Abstract
Identification of reliable and accessible biomarkers to characterize ischemic stroke patients’ prognosis remains a clinical challenge. Neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) are markers of brain injury, detectable in blood by high-sensitive technologies. Our aim was to measure serum NfL and GFAP after stroke, and to evaluate their correlation with functional outcome and the scores in rehabilitation scales at 3-month follow-up. Stroke patients were prospectively enrolled in a longitudinal observational study within 24 hours from symptom onset (D1) and monitored after 7 (D7), 30 ± 3 (M1) and 90 ± 5 (M3) days. At each time-point serum NfL and GFAP levels were measured by Single Molecule Array and correlated with National Institute of Health Stroke Scale (NIHSS), modified Rankin scale (mRS), Trunk Control Test (TCT), Functional Ambulation Classification (FAC) and Functional Independence Measure (FIM) scores. Serum NfL and GFAP showed different temporal profiles: NfL increased after stroke with a peak value at D7; GFAP showed an earlier peak at D1. NfL and GFAP concentrations correlated with clinical/rehabilitation outcomes both longitudinally and prospectively. Multivariate analysis revealed that NfL-D7 and GFAP-D1 were independent predictors of 3-month NIHSS, TCT, FAC and FIM scores, with NfL being the biomarker with the best predictive performance.
Keywords: Blood-derived biomarkers, GFAP, ischemic stroke, NfL, predictive factors
Introduction
One of the most challenging research field in cerebrovascular disease is to identify and validate reliable biomarkers to characterize the clinical evolution of ischemic stroke and patients’ prognosis. Various parameters are being studied, comprising different molecules, cell-types, neuroradiologic features, and it is now evident that a combination of them is required to give more useful information, providing higher sensitivity and specificity with respect to single biomarker molecules. 1
Ischemic stroke has high inter-individual variability as regards clinical presentation, etiology, infarct size and cerebral localization. 2 This clinical complexity is mirrored by heterogeneous physiopathological processes at cellular and molecular level: as a result of the acute interruption or severe reduction of cerebral blood flow, there is a deficit in energy substrate, triggering the so-called ischemic cascade.3,4
The brain parenchyma comprises several highly-specialized and interconnected cell types, which form the neurovascular unit (NVU). 5 After an ischemic stroke, the NVU releases molecules, which can be measured in patients’ cerebrospinal fluid and/or blood and could potentially present as biomarkers of ischemic injury. 6
In particular, blood biomarkers have the advantage of being minimally invasive, rapidly obtainable, quantitative and reproducible. Blood sampling can be easily repeated at distinct time-points, thus reflecting disease evolution in real-time. 7 Nevertheless, the clinical applicability of NVU biomarkers is constrained by their generally low serum concentration, requiring the use of ultra-sensitive detection techniques. Single Molecule Array (SiMoATM) is a digital immunoassay exploiting paramagnetic beads coated with specific antibodies to isolate and measure single target molecules present in a biofluid down to femtomolar concentrations. 8 In the last few years, SiMoATM platform has become the premier solution for quantifying neurofilament proteins in serum samples from patients with several neurological conditions.9–11
In this prospective observational study, the SiMoATM technology was applied to quantify the presence of Neurofilament Light Chain (NfL) and Glial Fibrillary Acidic Protein (GFAP) as biomarkers of ischemic brain injury, respectively derived from neuronal and glial cells. The aim of this study was to evaluate the NfL and GFAP concentrations in acute ischemic stroke patients’ sera within 24 hours and after 7, 30 ± 3 and 90 ± 5 days from the onset of neurological symptoms.
Although some recent studies have addressed the role of these biomarkers in predicting stroke severity and outcome,12–18 to date, none have given detailed insights into their correlation with more specific neurorehabilitation outcomes. In this study, the association of NfL and GFAP levels with overall functional outcome and stroke severity was studied in conjunction with patients’ functional recovery and their independency in the activities of daily living (ADLs), measured through ad hoc scales.
Materials and methods
Study population
For this longitudinal, prospective, observational study, patients admitted to the Stroke Unit department of the Policlinico San Matteo, Pavia, between August 2019 and March 2021 were screened for eligibility. Inclusion criteria were patients of both sexes aged more than 18 years, within 24 hours of ischemic stroke symptom onset, with scoring via the National Institute of Health Stroke Scale (NIHSS) ≥1. Exclusion criteria were: previous clinically symptomatic ischemic and/or hemorrhagic stroke, previous traumatic head injuries with residual deficits, active central or peripheral nervous system disease other than cerebrovascular disease, active oncological disease with life expectancy <12 months and pregnancy. A standard non-contrast head computed tomography (CT) scan was taken at admission in the emergency room (ER) on all patients as standard of care, to rule out hemorrhagic stroke.
Patients were treated as standard of care according to the national guidelines both in ER (performing IVT/EVT recanalization strategies when patients met the inclusion criteria) and in Stroke Unit. After patients’ enrolment, demographic and clinical data were recorded on the software Research Electronic Data Capture (REDCap®), pre-stroke mRS was assessed and the first blood sample was collected.
Patients needing neurorehabilitation at discharge were admitted to the rehabilitation units of the Istituti Clinici Scientifici Maugeri IRCCS, Pavia. Rehabilitation program was based on a multidisciplinary approach involving an interprofessional team composed of a physician with expertise in rehabilitation, nurses, physical therapists, occupational therapists, speech/language therapists and psychologist. The aim of the treatment was the rescue of lost neurological functions and/or optimization of residual capacities, reduction of disability and improvement of post-stroke quality of life.
A control group consisting of healthy volunteers not affected by a history of stroke was also enrolled, serving as a methodological control to check if analytical measurements of biomarkers were reliable and comparable with expected results.
The study was conducted following the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines and it was approved by the Ethical Committee of the Istituti Clinici Scientifici Maugeri IRCCS (Eudract number NCT05812846). All participants signed specific written informed consent before inclusion in this study.
Assessment of neurological deficit and disability
Time-points of evaluation were at D1 (within 24 hours from symptoms onset), D7 (day 7), M1 (day 30 ± 3) and M3 (day 90 ± 5) after stroke. At each time-point, the following scales were assessed by blinded investigators: NIHSS for stroke severity (score range: 0–42), 19 modified Rankin scale (mRS) for functional outcome (score range: 0–6), 20 Trunk Control Test (TCT, score range: 0–100) and Functional Ambulation Classification (FAC, score range: 0–5) for motor deficit,21,22 Functional Independence Measure (FIM, score range: 18–126; motor FIM sub-scale: 13–91; cognitive FIM sub-scale: 5–35) for a more comprehensive evaluation of patients’ disability in ADLs. 23
Neuroradiologic evaluation of chronic cerebrovascular disease
CT-scans acquired in ER were evaluated by a neuroradiologist blinded to the results of clinical and laboratory measurements. For each patient, the periventricular and deep white matter hypodensities were semi-quantitatively evaluated as a marker for chronic neuronal damage. Hypodensities were scored using a four-point scale derived from the Fazekas score routinely used for MRI, which was adapted for CT imaging: 24 0 (absence), 1 (punctuate foci), 2 (beginning confluence of foci), 3 (large confluent areas).
Blood collection
Venous blood samples (one vacutainer with clot activator) were obtained from the patients at all time-points (D1, D7, M1, M3) and centrifugated at 2000 × g for 10 minutes at 24°C within two hours of collection. Serum was aliquoted in cryovials and stored at −80°C until use. Due to sanitary restrictions imposed by the COVID-19 pandemic, complete longitudinal monitoring was not possible for all patients due to prevention of hospital access during a period concurrent with the period of the study. For all cases who had matched measurements at all the time-points, the profile levels of serum NfL and GFAP are displayed in Supplemental Materials (Figure S1 and S2).
NfL and GFAP measurement
Serum NfL (sNfL) and GFAP (sGFAP) concentrations were measured using the NF-light™ Advantage (Item 103400) and GFAP Discovery (Item 102336) kits for the SiMoA immunoassay SR-X (Quanterix, Lexington, MA, USA). Analysis was carried out using a 2-step assay following the manufacturer’s instructions. Calibrator points were run in duplicates while samples were analyzed in triplicates. Each sample was diluted with Sample Diluent before distributing to individual wells (for most of the samples a 4 × dilution was appropriate for the quantification of both sNfL and sGFAP). The lower limit of quantification (LLOQ) and the limit of detection (LOD) of the two assays were established according to the manufacturer’s instructions. The LLOQ of the NfL assay was 0.32 pg/mL, and the LOD was 0.06 pg/mL. The LLOQ of the GFAP assay was 1.37 pg/ml, and the LOD was 0.26 pg/ml.
Statistical analysis
Variables were reported as mean ± standard deviation (s.d.), median and interquartile range (IQR) or as absolute number and percentage. The non-normal distribution of the NfL and GFAP measurements was assessed by Shapiro-Wilk and Kolmogorov Smirnov tests in each group. Categorical variables were compared using χ2-test or exact Fisher’s, while continuous variables were compared using a non-parametric Mann-Whitney test. The Spearman’s rank correlation coefficient was used to assess the association between clinical/rehabilitation scales and levels of circulating biomarkers specific for each time-point. The statistical significance was set at a p-value <0.05. A multivariate analysis with a generalized linear model was performed in order to combine multiple measurements on each patient. Patients’ age, pre-stroke mRS and chronic cerebrovascular burden (CCVB) identified by adapted Fazekas scale were selected a priori as clinical variables to be included as possible source of bias when evaluating these biomarkers. 25 Patients’ outcome was evaluated through the following clinical/rehabilitation scales scored at M1 and M3: NIHSS, mRS, TCT, FAC, FIM. We conducted a preliminary sample size calculation in order to evaluate the correlation between biomarkers level after ischemic stroke and patient’s outcome: from literature we considered the correlation coefficients between sNfL and NIHSS score and between sNfL and mRS score as r = 0.50 and r = 0.45, respectively. 26 So, if we accept r = 0.45 (stricter situation) as expected correlation coefficient, with a power of 80% and a confidence level set at 95%, the required minimum sample size was of n = 36 patients. 27 Statistical analysis was performed using OriginLab, and SAS software [v. 9.4 SAS Institute Inc., Cary, USA]. Figures were prepared with OriginLab.
Results
Baseline features of the study population
The current study prospectively analyzed a cohort of 36 patients with stroke, enrolled within 24 hours from symptom onset. The clinical characteristics of the study population are displayed in Table 1. Mean age at study inclusion was 75.11 years (±11.80), 20 patients (55.6%) were women and median clinical stroke severity was 6 (IQR: 8.5) on the NIHSS. During the follow-up period, two patients died, none had a reinfarction of other neurovascular disease, one patient underwent carotid endarterectomy without peri- and after-surgery complications and/or reinfarction and therefore was not excluded. At baseline, sNfL median value in the stroke patient population was 31.9 pg/mL (IQR: 38.9), while sGFAP had a median concentration of 601.3 pg/mL (IQR: 2432.6). The levels of sNfL and sGFAP measured in acute stroke significantly correlated with patients’ age, NIHSS, and pre-stroke mRS assessed at the time of enrolment (Table S1).
Table 1.
Baseline characteristics of the stroke patients included in the study.
| Variable | Stroke patients (n = 36) |
|---|---|
| Gender | |
| Female, n (%) | 20 (55.6%) |
| Male, n (%) | 16 (44.4%) |
| Age (years), mean ± s.d. | 75.1 ± 11.8 |
| Hypertension, n (%) | 27 (79.4%) |
| HbA1c (%), mean ± s.d. | 6.2 ± 1.3 |
| LDL cholesterol level (mg/dL), mean ± s.d. | 119 ± 45.7 |
| Smoking habit, n (%) | 7 (20.0%) |
| Pre-existing co-morbidities, n (%) | 30 (83.3%) |
| Pre-mRS score, median [IQR] | 0 [1] |
| CCVB (CT-adapted Fazekas scale) | |
| 0, n (%) | 8 (22.2%) |
| 1, n (%) | 11 (30.6%) |
| 2, n (%) | 12 (33.3%) |
| 3, n (%) | 5 (13.9%) |
| NIHSS score baseline, median [IQR] | 6 [8.5] |
| Minor stroke (NIHSS <5), n (%) | 15 (41.7%) |
| Moderate stroke (NIHSS 5-14), n (%) | 17 (47.2%) |
| Severe stroke (NIHSS >14), n (%) | 4 (11.1%) |
| Injured hemisphere | |
| Right, n (%) | 15 (41.7%) |
| Left, n (%) | 18 (50.0%) |
| Bilateral, n (%) | 2 (5.6%) |
| Unknown, n (%) | 1 (2.7%) |
| Etiological diagnosis (TOAST classification) | |
| Atherosclerosis of large caliber arteries, n (%) | 13 (36.1%) |
| Cardio-aortic embolism, n (%) | 15 (41.7%) |
| Occlusion of small vessels, n (%) | 3 (8.3%) |
| Indeterminate cause, n (%) | 2 (5.6%) |
| Other cause, n (%) | 1 (2.8%) |
| Not known, n (%) | 2 (5.5%) |
| Revascularization therapy | 13 (36.1%) |
| Thrombolysis, n (%) | 4 (11.1%) |
| Mechanical thrombectomy, n (%) | 5 (13.9%) |
| Bridging, n (%) | 4 (11.1%) |
| sNfL-D1 (pg/mL), median [IQR] | 31.9 [38.9] |
| sGFAP-D1 (pg/mL), median [IQR] | 601.3 [2432.6] |
Temporal profile of sNfL and sGFAP
The temporal profiles of sNfL and sGFAP after stroke were assessed by measuring the concentrations of the two biomarkers in serum samples longitudinally collected at baseline (D1) and at D7, M1 and M3. Loss of some patients during follow-up was primarily due to the sanitary restrictions imposed by COVID-19 pandemic; moreover, 2 patients died (one after D1, the other after D7), and 3 patients did not manage to come to visit because transferred to other neurorehabilition facilities.
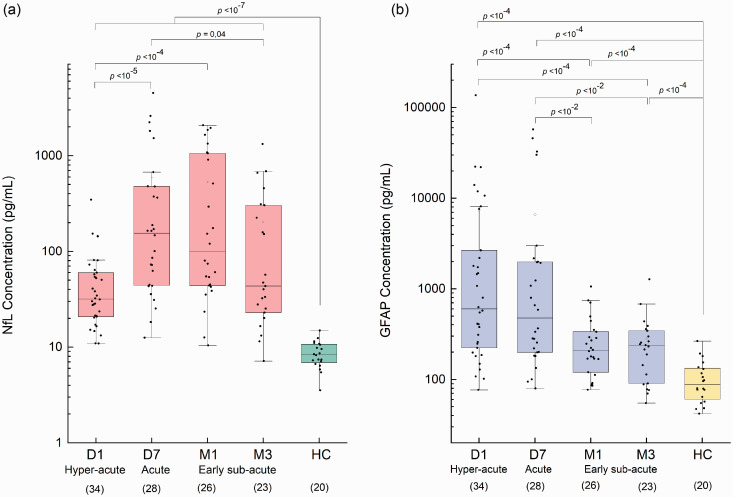
During patient’s follow-up, median sNfL significantly increased at D7 (p < 10−5) and M1 (p < 10−4) compared to D1; then decreased at M3 (p = 0.04 versus D7), returning similar to the baseline levels (Figure 1(a)). Time distribution revealed a maximum peak of median sNfL at D7 (155.7 pg/mL; IQR: 433.8).
Figure 1.
Trend of sNfL (a) and sGFAP (b) measured in stroke patients (n = 36) at D1, D7, M1, M3 after stroke. The stages of stroke corresponding to the different time-points are reported according to previous literature. 51 The number of patients completing each stage of follow-up is indicated under the x-axis. As reference values, sNfL and sGFAP levels were measured in a group of healthy controls (HC, n = 20). Each box represents the area between the 25th and 75th percentiles [interquartile range, IQR]. Lines inside the boxes represent the median values. White dots represent the mean value for each class. Whiskers extend to the lowest and highest values within 1.5 times the IQR from the box. Statistical analysis was performed by unpaired Mann-Whitney test.
In order to verify the reliability of the analytical measures, we analyzed in parallel a set of samples from healthy subjects (HC), not affected by a history of stroke and here used as internal controls. Baseline features of HC patients are reported in Table S2. Mean concentration of sNfL in HC was 8.3 pg/mL (IQR: 3.8), in line with reference values reported in the literature for healthy subjects with similar age. 28 The detectability of such a very low level of sNfL also confirmed the high sensitivity of SiMoA technology for the analysis of blood biomarkers.
In the same patient series, sGFAP was longitudinally measured at different time-points after stroke, and revealed a different kinetics of blood release as compared to sNfL. A maximum peak value was found at D1 (601.3 pg/mL; IQR: 2432.6); then, the levels of the protein decreased at the subsequent time-points (Figure 1(b)). The sGFAP concentrations were significantly higher in stroke patients than those measured in the HC group (p = 1.6 × 10−7). In HC, the mean sGFAP level was 87.9 pg/mL (IQR: 72.9), according to previous studies, 29 and further confirming the reliability of the analytical measurements performed in this study.
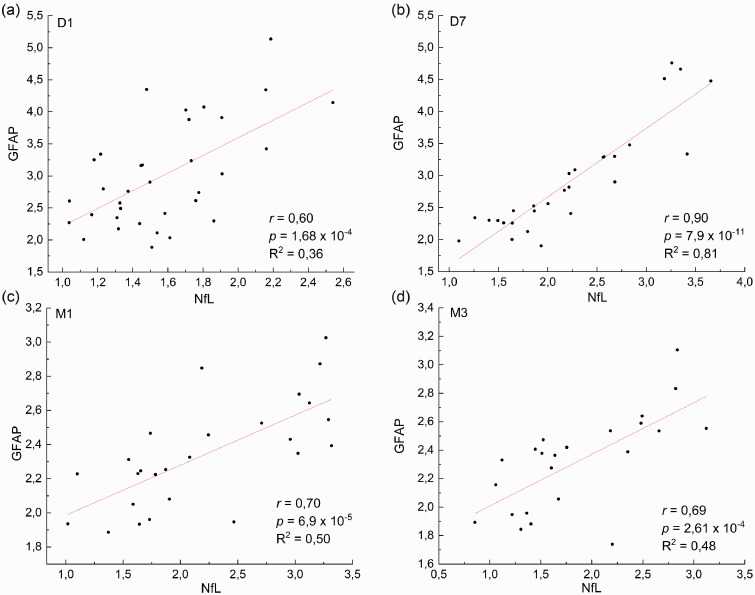
Then, we wanted to explore to which extent the measure of sNfL and sGFAP reflect ischemic damage in the central nervous system in the same way. To this aim we performed a correlation analysis between the two biomarkers at the different time-points (Figure 2). The obtained results show that at D7, the levels of the two proteins are highly correlated with r = 0.90 and a R2 = 0.81. As such, the measure of the two proteins obtained at this time is likely to correlate similarly with the clinical scales. Therefore, would not provide any additional information over a single protein. However, the correlation between sNfL and sGFAP is much lower at the other time points (D1, M1, M3). In particular, at D1 the R2 is 0.36, suggesting that the two proteins provide a different set of information.
Figure 2.
Correlation plots between sNfL and sGFAP at D1 (a, n = 34), D7 (b, n = 28), M1 (c, n = 26), M3 (d, n = 23) after stroke. Each point represents an individual subject analyzed. Data are shown upon logarithmic transformation of sNfL and sGFAP concentrations.
sNfL and sGFAP correlate with post-stroke clinical/rehabilitation scales
To investigate how the temporal profiles of sNfL and sGFAP reflect patients’ clinical status after stroke, we performed a longitudinal correlation analysis with the following clinical and rehabilitation scales: NIHSS, mRS, TCT, FAC and FIM (Table 2).
Table 2.
Correlation of sNfL and sGFAP levels with clinical and rehabilitation scales at D7, M1, M3 after ischemic stroke.
| Clinical/rehabilitation scale | Time-point | sNfL |
sGFAP |
||
|---|---|---|---|---|---|
| Spearman’s rho | p-value | Spearman’s rho | p-value | ||
| NIHSS | D7 | 0.74 | 6.4 × 10−6 | 0.65 | 0.0002 |
| M1 | 0.66 | 0.0003 | 0.65 | 0.0003 | |
| M3 | 0.50 | 0.01 | 0.55 | 0.01 | |
| mRS | D7 | 0.58 | 0.001 | 0.54 | 0.003 |
| M1 | 0.48 | 0.01 | 0.54 | 0.004 | |
| M3 | 0.26 | 0.24 | 0.28 | 0.2 | |
| Norm. mRS | D7 | 0.42 | 0.03 | 0.36 | 0.06 |
| M1 | 0.33 | 0.10 | 0.29 | 0.14 | |
| M3 | 0.003 | 0.99 | 0.12 | 0.59 | |
| TCT | D7 | −0.63 | 0.0003 | −0.58 | 0.001 |
| M1 | −0.62 | 0.0007 | −0.51 | 0.01 | |
| M3 | −0.44 | 0.04 | −0.47 | 0.03 | |
| FAC | D7 | −0.65 | 0.0002 | −0.57 | 0.001 |
| M1 | −0.50 | 0.01 | −0.52 | 0.01 | |
| M3 | −0.48 | 0.02 | −0.46 | 0.03 | |
| Total FIM | D7 | −0.78 | 1.19 × 10−6 | −0.68 | <0.0001 |
| M1 | −0.66 | 0.0003 | −0.64 | 0.0004 | |
| M3 | −0.59 | 0.004 | −0.55 | 0.01 | |
| Motor FIM | D7 | −0.72 | 1.4 × 10−5 | −0.66 | 0.0001 |
| M1 | −0.63 | 0.0006 | −0.61 | 0.0009 | |
| M3 | −0.51 | 0.02 | −0.46 | 0.03 | |
| Cognitive FIM | D7 | −0.84 | 1.7 × 10−8 | −0.77 | <0.0001 |
| M1 | −0.63 | 0.0005 | −0.61 | 0.0009 | |
| M3 | −0.53 | 0.02 | −0.63 | 0.001 | |
The levels of sNfL and sGFAP were both associated with NIHSS score at all the examined time-points and with mRS score assessed at D7 and M1. A significant inverse correlation was observed between sNfL and sGFAP with TCT, FAC, FIM scores at all time-points. We also found that sNfL and sGFAP inversely correlated with motor and cognitive FIM sub-scales considered separately, suggesting relevance for these biomarkers in both physical and mental recuperation of patients.
Then, we performed a prospective correlation analysis, taking into account the levels of sNfL and sGFAP measured at their maximum peak of blood release, i.e. D7 for NfL and D1 for GFAP. As reported in Table 3, sNfL-D7 correlated with NIHSS, TCT, FAC, FIM scales evaluated at M1 and M3. sNfL-D7 was also significantly associated with the mRS score assessed at M1, but this correlation was lost when mRS was evaluated at M3. sGFAP showed a trend comparable to sNfL, with some exceptions: sGFAP-D1 correlated with NIHSS, FAC and FIM scales evaluated at M1 and M3, but for motor FIM sub-scale the correlation was at the limits of significance at M3. Moreover, sGFAP-D1 concentrations correlated with mRS score measured at M1 and with TCT at M1, but not at M3.
Table 3.
Prognostic correlation between serum biomarkers measured at their maximum peak of blood release (D7 for sNfL, D1 for sGFAP) and patients’ outcome evaluated through clinical and rehabilitation scales scored at M1 and M3 after stroke.
| Clinical/rehabilitation scale | sNfL-D7 |
sGFAP-D1 |
|||
|---|---|---|---|---|---|
| Spearman’s rho | p-value | Spearman’s rho | p-value | ||
| NIHSS | M1 | 0.67 | 0.0004 | 0.75 | <0.0001 |
| M3 | 0.63 | 0.003 | 0.63 | 0.001 | |
| mRS | M1 | 0.51 | 0.01 | 0.53 | 0.005 |
| M3 | 0.35 | 0.14 | 0.24 | 0.29 | |
| Norm. mRS | M1 | 0.24 | 0.25 | 0.39 | 0.04 |
| M3 | 0.17 | 0.49 | 0.17 | 0.44 | |
| TCT | M1 | −0.51 | 0.01 | −0.62 | 0.0007 |
| M3 | −0.50 | 0.03 | −0.37 | 0.10 | |
| FAC | M1 | −0.48 | 0.02 | −0.63 | 0.0005 |
| M3 | −0.60 | 0.01 | −0.57 | 0.007 | |
| Total FIM | M1 | −0.67 | 0.0003 | −0.75 | <0.0001 |
| M3 | −0.65 | 0.003 | −0.55 | 0.01 | |
| Motor FIM | M1 | −0.62 | 0.001 | −0.72 | <0.0001 |
| M3 | −0.56 | 0.01 | −0.42 | 0.05 | |
| Cognitive FIM | M1 | −0.73 | 5.9 × 10−5 | −0.73 | <0.0001 |
| M3 | −0.56 | 0.01 | −0.64 | 0.002 | |
The prognostic role of sNfL and sGFAP
The prognostic role of sNfL and sGFAP was further explored with multivariate analysis with the following variables: age, pre-stroke mRS and CCVB. As shown in rows A and B of Table 4, both sNfL-D7 and sGFAP-D1 were independent prognostic factors for 3-month neurological severity (assessed through NIHSS) and patient’s functional recovery (assessed through TCT, FAC and FIM scales). No significant association was found between sNfL nor sGFAP with the mRS score evaluated at M3. Relevant associations were maintained with motor and cognitive FIM sub-scales considered separately for sNfL and sGFAP.
Table 4.
Multivariate analysis of serum biomarkers (sNfL-D7, sGFAP-D1) considered separately (raw A and B) or coupled in the same model (raw C) and clinical characteristics in relation to NIHSS, mRS, TCT, FAC, total FIM, motor FIM, cognitive FIM scores assessed at M3 in patients with ischemic stroke.
| NIHSS |
mRS |
TCT |
FAC |
Total FIM |
Motor FIM |
Cognitive FIM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß coeff. | p-value | ß coeff. | p-value | ß coeff. | p-value | ß coeff. | p-value | ß coeff. | p-value | ß coeff. | p-value | ß coeff. | p-value | ||
| A | Age | 0.02 | 0.69 | 0.009 | 0.76 | −0.19 | 0.3 | −0.01 | 0.43 | −0.15 | 0.51 | −0.18 | 0.44 | 0.04 | 0.31 |
| Pre-mRS | −0.24 | 0.64 | 0.61 | 0.1 | 10.15 | 0.0004 | 0.57 | 0.02 | 2.92 | 0.31 | 4.44 | 0.15 | −1.51 | 0.004 | |
| CCVB | 0.93 | 0.06 | 0.32 | 0.34 | −2.92 | 0.18 | −0.19 | 0.34 | −2.98 | 0.27 | −3.98 | 0.17 | 1 | 0.03 | |
| sNF-L-D7 | 0.003 | 0.0001 | 0.0003 | 0.44 | −0.02 | <0.0001 | −0.002 | <0.0001 | −0.02 | <0.0001 | −0.02 | <0.0001 | −0.002 | 0.0007 | |
| B | Age | 0.03 | 0.5 | 0.01 | 0.71 | −0.38 | 0.21 | −0.03 | 0.29 | −0.32 | 0.31 | −0.34 | 0.26 | 0.03 | 0.54 |
| Pre-mRS | −0.30 | 0.6 | 0.63 | 0.08 | 8.51 | 0.05 | 0.47 | 0.16 | 1.83 | 0.65 | 3.27 | 0.42 | −1.44 | 0.02 | |
| CCVB | 0.6 | 0.21 | 0.35 | 0.25 | −0.57 | 0.87 | 0.04 | 0.87 | −0.83 | 0.81 | −1.68 | 0.62 | 0.85 | 0.09 | |
| sGFAP-D1 | 0.0003 | 0.0006 | −0.00005 | 0.92 | −0.001 | 0.01 | 0.0002 | 0.002 | −0.002 | 0.009 | −0.002 | 0.002 | −0.0002 | 0.006 | |
| C | CCVB | 0.94 | 0.05 | 0.48 | 0.1 | −4.32 | 0.05 | −0.24 | 0.23 | −3.21 | 0.22 | −4.47 | 0.12 | 1.25 | 0.009 |
| Pre-mRS | −0.25 | 0.62 | 0.74 | 0.03 | 9.13 | 0.001 | 0.54 | 0.03 | 2.85 | 0.32 | 4.18 | 0.19 | −1.33 | 0.01 | |
| sGFAP-D1 | 0.00008 | 0.52 | −0.0002 | 0.07 | 0.0005 | 0.35 | −0.0002 | 0.72 | −0.0006 | 0.41 | −0.0004 | 0.5 | −0.0008 | 0.49 | |
| sNF-L-D7 | 0.002 | 0.01 | 0.001 | 0.05 | −0.02 | <0.0001 | −0.003 | 0.0007 | −0.02 | 0.001 | −0.02 | 0.004 | −0.001 | 0.09 | |
We then coupled sNfL-D7 and sGFAP-D1 in the same prediction model (Table 4, row C) together with pre-stroke mRS and CCVB. sNfL, but not sGFAP, showed significant prognostic value on NIHSS, TCT, FAC and total FIM scores. In this model, sNfL concentrations retained statistical significance for the prediction of motor FIM, but not cognitive FIM.
The results of the same analysis considering the scores assessed in M1 are shown in Table S3.
Discussion
Given the complexity of ischemic stroke physiopathology, a paradigm shift is required from the search for a single to a panel of biomarkers. 2 In this perspective, we simultaneously assessed the role of sNfL and sGFAP in the same cohort of patients, to decipher if they reflect ischemic damage in the same way or provide differential information on patient’s prognosis.
NfL is an intermediate filament protein of the neuronal cytoskeleton and constitutes a determinant structure for dendritic branching and growth. 30 It is released upon axonal injury in the extracellular space, reaching the cerebrospinal fluid and, to a lesser extent, the blood. For this reason, NfL is one of the most studied biomarkers of neuronal damage in various neurological disorders.31,32 GFAP is the main intermediate filament of mature astrocytes in both the grey and white matter. 33 It is released by the NVU in many neurological diseases, 34 as an indicator of glial stress or injury. Many studies have highlighted a role for sNfL and sGFAP as biomarkers of cerebrovascular disease, but none have evaluated them together in relation to specific motor and disability outcomes in patients with acute ischemic stroke.
As shown in Figure 1, sNfL and sGFAP significantly increased after stroke but with different kinetics: sNfL concentrations peaked at D7, while sGFAP reached its maximal concentration on D1. These results are in accordance with previous studies allowing the consideration of sNfL as a candidate prognostic biomarker,26,35–38 while sGFAP as an acute-phase marker to differentiate ischemic stroke from intracerebral hemorrhage. 39
Our results showed that serum levels of both biomarkers correlated with stroke severity at each time-point, a result coherent with literature data.13,40 Differently, sNfL and sGFAP concentrations correlated with the mRS scores only at D7 and M1. Moreover, mRS normalized with pre-stroke values was not significant, except sNfL at D7. This discrepancy could be due to few blood samples available at M3, but the existence of other confounding factors could not be ruled out. In particular, small infarcts occurring in eloquent brain areas may result in severe disability even if releasing a small amount of biomarkers. Moreover, the impact of timely and successful recanalization therapy should be considered as well. Finally, our population is characterized by low mRS scores (see Table S4) and it is known that this scale is more reliable when higher scores are detected. 20 All these potential confounding factors should be evaluated in larger cohorts of patients.
A strong linear correlation between sNfL and sGFAP concentrations was observed at D7, while the correlation was much lower at the other time points (D1, M1, M3, Figure 2). This is likely due (i) to the fact that both sNfL and sGFAP were higher at D7 as compared to HC, and (ii) to the ensuing neuro repair mechanisms that are initiated at D7, and whose biomarkers are distinct from those of acute injury. 41 This observation highlights the importance of assessing a panel of biomarkers reflecting ischemic stroke physiopathology and timing. Considering sNfL, it seems that their release is a consequence not only of direct axonal injury, but also of secondary neurodegenerative processes at the synaptic structures, 42 which could explain also why sNfL concentrations peak at D7.
The main and innovative finding of this study was that sNfL and sGFAP levels correlated with the scales ad hoc assessing patients’ recovery in key motor and functional domains, i.e. TCT, FAC, FIM. In particular, considering FIM scores subdivided in the motor and cognitive sub-scales, both sNfL and sGFAP levels retained the correlation up to M3, suggesting an additional role for these biomarkers in assessing post-stroke cognitive alterations. In this regard, higher sNfL concentrations were reported to increase the risk of ensuing cognitive deterioration and the progression of the already established mild cognitive impairment (MCI). 43 Also, GFAP was previously suggested to be linked with cognitive impairment, being a candidate biomarker for detecting and tracking reactive astrogliosis and Aβ pathology across the Alzheimer’s Disease continuum. 44
Longitudinal results were confirmed when we prospectively analyzed the prognostic role of sNfL and sGFAP at their respective concentration peaks, with sNfL-D7 being the biomarker with stronger correlations to the neurorehabilitation scales. Interestingly, when we performed a multivariate analysis with sNfL-D7 and sGFAP-D1 in the same prediction model, sNfL was discovered to have the best predictive performance, in accordance with the more prognostic role of this molecule as compared to sGFAP. In this model, sNfL retained statistical significance only with motor FIM prediction and not with the cognitive one: this could be influenced by the involvement of brain areas determinant for motor versus cognitive functions. However, we can not rule out that increasing the sample size could improve sNfL-D7 performance (at present p = 0.09).
In this regard, given the putative role of sNfL and sGFAP as prognostic markers of post-stroke cognitive impairment, a future dimension of this study will be to correlate these biomarkers with specific scales for the evaluation of patients’ cognitive domain functionality and the localization of the injured brain area. In fact, it was reported that patients with post-stroke cognitive impairment had significantly higher plasma NfL concentrations than patients that recovered to a normal cognitive function 3 months after stroke. 45
Strengths of this study include its prospective design, simultaneous evaluation of sNfL and sGFAP concentrations, accurate biomarkers quantification via ultra-sensitive SiMoATM technology, and correlation with specific neurorehabilitation scales, comprising both motor and cognitive domains. Compared to cerebrospinal fluid biomarkers, blood-based biomarkers are time- and cost-effective, as well as less invasive and easily accepted by patients. By SiMoATM technology, which dramatically improved the sensitivity of detection as compared to traditional methods, it was possible to measure reliable biomarkers through a simple blood sampling. This may become a precious opportunity for straightforward clinical translation, being hopefully employed in the emergency setting of cerebrovascular disease.
As pointed out by Rust, 46 the additional pieces of information given by biomarkers like NfL and GFAP, could be useful also to refer patients to more intensive rehabilitation units, and to monitor the efficacy of pharmacological and rehabilitative treatments. These future perspectives could be furtherly boosted combining these biomarkers with others under study, to envisage a multi-biomarker panel for monitoring brain injury/repair.47,48,
Otherwise, limitations of this study are the small sample size for which patients belong to the same ethnic group (caucasian) and it is not possible to statistically stratify them according to the presence/absence of risk factors that could influence biomarkers serum concentration (e.g., smoking habit, renal function, body-mass index, blood volume), the overall advanced age of the patients and the absence of a complete longitudinal monitoring for all patients due to the sanitary restrictions imposed by the COVID-19 pandemic, which has determined loss at follow-up.
In conclusion, this study provides novel information about the prognostic relevance of sNfL and sGFAP as blood-derived biomarkers of patient recovery after ischemic stroke. We demonstrated that these molecules are released with different kinetics in the blood circulation, reflecting ischemic stroke damage at different timing. Both biomarkers correlate not only with stroke severity but also with patients’ functional recovery assessed through specific motor and disability scales over a 3-month follow-up. As a future plan we intend to study other possible biomarkers such as factors that might reflect the central nervous system post-ischemic recovery49,50, to develop a panel for the close monitoring of patient recovery after an ischemic event.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231172520 for Quantification and prospective evaluation of serum NfL and GFAP as blood-derived biomarkers of outcome in acute ischemic stroke patients by Federica Ferrari, Daniela Rossi, Alessandra Ricciardi, Carlo Morasso, Liliana Brambilla, Sara Albasini, Renzo Vanna, Chiara Fassio, Tatjana Begenisic, Marianna Loi, Daniela Bossi, Alberto Zaliani, Elisa Alberici, Claudio Lisi, Andrea Morotti, Anna Cavallini, Federico Mazzacane, Antonio Nardone, Fabio Corsi and Marta Truffi in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors are grateful to all the patients who entered the study and to the medical staff for patients’ enrolment and management of informed consent. We are also grateful to Chiara Scarlata, Viola Chiavetta and Agostino Possenti for experimental help.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Italian Ministry of Health [EuroNanoMedIII-JTC2018-ABISens Project to Istituti Clinici Scientifici Maugeri IRCCS] and by Fondazione Collegio Ghislieri for the fellowship of FF.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article..
Authors' contributions
FF, DR, AR: investigation, data curation, writing-original draft preparation; CM: methodology, data curation, writing-review and editing; LB: investigation, data curation; SA: formal analysis; RV: methodology, conceptualization; EA: investigation; CF, TB, ML, DB, AZ: resources; CL, AM, FM: writing-review and editing; AC, AN: resources, writing-review and editing; FC: conceptualization, funding acquisition; MT: conceptualization, supervision, writing-original draft preparation. All authors read and approved the final manuscript.
ORCID iDs
Andrea Morotti https://orcid.org/0000-0002-6558-1155
Marta Truffi https://orcid.org/0000-0002-1095-4188
Supplemental material
Supplemental material for this article is available online.
References
- 1.Montellano FA, Ungethüm K, Ramiro L, et al. Role of Blood-Based biomarkers in ischemic stroke prognosis: a systematic review. Stroke 2021; 52: 543–551. [DOI] [PubMed] [Google Scholar]
- 2.Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke 2015; 46: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endres M, Dirnagl U, Moskowitz MA. The ischemic Cascade and mediators of ischemic injury. Handb Clin Neurol 2009; 92: 31–41. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 2011; Oct 26 14: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Zoppo GJ. Aging and the neurovascular unit. Ann N Y Acad Sci 2012; 1268: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steliga A, Kowiański P, Czuba E, et al. Neurovascular unit as a source of ischemic stroke biomarkers-limitations of experimental studies and perspectives for clinical application. Transl Stroke Res 2020; 11: 553–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawata K, Liu CY, Merkel SF, et al. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev 2016; 68: 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010; 28: 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol 2022; 21: 246–257. [DOI] [PubMed] [Google Scholar]
- 10.Witzel S, Frauhammer F, Steinacker P, et al. Neurofilament light and heterogeneity of disease progression in amyotrophic lateral sclerosis: development and validation of a prediction model to improve interventional trials. Transl Neurodegener 2021; 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Yang B, Wang F, et al. Association between plasma neurofilament light chain levels and cognitive function in patients with parkinson’s disease. J Neuroimmunol 2021; 358: 577662. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Geng J. Glial fibrillary acidic protein as a prognostic marker of acute ischemic stroke. Hum Exp Toxicol 2018; 37: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen A, Stanne TM, Nilsson S, et al. Circulating neurofilament light in ischemic stroke: temporal profile and outcome prediction. J Neurol 2019; 266: 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stokowska A, Bunketorp Käll L, Blomstrand C, et al. Plasma neurofilament light chain levels predict improvement in late phase after stroke. Eur J Neurol 2021; 28: 2218–2228. [DOI] [PubMed] [Google Scholar]
- 15.Uphaus T, Bittner S, Gröschel S, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke 2019; 50: 3077–3084. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Chu HJ, Hwang YT, et al. Plasma neurofilament light chain level predicts outcomes in stroke patients receiving endovascular thrombectomy. J Neuroinflammation 2021; 18: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabezas JA, Bustamante A, Giannini N, et al. Discriminative value of glial fibrillar acidic protein (GFAP) as a diagnostic tool in acute stroke. Individual patient data meta-analysis. J Investig Med 2020; 68: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Cheng YW, Chen YF, et al. Plasma neurofilament light chain and glial fibrillary acidic protein predict stroke in CADASIL. J Neuroinflammation 2020; 17: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams HP, Davis PH, Leira EC, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology 1999; 53: 126–131. [DOI] [PubMed] [Google Scholar]
- 20.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007; 38: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 21.Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry 1990; 53: 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viosca E, Martínez JL, Almagro PL, et al. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch Phys Med Rehabil 2005; 86: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 23.Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the functional independence measure. Arch Phys Med Rehabil 1994; 75: 127–132. [PubMed] [Google Scholar]
- 24.Rudilosso S, San Román L, Blasco J, et al. Evaluation of white matter hypodensities on computed tomography in stroke patients using the Fazekas score. Clin Imaging 2017; 46: 24–27. [DOI] [PubMed] [Google Scholar]
- 25.Qu Y, Tan CC, Shen XN, et al. Association of plasma neurofilament light with small vessel disease burden in nondemented elderly: a longitudinal study. Stroke 2021; 52: 896–904. [DOI] [PubMed] [Google Scholar]
- 26.Rahmig J, Akgün K, Simon E, et al. Serum neurofilament light chain levels are associated with stroke severity and functional outcome in patients undergoing endovascular therapy for large vessel occlusion. J Neurol Sci 2021; 429: 118063. [DOI] [PubMed] [Google Scholar]
- 27.Hulley SB, Cummings SR, Browner WS, et al. Designing clinical research: an epidemiologic approach. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2013. Appendix 6C, p.79. [Google Scholar]
- 28.Simrén J, Andreasson U, Gobom J, et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5-90 years. Brain Commun 2022; 4: fcac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tybirk L, Hviid CVB, Knudsen CS, et al. Serum GFAP – reference interval and preanalytical properties in Danish adults. Clin Chem Lab Med 2022; 60: 1830–1838. [DOI] [PubMed] [Google Scholar]
- 30.Yuan A, Rao MV, Nixon RA, et al. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 2017; 9: a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019; 90: 870–881. [DOI] [PubMed] [Google Scholar]
- 32.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14: 577–589. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Wang KKW. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci 2015; 38: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol 2022; 18: 158–172. [DOI] [PubMed] [Google Scholar]
- 35.Onatsu J, Vanninen R, Jäkälä P, et al. Serum neurofilament light chain concentration correlates with infarct volume but not prognosis in acute ischemic stroke. J Stroke Cerebrovasc Dis 2019; 28: 2242–2249. [DOI] [PubMed] [Google Scholar]
- 36.Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology 2018; 91: e1338–e1347. [DOI] [PubMed] [Google Scholar]
- 37.Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 2012; 58: 237–245. [DOI] [PubMed] [Google Scholar]
- 38.Wunderlich MT, Wallesch CW, Goertler M. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol 2006; 13: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 39.Undén J, Strandberg K, Malm J, et al. Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J Neurol 2009; 256: 72–77. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Chen J, Wang X, et al. Serum neurofilament light chain as a predictive biomarker for ischemic stroke outcome: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2020; 29: 104813. [DOI] [PubMed] [Google Scholar]
- 41.Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 2012; 1268: 21–25. [DOI] [PubMed] [Google Scholar]
- 42.Pekny M, Wilhelmsson U, Stokowska A, et al. Neurofilament light chain (NfL) in Blood-A biomarker predicting unfavourable outcome in the acute phase and improvement in the late phase after stroke. Cells 2021; 10: 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mielke MM, Syrjanen JA, Blennow K, et al. Comparison of variables associated with cerebrospinal fluid neurofilament, total-tau, and neurogranin. Alzheimers Dement 2019; 15: 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedet AL, Milà-Alomà M, Vrillon A, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol 2021; 78: 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Wang R, Li Y, et al. Plasma neurofilament light chain as a predictive biomarker for post-stroke cognitive impairment: a prospective cohort study. Front Aging Neurosci 2021; 13: 631738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rust R. Towards blood biomarkers for stroke patients. J Cereb Blood Flow Metab 2021; 41: 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fullerton JL, Thomas JM, Gonzalez-Trueba L, et al. Systematic review: association between circulating microRNA expression & stroke. J Cereb Blood Flow Metab 2022; 42: 935–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Huang Y, Liu Y, et al. Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J Cereb Blood Flow Metab 2021; 41: 2280–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ploughman M, Granter-Button S, Chernenko G, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res 2007; 1150: 207–216. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Detloff MR, Miller KN, et al. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol 2012; 233: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231172520 for Quantification and prospective evaluation of serum NfL and GFAP as blood-derived biomarkers of outcome in acute ischemic stroke patients by Federica Ferrari, Daniela Rossi, Alessandra Ricciardi, Carlo Morasso, Liliana Brambilla, Sara Albasini, Renzo Vanna, Chiara Fassio, Tatjana Begenisic, Marianna Loi, Daniela Bossi, Alberto Zaliani, Elisa Alberici, Claudio Lisi, Andrea Morotti, Anna Cavallini, Federico Mazzacane, Antonio Nardone, Fabio Corsi and Marta Truffi in Journal of Cerebral Blood Flow & Metabolism