Abstract
Introduction
The Understanding New Interventions with GBM ThErapy (UNITE) study was designed to assess the effect of prophylaxis for ocular side effects (OSEs) in patients with glioblastoma receiving the antibody-drug conjugate (ADC) depatuxizumab mafodotin. UNITE (NCT03419403) was a phase 3b, open-label, randomized, exploratory study performed at 18 research sites in 5 countries.
Methods
The study enrolled adult patients with epidermal growth factor receptor-amplified, histologically confirmed, newly diagnosed supratentorial glioblastoma or grade IV gliosarcoma, and a Karnofsky Performance Status ≥70, receiving depatuxizumab mafodotin. All patients were administered depatuxizumab mafodotin during concurrent radiotherapy and temozolomide and with adjuvant temozolomide. Ninety patients were to be randomized (1:1:1) to OSE prophylactic treatments with each depatuxizumab mafodotin infusion: (a) standard steroid eye drops, (b) standard steroid eye drops plus vasoconstrictor eye drops and cold compress, or (c) enhanced steroids plus vasoconstrictor eye drops and cold compress. A Corneal Epitheliopathy Adverse Event (CEAE) scale was devised to capture symptoms, grade OSEs (scale of 0–5), and inform ADC dose modifications. The primary endpoint was the frequency of a required change in OSE management due to inadequate control of OSEs, defined as decline from baseline in visual acuity (using logarithm of the minimum angle of resolution [LogMAR] scale) or a Grade ≥3 CEAE event, in the worst eye in the first 8 weeks of treatment; unless otherwise specified, the treatment period refers to both the chemoradiation and adjuvant phases.
Results
The UNITE study was stopped early after interim analysis of separate phase III trial showed no difference in survival from depatuxizumab mafodotin. Forty patients were randomized (38 received depatuxizumab mafodotin). Overall, 23 patients experienced inadequate control of OSEs that required change in OSE management within 8 weeks of treatment, with 21 (70.0%) experiencing ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity and 12 reporting a grade ≥3 CEAE. There were no definitive differences among prophylactic treatments.
Conclusions
The premature cessation of the study precludes definitive conclusions regarding the OSE prophylaxis strategies. No new clinically significant safety findings were noted. Despite these limitations, this study highlights the need for novel assessment tools to better understand and mitigate OSEs associated with ADCs.
Keywords: Ocular side effect, Antibody-drug conjugate, Depatuxizumab mafodotin, Corneal epitheliopathy, Epidermal growth factor receptor
Introduction
Antibody-drug conjugates (ADC) have become standard treatments in a variety of cancers [1]. By linking antibodies to a cytotoxic payload, ADCs can target specific cells while sparing others, improving the safety profile of systemic cytotoxic agents. Adverse events (AEs) associated with ADC treatment include ocular side effects (OSEs) such as symptoms of dry eye and blurred vision, and clinical findings of corneal epitheliopathy that can interfere with activities of daily living (ADL), but these are generally dose dependent and reversible [2, 3]. OSEs have been reported in 14–85% of patients in clinical studies of ADCs, reflecting differences in drug activity, study designs, AE reporting, and patient populations [3–13]. Variation in reporting OSEs precludes characterization of ADC-related OSEs in a consistent manner, highlighting the need for a rating scale that consolidates a number of these disparately used terms as well as standardization of ocular grading system to expand from the Common Terminology Criteria.
Depatuxizumab mafodotin (ABT-414) is a novel ADC that consists of 3 components: an epidermal growth factor receptor (EGFR)-targeting humanized monoclonal antibody (depatuxizumab); a potent microtubule toxin, monomethyl auristatin F (MMAF, mafodotin); and a noncleavable maleimidocaproyl linker, which connects MMAF to the antibody (see online suppl. Fig. 1; for all online suppl. material, see https://doi.org/10.1159/000531142) [14]. Depatuxizumab mafodotin binds to a unique epitope of the activated EGFR. Abnormalities of EGFR occur in several solid tumors, including glioblastoma (GBM) where focal gene amplification occurs in about 50% of patients with newly diagnosed GBM [14]. The clinical development of depatuxizumab mafodotin has included 6 trials in patients with solid tumors, with preliminary safety data available for 809 patients receiving at least 1 dose of depatuxizumab mafodotin [15–19]. In the INTELLANCE-2 study (NCT02343406), a phase 2 trial in patients with recurrent GBM, 77% of patients who received depatuxizumab mafodotin experienced at least 1 treatment-emergent AE coded to the system organ class of eye disorders. The preferred term frequently reported in association with depatuxizumab mafodotin-related OSEs was reversible corneal epitheliopathy, for which the rate of grade 3/4 events was 24% for patients receiving depatuxizumab mafodotin monotherapy and 33% for patients receiving depatuxizumab mafodotin in combination with chemotherapy [19]. In the subsequent placebo-controlled phase 2/3 INTELLANCE-1 study (NCT02573324), depatuxizumab mafodotin was administered with concurrent radiation and temozolomide and with adjuvant temozolomide to patients with newly diagnosed EGFR-amplified GBM. Of patients randomized to depatuxizumab mafodotin, 95% experienced at least 1 OSE of any grade, 61% experienced grade 3/4 OSEs, and 12% of patients discontinued treatment because of OSEs [8].
Data on the clinical management of ADC-induced OSEs are limited. The first in-human study of SAR3419, an ADC targeting CD19, provided initial observations of the rapid reversibility of corneal microcystic epitheliopathy, which occurred with high frequency in patients receiving higher dose ADC [3]. In two studies of denintuzumab mafodotin with MMAF, the same toxin as depatuxizumab mafodotin, prophylactic topical ophthalmic steroid application, with other mitigation strategies, appeared to reduce OSE duration and severity [2, 5, 10]. A hypothesized mechanism of ADC-induced microcystic epitheliopathy/keratopathy is the uptake of ADC at the level of transient amplifying cells (TACs), which are the dividing daughter cells of the corneal limbal stem cells, and release of the ADC payload, resulting in apoptosis of the affected corneal epithelial cells, possibly giving rise to what may appear clinically as epithelial microcystic lesions [3, 4, 9]. The affected epithelial cells, which are observed in the corneal periphery initially, often do not cause ocular symptoms; however as these affected cells migrate centrally (following the natural course of corneal epithelial turnover), patients often experience a myriad of ocular surface disease symptoms, such as dry eyes, sensitivity to light, and blurred vision [4, 15, 16, 20].
Given the well-accepted clinical observation of decreased rate of corneal re-epithelialization when treated with topical steroid [21, 22], it is hypothesized that topical steroids may reduce the rate of TAC division, thereby reducing exposure of TACs to ADC, potentially resulting in reduced signs and symptoms of ADC-keratopathy. Thus, steroid ophthalmic solutions such as prednisolone acetate 1% suspension and dexamethasone 0.1% solution have been required in depatuxizumab mafodotin clinical trials [4, 16].
The Understanding New Interventions with GBM ThErapy (UNITE) study (NCT03419403) was a phase 3b exploratory study of depatuxizumab mafodotin in combination with a standard chemoradiotherapy/adjuvant regimen (radiotherapy and temozolomide) in patients with EGFR-amplified newly diagnosed GBM (following surgical resection/biopsy). The study evaluated 3 prophylactic treatment options for OSEs associated with depatuxizumab mafodotin (online suppl. Fig. 2). The primary objective of this study was to assess the effect of each prophylactic treatment by estimating the rate of change in OSE management due to inadequate control, to explore how to manage and mitigate OSEs that arise during treatment with ADCs, which became evident from prior studies. During the course of the UNITE study, interim results from the phase 2/3 INTELLANCE-1 trial reported no survival benefit for depatuxizumab mafodotin versus placebo [23]. Therefore, enrollment into the UNITE study was stopped early after 40 patients were randomized out of the planned sample size of 90. We report descriptive results from 38 patients who received depatuxizumab mafodotin in the UNITE study.
Materials and Methods
Patients
The study enrolled patients aged ≥18 years with EGFR-amplified (as identified centrally by fluorescent in situ hybridization) [24], histologically confirmed (locally), newly diagnosed supratentorial GBM or Grade IV gliosarcoma (including subtypes following diagnostic biopsy or resection), and a Karnofsky Performance Status [25] (KPS) ≥70. Patients were required to have adequate bone marrow, renal, and hepatic function. Patients were excluded from the study if they had a visual condition that compromised the ability to accurately measure a patient’s visual acuity or assess visual ADL (e.g., central tumor affecting visual pathways), prior laser-assisted in situ keratomileusis procedure within the prior year, cataract surgery within the prior 3 months, or prior anticancer therapy within 5 years of study day 1.
The study was conducted in accordance with the International Council for Harmonisation (ICH) guidelines, applicable regulations and guidelines governing clinical study conduct, and the ethical principles within the Declaration of Helsinki. The study protocol was approved by the Independent Ethics Committee/Institutional Review Board of all participating institutions. All patients provided written informed consent, and the study is registered at ClinicalTrials.gov (NCT03419403).
Study Design
UNITE was a phase 3b, open-label, randomized, exploratory study performed at 18 research sites in 5 countries (Australia, Germany, The Netherlands, United Kingdom, and USA). All patients were administered depatuxizumab mafodotin during radiation combined with temozolomide (chemoradiation) and also with post-radiotherapy (adjuvant) temozolomide. Patients were randomized in a 1:1:1 ratio to one of three OSE-related prophylactic eye treatment arms to be administered with each infusion of depatuxizumab mafodotin. Patients in Arm A were treated with standard steroids (SS; e.g., prednisolone acetate 1% suspension or equivalent), 1 drop in each eye 3 times per day starting 2 days before depatuxizumab mafodotin infusion and continuing until 4 days after infusion, for a total of 7 days. Alternate steroid eye drops (e.g., 0.1% dexamethasone phosphate solution or equivalent) were used if prednisolone acetate was not tolerated. Patients in Arm B received SS plus vasoconstrictor eye drops and cold compress (SS/VC). The vasoconstrictor eye drops (e.g., naphazoline hydrochloride 0.012% solution or equivalent) were administered 1 drop in each eye 4 to 6 times on the day of infusion (5–10 min before infusion, at end of infusion, and 2–4 times during the remainder of the infusion day) and on day 1 and day 2 after infusion. The cold compress was provided 5 min before the start of infusion and continued for 30 min past the end of infusion and for at least 2 h total per day (in increments no longer than 30 min) during the remainder of the infusion day and for 2 days after infusion. Arm C treatment was comprised of enhanced steroids with vasoconstrictor and cold compress (ES/VC). The ES regimens included 1 eye drop (SS as previously described) in each eye 6 times a day and low-dose ophthalmic steroid ointment (e.g., fluorometholone ophthalmic 0.1%, betamethasone eye ointment 0.1%, or equivalent) in each eye at bedtime, both starting 2 days before depatuxizumab mafodotin infusion and continuing until 4 days after infusion, for a total of 7 days. Unrestricted supportive care measures were allowed at any time, as recommended by the treating ophthalmologist, including lubricant eye drops, topical antibiotic drops, and other measures for comfort, such as sunglasses if needed.
Chemoradiation began ≤7 weeks after GBM diagnosis. Radiotherapy (∼60 Gray [Gy] over ∼6 weeks) and temozolomide (75 mg/m2 continuously during radiotherapy) were administered as standard of care. Depatuxizumab mafodotin 2.0 mg/kg was administered via intravenous infusion once every 2 weeks (day 1 of weeks 1, 3, and 5) of chemotherapy.
After the chemoradiation phase, there was a recovery period of approximately 4 weeks before the adjuvant phase. During the adjuvant phase, patients received temozolomide (150–200 mg/m2 orally once daily on days 1–5 of 28 per local standard of care) and depatuxizumab mafodotin (1.25 mg/kg intravenously once every 2 weeks [day 1 and day 15 of 28]). Chemoradiation or adjuvant treatment was discontinued upon determination of tumor progression as defined by Response Assessment in Neuro-Oncology Working Group criteria [26], unacceptable toxicity, or after a maximum of 12 adjuvant 28-day cycles. Following treatment discontinuation, patients entered a follow-up phase, wherein they were monitored for safety (including follow-up of OSEs until symptom resolution) and overall survival.
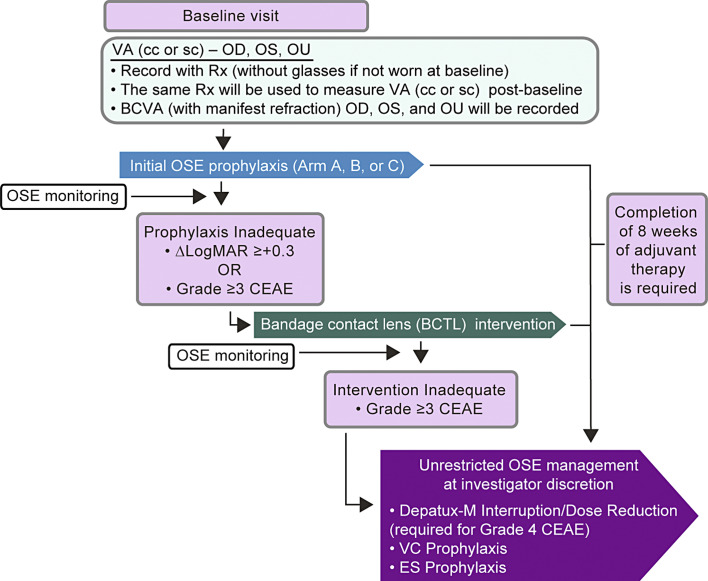
Scheduled ophthalmology examinations at baseline (Fig. 1), every 2 weeks during treatment, and then 35 and 49 days thereafter or until 30 days after resolution of any OSEs. Examinations included, at a minimum, assessment for ocular symptoms and visual acuity (using logarithm of the minimum angle of resolution [LogMAR] scale), intraocular pressure, and slit-lamp examination, as well as other examinations as deemed by the treating ophthalmologist.
Fig. 1.
OSE management during study. BCTL, bandage contact lens; BCVA, best corrected visual acuity; CEAE, corneal epitheliopathy adverse event; LogMAR, logarithm of the minimum angle of resolution; OD, right eye; OS, left eye; OSE, ocular side effect; OU, both eyes (oculus uterque); VA, visual acuity; VAcc, visual acuity with baseline glasses; VAsc, visual acuity without glasses; Rx, glasses prescription.
The Corneal Epitheliopathy Adverse Event (CEAE) scale was created by the authors to grade depatuxizumab mafodotin treatment eye events, including commonly reported symptoms (e.g., dry eye, eye pain, foreign body sensation, photophobia) which may complement clinical findings such as change in visual acuity, or corneal findings. The CEAE scale was used to assign OSE grade (on a scale of 0–5), which was utilized for depatuxizumab mafodotin dose modifications, and to systematically characterize the symptoms of corneal epitheliopathy related to depatuxizumab mafodotin (Table 1). CEAEs were assessed and scored at baseline, at each biweekly visit through the end of cycle 2 of the adjuvant phase, and then every 2 weeks thereafter until the final visit wherein adjuvant study drugs were administered. Given the nature of treating GBM patients, with possible decline in performance status and/or mental capacity to fully cooperate with ophthalmic examinations, CEAE assessment could be conducted by telephone and could incorporate information from caregivers. CEAE assessments were then conducted every 4 weeks beyond the day 49 follow-up visit, until symptom resolution, to provide detailed longitudinal information on severity overall and in specific domains.
Table 1.
Adverse event grading scales
| CEAE scale of visual ADLs | NCI CTCAE scale of adverse event severity |
|---|---|
| Grade 0 – asymptomatic | Grade 0 – no AE (or within normal limits) |
| Grade 1 – symptomatic, but no effect on visual ADLs | Grade 1 – mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated |
| Grade 2 – instrumental ADLsa affected, but can perform instrumental ADLs independently | Grade 2 – moderate; minimal, local, or noninvasive intervention (e.g., packing, cautery) indicated; limiting age-appropriate instrumental ADLs |
| Grade 3 – instrumental ADLsa require assistance | Grade 3 – severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADLs |
| Grade 4 – self-care ADLsb require assistance | Grade 4 – life-threatening consequences; urgent intervention indicated |
| Grade 5 – corneal perforation or corneal ulceration with impending perforation | Grade 5 – death related to AE |
ADLs, activities of daily living; AE, adverse event; CEAE, corneal epithelial adverse event. aInstrumental ADLs refer to preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc.
bSelf-care ADLs refer to bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden.
For patients who demonstrated inadequate control of OSEs with the initial prophylactic strategy, according to predefined criteria for loss of visual acuity (an increase on LogMAR of ≥+0.3 in both eyes) and/or OSE symptom of grade ≥3 on the CEAE scale, intervention with a bandage contact lens (BCTL) was used in addition to the assigned prophylactic regimen and the permitted supportive measures (Fig. 1). All BCTLs were approved by the respective study country’s regulatory authority and were inserted and changed in the clinic. All clinics used large diameter, zero power, soft lenses, which are approved for 30-day continuous use, along with topical antibiotic drops. Other topical treatments, including the assigned prophylactic steroid regimen, continued while the BCTL was in place. When a BCTL was introduced, considerations were made in relation to eye medications (particularly topical steroid), their frequency and potential medicamentosa from preservatives, and most importantly infection risks. Patients who demonstrated inadequate response (OSE of grade ≥3 on the CEAE scale, persisting for 2 weeks) with BCTL intervention were eligible for unrestricted OSE management according to investigator discretion, and the depatuxizumab mafodotin dose could be interrupted and/or reduced. Patients who completed 8 weeks of adjuvant therapy were allowed to switch to unrestricted OSE management per investigator regardless of CEAE grade, visual acuity, or BCTL intervention.
Nonocular AEs and some ocular AEs (those not involving the cornea or any serious AE) were assessed by investigators at every visit and graded and reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4; the last AE assessment was conducted at the day 49 follow-up visit. Laboratory profiles, physical examinations, and vital signs were also assessed throughout the study. The depatuxizumab mafodotin dose was interrupted in the event of grade 1/2 allergic reactions or grade 3/4 dermatologic toxicities.
Endpoints
The primary endpoint was the number of patients requiring change in OSE management due to inadequate control of OSEs, defined as a decline from baseline in visual acuity (≥+0.3 on LogMAR scale as assessed for the worse eye) or a grade ≥3 OSE event on the CEAE scale in the first 8 weeks after initiation of treatment with depatuxizumab mafodotin. It is important to note that visual acuity change utilized in the study was defined as the change from the patient’s baseline vision, which is more strict criteria than the change in the best corrected visual acuity at each visit. In addition, the endpoint used the worst eye, which is a stricter endpoint than bilateral vision (given that patients function at the level of the better eye). Secondary endpoints included maximum change from baseline in visual acuity on LogMAR scale, time to BCTL intervention [27], cumulative dose of depatuxizumab mafodotin received (during chemoradiation and adjuvant treatment), and CEAE grade at each visit. The effectiveness of the initial prophylactic regimen and the BCTL intervention were assessed separately. Evaluation of other secondary endpoints, including OSE resolution and symptom resolution after study drug discontinuation (reversibility), was limited due to early study termination.
Statistical Analyses
Analyses were conducted among all patients who received at least 1 dose of depatuxizumab mafodotin. Baseline characteristics, control of OSEs, and safety endpoints were summarized descriptively. For time to BCTL intervention, the Kaplan-Meier method was used to generate time-to-event curves and to calculate medians. Analyses were done with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) under the UNIX operating system. The database was locked on April 28, 2020.
Results
Patient Disposition and Baseline Characteristics
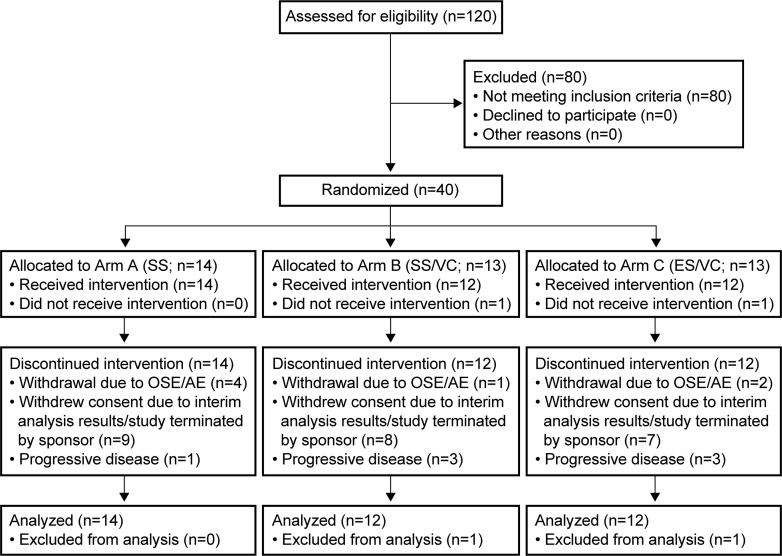
A total of 40 patients (of 90 planned) were randomized and 38 received study treatment (first dose administered between August 4, 2018, and May 15, 2019); 14 patients were assigned to Arm A and 12 patients to Arms B and C each (Fig. 2). Of these 38 patients, 16 (42.1%) entered the adjuvant phase. Median age was 54 years (range: 28–69 years) and 78.9% of patients were male. Most patients (81.6%) had a KPS ≥90, and 28 patients (73.7%) had a gross total resection. Baseline demographics and clinical characteristics were generally similar among treatment arms (online suppl. Table 1), although KPS score ≥90 was more common in Arms A and B (92.9% and 91.7%) than in Arm C (58.3%). Thirty-eight patients discontinued depatuxizumab mafodotin: 7 patients (18.4%) due to AEs (including 5 patients due to OSEs), 7 patients (18.4%) due to disease progression, and 24 patients (63.2%) due to early study termination.
Fig. 2.
CONSORT diagram of patient disposition. ES, enhanced steroids; SS, standard steroids; VC, vasoconstrictor and cold compress.
Exposure to Depatuxizumab Mafodotin
Overall, median treatment exposure to depatuxizumab mafodotin was 1.4 months (range: 0.5–9.0 months; n = 38). Median cumulative dose was 6.0 mg/kg (range: 2.0–24.7 mg/kg; Table 2) overall, 6.0 mg/kg (range: 2.0–20.9 mg/kg) for Arm A, 8.6 mg/kg (range: 2.0–24.7 mg/kg) for Arm B, and 6.0 mg/kg (range: 4.0–14.9 mg/kg) for Arm C. Median cumulative dose was 6.0 mg/kg (range: 2.0–6.2 mg/kg; n = 38) for the chemoradiation phase and 7.7 mg/kg (range: 1.2–18.7 mg/kg; n = 16) for the adjuvant phase.
Table 2.
Treatment exposure
| Parameter, median (range) | Arm A (SS) (n = 14) |
Arm B (SS/VC) (n = 12) |
Arm C (ES/VC) (n = 12) |
All patients (N = 38) |
|---|---|---|---|---|
| Treatment exposure of temozolomide, months | 1.4 (0.3–7.6) | 2.1 (0.6–8.5) | 1.3 (0.7–6.2) | 1.4 (0.3–8.5) |
| Treatment exposure of depatuxizumab mafodotin, months | 1.5 (0.5–8.3) | 3.0 (0.5–9.0) | 1.4 (1.0–6.9) | 1.4 (0.5–9.0) |
| Cumulative dose of temozolomide, mg | 1,800.1 (525.0–7,397.8) | 3,181.3 (771.8–7,292.9) | 1,707.2 (949.4–4,179.5) | 2,329.6 (525.0–7,397.8) |
| Cumulative dose of depatuxizumab mafodotin, mg/kg | 6.0 (2.0–20.9) | 8.6 (2.0–24.7) | 6.0 (4.0–14.9) | 6.0 (2.0–24.7) |
ES/VC, enhanced steroids with vasoconstrictor eye drops; SS, standard steroids; SS/VC, standard steroids plus vasoconstrictor eye drops.
Effectiveness of Prophylactic Regimen
Chemoradiation Phase
Overall, 23 patients experienced inadequate control of OSEs meeting criteria for the primary endpoint of change in OSE management within 8 weeks from first depatuxizumab mafodotin dose, with 21 patients (70.0%) experiencing +≥0.3 change on LogMAR scale in baseline-adjusted visual acuity and 12 reporting a grade ≥3 CEAE (Table 3). These 23 patients comprised 9 of 14 (64.3%) in Arm A, 8 of 11 (72.7%) in Arm B, and 6 of 12 (50.0%) in Arm C. Of the 23 patients meeting criteria for a change in OSE management, 14 received BCTL intervention. The overall median (range) maximum change from baseline on LogMAR scale of visual acuity was +0.5 (−0.58 to +1.20; n = 31).
Table 3.
Change in OSE management due to inadequate control of OSEs (worst eye)
| Arm A (SS) (n = 14) |
Arm B (SS/VC) (n = 12) |
Arm C (ES/VC) (n = 12) |
Total (N = 38) | |
|---|---|---|---|---|
| Overall | ||||
| ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity OR grade ≥3 CEAEa | 9/14 (64.3) | 8/11 (72.2) | 6/12 (50.0) | 23/37 (62.2) |
| ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity | 8/11 (72.7) | 8/10 (80.0) | 5/9 (55.6) | 21/30 (70.0) |
| Grade ≥3 CEAE | 5/14 (35.7) | 2/11 (18.2) | 5/10 (50.0) | 12/35 (34.3) |
| Adjuvant phase | ||||
| ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity OR grade ≥3 CEAEa | 4/6 (66.7) | 5/6 (83.3) | 1/4 (25.0) | 10/16 (62.5) |
| ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity | 3/5 (60.0) | 5/6 (83.9) | 1/2 (50.0) | 9/13 (69.2) |
| Grade ≥3 CEAE | 1/6 (16.7) | 2/5 (80.0) | 0/2 | 3/13 (23.1) |
| Post-BCTL intervention | ||||
| Patients who met the primary endpoint and received BCTL intervention | 5/11 (45.5) | 6/9 (66.7) | 3 (60.0) | 14 (56.0) |
| ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity OR grade ≥3 CEAEa | 4/7 (57.1) | 4/6 (66.7) | 5/6 (83.3) | 13/19 (68.4) |
| ≥+0.3 change on LogMAR scale in baseline-adjusted visual acuity | 2/7 (28.6) | 3/5 (60.0) | 4/5 (80.0) | 9/17 (52.9) |
| Grade ≥3 CEAE | 3/7 (42.9) | 2/5 (40.0) | 3/6 (50.0) | 8/18 (44.4) |
Data are presented as n/N (%).
BCTL, bandage contact lens; CEAE, corneal epitheliopathy adverse event; ES/VC, enhanced steroids with vasoconstrictor eye drops; LogMAR, logarithm of the minimum angle of resolution; OSE, ocular side effect; SS, standard steroids; SS/VC, standard steroids plus vasoconstrictor eye drops.
aAmong patients with at least one post-baseline assessment within 8 weeks for either LogMAR or CEAE; not all patients had values for both LogMar and CEAE.
Adjuvant Phase
Overall, 16 patients received depatuxizumab mafodotin in the adjuvant phase. Of these, 10 patients developed an event meeting the criteria for a change in OSE management within 8 weeks from first adjuvant dosing of depatuxizumab mafodotin, with 9 patients (69.2%) experiencing +≥0.3 change on LogMAR scale in baseline-adjusted visual acuity (3 of 5 patients in Arm A, 5 of 6 patients in Arm B, and 1 of 2 patients in Arm C). Three patients experienced ≥ grade 3 CEAEs (1 patient in Arm A and 2 in Arm B).
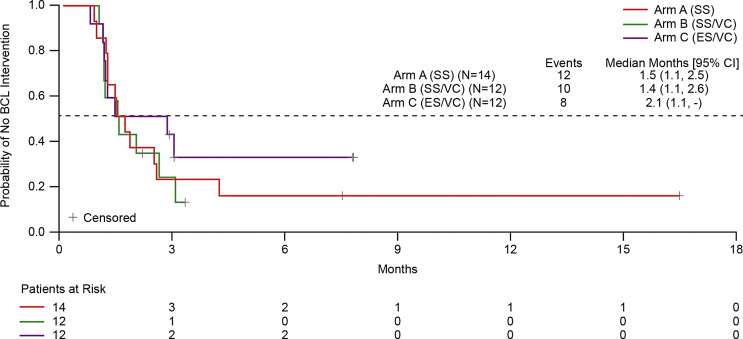
Time to BCTL Intervention
Addition of vasoconstrictor/cold compress and enhanced steroids (Arm C), but not vasoconstrictor/cold compress alone (Arm B), to the standard prophylaxis regimen (Arm A) modestly prolonged the time of adequate OSE control maintenance as assessed for the worst eye (median months [95% CI] of 2.1 [1.1–NE] for Arm C, 1.4 [1.1–2.6] for Arm B, and 1.5 [1.1–2.5] for Arm A; Figure 3).
Fig. 3.
Time to BCTL intervention as assessed using Kaplan-Meier method. BCTL, bandage contact lens; ES, enhanced steroids; SS, standard steroids; VC, vasoconstrictor and cold compress.
Effectiveness of BCTL Intervention
Overall, 8 of 18 evaluable patients required a second change in OSE management after BCTL intervention owing to inadequate control of OSEs. Three of 7 patients in Arm A, 2 of 5 in Arm B, and 3 of 6 in Arm C experienced a grade ≥3 CEAE, and 9 of 17 evaluable patients (52.9%) experienced +≥0.3 change on LogMAR scale in baseline-adjusted visual acuity (2 of 7 patients in Arm A, 3 of 5 patients in Arm B, and 4 of 5 patients in Arm C).
Safety
Ophthalmologic Side Effects
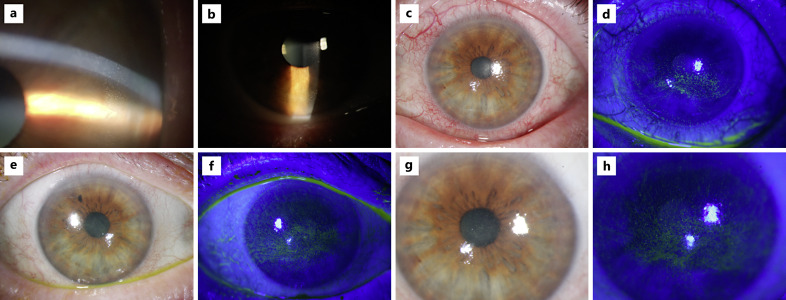
Overall, treatment-emergent CEAEs of any grade and grade ≥3 were reported by 94.7% and 39.5% of patients, respectively (Table 4). Representative images of the CEAEs observed during the trial are shown in Figure 4. CEAEs occurred with similar frequency among patients in each treatment arm. Of the 5 patients who discontinued depatuxizumab mafodotin due to OSEs, 3 patients were in Arm A and 2 patients were in Arm C.
Table 4.
OSEs summarized according to CEAE
| Arm A (SS) (n = 14) |
Arm B (SS/VC) (n = 12) |
Arm C (ES/VC) (n = 12) |
All patients (N = 38) | |
|---|---|---|---|---|
| Any OSE | 14 (100) | 11 (91.7) | 11 (91.7) | 36 (94.7) |
| Any OSE grade ≥3 | 5 (35.7) | 4 (33.3) | 6 (50.0) | 15 (39.5) |
| Any OSE leading to discontinuation of depatuxizumab mafodotin | 3 (21.4) | 0 | 2 (16.7) | 5 (13.2) |
Data are presented as n (%).
AE with onset date after the first study treatment and no more than 49 days after the last dose of depatuxizumab mafodotin are included.
AE, adverse event; ES/VC, enhanced steroids with vasoconstrictor eye drops; OSE, ocular side effect; SS, standard steroids; SS/VC, standard steroids plus vasoconstrictor eye drops.
Fig. 4.
CEAEs as observed during examinations. Images a and b are from the INTELLANCE-2 trial (NCT02343406) in which patients received depatuxizumab mafodotin at 1.0 mg/kg. Images c to h are from the INTELLANCE-1 trial (NCT02573324) and represent OSEs in patients who received depatuxizumab mafodotin at 1.25 mg/kg in the chemoradiotherapy phase and 2 mg/kg in the adjuvant phase; clinical presentation was graded using CTCAE keratitis criteria. c OS at week 7 with grade 3 OSE. d OS at week 7 with grade 3 OSE. e OD at week 8 with grade 3 OSE. f OD at week 8 with grade 3 diffuse epitheliopathy. g OS at week 8 with a grade 3 OSE. h OS at week 8 with grade 3 diffuse epitheliopathy. CTCAE, Common Terminology Criteria for Adverse Events; OD, right eye; OS, left eye; OSE, ocular side effect.
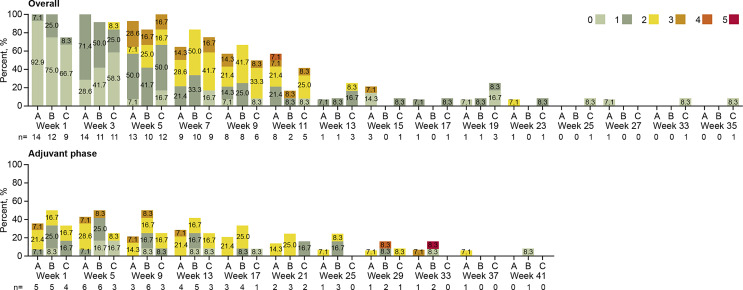
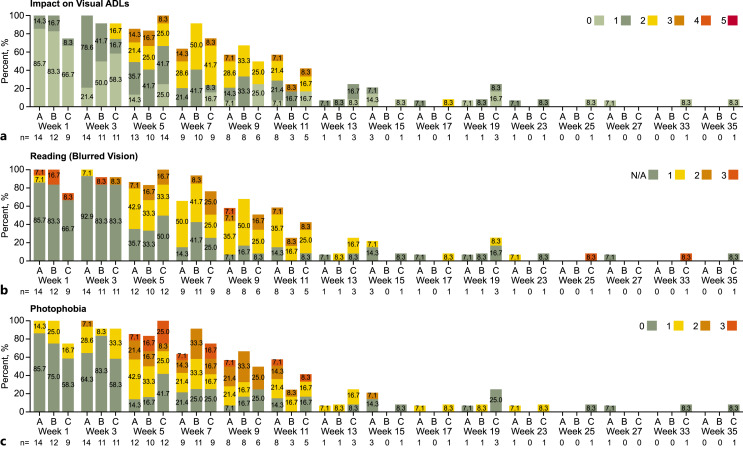
The event-free rates at month 6 of grade ≥3 CEAE by the Kaplan-Meier method were 63.5% (95% CI 33.1–83.0) for Arm A, 50.0% (95% CI 12.6–79.3) for Arm B, and 29.2% (95% CI 1.7–68.7) for Arm C (online suppl. Fig. 3). The majority of CEAEs were grade 0/1 during the first 3 weeks of chemoradiation, with grade ≥3 CEAEs occurring primarily between weeks 5 and 11; 1 patient in Arm A experienced a grade 4 CEAE (Fig. 5). Grade 2/3 CEAEs were reported throughout adjuvant treatment. One patient experienced a grade 4 event in week 29 and 1 patient experienced a grade 5 CEAE (corneal ulceration with impending perforation in a patient who did not use prophylactic topical antibiotic drops) in week 33 (this event was also recorded as serious AE of ulcerative keratitis [CTCAE grade 4]); both patients were in Arm B. CEAEs by ocular symptomatology are shown in Figure 6. Non-corneal ocular events included diplopia and eyelid ptosis in Arm B (1 patient each) and acquired epiblepharon and allergic conjunctivitis in Arm C (1 patient each); all were nonserious and NCI CTCAE grade <3.
Fig. 5.
CEAEs captured according to CEAE scale (overall severity of symptoms/findings related to corneal epithelial abnormalities) – safety analysis set. “Overall” includes all patients who entered the chemoradiation phase and did not enter the adjuvant phase but underwent follow-up until resolution of OSE (35 weeks); the “Adjuvant” phase includes patients who completed the chemoradiation phase and continued to the adjuvant phase. 0 = asymptomatic; 1 = symptomatic, but no effect on visual ADLs; 2 = instrumental ADLs affected, but can perform instrumental ADLs independently; 3 = instrumental ADLs require assistance; 4 = self-care ADLs require assistance 5 = corneal perforation or corneal ulceration with impending perforation. If a patient had >1 record in a visit window, the record closest to the nominal treatment day was chosen, and if there were multiple records on a day, then the worst result was chosen. Instrumental ADLs refer to activities such as preparing meals, shopping for groceries or clothes, using the telephone, managing money. Self-care ADLs refer to activities such as bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden. Numbers in bars represents percentage of patients. ADL, activities of daily living; CEAE, corneal epitheliopathy adverse events.
Fig. 6.
CEAEs captured according to CEAE scale by ocular symptomatology domain (combined chemoradiation and adjuvant phases) – safety analysis set. a Impact on visual ADLs. 0 = asymptomatic; 1 = symptomatic, but no effect on visual ADLs; 2 = instrumental ADLs affected but can perform instrumental ADLs independently; 3 = instrumental ADLs require assistance; 4 = self-care ADLs require assistance; 5 = corneal perforation or corneal ulceration with impending perforation. If a patient had >1 record in a visit window, the record closest to the nominal treatment day was chosen, and if there were multiple records on a day, then the worst result was chosen. b Reading (blurred vision). N/A = not applicable (patient does not read for reasons unrelated to blurred vision). 1 = no difficulties reading; 2 = reading requires large fonts for magnification; 3 = unable to read due to blurred vision. c Photophobia. 0 = none; 1 = mild (bright light); 2 = moderate (ambient light); 3 = severe (constant). Instrumental ADLs refer to activities such as preparing meals, shopping for groceries or clothes, using the telephone, managing money. Self-care ADLs refer to activities such as bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden. Numbers in bars represents percentage of patients. ADL, activities of daily living; CEAE, corneal epitheliopathy adverse events.
Nonocular Safety Profile
All 38 patients experienced a treatment-emergent AE, and 50% of patients had an AE possibly related to depatuxizumab mafodotin (online suppl. Table 2). The most frequently reported treatment-emergent AEs included fatigue (57.9%), nausea (39.5%), thrombocytopenia (36.8%), and headache (34.2%). Serious AEs were reported in 36.8% of patients. Less than half of the patients (44.7%) experienced a grade ≥3 treatment-emergent AE: 6 patients (15.8%) had thrombocytopenia, which was the only grade ≥3 AE to occur in >2 patients in the overall population. Two patients died within 49 days from the last dose of depatuxizumab mafodotin, both due to disease progression during treatment.
Potentially clinically significant hematology values categorized as NCI CTCAE grade ≥3 were observed for hemoglobin, leukocytes, neutrophils, lymphocytes, and platelets, with no parameter having clinically significant values reported for more than 20% of patients overall. The incidence of potentially clinically significant hematology values was similar across treatment arms, with the exception of an increased frequency of low lymphocyte values in Arm B (41.7%) versus Arm A (0%), and Arm C (8.3%). Potentially clinically significant chemistry values and vital signs (indicative of symptomatology) were observed in fewer than 10% of patients overall and were generally similar across treatment arms.
Discussion
This Phase 3b, randomized, open-label exploratory study evaluated the effectiveness of 3 different strategies for managing OSEs in patients with newly diagnosed EGFR-amplified GBM receiving depatuxizumab mafodotin in addition to standard chemoradiation with temozolomide followed by depatuxizumab mafodotin and adjuvant temozolomide. The UNITE study used a stepwise study design to better understand prophylactic management of OSEs and to characterize OSEs (rates, timing) using the protocol-specific CEAE rating scale. The CEAE scale was designed to describe the overall ocular symptoms experienced by the patient from ADC-related corneal epitheliopathy, accounting for both symptoms and clinical findings of keratopathy. Furthermore, the CEAE scale provided an additional, symptom-based means of assessing OSEs based on reported patient experience, without the requirement for a physical eye examination. Insight into the prevalence and impact of each symptom domain is lacking with NCI AE reporting, where investigators may report individual symptoms inconsistently or elect to use an overlying term (e.g., keratopathy) accounting for multiple symptoms. Systematic assessment of these domains with repeated administration of the CEAE provided detailed information on the longitudinal course of symptom severity. Treatment-emergent CEAEs of any grade were reported for 94.7% of patients, with 39.5% having CEAE Grade ≥3 events. While the small sample size limits interpretation of these data, no non-corneal OSE was reported by more than 1 patient (consistent with prior experience of depatuxizumab mafodotin) [15–18], suggesting that OSEs are restricted to effects on the cornea, in particular to the corneal epithelium. There was no consistent effect on the incidence of CEAEs (all grades or grade ≥3) over the course of the study for treatment with SS, SS/VC, or ES/VC.
Approximately, one half of evaluable patients required further OSE management with BCTL intervention to improve ocular symptoms and vision. For the overall prophylactic strategy, OSE control appears to show modest, numerical improvements with intensive prophylaxis.
Although the mechanisms for depatuxizumab mafodotin-associated OSEs are not fully understood, the effects appear to involve the corneal epithelium in a dose-dependent manner [16]. While EGFR expression is amplified in the cornea, it is hypothesized that depatuxizumab mafodotin exposure is similar to other ADCs causing microcystic keratopathy as it is taken up into rapidly dividing TACs/limbal stem cells, mainly by a nonselective, EGFR-unrelated mechanism; toxin is then released inside the cell, causing damage to TACs located within the basal cell layer that results in cellular damage/apoptosis and the formation of epithelial microcysts [2–4, 9]. Therefore, it is likely that OSEs are related to the cytotoxic payload component of the ADC, as opposed to the targeting of EGFR; this hypothesis is further supported by the observation of similar OSEs following treatment with ADCs that do not target EGFR [16, 28]. Other possible mechanisms for ADC-related corneal lesions may include altered centripetal differentiation as a result of focal separation of the epithelium and basement membrane, or stem cell toxicity, thereby disrupting corneal cell renewal [2]. These toxicities have been reported for ADCs with MMAF components [12, 29, 30]. OSEs with depatuxizumab mafodotin have demonstrated reversibility, but with a median time to resolution of ∼13 weeks after drug discontinuation despite a mean terminal half-life of <2 weeks for pharmacokinetic analytes of depatuxizumab mafodotin [15, 16]. Corneal epithelial regeneration comprises phases of cell migration, proliferation, and adhesion, which occur over the course of around 7–14 days, and each component of the process can be disrupted by a diverse range of clinical and pharmaceutical factors [31]. As the exposure of depatuxizumab mafodotin becomes less between doses, it is hypothesized that healthy corneal epithelial cells replace the damaged epithelial cells, with resolution of depatuxizumab mafodotin ADC-related keratopathy. The balance of these two events, fine-tuning the rate of each by possibly adjusting topical medications with change in their application duration, dose, and rate of taper, remains to be clarified.
Interpretation of these data is limited by the small study size; less than half of the participants had been accrued before study cessation (38/90 patients). Development of depatuxizumab mafodotin in GBM was stopped given the randomized controlled phase 3 study INTELLANCE-1 indicated no survival benefit for adding depatuxizumab mafodotin to standard chemoradiotherapy with temozolomide in patients with newly diagnosed GBM [23]. The premature cessation of data collection and resulting limited number of patients with data available preclude definitive conclusions regarding the OSE prophylaxis strategies assessed in this study. The current study utilized stricter criteria for grading ocular toxicity than is conventionally used (change in vision in the worst eye, rather than change in best corrected vision in the better eye), which leads to reporting of higher level of toxicity. Due to various method of grading ocular AEs, reported ocular toxicity may not always coincide with the severity of patients’ symptoms [13]. For example, the current study based grading on the worse eye whereas patients activity of daily living is performed with the vision out of their better eye. Even with the strict grading metric, the current study showed no new clinically significant safety findings. However, 1 patient required a corneal transplant, highlighting the requirement for judicious use of topical steroid eye drops with BCTL given the high risk for infectious keratopathy. Despite the limitations, this study highlights the concept of utilizing available ocular medication during the highest ADC drug exposure period. Although these ocular medications are not typically utilized in ophthalmology in this manner, topical steroids may slow down the epithelial proliferation [9, 21, 22] and decrease ADC uptake by corneal epithelium, and topical vasoconstriction may physically decrease the volume of ADC delivery to TACs. While dose modification and/or dose delay are the obvious strategy to decrease AEs, this must be balanced with delivery of a therapeutic dose. With improved strategies to mitigate ADC-induced keratopathy (OSEs), optimal dose may be possible for treatment of malignancies while improving the quality of life for patients. Further evaluation for ADC-induced keratopathy is ongoing.
Acknowledgments
Medical writing support was provided by Hayley Ellis, PhD, of Fishawack Facilitate Ltd., funded by AbbVie.
Statement of Ethics
The study was conducted in accordance with the International Council for Harmonisation (ICH) guidelines, applicable regulations and guidelines governing clinical study conduct, and the ethical principles within the Declaration of Helsinki. This study protocol was reviewed and approved by: East Midlands Leicester Central Research Ethics Committee (approval number: 15/EM/0324); Austin Health Human Research Ethics Committee (approval number: 5648006); Ethikkommission der Medizinischen Fakultaet Heidelberg (approval number: 2663001); Ethik-Kommisson an der Univ Regensburg (approval number: 26871002); Ethikkommission an der Medizinischen Fakultaet der Universitaet Leipzig (approval number: 30972002); Ethikkommission an der Med. Fakultaet der Eberhard-Karls-Universitaet Tuebingen; METC Utrecht; Baylor Scott and White Research (approval number: 30980001); Northwestern Memorial HealthCare IRB; UT Health (approval number: 4534002); Columbia University Medical Center (approval number: 7875009); North Shore University Health System (approval number: 22176004); Rush University Medical Center IRB (approval number: 1618053); University of Southern California Health Sciences Campus IRB; Advarra, Inc. (approval number: 28780006); and University of South Florida (approval number: 7874018). All patients provided written informed consent, and the study is registered at ClinicalTrials.gov (NCT03419403).
Conflict of Interest Statement
Colin J. Vize is a member of AbbVie advisory board. Stella K. Kim is ophthalmology consultant for AbbVie, Astellas, Cytomx, ImmunoGen, SeattleGenetics, Zymeworks. Tim Matthews is a member of AbbVie advisory board and receives Novartis educational stipend. Madan G. Kundu, Jennifer Yoon, Emma Kennedy, Madhavi Pai, and Earle Bain are AbbVie employees and may hold stock or options. Andrew B. Lassman reports grants, personal fees, and nonfinancial support from AbbVie; personal fees and nonfinancial support from Abbott Molecular, during the conduct of the study; personal fees and nonfinancial support from Bioclinica as an expert blinded independent reviewer of clinical and imaging data for a BMS-sponsored trial, grants, personal fees, and nonfinancial support from Karyopharm; personal fees from Sapience; personal fees and nonfinancial support from Novocure, grants, personal fees, and nonfinancial support from QED; personal fees and nonfinancial support from Forma, grants, personal fees, and nonfinancial support from Bayer, grants, personal fees, and nonfinancial support from Orbus, personal fees and nonfinancial support from NW Biotherapeutics, grants, personal fees, and nonfinancial support from Agios; personal fees from WebMD; personal fees, and nonfinancial support from Celgene; personal fees from prIME Oncology, grants and nonfinancial support from Kadmon, grants and nonfinancial support from VBI, grants and nonfinancial support from Beigene, grants and nonfinancial support from Oncoceutics, nonfinancial support from Tocagen, grants and nonfinancial support from Pfizer, grants and nonfinancial support from Genentech/Roche, grants and nonfinancial support from Millenium, grants and nonfinancial support from Celldex, grants and nonfinancial support from Novartis, nonfinancial support from Aeterna Zentaris, personal fees and nonfinancial support from PER, grants and nonfinancial support from BMS, outside the submitted work. Marian Macsai, Ryan Merrell, Sigmund Hsu, and Golnaz Moazami have nothing to disclose.
Funding Sources
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
A.B.L. was supported in part by Voices against Brain Cancer, The William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at New York-Presbyterian Hospital, and the National Institutes of Health (NIH)/National Cancer Institute NCI grants P30CA013696 and UG1CA189960. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NCI.
Author Contributions
All authors had access to relevant data and participated in the writing, review, and approval of the manuscript. Colin J. Vize, Stella K. Kim, Tim Matthews, Marian Macsai, Golnaz Moazami, Ryan Merrell, Sigmund Hsu, Madan G. Kundu, Jennifer Yoon, Emma Kennedy, Madhavi Pai, Earle Bain, and Andrew B. Lassman: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; and critical revision of the manuscript for important intellectual content.
Data Availability Statement
This study was sponsored by AbbVie. AbbVie and the authors are committed to responsible data sharing regarding clinical trial participation. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan (SAP) and execution of a data-sharing agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.
Funding Statement
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
A.B.L. was supported in part by Voices against Brain Cancer, The William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at New York-Presbyterian Hospital, and the National Institutes of Health (NIH)/National Cancer Institute NCI grants P30CA013696 and UG1CA189960. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NCI.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani AH. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol. 2019;11(1):3–23. [PMC free article] [PubMed] [Google Scholar]
- 2. Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31(10):589–604. 10.1089/jop.2015.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Younes A, Kim S, Romaguera J, Copeland A, Farial Sd C, Kwak LW, et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J Clin Oncol. 2012;30(22):2776–82. 10.1200/JCO.2011.39.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farooq AV, Degli Esposti S, Popat R, Thulasi P, Lonial S, Nooka AK, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9(4):889–911. 10.1007/s40123-020-00280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fathi AT, Borate U, DeAngelo DJ, O’Brien MM, Trippett T, Shah BD, et al. A phase 1 study of denintuzumab mafodotin (SGN-CD19A) in adults with relapsed or refractory B-lineage acute leukemia (B-ALL) and highly aggressive lymphoma. Washington, DC: American Society of Hematology; 2015. [Google Scholar]
- 6. Forero-Torres A, Moskowitz C, Advani RH, Shah BD, Kostic A, Albertson TM, et al. Interim analysis of a phase 1, open-label, dose-escalation study of SGN-CD19A in patients with relapsed or refractory B-lineage non-Hodgkin lymphoma (NHL). J Clin Oncol. 2014;32(15_Suppl):8505. 10.1200/jco.2014.32.15_suppl.8505. [DOI] [Google Scholar]
- 7. Hassan R, Blumenschein GR Jr, Moore KN, Santin AD, Kindler HL, Nemunaitis JJ, et al. First-in-Human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol. 2020;38(16):1824–35. 10.1200/JCO.19.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lassman AB, Pugh S, Wang T, Aldape K, Gan H, Preusser M, et al. ACTR-21. A randomized, double-blind, placebo-controlled phase 3 trial of depatuxizumab mafodotin (ABT-414) in epidermal growth factor receptor (EGFR) amplified (AMP) newly diagnosed glioblastoma (nGBM). Neuro Oncol. 2019;21(Supplement_6):vi17. 10.1093/neuonc/noz175.064. [DOI] [Google Scholar]
- 9. Matulonis UA, Birrer MJ, O'Malley DM, Moore KN, Konner J, Gilbert L, et al. Evaluation of prophylactic corticosteroid eye drop use in the management of corneal Abnormalities induced by the antibody-drug conjugate mirvetuximab soravtansine. Clin Cancer Res. 2019;25(6):1727–36. 10.1158/1078-0432.CCR-18-2474. [DOI] [PubMed] [Google Scholar]
- 10. Moskowitz CH, Fanale MA, Shah BD, Advani RH, Chen R, Kim S, et al. A phase 1 study of denintuzumab mafodotin (SGN-CD19A) in relapsed/refactory B-lineage non-Hodgkin lymphoma. Blood. 2015;126(23)(23):182. 10.1182/blood.v126.23.182.182. [DOI] [Google Scholar]
- 11. Tannir NM, Forero-Torres A, Ramchandren R, Pal SK, Ansell SM, Infante JR, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs. 2014;32(6):1246–57. 10.1007/s10637-014-0151-0. [DOI] [PubMed] [Google Scholar]
- 12. Thompson JA, Forero-Torres A, Heath EI, Ansell SM, Pal SK, Infante JR, et al. The effect of SGN-75, a novel antibody–drug conjugate (ADC), in treatment of patients with renal cell carcinoma (RCC) or non-Hodgkin lymphoma (NHL): a phase I study. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29(15_Suppl):3071. 10.1200/jco.2011.29.15_suppl.3071. [DOI] [Google Scholar]
- 13. Thompson JA, Motzer RJ, Molina AM, Choueiri TK, Heath EI, Redman BG, et al. Phase I trials of anti-ENPP3 antibody–drug conjugates in advanced refractory renal cell carcinomas. Clin Cancer Res. 2018;24(18):4399–406. 10.1158/1078-0432.CCR-18-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillips AC, Boghaert ER, Vaidya KS, Mitten MJ, Norvell S, Falls HD, et al. ABT-414, an antibody–drug conjugate targeting a tumor-selective EGFR epitope. Mol Cancer Ther. 2016;15(4):661–9. 10.1158/1535-7163.MCT-15-0901. [DOI] [PubMed] [Google Scholar]
- 15. Gan HK, Reardon DA, Lassman AB, Merrell R, van den Bent M, Butowski N, et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol. 2018;20(6):838–47. 10.1093/neuonc/nox202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goss GD, Vokes EE, Gordon MS, Gandhi L, Papadopoulos KP, Rasco DW, et al. Efficacy and safety results of depatuxizumab mafodotin (ABT-414) in patients with advanced solid tumors likely to overexpress epidermal growth factor receptor. Cancer. 2018;124(10):2174–83. 10.1002/cncr.31304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lassman AB, van den Bent MJ, Gan HK, Reardon DA, Kumthekar P, Butowski N, et al. Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial. Neuro Oncol. 2019;21(1):106–14. 10.1093/neuonc/noy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reardon DA, Lassman AB, van den Bent M, Kumthekar P, Merrell R, Scott AM, et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2017;19(7):965–75. 10.1093/neuonc/now257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Den Bent M, Eoli M, Sepulveda JM, Smits M, Walenkamp A, Frenel J-S, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020;22(5):684–93. 10.1093/neuonc/noz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parrozzani R, Lombardi G, Midena E, Leonardi F, Londei D, Padovan M, et al. Corneal side effects induced by EGFR-inhibitor antibody–drug conjugate ABT-414 in patients with recurrent glioblastoma: a prospective clinical and confocal microscopy study. Ther Adv Med Oncol. 2020;12:1758835920907543. 10.1177/1758835920907543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petroutsos G, Guimaraes R, Giraud JP, Pouliquen Y. Corticosteroids and corneal epithelial wound healing. Br J Ophthalmol. 1982;66(11):705–8. 10.1136/bjo.66.11.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomas-Barberan S, Fagerholm P. Influence of topical treatment on epithelial wound healing and pain in the early postoperative period following photorefractive keratectomy. Acta Ophthalmol Scand. 1999;77(2):135–8. 10.1034/j.1600-0420.1999.770203.x. [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov. A study of ABT-414 in subjects with newly diagnosed glioblastoma. (GBM) With Epidermal Growth Factor Receptor (EGFR) Amplification (Intellance1) – NCT02573324 Available from: https://clinicaltrials.gov/ct2/show/NCT02573324.
- 24. Lassman AB, Roberts-Rapp L, Sokolova I, Song M, Pestova E, Kular R, et al. Comparison of biomarker assays for EGFR: implications for precision medicine in patients with glioblastoma. Clin Cancer Res. 2019;25(11):3259–65. 10.1158/1078-0432.CCR-18-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1(4):634–56. . [DOI] [Google Scholar]
- 26. Wen P, Chang S, Van den Bent M, Vogelbaum M, Macdonald D, Lee E. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–49. 10.1200/JCO.2017.72.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macsai M, Nariani A, Gan H, Lassman A, Merrell R, Gomez E, et al. Corneal toxicity of ABT-414 in glioblastoma (GBM): clinical manifestations, ophthalmological findings and management. Invest Ophthalmol Vis Sci. 2016;57(12):269. [Google Scholar]
- 28. Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–21. 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 29. Fathi AT, Chen R, Trippett TM, O'Brien MM, DeAngelo DJ, Shah BD, et al. Interim analysis of a phase 1 study of the antibody-drug conjugate SGN-CD19A in relapsed or refractory B-lineage acute leukemia and highly aggressive lymphoma. Blood. 2014;124(21):963. 10.1182/blood.v124.21.963.963.24833353 [DOI] [Google Scholar]
- 30. Thompson JA, Motzer R, Molina AM, Choueiri TK, Heath EI, Kollmannsberger CK, et al. Phase I studies of anti-ENPP3 antibody drug conjugates (ADCs) in advanced refractory renal cell carcinomas (RRCC). J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(15_Suppl):2503. 10.1200/jco.2015.33.15_suppl.2503. [DOI] [Google Scholar]
- 31. Mannis MJ, Holland EJ. Cornea and sclera: anatomy and physiology. Cornea. 4th ed.Elsevier; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was sponsored by AbbVie. AbbVie and the authors are committed to responsible data sharing regarding clinical trial participation. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan (SAP) and execution of a data-sharing agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.