Abstract
Dengue hemorrhagic fever, the severe form of dengue virus infection, is believed to be an immunopathological response to a secondary infection with a heterologous serotype of dengue virus. Dengue virus capsid protein-specific CD4+ cytotoxic T-lymphocyte (CTL) clones were shown to be capable of mediating bystander lysis of non-antigen-presenting target cells. After activation by anti-CD3 or in the presence of unlabeled antigen-presenting target cells, these clones could lyse both Jurkat cells and HepG2 cells as bystander targets. Lysis of HepG2 cells suggests a potential role for CD4+ CTL in the liver involvement observed during dengue virus infection. Three CD4+ CTL clones were demonstrated to lyse cognate, antigen-presenting target cells by a mechanism that primarily involves perforin, while bystander lysis occurred through Fas/Fas ligand interactions. In contrast, one clone used a Fas/Fas ligand mechanism to lyse both cognate and bystander targets. Cytokine production by the CTL clones was also examined. In response to stimulation with D2 antigen, CD4+ T-cell clones produced gamma interferon, tumor necrosis factor alpha (TNF-α) and TNF-β. The data suggest that CD4+ CTL clones may contribute to the immunopathology observed upon secondary dengue virus infections through direct cytolysis and/or cytokine production.
Dengue virus (DV) is a mosquito-borne flavivirus which is prevalent in many tropical and subtropical areas of the world. Infecting up to 100 million individuals yearly (10), DV infection can be either asymptomatic or it can present as one of two forms of disease. The most common form, classical dengue fever (DF), presents as a flu-like syndrome, with symptoms including fever, retroorbital headache, muscle aches, and bone pain. Liver involvement is also a frequent manifestation of DV infection, characterized by hepatomegaly and increased plasma levels of liver transaminases (19, 24, 35). Patients with dengue hemorrhagic fever (DHF), the more severe form of disease, develop hemoconcentration, thrombocytopenia, and increased capillary permeability, resulting in plasma leakage and sometimes hemorrhage. At its most severe, DHF can culminate in circulatory shock and death.
DV exists as four distinct serotypes, called types 1, 2, 3, and 4. After infection with DV, lifelong protective immunity is induced against the infecting serotype, although immunity to heterologous serotypes is short-lived (38). Studies have suggested that the immune response mounted upon reinfection with a heterologous serotype of DV may have detrimental consequences for the host. The vast majority of DHF cases have been shown to occur in individuals who are undergoing a secondary DV infection, suggesting that preexisting immunity to one serotype of DV may predispose an individual to the development of more severe disease (10).
A number of research studies from our laboratory have shown that DV serotype-cross-reactive memory CD4+ and CD8+ T cells exist after primary DV infection (reviewed in reference 28). Reactivation of serotype-cross-reactive memory T cells upon secondary infection possibly results in the increased level of T-cell activation often observed in individuals with DHF (27). Induction of immunopathology by CD4+ T lymphocytes may occur by various mechanisms, including cell-mediated cytotoxicity and/or cytokine production.
CD4+ T-cell-mediated cytotoxicity is thought to occur via two main pathways: release of perforin and granzymes from the activated cytotoxic T lymphocytes (CTL) (32, 44) or the interaction of Fas ligand on the T cell with Fas on the target cell (18, 32, 42, 44). The Fas/FasL pathway could contribute to the destruction of both cells presenting viral antigens as well as non-antigen-presenting “innocent bystander” cells which are expressing Fas, as has been demonstrated in other systems (4, 31, 40). Although the existence of virus-specific CD4+ CTL in humans has been demonstrated after viral infection or immunization (16, 26, 36, 49), the mechanism of cytolysis utilized by human virus-specific CD4+ CTL clones has not been extensively characterized. The direct destruction of certain cell types, either by cognate or bystander lysis, may result in some of the pathology which occurs in DHF.
Liver disease is commonly observed in patients with DV infections. Elevated levels of liver enzymes are detectable in serum, indicating hepatocyte injury, and frequently the liver is enlarged. Kupffer cells appear to be the primary cell type supporting DV infection in the liver (9); however, there is some debate as to whether hepatocytes can be infected with DV (9, 15). The cause of hepatocyte injury during DV infection is unknown, and we postulate that CD4+ CTL may mediate liver damage through a mechanism involving bystander lysis.
In addition to direct cytolysis, cytokine production by activated T cells may contribute to severe dengue disease. Some reports have suggested that production of tumor necrosis factor alpha (TNF-α) is increased in cases of severe dengue illness (23, 48), although production by DV-specific T lymphocytes has not been demonstrated. When administered experimentally to human volunteers, TNF-α has been shown to induce symptoms similar to those observed in DHF, including hypotension, capillary leakage, and a flu-like syndrome consisting of headache, nausea, and fatigue (14, 39, 41). Additionally, it has been demonstrated that both interleukin-2 (IL-2) and gamma interferon (IFN-γ) are elevated in DV-infected individuals compared to healthy controls or children with other febrile illnesses (27).
Previously, a panel of seven CD4+ CTL clones was generated from a D4-immune donor (7). These clones were shown to recognize the DV capsid protein, as well as DV-infected B-lymphoblastoid cell lines (BLCL). Six of the seven clones were demonstrated to be cross-reactive between the D2 and D4 serotypes of DV, indicating their potential to be reactivated in vivo in the event of a secondary D2V infection. In this study, a functional analysis of these CD4+ T-cell clones was performed, in which we examined both their mechanisms of target cell lysis and secretion of IFN-γ, TNF-α, and TNF-β after stimulation. Results of these analyses suggest several potential pathological roles of DV-specific CD4+ T cells activated during DV infection.
MATERIALS AND METHODS
Establishment of CTL clones.
Leukocytes were obtained by leukopheresis from a human volunteer at 6 months postvaccination with an experimental live-attenuated D4V vaccine, 314750, and DV-specific CD4+ CTL clones were established by using a limiting dilution technique as previously described (7). Clones were restimulated every 14 days with autologous peripheral blood mononuclear feeder cells, recombinant human IL-2 (Collaborative Biomedical Products) at a final concentration of 20 U/ml, and the anti-CD3 antibody 12F6, kindly supplied by Johnson Wong (Massachusetts General Hospital, Boston, Mass.).
Recognition of the DV capsid protein by these T-cell clones was determined as described earlier (7). Clones 8G5, 6E2, and 7E4 are D2/D4-cross-reactive and have been shown to recognize an epitope located between amino acids (aa) 83 and 92 of the D4V capsid protein. An additional clone (5C8) was identified that was specific for D4, which recognized aa 43 to 55 of the D4V capsid protein.
Bystander lysis assay.
The expression of functional FasL on the T-cell clones was assessed by bioassay for lysis of Jurkat cells as described earlier (37). Briefly, a 24-well plate was coated with anti-CD3 antibody by adding monoclonal antibody 12F6 (Johnson Wong) at 5 μg/ml in phosphate-buffered saline (PBS) to the wells followed by incubation at 4°C overnight. Wells were then washed twice with PBS to remove unbound antibody, and 1 × 106 to 2 × 106 CTL clones were added to the wells for 4 h at 37°C for activation. After activation, clones were collected and used in a CTL assay against 5,000 51Cr-labeled Jurkat target cells per well at various effector/target (E/T) ratios. Lysis was assessed after 6 and 18 h of incubation (44). To assess the bystander lysis of liver cells, a similar experiment was performed in which the human hepatocellular carcinoma cell line HepG2 was used as a bystander target at 2,000 cells per well.
Analysis of cytotoxic mechanisms.
CTL assays were performed in which 2,500 Jurkat or HepG2 cells and 2,500 autologous BLCL were present in the same well with 2.5 μg of the relevant peptide per ml to examine the mechanism of lysis used by CTL clones when both bystander and cognate target cells are present in the same well. For analysis of cognate killing, BLCL were 51Cr-labeled and Jurkat cells were unlabeled, and the assay was harvested after a 4-h incubation. To analyze bystander lysis, Jurkat cells were 51Cr labeled, BLCL were left unlabeled, and lysis was measured after an 8-h incubation. Clones were used directly or preincubated in 96-well plates with either brefeldin A (BFA; catalog number B7651; Sigma, St. Louis, Mo.) or concanamycin A (CMA; catalog number C9705; Sigma) at various concentrations for 2 h at 37°C prior to the addition of target cells.
The anti-human FasL antibody 4H9 (Medical & Biological Laboratories Co., Ltd., Tokyo, Japan) was used to inhibit Fas/FasL-mediated lysis of D4 antigen-pulsed BLCL by adding between 0.1 and 10 μg/ml to each well at the start of a 5-h cytotoxicity assay.
RT-PCR for perforin and FasL gene expression.
Reverse transcriptase PCR (RT-PCR) to determine perforin gene expression was performed as described by Van Voorhis et al. (43). RT-PCR for Fas ligand gene expression was performed as described by Hargreaves et al. (11).
Cytokine production by CTL clones.
CTL clones were tested for cytokine production by a method similar to that used by Jassoy et al. (17). T-cell clones (105) were plated in 96-well V-bottom plates (Costar) in a final volume of 200 μl in AIM-V media with 10% human AB serum. Cells were stimulated with DV antigens or Vero antigen at 1:200 or 1:320 or with peptide (capsid protein, aa 84 to 92) at 6.25 μg/ml in the presence of 105 gamma-irradiated autologous PBMC as feeder cells. DV and Vero antigens were prepared as previously described (25). Cells were incubated at 37°C for 24 h, at which point plates were spun at 200 × g for 5 min, and 150 μl of supernatant was collected from each well. Supernatants from replicate wells were pooled and then divided into aliquots and stored at −70°C until use. Production of cytokines was analyzed with commercially available enzyme-linked immunosorbent assay (ELISA) kits (Endogen, Boston, Mass.) according to the manufacturers’ instructions.
RESULTS
DV-specific CD4+ CTL clones lyse bystander target cells.
In contrast to CD8+ T cells, which are believed to mediate lysis of only cognate target cells presenting the relevant peptide in the appropriate major histocompatibility complex molecule, studies indicate that CD4+ CTL can efficiently lyse both cognate and bystander target cells (40). Bystander target cells are defined as cells that neither present antigen nor activate CTL but instead are lysed due to their proximity to the antigen-presenting target cell.
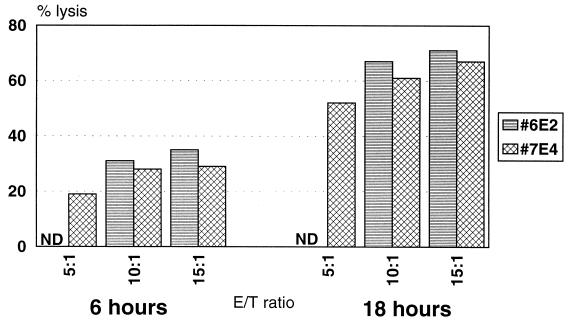
Two DV-specific CD4+ CTL clones were examined to determine whether they were capable of lysing Jurkat cells as bystander target cells. Jurkat cells are a human acute T-cell leukemia line that has been shown to be susceptible to Fas-mediated killing by T cells (37). The T-cell clones were activated on anti-CD3-coated plates for 4 h, and the lysis of Jurkat cells was then measured in a cytotoxicity assay. Figure 1 shows that clones 6E2 and 7E4 lysed Jurkat target cells after 6 h of incubation, and levels of lysis increased after 18 h of incubation. In the absence of preactivation, the clones showed no appreciable lysis (<5%) of Jurkat target cells (data not shown). These results indicate that clones 6E2 and 7E4 were able to lyse bystander target cells after nonspecific activation with anti-CD3.
FIG. 1.
DV-specific CD4+ CTL clones exhibit bystander lysis of Jurkat target cells. Clones 6E2 and 7E4 were preactivated for 4 h on an anti-CD3-coated plate and then used in either a 6- or an 18-h cytotoxicity assay with Jurkat target cells at the indicated E/T ratios. ND, not done. The spontaneous release was 9% at 6 h and 18% at 18 h.
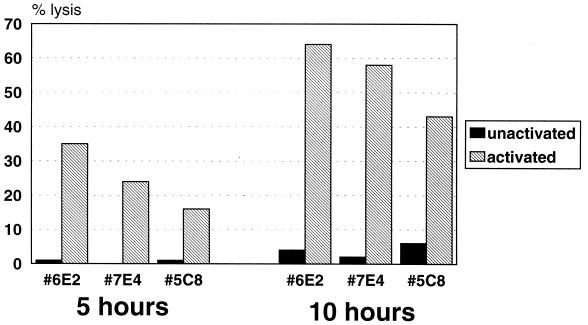
Evidence suggests that the macrophage-like Kupffer cells of the liver are sites of DV infection (9). Activated T cells responding to the infected Kupffer cells may also damage neighboring hepatocytes, potentially contributing to the liver damage observed in some cases of DV infection. In light of this possibility, the human hepatocellular carcinoma cell line HepG2 was tested for its susceptibility to bystander lysis by the CD4+ CTL clones. Figure 2 shows that three CD4+ CTL clones preactivated on anti-CD3-coated plates exhibited bystander lysis of 51Cr-labeled HepG2 cells, while no lysis was observed in the absence of preactivation.
FIG. 2.
DV-specific CD4+ CTL clones exhibit bystander lysis of HepG2 target cells. Clones 6E2, 7E4, and 5C8 were either unactivated or preactivated for 4 h on an anti-CD3-coated plate and then used in either a 5- or a 10-h cytotoxicity assay with HepG2 target cells. E/T ratios were 15:1 for 7E4, 9:1 for 6E2, and 4:1 for 5C8. The spontaneous release was 12% at 5 h and 18% at 10 h.
DV-specific CD4+ CTL clones kill antigen-presenting targets by either FasL or perforin.
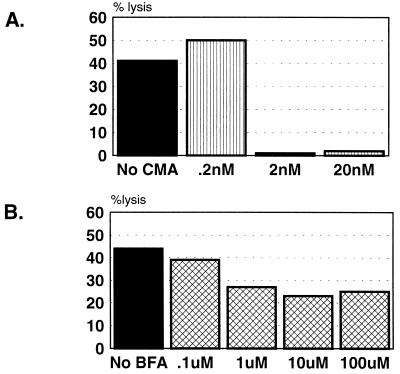
Experiments were performed to determine whether lysis of cognate, antigen-presenting target cells by the DV-specific CD4+ CTL clones was mediated by FasL or perforin by using chemical inhibitors of either pathway. BFA is an inhibitor of intracellular glycoprotein transport that has been shown to selectively inhibit Fas-based cytotoxicity (20). CMA is a specific inhibitor of vacuolar-type H+ ATPase, which acidifies vacuolar organelles (47). It inhibits the activity of perforin in dense granules, mostly due to the accelerated degradation of the protein (20). CMA was shown to almost completely inhibit the cytotoxicity of a human CD8+ CTL clone against peptide-presenting target cells (1). Figure 3 presents results from two assays in which a representative clone, 6E2, was preincubated with increasing concentrations of CMA or BFA to determine whether either inhibitor could decrease target cell lysis. Figure 3A demonstrates that a 2-h preincubation of clone 6E2 with 2 nM CMA completely abrogated lysis of dengue antigen-pulsed target cells. In contrast, Fig. 3B shows that a 2-h preincubation with BFA, even at a concentration of 100 μM, only inhibited lysis of cognate target cells by 50%. These results indicate that clone 6E2 lyses antigen-presenting target cells by a mechanism that is primarily mediated by perforin.
FIG. 3.
CD4+ CTL clone 6E2 lyses autologous D4 antigen-pulsed BLCL by a mechanism primarily involving perforin. (A) Clone 6E2 was preincubated with the indicated concentrations of CMA for 2 h prior to the addition of target cells. The E/T ratio was 35:1. (B) Clone 6E2 was preincubated with the indicated concentration of BFA for 2 h prior to the addition of target cells. The E/T ratio was 25:1. In both assays, lysis was assessed after a 5-h incubation. The spontaneous release was ≤20% for all conditions.
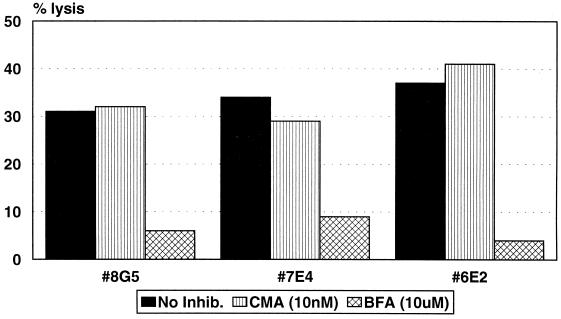
Experiments were performed to determine whether one mechanism of killing would be preferentially employed by the clones when both cognate and bystander target cells were present in the same well. To assess cognate antigen-presenting-cell lysis, autologous BLCL were 51Cr labeled, while Jurkat bystander cells present in the same well were unlabeled. Lysis was measured after 4 h of incubation. Figure 4 shows that, in the absence of inhibitors, all four clones tested were able to lyse cognate BLCL presenting the epitope peptide. CMA inhibited the lysis of BLCL 81% by clone 5C8, 94% by clone 7E4, and 100% by clone 6E2. Preincubation of clones with BFA blocked the lysis of BLCL to a lesser degree: 38% by clone 5C8, 20% by clone 7E4, and 41% by clone 6E2. The results indicate that these clones lyse cognate antigen-presenting cells by a mechanism primarily mediated by perforin. In contrast, lysis of labeled BLCL by the clone 8G5 was inhibited by 100% after preincubation with BFA, while CMA only decreased lysis by 46%, suggesting that this clone principally causes lysis of cognate, peptide-presenting BLCL via Fas/FasL interactions. Additionally, a monoclonal anti-human FasL antibody (4H9) also effectively blocked lysis of antigen-presenting target cells by clone 8G5. At 1 μg/ml, lysis was blocked by 43% and at 10 μg/ml an 86% inhibition of lysis was observed (14% lysis reduced to 8 and 2%, respectively).
FIG. 4.
CD4+ CTL clones lyse autologous target cells by two different mechanisms. Clones were incubated with CMA or BFA for 2 h prior to the addition of target cells, and lysis of autologous, peptide-presenting targets was measured in a 4-h cytotoxicity assay in the presence of unlabeled Jurkat cells as described in Materials and Methods. The E/T ratio was 12:1 for all clones. Clones 8G5 and 5C8 were tested in one experiment; 6E2 and 7E4 were tested in separate experiments. The spontaneous release was ≤25% in all experiments.
DV-specific CD4+ CTL clones lyse bystander target cells primarily via Fas/FasL interactions.
In the same experiments in which the mechanism of cognate target cell lysis was assessed (Fig. 4), the mechanism of bystander lysis was also investigated. In these assays, Jurkat or HepG2 bystander cells were 51Cr labeled, and autologous BLCL present in the same well were unlabeled. Lysis was measured after an 8-h incubation. These experiments could also determine whether bystander target cells would be killed if clones were activated by antigen-presenting cells, as well as after anti-CD3 stimulation as shown in Fig. 1.
Results presented in Fig. 5 show that the CD4+ CTL clones differ in their ability to kill Jurkat bystander target cells in this system. Clones 8G5 and 5C8 are able to mediate a significant degree of bystander lysis of Jurkat cells when activated by antigen-presenting BLCL; however, 7E4 and 6E2 show a much lower degree of bystander lysis in this experiment. Jurkat cell lysis by clones 8G5 and 5C8 was completely abrogated by preincubation of the clones with BFA, but preincubation with CMA only inhibited the lysis by 24 and 47%, respectively. This suggests that bystander lysis of Jurkat cells by clones 8G5 and 5C8 is dependent on Fas/FasL interactions.
FIG. 5.
CD4+ CTL clones 8G5 and 5C8 lyse bystander target cells via a FasL-mediated mechanism. Clones were incubated with CMA or BFA for 2 h prior to the addition of target cells, and lysis of labeled Jurkat cells was assessed after an 8-h incubation in the presence of unlabeled, autologous, peptide-presenting BLCL. The E/T ratio was 12:1 for all clones tested. Clones 8G5 and 5C8 were tested in one experiment; clones 6E2 and 7E4 were tested in separate experiments. The spontaneous release was ≤30% in all experiments.
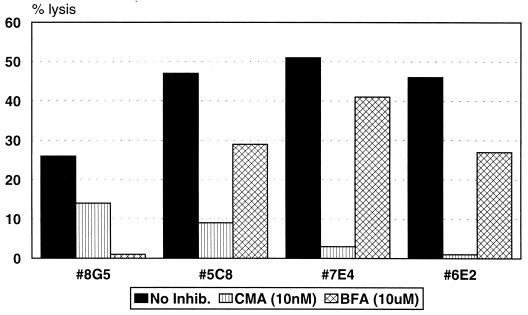
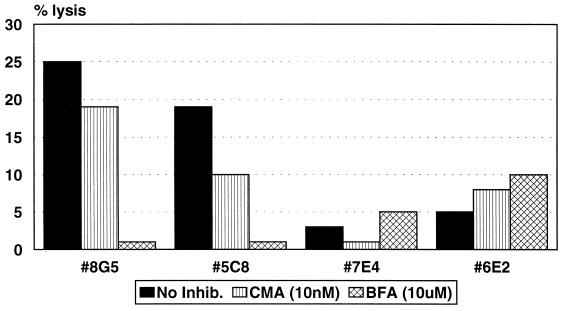
The mechanism of bystander lysis of HepG2 cells was assessed in the same manner. Figure 6 demonstrates that, in the presence of unlabeled BLCL and the epitope peptide, lysis of HepG2 cells was inhibited by greater than 74% for all clones tested after preincubation with BFA, but preincubation of clones with CMA resulted in only 15% inhibition of lysis by clone 7E4 and no inhibition with clones 8G5 and 6E2. These results indicate that, similar to Jurkat cells, bystander lysis of this human hepatoma cell line is likely to occur by a Fas/FasL-mediated mechanism.
FIG. 6.
CD4+ CTL clones lyse HepG2 bystander target cells via a Fas/FasL-mediated mechanism. Clones were incubated with either CMA at 10 nM or BFA at 10 μM for 2 h prior to the addition of target cells. Lysis of labeled HepG2 bystander targets in the presence of unlabeled peptide-presenting BLCL was assessed after an 8-h incubation. The E/T ratio was 10:1. The spontaneous release was ≤27% for all conditions.
IFN-γ, TNF-α, and TNF-β are produced by DV-specific CD4+ CTL clones upon activation.
Production of cytokines by serotype-cross-reactive memory T cells reactivated during a secondary DV infection could contribute to the immunopathology of DHF. In light of this possibility, CD4+ CTL clones were examined for cytokine production by ELISA after stimulation with heterologous DV antigen. Five of five serotype-cross-reactive CD4+ T-cell clones tested produced IFN-γ (700 to >1,000 pg/ml) after stimulation with D2 antigen. No IFN-γ production was observed upon stimulation with a Vero cell control antigen. IFN-γ production could also be observed after D2 antigen stimulation of the donor’s peripheral blood mononuclear cells in bulk culture.
In separate experiments, we also detected the production of TNF-α (9 to 412 pg/ml) by four of five CD4+ CTL clones and production of TNF-β (14 to 462 pg/ml) by five of five CD4+ CTL clones after stimulation with D2 antigen. In comparison, the levels of TNF-α and TNF-β after incubation with the control Vero cell Ag were <40 and 0 pg/ml, respectively.
DISCUSSION
Experiments presented here functionally characterize a panel of DV capsid-protein-specific CD4+ CTL clones with regard to their mechanism of target cell lysis and cytokine secretion. Chromium release assays with both Jurkat and HepG2 cells as bystander targets indicate that the DV capsid-specific CD4+ CTL clones are capable of mediating bystander lysis. Bystander lysis by human virus-specific CD4+ T-cell clones has not previously been demonstrated. In contrast, CD4+ CTL specific for human immunodeficiency virus (32) and herpes simplex virus (50) have been shown to kill only antigen-presenting targets. Our results are consistent with the observation that bystander lysis generally occurs via interaction of FasL on the T-cell clone with Fas, which is constitutively expressed on a number of cell types, including Jurkat and HepG2 cells (5, 31). The DV-specific CD4+ CTL clones express FasL mRNA after activation (data not shown), and the killing of both Jurkat and HepG2 target cells was abrogated by preincubation of the clones with BFA, an inhibitor of FasL-mediated lysis.
Patients with DV infections often exhibit evidence of hepatocyte injury, including increased plasma levels of liver enzymes (19, 24), as well as hepatomegaly and mild to moderate paracentral or zonal necrosis noted upon microscopic examination (3). Although bystander lysis mediated by T cells has not been demonstrated in vivo, the ability of the human hepatocellular cell line HepG2 to serve as a bystander target for the DV-specific T-cell clones suggests a potential role for activated DV-specific T-cell clones in this liver pathology. HepG2 is a well-differentiated liver cell line which retains certain liver-specific characteristics, including the synthesis of hepatic proteins (4, 22). Hepatocyte damage may occur when DV-specific CTL are activated by DV-infected Kupffer cells and subsequently lyse hepatocytes via a bystander mechanism. Fas is expressed constitutively at low levels in normal human liver (30), and upregulation of Fas in the liver occurs after some virus infections (8, 12). In DV-infected individuals, Bhamarapravati et al. (3) have histologically detected Councilman bodies in liver sections, and these structures have been postulated to be apoptotic cells (21, 29).
Only a limited number of studies have been performed examining the mechanism of lysis utilized by human CD4+ CTL and, to our knowledge, this is the first such characterization of human flavivirus-specific CD4+ T cells. Experiments presented here indicate that DV-specific CD4+ CTL clones isolated from the same donor exhibit heterogeneity with respect to their mechanisms of target cell lysis and further demonstrate that the perforin- and FasL-mediated mechanisms of target cell lysis are not mutually exclusive. Clones 7E4, 6E2, and 5C8 lyse cognate target cells via perforin, while lysis of HepG2 or Jurkat bystander target cells present in the same well appears to be FasL mediated. Lysis of antigen-presenting targets by a perforin-dependent mechanism suggests that some CD4+ CTL clones may be involved in the lysis of virally infected cells in a manner similar to that of CD8+ CTL. The importance of CD4+ CTL in protection and recovery from viral infection has been suggested previously by experiments performed in mice (34, 46).
A fourth CD4+ CTL clone, 8G5, appears to kill both cognate, peptide-pulsed targets and bystander targets by a FasL-mediated mechanism. This heterogeneity in the usage of killing mechanisms between clones recognizing the same viral epitope has not previously been observed with human virus-specific CD4+ T-cell clones, although similar results have been obtained with a panel of human autoreactive CD4+ T-cell clones specific for aa 83 to 99 of myelin basic protein (44). A recent study of human CD4+ purified-protein-derivative-specific clones by Lewinsohn et al. (31) suggested that the killing mechanism used by the clones was dependent on target cell susceptibility to Fas-mediated lysis. Although BLCL were shown to express Fas, they were relatively resistant to killing mediated by an anti-Fas antibody (31). Although variance in target cell susceptibility to bystander lysis is likely to explain some of our results as well, our work also suggests that the CTL clones themselves may have differential abilities to induce target cell death via the FasL- or perforin-mediated pathways. It is possible that upon activation clone 8G5 expresses a higher level of FasL on its surface than the other DV-specific CD4+ CTL clones and therefore can more efficiently cause FasL-mediated lysis of antigen-presenting BLCL.
The DV-specific T-cell clones produced IFN-γ, TNF-α, and TNF-β after antigenic stimulation. Although production of IFN-γ by DV-specific T-cell clones has been documented by our group (25, 26, 33), production of TNF-α and TNF-β by DV-specific T-cell clones has not been previously demonstrated. T-cell clones specific for other viruses, including human immunodeficiency virus (17), hepatitis B virus (2), and cytomegalovirus (6) have similarly been shown to produce TNF-α after stimulation. DV serotype-cross-reactive T-cell clones from this D4-immune individual produce cytokines after stimulation with D2 antigen, suggesting that a secondary infection of this individual with D2V would result in the production of TNF-α, TNF-β, and IFN-γ. Although we cannot exclude an effect of in vitro propagation on the pattern of cytokines detected, these results are consistent with the observations that plasma levels of IFN-γ and TNF-α are elevated in patients with DHF (13, 27, 45, 48). These cytokines are thought to contribute to the immunopathogenesis of DHF.
Overall, our results show that activated DV serotype-cross-reactive memory CD4+ T lymphocytes secrete cytokines, including IFN-γ, TNF-α, and TNF-β, and that these T cells exhibit lysis of both cognate antigen-presenting cells and bytander target cells. The serotype-cross-reactive nature of these CD4+ T cells suggests that, in vivo, a secondary infection of this individual with D2V infection could result in the elaboration of these activities, which might contribute to the enhanced pathology manifested as DHF.
ACKNOWLEDGMENT
This work was supported by a grant from the NIAID (RO1 AI30624).
REFERENCES
- 1.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 2.Barnaba V, Franco A, Paroli M, Benvenuto R, De Petrillo G, Burgio V L, Santilio I, Balsano C, Bonavita M S, Cappelli G, Colizzi V, Cutrona G, Ferrarini M. Selective expansion of cytotoxic T lymphocytes with a CD4+ CD56+ surface phenotype and a T helper type 1 profile of cytokine secretion in the liver of patients chronically infected with hepatitis B virus. J Immunol. 1994;152:3074–3087. [PubMed] [Google Scholar]
- 3.Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61:500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 4.Bouma M-E, Rogier E, Verthier N, Labarre C, Feldmann G. Further cellular investigation of the human hepatoblastoma-erived cell line HepG2: morphology and immunocytochemical studies of hepatic-secreted proteins. In Vitro Cell Dev Biol. 1989;25:267–275. doi: 10.1007/BF02628465. [DOI] [PubMed] [Google Scholar]
- 5.Cruikshank S M, Southgate J, Selby P J, Trejdosiewicz L K. Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established liver cell lines in vitro. J Hepatol. 1998;29:550–558. doi: 10.1016/s0168-8278(98)80149-9. [DOI] [PubMed] [Google Scholar]
- 6.Davignon J-L, Castanie P, Yorke J A, Gautier N, Clement D, Davrinche C. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J Virol. 1996;70:2162–2169. doi: 10.1128/jvi.70.4.2162-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagnon S J, Zeng W, Kurane I, Ennis F A. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T-lymphocyte clones. J Virol. 1996;70:141–147. doi: 10.1128/jvi.70.1.141-147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galle P R, Hofmann W J, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall W C, Crowell T P, Watts D M, Barros V L R, Kruger H, Pinheiro F, Peters C J. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 10.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 11.Hargreaves R G, Borthwick N J, Montani M S, Piccolella E, Carmichael P, Lechler R I, Akbar A N, Lombardi G. Dissociation of T cell anergy from apoptosis by blockade of Fas/Apo-1 (CD95) signaling. J Immunol. 1997;158:3099–3107. [PubMed] [Google Scholar]
- 12.Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Immunohistochemical detection of Fas antigen in liver tissues of patients with chronic hepatitis C. Hepatology. 1994;19:1354–1359. [PubMed] [Google Scholar]
- 13.Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere J-L, Vergez-Pascal R, Wattre P, Maniez-Montreuil M. Serum levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 14.Horvath C J, Ferro T J, Jesmok G, Malik A. Recombinant tumor necrosis factor increases pulmonary vascular permeability independent of neutrophils. Proc Natl Acad Sci USA. 1988;85:9219–9223. doi: 10.1073/pnas.85.23.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Innis B L, Myint K S A, Nisalak A, Ishak K G, Nimmannitya S, Laohapand T, Tanprasertsuk S, Pongritsakada V, Thisyakorn U. Acute liver failure is one important cause of fatal dengue infection (abstr.) Southeast Asian J Trop Med Public Health. 1990;21:695–696. [Google Scholar]
- 16.Jacobson S, Richert J R, Biddison W E, Satinsky A, Hartzmann R J, McFarland H F. Measles virus specific T4+ human cytotoxic T-cell clones are restricted by class II HLA antigens. J Immunol. 1984;133:754–757. [PubMed] [Google Scholar]
- 17.Jassoy C, Harrer T, Rosenthal T, Navia B A, Worth J, Johnson P R, Walker B D. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes release gamma interferon, tumor necrosis factor alpha (TNF-α), and TNF-β when they encounter their target antigens. J Virol. 1993;67:2844–2852. doi: 10.1128/jvi.67.5.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju S T, Cui H, Panka D J, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci. 1994;91:4185–4189. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalayanarooj S, Vaughn D W, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis B L, Rothman A L, Nisalak A, Ennis F A. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 20.Katoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 21.Kerr J F, Cooksley W G, Searle J, Halliday J W, Halliday W J, Holder I, Roberts I, Burnett W, Powell L W. The nature of piecemeal necrosis in chronic active hepatitis. Lancet. 1979;ii:827–828. doi: 10.1016/s0140-6736(79)92178-0. [DOI] [PubMed] [Google Scholar]
- 22.Knowles B B, Howe C C, Aden D P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:479–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 23.Kuno G, Bailey R E. Cytokine responses to dengue infection among Puerto Rican patients. Mem Inst Oswaldo Cruz. 1994;89:179–182. doi: 10.1590/s0074-02761994000200010. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C, Tai D, Chang-Chien C, Lan C, Chiou S, Liaw Y. Liver biochemical tests and dengue fever. Am J Trop Med Hyg. 1992;47:265–270. doi: 10.4269/ajtmh.1992.47.265. [DOI] [PubMed] [Google Scholar]
- 25.Kurane I, Innis B L, Nisalak C, Hoke C, Nimmanitya S, Meager A, Ennis F A. Human responses to dengue virus antigens. Proliferative responses and interferon gamma production. J Clin Invest. 1989;83:506–513. doi: 10.1172/JCI113911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurane I, Meager A, Ennis F A. Dengue virus-specific human T cell clones: serotype crossreactive proliferation, interferon-γ production, and cytotoxic activity. J Exp Med. 1989;170:763–775. doi: 10.1084/jem.170.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurane I, Innis B L, Nimmannitya S, Nisalak A, Meager A, Janus J, Ennis F A. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-γ in sera of children with dengue. J Clin Invest. 1991;88:1473–1480. doi: 10.1172/JCI115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurane I, Ennis F A. Cytotoxic T lymphocytes in dengue virus infection. Curr Top Microbiol Immunol. 1994;189:93–105. doi: 10.1007/978-3-642-78530-6_6. [DOI] [PubMed] [Google Scholar]
- 29.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 30.Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, Schmidt A, Debatin K-M, Krammer P H, Moller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 31.Lewinsohn D M, Bemet T T, Xu J, Lynch D H, Grabstein K H, Reed S G, Alderson M R. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–2379. [PubMed] [Google Scholar]
- 32.Miskovsky E P, Liu A Y, Pavlat W, Renate V, Stanhope P E, Finzi D, Fox W M I, Hruban R H, Podack E R, Siliciano R F. Studies of the mechanism of cytolysis by HIV-1-specific CD4+ human CTL clones induced by candidate AIDS vaccines. J Immunol. 1994;153:2787–2799. [PubMed] [Google Scholar]
- 33.Mori M, Kurane I, Janus J, Ennis F A. Cytokine production by dengue virus antigen-responsive human T lymphocytes in vitro examined using a double immunocytochemical technique. J Leukocyte Biol. 1997;61:338–345. doi: 10.1002/jlb.61.3.338. [DOI] [PubMed] [Google Scholar]
- 34.Neal Z C, Splitter G A. Picornavirus-specific CD4+ T lymphocytes possessing cytolytic activity confer protection in the absence of prophylactic antibodies. J Virol. 1995;69:4914–4923. doi: 10.1128/jvi.69.8.4914-4923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987;18:392–397. [PubMed] [Google Scholar]
- 36.Orentas R J, Hildreth J E K, Obah E, Polydefkis M, Smith G E, Clements M L, Siliciano R F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp120 subunit vaccine. Science. 1990;248:1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- 37.Ramsdell F, Seaman M S, Miller R E, Picha K S, Kennedy M K, Lynch D H. Differential ability of Th1 and Th2 T cells to undergo activation-induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 38.Sabin A B. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Sherman M L, Spriggs D R, Arthur K A, Imamura K, Frei E I, Kufe D W. Recombinant human tumor necrosis factor administered as a five-day continuous infusion in cancer patients: phase I toxicity and effects on lipid metabolism. J Clin Oncol. 1988;6:344–350. doi: 10.1200/JCO.1988.6.2.344. [DOI] [PubMed] [Google Scholar]
- 40.Smyth M J. Fas Ligand-mediated bystander lysis of syngeneic cells in response to an allogeneic stimulus. J Immunol. 1997;158:5765–5772. [PubMed] [Google Scholar]
- 41.Spriggs D R, Sherman M L, Michie H, Arthur K A, Imamura K, Wilmore D, Frei E I, Kufe D W. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase I and pharmacologic study. J Nat Cancer Inst. 1988;80:1039–1044. doi: 10.1093/jnci/80.13.1039. [DOI] [PubMed] [Google Scholar]
- 42.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- 43.Van Voorhis W C, Barrett L K, Nasio J M, Plummer F A, Lukehart S A. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect Immun. 1996;64:1048–1050. doi: 10.1128/iai.64.3.1048-1050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vergelli M, Hemmer B, Muraro P A, Tranquill L, Biddison W E, Sarin A, McFarland H F, Martin R. Human autoreactive CD4+ T cell clones use perforin- or Fas/Fas ligand-mediated pathways for target cell lysis. J Immunol. 1997;158:2756–2761. [PubMed] [Google Scholar]
- 45.Vitarana T, de Silva H, Withana N, Gunasekera C. Elevated tumour necrosis factor in dengue fever and dengue haemorrhagic fever. Ceylon Med J. 1991;36:63–65. [PubMed] [Google Scholar]
- 46.Wijburg O L C, Heemskerk M H M, Sanders A, Boog C J P, Van Rooijen N. Role of virus-specific CD4+ cytotoxic T cells in recovery from mouse hepatitis virus infection. Immunology. 1996;87:34–41. [PMC free article] [PubMed] [Google Scholar]
- 47.Woo J-T, Shinohara C, Sakai K, Hasumi K, Endo A. Isolation, characterization, and biological activities of concanamycins as inhibitors of lysosomal acidification. J Antibiot. 1992;45:1108. doi: 10.7164/antibiotics.45.1108. [DOI] [PubMed] [Google Scholar]
- 48.Yadav M, Kamath K R, Iyngkaran N, Sinniah M. Dengue haemorrhagic fever and dengue shock syndrome: are they tumour necrosis factor-mediated disorders? FEMS Microbiol Immunol. 1991;89:45–50. doi: 10.1111/j.1574-6968.1991.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 49.Yasukawa M, Inatsuki A, Kobayashi Y. Differential in vitro activation of CD4+ CD8− and CD8+ CD4− herpes simplex virus-specific human cytotoxic T cells. J Immunol. 1989;143:2051–2057. [PubMed] [Google Scholar]
- 50.Yasukawa M, Yakushijin Y, Fujita S. Two distinct mechanisms of cytotoxicity mediated by herpes simplex virus-specific CD4+ human cytotoxic T cell clones. Clin Immunol Immunopathol. 1996;78:70–76. doi: 10.1006/clin.1996.0010. [DOI] [PubMed] [Google Scholar]