Abstract
Bovine babesiosis, caused by different Babesia spp. such as B. bovis, B. bigemina, B. divergens, and B. major, is a global disease that poses a serious threat to livestock production. Babesia bovis infections are associated with severe disease and increased mortality in adult cattle, making it the most virulent agent of bovine babesiosis. Babesia bovis parasites undergo asexual reproduction within bovine red blood cells, followed by sexual reproduction within their tick vectors, which transmit the parasite transovarially. Current control methods, including therapeutic drugs (i.e., imidocarb) have been found to lead to drug resistance. Moreover, changing environmental factors add complexity to efficient parasite control. Understanding the fundamental biology, host immune responses, and host–parasite interactions of Babesia parasites is critical for developing next-generation vaccines to control acute disease and parasite transmission. This systematic review analyzed available research papers on vaccine development and the associated immune responses to B. bovis. We compiled and consolidated the reported vaccine strategies, considering the study design and rationale of each study, to provide a systematic review of knowledge and insights for further research. Thirteen studies published since 2014 (inclusive) represented various vaccine strategies developed against B. bovis such as subunit, live attenuated, and viral vector vaccines. Such strategies incorporated B. bovis proteins or whole live parasites with the latter providing the most effective prophylaxis against bovine babesiosis. Incorporating novel research approaches, such as "omics" will enhance our understanding of parasite vulnerabilities.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-05885-z.
Keywords: Bovine babesiosis, Babesia bovis, Vaccine development, Immune response
Introduction
Babesia bovis is one of the most significant causative agents of babesiosis in cattle, with severe impacts on livestock industries. Substantial economic losses range from US$ 22 million in Australia to US$ 60 million in China [1]. Babesia bovis infection can cause significant economic losses due to its high morbidity and mortality rates in cattle. Because of its high prevalence in countries like China, Brazil, Turkey, and Thailand, poor identification and diagnosis of the parasites leads to cattle losses. The disease is transmitted by the tick Rhipicephalus microplus species complex, prevalent in tropical and subtropical regions in America, Africa, Australia, and Asia, where it is the most important ectoparasite of cattle [2]. Calves possess a level of immunity related to innate immune and age-related factors that remains for 6–8 months, while adult cattle are most at risk and are susceptible to clinical disease. Cattle infected with B. bovis can exhibit clinical signs which include fever, anemia, hemoglobinuria, lethargy, loss of appetite, and weight loss, leading to decreased milk and meat production [2, 3]. Infection with B. bovis can result in a high mortality rate, especially in susceptible breeds or animals not previously exposed to the pathogen [2]. However, certain Bos indicus cattle breeds indigenous to the Babesia endemic regions often possess a certain degree of natural resistance to the disease, thus resulting in mild to moderate disease clinical signs [2].
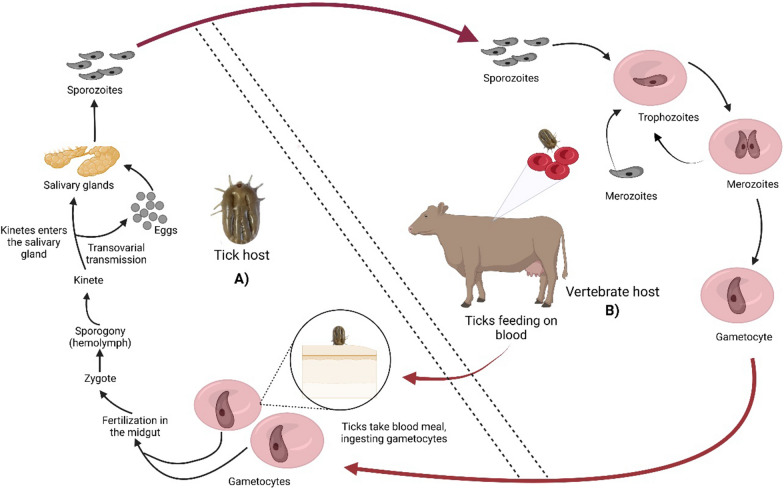
The life cycle of B. bovis infection is complex, involving sexual and asexual phases. The sexual reproductive phase occurs in the tick vector, whereas the asexual reproductive phase happens in the mammalian host [4, 5] (Fig. 1), specifically in the red blood cells (RBCs) [4]. When the zygote develops into an ookinete that migrates to the tick’s salivary glands as a kinete, then these kinetes develop into invasive sporozoites, which are transmitted by the tick to a new vertebrate host during a blood meal. Furthermore, transovarial transmission of B. bovis can also occur within the tick. The infection begins when Babesia spp. sporozoites enter the RBCs and multiply through asexual reproduction. When merozoites are released from these RBCs, they can reinfect other RBCs within the bovine host. Some of these parasites transform into male and female gametocytes. Once the tick ingests bovine blood containing these gametocytes, the sexual phase of the infection begins in the tick. The zygote, which is formed in the tick midgut when male and female gametocytes fuse, undergoes sporogony, a process that leads to the production of sporozoites [6].
Fig. 1.
Life cycle of Babesia bovis. Babesia bovis has two main phases: A The sexual cycle takes place in the tick host. The sexual cycle of B. bovis is initiated when a tick ingests gametocytes of the parasite during its blood meal on a mammalian host. Following ingestion, the gametocytes differentiate into male and female gametes within the tick's gut, which fuse to form a zygote. The zygote develops into an ookinete that migrates to the tick's salivary glands as a kinete, where it may be transmitted to a new vertebrate host during tick feeding. Kinetes gain access to the hemolymph of the tick, replicate, and invade various organs. Additionally, transovarial transmission of B. bovis can also occur within the tick. Subsequently, the kinetes develop into invasive sporozoites, which are transmitted by the tick to a new vertebrate host during a blood meal. B The asexual cycle occurs in the mammalian (vertebrate) host. During the asexual cycle of B. bovis, the sporozoites invade the red blood cells (RBCs) of the bovine host and develop into trophozoites and divide by binary fission, resulting in the formation of merozoites. These merozoites continue to proliferate, developing into trophozoites and eventually give rise to new merozoites. Within the RBCs, certain merozoites undergo differentiation into male and female gametocytes, which remain within the RBCs of the bovine host. These gametocytes are then acquired by ticks during their feeding process
Effective control measures against B. bovis infection include tick control through acaricides, vaccination, and babesicidal drugs [7–10]. However, they all have critical limitations. Acaricides are used to target tick vectors, but there is evidence that ticks are developing resistance [11, 12]. Also, in endemic regions, the use of drugs such as imidocarb is limited as they are expensive and cannot be used to prevent infection; in addition, suboptimal dosing might lead to the emergence of drug-resistant parasites, and could leave residual metabolites [13]. In extensive cattle production systems, the timely administration of babesicidal drugs poses a considerable challenge in the presence of clinical cases. Therefore, the combination of these control methods needs to be handled carefully. Countries such as Australia, South Africa, Argentina, Brazil, and Uruguay have utilized and produced live attenuated vaccines [14]. In Australia, prevention is mainly based on the use of a trivalent live vaccine which contains attenuated B. bovis and Babesia bigemina Australian strains, and Anaplasma centrale originally imported from South Africa is used to protect against Anaplasma marginale [15]. However, this has many significant limitations such as its short shelf-life of only 4 days from the production date when stored between 2 and 8 °C, possible reversion of the organisms to their virulent form, and issues in standardizing the dose [15]. Thus, further research is needed to develop new and effective control strategies against this disease. Developing an effective vaccine against B. bovis involves an understanding of the parasite’s biology and the immune response to infection. Several potential vaccine candidates have been identified including heat shock proteins [16], surface antigens [17, 18], and apical membrane proteins [19]. These different antigens have been shown to induce immune responses, but none to date have demonstrated protection against live pathogenic B. bovis challenge. The host’s immune response to the parasite is also a critical factor in the disease prognosis, with the development of immunity being a vital component in controlling the disease. In this systematic review, available research papers on vaccine development studies and the associated immune response to B. bovis were critically analyzed. The reported vaccine strategies were compiled and consolidated considering the study design and rationale of each study to provide a systematic review of knowledge and insights for further investigation.
Methods
Research selection and search criteria
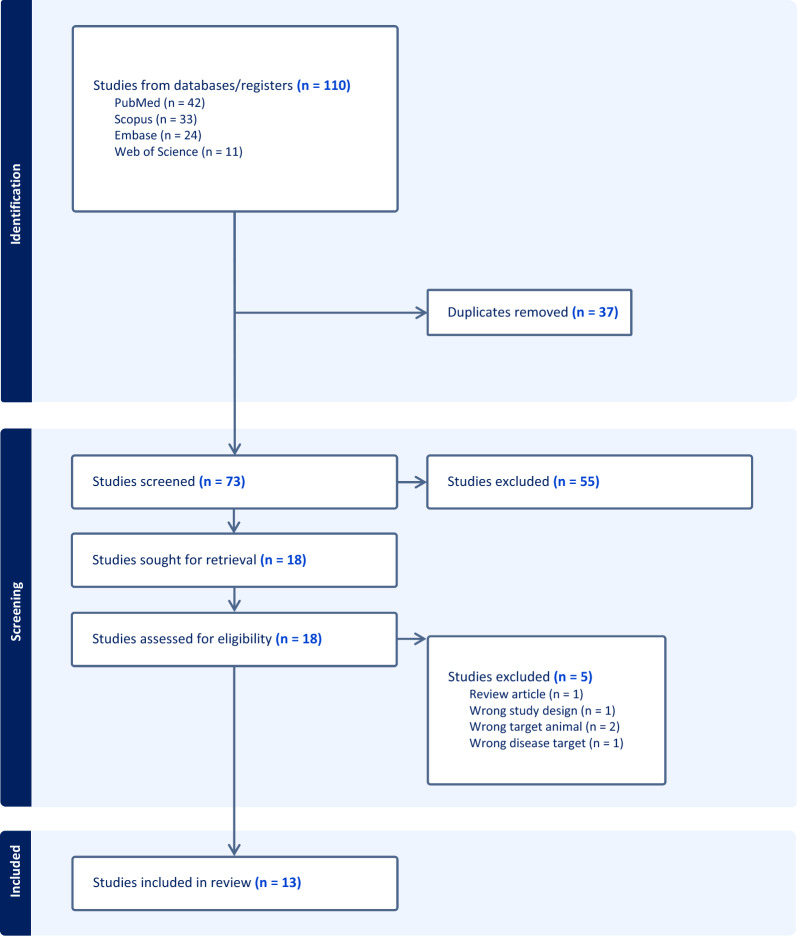
To collate the available research articles published within the last 10 years reporting B. bovis vaccine development and testing in bovines, searches were conducted in four electronic databases (PubMed, Web of Science, Embase, and Scopus) on 23 February 2023. This systematic review was framed around the review title “vaccine candidates for Babesia bovis” using the keywords “cattle,” “vaccine,” “babesiosis,” and “Babesia bovis” for the searches. The detailed search strategies for each database are listed in Additional file 1. The results generated by the databases were imported into EndNote and the duplicates were removed. In this systematic review, results that did not report original data were excluded, including reviews, conference abstracts, and book chapters. The research articles were downloaded, and articles that did not have English full text available were excluded. The titles and abstracts of all records were screened to filter out studies that performed vaccine tests and longitudinal evaluation and in hosts other than cattle and mice along with antigens derived from pathogens other than Babesia spp. The procedure in this systematic review was adopted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Fig. 2).
Fig. 2.
Flow chart of the study selection and identification process. The steps were adopted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines
Data extraction
Studies that met our inclusion criteria were subjected to the next phase of screening to extract the following information: journal name, year of publication, and vaccine type.
Results
Characteristics of studies included in the review
In total, 110 studies were retrieved from the customized searches. After removing the duplicates, the first screening was performed on 73 studies. Fifty-five studies were removed, including records that did not report original data, records that did not have an English full text, and the inclusion of animals that were not cattle. The full text of the remaining studies (n = 18) was further screened for eligibility, and 13 studies were eventually subjected to data extraction. The systematic review identified 13 studies on vaccine development for B. bovis that met the inclusion and eligibility criteria.
Table 1 shows the academic journals in which each study was published and the distribution of published research articles across several peer-reviewed journals that focus on the development of a vaccine for B. bovis. According to the data, Parasites & Vectors and Vaccine were the most prolific journals, publishing three papers on the topic, followed closely by Pathogens with two publications. The remaining journals, namely Ticks and Tick-borne Diseases, Veterinary Parasitology, Frontiers in Immunology, Frontiers in Veterinary Science, and Revista Brasileira de Parasitologia Veterinária, had only one publication each. This suggests that researchers have published a modest number of papers on B. bovis vaccine development in recent years.
Table 1.
Number of published papers by journal from 2014 to 2022
| Journal name | Number of papers |
|---|---|
| Parasites & Vectors | 3 |
| Vaccine | 3 |
| Pathogens | 2 |
| Ticks and Tick-borne Diseases | 1 |
| Veterinary Parasitology | 1 |
| Frontiers in Immunology | 1 |
| Frontiers in Veterinary Science | 1 |
| Revista Brasileira de Parasitologia Veterinária | 1 |
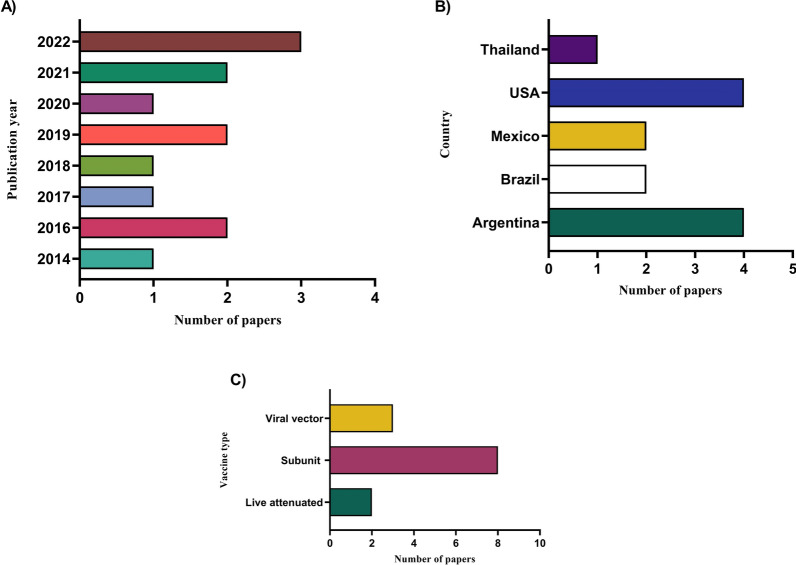
The B. bovis vaccine development papers included in this systematic review were published between 2014 and 2022. Figure 3A presents the number of papers published, indicating a general upward trend in the number of publications, with a marked increase between 2020 and 2022. Geographically, the studies reviewed were mainly from Argentina (n = 4) and the USA (n = 4), which together accounted for 62% (8/13) of the papers included in this review (Fig. 3B). Figure 3C depicts the number of papers stratified by vaccine type, indicating that subunit vaccines (n = 8) were the most frequently reported vaccine strategy, accounting for 62% of the reviewed papers. In summary, Fig. 3 suggests that research focused on B. bovis vaccine development is expanding, with a growing number of publications in recent years. Geographically, the Americas are at the forefront of this research, and recombinant vaccines appear to be the most widely studied vaccine strategy.
Fig. 3.
Number of studies categorized into A publication year, B geographical region, and C type of vaccine. The 13 papers were stratified according to their publication year, geographical region, and vaccine type used in the study
Characterization of B. bovis vaccine development studies
Most studies included in our systematic review reported the development of subunit vaccines (Fig. 3C, Table 2) [7, 18–24]. These subunit vaccines consisted of B. bovis membrane proteins, oligosaccharides, and merozoite surface antigens. Regarding live attenuated vaccines, B. bovis S74-T3Bo was attenuated and used to immunize cattle [25]. Details of the included studies are provided in Tables 2 and 3.
Table 2.
Vaccine strategies developed against B. bovis
| Type of vaccine | Antigen(s)/strain(s) used | Findings | References |
|---|---|---|---|
| Live attenuated | B. bovis-attenuated S74 T3Bo strain | New alterations in the composition of immune cells in the bloodstream, as well as changes in the expression of cytokines, have been observed in peripheral blood. These changes are linked to the immune response against acute bovine babesiosis and are indicative of a protective effect | [25] |
| Subunit | B. bovis apical membrane antigen 1 (AMA-1), merozoite surface antigen 2c (MSA-2c), and rhoptry-associated protein 1 (RAP-1) proteins | AMA-1, MSA-2c, and RAP-1 contain conserved epitopes that are recognized by B and T cells. These epitopes trigger the production of neutralizing antibodies and promote a durable Th1 immune response | [21] |
| Subunit | Structural ectodomains I and II of B. bovis apical membrane antigen 1 [BbAMA-1(I/II)] | Cattle that have been immunized with BbAMA-1(I/II) exhibit substantially elevated levels of total immunoglobulin (Ig)G antibodies, along with a heightened ratio of IgG2 to IgG1. Furthermore, this immunization has been found to induce a robust Th1 cell response in the vaccinated cattle | [19] |
| Live attenuated | Stable transfected strain of B. bovis expressing an enhanced green fluorescent protein (eGFP) and a chimeric version of Bm86 (B. bovis/Bm86/eGFP) | Post-mortem analysis did not reveal any indication of parasites sequestering in the cerebral capillaries, which confirms that the strain has been attenuated. Additionally, this is the first documented case of B. bovis that has been genetically modified to express the tick antigen Bm86 on the surface of merozoites, triggering an antibody response against native Bm86 | [26] |
| Subunit | Synthetic ß-(1 → 6)-linked glucosamine oligosaccharides conjugated to tetanus toxoid (5GlcNH2-TT) | Experienced acute babesiosis, characterized by the adherence of infected erythrocytes to capillary vessels in the brain. Despite the production of antibodies against this antigen, they were unable to prevent the onset of the disease | [24] |
| Subunit | B. bovis 6-cysteine proteins A and B | Cattle that were immunized produced antibodies against r6cys A and r6cys B, but these antibodies were ineffective in inhibiting the sexual reproduction of the parasite in ticks | [7] |
| Subunit | B. bovis GPI-anchored surface antigen 1 (GASA-1) | When B. bovis in vitro cultures were exposed to anti-GASA-1 antibodies, there was a partial yet significant reduction in erythrocyte invasion. This suggests that the protein GASA-1 contains epitopes that are sensitive to neutralization by antibodies | [20] |
| Viral vector | B. bovis merozoite surface antigen 2c (MSA-2c), rhoptry-associated protein 1 (RAP-1), and heat shock protein 20 (HSP20) | The absence of protective effects observed with this recombinant formulation underscores the importance of conducting additional basic and clinical investigations in the bovine model to attain the desired level of effectiveness | [27] |
| Subunit | B. bovis rhoptry neck protein 2 (RON2) | RON2 as a novel B. bovis vaccine candidate antigen that contains conserved B-cell epitopes that elicit partially neutralizing antibodies | [22] |
| Subunit | B. bovis merozoite surface antigens (MSA-1, MSA-2b, and MSA-2c) | Calves up to 6 months of age, all the calves developed active immunity against B. bovis | [23] |
| Subunit | B. bovis merozoite surface antigens: MSA-2a1, MSA-2b, and MSA-2c | Elicited invasion-inhibitory antibodies and IFN-γ-producing cells | [18] |
| Viral vector | Chimeric multi-antigen of DNA fragments containing B- and T-cell epitopes of merozoite surface antigen 2c (MSA-2c), rhoptry-associated protein 1 (RAP-1) and heat shock protein 20 (HSP20) genes | Elevated levels of IgG, IFN-γ, CD4+ and CD8+ T cells were successfully attained | [28] |
| Viral vector | Immunodominant B- and T-cell epitopes of three B. bovis proteins: merozoite surface antigen 2c (MSA-2c), rhoptry-associated protein 1 (RAP-1), and heat shock protein 20 (HSP20) | The viral-vectored approach alone induced significant levels of IFN-γ and resulted in a higher ratio of IgG2a subclass | [29] |
Table 3.
Promising vaccine development studies and their prospects
| Vaccine type | Study | Limitation | Future research direction |
|---|---|---|---|
| Live attenuated | [25] | The absence of significant changes in immune cell behavior and cytokine expression patterns in animals infected with Att-S74-T3Bo and then superinfected with Vir-S74-T3Bo suggests that antibodies might play a role in providing protection during reinfection |
• In future vaccine trials, it is recommended to prioritize certain approaches such as using live attenuated parasites, genetically modified live parasites, Babesia antigens, and novel adjuvants • These methods should focus on activating myeloid cells (monocytes and neutrophils) and CD4+ T cells early on. It is also important to assess the balance of pro- and regulatory cytokines (TNF-α, CXCL10, IFN-γ, IL-10, and IL-4) in the peripheral blood as a potential indicator of protection against acute Babesia infection |
| Subunit | [21] | Despite using peptides of B. bovis antigens in vaccines that generate neutralizing immunity and involve CD4+ T cells, the resulting Th1 immune response was not effective in protecting vaccinated cattle against a virulent strain of B. bovis. This failure may be due to using a single antigen in the vaccine, which may not generate a strong enough immune response to block multiple stages of the invasion process |
• In this research, the scientists discovered peptides of MSA2c and AMA-1 that could generate neutralizing antibodies and IFN-γ, which is a Th1 cytokine associated with protection • However, it remains to be determined whether a combination of these peptides in a multi-antigen vaccine could enhance the effectiveness of the immune response, in terms of both humoral and cellular responses, or during exposure to a virulent strain |
| Subunit | [19] | Different B. bovis strains that significantly hinder the effectiveness of live vaccines and pose a challenge in creating subunit vaccines |
• Considering that BbAMA-1's structural domains I and II can elicit both humoral and cellular immune responses, especially Th1 responses, BbAMA-1 is a promising vaccine candidate for bovine babesiosis • This is significant because strain variations pose challenges to live vaccines and subunit vaccine development. BbAMA-1, being highly conserved, has the potential to overcome strain-related issues by providing protective immunity against various B. bovis strains in real-world conditions |
| Subunit | [7] | It is worth noting that B. bovis may not have all the necessary enzymes for complete N-linked glycosylation, unlike the Plasmodium parasite. As a result, the native 6cys proteins in B. bovis are expected to differ in terms of protein folding, surface glycan profile, stability, and other characteristics compared to recombinant 6cys proteins that undergo significant glycosylation when expressed in HEK 293 cells, which is a eukaryotic system |
• Peptides derived from B. bovis cys proteins that were used to generate rabbit antibodies may provide protective epitopes • As a result, future research efforts will be directed towards further investigating these specific regions of the 6cys A and 6cys B proteins to gain deeper insights into their immunogenicity and potential for protective immunity |
The significance of understanding immune responses against B. bovis
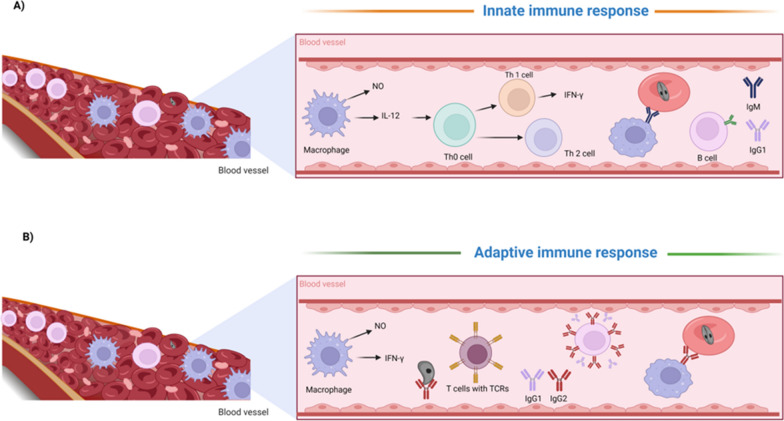
The induction of strong innate immune responses is necessary for immunity to Babesia parasites in young animals, while the development of effective adaptive immune responses is necessary for adult animals (Fig. 4). Studies have indicated that animals that have been successfully immunized with a subunit or live attenuated B. bovis or are persistently infected and have survived the acute stage of infection may rely on CD4+ T cells that are specific to the antigen and produce interferon gamma (IFN-γ) [18, 28, 29]. IFN-γ plays a crucial role in the immune response, as it can activate macrophages, is necessary for the elimination of parasites, and enhances the production of the neutralizing immunoglobulin (Ig)G2 antibody [30, 31]. Moreover, IFN-γ initiates and improves the adaptive immune response through IgG2 production by B cells, which, when combined with IgG1, protected cattle against homologous challenge [32]. Although protective neutralizing antibodies have been observed in live attenuated vaccines and persistently infected animals, the specific antigens that are targeted by these antibodies are still unknown. Moreover, the mechanism of T-cell activation and the role of distinct T-cell populations (such as γδ-T cells) in facilitating a successful adaptive immune response in vaccinated or persistently infected adult cattle requires further exploration.
Fig. 4.
Scheme of immune responses in bovines infected with Babesia parasites. A Schematic of innate immunity. Innate immune responses in young calves are characterized by the swift activation of macrophages and the abundant release of interferon-γ (IFN-γ) and nitric oxide (NO). Young, naive calves have a natural resistance to Babesia infection and typically survive exposure to Babesia-infected ticks in endemic areas. In contrast, adult animals are more susceptible to Babesia infection, which often results in acute and fatal babesiosis. However, if animals survive the acute infection, they may develop chronic babesiosis and produce life-long protective immune responses. Additionally, the innate immune response is more pronounced in young animals compared to adult animals. B Schematic of adaptive immunity in persistently infected or vaccinated animals with live attenuated vaccine. Macrophages and protective neutralizing antibodies are deemed critical for controlling parasitemia in these animals
Discussion
The cattle’s immune response to B. bovis infection plays a vital role in the outcome of the disease, influencing the severity of clinical signs, the level of parasitemia, and the development of immunity. In this systematic review of 13 studies, which included three vaccine strategies (subunit, live attenuated, and viral vector), we found evidence of promising vaccine candidates that could be further developed to improve efficacy and temporal immunity against B. bovis.
Our current knowledge and understanding of the immune mechanisms underlying protection against bovine babesiosis remains inadequate due to a range of practical, ethical, and economic constraints associated with conducting bovine experiments and the lack of dependable small animal models for B. bovis studies. The data from our systematic review suggests that this knowledge gap impairs the development of effective vaccines since the absence of practical and reliable experimental systems limits the investigation of the underlying protective mechanisms. Despite significant advancements in comprehending the immune responses to B. bovis infections, this challenge persists. The ability to survive the acute stages of infection is dependent on age and robust innate immune responses, which must be followed by effective stimulation of immune mechanisms that lead to the production of antibodies. These antibodies play a critical role in controlling infection in vaccinated and persistently infected animals.
There is also an age-related immunity to primary infection with B. bovis. Upon initial infection with B. bovis, young calves (less than 6 months) are generally immune to developing serious disease as seen in vulnerable adults [31]. Initially, this phenomenon was thought to be due to passive immunity from the protective antibodies in the colostrum [33]. However, subsequent studies found that the immune response of young calves to B. bovis infection includes the early activation of IFN-γ and interleukin (IL)-12 and the existence of inducible nitric oxide synthase (iNOS) messenger RNA (mRNA) expression in the spleen [34]. In contrast, iNOS was not induced, and IFN-γ and IL-12 were activated later in B. bovis infection in adult cattle. Another possible explanation for increased resistance in calves is the presence of a high proportion of γδ-T cells, which encompasses up to 70% circulating T cells in calves and 30% in adult cattle [35] or reduced pro-inflammatory cytokines in response to infection which may aid in pathogenesis [36]. In addition, the spleen is an important organ in controlling the infection as indicated by splenomegaly during acute babesiosis, and elevated levels of parasitemia in splenectomized animals [34]. The effective activation of innate immune mechanisms in young animals can result in the development of a protective adaptive immune response in adult animals, which prevents the establishment of persistent disease or death due to the detrimental effects of uncontrolled acute infection. A deeper understanding of the mechanisms that confer resistance against acute B. bovis infection in young and naïve animals (i.e., innate immunity), as well as those necessary to control parasitemia to persistently low levels in adult cattle that have survived the infection (i.e., adaptive immunity), is crucial for the development of vaccination strategies. Further research is essential to address this important research gap.
Progress and challenges in B. bovis vaccine development
As babesiosis significantly affects the animal and livestock industry, more effective control of this parasite would decrease the burden of disease [13]. The primary obstacle in controlling babesiosis is the lack of effective vaccines and the development of anti-babesial drug resistance [8, 13].
Common strategies for controlling babesiosis include tick management, anti-babesial drugs, and administration of live attenuated vaccines [26]. In Australia, live attenuated vaccines are generated in splenectomized calves, while in South America, in vitro culture of the Babesia parasites has been successfully utilized and has paved the way for vaccine development. Currently, available live attenuated vaccines that contain viable Babesia-infected RBCs have several limitations, such as the transmission risk of contaminating blood-borne pathogens and the risk of reversion of the Babesia parasite to a virulent phenotype [26]. Additionally, it is also a problem to keep the donor cattle Babesia spp.-free when vaccine preparations or production are carried out in tick-endemic countries [9]. Lastly, the vaccine needs a longer shelf-life and a cold chain to retain its efficiency, which is a challenge in tropical regions [18].
Babesia parasites produced in vitro have been a novel strategy for developing a vaccine against B. bovis [9]. Vaccines produced by in vitro methods are less likely to transmit pathogens, and at the same time, they allow more controlled and standardized conditions [37]. However, the critical limitation of this method is that it needs a constant supply of serum and erythrocytes from donor animals as well as sufficient laboratory equipment and trained staff [9]. It was found that vaccines based on live B. bovis did not result in an immune response that could eliminate the parasite, but rather generated a disease-resistant carrier that could serve as a reservoir for tick transmission [38]. Despite the progress made in vaccine development against B. bovis, several gaps in knowledge remain. A significant gap is the lack of understanding of cattle's immune response to B. bovis infection. The role of T cells and cytokines in protective immunity is not well understood, and further research is needed to elucidate the mechanisms of immune protection. As highlighted above, immune responses to Babesia involve innate and adaptive immune systems.
In comparison with mammals, arthropods lack the capacity to develop adaptive immune responses, and their immune system is less complex. Moreover, Babesia parasites have co-evolved with ticks and with their hosts by developing the ability to undergo biological amplification and sexual reproduction without affecting and stimulating the tick immune system. However, studies focusing on the modulation of Babesia proteins in tick immunity are lacking and some research with other invertebrates has provided insights into the immune system of ticks [39]. Altogether, it is tempting to speculate that Babesia proteins that regulate the tick’s immune system to initiate infection could be ideal candidates for transmission-blocking vaccines, but these antigens are yet to be identified. Further, more research is needed on host antigen presentation to stimulate CD4+ T cells and provide helper B cells. Better knowledge and understanding of the innate immune responses in naïve and young animals infected with B. bovis, antibody responses in adult animals with high levels of parasitemia, and tick immune responses to Babesia parasites are vital to developing and designing strategies to induce protective immunity, and thus further research is warranted to close this critical gap of knowledge. Furthermore, it is important to note that the limited understanding of the genetic diversity of B. bovis strains is another knowledge gap. The efficacy of vaccines could be impacted by the genetic diversity of the parasite, underscoring the need for further research to characterize the genetic diversity of B. bovis strains worldwide. Therefore, closing these critical knowledge gaps is essential to design effective strategies for inducing protective immunity against B. bovis infection.
The safety and efficacy of vaccines in field conditions are also critical gaps in knowledge. Vaccine efficacy studies often rely on experimental challenge studies in controlled environments, which may not reflect the real-world conditions of cattle production. Further studies are needed to evaluate the safety and efficacy of vaccines under field conditions and in different cattle populations.
Modern molecular toolkit and future directions
The prevention and management of bovine babesiosis present significant biological challenges and research gaps that need to be addressed for the development of effective control methods. However, the continuous influx of breakthroughs and technological advancements in the field of both bovine babesiosis and closely related apicomplexans supports the successful development of innovative methods aimed at more efficient control of bovine babesiosis. For instance, the sequencing of relevant Babesia spp. genomes, beginning with the publication of the first complete B. bovis genome in 2007, has resulted in important advances in our understanding of parasite biology [40]. These advancements have been facilitated by the development of transfection systems for gene modification and functional analysis, accelerating vaccine candidate discovery [13]. As a result of genomic advancements, critical genes and gene families involved in host immune evasion and sexual stage development, such as the large and antigenically variable ves1 gene family [41], 6-Cys [42], CCp1-3 [43], CPW-WPC [44], and HAP2 [45], have been completely identified [7]. Additionally, conserved master regulatory genes such as AP2 [46] have been identified and found to be responsible for transcriptional control of genes involved in parasite stage transitions, similar to what has been observed in Plasmodium and Theileria parasites [46, 47].
The use of “omic” techniques, such as transcriptomics, enabled the comparison of virulent and attenuated Babesia spp. strains to identify virulence factors and attenuation markers, a critical research gap in developing effective vaccines [13]. The implementation of these techniques holds promise in identifying the peptides presented by major histocompatibility complex (MHC) class II molecules to CD4+ T cells and genes that are differentially expressed during the different stages of the parasite's life cycle, thereby facilitating the discovery of novel and promising vaccine targets.
Altogether, the information and advanced genetic manipulation techniques will play a crucial role in creating innovative vaccines that could target the different stages of the Babesia parasite's life cycle. This approach may be critical for effectively managing the disease in the future.
Conclusions
The biology of Babesia parasites has been a subject of intense research interest over the years, owing to their ability to cause babesiosis in humans and animals. The advancement of research in this area has been heavily influenced by the advances in molecular and cellular biology, immunology, computational sciences, and vaccinology. In this systematic review, we provided an overview of the current state of knowledge regarding B. bovis vaccine development studies and highlighted the areas that require further investigation.
A complete understanding of the biology of Babesia parasites is critical for the development of effective strategies for controlling bovine babesiosis. This includes a better and more detailed characterization of the different distinct phases in the parasite’s life cycle, and the interactions between Babesia parasites and their host. Recent advances in genomic and proteomic technologies have provided a wealth of information on the biology of Babesia parasites, including their virulence factors, antigenic variation, and host–parasite interactions. However, several challenges need to be addressed. In practical terms, research in the biology of Babesia parasites will require multidisciplinary approaches, including the integration of molecular and cellular biology, immunology, computational sciences, and vaccinology. The availability of these tools provides an appropriately equipped toolbox for guiding researchers on a successful journey towards bovine babesiosis control. Overall, the advancement of research in the biology of Babesia parasites is critical for the development of effective strategies for controlling bovine babesiosis. The multidisciplinary approaches and the availability of advanced technologies provide an optimistic outlook for future research in this area. However, the development of an effective vaccine remains a critical challenge that warrants further attention.
Supplementary Information
Additional file 1: Keywords and search strategies for each database
Acknowledgements
The authors would like to acknowledge BioRender used to create the figures presented in this article. The authors would also like to express their gratitude to The University of Queensland-Research Training Program (UQ-RTP) for providing the PhD scholarship to John Harvey Santos. We thank Mst. Sogra Banu Juli (UQ) for her invaluable suggestions for keywords and paper search for systematic review.
Author contributions
JHMS wrote and revised the manuscript, and prepared the figures and tables. HVS, AR, DIS, MFG, and AT reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
PhD scholarship awarded to John Harvey Santos by The University of Queensland Graduate School.
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors provide this consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bal MS, Mahajan V, Filia G, Kaur P, Singh A. Diagnosis and management of bovine babesiosis outbreaks in cattle in Punjab state. Vet World. 2016;9:1370–1374. doi: 10.14202/vetworld.2016.1370-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock R, Jackson L, De Vos A, Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:S247–S269. doi: 10.1017/S0031182004005190. [DOI] [PubMed] [Google Scholar]
- 3.Kirupananthan R, Kamaral LCJ, Galhena GH, Perera KLN, Magamage MPS. Address the public health and food security concerns of babesiosis through molecular detection of Babesia bovis in suspected carrier cattle of selected localities in Sri Lanka. Procedia Food Sci. 2016;6:213–219. doi: 10.1016/j.profoo.2016.02.053. [DOI] [Google Scholar]
- 4.Bock R, De Vos A, Kingston T, Shiels I, Dalgliesh R. Investigations of breakdowns in protection provided by living Babesia bovis vaccine. Vet Parasitol. 1992;43:45–56. doi: 10.1016/0304-4017(92)90047-D. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalovecka M, Hajdusek O, Sojka D, Kopacek P, Malandrin L. The complexity of piroplasms life cycles. Front Cell Infect Microbiol. 2018;8:248. doi: 10.3389/fcimb.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzan HF, Bastos RG, Ueti MW, Laughery JM, Rathinasamy VA, Cooke BM, et al. Assessment of Babesia bovis 6cys A and 6cys B as components of transmission blocking vaccines for babesiosis. Parasites Vectors. 2021;14:210. doi: 10.1186/s13071-021-04712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuvshintulga B, Sivakumar T, Yokoyama N, Igarashi I. Development of unstable resistance to diminazene aceturate in Babesia bovis. Int J Parasitol Drugs Drug Resist. 2019;9:87–92. doi: 10.1016/j.ijpddr.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florin-Christensen M, Suarez CE, Rodriguez AE, Flores DA, Schnittger L. Vaccines against bovine babesiosis: where we are now and possible roads ahead. Parasitology. 2014;141:1563–1592. doi: 10.1017/S0031182014000961. [DOI] [PubMed] [Google Scholar]
- 10.Kiss T, Cadar D, Spînu M. Tick prevention at a crossroad: new and renewed solutions. Vet Parasitol. 2012;187:357–366. doi: 10.1016/j.vetpar.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Obaid MK, Islam N, Alouffi A, Khan AZ, da Silva VI, Jr Tanaka T, et al. Acaricides resistance in ticks: selection, diagnosis, mechanisms, and mitigation. Front Cell Infect Microbiol. 2022;12:941831. doi: 10.3389/fcimb.2022.941831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vudriko P, Okwee-Acai J, Tayebwa DS, Byaruhanga J, Kakooza S, Wampande E, et al. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Paras Vectors. 2016;9:4. doi: 10.1186/s13071-015-1278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez CE, Alzan HF, Silva MG, Rathinasamy V, Poole WA, Cooke BM. Unravelling the cellular and molecular pathogenesis of bovine babesiosis: is the sky the limit? Int J Parasitol. 2019;49:183–197. doi: 10.1016/j.ijpara.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Castro JJ. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet Parasitol. 1997;71:77–97. doi: 10.1016/S0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- 15.Standfast NF, Bock RE, Wiecek MM, Devos AJ, Jorgensen WK, Kingston TG. Overcoming constraints to meeting increased demand for Babesia bigemina vaccine in Australia. Vet Parasitol. 2003;115:213–222. doi: 10.1016/S0304-4017(03)00223-1. [DOI] [PubMed] [Google Scholar]
- 16.Norimine J, Mosqueda J, Palmer GH, Lewin HA, Brown WC. Conservation of Babesia bovis small heat shock protein (Hsp20) among strains and definition of T helper cell epitopes recognized by cattle with diverse major histocompatibility complex class II haplotypes. Infect Immun. 2004;72:1096–1106. doi: 10.1128/iai.72.2.1096-1106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines SA, Palmer GH, Jasmer DP, McGuire TC, McElwain TF. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol Biochem Parasitol. 1992;55:85–94. doi: 10.1016/0166-6851(92)90129-8. [DOI] [PubMed] [Google Scholar]
- 18.Gimenez AM, Françoso KS, Ersching J, Icimoto MY, Oliveira V, Rodriguez AE, et al. A recombinant multi-antigen vaccine formulation containing Babesia bovis merozoite surface antigens MSA-2a1, MSA-2b and MSA-2c elicits invasion-inhibitory antibodies and IFN-γ producing cells. Parasites Vectors. 2016;9:1–13. doi: 10.1186/s13071-016-1862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rittipornlertrak A, Nambooppha B, Muenthaisong A, Apinda N, Koonyosying P, Srisawat W, et al. Immunization of cattle with recombinant structural ectodomains I and II of Babesia bovis apical membrane antigen 1 [BbAMA-1(I/II)] induces strong Th1 immune response. Front Vet Sci. 2022;9:917389. doi: 10.3389/fvets.2022.917389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores DA, Rodriguez AE, Tomazic ML, Torioni de Echaide S, Echaide I, Zamorano P, et al. Characterization of GASA-1, a new vaccine candidate antigen of Babesia bovis. Vet Parasitol. 2020;287:109275. doi: 10.1016/j.vetpar.2020.109275. [DOI] [PubMed] [Google Scholar]
- 21.Hidalgo-Ruiz M, Mejia-López S, Pérez-Serrano RM, Zaldívar-Lelo de Larrea G, Ganzinelli S, Florin-Christensen M, et al. Babesia bovis AMA-1, MSA-2c and RAP-1 contain conserved B and T-cell epitopes, which generate neutralizing antibodies and a long-lasting Th1 immune response in vaccinated cattle. Vaccine. 2022;40:1108–1115. doi: 10.1016/j.vaccine.2022.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo-Ruiz M, Suarez CE, Mercado-Uriostegui MA, Hernandez-Ortiz R, Ramos JA, Galindo-Velasco E, et al. Babesia bovis RON2 contains conserved B-cell epitopes that induce an invasion-blocking humoral immune response in immunized cattle. Parasites Vectors. 2018;11:575. doi: 10.1186/s13071-018-3164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matos CA, Gonçalves LR, Alvarez DO, Freschi CR, Silva JBD, Val-Moraes SP, et al. Longitudinal evaluation of humoral immune response and merozoite surface antigen diversity in calves naturally infected with Babesia bovis, in São Paulo. Brazil Rev Bras Parasitol Vet. 2017;26:479–490. doi: 10.1590/s1984-29612017069. [DOI] [PubMed] [Google Scholar]
- 24.Taus NS, Cywes-Bentley C, Johnson WC, Pier GB, Fry LM, Mousel MR, et al. Immunization against a conserved surface polysaccharide stimulates bovine antibodies with opsonic killing activity but does not protect against Babesia bovis challenge. Pathogens. 2021;10:1598. doi: 10.3390/pathogens10121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastos RG, Laughery JM, Ozubek S, Alzan HF, Taus NS, Ueti MW, et al. Identification of novel immune correlates of protection against acute bovine babesiosis by superinfecting cattle with in vitro culture attenuated and virulent Babesia bovis strains. Front Immunol. 2022;13:1045608. doi: 10.3389/fimmu.2022.1045608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazuz ML, Laughery JM, Lebovitz B, Yasur-Landau D, Rot A, Bastos RG, et al. Experimental infection of calves with transfected attenuated Babesia bovis expressing the Rhipicephalus microplus Bm86 antigen and eGFP marker: preliminary studies towards a dual anti-tick/Babesia vaccine. Pathogens. 2021;10:135. doi: 10.3390/pathogens10020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaramillo Ortiz JM, Paoletta MS, Gravisaco MJ, López Arias LS, Montenegro VN, de la Fournière SAM, et al. Immunisation of cattle against Babesia bovis combining a multi-epitope modified vaccinia Ankara virus and a recombinant protein induce strong Th1 cell responses but fails to trigger neutralising antibodies required for protection. Ticks Tick Borne Dis. 2019;10:101270. doi: 10.1016/j.ttbdis.2019.101270. [DOI] [PubMed] [Google Scholar]
- 28.Jaramillo Ortiz JM, Molinari MP, Gravisaco MJ, Paoletta MS, Montenegro VN, Wilkowsky SE. Evaluation of different heterologous prime–boost immunization strategies against Babesia bovis using viral vectored and protein-adjuvant vaccines based on a chimeric multi-antigen. Vaccine. 2016;34:3913–3919. doi: 10.1016/j.vaccine.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 29.Jaramillo Ortiz JM, Del Médico Zajac MP, Zanetti FA, Molinari MP, Gravisaco MJ, Calamante G, et al. Vaccine strategies against Babesia bovis based on prime-boost immunizations in mice with modified vaccinia Ankara vector and recombinant proteins. Vaccine. 2014;32:4625–4632. doi: 10.1016/j.vaccine.2014.06.075. [DOI] [PubMed] [Google Scholar]
- 30.Homer MJ, Aguilar-Delfin I, Telford SR, III, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/CMR.13.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown WC, Norimine J, Knowles DP, Goff WL. Immune control of Babesia bovis infection. Vet Parasitol. 2006;138:75–87. doi: 10.1016/j.vetpar.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney D. Studies on the protection of cattle against Babesia bovis infection. In: Morrison WI, editor. The Ruminant Immune System in Health and Disease. Cambridge, UK: Cambridge University Press; 1986. pp. 539–554. [Google Scholar]
- 33.Mahoney D. Bovine babesiosis: the passive immunization of calves against Babesia argentina with special reference to the role of complement fixing antibodies. Exp Parasitol. 1967;20:119–124. doi: 10.1016/0014-4894(67)90029-X. [DOI] [PubMed] [Google Scholar]
- 34.Goff WL, Johnson WC, Parish SM, Barrington GM, Tuo W, Valdez RA. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-γ and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 2001;23:463–471. doi: 10.1046/j.1365-3024.2001.00402.x. [DOI] [PubMed] [Google Scholar]
- 35.Hein WR, Mackay CR. Prominence of γδ T cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 36.Clark I, Jacobson L. Do babesiosis and malaria share a common disease process? Ann Trop Med Parasitol. 1998;92:483–488. doi: 10.1080/00034989859456. [DOI] [PubMed] [Google Scholar]
- 37.Shkap V, de Vos AJ, Zweygarth E, Jongejan F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: the continuing necessity. Trends Parasitol. 2007;23:420–426. doi: 10.1016/j.pt.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajdusek O, Sima R, Ayllon N, Jalovecka M, Perner J, de la Fuente J, et al. Interaction of the tick immune system with transmitted pathogens. Front Cell Infect Microbiol. 2013;16:26. doi: 10.3389/fcimb.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brayton K, Lau A, Herndon D, Hannick L, Kappmeyer L. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–1413. doi: 10.1371/journal.ppat.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allred DR, Carlton JM-R, Satcher RL, Long JA, Brown WC, Patterson PE, et al. The ves multigene family of B. bovis encodes components of rapid antigenic variation at the infected erythrocyte surface. Mol Cell. 2000;5:153–162. doi: 10.1016/S1097-2765(00)80411-6. [DOI] [PubMed] [Google Scholar]
- 42.Alzan HF, Cooke BM, Suarez CE. Transgenic Babesia bovis lacking 6-Cys sexual-stage genes as the foundation for non-transmissible live vaccines against bovine babesiosis. Ticks Tick Borne Dis. 2019;10:722–728. doi: 10.1016/j.ttbdis.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Bastos RG, Suarez CE, Laughery JM, Johnson WC, Ueti MW, Knowles DP. Differential expression of three members of the multidomain adhesion CCp family in Babesia bigemina, Babesia bovis and Theileria equi. PLoS ONE. 2013;8:e67765. doi: 10.1371/journal.pone.0067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kangwanrangsan N, Tachibana M, Jenwithisuk R, Tsuboi T, Riengrojpitak S, Torii M, et al. A member of the CPW-WPC protein family is expressed in and localized to the surface of developing ookinetes. Malar J. 2013;12:129. doi: 10.1186/1475-2875-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussein HE, Bastos RG, Schneider DA, Johnson WC, Adham FK, Davis WC, et al. The Babesia bovis hap2 gene is not required for blood stage replication, but expressed upon in vitro sexual stage induction. PLoS Negl Trop Dis. 2017;11:e0005965. doi: 10.1371/journal.pntd.0005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alzan HF, Knowles DP, Suarez CE. Comparative bioinformatics analysis of transcription factor genes indicates conservation of key regulatory domains among Babesia bovis, Babesia microti, and Theileria equi. PLoS Negl Trop Dis. 2016;10:e0004983. doi: 10.1371/journal.pntd.0004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pieszko M, Weir W, Goodhead I, Kinnaird J, Shiels B. ApiAP2 factors as candidate regulators of stochastic commitment to merozoite production in Theileria annulata. PLoS Negl Trop Dis. 2015;9:e0003933. doi: 10.1371/journal.pntd.0003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Keywords and search strategies for each database
Data Availability Statement
Not applicable.