Abstract
Background:
Demographic characteristics, social determinants of health (SDoH), health inequities, and health disparities substantially influence the general and disease-specific health outcomes of people with multiple sclerosis (MS). Participants in clinical trials do not represent all people with MS treated in practice.
Objective:
To provide recommendations for enhancing diversity and inclusion in clinical trials in MS.
Methods:
We held an international workshop under the Auspices of the International Advisory Committee on Clinical Trials in MS (the “Committee”) to develop recommendations regarding diversity and inclusivity of participants of clinical trials in MS. Workshop attendees included members of the Committee as well as external participants. External participants were selected based on expertise in trials, SDoH, health equity and regulatory science, and diversity with respect to gender, race, ethnicity, and geography.
Results:
Recommendations include use of diversity plans, community engagement and education, cultural competency training, biologically justified rather than templated eligibility criteria, adaptive designs that allow broadening of eligibility criteria over the course of a trial, and logistical and practical adjustments to reduce study participant burden. Investigators should report demographic and SDoH characteristics of participants.
Conclusion:
These recommendations provide sponsors and investigators with methods of improving diversity and inclusivity of clinical trial populations in MS.
Keywords: Multiple sclerosis, clinical trials, diversity, social determinants of health
Introduction
Diversity is multidimensional and encompasses sex, gender, age, race, ethnicity, comorbidity, ability, and social determinants of health (SDoH; Table 1, Figure 1); these dimensions overlap. SDoH are the social or economic factors or conditions in which people are born, develop, live, learn, work, play, and age that influence health. 1 Among these factors are educational access, quality, and achievement, economic stability; food security and stability; neighborhood and built environment; health care access and quality; and social and community contexts, among others. SDoH are not distributed evenly across the population. Health inequities and disparities often disproportionately affect members of the population of particular genders, sexual orientations, racial, ethnic, and religious identities, and abilities.
Table 1.
Terminology. 2
| Terminology | Definitions |
|---|---|
| Diversity | Multidimensional construct: encompasses sex, gender, race, ethnicity, comorbidity, ability, social determinants of health |
| Social determinants of health | Social or economic factors or conditions in which people are born, live, work, play, age that influence health |
| Equality | Each individual is provided with the same resources and opportunities irrespective of their circumstances |
| Equity | Resources and opportunities are allocated to individuals based on their specific circumstances to achieve equal outcomes |
| Health disparities | Health disparities refer to differences in health among different subgroups of the population without an identified etiology |
| Health care disparities | Differences in health care quality that not caused by differences in clinical needs, preferences, access-related issues, or whether the intervention is appropriate. They are underpinned by inequities. |
| Health inequalities | Differences in health status, or differences in the distribution of determinants of health among different subgroups of the population |
| Health inequities | Differences in health outcomes that are avoidable and unjust, and are underpinned by social determinants of health |
| Cultural competence | Provision of services/care that shows respect for culture and identity, incorporate a person’s needs and rights, free of discrimination, and enhance effectiveness of patient care |
Figure 1.
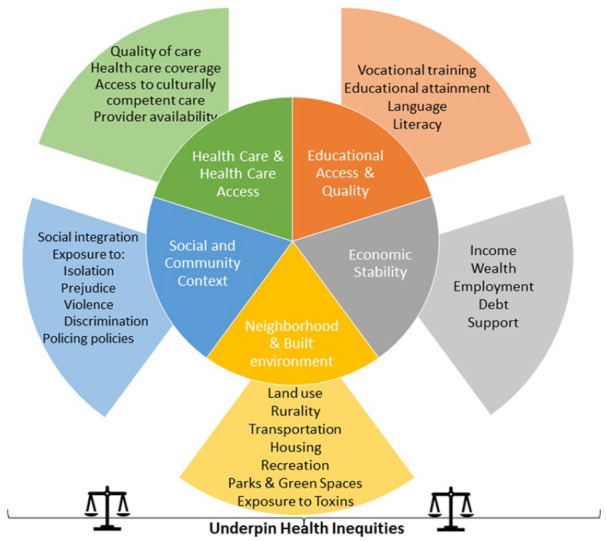
Social determinants of health.
Among people with multiple sclerosis (MS), demographic characteristics such as sex, race, ethnicity, and SDoH importantly influence health outcomes. 3 For example, Hispanic/Latinx American and African American persons with MS attending two American centers had higher MS Severity Scores after adjusting for age and gender than those who were White Americans. 4 Hypertension is more likely to be underdiagnosed among Hispanic/Latinx persons with MS. 5 Immigrants to Canada with MS have more comorbidities 6 and are hospitalized more often in the year of diagnosis than long-term Canadian residents. 7 In one survey, participants with MS who identified as transgender reported lower comfort discussing sexual health with their physician. 8 Another survey found that people with MS who identified as lesbian, gay, bisexual, and/or transgender were more likely to change MS centers, a change attributed to the perception of homophobic behaviors. 9 A sample of African American women with MS reported their diagnoses had been delayed due to misbeliefs by physicians about the risk of MS in their racial group. 10 Less is known about the influence of race and ethnicity outside North America; half of Organisation for Economic Cooperation and Development countries do not routinely collect racial or ethnic identity data, and collection of such data is considered sensitive in Europe. 11 Among people with MS, lower as compared to higher area-level socioeconomic status (SES) is associated with increased hospitalization rates, 12 greater disability progression, 13 and increased mortality. 14
In December 2022, an international group held a workshop, sponsored by the European Committee on Treatment and Research in MS and the US National MS Society. Participants included members of the International Advisory Committee on Clinical Trials in MS whose members are selected to represent different disciplines relevant to MS, geographic regions, and stages of career, while balancing gender and considering race and ethnicity. External participants were selected based on expertise in trials, SDoH, health equity, regulatory science, and diversity with respect to gender, race, ethnicity, and geography.
Herein, we review evidence regarding the diversity of participants enrolled in clinical trials in MS, barriers to more inclusive trial populations, and regulatory perspectives. Finally, we make recommendations to enhance the diversity of clinical trial populations and the inclusiveness of clinical trials going forward.
Health inequities and disparities
Health and health care disparities refer to differences in health and health care quality among different subgroups of the population. These disparities are underpinned by inequities, that is, differences in health outcomes that are avoidable, and the inequities are underpinned by SDoH. These disparities can be viewed from multiple, often overlapping perspectives commonly related to population type, geography, and risk factors. Population could refer to race, ethnicity, age, gender, sexual orientation, or ability. Geography could refer to urban versus rural, high-income versus low- or middle-income country as defined by the World Bank, or degree of neighborhood privilege versus deprivation. Risk factors could refer to access to care or environmental risks. The formulation of these perspectives may differ across countries because they are shaped by power, wealth, and systemic racism (Table 2).15,16
Table 2.
Populations at risk of health inequities.
| National Institutes of Health (United States) 15 | Public Health Agency of Canada (Canada) 16 | Australia 17 | European Union 11 |
|---|---|---|---|
| Socioeconomically disadvantaged | Socioeconomically disadvantaged | Socioeconomically disadvantaged | Socioeconomically disadvantaged |
| Sexual and gender minority group | Sexual orientation minority group | Women and sexual orientation minority groups | |
| African American/Black | Culturally and linguistically diverse background | Ethnic minorities | |
| Hispanic/ Latino | |||
| American Indian/Alaska Native/Native Hawaiian/Pacific Islander | Indigenous group | Aboriginal and Torres Strait Islander peoples | |
| Asian | |||
| Residence in underserved rural community | Residence in rural community | Residence outside major city | |
| Immigrants | Refugees | Refugees and migrants | |
| People with disabilities | Old people and people with disabilities |
Health disparities and inequities affect individuals, health systems, and societies. An actuarial analysis of the relationship between health care disparities secondary to sex, gender, race, SES, and health care spending in the context of diabetes, asthma, cardiovascular disease, and breast and colorectal cancers 18 found that consequent health inequities accounted for $320 billion (USD) in health care spending and $42 billion in lost productivity annually in the United States. In the European Union, the economic burden of socioeconomic-related health inequities was ~10% of the gross domestic product in 2011. 19 The causal pathways between health care disparities and costs are complex, but the high costs emphasize the importance of addressing health inequities related to SDoH, including in the context of clinical trials.
The consequences of failure to recruit and retain diverse, representative populations in clinical trials are manifold. First, the findings may lack generalizability to the entire population of interest. Second, the lack of variation reduces the ability to evaluate the heterogeneity of disease biology and treatment effects. Third, it creates unequal access to the benefits of research and the perpetuation of health inequities, mistrust, lack of uptake of interventions, and harm from the use of ineffective or unsafe therapies. 20 As noted in a report by the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard, “Race, ethnicity, sex, gender, age, and geographic ancestry do not define distinct genetic or biological groups; yet along with social, cultural, and economic factors, these factors can be associated with important differences in disease susceptibility and manifestation, [and] treatment response . . .” 20 Potential benefits of diversifying trial populations (e.g. for participants at extremes of age, those with comorbidities, and underrepresented racial and ethnic groups) include improvements in scientific credibility, social responsibility, compliance with regulatory guidelines and funder expectations, 20 and improved clinical decision-making and outcomes.
Diversity of clinical trial populations in MS: current state
Clinical trials suffer from a lack of diversity with respect to leadership, 21 stakeholder involvement, and participant characteristics. Generally, women who lead research teams are more likely to consider sex and gender effects in study design and analysis than men who lead research teams. 22 A review of heart failure trials found that those led by women were more likely to report race and ethnicity data and enrolled a higher percentage of participants identifying as Black, Indigenous, or people of color (BIPOC). 23 Yet, women are underrepresented as authors of seminal clinical trials for MS disease-modifying therapies (DMT). 21 Leadership of MS trials by BIPOC individuals has not been described, possibly due to a paucity of data.
Explicit and implicit exclusion criteria for trials are problematic. In trials published in high-impact general medical journals, only 47.2% of the exclusion criteria were strongly justified. 24 A review of trials in cardiology, mental health, and oncology 25 found that trial populations were highly selected, and excluded older adults, those with comorbidities, and those with a lower SES. These exclusions result in the ineligibility of 50%–80% of typical clinical populations. A review of 45 phase 3 clinical trials in MS conducted for DMT approved between 1995 and 2020 found that 17 (37.8%) of trials did not report race or ethnicity, 26 14 (31.1%) trials reported the proportion of the study participants who were White, and the remaining 14 (31.1%) reported ⩾2 ethnicities. At the individual level, the median percentage of participants who did not identify as White was only 6.2% (1.9% Black, 0.5% Asian); the remainder were largely classified as “other” (unspecified). That review did not address reporting of factors such as educational attainment, annual income, or distance lived from the trial site.
A scoping review that examined the extent to which SDoH are considered in the recruitment of participants in MS rehabilitation trials found that exclusions due to implicit and explicit factors were common. 27 Implicit factors included living in rural or remote areas, limited access to an MS clinic, and inability to reach the study site or pay for study-related costs (such as missed time from work). Explicit factors included greater disability, cognitive impairment, physical and mental comorbidities, older age, and language or literacy. The extent of the problem was difficult to evaluate because, other than age and biological sex, most SDoH were not reported. Thus, the lack of diversity in trial populations extends across the full spectrum of clinical trials in MS, and inconsistent reporting hinders understanding of the problem.
Regulatory perspectives
Regulatory agencies have emphasized the need to increase diversity and inclusion of underrepresented populations in clinical trials,28,29 to ensure that participant characteristics reflect clinically relevant populations with respect to age, sex, race, ethnicity, comorbidity, and disease severity. Exclusion criteria must be well-justified based on evidence of lower efficacy or higher risks of adverse events in the excluded group. Trials in many fields have historically excluded participants in whom the risks are perceived to outweigh the benefits of treatment, or in whom the risks are not known. This has resulted in common, templated criteria across clinical trials.30,31 For example, trials in MS have often had common exclusion criteria based on age (<18 or >55 years), level of disability (Expanded Disability Status Scale score > 5.5), and comorbidities (e.g. any other disease or condition that could interfere with participation). 30 This requires clinicians and people with MS who do not meet trial criteria to make decisions about treatment in which benefits and risks are uncertain.
Inclusive practices in trial design that are supported by the US Food and Drug Administration and European Medicines Agency can address challenges related to explicit exclusions on the basis of uncertain risk and benefit. First, eligibility criteria can be modified as the evaluation of an intervention progresses along the clinical trial continuum. For example, age criteria can be broadened in phase 3 trials to include older adults and youth based on accumulated safety and pharmacology data from earlier phase studies, and should not simply be carried forward from earlier phase studies. 32 Second, for individuals with organ dysfunction and other comorbidities, distinctions can be made by severity, so that individuals with mild dysfunction are included, whereas those with moderate or severe dysfunction are excluded. Exclusions for comorbidities should be based on specific rationales, rather than blanket exclusions. For example, excluding individuals with cardiac arrhythmias from trials of fingolimod is justifiable based on the drug’s mechanism of action as a modulator of sphingosine-1-phosphate receptors expressed in cardiac tissue. Third, drug metabolism studies can be conducted earlier in the drug development pipeline to gain a better understanding of the potential for adverse drug reactions in sub-populations such as older adults or those with organ (e.g. liver, kidney) dysfunction that is often relevant to drug safety. Fourth, adaptive clinical trial designs could be used to expand or contract eligibility using pre-specified criteria and interim analyses that rely on safety data accrued during the trial.33,34 Similarly, individuals unlikely to benefit from the intervention could be excluded (discussed further elsewhere). 35 These approaches also address challenges related to conducting clinical trials in pediatric MS, a rare condition which faces difficulties achieving timely adequately powered trials. 36 Specifically, they would allow staggered enrollment of persons with MS aged <18 years, beginning with adolescents followed by younger children as well as older adults. Other strategies to enhance clinical trial design in pediatric MS are discussed in a companion paper. 35 Finally, mixed methods designs may be useful for clinical trials of non-pharmacologic interventions. Qualitative data may provide insight when valid and reliable instruments are lacking for the groups of interest, or when the applicability of concepts or questions to ask are unclear.
Specific action is needed to improve the enrollment of minority racial and ethnic and other disadvantaged populations who are not explicitly excluded. On 29 December 2022, the US Federal Food Drug and Cosmetic Act was amended to require that sponsors submit a race and ethnicity diversity action plan for clinical investigation of a new drug in phase 3 or another pivotal study; prior draft guidance was issued in April 2022. 37 The plan should provide (1) an overview of the disease, including what is known about it in underrepresented populations; (2) the scope of the development program, including planned clinical trials and their design elements, how inclusion of underrepresented populations will be addressed, and summarize data regarding differential treatment response in underrepresented populations; (3) an indication of the underrepresented populations of interest and the justification of specific enrollment targets for these populations; and (4) an operational plan for recruitment and retention that addresses intended site locations, measures to limit participant burden, the community engagement strategy, and the evaluation plan to measure progress.
Barriers to participation of underrepresented/underserved groups
In the context of historical injustices and ongoing experiences of discrimination in research and health care,38,39 multiple potential barriers affect the participation of underserved groups in clinical trials. 40 Broadly, these barriers can be categorized as those related to language and communication, lack of trust, access to trials, eligibility criteria, attitudes and beliefs, lack of knowledge regarding clinical trials, and logistical challenges. 41 Barriers can also be classified by whether they act at the system, individual, or interpersonal levels. 42 System factors, for example, include the availability of trials being limited to tertiary care centers, restrictive inclusion criteria, lack of community engagement, and financial burden related to participation. Individual factors can relate to the patient, such as language, or the provider/investigator, such as implicit bias. Interpersonal factors may include the physician–patient relationship.
A systematic review of 44 studies identified multiple shared barriers to participation across African American, Hispanic/Latinx, Asian American, or Pacific Islander 43 groups. These included mistrust and fear of participation, lack of access to information, competing demands related to time and financial resources, and logistical concerns related to scheduling, childcare, and lack of transportation. Similarly, a recent survey of 2599 persons with MS identifying as African American, White, Hispanic/Latinx, non-Hispanic, non-disclosed, and living in the United States found that all groups supported research. 44 However, research priorities differed as did preferred sources of information about research opportunities and which sources were most trusted. All groups had concerns about potential harms to their health, confusing study information, not being fully informed about a study when participating, and difficulty accessing the study site. Hispanic/Latinx participants and those of undisclosed ethnicity were more concerned about the effects of research participation on employment, legal status, and loss of health insurance compared to non-Hispanic/Latinx participants. As compared to White participants, African American participants were more concerned about privacy, receiving poor quality medical care, and being taken advantage of by the research team, consistent with observations in the general population and stemming from negative historical and sociopolitical perspectives.
Recommendations
Workshop attendees made recommendations to enhance the diversity and inclusiveness of trials in MS (Table 3). The dimensions of diversity that are relevant may vary with the intervention and by country. 20 For example, the characteristics of people with MS vary by region, and thus the underrepresented groups may also vary. Broadly, sponsors and investigators should develop a formal diversity plan for their trials as described earlier. Sponsors, investigators and their study teams should engage with the community on an ongoing basis, and expand trial sites to underserved communities. Investigators and study staff should undergo cultural competency and implicit bias training, and teams should include people who identify with the underrepresented groups. Explicit eligibility criteria need to be biologically justified and can be modified as the trial progresses using adaptive designs. Sponsors, funders, and investigators must minimize the logistical and practical burdens of study participation to avoid implicit exclusions related to geography and SES. For example, use of digital technology, data collection through linked data sources, conducting evening or weekend study visits, and providing appropriate financial supports may help. 45 Table 3 outlines more recommendations (by population group). Generally, multiple strategies are needed to increase inclusion, and the optimal set of strategies will vary across studies and even across sites (both within and between countries) within multi-site studies.41,46 –51 We also refer the reader to other resources.20,52 Concerns that focusing on enhancing diversity of trial populations is too time-consuming, costly, or may adversely affect trial outcomes due to heterogeneity of treatment effect are not supported by evidence. 53
Table 3.
| Target population | General recommendation | Specific strategies | Barriers targeted |
|---|---|---|---|
| Racial and ethnic minority | Develop a formal diversity plan | • Outline how inclusion of underrepresented populations will be addressed • Specify enrollment targets • Develop operational plan that addresses intended site locations, measures to limit participant burden, the community engagement strategy, and the evaluation plan to measure progress |
• Access to trials • Logistical and practical challenges |
| Engage with community groups | • Engage clinicians, clinic staff, research staff from racial and ethnic minority groups. • Establish community advisory board • Patient partners • Attend community events • Establish a presence at community centers and clinics, instead of remaining at tertiary care sites • Host health fairs/education sessions • Reach out to clinicians in the community to ensure they are aware of ongoing trials |
• Attitudes and beliefs • Lack of trust • Access to trials • Lack of knowledge regarding clinical trials • Enhance investigators’ awareness of barriers |
|
| Train site principal investigators and study staff to understand cultural norms | • Take an Implicit Association Test (IAT) such as through Project Implicit • Implicit bias training • Cultural competency training • Recruit study staff from underrepresented groups |
• Language and communication • Lack of trust |
|
| Develop study materials and means of advertising study appropriate to target population | • Translate study materials into multiple languages • Use culturally appropriate study materials (e.g. person-first language, images from the target population) • Ensure patient-facing materials are reviewed by laypersons from the target group • Use multiple modalities to reach potential participants (e.g. traditional print media, social media, video) |
• Access to trials • Language and communication • Lack of trust |
|
| Lower socioeconomic status | Address financial and logistical barriers to participation | • Assess SDoH at baseline • Provide care for dependents • Provide compensation for time off work • Enable visits on weekends and after hours for those who cannot miss work • Reimburse caregivers/parents for time, travel, parking, meals to support patient’s participation in the trial |
• Logistical and practical challenges |
| Rural and remote | Minimize burden in the study procedures | • Digital technologies ○ Telehealth assessments ○ E-consent ○ E-collection of patient-reported outcomes ○ Wearables ○ Pay for access to internet if needed • Mobile health professionals • Home blood collection • Provide transportation or reimbursement for transportation costs regardless of mode of transportation (public transit, private vehicle, shared transport, accessible transit) • Use linked data sources to reduce • primary data collection needed |
• Access to trials • Logistical and practical challenges |
| Extremes of age and comorbidity | Include biologic rationale for exclusions and consider alternative diseases | • Rationale should be specific to the age or comorbidity • Provide plan to address excluded groups in subsequent trial phases or using adaptive designs. If this is not possible provide strategy for how data regarding risks and benefits will be gathered (such as using real-world data) |
• Eligibility criteria |
| Sex and gender | • Collect data on sex at birth and gender identity | • Communication |
SDoH: social determinants of health.
Both recruitment methods and the transparent reporting of the characteristics of populations enrolled in clinical trials must improve to ensure trial populations represent those affected by the disease and to measure progress toward the goal of improved diversity and inclusion. Currently, reporting of race and ethnicity information is infrequent and not relevant worldwide, 26 and other SDoH are not reported. 27 The CONSORT-Equity statement, an extension of the CONSORT statement used for reporting of clinical trials, was developed to improve reporting in clinical trials in which health equity is relevant. Health equity was considered to be relevant when the target population was one experiencing social disadvantage, or when heterogeneity of treatment effect between two groups with differing levels of social disadvantage was of interest. The statement used the PROGRESS-Plus framework to define potentially disadvantaged groups. PROGRESS indicates place of residence, race/ethnicity/culture/language, occupation, gender/sex, religion, education, SES and social capital. 54 PROGRESS-Plus adds personal characteristics associated with discrimination (e.g. age), features of relationships between people and their settings, and time-dependent relationships (e.g. recent immigration) that may impose temporary disadvantage. Investigators should report gender, race and ethnicity, education, income, and place of residence for all trials, sex where biologically relevant, and recruitment strategies used. Potential reporting standards are proposed in Table 4.
Table 4.
Participant characteristics relevant to health equity.
| PROGRESS element | Potential reporting approaches | Details |
|---|---|---|
| Place of residence a | Urban, rural, suburban Low-, middle-, or high-income country |
Country classification based on World Bank income group 55 |
| Race, ethnicity, culture, language a | Clinical Data Interchange Standards Consortium (CDISC) | Race and ethnicity classifications commonly used in the United States are not meaningful elsewhere and lack granularity. CDISC allows more granular collection of information to meet global needs, but can be aggregated to meet FDA reporting standards |
| Occupation | International Standard Classification of Occupations (ISCO) 56 | International classification structure for organizing information on labor and jobs |
| Gender, sex a | Always ask about gender Ask about sex if biologically relevant |
Sex and gender are conceptually distinct.
57
Gender encompasses gender identity and gender expression. Example questions: What sex were you assigned at birth on your original birth certificate? (Female/Male/Don’t Know/Prefer not to Answer) What is your current gender? (Female/Male/Transgender/Two-spirit/I use a different term specify) If gender not socially and legally acceptable to collect, document |
| Religion | Categories proposed by Pew Forum on Religion & Public Life 58 | Classifies individuals based on the most common religions globally (Christian, Muslim, Hindu, Buddhist, Jewish, Folk religionist, unaffiliated, and other), based on a review of data from 232 countries |
| Education a | International Standard Classification of Education (ISCED) 59 | Internationally agreed definitions to aid comparisons worldwide |
| Socioeconomic status | Annual household disposable income
60
Number of people in household Is annual household income below poverty line |
Income does not fully reflect the construct of socioeconomic status but is accessible information. Here income includes earnings, self-employment and capital income and public cash transfers. Taxes and social security contributions are excluded from the total. Poverty line is half of the median household income of the total population, thus comparable across regions |
| Social capital | Tailored to research question | Usually assessed by questionnaire |
FDA: Food and Drug Administration.
Indicates characteristics to be reported in all clinical trials.
The CHIMES (Prospective Study to Assess Disease Activity and Biomarkers in Minority Participants with Relapsing Multiple Sclerosis After Initiation and During Treatment with Ocrelizumab) trial (NCT04377555) provides a salient example of timely and successful recruitment of underrepresented racial and ethnic minority populations in MS who were not well-represented in the original phase 3 trials. The CHIMES trial is a phase 4 clinical trial study of the safety and efficacy of ocrelizumab in African American and Hispanic/Latinx persons with MS, sponsored by Genentech. 61 The trial was designed collaboratively, engaging people with MS, researchers, and advocacy groups. By design, it addressed historical SDoH that act as barriers to enrollment of underrepresented populations. The trial protocol provides compensation for loss of earnings, transportation to the study site, reimbursement for childcare expenses, reimbursement for travel costs and meals, and greater flexibility with respect to study visits to assist with recruitment and retention of participants.
Conclusion
Clinical trial populations in MS are not adequately diverse to support effective shared decision-making by people with MS and their health care providers. Opportunities exist to refocus and amend trial processes to be more inclusive and equitable. Effective strategies are available to address this deficit, and we recommend concerted action by investigators, funders, advocacy groups, people affected by MS, and ethics committees. More comprehensive reporting of participant characteristics with respect to race, ethnicity, and SDoH will inform these efforts.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231189677 for Enhancing diversity of clinical trial populations in multiple sclerosis by Ruth Ann Marrie, Jeremy Chataway, Barbara E Bierer, Marcia Finlayson, Elena H Martinez-Lapiscina, Jennifer Panagoulias, Maria Pia Sormani, Mitzi Joi Williams and Lilyana Amezcua in Multiple Sclerosis Journal
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ruth Ann Marrie receives research funding from: Canadian Institutes of Health Research, Research Manitoba, MS Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, Consortium of MS Centers, the Arthritis Society, U.S. Department of Defense. She is supported by the Waugh Family Chair in Multiple Sclerosis. She is a co-investigator on a study funded in part by Biogen Idec and Roche (no funds to her or her institution). In the last 3 years, Jeremy Chataway has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC), and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Program (NIHR), the UK MS Society, the US National MS Society, and the Rosetrees Trust. He is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by the MS Canada. He is a local principal investigator for commercial trials funded by Ionis, Novartis, and Roche, and has taken part in advisory boards/consultancy for Azadyne, Biogen, Lucid, Janssen, Merck, NervGen, Novartis, and Roche. Barbara Bierer reports receiving research funding from the National Institutes of Health, US Food and Drug Administration, World Health Organization, Bill & Melinda Gates Foundation, The Greenwall Foundation, and Comprehensive and Integrative Medicine Institute (South Korea). She has served as a consultant on bioethical issues for Merck and Lilly. She serves on the Board of Directors of Clinithink, Edward P. Evans Foundation, Vivli, and North Star Research Board. Marcia Finlayson is a co-investigator on projects funded by the Patient-Centered Outcomes Research Institute, the University Hospitals Kingston Foundation, and the National Multiple Sclerosis Society. She has received consulting/speaker fees from Novartis and Biogen and serves on the editorial board of the IJMSC. Jennifer Panagoulias does not have any disclosures to declare. MP Sormani has received consulting fees from Biogen, Genzyme, GeNeuro, MedDay, Merck, Novartis, Roche, and Teva. Elena Martinez-Lapiscina is an employee of the European Medicines Agency. The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties. Lilyana Amezcua received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, EMD Serono and research support from the National MS Society, NIH NINDS, Bristol Myer Squibb Foundation, Race to Erase MS and Biogen Idec. She is a local PI for commercial trials funded by Genentech and Sanofi, Genzyme.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The International Advisory Committee on Clinical Trials in Multiple Sclerosis and the International Conference on Innovations in Clinical Trial Design & Enhancing Inclusivity of Clinical Trial Populations were supported by the National Multiple Sclerosis Society and the European Committee for Treatment and Research in Multiple Sclerosis. There was no involvement of the sponsors in the design, collection, analysis, or interpretation of data discussed at the Conference. The opinions expressed are those of the authors.
ORCID iDs: Ruth Ann Marrie  https://orcid.org/0000-0002-1855-5595
https://orcid.org/0000-0002-1855-5595
Marcia Finlayson  https://orcid.org/0000-0002-1774-4810
https://orcid.org/0000-0002-1774-4810
Maria Pia Sormani https://orcid.org/0000-0001-6892-104X
Lilyana Amezcua https://orcid.org/0000-0003-1542-7819
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Ruth Ann Marrie, Departments of Internal Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada.
Jeremy Chataway, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK/National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK/Medical Research Council Clinical Trials Unit at UCL, Institute of Clinical Trials and Methodology, University College London, London, UK.
Barbara E Bierer, The Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard, Cambridge, MA, USA/Harvard Medical School, Boston, MA, USA.
Marcia Finlayson, School of Rehabilitation Therapy, Faculty of Health Sciences, Queen’s University, Kingston, ON, Canada.
Elena H Martinez-Lapiscina, Center of Neuroimmunology, Laboratory of Advanced Imaging in Neuroimmunological Diseases, Hospital Clinic Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain/Office of Therapies for Neurological and Psychiatric Disorders, Human Medicines Division, European Medicines Agency, Amsterdam, The Netherlands.
Jennifer Panagoulias, Foundation for Angelman Syndrome Therapeutics, Austin, TX, USA.
Maria Pia Sormani, Department of Health Sciences, University of Genoa, Genoa, Italy.
Mitzi Joi Williams, Joi Life Wellness Group MS Center, Atlanta, GA, USA.
Lilyana Amezcua, Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
References
- 1. Dobson R, Rice DR, D’hooghe M, et al. Social determinants of health in multiple sclerosis. Nat Rev Neurol 2022; 18: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braveman P. Health disparities and health equity: Concepts and measurement. Annu Rev Public Health 2006; 27: 167–194. [DOI] [PubMed] [Google Scholar]
- 3. Amezcua L, Rivera VM, Vazquez TC, et al. Health disparities, inequities, and social determinants of health in multiple sclerosis and related disorders in the US: A review. JAMA Neurol 2021; 78: 1515–1524. [DOI] [PubMed] [Google Scholar]
- 4. Ventura RE, Antezana AO, Bacon T, et al. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult Scler 2017; 23(11): 1554–1557. [DOI] [PubMed] [Google Scholar]
- 5. Robers MV, Chan C, Vajdi B, et al. Hypertension and hypertension severity in Hispanics/Latinx with MS. Mult Scler 2021; 27(12): 1894–1901. [DOI] [PubMed] [Google Scholar]
- 6. Rotstein D, Maxwell C, Tu K, et al. High prevalence of comorbidities at diagnosis in immigrants with multiple sclerosis. Mult Scler 2021; 27(12): 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rotstein DL, Marrie RA, Tu K, et al. Health service utilization in immigrants with multiple sclerosis. PLoS ONE 2020; 15(7): e0234876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khayambashi S, Salter A, Tyry T, et al. Gender identity and sexual orientation affect health care satisfaction, but not utilization, in persons with Multiple Sclerosis. Mult Scler Relat Disord 2020; 37: 101440. [DOI] [PubMed] [Google Scholar]
- 9. Lavorgna L, Moccia M, Russo A, et al. Health-care disparities stemming from sexual orientation of Italian patients with Multiple Sclerosis: A cross-sectional web-based study. Mult Scler Relat Disord 2017; 13: 28–32. [DOI] [PubMed] [Google Scholar]
- 10. Stuifbergen A, Becker H, Phillips C, et al. Experiences of African American women with multiple sclerosis. Int J MS Care 2021; 23(2): 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chopin I, Farkas L, Germaine C, et al. Ethnic origin and disability data collection in Europe: Measuring inequality—Combating discrimination. Hermanin C, Atanasova A. (eds). New York: Open Society Foundations, 2014, pp. 11–13. [Google Scholar]
- 12. Marrie RA, Elliott L, Marriott J, et al. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology 2014; 83: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harding KE, Wardle M, Carruthers R, et al. Socioeconomic status and disability progression in multiple sclerosis: A multinational study. Neurology 2019; 92: e1497–e1506. [DOI] [PubMed] [Google Scholar]
- 14. Calocer F, Ng HS, Zhu F, et al. Low socioeconomic status was associated with a higher mortality risk in multiple sclerosis. Mult Scler 2023; 29: 466–470. [DOI] [PubMed] [Google Scholar]
- 15. Alvidrez J, Castille D, Laude-Sharp M, et al. The National Institute on Minority Health and Health Disparities research framework. Am J Public Health 2019; 109: S16–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Public Health Agency of Canada. Reducing health inequalities: A challenge for our times. Ottawa, ON, Canada: Public Health Agency of Canada, 2011. [Google Scholar]
- 17. Australian Institute of Health and Welfare. Australia’s health 2016. Australia’s health series. Canberra, ACT, Australia: Australian Institute of Health and Welfare, 2016. [Google Scholar]
- 18. Dhar A, Bhatt J, Batra N, et al. US health care can’t afford health inequities. New York: Deloitte Insights, 2022. [Google Scholar]
- 19. Mackenbach JP, Meerding WJ, Kunst AE. Economic costs of health inequalities in the European Union. J Epidemiol Community Health 2011; 65(5): 412–419. [DOI] [PubMed] [Google Scholar]
- 20. Bierer BE, White SA, Meloney LG, et al. Achieving diversity, inclusion and equity in clinical research: Guidance document. Boston, MA: The MRCT Center of Brigham and Women’s Hospital and Harvard, 2021, pp. 1–306. [Google Scholar]
- 21. Moneim J, Coles A, Giovannoni G, et al. Women on multiple sclerosis clinical trial steering committees. Ann Neurol 2018; 84(2): 329–330. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen MW, Andersen JP, Schiebinger L, et al. One and a half million medical papers reveal a link between author gender and attention to gender and sex analysis. Nat Hum Behav 2017; 1(11): 791–796. [DOI] [PubMed] [Google Scholar]
- 23. Wei S, Le N, Zhu JW, et al. Factors associated with racial and ethnic diversity among heart failure trial participants: A systematic bibliometric review. Circ Heart Fail 2022; 15(3): e008685. [DOI] [PubMed] [Google Scholar]
- 24. Van Spall HGC, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: A systematic sampling review. JAMA 2007; 297: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 25. Kennedy-Martin T, Curtis S, Faries D, et al. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015; 16: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Onuorah H-M, Charron O, Meltzer E, et al. Enrollment of non-white participants and reporting of race and ethnicity in phase III trials of multiple sclerosis DMTs: A systematic review. Neurology 2022; 98: e880–e892. [DOI] [PubMed] [Google Scholar]
- 27. Finlayson M, Al-Mashita L, Sandhu R. Participant diversity in clinical trials of rehabilitation interventions for people with multiple sclerosis: A scoping review. Mult Scler 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services. Enhancing the diversity of clinical trial populations—Eligibility criteria, enrollment practices and trials designs: Guidance for industry. Silver Spring, MD: US Department of Health and Human Services, 2020, pp. 1–19. [Google Scholar]
- 29. European Medicines Agency. EMA regulatory science to 2025: Strategic reflection. Amsterdam: European Medicines Agency, 2020, pp. 1–77. [Google Scholar]
- 30. Marrie RA, Miller A, Sormani MP, et al. The challenge of comorbidity in clinical trials for multiple sclerosis. Neurology 2016; 86: 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fakolade A, Bisson EJ, Petrin J, et al. Little is known about the impact of comorbidities on outcomes of neurorehabilitation interventions in MS. Int J MS Care 2016; 18: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cerreta F, Vučić K, Laslop A. Assessing medicines for use in the geriatric population. Clin Pharmacol Ther 2023; 113(3): 536–540. [DOI] [PubMed] [Google Scholar]
- 33. Simon N, Simon R. Adaptive enrichment designs for clinical trials. Biostatistics 2013; 14: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. US Department of Health and Human Services. Adaptive designs for clinical trials of drugs and biologics: Guidance for industry. Silver Spring, MD: US Department of Health and Human Services, 2019, pp. 1–33. [Google Scholar]
- 35. Marrie R, Sormani MP, Apap Mangion S, et al. Improving the efficiency of clinical trials in multiple sclerosis (Submitted). 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bovis F, Ponzano M, Signori A, et al. Reinterpreting clinical trials in children with multiple sclerosis using a Bayesian approach. JAMA Neurol 2022; 79: 821–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. US Department of Health and Human Services. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials: Guidance for industry. Silver Spring, MD: US Department of Health and Human Services, 2022, pp. 1–12. [Google Scholar]
- 38. Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med 2021; 19(1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health 1997; 87(11): 1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shea L, Pesa J, Geonnotti G, et al. Improving diversity in study participation: Patient perspectives on barriers, racial differences and the role of communities. Health Expect 2022; 25(4): 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodicoat DH, Routen AC, Willis A, et al. Promoting inclusion in clinical trials—A rapid review of the literature and recommendations for action. Trials 2021; 22: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamel LM, Penner LA, Albrecht TL, et al. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control 2016; 23(4): 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health 2014; 104(2): e16–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pimentel Maldonado DA, Moreno A, Williams MJ, et al. Perceptions and preferences regarding multiple sclerosis research among racial and ethnic groups. Int J MS Care 2021; 23(4): 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bove R, Poole S, Cuneo R, et al. Remote observational research for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2023; 10(2): e200070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wells JS, Pugh S, Boparai K, et al. Cultural competency training to increase minority enrollment into radiation therapy clinical trials-an NRG oncology RTOG study. J Cancer Educ 2017; 32(4): 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borno HT, Lin TK, Zhang S, et al. Accelerating cancer clinical trial recruitment through a financial reimbursement program integrated with patient navigation: An interrupted time series analysis. J Cancer Policy 2021; 30: 100305. [DOI] [PubMed] [Google Scholar]
- 48. Nipp RD, Lee H, Powell E, et al. Financial burden of cancer clinical trial participation and the impact of a cancer care equity program. Oncologist 2016; 21(4): 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart J, Krows ML, Schaafsma TT, et al. Comparison of racial, ethnic, and geographic location diversity of participants enrolled in clinic-based vs 2 remote COVID-19 clinical trials. JAMA Netw Open 2022; 5: e2148325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Getz K, Florez M, Botto E, et al. Global investigative site personnel diversity and its relationship with study participant diversity. Ther Innov Regul Sci 2022; 56(5): 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andrasik MP, Broder GB, Wallace SE, et al. Increasing black, indigenous and people of color participation in clinical trials through community engagement and recruitment goal establishment. PLoS ONE 2021; 16(10): e0258858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bierer BE, White SA, Meloney LG, et al. Achieving diversity, inclusion and equity in clinical research: Toolkit. Boston, MA: Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard, 2021, p. 145. [Google Scholar]
- 53. Chaudhry MS, Spahn J, Patel S, et al. Myths about diversity in clinical trials reduce return on investment for industry. Nat Med 2022; 28: 1520–1522. [DOI] [PubMed] [Google Scholar]
- 54. O’Neill J, Tabish H, Welch V, et al. Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol 2014; 67(1): 56–64. [DOI] [PubMed] [Google Scholar]
- 55. The World Bank. World Bank Country and Lending Groups [online]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. (accessed 29 December 2022).
- 56. International Labour Office. International Standard Classification of Occupations (ISCO-8). Structure, group definitions and correspondence tables. Geneva: International Labour Office, 2012. [Google Scholar]
- 57. National Academies of Sciences E, and Medicine. Measuring sex, gender identity, and sexual orientation. Washington, DC: The National Academies Press, 2022. [PubMed] [Google Scholar]
- 58. Pew Research Center’s Forum on Religion and Public Life. The Global Religious Landscape. A report on the size and distribution of the world’s major religious groups as of 2010. Washington, DC: Pew Research Center, 2012. [Google Scholar]
- 59. UNESCO Institute for Statistics. International Standard Classification of Education ISCED 2011. Montreal, QC: UNESCO Institute for Statistics, 2012 [Google Scholar]
- 60. Organisation for Economic Co-operation and Development. Income inequality (indicator). Paris, France: OECD, 2023. [Google Scholar]
- 61. Williams MJ, Amezcua L, Vartanian T, et al. Treating minority patients with multiple sclerosis: Development of the CHIMES trial. Hackensack, NJ: Consortium of Multiple Sclerosis Centers, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231189677 for Enhancing diversity of clinical trial populations in multiple sclerosis by Ruth Ann Marrie, Jeremy Chataway, Barbara E Bierer, Marcia Finlayson, Elena H Martinez-Lapiscina, Jennifer Panagoulias, Maria Pia Sormani, Mitzi Joi Williams and Lilyana Amezcua in Multiple Sclerosis Journal


