Abstract
The development of synthetic strategies for the preparation of bioisosteric compounds is a demanding undertaking in medicinal chemistry. Numerous strategies have been developed for the synthesis of bicyclo[1.1.1]pentanes (BCPs), bridge-substituted BCPs and, bicyclo[2.1.1]hexanes. However, progress on the synthesis of bicyclo[3.1.1]heptanes, which serve as meta-substituted arene bioisosteres, has not been previously explored. Herein, we disclose the first photoinduced [3σ+2σ] cycloaddition for the synthesis of trisubstituted bicyclo[3.1.1]heptanes using bicyclo[1.1.0]butanes and cyclopropylamines (CPAs). This transformation not only uses mild and operationally simple conditions, but also provides unique meta-substituted arene bioisosteres. The applicability of this method is showcased by some derivatization reactions.
Keywords: Bicyclo[3.1.1]heptanes, Photoredox, Cyclopropylamines, Bicyclo[1.1.0]butane, [3σ+2σ]-Cycloaddition
Graphical Abstract
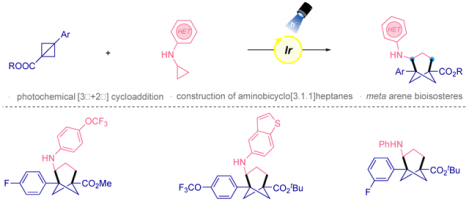
Bicyclic hydrocarbons have demonstrated the ability to replace aromatic rings in therapeutic molecules, providing greater solubility and metabolic stability, along with enhanced pharmacokinetic properties. Because of these enhanced properties, these bioisosteric scaffolds have received considerable attention in drug molecular design.1 One of the most studied among small-ring cage hydrocarbons are bicyclo[1.1.1]pentanes (BCPs),2 which serve as a bioisosteric replacement of ortho- and para-substituted phenyl rings as well as the tert-butyl group.3 Synthetic organic chemists have also made great progress in the development of new synthetic methods that provide geometrically complementary meta-substituted arene bioisosteres. However, these architectures have been explored using mainly bridge-substituted BCPs4 and bicyclo[2.1.1]hexanes,4,5 although these motifs do not exactly mimic the bond vectors displayed in meta-substituted arenes. The preparation of bioisosteres that precisely reproduce the geometrics of meta-substituted arenes is a challenge from a synthetic point of view. Recently, the preparation of difunctionalized bicyclo[3.1.1]heptanes using [3.1.1]propellane as a feedstock demonstrates that these substructures can serve as meta-substituted arene analogues (Figure 1).6
Figure 1.
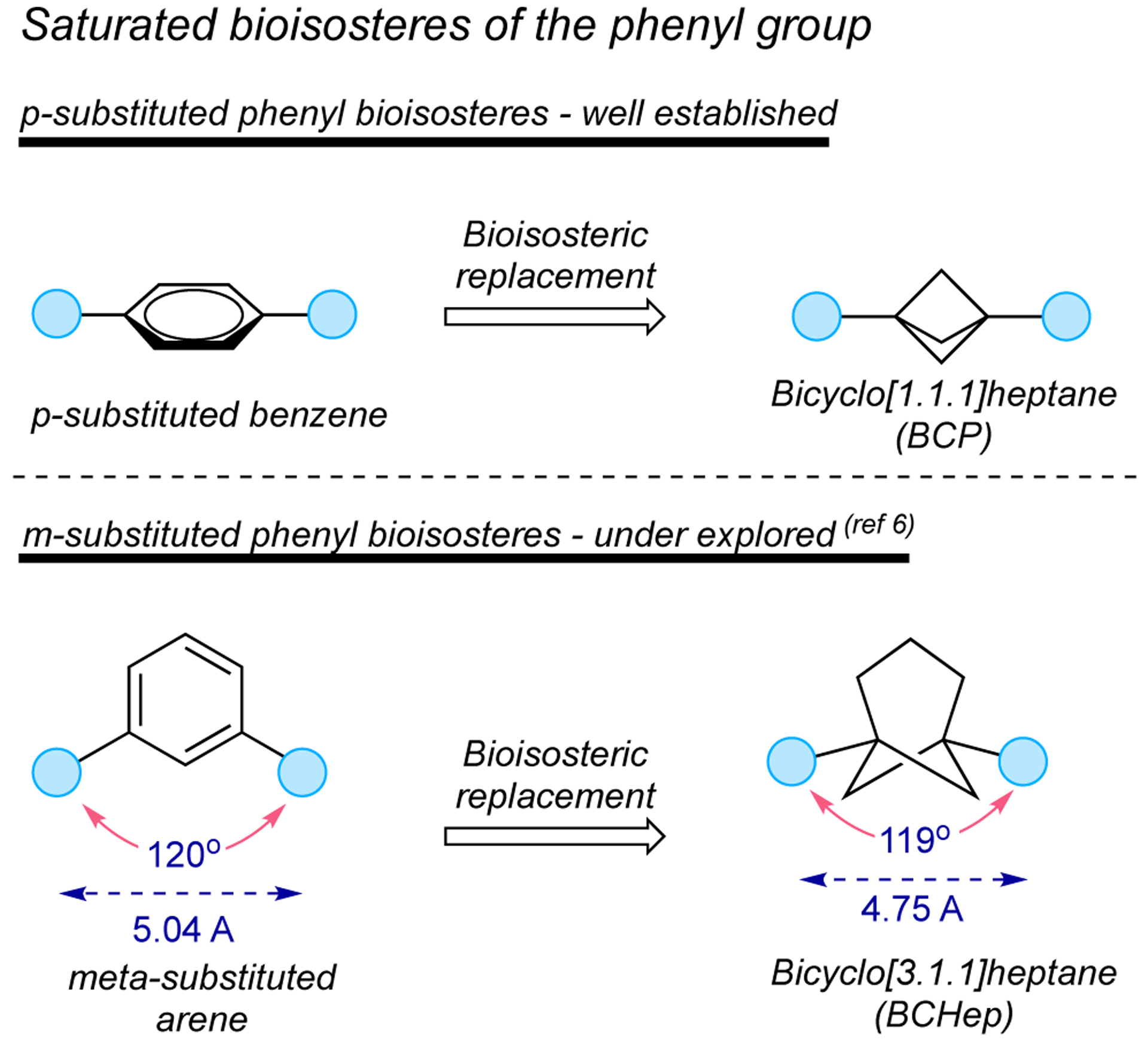
Comparison of para- and meta-substituted arene ioisosteres
Cyclopropylamines (CPAs) have shown their versatility as building blocks to access highly valuable nitrogenated organic motifs.7 They are extremely useful in [3+2] annulation reactions with alkenes for the construction of more complex cyclic amines.7c Their use as synthetic scaffolds arises from an irreversible ring-opening upon an initial oxidation to the nitrogen radical cation.7f In 1998 Cha8 and Iwata9 disclosed the use of N,N-dialkyl aminocyclopropanes bearing an alkene group for the construction of amino octahydropentalenes. In the development of photochemical [3+2] cycloadditions for the synthesis of bicycloaminoalkanes, bicyclic cyclopropylamines were used in an intermolecular approach using a ruthenium photocatalyst.7a Later, intra-10 and intermolecular11 cycloaddition reactions utilizing amino/iminocyclopropanes and alkenes (Figure 2) were reported.
Figure 2.
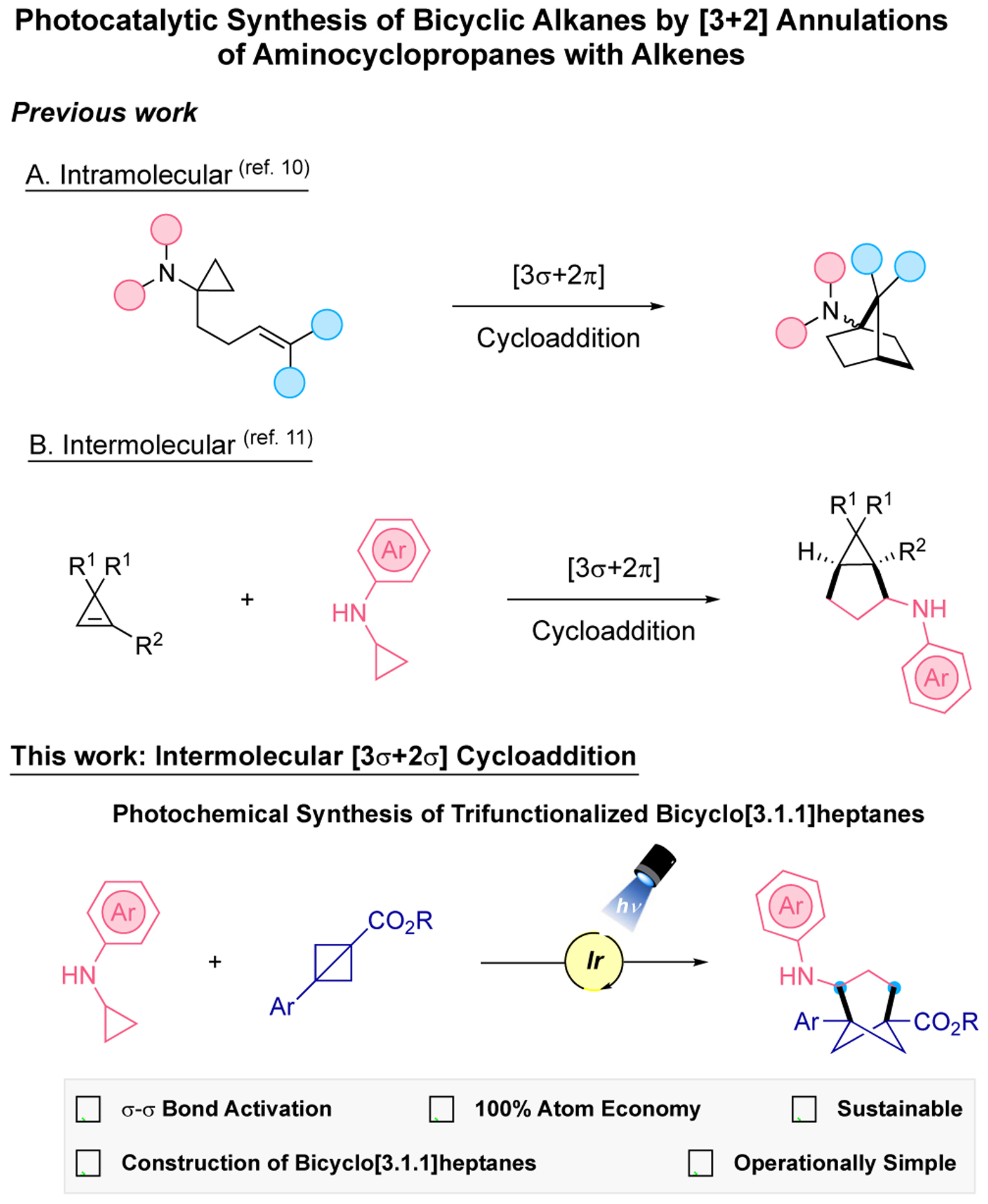
Photocatalytic [3+2] annulations of alkenes using aminocyclopropanes for the construction of bicyclic systems.
Bicyclo[1.1.0]butanes (BCBs) have received increasing attention12 by the chemistry community for the synthesis of small ring systems because of their ability to engage in ring-opening reactions with different partners,13 including radical species. We envisioned that a photoredox [3σ+2σ] cyclization reaction could be applied using cyclopropylamines and bicyclo[1.1.0]butanes. This reaction design would provide unique functionalized bicyclo[3.1.1]heptanes, which expands chemical space because of the several diversifiable positions on the bicyclo[3.1.1]heptane ring. Additionally, this bicyclic motif can potentially act as a precise meta-substituted arene bioisostere.6 To the best of our knowledge, the photoinduced construction of bicyclo[3.1.1]heptanes has never been previously explored. This photocatalytic approach would overcome the challenge for the construction of multifunctionalized bicyclo[3.1.1]heptanes in a sustainable and straightforward manner.
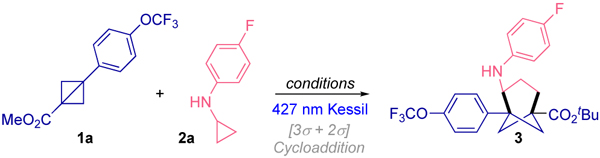
BCB 1a and CPA 2a were chosen as representative substrates for optimization of reaction conditions. First, considering that the choice of photocatalyst significantly impacts the product formation, and that the oxidation potential of cyclopropylanilines is around 0.80 V vs SCE,7a,f we initiated the studies utilizing Ir[dF(CF3)ppy]2(dtbpy)PF6 (E1/2 *IrIII/IrII = 1.21 V vs SCE)14 in DMSO as solvent. Under these conditions the bicyclic compound 3 was obtained in 70% yield (Table 1 entry 1). The exploration of other photocatalysts (Table 1 entries 2–5) did not provide better efficiency toward the formation of the desired product. Next, other solvents were examined using the optimal photocatalyst, but no improvement was achieved (Table 1 entries 6–9). The photochemical nature of this transformation was confirmed when the reaction was performed either in the absence of photocatalyst (Table 1, entry 10) or light (Table 1, entry 11). Finally, the presence of the radical scavenger TEMPO in the reaction completely inhibited the formation of compound 3 (Table 1, entry 12) and unreacted 1a was observed by GCMS analysis (see Supporting Information). Of note, this transformation provides unprecedented access to functionalized, fully assembled bicyclo[3.1.1]heptane 3 in a single step from readily available BCB15 1a and CPA 2a.
Table 1.
Exploration of the reaction conditionsa for the photochemical synthesis of functionalized bicyclo[3.1.1]heptanes.
| |||
|---|---|---|---|
| Entry | Solvent | PC | Yield of 3 (%)b |
| 1 | DMSO | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 72 (70)e |
| 2 | DMSO | Ir(ppy)3 | <10 |
| 3 | DMSO | Ru(bpy)3(PF6)2 | 30 |
| 4 | DMSO | MesAcr | traces |
| 5 | DMSO | 4CzIPN | 27 |
| 6 | DMA | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 46 |
| 7 | MeCN | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 40 |
| 8 | 1,4-dioxane | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 0 |
| 9 | MeNO2 | Ir[dF(CF3)ppy]2(dtbpy)PF6 | traces |
| 10 | DMSO | none | 0 |
| 11c | DMSO | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 0 |
| 12d | DMSO | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 0 |
Reaction conditions: 1a (0.1 mmol, 1 equiv), 2a (0.2 mmol, 2 equiv), photocatalyst (2 mol % metal-based PC or 5 mol % organic based PC), in dry and degassed solvent (0.2 M) under blue Kessil irradiation (λmax = 427 nm) at rt.
Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
Reaction in the absence of light irradiation.
Reaction as in entry 5 but in the presence of 5 equiv of TEMPO.
Isolated yield from 0.2 mmol scale.
Once the optimization reaction conditions were suitable for the development of this [3σ+2σ] cyclization reaction, the scope of the process using different CPAs was explored. This transformation works especially well with electron-withdrawing groups in the aryl ring of the N-cyclopropylaniline. The p-chlorophenyl-substituted CPA gave the desired compound 4 in 36% yield, but the presence of a fluorine atom in the meta position (5) as well as in the ortho- and para positions (6) provided the desired products in much higher yield. The presence of strongly deactivating groups such as trifluoromethyl (7) and difluoromethyl (8) gave the corresponding aminobicyclo[3.1.1]heptanes in 63 and 65%, respectively. Incorporation of the medicinally relevant trifluoromethoxy group afforded access to compound 9 in 62% yield. Of note, detailed NMR studies as well as the single crystal X-ray analysis of compound 9 (see Supporting Information) confirm the 3-dimensional structures of the 4-aminobicyclo[3.1.1]heptanes. Phenyl- and 1,3-dioxolanyl-substituted CPAs provided 10 and 11, respectively, in moderate yields. An electron-rich methoxy-substituted derivative group showed reactivity, although the desired product (12) was accessed in low yield. This result is not surprising given that the more electronically rich cyclopropylanilines are known to promote easier SET, while at the same time undergoing CPA ring-opening at a slower rate.16 Alkyl groups in the para- or ortho position showed good reactivity (13 and 14). Finally, some heteroarene-based amines were tested because of their outsized use in medicinal chemistry. Of note, benzothiophene (15) and benzofuran (16) motifs were well tolerated, providing the desired products in 51 and 47% yield, respectively. To explore further the use of heterocycles in this transformation, quinoline (17) and pyridine (18) derivatives were incorporated, although the desired products were isolated in lower yields.
Subsequently, the scope was further extended to the modification of the BCB ring. In general, we observed good reactivity toward the formation of the desired 4-aminobicyclo[3.1.1]heptanes when the BCB was tethered to an electron-poor arene. Thus, the presence of fluorine (20 and 21) and chlorine (22) substituents, as well as the p-trifluoromethylphenyl derivative (23), gave the corresponding products in good yields. Finally, m-CF3 (24) and more electronically rich substituents (25-28) furnished the final compounds in moderate yields.
Given previous literature precedents,10,11,17 a mechanism for the presented photoinduced aminobicyclo[3.1.1]heptane synthesis is postulated in Scheme 1. After photoexcitation of the IrIII photocatalyst by blue light irradiation, the photoexcited state (IrIII*) (E1/2 *IrIII/IrII = 1.21 V vs SCE) is accessed.14 Single-electron transfer to the cyclopropylaniline (2) (E1/2 = 0.80 V vs SCE)7a,f induces the formation of the radical cation species (I) followed by ring opening via β-scission to the distonic radical cation (II). Subsequent addition of this reactive intermediate to BCB 1 furnishes another relatively stabilized distonic radical cation where the radical is localized in a secondary benzylic position (III). Subsequently, this species (III) undergoes cyclization, providing access to the radical cation (IV). At this stage, (IV) could be reduced by the IrII species generated in the reductive quenching photoredox cycle or it can be reduced by the presence of the cyclopropylaniline 2a. Given these two mechanistic scenarios, we explored the photochemical quantum yield of this transformation. We observed a quantum yield (ϕ) value of 0.47. Although the quantum yield is lower than 1, it does not guarantee a closed catalytic cycle.18 Given that single-electron transfer processes with amines are highly influenced by post-oxidation reactivity, a propagative mechanistic pathway is a more likely process because the reduction of IV by 2a is an enthalpically-driven step.19 This artificially low quantum yield value was also observed in other photochemical [3+2] cycloadditions reactions based on the use of CPA.10a
Scheme 1.
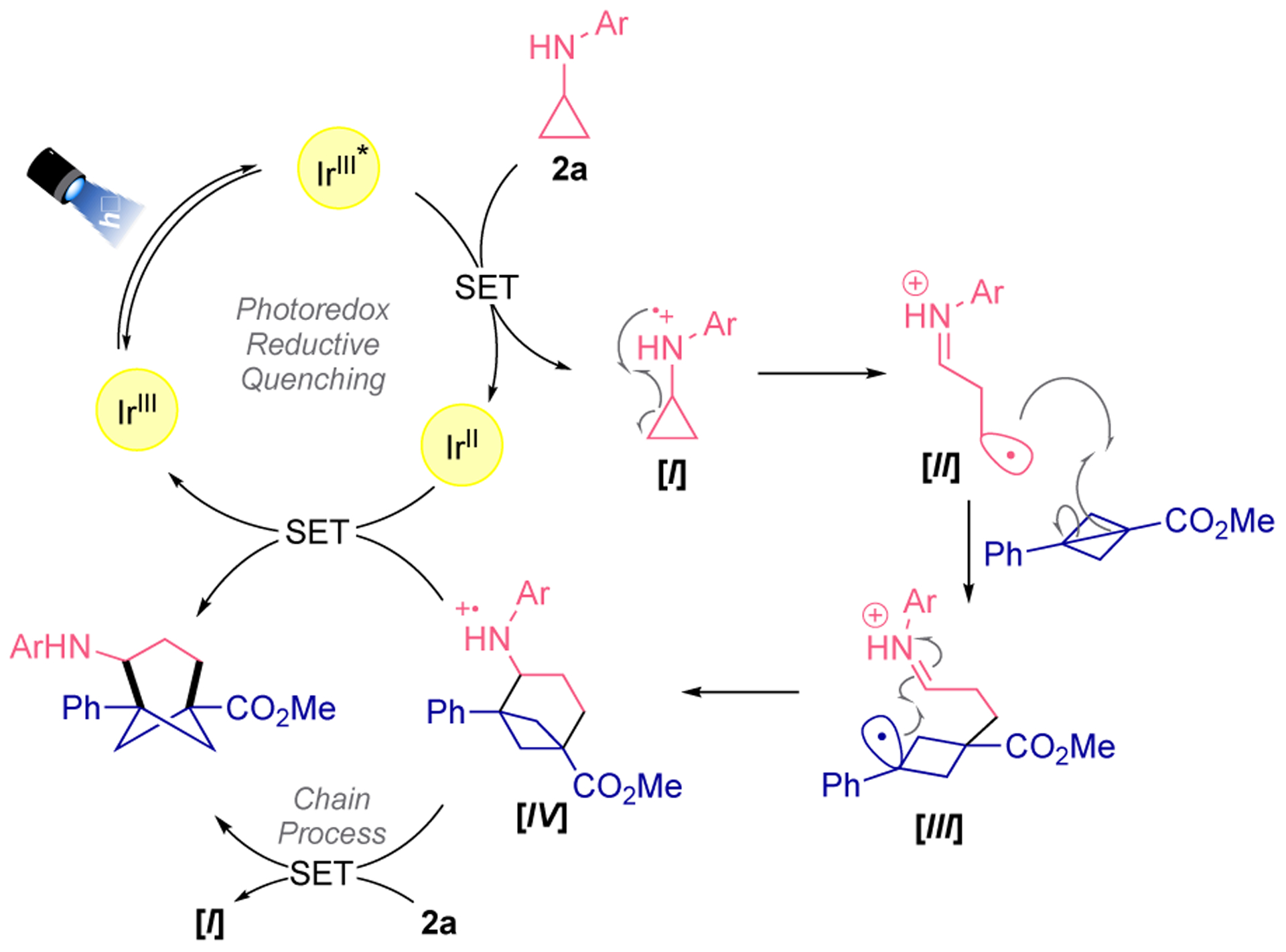
Proposed reaction mechanism for the photoinduced synthesis of 4-aminobicyclo[3.1.1]heptanes.
To showcase the applicability of the preparation of bicyclo[3.1.1]heptanes toward further functionalization, we prepared four different 4-anilinyl bicyclo[3.1.1]heptane derivatives (Scheme 2, 29-32), forming new C-C, C-O and C-N bonds. First, compound 25 was functionalized to the corresponding 3-oxobutanenitrile 29 in 73% yield using a combination of acetonitrile and LDA. This functional unit, containing α-methylene active protons, offers further opportunities for postfunctionalization. Subsequently, 25 was hydrolyzed to the corresponding carboxylic acid (30) in excellent yield, which was further coupled with estrone to provide 31 in 80% yield. Additionally, 30 was transformed to amide 32 in 85% yield. This Weinreb-type20 amide 32 serves as a carbonyl precursor for the formation of ketones, aldehydes, or alcohols.
Scheme 2.
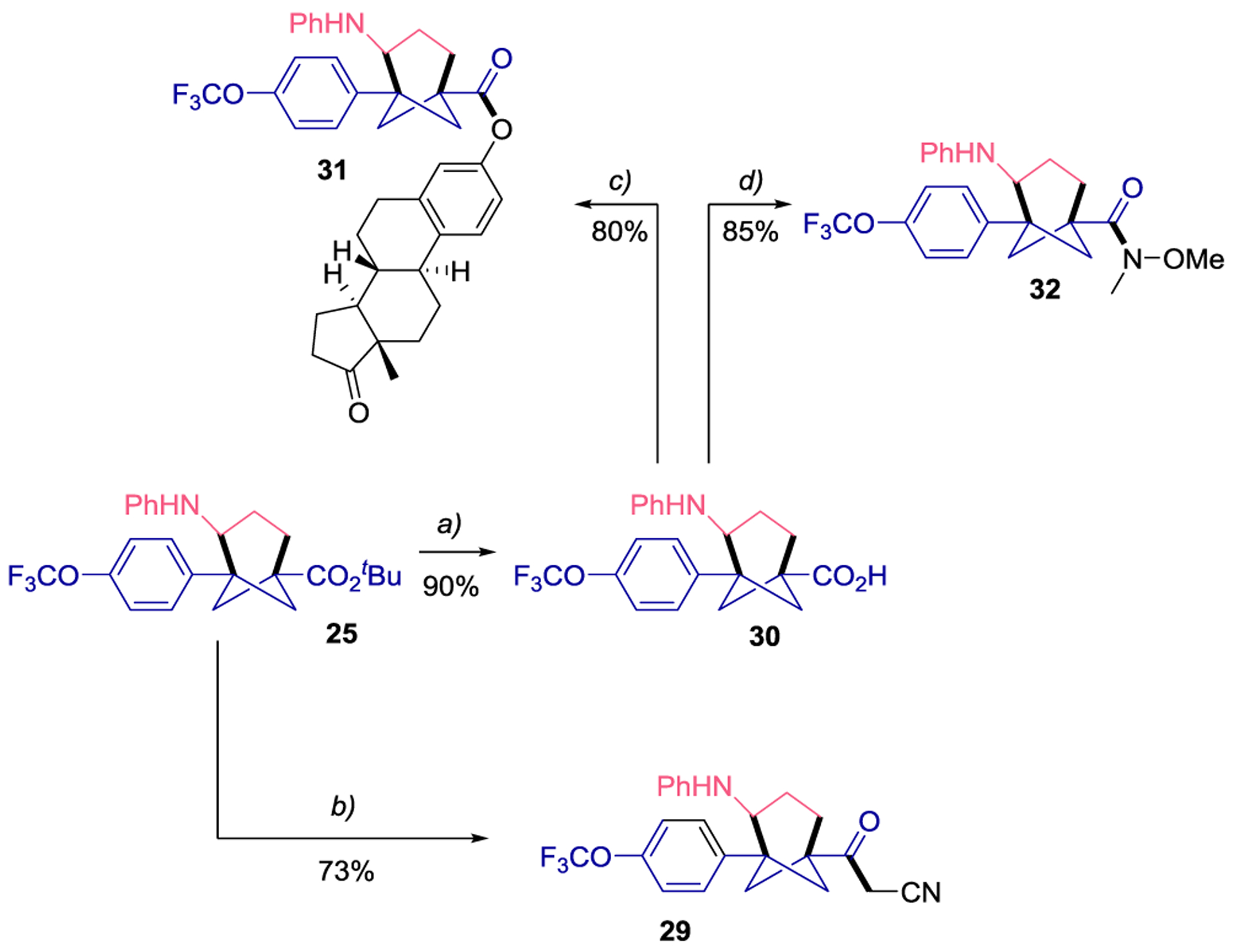
Derivatization reactions of 25. Reaction conditions: a) LiOH in MeOH, then HCl 1 M. b) LDA (2.2 equiv) and MeCN (2 equiv). c) Estrone (1 equiv), DMAP (0.05 equiv), DIC (1.2 equiv) in CH2Cl2. d) N,O-dimethylhydroxylamine (1 equiv), DMAP (0.05 equiv), DIC (1.2 equiv) in CH2Cl2. See Supporting Information for more details.
In summary, a general and highly practical method for the construction of 4-aminobicyclo[3.1.1]heptanes under mild conditions has been developed. The well-orchestrated and serialized mechanism steps furnished new bicyclo[3.1.1]heptanes containing a wide range of functional groups. Derivatization to other functional groups has also been achieved. Overall, the photochemical [3σ+2σ] annulation reaction presented herein enables access to unprecedented 4-aminobicyclo[3.1.1]heptanes assembled from BCBs and CPAs. These structures appear likely to serve as useful meta-disubstituted arene bioisosteres in the drug discovery field.
Supplementary Material
Table 2.
Substrate Scope Exploration for the Synthesis of Bicyclo[3.1.1]heptanesa
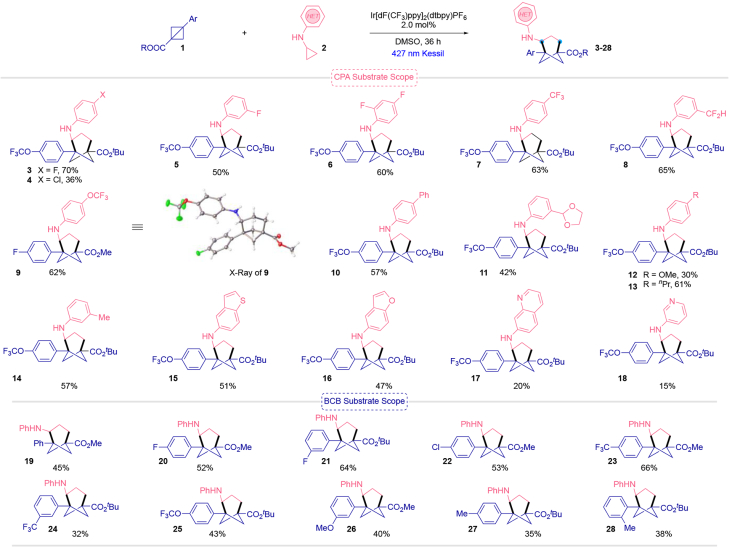
|
Reaction conditions: BCB 1 (0.2 mmol, 1 equiv), CPA 2a (0.4 mmol, 2 equiv), Ir[dF(CF3)ppy]2(dtbpy)PF6 (2 mol %), in dry and degassed DMSO (0.2 M) under blue Kessil irradiation (λmax = 427 nm) at room temperature.
ACKNOWLEDGMENT
The authors thank Dr. Jun Gu for assistance in carrying out 2D NMR studies, Dr. Charles W. Ross, III (University of Pennsylvania) for obtaining HRMS data, and Kessil Lighting for lights used in this study.
Funding Sources
The authors are grateful for financial support provided by NIGMS (R35 GM 131680 to G.M.) and NIH NCI (R21 EB 032027 to M.M.). Financial support for this research was also provided in part by AbbVie. The NSF Major Research Instrumentation Program (Award NSF CHE-1827457), the NIH Supplement Awards 3R01GM118510-03S1 and 3R01GM087605-06S1, and the Vagelos Institute for Energy Science and Technology supported the purchase of the NMRs used in this study.
Footnotes
Supporting Information.
A general procedure for the preparation of starting materials as well as the protocol for the [3+2] cycloaddition is described. Derivatization reactions details, quantum yield experiment, TEMPO experiment, X-ray details and full characterization (including NMR spectra) of all materials prepared is also included.
REFERENCES
- 1.(a) Bauer MR; Fruscia PD; Lucas SCC; Michaelides IN; Nelson JE; Storer RI Whitehurst, B. C. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem 2021, 12, 448–471. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mykhailiuk PK Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem 2019, 17, 2839–2849. [DOI] [PubMed] [Google Scholar]; (c) Subbaiah MAM; Meanwell NA Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. J. Med. Chem 2021, 64, 14046–14128. [DOI] [PubMed] [Google Scholar]; (d) Chalmers BA; Xing H; Houston S; Clark C; Ghassabian S; Kuo A; Cao B; Reitsma A; Murray C-EP; Stok JE; Boyle GM; Pierce CJ; Littler SW; Winkler DA:; Bernhardt PV; Pasay C; De Voss JJ; McCarthy J; Parsons PG; Walter GH; Smith MT; Cooper HM:; Nilsson SK; Tsanaktsidis J; Savage GP; Williams CM Validating Eaton’s Hypothesis: Cubane as a Benzene Bioisostere. Angew. Chem., Int. Ed 2016, 55, 3580–3585. [DOI] [PubMed] [Google Scholar]; (e) Auberson YP; Brocklehurst C; Furegati M; Fessard TC; Koch G; Decker A; La Vecchia L; Briard E Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem 2017, 12, 590–598. [DOI] [PubMed] [Google Scholar]; (f) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med.Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]
- 2.Selected review on functionalized BCP preparation:; Anderson JM; Measom ND; Murphy JA; Poole DL Bridge functionalisation of bicyclo[1.1.1]pentane derivatives. Angew. Chem., Int. Ed 2021, 60, 24754–24769. [DOI] [PMC free article] [PubMed] [Google Scholar]; Selected examples on BCP functionalization:; (b) Nugent J; Arroniz C; Shire BR; Sterling AJ; Pickford HD; Wong MLJ; Mansfield SJ; Caputo DFJ; Owen B; Mousseau JJ; Duarte F; Anderson EA A General Route to Bicyclo[1.1.1]Pentanes through Photoredox Catalysis. ACS Catal. 2019, 9 9568–9574. [Google Scholar]; (c) Yen-Pon E; Li L; Levitre G; Majhi J; McClain EJ; Voight EA; Crane EA; Molander GA On-DNA Hydroalkylation to Introduce Diverse Bicyclo[1.1.1]pentanes and Abundant Alkyls via Halogen Atom Transfer. J. Am. Chem. Soc 2022, 144, 12184–12191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huang W; Keess S; Molander GA Dicarbofunctionalization of [1.1.1]Propellane Enabled by Nickel/Photoredox Dual Catalysis: One-Step Multicomponent Strategy for the Synthesis of BCP-Aryl Derivatives. J. Am. Chem. Soc 2022, 144, 12961–12969. [DOI] [PubMed] [Google Scholar]; (e) Dong W; Yen-Pon E; Li L; Bhattacharjee A; Jolit A; Molander GA Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane. Nat. Chem 2022, 10.1038/s41557-022-00979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Polites VC; Badir SO; Keess S; Jolit A; Molander GA Nickel-Catalyzed Decarboxylative Cross-Coupling of Bicyclo[1.1.1]pentyl Radicals Enabled by Electron Donor–Acceptor Complex Photoactivation. Org. Lett 2021, 23, 4828–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Stepan AF; Subramanyam C; Efremov IV; Dutra JK; O’Sullivan TJ; Dirico KJ; McDonald WS; Won A; Dorff PH; Nolan CE; Becker SL; Pustilnik LR; Riddell DR; Kauffman GW; Kormos BL; Zhang L; Lu Y; Capetta SH; Green ME; Karki K; Sibley E; Atchison KP; Hallgren AJ; Oborski CE; Robshaw AE; Sneed B; O’Donnell CJ Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem 2012, 55, 3414–3424. [DOI] [PubMed] [Google Scholar]; (b) Westphal MV; Wolfstädter BT; Plancher J-M; Gatfield J; Carreira EM Evaluation of tert-Butyl Isosteres: Case Studies of Physicochemical and Pharmacokinetic Properties, Efficacies, and Activities. ChemMedChem 2015, 10, 461–469. [DOI] [PubMed] [Google Scholar]; (c) Kanazawa J; Uchiyama M Recent Advances in the Synthetic Chemistry of Bicyclo[1.1.1]pentane. Synlett 2019, 30 ,1–11. [Google Scholar]
- 4.Yang Y, Tsien J, Hughes JME Peters BK; Merchant RR; Qin T An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem 2021, 13, 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Guo R; Chang Y-C; Herter L; Salome C; Braley SE; Fessard TC; Brown MK Strain-Release [2π+2σ] Cycloadditions for the Synthesis of Bicyclo[2.1.1]hexanes Initiated by Energy Transfer. J. Am. Chem. Soc 2022, 144, 7988–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kleinmans R, Pinkert T, Dutta S Paulisch TO; Keum H; Daniliuc CG; Glorius F Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature, 2022, 605, 477–482. [DOI] [PubMed] [Google Scholar]; (c) Dhake K; Woelk KJ; Becica J; Un A; Jenny SE; Leitch DC Beyond Bioisosteres: Divergent Synthesis of Azabicyclohexanes and Cyclobutenyl Amines from Bicyclobutanes. Angew. Chem. Int. Ed 2022, 61, e202204719. [DOI] [PubMed] [Google Scholar]; (d) Agasti S; Beltran F; Pye E; Kaltsoyannis, Crisenza GEM; Procter DJ A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. ChemRxiv. 2022, 10.26434/chemrxiv-2022-v93kv. [DOI] [PubMed] [Google Scholar]
- 6.Frank N; Nugent J; Shire B; Pickford H; Rabe P; Sterling A; Zarganes-Tzitzikas T; Grimes T; Thompson A; Smith R; Schofield C; Brennan P; Duarte F; Anderson E Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature, 2022, doi.org/ 10.1038/s41586-022-05290-z. [DOI] [PubMed] [Google Scholar]
- 7.Selected examples:; (a) Maity S; Zhu M; Shinabery RS; Zheng N Intermolecular [3+2] Cycloaddition of Cyclopropylamines with Olefins by Visible-Light Photocatalysis. Angew. Chem. Int. Ed 2012, 51, 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang M-M; Waser J Oxidative Fluorination of Cyclopropylamides through Organic Photoredox Catalysis. Angew. Chem. Int. Ed 2020, 59, 16420–16424. [DOI] [PubMed] [Google Scholar]; (c) Wang M-M; Nguyen TVT; Waser J Activation of aminocyclopropanes via radical intermediates. Chem. Soc. Rev, 2022, cs/d2cs00090c. [DOI] [PubMed] [Google Scholar]; (d) Kuang Y; Ning YB; Zhu J; Wang YH Dirhodium(II)-Catalyzed (3 + 2) Cycloaddition of the N-Arylaminocyclopropane with Alkene Derivatives. Org. Lett 2018, 20, 2693–2697. [DOI] [PubMed] [Google Scholar]; (e) Nguyen TH; Maity S; Zheng N Visible light mediated intermolecular [3 + 2] annulation of cyclopropylanilines with alkynes. Beilstein J. Org. Chem 2014, 10, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Morris SA; Wang J; Zheng N The Prowess of Photogenerated Amine Radical Cations in Cascade Reactions: From Carbocycles to Heterocycles. Acc. Chem. Res 2016, 49, 1957–1968. [DOI] [PubMed] [Google Scholar]
- 8.Ha JD; Lee J; Blackstock SC; Cha JK Intramolecular [3 + 2] Annulation of Olefin-Tethered Cyclopropylamines. J. Org. Chem 1998, 63, 8510–8514. [Google Scholar]
- 9.Takemoto Y; Yamagata S; Furuse S-I Iwata, C. CAN-mediated tandem 5-exo-cyclisation of tertiary aminocyclopropanes: novel accelerative effect of an N-benzyl group for oxidative ring-opening. Chem. Commun 1998, 651–652. [Google Scholar]
- 10.(a) Staveness D Sodano TM; Li K; Burnham EA; Jackson KD; Stephenson CRJ Providing a New Aniline Bioisostere through the Photochemical Production of 1-Aminonorbornanes. Chem 2019, 5, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Staveness D; Collins JL; McAtee RC; Stephenson CRJ Exploiting Imine Photochemistry for Masked N-Centered Radical Reactivity. Angew. Chem. Int. Ed 2019, 58, 19000–19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muriel B; Gagnebin A; Waser J Synthesis of bicyclo[3.1.0]hexanes by (3 + 2) annulation of cyclopropenes with aminocyclopropanes. Chem. Sci 2019, 10, 10716–10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) McNamee RE; Thompson AL; Anderson EA Synthesis and Applications of Polysubstituted Bicyclo[1.1.0]butanes. J. Am. Chem. Soc 2021, 143, 21246–21251. [DOI] [PubMed] [Google Scholar]; (b) Walczak MAA; Krainz T; Wipf P Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res 2015, 48, 1149–1158. [DOI] [PubMed] [Google Scholar]
- 13.Selected example using nucleophiles:; Guo L; Noble A; Aggarwal VK α-Selective Ring Opening Reactions of Bicyclo[1.1.0]butyl Boronic Ester with Nucleophiles. Angew. Chem., Int. Ed 2021, 60, 212–216. [DOI] [PubMed] [Google Scholar]; Selected example using radicals:; Ociepa M; Wierzba AJ; Turkowska J; Gryko D Polarity-Reversal Strategy for the Functionalization of Electrophilic Strained Molecules via Light-Driven Cobalt Catalysis. J. Am. Chem. Soc 2020, 142, 5355–5361. [DOI] [PubMed] [Google Scholar]; Selected example using electrophiles:; Fawcett A; Biberger T; Aggarwal VK Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem 2019, 11, 117–122. [DOI] [PubMed] [Google Scholar]
- 14.Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly CB; Milligan JA; Tilley L; Sodano TM Bicyclobutanes: From Curiosities to Versatile Reagents and Covalent Warheads. Chem. Sci 2022, 13, 11721–11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Wong HNC; Hon M-Y; Tse C-W; Yip Y-C; Tanko JM; Hudlicky T Use of cyclopropanes and their derivatives in organic synthesis. Chem. Rev 1989, 89, 165–198. [Google Scholar]; (b) Cooksy AL; King HF; Richardson WH Molecular orbital calculations of ring opening of the isoelectronic cyclopropylcarbinyl radical, cyclopropoxy radical, and cyclopropylaminium radical cation series of radical clocks. J. Org. Chem 2003, 68, 9441–9452. [DOI] [PubMed] [Google Scholar]; (c) Grimm ML; Suleman NK; Hancock AN; Spencer JN; Dudding T; Rowshanpour R; Castagnoli N; Tanko JM Stereoelectronic and Resonance Effects on the Rate of Ring Opening of N-Cyclopropyl-Based Single Electron Transfer Probes. J. Am. Chem. Soc 2020, 142, 2640–2652. [DOI] [PubMed] [Google Scholar]
- 17.Cai Y; Wang J; Zhang Y; Li Z; Hu D; Zheng N; Chen H Detection of Fleeting Amine Radical Cations and Elucidation of Chain Processes in Visible-Light-Mediated [3 + 2] Annulation by Online Mass Spectrometric Techniques J. Am. Chem. Soc, 2017, 139, 12259–12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Cismesia MA; Yoon TP Characterizing Chain Processes in Visible Light Photoredox Catalysis. Chem. Sci 2015, 6, 5426–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buzzetti L; Crisenza GEM; Melchiorre P Mechanistic Studies in Photocatalysis. Angew. Chem., Int. Ed 2019, 58, 3730–3747. [DOI] [PubMed] [Google Scholar]
- 19.Carey FA; Sundberg RJ Advanced Organic Chemistry, 3rd ed.; Plenum Press: New York, 1990; p 2. [Google Scholar]
- 20.Nahm S; Weinreb SM N-methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


