Abstract
Psoriasis is an incurable, chronic and auto-immune skin disorder with a global prevalence rate of approximately 2–3%. The study investigated the antipsoriasis activities of Deprungsith formulation and its bioactive components and their potential for inhibitory activities on human cytochrome P450 (CYP450). HaCaT and peripheral blood mononuclear cells (PBMCs) from healthy volunteers (n = 9) and psoriasis patients (n = 10) were exposed to Deprungsith formulation (Thai traditional medicine for psoriasis consisting of 16 plants), ethyl p-methoxycinnamate (EPMC), ligustilide and cyclosporin for 24 and 48 h. The antiproliferative, cell apoptosis and cell cycle arrest activities were evaluated using MTT assay and flow cytometry, respectively. The pro-inflammatory cytokine mRNA expression levels were measured using real-time polymerase chain reaction (RT-PCR). The CYP450 inhibitory effect was investigated using a bioluminescent-based CYP450 assay. Deprungsith formulation and the bioactive compounds inhibited HaCaT cells and PBMCs with weak to moderate potencies. EPMC and ligustilide combination produced an additive effect. Most substances arrested cell transition at sub-G1 and S phases, leading to early and late apoptosis induction. With prolonged exposure (48 h), all test substances decreased PBMCs necrosis. The mRNA expression of all pro-inflammatory cytokines was downregulated. Deprungsith formulation, EPMC, ligustilide and ferulic acid inhibited CYP1A2, CYP2C9, CYP2D6 and CYP3A4 activities with weak to moderate potencies. Deprungsith formulation and bioactive components induced cell apoptosis by inhibiting cell transition at specific cell cycle phases, which was correlated with the mRNA downregulation of interleukin (IL-6, IL-12p19, IL-23) and tumor necrosis factor (TNF-α). There is a low risk of potential adverse drug reactions and toxicity due to CYP450 interaction when Deprungsith formulation is concurrently administered with modern medicines.
Keywords: deprungsith formulation, ethyl p-methoxycinnamate, ligustilide, antipsoriasis, cytochrome P450 interaction
Introduction
Psoriasis is an incurable, chronic, auto-immune skin disorder with a global prevalence rate of approximately 2–3%. 1 Based on the statistics from the Dermatology Institutes, the worldwide incidence of psoriasis ranks fifth out of 10th in dermatological diseases. 2 The pathogenesis of psoriasis has been linked with several factors, such as genetics, inflammation, autoimmunity, metabolism, environment, and infection. 3 The main clinical presentations are erythematous scaly rash patches (itching and flaking skin) and mostly are classified as plaque psoriasis, which is further classified according to the target organs/areas, eg, hands, feet, nails, scalp, trunk, extensor surfaces of the limbs, and genital area. Although the disease seldom leads to death, chronic complications significantly impair the patients’ quality of life (QoL). The severity of the disease is classified as mild, moderate, and severe based on the degree of lesionpresented on the body surface area (BSA), which affects patients’ QoL, disease control/progression, and disease management (topical or systematic drug administration). 4 The key pathological alterations are malformed epidermal keratinocyte proliferation and differentiation, extreme infiltration of the immune/inflammatory cells such as T cells (Th17, Th1, and Th2), dendritic cells (DCs), macrophages, and neutrophils, and increased angiogenesis. 1 Several triggering factors, eg, skin trauma, infection, drugs, intense sunlight, physiological stress, and smoking, lead to activation of the immune system, followed by chronic disease progression. A network of immunological pathways, particularly skin inflammation via various pro-inflammatory cytokines have been reported to be associated with psoriasis pathogenesis and clinical manifestations through T-cell and NF-κB activation.5,6 The premature maturation of keratinocytes triggered by an inflammatory cascade in the dermis results in rapid changes in skin cells. The movement from the dermis to the epidermis of the immune cells and secretion of pro-inflammatory cytokines, eg, interleukin-1β (IL-1β), IL-6, IL-12, IL-22, IL-23, IL-17A and IFN-γ stimulate keratinocytes to proliferate and secrete IL-1, IL-6, and TNF-α. 7 These cytokines signal downstream inflammatory cells to reach the inflammation site, thus stimulating further inflammation. 5 Furthermore, a defect in regulatory T cells and regulatory cytokine IL-10 has also been linked with psoriasis pathogenesis. 5 The risks of adverse drug reactions/toxicity, disease recurrence, and drug resistance limit the current treatment of psoriasis. Available local and systemic treatments include coal tar, dithranol (anthralin), calcipotriol, corticosteroids, photochemotherapy (PUVA, psoralens with long-wave ultraviolet radiation), retinoids, methotrexate, and other cytostatic drugs (eg, hydroxyurea and cyclosporin).8,9 Effective alternative antipsoriatic agents with acceptable safety profiles are required.
Self-medication with traditional medicine is common in psoriasis patients, of which about 30–40% are being used or have used these remedies in combination with conventional psoriasis therapy. The practice is mainly focused in Asia, particularly in China, 10 India, 11 and Thailand. 12 In most cases, these traditional uses are arbitrary, with no scientific support for their clinical effectiveness. Deprungsith formulation is one of the Thai traditional medicine that traditional practitioners have used to treat psoriasis. The formulation consists of 16 plants (Table 1). 13 A preliminary in vitro investigation demonstrated superior anti-inflammatory activity of the ethanolic extract compared with water extract. 14 The expression of the pro-inflammatory cytokines was significantly downregulated. 14 In the psoriasis-like skin inflammation model, the ethanolic extract significantly reduced clinical psoriasis area and severity index (PASI) with comparable potency to the reference compound cyclosporin. 15 Acute toxicity testing in Sprague Dawley rats showed no toxicity at the highest dose level (5000 mg/kg body weight). In the imiquimod (IMQ)-induced psoriasis-like model in mice, Deprungsith extract at the dose levels of 25, 50, and 125 mg/kg body weight significantly decreased PASI and pro-inflammatory levels – IL-17A, IL-22 and IL-23 in both serum and skin samples. The estimated optimal dose used in patients is 250 mg per day. 13
Table 1.
Composition of Deprunsith Formulation. 13
| Scientific name/Family | Thai name | Part used (Proportion) | Isolated compounds | Pharmacological activities |
|---|---|---|---|---|
| Angelica dahurica/Apiaceae | Kod Sor | rhizome | Z-ligustilide, imperatorin, iso-imperatorin | anti-inflammatory, anticancer, neuroprotective |
| Angelica sinensis/Apiaceae | Kod Chiang | root | Z-ligustilide | vasodilation and improving microcirculation, anti-arthrosclerosis, antiplatelet aggregation, anti-inflammatory, anti-oxidative |
| Aquilaria malaccensis/Thymelaeaceae | Krisana | wood | phytol, squalene, n-hexadecanoic acid and octadecatrienoic acid. tetramethyl-2-hexadecen-1-1 | antioxidant, anti-inflammatory, anticancer |
| Aucklandia lappa/Asteraceae | Kod Kra Dook | root | costunolide, isodihydrocostunolide, cynaropicrin | anti-ulcer, anticonvulsant, anticancer, hepatoprotective, anti-arthritic, antiviral |
| Cuminum cyminum/Apiaceae | Tein Kao | seed | cumin aldehyde, limonene, α- and β-pinene, 1,8-cineole, | antioxidant, antimicrobial, antidiabetic, diuretic, immunomodulatory |
| Diospyros decandra/Ebenaceae | Chan Kao | bark | phenolic compounds | blood tonic, antimycobacterial, antimalarial, antifungal |
| Eupatorium fortunei/Compositae | Sun Pra Hom | leaf | 2-hydroxy-4 -met hylacetophenone, 8, 1O-Epoxy-9-acetoxythymol angelate, 9-isobutyryloxy-8. 10-dihydroxythymol, 9-angelo yloxy-8. 1 0-dihydroxythymol | anticancer |
| Euphorbia antiquorum/Euphorbiaceae | Sa Lad Dai Pa | whole plant | ingenol 3-angelate | analgesic, anti-inflammatory |
| Foeniculum vulgare/Apiacae | Tein Kao Pleuk | seed | trans-anethole, fenchone, estragole (methyl chavicol), α-phellandrene | diuretic, antihypertensive |
| Kaempferia galanga/Zingiberaceae | Proa Hom | rhizome | ethyl-p-methoxycinnamate (EPMC) | antimicrobial, antifungal, anti-inflammatory, analgesic, anticancer |
| Ligusticum sinense/Apiaceae | Kod Hua Bua | Root | ligustrazine, ferulic acid, cnidilide, ligustilide | cardiovascular protective, anti-inflammatory |
| Mimusops elengi/Sapotaceae | Khon Dok | wood | α-spinasterol, taraxerol | antihemorrhoidal, antifungal, anticariogenic, free radical scavenging, antihyperglycemic |
| Nigella sativa/Ranunculaceae | Tien Dumm | seed | thymoquinone (TQ) | antidiabetic, anticancer and immunomodulatory, analgesic, antimicrobial, anthelmintic, analgesics and anti-inflammatory |
| Pogostemon cablin/Labiatae | Pim Sen Ton | whole plant | α-patchoulene, β-patchoulene, α-bulnesene | antioxidant, analgesic, anti-inflammatory |
| Draceana loureiri/Dracaenaceae | Chan daeng | Wood | rihydroxystilbene , 4,3′-dihydroxy-5′-methoxystilbene and 4-hydroxy-3′,5′-dimethoxystilbene | antimalarial |
| Limnophilia rugosa/ Plantaginaceae | Phak kachom | whole plant | Nevadensin (5,7-dihydroxy-6,8,4'-trimethoxyflavone) | antibacterial, antifungal activities, anti-inflammatory activity, diuretic |
Deprungsith formulation consists of several medicinal plants, and therefore, the major bioactive constituents found in these plants were selected as chemical markers for quality control during material preparation. Ethyl-p-methoxycinnamate (EPMC) is an essential oil found in Kaempferia galanga L. with anti-inflammatory activity. 16 The essential oil ligustilide and the phenolic compound ferulic acid are bioactive components found in Ligusticum sinense, 17 which possess antioxidant and anti-inflammatory activities.18,19 Metabolism of xenobiotics via CYP450 enzymes is the key pharmacokinetic process that contributes to the risks of drug-drug or herbal-drug interactions, resulting in drug-related or herbal-related toxicities. CYP450 plays a critical role in the biotransformation of a wide range of xenobiotics, including therapeutic drugs, carcinogens, and toxicants. 20 In humans, three CYP450 families, CYP1, CYP2 and CYP3 are the most abundant CYP450, and they are active in the metabolism of most xenobiotics. Among the total 57 isozymes, 6 of these are responsible for 90% of drug metabolism. These six isozymes include CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4. 21
The present study investigated the antiproliferative and anti-inflammatory activities of Deprungsith formulation and its bioactive components in the in vitro model using immortalized human keratinocyte cell line (HaCaT) and human mononuclear cells (PBMCs) from psoriasis patients and healthy volunteers. In addition, potential herb-drug interaction for the inhibitory effects on major drug-metabolizing enzyme cytochrome (CYP450) was also investigated using a bioluminescent-based CYP450 assay.
Materials and Methods
Chemicals
The bioactive constituents of Deprungsith formulation, ie, ethyl-p-methoxycinnamate (EPMC), ligustilide, ferulic acid, imperatorin and iso-imperatorin, including the reference compound cyclosporin were supplied by Wako Co. Ltd (Osaka, Japan). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was supplied by Sigma Chemical Co. (St. Louis, Mo, USA). Commercial standards of the selective CYP450 inhibitors α-naphthoflavone and quinidine were purchased from Sigma Chemical Co. Sulfaphenazole and troglitazone were obtained from Cayman Chemical Company (Ann Arbor, MI, USA). Ketoconazole was supplied by Tokyo Chemical Industries (Tokyo, Japan) and acetonitrile, methanol and formic acid were supplied by Fisher Scientific (London, UK).
The stock solution of Deprungsith extract (100 mg/ml) was prepared in 50% ethanol. EPMC was dissolved with absolute ethanol to obtain 50 mM stock solution. Ligustilide was dissolved with DMSO to obtain 100 mM stock solution. All working solutions (contained < 0.1% ethanol or DMSO) were prepared by diluting the stock solutions with DMEM or RPMI 1640 supplemented with 10% fetal bovine serum albumin.
TRIzolTM reagent, SuperScriptTM III Reverse Transcriptase kit and DEPC-treated water were purchased from Invitrogen Life Technologies Inc. (Carlsbad, CA, USA). SYBRTM Green Real-time PCR Supermix was purchased from Bio-Rad Laboratories Inc. (CA, USA). Other reagents were purchased from conventional commercial sources.
Plant Extract
Deprungsith formulation is composed of 16 Thai herbs ( Table 1 ). 13 Each plant part was purchased from Vejpongosot Holding Co., Ltd, Thailand. All plants were authenticated by experienced traditional practitioners. The chemical markers EPMC, ligustilide, imperatorin and iso-imperatorin were analyzed qualitatively and quantitatively according to the Thai Herbal Pharmacopoeia and the World Health Organization (WHO) guidelines. 22
Deprungsith formulation was prepared according to the previously described protocol. 13 The plant materials (1 kg) were dried at 60 °C for 48 h after washing with water, and the seeds were removed. All dried materials were blended using the electric blender and passed through sieve No.14. The powder (15 g) was macerated in 1000 ml of 50% ethanol for 6 h to extract both polar and non-polar chemical compounds (cinnamic acid esters, phenolic acids, flavonoids, isobenzofurans, terpenoids, coumarins and sterols, etc) found in Deprungsith formulation, then filtered (Whatman No.1), and freeze-dried. Gamma-irradiation was used to control microbial contamination. 13 The yield was 10.53% of the fresh weight. The chemical markers were selected based on the proportion of medicinal plants in the formulation. EPMC (cinnamic esters) is a major constituent of Kaempferia galanga L. and was used at a ratio of 2:20 (w:w) of Deprungsith formulation. Ligustilide (isobenzofurans) is a bioactive compound found in Ligusticum sinense and was used at a ratio of 6:20 (w:w) of Deprungsith formulation. Imperitorin and iso-imperitorin (coumarins) are chemical constituents found in Angelica dahurica Benth. and was used at a ratio of 1:20 (w:w) of Deprungsith formulation. 13 The amounts (mean ± SD) of the chemical markers EPMC, ligustilide, imperatorin and iso-imperatorin were 6.91 ± 0.00 (0.69%), 1.00 ± 0.00 (0.1%), 0.03 ± 0.00 (0.003%) and 0.06 ± 0.00 (0.006%) μg/mg of the extract, respectively.
Assessment of Antiproliferative and Anti-Inflammatory Activities
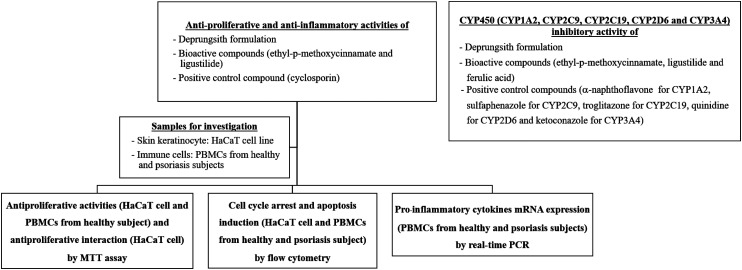
Study Design
The diagram of the study framework is presented in Figure 1. The antiproliferative and anti-inflammatory activities of Deprungsith formulation and its bioactive components were evaluated in an in vitro model using immortalized human keratinocyte cell line (HaCaT) for the antiproliferative and anti-inflammatory activities, and human peripheral blood mononuclear cells (PBMCs) from psoriasis patients and healthy volunteers for the immunomodulatory activity. The study was performed and interpreted together with the positive control, cyclosporin. In addition, potential herb-drug interactions of Deprungsith formulation and its bioactive components for the inhibitory effect on CYP450 was also investigated. Positive control compounds for the experiment included α-naphthoflavone for CYP1A2, sulfaphenazole for CYP2C9, troglitazone for CYP2C19, quinidine for CYP2D6 and ketoconazole for CYP3A4.
Figure 1.
Study framework to investigate antiproliferative and anti-inflammatory activities as well as herb-drug interactions of Deprungsith extract formulation and its bioactive compounds.
Cell Culture
The HaCaT (immortalized human keratinocyte) cell line was obtained from Cell Lines Service GmbH (Eppelheim, Baden-Wurttemberg, Germany). The cell was cultured in Dulbecco's Modified Eagle's Medium (DMEM: Gibco Co. Ltd, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum and 100 IU/ml of antibiotic-antimycotic solution (Gibco Co. Ltd). All cells were incubated at 37 °C with 5% CO2% and 95% humidity (HERACELL 150i, Thermo Scientific, Waltham, MA, USA). 23
Blood Sample Collection
Written informed consent was obtained from all research participants before blood collection. Heparinized blood (10 ml each) was obtained from (i) 9 healthy volunteers (4 and 5 males and non-pregnant females, aged 20-60 years) with no history of using anti-inflammatory drugs within the past two months; and (ii) 10 patients (5 and 5 males and females, aged 20-60 years) with mild degree psoriasis (Psoriasis Area Severity Index 2-8) 24 with no history of using anti-inflammatory drugs, phytotherapy, or treatments of psoriasis within the past two months.
Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were separated from each blood sample within 6 h after collection using Ficoll-PaqueTM (GE Healthcare, NJ, USA). In brief, the sample was diluted 2x volume with PBS (1x, pH 7.4) and layered over an equal volume of Ficoll-Paque in a 15 ml-conical tube. The mononuclear cells (lymphocytes, monocytes, and thrombocytes) were separated through centrifugation at 400xg for 30 min (20°C) and transferred to a new conical tube. PBS (1x) was added, and the cell suspension was centrifuged at 300xg for 10 min (20°C). After removing the supernatant, cell pellets were resuspended with PBS (x1). The PBMC pellets were obtained through centrifugation over Ficoll–PaqueTM cushions of buffy-coat (200 xg, 10 min, 20°C). The cell suspension (5 × 105 cells/ml) was prepared in RPMI 1640 medium supplemented with 10% fetal bovine serum and incubated at 37 °C for 24 h. 25
Cell Proliferation Assay
Antiproliferative activity against HaCaT and PBMCs: HaCaT and PBMCs (from healthy subjects) (100 µl of 1.5 × 104 cells/well and 2.0 × 104 cells/well, respectively) were seeded onto a 96-well plate. The final concentrations of Deprungsith extract in complete medium were 4000, 2000, 1000, 500, 250, 125, 62.5 and 31.25 μg/ml (200 µl/well). The final concentrations of EPMC were 2000, 1000, 500, 250, 125, 62.5, 31.25 and 15.63 μM. The final concentrations of ligustitide were 1000, 500, 250, 125, 62.5, 31.25, 15.63 and 7.81 μM. The final concentrations of cyclosporin were 500, 250, 125, 62.5, 31.25, 15.63, 7.81 and 3.91 μM. The plates were incubated at 37 °C under 5% CO2 for 48 h. Freshly prepared MTT reagent (20 µl of 5 mg/ml MTT in PBS) was added to each well and incubated for 3 h. The supernatant was removed and DMSO reagent (100 µl) was added with swirling. The absorbance was measured at 590 nm (Varioskan flash microplate reader, Thermo Scientific, USA). The experiment was performed in triplicate. The percentage of cell viability was calculated as follow:
The concentration-effect curve was analyzed, and the IC25 and IC50 (concentrations that inhibits cell growth by 25% and 50%, respectively) were determined using Calcusyn® version 1.1 (Biosoft, Cambridge, UK). 25
Antiproliferative Interaction in HaCaT Cell Line
The antiproliferative interaction of EPMC and ligustitide combination was evaluated using the MTT assay as described above at the concentration ratios of 10:0, 7:3, 5:5, 3:7 and 0:10, with a serial dilution for each combination pair. The highest concentration used was 1031.20 µg/ml (5000 µM) for EPMC and 380.48 µg/ml (2000 µM) for ligustilide. The experiment was performed at least three times, triplicate each. The IC50 of each compound was estimated as described above. The IC25 and IC50 of cyclosporin for both cells were obtained from a previous study. 26 The FIC (fractional inhibitory concentration) index of each combination pair and the sum FIC were calculated as the ratio of IC50 of the combination and each compound. The isobologram was generated from the average sum FIC index (sum of the IC50 of each combination pair divided by the IC50 of a single compound). Sum FIC values of FIC < 0.1, > 0.1–0.3, > 0.3–0.7, > 0.7–0.85, > 0.85–0.9, > 0.9–1.10, > 1.10–1.20, > 1.20–1.45, > 1.45–3.30, > 3.30–10.0 and > 10.0 indicate very strong synergistic, strong synergistic, synergistic, moderate synergistic, weak synergistic, additive/no interaction, weak antagonistic, moderate antagonistic, antagonistic, strong antagonistic and very strong antagonistic cytotoxic interaction, respectively. 27
Cell Cycle Arrest
The HaCaT cell line and PBMCs were grown overnight in a 6-well plate (5 × 105 cells/well) before the experiment. The cells were incubated with Deprungsith extract, EPMC, ligustilide and the reference compound cyclosporin at the IC25 and IC50 concentrations for 24 and 48 h. Cells were harvested by trypsinization or scrapping. DNA content was stained by fluorescence dye (BD CycletestTM Plus DNA Reagent Kit, BD Biosciences, CA, USA). Briefly, cell pellets were collected through centrifugation (250 xg, 5 min) and incubated with the provided reagent before staining with propidium iodide (PI). Cell number and DNA copy number was determined using a FACTverse flow cytometer (BD Biosciences, CA, USA) at 278 energy voltage. The development of cells to G0/G1, S and G2/M phases in the tested samples was compared to the control. 28
Cell Apoptosis
The HaCaT cells and PBMCs (5 × 105 cells) were cultured overnight in a 25-ml filter flask and exposed to Deprungsith extract, EPMC, ligustilide and cyclosporin at the IC25 and IC50 concentrations. Following 24 and 48 h of exposure, cells were harvested by trypsinization or scrapping. The apoptotic protein marker phosphatidylserine and intracellular DNA content were stained with fluorescence dye (BD PharmingenTM FITC Annexin V Apoptosis Detection Kit, BD Biosciences, CA, USA). The numbers of living and apoptotic (early and late) cells were determined using a FACTverse flow cytometer (BD Biosciences, CA, USA) at 200–240 and 270–390 energy voltages for FITC and PI, respectively. The experiment was performed in triplicate. 28
Pro-inflammatory Cytokines mRNA Expression Levels
The RNA contents of the exposed PBMCs were extracted with TRIzolTM (ThermoFisher Scientific, MA, USA). Briefly, TRIzol reagent (1000 µl) and chloroform (200 µl) were added supernatant was separated through centrifugation at 12,000xg for 15 min (4 °C). Cold isopropanol (500 µl) was added, and the RNA pellets were separated through centrifugation at 12,000 xg for 15 min (4 °C). The pellets were washed with 75% cold ethanol (1 ml) in DEPC water, left to dry, and dissolved with DEPC water (20 µl). Total RNA content was measured by spectrophotometer (ThermoFisher Scientific, MA, USA) at the wavelength of 260 nm. 25
The cDNA was prepared using the SuperScript® III First-Strand Synthesis System for RT-PCR. Briefly, the mixture of RNA from PBMCs (2 µl, 500 ng/ml), 50 µM oligo dT (0.5 µl), and dNTP mix (10 µM) were incubated at 65 °C for 5 min and plated on ice for at least 1 min. The cDNA synthesis mixture was prepared by adding RT buffer (10x, 2 µl), MgCl2 (25 mM, 4 µl), DTT (0.1 M, 2 µl), RNase OUT (40 U/µl, 1 µl), and SuperScriptTM III RT (200 U/µl, 1 µl). The cDNA synthesis mixture (10 µl) was added to each RNA template and incubated at 50 °C for 50 min and at 85 °C for 5 min to terminate the reaction. RNase H (1 µl) was added and incubated at 37 °C for 20 min. The synthesized cDNA was stored at −20 °C until use. 25
The mRNA expression levels of IL-6, IL-23A, TNF-α and IL-12p19 were measured (iTaq™ Universal SYBRTM Green Supermix (Bio-Rad Laboratories Inc., CA, USA) and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The cDNA was amplified (Bio-Rad CFX, Bio-Rad, CA, USA) using the forward and reverse primers for IL-6, IL-23A, TNF-α, and IL-12p19 (Table 2).25,29–31 The real-time PCR conditions were as follows: denaturing at 95 °C for 10 s, annealing at 60 °C for 10 s, and extension at 72 °C for 20 s (49 cycles). Three independent experiments (triplicate each) were performed for each analysis.
Table 2.
Primer Sequences and Analysis of the Expression of the Cytokines IL-6, IL-23A, TNF-α and IL-12p19 by RT-PCR.
| Primer sequence (5′→3′) | Step | Condition | |
|---|---|---|---|
| IL-6- F: 5′- TTGTTTGTGAGTGGGGTCCT-3′ IL-6- R: 5′- TGGGACTCCTGGGAATACTG-3′ IL-23A- F: 5′-ATGATGTTCCCCATATCCAGTGTGG-3′ IL-23A- R: 5′-GCAAGCAGAACTGACTGTTGTCCCT-3′ TNF-α - F: 5′-TGCTTGTTCCTCAGCCTCTT-3′ TNF-α - R: 5′- ATGGGCTACAGGCTTGTCACT-3′ IL-12p19- F: 5′-GAGCCTTCTCTGCTCCCTGAT-3′ IL-12p19- R: 5′-AGTTGGCTGAGGCCCAGTAG-3′ GAPDH-F: 5′-CAACAGCCTCAAGATCATCAGC-3′ GAPDH-R: 5′-TTCTAGACGGCAGGTCAGGTC-3′ | Pre-denaturation | 95°C, 5 min | |
| Denaturation | 95°C, 15 s | 49 cycles with fluorescent detection | |
| Annealing | 60°C, 1 min | ||
| Melting curve plotting | 95°C, 1 min | ||
| 60°C, 1 min | |||
| Melting curve: increment of temperature from 0.5°C to 95°C | |||
The relative quantification of the target gene was estimated as follows:
Where ΔCt (1) = delta Ct of unknown sample, ΔCt (2) = delta Ct of control, IL-6, IL-23A, TNF-α or IL-12p19 = target gene, and GAPDH = housekeeping gene.25,29
Assessment of CYP450 Inhibitory Activities
The inhibitory potentials of Deprunsith extract, EPMC, ligustilide and ferulic acid on CYP1A2, CYP3A4, CYP2C9, CYP2C19, and CYP2D6 enzymes were investigated using P450-GloTM Screening Systems (Promega Corp, Madison, USA). The system consisted of recombinant human CYP1A2 (rCYP1A2), CYP2C9 (rCYP2C9), CYP2C19 (rCYP2C19), CYP2D6 (rCYP2D6), and CYP3A4 (rCYP3A4) enzymes, which were produced by a baculovirus expression system, specific luminogenic CYP450 substrates (luciferin-H, luciferin-H EGE, luciferin-ME, luciferin-ME EGE, and luciferin-IPA), negative control membranes without CYP450 activity, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) regeneration system, reaction buffer, luciferin detection reagent, and luciferin-free water. The assay was performed according to the manufacturer's protocol (Technical Bulletin, P450-Glo Assays, Promega Corp., Madison, USA) in a white opaque 96-well plate. 32 Each test compound (12.5 αl) was prepared using luciferin-free water. The final concentrations of Deprunsith extract were 125, 50, 12.5, 5, 1.25, and 0.5 αg/ml. The final concentrations of EPMC were 1000, 500, 100, 50, 5, and 1 αM. The final concentrations of ligustitide and ferulic acid were 2000, 1000, 500, 100, 50, and 5 αM. The concentration ranges of the selective CYP450 inhibitors (12.5 αl) were: 0–5 αM ∝-naphthoflavone (CYP1A2 inhibitor), 0–50 αM sulfaphenazole (CYP2C9 inhibitor), 0–50 αM troglitazone (CYP2C19 inhibitor), 0–25 αM quinidine (CYP2D6 inhibitor), and 0–12.5 αM ketoconazole (CYP3A4 inhibitor). Luciferin-free water (12.5 αl) was added to the untreated and minus-P450 control wells. The control reaction mixture containing the luminogenic CYP450-specific substrate, control membranes, and potassium phosphate buffer (12.5 αl each) was added to the minus-P450 control wells. The reaction mixture (12.5 αl) containing human CYP450 membrane preparations, a luminogenic CYP450-specific substrate, and potassium phosphate buffer was added to other wells. Each plate was pre-incubated at 37 °C for ten minutes. The reactions were initiated by adding the NADPH regeneration system (25 αl) and incubated for 10 (CYP1A2 and CYP3A4), 20 (CYP2C19 and CYP2C9) or 30 (CYP2D6) minutes. Luciferin detection reagent (50 αl) was added, and the luminescence of all samples was measured using a microplate reader. Each experiment was performed as three independent assays. The total light produced is proportional to the CYP450 enzyme activity. The signals from untreated CYP450 reactions signify the total CYP450 activity (100%). Modulation of the CYP450 activity by the test materials was evaluated by comparing the alterations from the average net signal of untreated CYP450 reactions with the changes observed from the test compound. The IC50 (concentration that inhibits 50% of the CYP450 activity) values were estimated from concentration-response curves using CalcuSyn software (Biosoft, Cambridge, UK). The inhibitory potency of each test compound was classified based on the IC50 value for each CYP450 isoform as potent, moderate, and weak if the IC50 was ≤20 μg/ml, > 20–100 μg/ml and > 100 μg/ml, respectively. 32
Statistical Analysis
Quantitative data are expressed as the median and range values. The difference between the two quantitative groups of variables was determined using the Mann-Whitney U test (SPSS version 19, IBM, USA) at a statistical significance level of α = 0 .05.
Results
Antiproliferative and Anti-Inflammatory Activities
Antiproliferative Activities
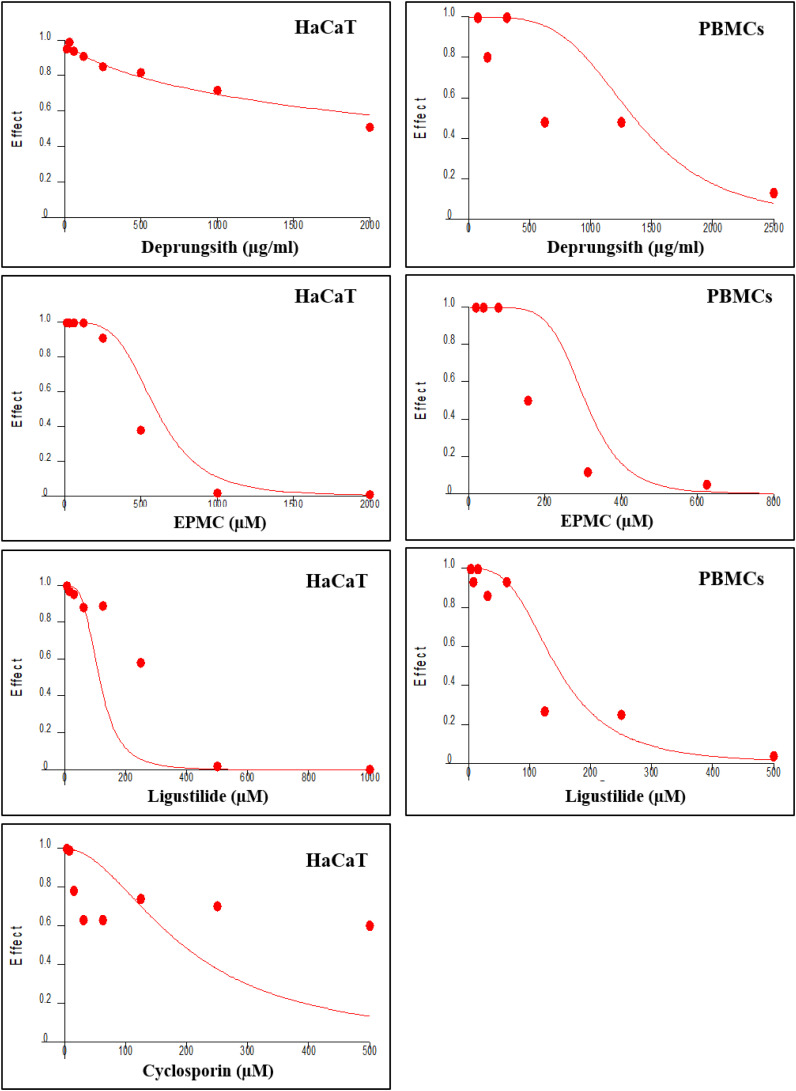
Deprungsith formulation and all test compounds suppressed HaCaT and PBMCs growth in a concentration-dependent manner, with the maximum inhibition of 0.72% and 17.93%, respectively, at the highest concentrations. The IC25 and IC50 values of each test substance for each cell are summarized in Table 3, and concentration-inhibitory effect curves are presented in Figure 2. The inhibitory effect of Deprungsith formulation and the bioactive compounds on both cells were relatively weak compared with cyclosporin. The activities of all test materials were relatively more potent against the PBMCs compared with HaCaT cells.
Table 3.
Antiproliferative Activities of Deprungsith Formulation, EPMC, Ligustilide and Cyclosporin in HaCaT Cells and PBMCs (from Healthy Subjects). Data are Presented as IC25 and IC50 Values [Median (Range)].
| Tests | PBMCs | HaCaT | ||
|---|---|---|---|---|
| IC25 | IC50 | IC25 | IC50 | |
| Deprungsith extract (μg/ml) | 1033 (991-1077) | 1361 (1218-1413) | 2114 (1764-2137) | 2811 (2604-2821) |
| EPMC (μM) | 254 (234-258) | 305 (280-310) | 432 (395-458) | 554 (508-601) |
| Ligustitide (μM) | 105 (101-106) | 143 (137-151) | 151 (138-155) | 188 (214-236) |
| Cyclosporin (μM) 10 | 5 | 10 | 110 (106-129) | 192 70–203) |
Figure 2.
Representative concentration-inhibitory effect curves of HaCaT cell and PBMCs (obtained from healthy subject) following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin. The inhibitory concentration of cyclosporin in PBMCs was adopted from previous study. 26
The antiproliferative interaction of the two bioactive compounds EPMC and ligustilide was further investigated in HaCaT cells. The median (range) sum FIC50 was 1.036 (0.884-1.49), suggesting additive or no interaction between the two bioactive compounds against HaCaT cells (Figure 3).
Figure 3.
Isobologram of the interaction between EPMC and ligustilide (additive interaction with sum FIC 0.9-1.1).
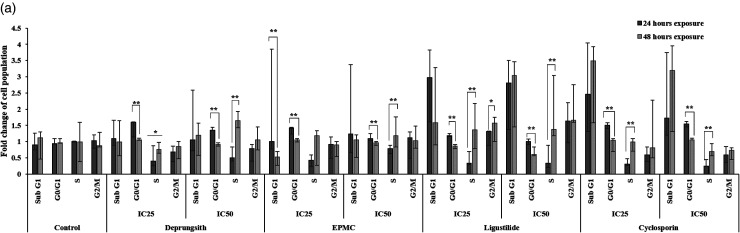
Effects on Cell Cycle Arrest
Effects of all test materials on the cell cycles of HaCaT cells and PBMCs (from psoriasis patients and healthy subjects) were investigated for a better understanding of their mechanisms of antiproliferative activities. The histograms of cell cycle arrest are presented in Figure 4. The patterns of cell cycle arrest following exposure of HaCaT cells varied with test materials in a prominent time-dependent manner (Figure 4a). At 24 h of exposure, all test materials, including cyclosporin at both concentrations (IC25 and IC50), decreased cell transition to the S phase. The decrease in cell transition to the sub-G1 phase was found with ligustilide at the IC50 concentration and cyclosporin at both concentrations. The reduction in cell transition to G2/M was also observed with Deprungsith formulation and ligustilide at both concentrations. Deprungsith formulation at the IC25 inhibited the G0/G1 phase of the cell cycle. EPMC at the IC25 and IC50 interrupted the G0/G1 and sub-G1 phases of the cell cycle, respectively. Ligustilide and cyclosporin at both concentrations interrupted the sub-G1 phase of the cell cycle. With prolonged exposure for 48 h, all significantly decreased cell transition to G0/G1 phase. The decrease in cell transition to the sub-G1 phase was found with EPMC at both concentrations and ligutilide at the IC25 concentration. The reduction in cell transition to G2/M was also found with EPMC at both concentrations. Cell cycle arrest was found in the S phase after exposure to Deprungsith formulation at the IC50 and EPMC at the IC25 and hase. Ligustilide at the IC25 interrupted the G2/M phase of the cell cycle, while at the IC50 interrupted the sub-G1 phase of the cell cycle. Cyclosporin at both concentrations inhibited the sub-G1 phase of the cell cycle.
Figure 4.
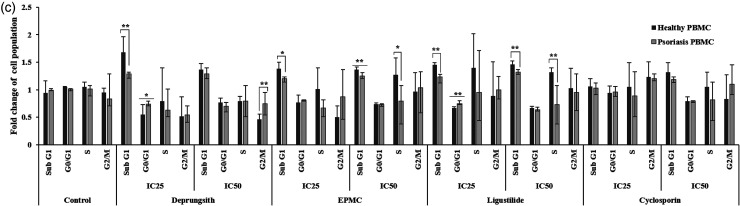
Representative cell cycle analysis histogram of HaCaT cell and PBMCs following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 and 48 h. Figure 4a. Cell cycle analysis of HaCaT cells following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 and 48 h (* and ** represent significant differences of cell population changing between H24 and H48, p-value ≤ 0.05 and ≤ 0.01). Figure 4b. Cell cycle analysis of PBMCs from psoriasis patients and healthy subjects following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 h (* represents significant difference of cell population changing between healthy and psoriasis PBMC, p-value ≤ 0.01). Figure 4c. Cell cycle analysis of PBMCs from psoriasis patients and healthy subjects following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 48 h (* and ** represent significant difference of cell population changing between healthy and psoriasis PBMC, p-value ≤ 0.01 and 0.05, respectively).
For the PBMCs collected from patients with psoriasis, all test materials, including cyclosporin at both concentrations, reduced cell transition at all phases, ie, sub-G1, G0/G1, S and G2/M) compared with control (PBMCs from healthy subjects), but the effects were predominantly observed at the S phase following 24 h of exposure. In addition, cell cycle arresting activities of all substances at both concentrations (IC25 and IC50) in PBMCs from healthy subjects were observed at the S phase. For PBMCs from patients with psoriasis, Deprungsith formulation at both concentrations and EPMC at IC50 inhibited the cell cycle at sub-G1. Ligustilide at both concentrations induced cell cycle arrest at the S phase (Figure 4b). The effects were time- and concentration-dependent. With the prolonged exposure to 48 h, the test substances and cyclosporin at both concentrations significantly reduced cell cycle arrest at specific phases (Figure 4c). The reduction of cell transition to sub G1 and S phases was found with all test substances and cyclosporin at both concentrations. Deprungsith formulation at the IC25 increased, while the IC50 decreased cell transition to G0/G1 phase. EPMC at both concentrations increased cell transition to G0/G1 and G2/M phases. Ligustilide at both concentrations significantly reduced cell transition to the S phase. Cell transition to G0/G1 and G2/M was increased at the IC25 concentration but decreased at the IC50 concentration. Cyclosporin at both concentrations increased cell transition to G0/G1, while decreasing cell transition at the S phase. Cell transition to G2/M was reduced at the IC25 concentration but increased at the IC50 concentration. Cell cycle arrest was predominantly found at the sub-G1 phase following a 48-h exposure.
Effects on Cell Death
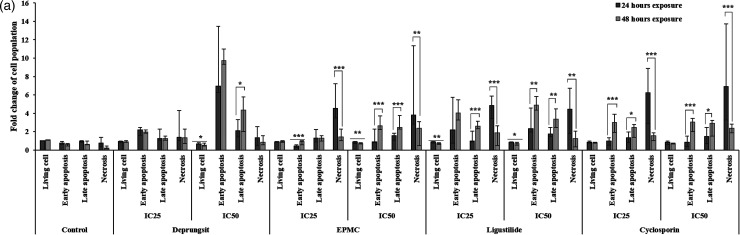
Investigation of cell apoptosis using FITC-Annexin V and propidium iodide (PI) staining can classify HaCaT and PBMCs and cell populations as live (healthy cells), early apoptotic cells, late apoptotic cells, and necrotic cells. The scatter plot of the cell population is presented in Figure 5. The patterns of cell apoptosis/necrosis following exposure of HaCaT cells varied with test materials in a time- and concentration-dependent manner (Figure 5a). The number of cells with early apoptosis following 24-h exposure to EPMC, ligustilide and cyclosporin at both concentrations was significantly lower than 48-h exposure. The proportion of living cells was decreased with all test materials, including cyclosporin. The effect on late apoptosis depended on the exposure concentrations. All test materials, including cyclosporin, with prolonged exposure to 48 h, decreased living and necrotic cells. The proportions of early and late apoptotic cells were increased after exposure to Deprungsith formulation and EPMC at the IC50, as well as ligustilide and cyclosporin at the IC25 and IC50.
Figure 5.
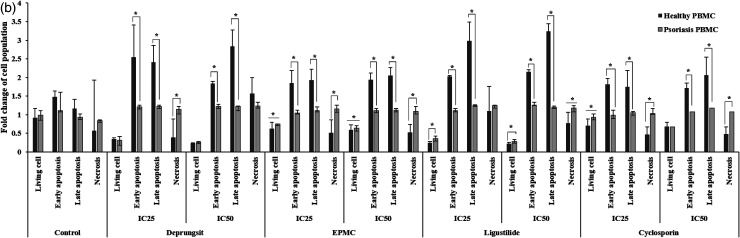
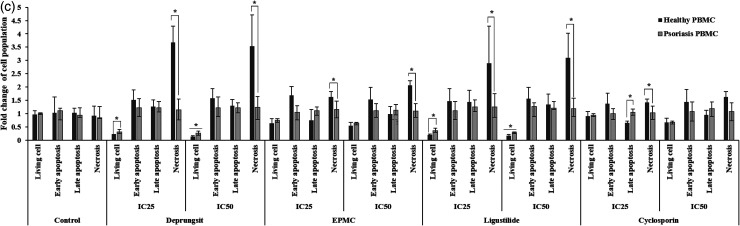
Representative cell death (apoptosis and necrosis) scatter plot of HaCaT cell and PBMCs following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 and 48 h. Figure 5a. HaCaT cell death (apoptosis and necrosis) following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 and 48 h (*, ** and *** represent significant difference of cell population changing between H24 and H48, p-value ≤ 0.05, ≤ 0.01 and ≤ 0.001). Figure 5b. The death (apoptosis and necrosis) of PBMCs following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 h (* represents significant difference of cell population changing between healthy and psoriasis PBMC, p-value ≤ 0.01). Figure 5c. The death (apoptosis and necrosis) of PBMCs following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 48 h (* represents significant difference of cell population changing between healthy and psoriasis PBMC, p-value ≤ 0.01).
The patterns of cell apoptosis/necrosis following exposure to PBMCs varied with test substances in a time-dependent manner (Figures 5b and 5c). Following 24-h exposure to all test substances and cyclosporin at both concentration exposure, the number of living PBMCs from psoriasis patients was higher than healthy subjects. All test substances and cyclosporin at both concentrations decreased the apoptosis of PBMCs from psoriasis patients compared with healthy subjects at both early and late stages. The proportion of necrotic PBMCs from psoriasis patients after exposure to Deprungsith formulation at the IC25, ligutilide at the IC50, and EPMC and cyclosporin at both concentrations were higher than the PBMCs from healthy subjects (Figure 5b). With the prolonged exposure for 48 h, the proportions of living PBMCs from psoriasis patients following exposure to Deprungsith formulation and ligustilide were significantly higher than the PBMCs from healthy volunteers. The proportions of PBMCs with early apoptosis and necrosis after exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin at both concentrations were significantly lower than the PBMCs from healthy volunteers (Figure 5c).
Effects on mRNA Expression Levels of Pro-inflammatory Cytokines
IL-6, IL-23A, IL-12p19 and TNF-α mRNA expression levels were downregulated following exposure of the PBMCs from psoriasis to Deprungsith formulation and bioactive compounds at both concentrations for 24 and 48 h, compared with the PBMCs from healthy subjects (Figures 6a and 6b). The effects were time-dependent and test material-specific. Exposure to Deprungsith formulation, EPMC and cyclosporin at both concentrations for 24 h downregulated IL-23A and TNF-α expression while downregulating the expression of IL-12p19 only at IC50. Ligustilide at both concentrations reduced the expression of IL-23A, IL-12p19 and TNF-α (Figure 6a). With prolonged exposure to 48 h, IL-6, IL-23A, IL-12p19 and TNF-α were downregulated after exposure to Deprugsith formulation at the IC50 (Figure 6b). Exposure to EPMC at IC50 reduced the expression of IL-6 and IL-23A. Exposure to ligustilide at the IC25 downregulated IL-23A, IL-12p19 and TNF-α, while downregulating the expression of IL-6 only at IC50. Exposure to cyclosporin at both concentrations downregulated the expression of IL-6 and IL-12p19.
Figure 6.
(a) mRNA expression of the pro-inflammatory cytokines (IL-6, IL-23A, TNF-α and IL-12p19) released from the PBMCs of psoriasis patients and healthy subjects following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 24 h. (b) mRNA expression of the pro-inflammatory cytokines (IL-6, IL-23A, TNF-α and IL-12p19) released from the PBMCs of psoriasis patients and healthy subjects following exposure to Deprungsith formulation, EPMC, ligustilide and cyclosporin for 48 h.
Inhibitory Effects on CYP450 Enzymes In Vitro
The potential inhibitory effects (indicated by IC50 values) of Deprungsith formulation, EPMC, ligustilide, and ferulic acid, including selective CYP450 isoform inhibitors, on the activities of CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 are summarized in Table 4. Representative concentration-inhibitory effect curves of the test compounds with moderate inhibitory activities on each CYP450 enzyme are shown in Figure 7. The potency of the inhibitory activity was classified into three levels: potent (IC50 < 20 μg/ml), moderate (IC50 > 20-100 μg/ml), and weak (IC50 > 100 μg/ml). 32 All reference inhibitors potently inhibited CYP450 enzymes with IC50 ranging from 0.01–2.33 μM. All the test materials, on the other hand, inhibited CYP450 activities with weak to moderate potencies. CYP1A2 was moderately inhibited by Deprungsith formulation and ligustilide. CYP2D6 was moderately inhibited by EPMC and ferulic acid. CYP2C9 and CYP3A4 were moderately inhibited by Deprungsith formulation and ligustilide, respectively. None inhibited the activity of CYP2C19.
Table 4.
Inhibitory Effects of Deprungsith Formulation, PBMC, Ligustilide and Ferulic Acid, Including Reference Standards on CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 Activities. Data are Presented as Median (Range) Values.
| CYP | Reference inhibitor | Extract (μg/ml) | EPMC (μM) | Ligustilide (μM) | Ferulic acid (μM) |
|---|---|---|---|---|---|
| CYP1A2 | α-Napthoflavone 0.18 (0.13-0.19) Potent | 36.83 (29.58-42.79) Moderate | 117.91 (88.99-131.82) Weak | 20,61 (19.63-21.26) Moderate | 5245.5 (4423.0-5643.78) Weak |
| CYP2C9 | Sulfaphenazole 0.24 (0.21-0.25) Potent | 56.81 (52.37-58.06) moderate | 87.45 (85.20-87.46) Weak | 114.33 (86.29-119.75) Weak | 469.47 (465.21-488.49) Weak |
| CYP2C19 | Troglitazone 2.07 (1.96-2.33) Potent | 429.15 (363.51-441.99) Weak | 80.35 (76.76-83.27) Weak | 89.15 (83.99-90.11) Weak | 1454.45 (1452.36-1670-14) Weak |
| CYP2D6 | Quinidine 0.01 (0.01-0.02) Potent |
879.35 (827.82-1141.37) Weak | 17.76 (15.23-17.76) Moderate | 217.87 (165.48-244.66) Weak | 11.40 (11.21-14.42) Moderate |
| CYP3A4 | Ketoconazole 0.31 (0.29-0.33) Potent | 136.25 (136.11-141.72) Weak | 865.16 (779.69-890.38) Weak | 39.75 (36.56-40.26) Moderate | 1153.43 (973.59-1406.65) Weak |
Figure 7.
Representative concentration-inhibitory effect curves of the test compounds with moderate inhibitory activity on CYP1A2 (Deprungsith formulation and ligustilide), CYP2C9 (Dephrungsit extract), 2D6 (ferulic acid and EPMC) and CYP3A4 (ligustilide).
Discussion
The study is part of a series of study that provides evidenced-based support for the traditional use of Deprungsith formulation for the treatment of psoriasis using modern scientific procedures.13–15 The antiproliferative and anti-inflammatory activities of Deprungsith formulation and bioactive compounds were investigated in the present study using HaCaT. Due to their highly similar physiological characteristics to normal human keratinocytes, it is a widely used model to investigate the proliferation and differentiation of human epidermal cells and the pharmacological activity of psoriasis treatment. 33 Apart from HaCaT, the effects on the inflammatory response of the PBMCs from psoriasis patients and healthy subjects were also investigated. Results showed that Deprungsith formulation and bioactive compounds, including cyclosporin inhibited keratinocyte cell proliferation and apoptosis by stimulating cell cycle arrest at specific phases. The antiproliferative activity of the Deprungsith formulation was weak (IC50 = 1361 and 2811 μg/ml for PBMCs and HaCaT cells, respectively). This could be due to the very low concentrations of both compounds in the Deprungsith formulation (0.1% and 0.69% of the fresh material for EPMC and ligustilide, respectively). The potencies of antiproliferative activities of EPMC and ligustilide were moderate (IC50: 554 μM or 114.25 μg/ml and 188.4 μM or 35.84 μg/ml, respectively). The antiproliferative effect of the EPMC and ligustilide combination was additive. Besides EPMC and ligustilide, other bioactive compounds could also contribute to the activity. The activities of Deprungsith formulation and the two compounds on PBMCs cell proliferation were relatively more potent than HaCaT. The IC50 of Deprungsith formulation, EPMC and ligustilide were 1361 μg/ml, 305.7 μM (63.09 μg/ml) and 143.8 μM (27.35 μg/ml). With the limitation of testing material and PBMCs collected from the volunteers, the MTT assay could be done only at a single time-point (at 48 h). This may not provide a progression of the inhibitory effects as well as a comprehensive understanding of the effects of exposure time. This time point is however, expected to cover the cell division periods of HaCaT cell line as well as the duration of action of the test extract. It was noted for a trend of the inhibitory effect of the extract and test compounds on cell proliferation at 24 h of incubation as reflected by the results of cell cycle and apoptosis analysis.
The transition of HaCaT and PBMC cells to the S phase was mainly inhibited by all test substances, suggesting the arrest of cells at the G0/G1 phase. Exposure to the test substances for 24–48 h significantly increased HaCaT cell apoptosis at early and late stages. With prolonged exposure for 48 h, cell necrosis was significantly decreased. Interestingly, the downregulation of mRNA expression of all cytokines (TNF-α, IL-6, IL-23 and IL-12p19) were found in the PBMCs from patients with psoriasis compared with healthy subjects after exposure (24 and 48 h) to Deprungsith formulation and bioactive compounds EPMC and ligustilide. Ligustilide downregulated the expression of TNF-α, IL-23 and IL-12p19 following 24 h of exposure, while Deprungsith formulation at IC50 downregulated the expression of all cytokines following 48 h of exposure. The inhibitory effects of EPMC and cyclosporin varied with the exposure concentrations and time. The activities of the test material and incubation time could impact mRNA expression levels of the target genes. For slow-action or low-concentration test materials, cellular response to the treatment may not be fully manifested if the incubation time is too short. On the other hand, an excessively long incubation time could result in mRNA degradation or saturation of the cellular response for fast-acting or high-concentration test materials. Altogether, results suggest the anti-inflammatory activity of Deprungsith formulation and bioactive compounds via suppression of mRNA expression of the key pro-inflammatory cytokines involved in psoriasis pathogenesis. IL-6, IL-12p19 and IL-23 mRNA have been reported to be over-expressed in psoriasis patients. IL-6 is a pro-inflammatory cytokine produced by several types of immune and nonimmune cells, 34 including keratinocytes, 34 which are involved in the proliferative response. IL-23 is the important driver behind the T17, which is an intermediate molecule to trigger keratinocyte proliferation.35–37 IL-12 is a heterodimer of IL-23, which plays a role in stimulating Th1 cells. 38 TNF-α is prominent in normal skin, as well as in damaged keratinocytes. 34 Regulation of antigen-presenting cells appears to be the major role of TNF-α. 39
The antipsoriasis activities, including the molecular and cellular mechanisms of action of several herbal medicines used for psoriasis treatment, have been investigated. Some Thai herbs, including naringin isolated from citrus peel 40 and Angelica spps. 41 have been shown to exhibit antipsoriatic activity with potent anti-inflammatory and antioxidant activities. In addition to single plants, the antipsoriasis potential of the Thai herbal formulation –Wanachawee Recipe (consisting of 8 herbs) has also been reported.23,42 These herbs and herbal formulas have been shown to produce antipsoriasis effects by acting on inflammatory signaling molecules/pathways and immune cells (T-cells, dendritic cells, monocytes, neutrophils, and macrophages).
The potentials of Deprungsith formulation and bioactive components, EPMC, ligustilide and ferulic acid (a ubiquitous phenolic acid) to inhibit the activities of the five major CYP450 isoforms, ie, CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 were investigated in vitro using a bioluminescent-based CYP450 assay. 43 This approach is rapid and eliminates the requirement for sample preparation before detection by mass-spectrometry or high-performance liquid chromatography. In addition, the assay is substantially more sensitive than most fluorescent-based CYP450 assays and obviates any interference from fluorescent analytes in herbs containing phenols, alkaloids, and terpenoids. 44 The study was the first to investigate the CYP450 inhibitory potential of Deprungsith formulation. The results showed that Deprungsith formulation and bioactive compounds inhibited the activities of CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 with weak to moderate potencies. The potencies of inhibitory effects varied with the compounds. While the extract of the plant mixture moderately inhibited CYP1A2 and CYP2C9, ligustilide moderately inhibited CYP1A2 and CYP3A4, and EPMC and ferulic acid moderately inhibited only CYP2D6. The potencies of the inhibitory effects of all CYP450 selective inhibitors (α-naphthoflavone, sulfaphenazole, troglitazone, ketoconazole, and quinidine) were comparable with those reported in previous studies using different assay methods.45–47 The inhibitory potencies of all test substances on all investigated CYP450 enzymes were markedly low compared with the selective CYP450 inhibitors used in the experiments ( Table 4 ). CYP3A4 is involved in the metabolism of more than 50% of all prescription drugs and accounts for 30% of the total CYP450 protein content in the liver. 37 CYP2D6, second to CYP3A4, is responsible for the metabolism of more than 30 clinically important drugs, particularly those acting on the cardiovascular and nervous systems. 48 CYP2C9 makes up approximately 18–20% of the liver CYP450 proteins. It is involved in the biotransformation of narrow therapeutic index drugs, such as warfarin and phenytoin. 48 CYP1A1/2 are involved in the metabolism of several planar aromatic compounds. The enzymes are responsible for activating several chemical carcinogens that humans are exposed to in daily life via diet and environment, eg, polycyclic aromatic hydrocarbon, aromatic amines, and heterocyclic amines. 49 In a previous study, 20 EPMC, the major component of K. galanga, was shown to induce CYP1A1 and CYP1A2 mRNA expression in mouse hepatocytes. In the present study, on the other hand, it weakly inhibited CYP1A2. Ligustilide, the major component of L. sinensis, was reported to significantly inhibit CYP1A2 as well as CYP2C9 and CYP3A4. 50 Ferulic acid, the main component of A. sinensis, appears to be metabolized by CYP1A2, CYP3A4 and UGTs, 51 and coadministration of plants containing this compound may interfere with the metabolism of this compound in Deprungsith formulation, leading to toxicity or inadequate treatment. Other chemical constituents may also contribute to CYP450 inhibitory effects in Deprungsith formulation, and their potencies of inhibitory effects depend on the relative amount of each compound in the formulation. Considering the relatively low inhibitory potency of the extract and bioactive compounds, the risk of herb-drug interaction and toxicity following coadministration of Deprungsith formulation with other drugs metabolized by these CYP450 isoforms is likely to be low. From a beneficial therapeutic point of view, on the other hand, the inhibitory effect of the extract and ligustitide on CYP1A2 should be helpful in preventing the development of carcinogenesis. EPMC was also shown to produce relatively strong anticarcinogenic potential in an in vitro study. 52
Conclusion
The overall results suggested the novel activity of Deprungsith formulation and its active components on the downregulation of the pro-inflammatory cytokines IL-6, IL-12p19, IL-23 and TNF-α related to early inflammation of mild type of psoriasis. The risk of potential adverse drug reactions and toxicity due to CYP450 interaction when Deprunsith formulation is concurrently administered with modern medicines is low.
Acknowledgements
We would like to thank Dr Kanawut Kotawong and Ms. Arthitaya Tiengsusuk for their assistance in sample collection and technical support.
Footnotes
Author Contributions: KN- designed the study, performed data analysis, supervised laboratory analysis and revised and finalized the manuscript; MT- designed the study and revised the manuscript; PM- performed laboratory and statistical analyses and drafted the manuscript; YK, NS, KB, MT and TP - performed laboratory and data analyses; All persons who meet authorship criteria are listed as authors, and all the authors contributed substantially to the conception or design of the work or the acquisition, analysis or interpretation of the data for this manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The clinical part of the study was approved by the Ethics Committee of Thammasat University from July to August 2021 (number MTU-EC-OO-4-125/64). All the reagents used in the study were prepared, used, and disposed according to the required laboratory guidelines.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Research and Development Agency (ARDA) and Thammsat University (Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangicarcinoma). Kesara Na-Bangchang is supported by the National Research Council of Thailand under the Research Team Promotion grant (grant number 820/2563).
ORCID iDs: Kesara Na-Bangchang https://orcid.org/0000-0001-6389-0897
Phunuch Muhamad https://orcid.org/0000-0002-9974-5832
References
- 1.Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022;13(1):81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archananupab S. textbook of general physical examination on 350 diseases. Holistics Publishing; 2008, (in Thai). [Google Scholar]
- 3.Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl). 2016;6(1):7‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017 Apr;63(4):278‐285. [PMC free article] [PubMed] [Google Scholar]
- 5.Albanesi C. Immunology of psoriasis. In: Clinical immunology. Elsevier; 2019:871‐878. [Google Scholar]
- 6.Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 1682;2018;9(1):01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73(2):342‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain H, Bhat AR, Dalvi H, Godugu C, Singh B, Srivastava S. Repurposing approved therapeutics for new indication: Addressing unmet needs in psoriasis treatment. Curr Res Pharmacol Drug Discov. 2021;2(1):100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng S, Lin Z, Wang Y, Wang Z, Li P, Zheng Y. Psoriasis therapy by Chinese medicine and modern agents. Chin Med. 2018;13(2):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nille GC, Chaudhary AK. Potential implications of Ayurveda in Psoriasis: A clinical case study. J Ayurveda Integr Med. 2021;12(1):172‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chumchuen S. Thai Traditional medicines for psoriasis and their herbal pharmacological activities. Thai Pharm Health Sci J. 2020;15(2):130‐137. [Google Scholar]
- 13.Sireeratawong S. Final report: The second phase of the research project of efficacy and safety of Dephrangsith for psoriasis treatment. Chiang Mai. Faculty of Medicine, Chiang Mai University, 2019.
- 14.Sripanidkulchai B, Fangkrathok N. A complete report on the First phase of the research project of efficacy and safety of Dephrangsith for psoriasis treatment. Final report, Faculty of Pharmacy, Khon Kaen University.
- 15.Tungsukruthai P, Sriyakul K, Kongkiatpaiboon K. A complete report on the first phase of the research project of efficacy and safety of Dephrangsith for psoriasis treatment. Pathum Thani, The Chulabhorn International College of Medicine, Thammasat University, 2014.
- 16.Umar MI, Asmawi MZ, Sadikun Aet al. et al. Ethyl-p-methoxycinnamate isolated from Kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-α, and angiogenesis by blocking endothelial functions. Clinics (Sao Paulo). 2014;69(2):134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasasook J, Srimongkol W, Klangprapun S. Allium Sativum L. and Ligusticum Sinense Oliv. cv. chuanxiong : A Substitute Herb according to Thai Traditional Medicine. Journal of Health and Health Management. 2020;6(2):1‐15. [Google Scholar]
- 18.Yu-Wen S, Wen-Fei C, Shiou-Huei C, et al. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Inter Immunopharmacol. 2011;11(9):1166‐1172. [DOI] [PubMed] [Google Scholar]
- 19.Paiva Ld, Goldbeck R, Santos Wd, Squina FM. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Braz J Pharm Sci. 2013;49(3):395‐411. [Google Scholar]
- 20.Sirisangtragul W, Jarukamjorn K, Nemoto N, Yenjai C, Sripanidkulchai B. Effect of Ethyl-p-Methoxy cinnamate from Kaempferia galanga on cytochrome P450 enzymes expression in mouse hepatocytes. Chiang Mai J. Sci. 2011;38(3):453‐462. [Google Scholar]
- 21.Stavropoulou E, Pircalabioru GG, Bezirtzoglou E. The role of cytochromes P450 in infection. Front Immunol. 2018;9(4):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues, Geneva, Switzerland, 2007.
- 23.Na Takuathung M, Wongnoppavich A, Pitchakarn P, et al. Effects of wannachawee recipe with antipsoriatic activity on suppressing inflammatory cytokine production in HaCaT human keratinocytes. Evid-based Complement Altern Med. 2017;2017(1):5906539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado-Boquete L, Carrascosa JM, Llamas-Velasco M, Ruiz-Villaverde R, de la Cueva P, Belinchón I. A new classification of the severity of psoriasis: What's moderate psoriasis? Life (Basel). 2021 Jun 29;11(7):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulma I, Panrit L, Plengsuriyakarn T, Chaijaroenkul W, Warathumpitak S, Na-Bangchang K. A randomized placebo-controlled phase I clinical trial to evaluate the immunomodulatory activities of Atractylodes lancea (Thunb) DC. in healthy Thai subjects. BMC Complement Med Ther. 2021;61(1):122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano T, Oka K, Takeuchi H, et al. Immunosuppressant pharmacodynamics on lymphocytes from healthy subjects and patients with chronic renal failure, nephrosis, and psoriasis: Possible implications for individual therapeutic efficacy. Clin Pharmacol Ther. 1997;62(6):652‐664. [DOI] [PubMed] [Google Scholar]
- 27.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621‐681. [DOI] [PubMed] [Google Scholar]
- 28.Muhamad P, Panrit L, Chaijaroenkul W, Na-Bangchang K. Cytotoxicity, cell cycle arrest, and apoptosis induction activity of ethyl-p-methoxycinnamate in cholangiocarcinoma cell. Asian Pac J Cancer Prev. 2020;21(2):927‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarasuk M, Songprakhon P, Chieochansin T, Choomee K, Na-Bangchang K, Yenchitsomanus PT. Alpha-mangostin inhibits viral replication and suppresses nuclear factor kappa B (NF-κB)-mediated inflammation in dengue virus infection. Sci Rep. 2022;22(2):16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee E, Trepicchio WL, Oestreicher JLet al. et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;14(3):125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myhr CB, Hulme MA, Wasserfall CHet al. The autoimmune disease-associated SNP rs917997 of IL18RAP controls IFNγ production by PBMC. J Autoimm. 2013;1(2):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasamy S, Kiew LV, Chung LY. Inhibition of human cytochrome P450 enzymes by Bacopa monnieri standardized extract and constituents. Molecules. 2014;19(2):2588‐2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deyrieux AF, Wilsonm VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotech. 2017;14(1):77‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8(6):E67078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuruta D. NF-kappaB links keratinocytes and lymphocytes in the pathogenesis of psoriasis. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):40‐48. [DOI] [PubMed] [Google Scholar]
- 37.Summers deLuca L, Gommerman JL. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol. 2012;12(5):339‐351. [DOI] [PubMed] [Google Scholar]
- 38.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing gamma-delta T cells in skin inflammation. Immunity. 2011;35(4):596‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deenonpoe R, Prayong P, Thippamom N, Meephansan J, Na-Bangchang K. Anti-inflammatory effect of naringin and sericin combination on human peripheral blood mononuclear cells (hPBMCs) from patient with psoriasis. BMC Complement Altern Med. 2019;19(1):168‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai YJ, Li YY, Zeng HMet al. et al. Effect of Yinxieling decoction on PASI, TNF-α and IL-8 in patients with psoriasis vulgaris. Asian Pac J Trop Med. 2014;7(8):668‐670. [DOI] [PubMed] [Google Scholar]
- 42.Na Takuathung M, Wongnoppavich A, Panthong A, Khonsung P, Chiranthanut N, Soonthornchareonnon N. Sireeratawong S.antipsoriatic effects of wannachawee recipe on imiquimod-induced psoriasis-like dermatitis in BALB/c mice. Evid Based Complement Alternat Med. 2018;2018(2):7931031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cali JJ, Niles A, Valley MP, O'Brien MA, Riss TL, Shultz J. Bioluminescent assays for ADMET. Expert Opin Drug Metab Toxicol. 2008;4(1):103‐120. [DOI] [PubMed] [Google Scholar]
- 44.Cali JJ, Ma D, Sobol M, et al. Luminogenic cytochrome P450 assays. Expert Opin Drug Metab Toxicol. 2006;2(4):629‐645. [DOI] [PubMed] [Google Scholar]
- 45.Ponnusankar S, Pandit S, Babu R, Bandyopadhyay A, Mukherjee PK. Cytochrome P450 inhibitory potential of Triphala–a Rasayana from Ayurveda. J Ethnopharmacol. 2011;133(1):120‐125. [DOI] [PubMed] [Google Scholar]
- 46.Vijayakumar TM, Kumar RM, Agrawal A, Dubey GP, Ilango K. Comparative inhibitory potential of selected dietary bioactive polyphenols, phytosterols on CYP3A4 and CYP2D6 with fluorometric high-throughput screening. J Food Sci Technol. 2015;52(7):4537‐4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jumpa-ngern P, Plengsuriyakarn T, Mahavorasirikul W, Na-Bangchang K. Potential inhibitory and inducing effects of Triphala formulation on cytochrome P450 enzymes. Walailak J Sci Tech. 2022;7(1):241‐252. [Google Scholar]
- 48.Zhao M, Ma J, Li Met al. Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci. 2021;22(23):12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan Z, Caldwell GW. Metabolism profiling, and cytochrome P450 inhibition and induction in drug discovery. Curr Top Med Chem. 2001;1(5):403‐425. [DOI] [PubMed] [Google Scholar]
- 50.Qian M, Shi LF, Hu JH. Enzyme kinetics of ligustilide metabolism in rat liver microsomes. Yao Xue Xue Bao. 2009;44(4):395‐400. [PubMed] [Google Scholar]
- 51.Zhuang XM, Chen L, Tan Yet al. et al. Identification of human cytochrome P450 and UGT enzymes involved in the metabolism of ferulic acid, a major bioactive component in traditional Chinese medicines. Chin J Nat Med. 2017;15(9):695‐702. [DOI] [PubMed] [Google Scholar]
- 52.Xue Y, Chen H. Study on the anticarcinogenic effects of three compounds in Kaempferia galanga L. Xei Shen Yan Jui. 2002;341(4):247‐248. [PubMed] [Google Scholar]