health and disease. Am J Obstet Gynecol. 2015;213(4 Suppl):S163–172. doi: 10.1016/j.ajog.2015.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gammill HS, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol. 2010;54(2–3):531–543. doi: 10.1387/ijdb.082767hg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93(2):705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams Waldorf KM, Gammill HS, Lucas J, et al. Dynamic changes in fetal microchimerism in maternal peripheral blood mononuclear cells, CD4+ and CD8+ cells in normal pregnancy. Placenta. 2010;31(7):589–594. doi: 10.1016/j.placenta.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson SE, Nelson JL, Guthrie KA, et al. Prospective assessment of fetal-maternal cell transfer in miscarriage and pregnancy termination. Hum Reprod. 2012;27(9):2607–2612. doi: 10.1093/humrep/des244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shree R, Harrington WE, Kanaan SB, et al. Fetal microchimerism by mode of delivery: a prospective cohort study. BJOG. 2019;126(1):24–31. doi: 10.1111/1471-0528.15432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadi VK. Fetal microchimerism and cancer. Cancer Lett. 2009;276(1):8–13. doi: 10.1016/j.canlet.2008.07.025 [DOI] [PubMed] [Google Scholar]
- 38.Kamper-Jørgensen M, Hjalgrim H, Andersen AMN, Gadi VK, Tjønneland A. Male microchimerism and survival among women. Int J Epidemiol. 2014;43(1):168–173. doi: 10.1093/ije/dyt230 [DOI] [PubMed] [Google Scholar]
- 39.Hallum S, Gerds TA, Sehested TSG, Jakobsen MA, Tjønneland A, Kamper-Jørgensen M. Impact of Male-Origin Microchimerism on Cardiovascular Disease in Women: A Prospective Cohort Study. Am J Epidemiol. 2021;190(5):853–863. doi: 10.1093/aje/kwaa250 [DOI] [PubMed] [Google Scholar]
- 40.Gammill HS, Aydelotte TM, Guthrie KA, Nkwopara EC, Nelson JL. Cellular fetal microchimerism in preeclampsia. Hypertension. 2013;62(6):1062–1067. doi: 10.1161/HYPERTENSIONAHA.113.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunku CC, Gadi VK, de Laval de Lacoste B, Guthrie KA, Nelson JL. Maternal and fetal microchimerism in granulocytes. Chimerism. 2010;1(1):11–14. doi: 10.4161/chim.1.1.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93(6):2033–2037. [PubMed] [Google Scholar]
- 43.Scaletti C, Vultaggio A, Bonifacio S, et al. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002;46(2):445–450. [DOI] [PubMed] [Google Scholar]
- 44.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133(1):e1–e25. doi: 10.1097/AOG.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 45.Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther. 2014;36(2):117–128. doi: 10.1159/000359969 [DOI] [PubMed] [Google Scholar]
- 46.Kanaan SB, Gammill HS, Harrington WE, et al. Maternal microchimerism is prevalent in cord blood in memory T cells and other cell subsets, and persists post-transplant. Oncoimmunology. 2017;6(5):e1311436. doi: 10.1080/2162402X.2017.1311436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239(4839):487–491. doi: 10.1126/science.2448875 [DOI] [PubMed] [Google Scholar]
- 48.Guthrie KA, Gammill HS, Kamper-Jørgensen M, et al. Statistical Methods for Unusual Count Data: Examples From Studies of Microchimerism. Am J Epidemiol. 2016;184(10):779–786. doi: 10.1093/aje/kww093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1(1):61–76. doi: 10.1016/s0143-4004(80)80016-6 [DOI] [PubMed] [Google Scholar]
- 50.Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta. 2012;33(5):352–359. doi: 10.1016/j.placenta.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D, Gao Q, Wang Y, Xiong T. Placental dysfunction: The core mechanism for poor neurodevelopmental outcomes in the offspring of preeclampsia pregnancies. Placenta. 2022;126:224–232. doi: 10.1016/j.placenta.2022.07.014 [DOI] [PubMed] [Google Scholar]
- 52.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381 [DOI] [PubMed] [Google Scholar]
- 53.Gammill HS, Adams Waldorf KM, Aydelotte TM, et al. Pregnancy, microchimerism, and the maternal grandmother. PLoS ONE. 2011;6(8):e24101. doi: 10.1371/journal.pone.0024101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burt TD. Fetal Regulatory T Cells and Peripheral Immune Tolerance in utero: Implications for Development and Disease. Am J Reprod Immunol. 2013;69(4):346–358. doi: 10.1111/aji.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32(7):622–629. doi: 10.1016/j.exphem.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 56.Adams KM, Lambert NC, Heimfeld S, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102(10):3845–3847. doi: 10.1182/blood-2003-05-1570 [DOI] [PubMed] [Google Scholar]
- 57.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292(1):75–80. doi: 10.1001/jama.292.1.75 [DOI] [PubMed] [Google Scholar]
- 58.Lee MW, Jang IK, Yoo KH, Sung KW, Koo HH. Stem and progenitor cells in human umbilical cord blood. Int J Hematol. 2010;92(1):45–51. doi: 10.1007/s12185-010-0619-4 [DOI] [PubMed] [Google Scholar]
- 59.Nassar D, Droitcourt C, Mathieu-d’Argent E, Kim MJ, Khosrotehrani K, Aractingi S. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. The FASEB Journal. 2012;26(1):149–157. doi: 10.1096/fj.11-180695 [DOI] [PubMed] [Google Scholar]
- 60.Cómitre-Mariano B, Martínez-García M, García-Gálvez B, et al. Feto-maternal microchimerism: Memories from pregnancy. iScience. 2021;25(1):103664. doi: 10.1016/j.isci.2021.103664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bustos ML, Frías S, Ramos S, et al. Local and Circulating Microchimerism Is Associated with Hypersensitivity Pneumonitis. Am J Respir Crit Care Med. 2007;176(1):90–95. doi: 10.1164/rccm.200608-1129OC [DOI] [PubMed] [Google Scholar]
- 62.Kuroki M, Okayama A, Nakamura S, et al. Detection of maternal-fetal microchimerism in the inflammatory lesions of patients with Sjögren’s syndrome. Ann Rheum Dis. 2002;61(12):1041–1046. doi: 10.1136/ard.61.12.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murray EJ, Gumusoglu SB, Santillan DA, Santillan MK. Manipulating CD4+ T Cell Pathways to Prevent Preeclampsia. Front Bioeng Biotechnol. 2021;9:811417. doi: 10.3389/fbioe.2021.811417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cornelius DC, Amaral LM, Harmon A, et al. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309(8):R884–891. doi: 10.1152/ajpregu.00154.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149(1):139–145. doi: 10.1111/j.1365-2249.2007.03397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011;91(1–2):76–82. doi: 10.1016/j.jri.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 67.Steinborn A, Schmitt E, Kisielewicz A, et al. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol. 2012;167(1):84–98. doi: 10.1111/j.1365-2249.2011.04493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu P, Santner-Nanan B, Dahlstrom JE, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181(6):2149–2160. doi: 10.1016/j.ajpath.2012.08.032 [DOI] [PubMed] [Google Scholar]
- 69.Fukui A, Yokota M, Funamizu A, et al. Changes of NK cells in preeclampsia. Am J Reprod Immunol. 2012;67(4):278–286. doi: 10.1111/j.1600-0897.2012.01120.x [DOI] [PubMed] [Google Scholar]
- 70.Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. 2016;12(2):209–227. doi: 10.1586/1744666X.2016.1105740 [DOI] [PubMed] [Google Scholar]
- 71.Aghaeepour N, Ganio EA, Mcilwain D, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2(15). doi: 10.1126/sciimmunol.aan2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Apps R, Kotliarov Y, Cheung F, et al. Multimodal immune phenotyping of maternal peripheral blood in normal human pregnancy. JCI Insight. 2020;5(7). doi: 10.1172/jci.insight.134838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):103–108. [DOI] [PubMed] [Google Scholar]
- 74.Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351(9102):559–562. doi: 10.1016/S0140-6736(97)08357-8 [DOI] [PubMed] [Google Scholar]
- 75.Nelson JL. Microchimerism and autoimmune disease. N Engl J Med. 1998;338(17):1224–1225. doi: 10.1056/NEJM199804233381711 [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez RM, Suarez-Alvarez B, Salvanés R, et al. DNA Methylation Dynamics in Blood after Hematopoietic Cell Transplant. PLoS One. 2013;8(2). doi: 10.1371/journal.pone.0056931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mattina GF, Van Lieshout RJ, Steiner M. Inflammation, depression and cardiovascular disease in women: the role of the immune system across critical reproductive events. Ther Adv Cardiovasc Dis. 2019;13. doi: 10.1177/1753944719851950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujiki Y, Johnson KL, Peter I, Tighiouart H, Bianchi DW. Fetal Cells in the Pregnant Mouse Are Diverse and Express a Variety of Progenitor and Differentiated Cell Markers1. Biology of Reproduction. 2009;81(1):26–32. doi: 10.1095/biolreprod.108.074468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khosrotehrani K, Leduc M, Bachy V, et al. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol. 2008;180(2):889–897. doi: 10.4049/jimmunol.180.2.889 [DOI] [PubMed] [Google Scholar]
- 80.Sedov E, McCarthy J, Koren E, Fuchs Y. Fetomaternal microchimerism in tissue repair and tumor development. Dev Cell. 2022;57(12):1442–1452. doi: 10.1016/j.devcel.2022.05.018 [DOI] [PubMed] [Google Scholar]
- 81.Fugazzola L, Cirello V, Beck-Peccoz P. Fetal microchimerism as an explanation of disease. Nat Rev Endocrinol. 2011;7(2):89–97. doi: 10.1038/nrendo.2010.216 [DOI] [PubMed] [Google Scholar]
- 82.Boyon C, Collinet P, Boulanger L, Rubod C, Lucot JP, Vinatier D. Fetal microchimerism: benevolence or malevolence for the mother? Eur J Obstet Gynecol Reprod Biol. 2011;158(2):148–152. doi: 10.1016/j.ejogrb.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 83.Bianchi DW, Khosrotehrani K, Way SS, MacKenzie TC, Bajema I, O’Donoghue K. Forever Connected: The Lifelong Biological Consequences of Fetomaternal and Maternofetal Microchimerism. Clin Chem. 2021;67(2):351–362. doi: 10.1093/clinchem/hvaa304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kinder JM, Stelzer IA, Arck PC, Way SS. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol. 2017;17(8):483–494. doi: 10.1038/nri.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]