Abstract
Background:
Although often overlooked, patient and public involvement (PPI) is vital when considering the design and delivery of complex and adaptive clinical trial designs for chronic health conditions such as multiple sclerosis (MS).
Methods:
We conducted a rapid review to assess current status of PPI in the design and conduct of clinical trials in MS over the last 5 years. We provide a case study describing PPI in the development of a platform clinical trial in progressive MS.
Results:
We identified only eight unique clinical trials that described PPI as part of articles or protocols; nearly, all were linked with funders who encourage or mandate PPI in health research. The OCTOPUS trial was co-designed with people affected by MS. They were central to every aspect from forming part of a governance group shaping the direction and strategy, to the working groups for treatment selection, trial design and delivery. They led the PPI strategy which enabled a more accessible, acceptable and inclusive design.
Conclusion:
Active, meaningful PPI in clinical trial design increases the quality and relevance of studies and the likelihood of impact for the patient community. We offer recommendations for enhancing PPI in future MS clinical trials.
Keywords: Multiple sclerosis, clinical trials, involvement, engagement, patient and public involvement
Introduction
Interest is growing in patient and public involvement (PPI) in health research in regions around the world, often supported by specific organizations or research frameworks, 1 although the terminology used differs. For example, in Canada, the Canadian Institutes for Health Research lead the development of the Strategy for Patient-Oriented Research. This strategy refers to “patient engagement” in research, whereas in the United Kingdom, the term PPI is used to indicate active inclusion of patients, their families and caregivers, or the lay public as research partners or representatives throughout the research process. In the rehabilitation literature, the term “participatory action research” is used, 2 while the US-based organization, the Agency for Healthcare Research has referred to community-based participatory research. The slogan “nothing about us without us,” a motto ascribed to the disability rights movement, 3 is sometimes used to highlight a key rationale for PPI in research. That is, people living with or affected by (i.e. family members or caregivers) a condition, such as multiple sclerosis (MS), have a right to be involved in research regarding their condition because they will receive the findings of the health research. Moreover, PPI improves the relevance of the research and enhances accountability. For this article, we will use the term people affected by multiple sclerosis (paMS) to be inclusive of people living with and affected by the condition.
Patients, caregivers, and the general public may be engaged throughout all clinical trial stages including setting of priorities, study design, recruitment, and dissemination and implementation of the findings; this has several potential benefits.4 –6 A systematic review of 26 studies found that PPI increased the odds of participant enrollment by 16% (odds ratio (OR) = 1.16; 1.10–1.34). 7 An examination of the value of PPI using financial modeling techniques that accounted for time, cost, revenue, and risk suggested substantial financial benefits. 8 Specifically, if PPI was incorporated into a pre-phase 2 project, and led to avoiding one protocol amendment as well as improved enrollment, adherence, and retention, then a $100,000 involvement activity would provide at least a 500-fold return on investment. 8
In December 2022, an international group of investigators in MS, epidemiology, biostatistics, rehabilitation and clinical trials, and people with MS met under the auspices of the International Advisory Committee on Clinical Trials in MS, sponsored by the European Committee on Treatment and Research in MS and the US National MS Society (for attendees, see Supplemental Appendix I). One of the workshop goals was to discuss strategies to enhance involvement of paMS in clinical trial design. Herein, we: (1) briefly review the current status of PPI in the design and conduct of clinical trials in MS, (2) provide a case study of PPI in the OCTOPUS platform, and (3) and offer recommendations for enhancing PPI in future MS clinical trials.
Current status of PPI in MS trials
In preparation for the workshop, we conducted a rapid review, 9 rather than a definite, comprehensive systematic review to gain insight into the use of PPI in clinical trials in MS in the last 5 years (for details regarding methods, see Supplemental Appendix II). The search identified 44 articles, of which 43 articles were retrieved successfully (Supplemental Appendix II). Of these, nine described clinical trials or constituted clinical trial protocols,10 –18 two of which referred to the same trial.16,17 Seven were conducted in the United States, at least in part. Notably, of the eight unique studies, six were funded by the Patient-Centered Outcomes Research Institute (PCORI), an organization which mandates stakeholder involvement and a focus on relevance to the end-user. 5 This highlights the key role of funders in encouraging PPI in health research, either by mandating PPI or being unlikely to fund research that does not use it.
The studies varied with respect to the degree of detail reported regarding PPI (Table 1). Most described the general activities which involved paMS and other stakeholders, such as in study design, or development of recruitment strategies. Specific examples of the ways in which that input altered study design or operations were usually not described with one exception. The COMBO-MS trial tested the comparative effectiveness of cognitive behavioral therapy, modafinil, and combination therapy. 12 PaMS as well as clinicians, individuals from advocacy groups, and payers participated in meetings four times per year to provide input into study design and operations. This input resulted in meaningful changes. For example, outcome measures were expanded to add social participation measures. The various stakeholders also provided guidance to the investigators regarding information sharing with study participants, such as providing letters reporting sleep disorder risk.
Table 1.
Clinical trials that reported patient and public involvement in their design and conduct.
| Author (years) | Name | Description | Funder/country | People affected by MS/members of public | Other stakeholders | Nature of stakeholder involvement a |
|---|---|---|---|---|---|---|
| Ehde et al. 10 | MS-care trial | Test of a collaborative care intervention vs usual care in an outpatient specialty clinic for participants with chronic pain and/or major depressive disorder | PCORI/United States | Individuals with MS, partners/family members of individuals with MS | MS clinic providers/staff, representatives of community advocacy groups | Shaping study design and intervention, identifying outcomes of interest to stakeholders, troubleshooting problems during study implementation, monitoring study progress, participating in dissemination of study results |
| Kratz et al. 12 | COMBO-MS trial | Test of cognitive behavioral therapy (CBT), modafinil, or both for fatigue in MS | PCORI/United States | Individuals with MS, family members and partners of people with MS | MS neurologists and clinical staff, payer representatives from Blue Care Network, individuals from community advocacy groups | Research questions developed from clinician stakeholders’ experiences, and input from patient and payer stakeholders. Protocol developed with stakeholder input and consensus stakeholder approval before finalization. Quarterly team meetings involve all stakeholders and address study operations including regulatory issues, recruitment and retention strategies, adverse events, treatment adherence. In the first year of study, stakeholders chose layout and photos for recruitment materials, made eligibility criteria broader and more inclusive, added outcome measures, provided guidance as to how to return information to study participants |
| Kever et al. 10 | ASPIRE trial | Test of aspirin as a means of preventing overheating and improving exercise performance in MS as compared to acetaminophen and placebo | NIH/United States | Individuals with MS involved throughout development and conduct of the study | Input regarding outcome variables and study design. Incorporated participant feedback from a prior pilot trial | |
| Motl et al. 13 | STEP for MS | To compare a supervised, facility-based setting vs a remotely coached/guided, home-based setting using telerehabilitation for walking dysfunction and mobility disability | PCORI/United States | People with MS, caregivers | Clinicians (physicians and rehabilitation providers with experience in MS), community exercise specialists, representatives from MS advocacy groups including the National MS Society and iConquerMS, a policymaker, insurance representative | Person with MS included as co-investigator, member of study steering committee, chair of the advisory board. The advisory board provided input regarding delivery and evaluation methods of the intervention, including selection of screening tools, primary and secondary outcome measures, testing the web portal, decisions regarding frequency and length of assessment visits, strategies for recruitment and revisions of the training manual and other study materials. The board met monthly during the planning phase of the study and will meet every 2 months during the intervention phase. During the final phase, the board will engage in data interpretation and dissemination of findings |
| Plow et al. 18 | REFRESH | To compare three formats of delivering the Managing Fatigue intervention: teleconference, Internet, and in person | PCORI/United States | Individuals with MS | Policy advocates, insurance representatives, clinicians, researchers | Inform all aspects of the study including drafting and reviewing recruitment and intervention material, study branding, selecting the delivery formats to test, assessing acceptability of online questionnaires, and review of the informed consent process. Stakeholders will continue to meet throughout the study to guide recruitment, disseminate findings and create an infrastructure for implementation |
| Ontaneda et al. 15 | DELIVER-MS | To compare the effectiveness of early intensive vs escalation approaches for the treatment of relapsing remitting MS | PCORI/United States and United Kingdom | People with MS, caregivers of people with MS | Insurance industry representatives, healthcare agency regulators, advocacy group representatives, investigators constitute advisory committee | Advisory committee informs, guides planning, conduct, dissemination of the clinical trial. Works with the steering committee. |
| Nourbakhsh et al.16,17 | TRIUMPHANT-MS | To test modafinil, amantadine, methylphenidate for MS fatigue | PCORI/United States | Patients with MS | Advisory committee including academic and community neurologists, experts in MS fatigue, representative from National MS Society | Provided guidance and feedback during design, execution and reporting of trial results |
| Nordfalk et al. 14 | To evaluate the effect of specific communication training for neurologists on how to provide complex information about treatment options to patients with MS | Stiftelsen Dam/Norway | Patient with MS | Professor of medical ethics | Served as advisory group |
MS: multiple sclerosis; NIH: National Institutes of Health; PCORI: Patient-Centered Outcomes Research Institute.
No distinctions were described in the nature of the involvement of people affected by multiple sclerosis (MS) versus other stakeholders.
This review may have underestimated the degree of PPI in MS clinical trials for several reasons. First, rapid reviews should be interpreted cautiously as they are less comprehensive and seek to answer questions more rapidly than systematic reviews. Ongoing PPI activities will not be in published literature yet or remain in the gray literature. Second, the inconsistent terminology used to describe PPI may reduce the ability to detect all studies that had PPI. Third, PPI may not be reported even when it occurs due to barriers such as journal word limitations, the lack of recommendation to report PPI in the CONSORT statement for clinical trials, and general lack of consensus as to what type of information should be reported and in what format.
Potential approaches for PPI
Multiple methodological strategies can be employed to achieve effective PPI, 6 and within a given study, this can vary by study element. For example, identifying key priorities could involve including patients or caregivers as members of trial steering committees or advisory groups. Alternative strategies include surveys, workshops, or focus groups. This ensures that the questions and outcomes are meaningful to patients and enhances the relevance to clinical practice and policy. Involvement in study design can be achieved through inclusion in the study team, interviews, surveys, focus groups, and choice experiments. For example, surveys and focus groups can be used to identify priorities and framing of research questions during the conceptual phase of trial development. Focus groups can be used to provide insight into potential barriers and facilitators to proposed interventions or feasibility of different dosing regimens (e.g. weekly vs thrice-weekly supervised exercise regimes). When multiple potential interventions are being considered, choice experiments can help to elucidate preferences of potential participants.
Involvement in the design enables the development of interventions that are acceptable to patients and feasible, as well as the selection of outcomes that are important to patients, enhancing future uptake in clinical practice. Patient feedback regarding information provided to potential participants; 19 the consent process and study burden may facilitate recruitment and retention. PPI may be particularly important when devising materials and strategies, such as transportation support, to engage underrepresented groups as discussed further in a companion paper. 20 Patient partners/co-researchers can assist with the development of lay friendly summaries of trial results, presentation of findings to their communities, and post-trial advocacy for implementation of the findings.
Researchers need to be supported as they seek to add PPI to their work. The MULTI-ACT project was funded by the European Commission to enhance the impact of health research for individuals living with brain disorders, via a participatory and anticipatory governance model. 21 MULTI-ACT provides a toolkit of resources to assist with developing appropriate engagement plans. PCORI-funded and other efforts have also created patient engagement toolkits for researchers.22,23 Funding to address the additional expenses to successfully conduct studies with meaningful PPI is also critical.24,25
The characteristics of successful PPI in health research include (1) involvement of people and their caregivers begins as early as possible in the project, so they are involved in conception of the project; (2) involvement is maintained throughout the project; (3) the plan for involvement of patients and caregivers should be well-defined with an articulated purpose, role and structure; (4) orientation and education about PPI in research for researchers and patients; (5) provision of support and recognition for the contributions of patients and caregivers such as reimbursement for time and authorship; and (6) evaluation and reporting of PPI. 1
Because we have focused on PPI we have not discussed the role of other potential stakeholders such as healthcare providers, payers, policymakers, or advocacy organizations extensively. A commentary regarding MULTI-ACT 21 highlights the importance of identifying and involving all relevant stakeholders to enable consideration and integration of a breadth of perspectives and achieving the research goal successfully.
Case study from OCTOPUS: involvement of people affected by MS
Optimal Clinical Trials Platform for Progressive Multiple Sclerosis (OCTOPUS, ISRCTN140448364) is a multi-arm multi-stage (MAMS) adaptive platform trial that aims to accelerate the development of re-purposed or novel treatments to slow or stop the accumulation of disability progression, relative to other clinical trial designs.26,27 In MAMS trials flexibility is planned, such that interventions being tested can change over time, all of which are compared to a common control arm (multi-arm); interventions that appear effective in an early stage can continue on into a later stage (multi-stage). OCTOPUS will incorporate phase 3 evaluations of selected treatments in double-blind, randomized, comparison to standard of care. Adaptive elements incorporated into OCTOPUS include the ability to drop treatment arms at the planned interim stage, based on lack of sufficient activity against pre-specified targets, and the ability to add arms based on the pre-specified process for treatment selection.
The methods, benefits and challenges of PPI in clinical trial design are well-established.4 –6 Although PPI in the design of MAMS trials presents new challenges, it is more important for their success. MAMS trials are more complex in design and less familiar to the general public. Therefore, careful communication is needed to support participant recruitment and retention. PaMS have been involved in co-designing OCTOPUS from the early planning stages through to the ongoing management. Multiple methods have been used to ensure paMS could provide input regarding different aspects of the trial and in a variety of ways.
Methods of involvement
In 2018, the MS Society (the United Kingdom) established an Expert Consortium for progression in MS clinical trials composed of clinicians, clinical trial methodologists and statisticians, basic scientists, healthcare professionals internal and external to the MS research community, and paMS (recruited from the MS Society’s Research Network, MSRN). 28 Its objective was to design all the components of an efficient clinical trials platform for progression in MS, including infrastructure, methodology, and treatment selection that would form a program grant application to the MS Society for funding. All Expert Consortium members were equal partners and had to agree to a Charter of behavior, defined objectives, and timelines.
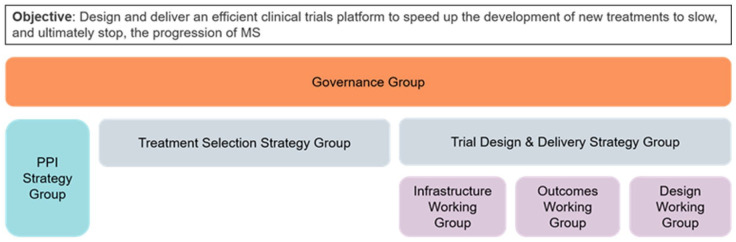
Initially, six paMS from MSRN joined the Governance Group (leading the direction and strategy of the Expert Consortium), the Treatment Selection Group, and the Trial Design and Delivery Group. The Trial Design and Delivery Group was further split into three working groups: (1) design, (2) outcomes, and (3) infrastructure (Figure 1). Each of these groups had paMS in their core membership. Four paMS formed a PPI Strategy Group alongside MS Society staff and a researcher with PPI experience. One of the key deliverables of this group was to determine the PPI needs of all the groups and to organize and lead a series of workshops across the United Kingdom to ensure that the trial design was shaped by a wider group than those involved in the strategy and working groups. All groups, including the PPI Strategy Group, were provided budgets to conduct the foundational work required to meet their objectives.
Figure 1.
Expert Consortium for progression in MS clinical trials governance structure.
In 2019, the program grant application to the MS Society was written with a person with MS as a co-applicant. Following the awarding of OCTOPUS funding, PPI has continued to play a key role in study design and set up, including paMS on the Trial Management Group and a communication subgroup. A separate PPI Forum was established, to engage paMS who had not been involved to date, widening the diversity of paMS involved. The PPI Forum was available for the trial team to consult with on a required basis about issues arising as OCTOPUS prepared to launch recruitment.
Results of involvement
Involving paMS in the Strategy Groups, Working Groups, and workshops throughout the design process confirmed the need for OCTOPUS in the field of MS. The co-design approach enabled the creation of an inclusive clinical trial design for people experiencing a complex condition.
Treatment Selection
The Treatment Selection Strategy Group established a systematic selection method for shortlisting initial candidate treatments to enter OCTOPUS, and ongoing identification of treatments to be considered as new evidence emerges. 29 The group decided on “Drug CVs” as the method for cataloging and comparing treatments. paMS helped design the templates for the CVs and reviewed patient leaflets for the candidate drugs, extracting key information that would be important to include in the CVs. This ensured the CVs included information that would help paMS contribute to the decision-making.
Multiple Drug CVs were developed and scored by all members of the Treatment Selection Group to create a shortlist. Once the shortlist was established, the group held two panel meetings, which were open to more scientific experts and paMS to maximize the representation of those within the MS community and to alleviate pressure felt by lay members of the group. In these meetings, each drug was presented, discussed, and given an overall score. The scientific members of the committee focused on safety and efficacy. paMS scored each drug, focusing on the ease of administration, tolerability of any adverse effects, safety, and risks. They also considered their willingness to take the drug if it slowed progression of their MS. While the scientific members and paMS focused on different aspects, they scored the drugs using comparable scales ensuring the scores from paMS held as much weight as those from experts and contributed to a spirit of co-production. The comments from paMS on the Drug CVs focused on the acceptability of these drugs, a perspective that would have been missed had they not been involved in the scoring (see Table 2).
Table 2.
Examples of how perspective of people affected by multiple sclerosis influenced shortlisting of OCTOPUS treatment arms.
| Therapy | Issue | Perspective |
|---|---|---|
| Glibenclamide | Strict eating schedule | Challenging for people with MS |
| Glibenclamide | High degree of monitoring required | Burdensome |
| Safinamide | Adverse effects/risks in those with eye problems | Visual symptoms common in MS |
| Safinamide | Interactions with antidepressants | High frequency of antidepressant use would reduce number of people eligible for therapy |
OCTOPUS: Optimal Clinical Trials Platform for Progressive Multiple Sclerosis; MS: multiple sclerosis.
Within the current OCTOPUS governance, an international Treatment Advisory Committee has taken over this area, with paMS at its core. It utilizes Drug CVs and international peer review and has recommended three additional future treatments arms for OCTOPUS.
Trial Design and Delivery
The Trial Design and Delivery Strategy Group focused on trial methodology, outcome measures, and infrastructure in close collaboration with the PPI Strategy Group.
During meetings, paMS raised important considerations about trial design. For example, fairness and access to the trial were highlighted as particularly important, as people with progressive MS often feel left out of the research process. paMS insisted that having trial sites across the United Kingdom and inclusive as possible eligibility criteria were essential. This contributed to the decision for the upper age limit to be higher than most clinical trials and aiming to have a broad distribution of sites across the United Kingdom with the creation of recruitment hubs. These hubs would receive extra funding to boost recruitment in areas where participation in trials could be improved and help build the infrastructure and relationships in different regions, helping people with progressive MS engage with research. paMS also raised how important it is to highlight the benefits of taking part in trials, such as access to nurses for symptom management, receiving standard of care, as well as having an magnetic resonance imaging (MRI) scan. The group discussed these benefits and how best to communicate them with potential participants.
As well as making valuable contributions themselves, as highlighted above, the PPI members of the Trial Design Group felt this topic needed input from a wider group of paMS. To this end, they collaborated with the PPI Strategy Group to host a series of workshops held in Edinburgh, Sheffield, and London. Workshop participants were recruited through the MSRN and via social media to attract a wider audience. The PPI Strategy Group hosted these sessions, with workshops co-presented by MS Society staff and paMS. The paMS who facilitated the discussions felt that their role improved the quality of the conversation, helped to put workshop attendees at ease, and created a more equal power dynamic.
The workshop discussions focused on designing an acceptable trial for paMS and selecting outcome measures they felt would address the key challenges of the condition (e.g. fatigue measures). One specific example they thought would make the trial attractive to paMS was the option for participants to be re-randomized from a non-performing treatment arm to an arm that was shown to demonstrate enough benefit on continue investigation. Workshop participants did not want people to be excluded from taking part in OCTOPUS in the future as a result of being part of an arm that is stopped. Other topics discussed during the workshops included eligibility criteria, engagement strategies (including regular communications via a range of channels), wearable devices, and improving the trial experience for participants.
PPI Forum
Since the late 2020, a PPI Forum was formed to provide additional input into all aspects of OCTOPUS. The group is facilitated by MS Society’s Public Involvement Manager and meets virtually on an ad hoc basis. Any member of the OCTOPUS team can bring topics for discussion to the PPI Forum, where the group can critique suggestions and develop solutions together.
The PPI Forum had several meetings with different members of the OCTOPUS team. Their first meeting was to discuss the acceptability of re-randomization. They felt overall it is a positive aspect of the design but was also important to carefully consider how this information is communicated to potential participants. Specifically, the PPI Forum raised that when people are first recruited, they should be informed that arms may be stopped but that this is not a failure, and that it means more resources can be put into arms that look more promising. Learning your treatment arm is being stopped may be concerning for some and without the right messaging might put people off from continuing to participate; the benefits of taking part in a different arm must be outlined and it must be highlighted that this is voluntary and there is no expectation that someone must continue on a different arm.
They also helped to design the expression of interest online recruitment portal on the UK MS Register, the content of the trial website and even its URL, the participant information sheet and consent form, and other external facing communications.
Lessons learned
Involving paMS at an early stage has ensured OCTOPUS has been shaped by the lived experience of paMS. OCTOPUS has shown that co-designing a clinical trial is an effective and efficient way of developing a trial that works for members of the public and the research team. This collaborative approach will be continued throughout the lifetime of OCTOPUS. Several lessons and takeaways from this experience are presented to support future involvement of paMS in clinical trial design (Box 1). We still have a way to go as a community to ensure involvement opportunities are inclusive and accessible to a more diverse range of paMS. This will involve specialized solutions and increased resourcing and time taken to deliver PPI activities.
Box 1.
Lessons learned for involvement of people affected by MS in development of the OCTOPUS trial.
| • People affected by MS are very effective co-investigators ○ With the right support and infrastructure in place people with MS are equal partners with researchers, grasping and accepting concepts of complex design quickly. ○ Therefore, through PPI activities in clinical research, the aim should be always be co-design. • Support and infrastructure for involvement is non-negotiable if there is to be meaningful and not tokenistic PPI. ○ We must strive for better than just a focus group or asking the opinion of a few familiar patients. ○ Effective PPI is a discipline in itself that requires expertise, training of people with MS and adequate financial resourcing. • To ensure effective communication and engagement with people with MS, all communications channels and methods are required – there is no one size fits all. ○ A key consideration is ensuring education around the benefits of being part of a clinical trial regardless of the arm you are on. ○ People with MS also really care about the ‘trial experience’ itself and the practicalities of taking part. • We still have a way to go as a community to ensure involvement opportunities are inclusive and accessible to a more diverse range of people with MS. ○ This will involve specialized solutions and increased resourcing and time taken to deliver PPI activities. |
Recommendations
Workshop attendees agreed that paMS should be involved in all aspects of clinical trials in MS, including the trial design and outcome measures, strategies for recruitment and retention of participants, as well as communication including study materials and dissemination of results. Attendees endorsed this as relevant for all clinical trials, regardless of the type of intervention or type of funder. Specific mention was made of the importance of enhancing PPI in clinical trials sponsored by Pharma. Such trials always include a steering committee of professionals who can insist on the inclusion of paMS. Table 3 outlines recommendations to support PPI in clinical trials from the perspective of the investigator, funder, consumer advocacy group, and journal editor; collective action is needed to ensure success.
Table 3.
Recommendations for enhancing patient and caregiver involvement in the design and conduct of clinical trials in multiple sclerosis.
| Investigator | Funder | Patient advocacy groups | Journal editors |
|---|---|---|---|
| Include patients in the design and management of the trial | Include stakeholder engagement (involvement) section in grant application | Identify, train, and support people with MS and caregivers to be patient representatives | Require stakeholder engagement section in reports of clinical trials, similar to how data-sharing statements are required by many journals |
| Include stakeholder engagement section in grant application | Grant progress reports should include section that describes ongoing patient/caregiver involvement throughout the trial | Support investigators in reaching underrepresented groups to serve as patient representatives | Require the use of the Guidance for Reporting Involvement of Patients and the Public (GRIPP)2 reporting checklist30 |
| Obtain feedback regarding lay summaries of study findings for publications and meetings from patient representatives | Require lay summaries of the results of every funded study. Provide support or consultancy with paMS in the process of preparing the grants especially around lay summary development |
Disseminate lay summaries of research | Publish lay summaries of research with each paper reporting results of a clinical study(ies) |
| Establish standard operating procedures for role of patient representatives throughout the project. Create terms of reference for patient representatives so expectations, roles, and responsibilities are clear | Include people with MS and/or caregivers in the grant review process | Provide clear written roles and responsibilities and expectations of contributions from paMS | |
| Include a budget for reimbursement of patient time and travel expenses | Make reimbursement of patient or caregiver representatives’ time and travel expenses an allowable budget expense | ||
| Use methods (e.g. phone call, video call) that reduce barriers to participation | Encourage investigators to use methods (e.g. phone call, video call) that may reduce barriers to participation by representatives in rural or remote communities, by people with physical impairments that make travel difficult, and those of lower socioeconomic status | ||
| Report PPI in detail in journal publications to promote an understanding of what works, for whom, where, and when (in what setting) so that other investigators can learn from the experience | Advocate to journals to require information about how patients or caregivers are involved in a study, similar to how data sharing statements are required now in many journals | Consider patients as lay reviewers for papers with potentially high clinical impact |
MS: multiple sclerosis; PPI: patient and public involvement.
Conclusion
Despite the knowledge of the benefits of quality PPI in all aspects of clinical trials, there remains an apparent paucity of PPI reported in the design and conduct of clinical trials in MS. This may reflect lack of PPI or lack of reporting PPI or both; methodological issues in our review may also contribute. To highlight the benefit of PPI in trial design, the OCTOPUS trial was described as an example of co-designing a complex trial with paMS, thus creating a more accessible, acceptable, and inclusive design with many lessons learnt along the way. We have offered recommendations for investigators, funders, and patient advocacy groups for enhancing PPI in future MS clinical trials knowing that this requires appropriate expertise, strategy, training, and resourcing for both researchers and paMS. As an international MS research community, PPI in clinical research must become part of the research process without exception if we are to maximize opportunity and impact for people living with and affected by MS.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231189678 for Enhancing involvement of people with multiple sclerosis in clinical trial design by Emma Gray, Anneesa Amjad, Jenny Robertson, Judy Beveridge, Susan Scott, Guy Peryer, Marie Braisher, Cheryl Pugh, Sara Peres, Ruth Ann Marrie, Maria Pia Sormani and Jeremy Chataway; in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585231189678 for Enhancing involvement of people with multiple sclerosis in clinical trial design by Emma Gray, Anneesa Amjad, Jenny Robertson, Judy Beveridge, Susan Scott, Guy Peryer, Marie Braisher, Cheryl Pugh, Sara Peres, Ruth Ann Marrie, Maria Pia Sormani and Jeremy Chataway; in Multiple Sclerosis Journal
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.A.M. received research funding from Canadian Institutes of Health Research, Research Manitoba, MS Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, Consortium of MS Centers, the Arthritis Society, and the US Department of Defense. She is supported by the Waugh Family Chair in Multiple Sclerosis. She is a co-investigator on a study funded in part by Biogen Idec and Roche (no funds to her or her institution). In the last 3 years, J.C. has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and the National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society, and the Rosetrees Trust. He is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by the MS Canada. A local principal investigator for commercial trials funded by Ionis, Novartis, and Roche and has taken part in advisory boards/consultancy for Azadyne, Biogen, Lucid, Janssen, Merck, NervGen, Novartis, and Roche. In the last 3 years, G.P. has worked as an independent contractor with the MS Society (UK) and Bristol-Myers Squibb. M.B. has received support from the NIHR partnership and the HTA Programme (NIHR), the UK MS Society, and the NIHR Local Clinical Network. M.P.S. has received consulting fees from Biogen, Genzyme, GeNeuro, MedDay, Merck, Novartis, Roche, and Teva. E.G., J.R., A.A., S.S., J.B., C.P., and S.P. have nothing to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The International Advisory Committee on Clinical Trials in Multiple Sclerosis and the International Conference on Innovations in Clinical Trial Design & Enhancing Inclusivity of Clinical Trial Populations were supported by the National Multiple Sclerosis Society and the European Committee for Treatment and Research in Multiple Sclerosis. There was no involvement of the sponsors in the design, collection, analysis, or interpretation of data discussed at the Conference. The opinions expressed are those of the authors. Thank you to all members of the Expert Consortium for progression in MS clinical trials including MS Society staff and in particular members of the MS Society Research Network and past and current members of the OCTOPUS PPI Forum. All underpinning work carried out by the Expert Consortium for progression in MS clinical trials were funded by grants from the MS Society (UK). OCTOPUS is funded by grant #135 from the MS Society (UK).
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Jenny Robertson  https://orcid.org/0000-0003-3271-7885
https://orcid.org/0000-0003-3271-7885
Marie Braisher  https://orcid.org/0000-0003-0634-8350
https://orcid.org/0000-0003-0634-8350
Cheryl Pugh  https://orcid.org/0000-0003-4584-9780
https://orcid.org/0000-0003-4584-9780
Ruth Ann Marrie  https://orcid.org/0000-0002-1855-5595
https://orcid.org/0000-0002-1855-5595
Maria Pia Sormani  https://orcid.org/0000-0001-6892-104X
https://orcid.org/0000-0001-6892-104X
Contributor Information
Emma Gray, Department of Research, MS Society UK, London, UK.
Anneesa Amjad, Department of Research, MS Society UK, London, UK.
Jenny Robertson, Department of Research, MS Society UK, London, UK.
Judy Beveridge, Research Network, MS Society UK, London, UK.
Susan Scott, Research Network, MS Society UK, London, UK.
Guy Peryer, Research Network, MS Society UK, London, UK/ Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, UK.
Marie Braisher, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK.
Cheryl Pugh, National Institute for Health Research, Biomedical Research Centre, University College London Hospitals, London, UK.
Sara Peres, National Institute for Health Research, Biomedical Research Centre, University College London Hospitals, London, UK.
Ruth Ann Marrie, Departments of Internal Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada.
Maria Pia Sormani, Biostatistics Unit, Department of Health Sciences, University of Genoa, Genoa, Italy/IRCCS Ospedale Policlinico San Martino, Genoa, Italy.
Jeremy Chataway, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK/National Institute for Health Research, Biomedical Research Centre, University College London Hospitals, London, UK/Medical Research Council Clinical Trials Unit at UCL, Institute of Clinical Trials and Methodology, University College London, London, UK.
References
- 1. Manafo E, Petermann L, Mason-Lai P, et al. Patient engagement in Canada: A scoping review of the “how” and “what” of patient engagement in health research. BMC Health Res Policy Syst 2018; 16: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ehde DM, Wegener ST, Williams RM, et al. Developing, testing, and sustaining rehabilitation interventions via participatory action research. Arch Phys Med Rehabil 2013; 94(Suppl. 1): S30–S42. [DOI] [PubMed] [Google Scholar]
- 3. Koontz A, Duvall J, Johnson R, et al. “Nothing about us without us”: Engaging at users in at research. Assist Tech 2022; 34: 499–500. [DOI] [PubMed] [Google Scholar]
- 4. South A, Hanley B, Gafos M, et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council Clinical Trials Unit at University College London: Findings from ten case studies. Trials 2016; 17: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frank L, Basch E, Selby JV, et al. The PCORI perspective on patient-centered outcomes research. JAMA 2014; 312: 1513–1514. [DOI] [PubMed] [Google Scholar]
- 6. Tong A, Scholes-Robertson N, Hawley C, et al. Patient-centred clinical trial design. Nat Rev Nephrol 2022; 18(8): 514–523. [DOI] [PubMed] [Google Scholar]
- 7. Crocker JC, Ricci-Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: Systematic review and meta-analysis. BMJ 2018; 363: k4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levitan B, Getz K, Eisenstein EL, et al. Assessing the financial value of patient engagement: A quantitative approach from CTTI’s Patient Groups and Clinical Trials Project. Ther Innov Regul Sci 2018; 52(2): 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khangura S, Konnyu K, Cushman R, et al. Evidence summaries: The evolution of a rapid review approach. Syst Rev 2012; 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehde DM, Alschuler KN, Sullivan MD, et al. Improving the quality of depression and pain care in multiple sclerosis using collaborative care: The MS-care trial protocol. Contemp Clin Trials 2018; 64: 219–229. [DOI] [PubMed] [Google Scholar]
- 11. Kever A, Nelson KE, Aguerre IM, et al. ASPIRE trial: Study protocol for a double-blind randomised controlled trial of aspirin for overheating during exercise in multiple sclerosis. BMJ Open 2020; 10: e039691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kratz AL, Alschuler KN, Ehde DM, et al. A randomized pragmatic trial of telephone-delivered cognitive behavioral-therapy, modafinil, and combination therapy of both for fatigue in multiple sclerosis: The design of the “COMBO-MS” trial. Contemp Clin Trials 2019; 84: 105821. [DOI] [PubMed] [Google Scholar]
- 13. Motl RW, Backus D, Neal WN, et al. Rationale and design of the STEP for MS Trial: Comparative effectiveness of Supervised versus Telerehabilitation Exercise Programs for Multiple Sclerosis. Contemp Clin Trials 2019; 81: 110–122. [DOI] [PubMed] [Google Scholar]
- 14. Nordfalk JM, Holmøy T, Thomas O, et al. Training physicians in providing complex information to patients with multiple sclerosis: A randomised controlled trial. BMJ Open 2022; 12: e049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ontaneda D, Tallantyre EC, Raza PC, et al. Determining the effectiveness of early intensive versus escalation approaches for the treatment of relapsing-remitting multiple sclerosis: The DELIVER-MS study protocol. Contemp Clin Trials 2020; 95: 106009. [DOI] [PubMed] [Google Scholar]
- 16. Nourbakhsh B, Revirajan N, Waubant E. Treatment of fatigue with methylphenidate, modafinil and amantadine in multiple sclerosis (TRIUMPHANT-MS): Study design for a pragmatic, randomized, double-blind, crossover clinical trial. Contemp Clin Trials 2018; 64: 67–76. [DOI] [PubMed] [Google Scholar]
- 17. Nourbakhsh B, Revirajan N, Morris B, et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: A randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol 2021; 20(1): 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plow M, Packer T, Mathiowetz VG, Preissner K, Ghahari S, Sattar A, et al. REFRESH protocol: A non-inferiority randomised clinical trial comparing internet and teleconference to in-person ‘Managing Fatigue’ interventions on the impact of fatigue among persons with multiple sclerosis. BMJ Open 2020; 10: e035470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dwyer CP, Joyce RA, Bane EM, et al. An examination of the effects of a patient-designed-and-informed participant information sheet in comparison with a standard, researcher-designed information sheet on recruitment, retention and understanding: Protocol for a study-within-a-trial. HRB Open Res 2020; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marrie RA, Chataway J, Bierer BE, et al. Enhancing diversity of clinical trial populations in multiple sclerosis. Mult Scler 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaratin P, Bertorello D, Guglielmino R, et al. The MULTI-ACT model: The path forward for participatory and anticipatory governance in health research and care. BMC Health Res Policy Syst 2022; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patient-Centered Outcomes Research Institute (PCORI). Patient engagement toolkit. Washington, DC: PCORI, 2011. [Google Scholar]
- 23. PIaET, Health and Care Research Wales. UK Standards for Public Involvement: Resources and support, https://healthandcareresearchwales.org/public-help-research/uk-standards-public-involvement
- 24. Schoch S, Oehrlein E, Perfetto E. Patient engagement fair-market value calculator & compensation tools. Washington, DC: National Health Council, 2020. [Google Scholar]
- 25. National Institute for Health and Care Research. Payment guidance for researchers and professionals. Version 1.3, 2022, https://www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392
- 26. Li V, Leurent B, Barkhof F, et al. Designing multi-arm multistage adaptive trials for neuroprotection in progressive multiple sclerosis. Neurology 2022; 98: 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ISRCTN Registry. Testing and comparing multiple drugs at once against the standard treatment for progressive multiple sclerosis treatment. BioMed Central, London, 2022. [Google Scholar]
- 28. MS Society. MS Society research network, London, https://www.mssociety.org.uk/research/take-part-in-ms-research/research-network [Google Scholar]
- 29. Cunniffe N, Vuong KA, Ainslie D, et al. Systematic approach to selecting licensed drugs for repurposing in the treatment of progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2021; 92(3): 295–302. [DOI] [PubMed] [Google Scholar]
- 30. Staniszewska S, Bertt J, Simera I, et al. GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. BMJ 2017; 358: j3453. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231189678 for Enhancing involvement of people with multiple sclerosis in clinical trial design by Emma Gray, Anneesa Amjad, Jenny Robertson, Judy Beveridge, Susan Scott, Guy Peryer, Marie Braisher, Cheryl Pugh, Sara Peres, Ruth Ann Marrie, Maria Pia Sormani and Jeremy Chataway; in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585231189678 for Enhancing involvement of people with multiple sclerosis in clinical trial design by Emma Gray, Anneesa Amjad, Jenny Robertson, Judy Beveridge, Susan Scott, Guy Peryer, Marie Braisher, Cheryl Pugh, Sara Peres, Ruth Ann Marrie, Maria Pia Sormani and Jeremy Chataway; in Multiple Sclerosis Journal