Abstract
Background
Medial sclerosis (MeS) is a chronic systemic vascular disease that mainly affects the arteries of the lower limb. Its prevalence in the general population is approximately 2.5% (range: 1.6% to 10.0%). It is more common in men than in women.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed.
Results
MeS is the final common pathway of a wide variety of diseases; its pathogenesis is not fully understood. It often remains clinically silent for decades and is usually diagnosed as an incidental finding or in a late stage. MeS with or without atherosclerosis is the most common histologic finding after limb amputation. MeS of the below-the-knee arteries is a major risk factor for chronic critical leg ischemia (OR:13.25, 95% confidence interval: [1.69; 104.16]) and amputation (RR 2.27, [1.89; 2.74]). Patients with peripheral arterial occlusive disease and marked calcification have a much higher risk of amputation (OR 2.88, [1.18; 12.72]) and a higher mortality (OR 5.16, [1.13; 21.61]). MeS is a risk factor for the failure of endovascular treatment of the pedal arteries (OR 4.0, [1.1; 16.6]). The more marked the calcification, the higher the risk of major amputation (HR 10.6 [1.4; 80.7] to HR 15.5 [2.0; 119]). Patients with vascular calcifications have been found to have lower patency rates and higher treatment failure rates two years after open surgical revascularization of the below-the-knee arteries. No pharmacotherapy for MeS is available to date.
Conclusion
MeS is an important risk factor for chronic critical lower limb ischemia, amputation, morbidity, and complications, particularly after endovascular and surgical procedures.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 29 May 2024.
Medial sclerosis (MeS) was first described by Johann Georg Mönckeberg in 1903 (1). However, it was not until the 1990s that MeS was identified as an independent risk factor for cardiovascular disease (2). Today, MeS has been recognized as an important cause of chronic critical leg ischemia and a major risk factor for complications, especially of endovascular treatment (3).
The disease is characterized by increasing deposition of hydroxyapatite crystals, leading to progressive medial destruction. The pathogenesis of MeS is not fully understood; most likely, it is a final common pathway of several different conditions. No pharmacotherapy for MeS is available to date.
In this review article, we provide information on the epidemiology and clinical significance of this disease whose importance has so far been rather underestimated in everyday clinical practice.
Methods
This review is based on pertinent publications retrieved by a selective search in the PubMed database and with the Google Scholar search engine, using the search terms “Mediasklerose“ (German) and “medial arterial calcification“ (English).
Epidemiology
The prevalence of MeS can be estimated based on ankle-brachial index (ABI) screening data. A prevalence rate of 2.5% (range 1.6% to 10.0%) was determined for the general population; MeS is more common in men than in women. The prevalence ranged from 9% to 34% in individuals at high cardiovascular risk, from 9% to 41% in patients with diabetes mellitus and was 25% in patients with severe chronic kidney disease, and up to 93% in patients with chronic critical leg ischemia (etable 1). In the scientific literature, however, a number of different threshold values have been used, including ABI>1.3, >1.4, >1.5, noncompressible, >260 mm Hg, as well as specific differences in blood pressure measurements. Furthermore, the accuracy of prevalence data is somewhat limited as a result of the limited sensitivity and specificity of blood pressure measurements in early stages of the disease and due to extravascular indurations. The occurrence of MeS lesions in general specimens varies with age; in patients aged ≤ 20, 21–30, 31–40, 41–50, 51–60, 61–70, and > 71 years, a histology-based prevalence rate of 7%, 17%, 16%, 48%, 46%, 64%, and 61%, respectively, was found (4). Given the high prevalence of MeS lesions in the general population, their clinical significance must be put into perspective and, for the time being, probably limited to patients with documented impaired perfusion. Yet, considering the present inconsistency in screening, a high number of unreported cases of MeS with potential clinical significance may be assumed as well. Typically, MeS affects the arteries of the lower limbs, but it may occur in other vascular regions as well (eTabelle 2). MeS is most commonly found in patients with diabetes mellitus or with chronic kidney disease; however, MeS is also part of the phenotype of other diseases (etable 3). Moreover, severe MeS can occur in patients with no known disease (5). In view of the rising incidence of diabetes mellitus and the impact of demographic change, the prevalence of MeS is expected to continue to increase further.
eTable 1. Compilation of available data on the epidemiology of medial sclerosis in different populations (selection)*.
| Population | Number of subjects | Prevalence of medial sclerosis (%) | Methods | References |
| Pelvis/legs | ||||
| General population | 4 735 | 10 (total) 13.3 (men) 6.9 (women) 0.6 (total) 1.1 (men) 0.5 (women) 0.6 (total) |
ABI >1.3 ABI >1.5 >260 mm Hg |
(e1) |
| 48 294 | 2.5 (total) 2.8 (men) 2.2 (women) |
ABI >1.4 | (e2) | |
| 6 647 | 1.6 (total) | ABI ≥ 1.4 or noncompressible | (e3) | |
| Cardiovascular disease or high risk | 718 | 25 (total) 8.7 (total) |
CT (extremities) ABI >1.4 |
(e4) |
| Diabetes mellitus | 623 | 9 (total) | BP difference >50 mm Hg or noncompressible | (e5) |
| 662 | 4.45 (total) | ABI ≥ 1.3 | (e6) | |
| 1 059 | 41.5 (total) | Radiography (plain) Femur |
(e7) | |
| 133 | 17.3 (total) 23 (men) 11 (women) |
Radiography (plain) Femur |
(e8) | |
| 250 |
20.4 (total) vs control 8 23.6 (total) vs control 8.8 |
Radiography (plain) Knee Feet |
(e9) | |
| Cardiovascular disease or high risk and diabetes | ||||
|
Risk Diabetes mellitus |
520 198 |
34 23.1 34.2 30.8 |
CT (dominant medial pattern) Femur Lower leg Femur Lower leg |
(e10) |
| Severe chronic kidney disease | 202 | 26.7 (total) | Radiography (plain) Pelvis and femur |
(e11) |
| Peripheral arterial occlusive disease (PAOD) | 204 |
75 93.5 80.5 57.9 85 78.6 |
CT (full body) Foot Lower leg Femur Pelvis Abdominal aorta Thoracic aorta |
(e12) |
| PAOD (critical chronic leg ischemia or amputation) | 1 413 | 24.8 | ABI > 1.4 | (e13) |
| Suspected stroke | ||||
| Internal carotid artery, intracranial | 1 132 | 46.9 | CT (head) | (e14) |
* The considerable differences in reported data presumably reflect different testing methods (ABI, radiography, CT), different cut-off values for ABI (1.3; 1.4; 1.5; noncompressible, pressure difference, >260 mm Hg), and different age distributions in the studied populations.
ABI, ankle-brachial index; CT, computed tomography; PAOD, peripheral arterial occlusive disease; vs, versus
eTable 2. Occurrence of medial sclerosis by vascular regions.
eTable 3. Occurrence of medial sclerosis in association with various diseases (examples) with references.
| Disease | Key feature | Additional soft-tissue calcifications | References |
| Diabetes mellitus | Carbohydrate metabolism disorders | No | (e7) |
| Chronic kidney disease | Impaired excretory and metabolic renal function | No | (e11) |
| Aging | Unknown; disorders of the extracellular matrix? Destruction of elastic fibers? |
No | (e22) |
| Primary medial sclerosis | Unknown, hereditary genetic disorder? | No | (e23) |
| Disorders of Vitamin K metabolism | Inhibition of anti-inflammatory factors, particularly mediated by the NF-kB pathway; inhibition of matrix Gla protein (MGP) carboxylation | No | (e24) |
| Disorders of Vitamin D metabolism | Stimulation of calcium absorption, synergistic effect with parathyroid hormone on bone resorption? | No | (e25) |
| Atherosclerosis | Intimal calcification | Yes | (e26) |
| Pseudoxanthoma elasticum | Defect of the ABCC6 gene ATP Binding Cassette Subfamily C Member 6 | Yes | (e27) |
| Rheumatoid arthritis? | Disorders of human leucocyte antigen (HLA), major histocompatibility complex (MHC), cytokine promotors, T-cell signaling pathway, and others | Yes | (e28) |
| β-thalassemia | Mutation of the β-globulin gene, resulting in absent or low production of β-globulin | Yes | (e29) |
| Calciphylaxis | Unknown | Yes | (e30) |
| Kawasaki disease | Unknown; generalized inflammatory reaction secondary to infection in predisposed children? | Yes | (e31) |
| Singleton-Merten syndrome and other forms of type I interferonopathies | Genetic mutation associated with activation of type I interferon activity | Yes | (e32) |
| Parathyroid gland dysfunction | Disorders of parathyroid hormone metabolism | Yes | (e33) |
| Generalized Arterial Calcification of Infancy (GACI) | Gene mutations affecting ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), which cleaves ATP to inorganic pyrophosphate (PPi) and adenosine monophosphate (AMP) (extracellular) | Yes | (e34) |
| Arterial Calcification due to CD73 Deficiency (ACDC) | Ecto-5‘-nucleotidase (NT5E) gene mutation which codes CD73—with its mutation, the conversion of adenosine monophosphate (AMP) into adenosine is defective | Yes | (e35) |
| Idiopathic Basal Ganglia Calcification (IBGC) | Impaired extracellular transport of inorganic phosphate by mutations of the SLC20A2, PDGFRB, PDGFB, XPR1, and MYORG genes. | Yes | (e36) |
| Scleroderma | Autoimmune disease frequently associated with changes in the human leukocyte antigen (HLA) complex | Yes | (e37) |
| Hutchinson-Gilford progeria syndrome | Single nucleotide substitution in the LMNA gene | Yes | (e38) |
Risk factors
Major risk factors for MeS include diabetes mellitus, chronic kidney disease and age. With the exception of diabetes mellitus, the typical risk factors of atherosclerosis are of no significance.
Pathogenesis
MeS is characterized by increasing calcifications in the middle layer of the arterial wall, the tunica media. The pathogenesis which involves the crystallization and accumulation of hydroxyapatite, a compound that contains phosphate and calcium (6), has not yet been well understood, with the exception of monogenetic disease (7). However, in patients with diabetes or chronic kidney disease, strong evidence for possible involvement of specific molecular cascades has been found. In patients with diabetes, for example, the accumulation of toxic advanced glycation end-products (TAGEs) is thought to be of pathogenic significance (8). TAGEs are thought to contribute to vessel wall injury through both their accumulation and their numerous cell-toxic intracellular effects which are mediated by the receptor for advanced glycation end-products (RAGE) (9). In patients with chronic kidney disease, one of the pathogenic mechanisms is the increased uptake of phosphate in vascular smooth muscle cells by means of inorganic phosphate transporter receptors (PiT-1,2) with subsequent increase in Runt-related transcription factor 2 (RUNX2) which initiates a number of toxic intracellular processes. Among these, osteogenic transdifferentiation of vascular smooth muscle cells is of particular significance. Recently, sirtuins (SIRTs), a group of enzymes with high metabolic activity, have been reported to prevent osteogenic smooth muscle cell transformation in patients with chronic kidney disease (10). The clinical significance of the numerous experimentally identified promoters and inhibitors of calcium phosphate precipitation and hydroxyapatite crystallization (etable 4) remains uncertain. Figure 1 provides an overview of the different hypotheses of the pathogenesis of MeS.
eTable 4. Promotors and inhibitors of vascular calcifications.
| Promotors | Inhibitors | Reference |
| Collagen I, fibronectin, BMP 2 (bone matrix protein), LDLox (oxidized LDL), leptin, vitamin D3, inorganic phosphate, calcium, osteocalcin, alkaline phosphatase, oncostatin, TNF-α (tumor necrosis factor-α), Wnt signal transduction cascade, parathyroid hormone 7–84, MSX-2 transcription factor, FGF-23 (fibroblast growth factor– 23)-clotho/and RUNX2 (Runt-related transcription factor 2) | Fetuin, collagen IV, osteoprotegerin, parathyroid hormone-related protein, matrix GLA protein, osteopontin, parathyroid hormone 1–34, pyrophosphate, and BMP 7 (bone morphogenetic protein) | (e39, e40) |
Histopathology
The histological appearance of MeS is characterized by disseminated, confluent, and, in advanced stages, ring-like calcifications of the media. In the intima, myointimal hyperplasia – hyperplasia without signs of atherosclerosis – is often noted. Additional ectopic bone formation is common. eFigure 1 compares the histologies of MeS and atherosclerosis side by side. eFigure 2 shows the histological stages of MeS (11).
Hemodynamics
Progressive stiffening of the affected arteries impairs the buffering (Windkessel) function of the aorta and downstream arteries, increases the afterload of the left ventricle and promotes the development of left heart failure. The increased pulsatility leads to additional damage to parenchymal organs, such as the brain, liver, and kidney (12). The hemodynamic impact of MeS on small artery function and microcirculation is an area of on-going research.
Diagnosis
Clinical picture
MeS is usually clinically silent for a prolonged period of time and may even remain undiagnosed for life. The initial lack of clinical signs and symptoms in patients with overt MeS is explained by the absence of hemodynamically significant stenoses. Moreover, the clinical manifestation of the disease is mitigated by the development of collaterals. We, as the authors, have many years of experience in the management of patients with tissue necrosis as the initial manifestation of MeS. Such late initial manifestations presumably reflect the exhaustion of collateral reserves and the resulting microcirculatory decompensation. Consequently, these patients may not always present with intermittent claudication, a typical symptom of Fontaine stages IIa/IIb peripheral arterial occlusive disease.
Ankle-brachial index
Screening for MeS is performed by determining the ankle-brachial index (ABI). Here, the threshold is an ABI >1.4 (13). In patients with MeS, determining the ABI after a defined stress (for example, rising up on the toes for 20 times) is of no significance. In case of inconclusive findings, the great toe-brachial index can be determined; however, its significance in clinical practice is limited.
Pulse wave velocity
Due to vascular stiffening, pulse wave velocity (PWV) is increased in patients with MeS. According to the standardized and reproducible measurements, a PWV of 10 m/s is considered as the threshold (14).
Conventional radiography
In MeS, plain radiographs show a typical “railroad track” pattern of calcification. In contrast to the coarse granular pattern of calcification in atherosclerosis, MeS calcifications present as uniform and continuous fine granular stripes (15).
Vascular ultrasound
On B-scan ultrasound, MeS is diagnosed by homogeneous, hyperechoic streaks within the arterial wall with smooth endothelial interfaces. In routine clinical practice, ultrasound scoring methods are rarely used to determine the severity of MeS (16).
Computed tomography
Computed tomography (CT) shows MeS calcifications as narrow, uniform stripes within the arterial wall which are typically circular in cross-section and almost continuous.
When compared with histological findings, the sensitivity of CT scans for calcifications in MeS and in atherosclerosis was determined to be approximately 70%. This sensitivity equals the sensitivity of conventional radiography (17).
Intravascular ultrasound
Intravascular ultrasound (IVUS), with a spatial resolution of approximately 100 µm, show MeS calcifications as uniform stripes or rings in contrast to the coarse, lump-like pattern of calcification seen in atherosclerosis. Unlike the calcifications in atherosclerosis, acoustic shadowing is rarely observed with MeS calcifications.
Optical coherence tomography
Optical coherence tomography (OCT), with a spatial resolution of approximately 10 µm, shows MeS calcifications as dark, low-intensity signal, often inhomogeneous, but always sharply demarcated arc- to ring-shaped stripes or rings. Typically, these calcifications can be clearly located in the middle layer of the vessel wall. In Figure 1 a-g, the imaging findings in MeS are illustrated with typical examples.
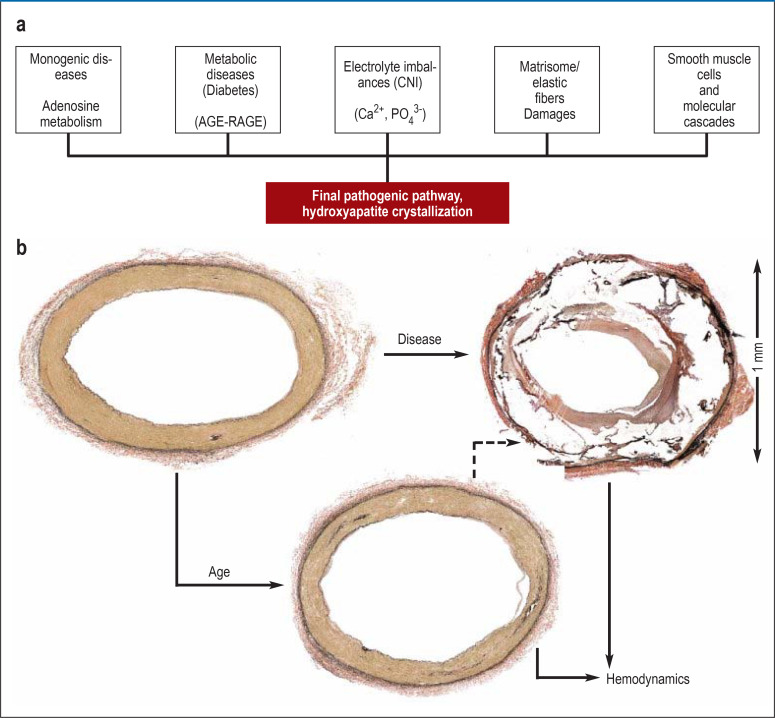
Figure 1.
Hypothesis of the pathogenesis of medial sclerosis, greatly simplified diagram
a) Medial sclerosis is the final common pathway of diverse conditions with distinct pathogenesis. As a consequence of mechanisms not yet fully understood, a pathogenetic cascade of medial calcification is triggered. In monogenic diseases, there is a defect in the metabolism of adenosine. Especially in diabetes mellitus, advanced glycation end products (AGEs) are thought to play a key role. In patients with chronic kidney disease, disturbances of electrolyte metabolism and electrolyte homeostasis in the medial layer of the arterial wall are of special importance. Damage to elastic fibers and biochemical changes in the extracellular matrix may occur, especially with advanced age. Activations of a number of molecular mechanisms, including transdifferentiation of vascular smooth muscle cells from a contractile to a secretory phenotype and disturbances in the balance between promoters and inhibitors, are thought to be involved in the calcification processes seen in all types of the disease. Common to all processes is the crystallization of hydroxyapatite.
b) Onset of medial calcification already occurs in younger individuals and the process of calcification increases with age. In some cases, severe, abnormal medial calcifications may develop even in otherwise healthy individuals. Non-age-related medial sclerosis is characterized by progressive destruction of the media. The effects of medial sclerosis on hemodynamics are an area of ongoing research.
Histology images by courtesy of Dr. Alexey Kamenskiy, University Nebraska, Omaha, U.S.A.
Laboratory testing
In MeS, clinical laboratory testing is limited to screening for risk factors (etable 5). Testing for specific biomarkers of vascular calcifications (18) has not yet been adopted for clinical use.
eTable 5. Overview of clinically relevant and preclinical laboratory tests.
| Biomarker | References | |
| Clinical | ||
| Type 2 diabetes mellitus | Glucose (fasting) ≥ 126 mg/dL (2×) or HbA1C ≥ 7% or glucose (postprandial) >198 mg/dL | (e41) |
| Chronic kidney disease | Glomerular filtration rate ≥ 90 mL/min /1.73 m2; urinary albumin excretion <2 mg/l or <80 mg/24 h | (e41) |
| Parathyroid diseases | Parathyroid hormone <10–55 pg/ml | (e41) |
| Vitamin D metabolism | 25-hydroxy vitamin D <20 ng/ml (50 nmol/l) and between 21–29 ng/ml (52.5–72.5 nmol/l), respectively; 1,25-dihydroxy vitamin d < 20 or 45 pg/ml; (<48 and >108 pmol/L) | (e41) |
| Electrolyte imbalances | Calcium (total) [<8.5 or >10.2 mg/dL], phosphate [<2.4 or >4.1 mg/dL], magnesium [<1.8 or >3.6 mg/dL] |
(e41) |
| Experimental | ||
| Vitamin K metabolism | Dp-ucMGP, PIVKA II | (e42) |
| Bone metabolism | Osteocalcin, osteonectin, osteopontin | (e43, e44) |
| Inflammation | IL-6, IL-8, TNFα | (e43, e44) |
| Other | Fibroblast growth factor-23, Klotho, Fetuin-A, bone morphogenetic proteins, pyrophosphate, fibrillin, SMADs, carbonic anhydrase, calcium-sensing receptor, sclerotin | (e43, e44) |
Dp-ucMGP, dephosphorylated uncarboxylated matrix Gla-protein; IL, interleukin; TNF-α, tumor necrosis factor alpha; PIVKA II, protein synthesized in vitamin K deficiency; Smads, suppressor of mothers against decapentaplegic
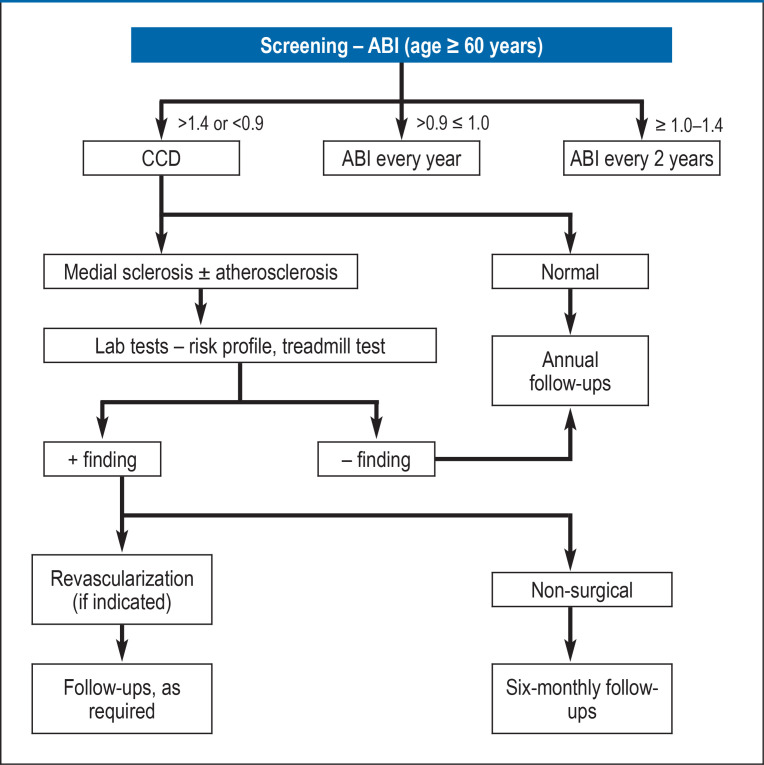
Figure 2 shows the recommended diagnostic algorithm for patients with suspected MeS.
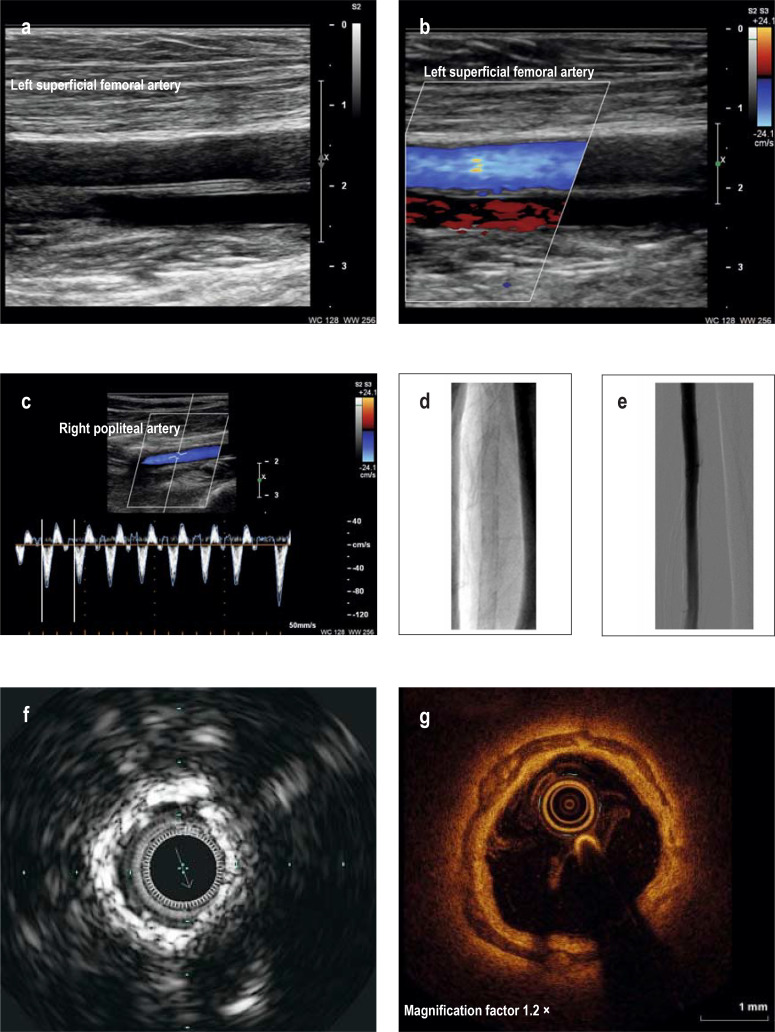
Figure 2.
Imaging diagnosis of medial sclerosis
a)B-scan ultrasound shows medial sclerosis as stripes with increased echogenicity of the arterial wall; the endothelial interfaces are smooth and free of atherosclerosis.
b) Color duplex imaging shows laminar blood flow.
c) Color duplex imaging using pulse wave (PW) technology shows normal triphasic arterial flow.
d) Plain radiograph of the brachial artery shows the railroad track pattern of calcification characteristic of medial sclerosis.
e) Corresponding x-ray angiography reveals smooth endothelial interfaces.
f) In intravascular ultrasound of the superficial femoral artery, the medial calcifications appear as annular, echogenic structures.
g)On optical coherence tomography of the popliteal artery, the calcifications of the media appear as dark stripes or rings.
Clinical significance
In patients with chronic critical leg ischemia, MeS is a common finding. It can occur both with and without atherosclerosis. In histological studies of distal artery specimens from amputated limbs, MeS was found significantly more frequently than atherosclerosis (11). Patients with critical leg ischemia and an ABI of > 1.3 are at an increased risk of amputation (19, 20). MeS of below-the-knee arteries is a major risk factor for both chronic critical leg ischemia (odds ratio [OR] 13.25, 95% confidence interval [1.69; 104.16]) (21) and amputation (relative risk [RR] 2.27, [1.89; 2.74]) (22). Patients with peripheral arterial occlusive disease and high-grade compared to low-grade calcifications have significantly increased risks for both amputation (OR 2.88, [1.18; 12.72]) and mortality (OR 5.16, [1.13; 21.61]) (23). In Germany, 63 430 amputations were performed in 2018 (24). Thus, MeS in below-the-knee and foot arteries is a common cause of amputation in Germany according to the literature.
Although patients with chronic critical leg ischemia typically present with a slowly progressive course, MeS can also be fulminant in individual cases (25). The causes of a fulminant course are still not known.
In cases with asymptomatic disease, MeS is probably a benign condition secondary to degenerative, age-related changes in the media; however, with increasing age, severely impaired peripheral perfusion may develop in some cases, even in the absence of additional risk factors (4).
In patients with acute or chronic dissection of the thoracic aorta, MeS was frequently diagnosed based on chest CT findings (26). However, a causal relationship was not concluded from these radiographic findings.
Arterial wall stiffening due to MeS could impair positive remodeling and thereby contribute to the clinical manifestation of atherosclerosis (27).
Management
There is no known causal therapy for MeS. The clinical studies performed so far, evaluating the use of phosphate binders, vitamin K preparations, pyrophosphate, tenapanor, acetazolamide, and aldosterone, have not shown convincing results (3). In patients with advanced chronic kidney disease, treatment with myo-inositol hexaphosphate, aimed at stopping the progression of MeS by inhibiting appositional HAP crystal growth, showed benefits in first randomized clinical trials (28). Likewise, the administration of magnesium appears to offer therapeutic advantages in this patient population (29). In view of the potential promotion of the clinical manifestation of atherosclerosis, strict adherence to prevention should be recommended in patients with MeS.
With endovascular treatments, MeS in the foot is a significant risk factor for treatment failure (OR 4.0, [1.1; 16.6] and for the risk of major amputation, with significant increases in risk with increasing severity of calcification (HR 10.6 [1.4; 80.7] to HR 15.5 [2.0; 119]) (30). In patients with vascular calcifications of below-the-knee arteries who underwent open surgical revascularization, lower patency rates and higher treatment failure rates were found during follow-up (31). Considering the distinctive features of MeS compared to atherosclerosis, especially with regard to the massive calcifications, to the marked myointimal hyperplasia, and to the largely exhausted perfusion reserves in these patients, endovascular treatments, in particular, should be performed exclusively by surgeons who are familiar with this condition in order to avoid unnecessary complications. Figure 4 shows a typical example of endovascular treatment in a patient with peripheral arterial occlusive disease secondary to MeS.
Outlook
Pharmacotherapy of MeS requires a thorough understanding of the pathogenesis of the disease. Despite advances in basic research over the past 20 years, the pathogenesis of MeS has remained largely hypothetical until today. As an additional drawback, lack of interest, partly due to the frequent confusion with atherosclerosis, has meant that MeS has rarely been the focus of clinical research.
In the future, the new possibilities offered by molecular biological single-cell omics technologies in combination with electronic data processing will provide the opportunity to comprehensively analyze the genome, transcriptome and metabolome of single, topographically well identified cells in a targeted manner and then integrate the results into the network of molecular cascades (33). Such precise and targeted techniques would be particularly beneficial in MeS, given that the media is well demarcated anatomically. Clinical research should provide understanding of conditions exhibiting the MeS phenotype and enable their differentiation from age-related medial calcifications. It would be of particular interest to shed light on the significance of so-called “deep” calcifications in the coronary arteries. Last but not least, broader clinical awareness of this as yet underestimated disease should contribute to improving the management of patients with MeS.
Conclusion
Medial sclerosis has historically been considered a rare nosological entity without major clinical significance (34). Today, it is well established that medial sclerosis is an important risk factor for chronic critical leg ischemia, amputations, and primarily endovascular treatment-related complications. Reorientation of basic research and greater clinical awareness are crucial prerequisites for successfully preventing and treating medial sclerosis in the future..
Figure 3.
Diagnostic algorithm for medial sclerosis
ABI, ankle-brachial index; CCD, color-coded duplex sonography
Figure 4.
Percutaneous transluminal angioplasty in patients with type 2 diabetes and marked medial sclerosis of the below-the-knee and foot arteries
a) The latero-lateral projection of the foot on plain radiograph shows the typical railroad track pattern of medial calcifications of the dorsalis pedis artery and lateral plantar artery.
b) The anteroposterior projection of the foot on plain radiograph shows the medial calcifications of the metatarsal and digital arteries.
c) Lateral imaging of the foot using digital subtraction angiography (DSA) shows marked rarefaction of the entire arterial bed with numerous stenoses and vascular occlusions prior to percutaneous transluminal angioplasty (PTA).
d) The lateral DSA image of the foot after PTA shows improved vascularization of the forefoot.
e) The corresponding antero-posterior image shows the persisting marked rarefaction of the arterial terminal vessels.
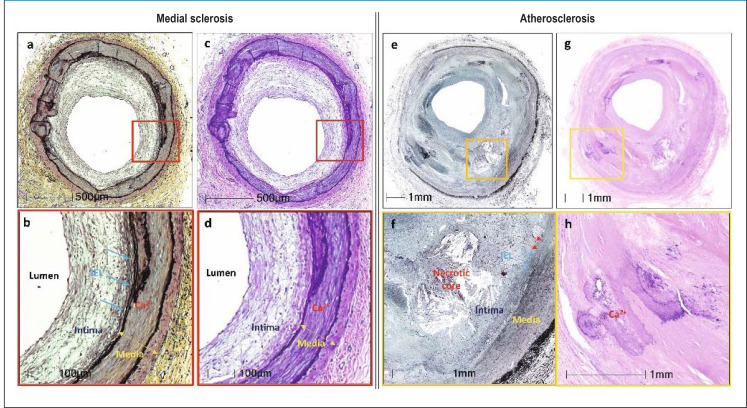
eFigure 1.
Comparison of typical histological features of medial sclerosis (a–d) and atherosclerosis (e–h)
Both diseases are shown in lower (a, b and e, f, respectively) and higher (c, d and g, h, respectively) magnification.
The typical histological picture of medial sclerosis of the popliteal artery is depicted. Medial sclerosis is characterized by circular calcification within the media and moderate intimal hyperplasia. The typical histological picture of atherosclerosis of the superficial femoral artery is shown. Intimal lesion with necrotic nucleus, penetration of the internal elastic lamina and calcified focus, clearly assigned to the intima, are shown. Internal elastic lamina (IEL) marked with arrows.
Adopted from Lanzer et al. Eur Heart J 2022; 43: 2824–6.
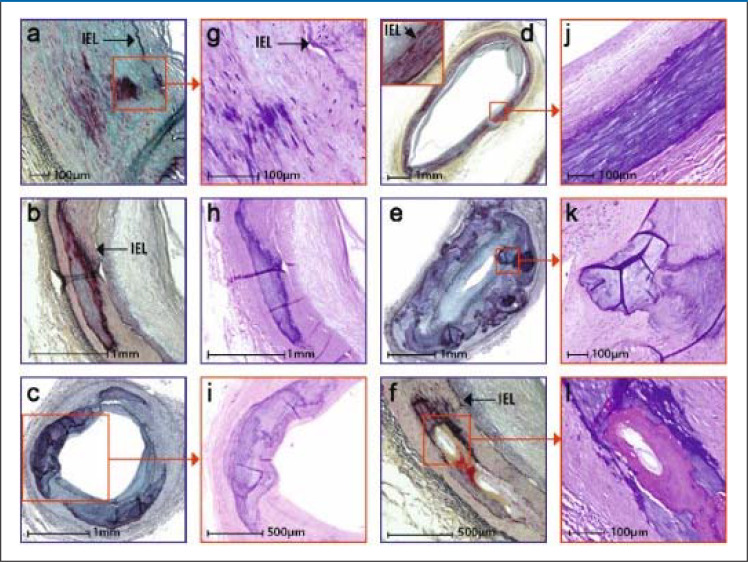
eFigure 2.
Histological stages and histological characteristics of medial sclerosis
Staging is based on the extent of calcifications.
The various stages and morphological characteristics are shown in lower (Figures a, b, c, d, e, f) and higher (Figures g, h, i, j, k, l) magnifications.
Stage I: Calcifications of the elastica interna with or without extension into the media (Figures a, g).
Stage II: Formation of larger calcifications (1 to 3 mm) (Figures b, h)
Stage III: Calcifications >3 mm to >90° of medial circumference (Figures c, i)
Stage IV: Annular calcifications of the entire circumference (Figures d, j)
Nodular calcifications as globular calcification foci with fibrin deposits (Figures e, k)
nd bone formation as proper bone foci, rarely associated with chondroid metaplasia, occur mostly in stages III/IV, rarely in stage II (Figures f, l).
Lanzer et al. J Am Coll Cardiol 2021; 78:1145–65 by courtesy of Elsevier)
Questions on the article in issue 21–22/2023:
Medial Sclerosis—Epidemiology and Clinical Significance
The submission deadline is 29 May 2024. Only one answer is possible per question.
Please select the answer that is most appropriate.
Question 1
What is the prevalence of medial sclerosis in the general population?
0.8 %
2.5 %
15.1 %
32.5 %
52.1 %
Question 2
In patients with medial sclerosis, what is deposited in the tunica media of the arterial wall?
Hydroxyapatite crystals
Oxalic acid crystals
Uric acid crystals
Calcium carbonate crystals
Sodium chloride crystals
Question 3
What does the abbreviation ABI stand for in the text?
Arm-leg index
Ankle-body index
Anterior leg index
Ankle-brachial index
Arm-body index
Question 4
Which of the following factors are listed as major risk factors for medial sclerosis in the text?
Pulmonary hypertension, kidney stones, age
Emphysema, gout, hypertension
Diabetes mellitus, chronic kidney disease, age
Monoclonal gammopathy, pulmonary hypertension, gout
Kidney stones, age, hypertension
Question 5
Which of the following elements is found in the calcification of the vessel wall in medial sclerosis?
Calcium
Potassium
Magnesium
Sodium
Iron
Question 6
What term is used to describe the change of the intima frequently observed in medial sclerosis?
Macro-intimal hyperplasia
Micro-intimal necrosis
Myointimal hyperplasia
Media-intima hyperplasia
Minor intima necrosis
Question 7
According to the text, what are the effects of medial sclerosis-related changes in hemodynamics on cardiac function?
Increase in right ventricular afterload
Increase in left ventricular preload
Increase in right ventricular preload
Increase in left ventricular afterload
Increase in biatrial preload
Question 8
In screening for medial sclerosis, what is the threshold used?
ABI >1.4
ABI <0.8
ABI >4.3
ABI <1.0
ABI >0.5
Question 9
With regard to the diagnosis of medial sclerosis, which of the following statements is true?
There is usually a lack of collateral vessels.
Transient claudication is always the first symptom.
Medial sclerosis is usually diagnosed at a young age.
At times, tissue necrosis emerges as the first symptom.
In medial sclerosis, imaging reveals coarse granular calcifications.
Question 10
Which of the following methods cannot be used to diagnose medial sclerosis?
Intravascular ultrasound
Optical coherence tomography
Computed tomography
Radiographic assessment
Testing for specific biomarkers
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Mönckeberg JG. Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virch Arch. 1903;169:141–167. [Google Scholar]
- 2.Lanzer P, Boehm M, Sorribas V, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzer P, Hannan FM, Lanzer JD, et al. Medial arterial calcification: JACC state-of-the-art review. J Am Coll Cardiol. 2021,;78:1145–1165. doi: 10.1016/j.jacc.2021.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamenskiy A, Poulson W, Sim S, et al. Prevalence of calcification in human femoropopliteal arteries and its association with demographics, risk factors, and arterial stiffness. Arterioscler Thromb Vasc Biol. 2018;38:e48–e57. doi: 10.1161/ATVBAHA.117.310490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanzer P. Mediakalzinose Mönckeberg. Z Kardiol. 1998;87:586–593. doi: 10.1007/s003920050217. [DOI] [PubMed] [Google Scholar]
- 6.Millán Á, Lanzer P, Sorribas V. The thermodynamics of medial vascular calcification. Front Cell Dev Biol. 2021:;9 doi: 10.3389/fcell.2021.633465. 633465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi M. Toxic AGEs (TAGE) theory: a new concept for preventing the development of diseases related to lifestyle. Diabet Metabol Syndr. 2020;12 doi: 10.1186/s13098-020-00614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twarda-Clapa A, Olczak A, Bialkowska AM. Koziolkiewicz: Advanced glycation end-products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells. 2022;11 doi: 10.3390/cells11081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Feng W, Su X, et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdiffentiation via Runc2 in chronic kidney disease. J Clin Invest. 2022;132 doi: 10.1172/JCI150051. e150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O‘Neill WC, Han KH, Schneider TM, Hennigar RA. Prevalence of nonatheromatous lesions in peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2015;35:439–447. doi: 10.1161/ATVBAHA.114.304764. [DOI] [PubMed] [Google Scholar]
- 12.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboyans V, Ricco J-B, Bartelink M-L EL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. endorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 14.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindbom A. Arteriosclerosis and arterial thrombosis in the lover limb; a roentgenological study. Acta Radiol Suppl. 1950;80:1–80. [PubMed] [Google Scholar]
- 16.Tian J, Tang G, Xu X, et al. Different ultrasound scoring methods for assessing medial arterial calcification: association with diabetic complications. Ultrasound Med Biol. 2020;46:1365–1372. doi: 10.1016/j.ultrasmedbio.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Vos A, Vink A, Kockelkoren R, et al. Radiography and computed tomography detection of intimal and medial calcifications in leg arteries in comparison to histology. J Pers Med. 2022;12 doi: 10.3390/jpm12050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golüke NMS, Schoffelmeer MA, de Jonghe A, et al. Serum biomarkers for arterial calcification in humans: a systematic review. Bone Reports. 2020;17 doi: 10.1016/j.bonr.2022.101599. 101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvestro A, Diehm N, Savolainen H, et al. Falsely high ankle-brachial index predicts major amputation in critical limb ischemia. Vasc Med. 2006;11:69–74. doi: 10.1191/1358863x06vm678oa. [DOI] [PubMed] [Google Scholar]
- 20.Lew E, Nicolosi N, Botek G. Lower extremity amputation risk factors asociated with elevated ankle brachial indices and radiographic arterial calfication. J Foot Ankle Surg. 2015;54:473–477. doi: 10.1053/j.jfas.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Ferraresi R, Mauri G, Losurdo E, et al. BAD transmission and SAD distribution: a new scenario for critical limb ischemia. J Cardiovasc Surg (Torino) 2018;59:655–664. doi: 10.23736/S0021-9509.18.10572-6. [DOI] [PubMed] [Google Scholar]
- 22.Losurdo F, Ferraresi R, Ucci A, et al. Association of infrapopliteal medial arterial calcification with lower-limb amputations in high-risk patients: a systematic review and meta-analysis. Vasc Med. 2021;26:164–173. doi: 10.1177/1358863X20979738. [DOI] [PubMed] [Google Scholar]
- 23.Skolnik J, Weiss R, Meyr AJ, et al. Evaluating the impact of medial arterial calcification on outcomes of infrageniculate endovascular interventions for treatment of diabetic foot ulcers. Vasc Endovasc Surg. 2021;55:382–388. doi: 10.1177/1538574421993314. [DOI] [PubMed] [Google Scholar]
- 24.Kühnl A, Knipfer E, Lang T, et al. Krankenhausinzidenz, stationäre Versorgung und Outcome der peripheren arteriellen Verschlusskrankheit und arteriellen Thrombose/Embolie in Deutschland von 2005 bis 2018. Gefäßchir. 2020;25:433–445. [Google Scholar]
- 25.Mowafy KA, Soliman M, Hammoda AM, Soliman RM. Bilateral lower limb disabling claudication in a young man: A case of Mönckeberg’s Arteriosclerosis. Vascular & Endovascular Review. 2019;2:48–52. [Google Scholar]
- 26.de Jong PA, Hellings WE, Takx RA, Išgum I, van Herwaarden JA, Mali WP. Computed tomography of aortic wall calcifications in aortic dissection patients. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102036. e102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fok PW, Lanzer P. Media sclerosis drives and localizes atherosclerosis in peripheral arteries. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205599. e0205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raggi P, Bellasi A, Bushinsky D, et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized phase 2b study. Circulation. 2020;141:728–739. doi: 10.1161/CIRCULATIONAHA.119.044195. [DOI] [PubMed] [Google Scholar]
- 29.ter Braake AD, Vervloet MG, de Baaij JHF, Hoenderop JGJ. Magnesium to prevent kidney disease-associated vascular calcification: crystal clear? Nephrol Dial Transpl. 2022;37:421–429. doi: 10.1093/ndt/gfaa222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu IH, Wu B, Krepkiy V, et al. Pedal arterial calcification score is associated with hemodynamic change and major amputation after infrainguinal revascularization for chronic limb-threatening ischemia. J Vasc Surg. 2022;76:1688–1697. doi: 10.1016/j.jvs.2022.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Misare BD, Pomposelli FB, Gibbons GW, Campbell DR, Freeman DV, LoGerfo FW. Infrapopliteal bypasses to severely calcified, unclampable outflow arteries: two-year results. J Vasc Surg. 1996;24:6–16. doi: 10.1016/s0741-5214(96)70139-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Hyeon DY, Hwang D. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med. 2020;52:1428–1442. doi: 10.1038/s12276-020-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanathan GA, Seto J, Patil S, et al. Getting started in biological pathway construction and analysis. PLoS Comput Biol. 2008;4 doi: 10.1371/journal.pcbi.0040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanzer P, Schanahan C. Morbus Mönckeberg: Gefäße als zweites Skelett. Dtsch Arztebl. 2000;97 A-1746. [Google Scholar]
- E1.Kröger K, Stang A, Kondratieva J, et al. Prevalence of peripheral arterial disease—results of the Heinz Nixdorf Recall study. Eur J Epidemiol. 2006;21:279–285. doi: 10.1007/s10654-006-0015-9. [DOI] [PubMed] [Google Scholar]
- E2.Fowkes FGR, Murray GD, Murray D, et al. Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study ofAtherosclerosis) J Amer Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Hoek AA, Zwakenberg SR, Elders PJM, et al. An elevated ankle-brachial index is not valid proxy for peripheral medial arterial calcification. Atherosclerosis. 2021;323:13–19. doi: 10.1016/j.atherosclerosis.2021.03.010. [DOI] [PubMed] [Google Scholar]
- E5.Janka HU, Standl E, Mehnert H. Peripheral vascular disease in diabetes mellitus and its relation to cardiovascular risk factors: screening with the Doppler ultrasonic technique. Diab Care. 1980;3:207–213. doi: 10.2337/diacare.3.2.207. [DOI] [PubMed] [Google Scholar]
- E6.Hirschl M, Francesconi M, Hirschl MM. Moenckebergsche Mediasklerose: Klinische Aspkte bei Diabetikern. Vasa. 1991;20:216–221. [PubMed] [Google Scholar]
- E7.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 19961;6:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- E8.Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabet Care. 1994;17:1252–1256. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- E9.Ferrier TM. Radiologically demonstrable arterial calcification in diabetes mellitus. Australas Ann Med. 1964;13:222–228. doi: 10.1111/imj.1964.13.3.222. [DOI] [PubMed] [Google Scholar]
- E10.Zwakenberg SR, de Jong PA, Hendriks EJ, et al. Intimal and medial calcification in relation to cardiovascular risk factors. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235228. e0235228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- E12.Konijn LCD, van Overhagen H, Takx RAP, et al. CT calcification patterns of peripheral arteries in patients without known peripheral artery disease. Eur J Radiol. 2020;128 doi: 10.1016/j.ejrad.2020.108973. 108973. [DOI] [PubMed] [Google Scholar]
- E13.Moussa Pacha H, Mallipeddi VP, Afzal N, et al. Association of ankle-brachial indices with limb revascularization or amputation in patients with peripheral artery disease. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.5547. e185547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Vos A, Kockelkoren R, de Vis JB, et al. Risk factors for atherosclerotic and medial calcifications of the intracranial internal carotid artery. Atherosclerosis. 2018;276:44–49. doi: 10.1016/j.atherosclerosis.2018.07.008. [DOI] [PubMed] [Google Scholar]
- E15.Lachman AS, Spray TL, Kerwin DM, Shugoll GI, Robert WC. Medial calcinosis of Mönckeberg: a review of the problem and a description of a patient with involvement of peripheral, visceral and coronary arteries. Am J Med. 1977;63:615–622. doi: 10.1016/0002-9343(77)90207-8. [DOI] [PubMed] [Google Scholar]
- E16.Castillo BV, Torczynski E, Edward DP. Mönckeberg’s sclerosis in a temporal artery biopsy specimen. Br J Ophthalmol. 1999;83:1091–1092. doi: 10.1136/bjo.83.9.e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E17.Lee SJ, Choe YS, Lee JC, Park BC, Lee WJ, Kim DW. Two cases of Mönckeberg’s medial sclerosis on the face. Ann Dermatol (Soul) 2007;19:31–34. [Google Scholar]
- E18.Kim HJ, Greenerg JS, Javitte MC. Breast calcification due to Mönckeberg medial calcific sclerosis. Radiographics. 1999;19:1401–1403. doi: 10.1148/radiographics.19.5.g99se221401. [DOI] [PubMed] [Google Scholar]
- E19.Vos A, Kockelkoren R, de Vis RB, et al. Risk factors for atherosclerotic and medial arterial calcification of the intracranial internal carotid artery. Atherosclerosis. 2018;276:44–49. doi: 10.1016/j.atherosclerosis.2018.07.008. [DOI] [PubMed] [Google Scholar]
- E20.Pisani I, De Troia A, Allegri L, Corradi D, Vaglio A. Malignant Mönckeberg medial calcific sclerosis. Intern Emerg Med. 2018;13:615–617. doi: 10.1007/s11739-018-1794-1. [DOI] [PubMed] [Google Scholar]
- E21.de Jong PA, Hellings WE, Takx RAP, Išgum I, van Herwaarden JA, Mali WP. Computed tomography of aortic wall calcifications in aortic dissection patients. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102036. e102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.Pescatore LA, Gamarra LF, Liberman M. Multifaceted mechanisms of vascular calcification in aging. Arterioscler Thromb Vasc Biol. 2019;39:1307–1316. doi: 10.1161/ATVBAHA.118.311576. [DOI] [PubMed] [Google Scholar]
- E23.Lanzer P. Mediakalzinose Mönckeberg. Z Kardiol. 1998;87:586–593. doi: 10.1007/s003920050217. [DOI] [PubMed] [Google Scholar]
- E24.Wen L, Chen J, Duan L, Shuzhuang Li S. Vitamin K-dependent proteins involved in bone and cardiovascular health (review) Mol Med Rep. 2018;18:3–15. doi: 10.3892/mmr.2018.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Wang J, Zhou JJ, Robertson GR, Lee VW. Vitamin D in vascular valcification: a double-edged sword? Nutrients. 2018;10 doi: 10.3390/nu10050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- E27.Li Q, van de Wetering K, Uitto J. Pseudoxanthoma elasticum as a paradigm of heritable ectopic mineralization disorders: pathomechanisms and treatment development. Am J Pathol. 2019;189:216–225. doi: 10.1016/j.ajpath.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Ruscitti P, Cipriani P, Liakouli V, et al. Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther. 2019;21 doi: 10.1186/s13075-019-1975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Aessopos A, Samarkos M, Voskaridou E, et al. Arterial calcifications in ß-Thalassemia. Angiology. 1998;49:137–143. doi: 10.1177/000331979804900206. [DOI] [PubMed] [Google Scholar]
- E30.Moe SM, Chen NX. Calciphylaxis and vascular calcification: a continuum of extra-skeletal osteogenesis. Pediatr Nephrol. 2003;18:969–975. doi: 10.1007/s00467-003-1276-0. [DOI] [PubMed] [Google Scholar]
- E31.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health rofessionals from the American Heart Association On behalf of the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- E32.Feigenbaum A, Müller C, Yale C, et al. Singleton-Merten Syndrome: an autosomal dominant disorder with variable expression. Am J Med Genet A. 2013;161A:360–370. doi: 10.1002/ajmg.a.35732. [DOI] [PubMed] [Google Scholar]
- E33.Goettsch C, Iwata H, Aikawa E. Parathyroid hormone: critical bridge between bone metabolism and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1333–1335. doi: 10.1161/ATVBAHA.114.303637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Wang C, Li Y, Shi L, et al. Mutations in slc20a2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet. 2012;44:254–256. doi: 10.1038/ng.1077. [DOI] [PubMed] [Google Scholar]
- E35.St Hilaire C, Ziegler SG, Markello TC, et al. Nt5e mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Rutsch F, Ruf N, Vaingankar S, et al. Mutations in ENPP1 are associated with ‚idiopathic‘ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- E37.Cannarile F, Valentini V, Mirabelli G, et al. Cardiovascular disease in systemic sclerosis. Ann Transl Med. 2015;3 doi: 10.3978/j.issn.2305-5839.2014.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Kreienkamp R, Gonzalo S. Metabolic dysfunction in Hutchinson-Gilford progeria syndrome. Cells. 2020;9 doi: 10.3390/cells9020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E39.Boström KI. Where do we stand on vascular calcification? Vascul Pharmacol. 2016;84:8–14. doi: 10.1016/j.vph.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E40.Pan W, Jie W, Huang H. Vascular calcification: Molecular mechanisms and therapeutic interventions. MedComm. 2023;4 doi: 10.1002/mco2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Lanzer P. Primary media sclerosis Mönckeberg: diagnostic criteria. Cor et Vasa. 2018;60:2205. e8. [Google Scholar]
- E42.Roumeliotis S, Roumeliotis A, Dounousi E, Eleftheriadis T, Liakopoulos V. Biomarkers of vascular calcification in serum. Adv Clin Chem. 2020;98:91–147. doi: 10.1016/bs.acc.2020.02.004. [DOI] [PubMed] [Google Scholar]
- E43.Smith ER, Hewitson TD, Holt SG. Diagnostic tests for vascular calcification. Adv Chronic Kidney Dis. 2019;26:445–463. doi: 10.1053/j.ackd.2019.07.001. [DOI] [PubMed] [Google Scholar]
- E44.Zwankenberg SR, de Jong PA, Hendicks EJ, et al. Intimal and medial calcification in relation to cardiovascular risk factors. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235228. e0235228. [DOI] [PMC free article] [PubMed] [Google Scholar]