Abstract
Both monocyte-derived macrophages (MDMs) and brain resident microglia participate in hematoma resolution after intracerebral hemorrhage (ICH). Here, we utilized a transgenic mouse line with enhanced green fluorescent protein (EGFP) labeled microglia (Tmem119-EGFP mice) combined with a F4/80 immunohistochemistry (a pan-macrophage marker) to visualize changes in MDMs and microglia after ICH. A murine model of ICH was used in which autologous blood was stereotactically injected into the right basal ganglia. The autologous blood was co-injected with CD47 blocking antibodies to enhance phagocytosis or clodronate liposomes for phagocyte depletion. In addition, Tmem119-EGFP mice were injected with the blood components peroxiredoxin 2 (Prx2) or thrombin. MDMs entered the brain and formed a peri-hematoma cell layer by day 3 after ICH and giant phagocytes engulfed red blood cells were found. CD47 blocking antibody increased the number of MDMs around and inside the hematoma and extended MDM phagocytic activity to day 7. Both MDMs and microglia could be diminished by clodronate liposomes. Intracerebral injection of Prx2 but not thrombin attracted MDMs into brain parenchyma. In conclusion, MDMs play an important role in phagocytosis after ICH which can be enhanced by CD47 blocking antibody, suggesting the modulation of MDMs after ICH could be a future therapeutic target.
Keywords: Intracerebral hemorrhage, microglia, monocyte-derived macrophages, mouse, peroxiredoxin 2
Introduction
Intracerebral hemorrhage (ICH) is one of the most severe types of stroke. It results in high mortality and morbidity, imposing a huge economic burden on the individual, families and society. 1 However, treatment is limited to the prevention of secondary injury by limiting the hematoma expansion, intracranial pressure management, and supportive care. 2 After blood enters the parenchyma, it forms a hematoma, causing a mechanical “mass effect” to the brain, which may compress and damage the adjacent brain tissue and increase the intracranial pressure to a point that further damage the distal brain regions. Additionally, release of neurotoxic and pro-inflammatory blood components such as thrombin as well as red blood cell degradants, like iron, and peroxiredoxin 2 (Prx2), from the hematoma leads to secondary brain damage. 3 To potentially reduce hematoma-induced injury, the STICH 4 and STICH II 5 trials examined the effects of surgical clot evacuation, but both showed a nonsignificant benefit of early surgery on long-term functional outcomes. The MISTIE III trial explored minimally invasive surgical evacuation for ICH and only found reduced mortality, but no improvement in functional outcomes compared to the conservative treatment group. 6 Therefore, greater efforts are needed to look for approaches to mitigate brain damage after ICH.
One promising strategy for speeding hematoma clearance is accelerating intrinsic hematoma resolution. Phagocytosis plays a key role in hematoma resolution and brain recovery following ICH. 7 Phagocytotic removal of the hematoma not only reduces the initial mechanical “mass effect” but it also removes erythrocytes and neurotoxic components tapering the secondary neuroinflammation, 8 which could be a potential therapeutic target for ICH. 9 Our previous studies found that enhancing phagocytosis by blocking erythrocytes surface CD47, a glycoprotein that interacts with signal regulatory protein alpha (SIRP-α) on phagocytes, can boost hematoma clearance, alleviate brain damage and improve functional outcome in both mice and rat ICH models.10,11 Those findings indicate that modulating phagocytosis and accelerating hematoma clearance can be beneficial to brain recovery after ICH and hence is an important avenue for ICH research.
Both microglia and non-microglial phagocytes are involved in hematoma clearance after brain hemorrhage. 12 Microglia, which account for approximately 10% of the brain cells, are resident macrophages that play important roles in brain development and homeostasis and are the predominant brain cells responding to various pathological changes in the brain.13,14 However, besides microglia, other phagocytes contribute to neuro-inflammation, brain injury and recovery after ICH, including monocyte-derived macrophages (MDMs). 15 Differences between microglia and MDM responses after ICH are difficult to investigate due to lack of ideal cell-specific markers. 16 Previously, we have utilized a transgenic mouse line17 that has an enhanced green fluorescent protein (EGFP) gene inserted into the stop codon of the microglia-specific transmembrane protein 119 (Tmem 119) 18 locus to visualize changes in microglia after ICH. The EGFP-Tmem119 mouse line has a highly efficient and specific expression of EGFP in microglia. We found the number of perihematomal microglia decreased 1 day after ICH, while markedly increasing in number and being highly activated by day 3 and day 7. 19
To compare the unique characteristics of microglia and MDMs after ICH, the current study utilized the Tmem119-EGFP mice combined with F4/80 immunohistochemistry (a pan-macrophage marker) to distinguish brain resident microglia and MDMs, as well as studying changes after ICH and injection of blood components in mice. This study also focused on changes of microglia vs. MDMs after injection of clodronate liposomes to induce phagocyte depletion and CD47 blockade to enhance phagocytosis after ICH. Understanding these differences may lead to a potential therapeutic target to enhance hematoma clearance.
Methods
The data supporting this study’s findings are available from the corresponding authors, F.Y., and G.X., upon reasonable request.
Animals and ICH model
All the animal studies were approved by the University of Michigan Committee on the Use and Care of Animals and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, (8th edition, revised 2011). The study followed the ARRIVE guidelines for reporting in vivo animal experiments. 20 In total, 75 male Tmem119-EGFP (C57BL/6-Tmem119em2Gfng/J) mice (The Jackson Laboratory, strain #: 031823) aged of 2–4 months were utilized in this study. The ICH modeling was performed as described previously. 10 In brief, mice were anesthetized with ketamine (80 mg/kg) and xylazine (5 mg/kg) intraperitoneally, and carprofen (5 mg/kg subcutaneously) was used as an analgesic. A feedback heated blanket was used to keep the animal core body temperature at 37°C during surgical procedures. Autologous arterial blood for intracerebral injection was collected individually from the right femoral artery. For intracerebral injection, mice were positioned in a stereotaxic frame (Kopf Instruments), a 5 mm midline scalp incision was made, and a 1 mm cranial burr hole was drilled (0.6 mm anterior, 2.5 mm lateral to bregma). A 26-gauge needle was inserted through the burr hole (3.5 mm depth) for the injection of 30 µl autologous arterial blood, rat Prx2 (10 µL, 1 mg/ml; Novus Biological; NBP2–52150) or rat thrombin in saline (0.5 U, 10 µl, Sigma, T5772, n = 6) into the right basal ganglia. The injection was performed with a micro-infusion pump (Harvard Apparatus Inc.) over a 10 min period, and the needle was left in place for another 5 min to prevent reflux. Sham surgeries omitted the injection. Randomization was carried out using random numbers or odd and even numbers. Two mice died after ICH in this study, no mice died in the Prx2, thrombin group or sham groups. One animal was excluded for small hematoma because of intra-ventricular hemorrhage extension. Mice were euthanized at days 1, 3, and 7after ICH, and their harvested brains fixed in 4% paraformaldehyde. Sham surgeries, thrombin and Prx2 injection mice were euthanized on day 1.
Experimental groups
This study had four parts. In the first part, mice received an intracerebral injection of 30 µL autologous arterial blood into the right basal ganglia (ICH group) and the sham group underwent needle insertion and was used as control. The ICH mice were euthanized on days 1, 3, and 7 (n = 6 for each group). Sham group mice were euthanized on day 1 (n = 6). In the second part, mice received an intracerebral injection of 30 µL autologous blood mixed with functional grade anti-CD47 antibody (Clone: B6H12, Invitrogen 16-0479-85, 10 µg/mL) or mouse IgG1 isotype control (Invitrogen, 16-4714-82, 10 µg/mL). Mice were euthanized at days 3 or 7 (n = 6 for each group). In the third part, mice received an intracerebral injection of 30 µl autologous blood mixed with 5 µL clodronate liposomes (FormuMax, F70101C-A) or control liposomes (FormuMax, F70101-A). Mice were euthanized on day 3 (n = 6 for each group). In the fourth part, rat Prx2 10 µL (n = 6) or rat thrombin 10 µL (n = 6) was injected into the basal ganglia. Mice were euthanized 1 day after injection. The brains were harvested for histology.
Immunofluorescence staining
The protocols used for immunofluorescence staining were as described previously. 21 Mice were euthanized with a fatal dose of pentobarbital (100 mg/kg, intraperitoneally) and underwent trans-cardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, PH = 7.4). The mice brains were then harvested and immersed in 4% paraformaldehyde for 24 hours before transferal to 30% sucrose in 0.1 M PBS at 4°C until they sank to the bottom. Brains were embedded before being sectioned coronally (18 µm) on a cryostat (Thermo Fisher Scientific). Cryosections were observed with or without immunofluorescence staining. The primary antibodies were rat anti-F4/80 antibody (1:200, abcam, ab6640), rabbit anti-DARPP-32 antibody (1:500, Cell Signaling, 2306), rabbit anti-HEXB antibody (1:100, Proteintech, 16229-1-AP), rat anti-Sall1 antibody (1:100, Invitrogen, 14-9729-82), rabbit anti-GFP antibody (1:1000, Invitrogen, A11122 or 1:500, Aves labs, GFP-1020). Secondary antibodies were Alexa Fluor 594 donkey anti-rat IgG (H + L) (1:500; Invitrogen, A-21209), Alexa Fluor 488 donkey anti-rabbit IgG (H + L) (1:500; Invitrogen, A-21206).
Cell counting
Cell counting was conducted on coronal brain sections by 2 blinded researchers. The number of EGFP-positive cells was calculated. Brain slices from bregma 0.20 mm were used for cell counting. The hematoma core was defined as the area within the hematoma border. The perihematomal area was defined as the area within 250 µm of the hematoma border. The ipsilateral basal ganglia (BG) area was selected from ipsilateral BG beyond 250 µm from the hematoma border. The contralateral BG was the selected area in the contralateral BG corresponding to the hematoma. Cells having diameters over 20 µm were considered giant cells. In the Prx2 and thrombin injection group, area of interest was within 250 µm of the needle tract. On each slice, three high-power images (x40 magnification) were taken by a digital camera from each pre-designated target area to minimalize selection bias. All measurements were repeated three times, and a mean value was calculated.
Power analysis
Based on our prior study, 19 we chose a sample size of 6 animals per group to provide 90% power to demonstrate a 40% change in microglia number.
Data and statistical analysis
All data are described as means ± standard deviation (SD) or median (quartile 1, quartile 3). The normality of the data was assessed using Kolmogorov-Smirnov tests. When assessing data between two groups non-paired two-tailed Student’s t-tests or Mann-Whitney tests were used. One-way ANOVA with a Tukey post hoc test with effect estimates with a 95% confidence interval was used for multiple group comparisons. Significance levels were set at p < 0.05. Statistics were performed using Prism.
Results
MDM Infiltration and phagocytosis after ICH
F4/80, a monocyte-macrophage marker was used to identify all phagocytes including monocytes, macrophages as well as microglia. Cells that were F4/80 positive but GFP negative (F4/80(+)GFP(−)) were considered to be non-microglia macrophages, most likely MDMs. Here we observed the natural history of MDMs infiltration in the mouse model of ICH. The robust and specific expression of EGFP in microglia 3 days after ICH was confirmed by co-staining with microglia specific markers Hexb and Sall1 (supplement Figure 1). An absence of peri-hematomal cells that were Hexb(+) or Sall1(+) but GFP(−) also indicates that the observed F4/80(+)GFP(−) cells were unlikely to be microglia that had stopped expressing GFP due to Tmem119 downregulation. Such cells would have to had to down regulate all three microglial markers (Tmem119, Hexb and Sall1). Masuda et al. 22 particularly identified HexB as being stably expressed in brain pathology.
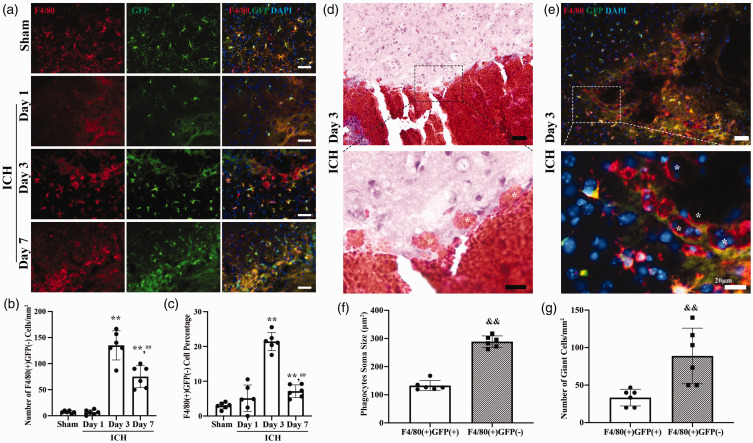
Figure 1.
Monocyte-derived macrophages (MDMs) infiltrate the brain and form a cell layer after intracerebral hemorrhage (ICH); (a) Representative image of GFP (green fluorescent protein; green) and F4/80 (red) double staining in the perihematomal area in posthemorrhagic Tmem119-EGFP mice from day 1, day 3, day 7 and sham group mice. Sham mice received a needle insertion. Scale bars are 50 µm. (b) Quantification data on the number of perihematomal F4/80(+)GFP(−) cells (MDMs). (c) Quantification data on the percentage of perihematomal F4/80(+)GFP(−) cells. (d) Representative hematoxylin and eosin stained image of giant cells engulfed red blood cells (indicated with *) around hematoma. High magnification images correspond to the hematoma border. Scale bar is 50 µm in the upper panel, 20 µm in the lower panel. (e) Representative image of MDMs gathering around hematoma and forming giant phagocytes. High magnification images correspond to the hematoma border. * indicates F4/80(+)GFP(−) cells. Scale bar is 50 µm in the upper panel, 20 µm in the lower panel. (f) Quantification data on soma size of perihematomal F4/80(+)GFP(+) and F4/80(+)GFP(−) cells and (g) Quantification data on number of giant cells in perihematomal area. **P < 0.001 vs the sham group, ##P < 0.001 vs 3 days. &&P < 0.001 vs the F4/80(+)GFP(+) cells. Values are means ± SD or median (quartile 1, quartile 3); n = 6 per group.
The number of F4/80(+)GFP(−) cells was minimal in sham brains (7 ± 3 cells/mm2) and in post-hemorrhage brains at day 1 (7 ± 5 cells/mm2, n = 6 per group, Figure 1(a) and (b)). However, at post hemorrhage day 3, a layer of F4/80(+)GFP(−) cells gathered just immediately around the hematoma forming a noticeable hematoma margin (Figure 1(d)). Thus, the number of perihematomal MDMs was significantly increased by day 3 (135 ± 28 cells/mm2, n = 6, p < 0.001 vs. Sham group). F4/80(+)GFP(−) cells were also found in the hematoma core and ipsilateral BG. At post hemorrhage day 7, the number of MDMs was still increased compared to baseline (75 ± 21 cells/mm2, n = 6, p < 0.001 vs. Sham group), but less than that at day 3 (p < 0.001 vs. ICH day 3, Figure 1(a) and (b)). In addition, MDMs as a percentage of all F4/80(+) cells changed with time after ICH. A significant increase was found at post hemorrhage day 3 (21.4 ± 2.6 vs. 3.0 ± 0.9% in sham group and 5.1 ± 3.8% at ICH day 1, n = 6, p < 0.001), but it dropped markedly by day 7 post ICH (7.1 ± 1.8%, n = 6, p < 0.001 vs. ICH day 3, Figure 1(a) and (c)).
The morphology of phagocytes in the perihematomal area was distinct between microglia and MDMs after ICH. Microglia are the most abundant resident immune cells of the brain, 13 and when reacting to stress, their morphology changes accordingly. 23 On hematoxylin and eosin-stained tissue samples three days after ICH, we identified a group of giant cells engulfed red blood cells surrounding the hematoma (Figure 1(d)). Giant cells were also found with F4/80 and GFP co-staining and were F4/80 positive (Figure 1(e)). We defined those cells with diameters over 20 µm as giant cells, which are likely macrophages/microglia, as previously described. 24 Similar giant cells are also found in human spontaneous ICH. 25 Most perihematomal microglia were bushy with a round enlarged cell body and processes, a small group of microglia were amoeboid-like. In contrast, most of the perihematomal MDMs were disc-like giant cells. The size of MDMs was significantly larger than the microglia (289 ± 20 vs. 133 ± 18 µm2 for microglia, n = 6 per group, p < 0.001, Figure 1(e) and (f)). The number of giant cells that originated from MDMs (F4/80(+)GFP(−)) was almost three times higher than the number that originated from microglia (F4/80(+)GFP(+)) (89 ± 37 vs. 33 ± 11/mm2 for microglia, n = 6 per group, p = 0.0056, Figure 1(e) and (g)).
CD47 blocking antibody enhanced the number and persistence of perihematomal MDMs
We assessed the effect of a CD47 blocking antibody on MDMs after ICH by co-injecting either CD47 blocking antibody or control IgG with autologous blood in the Tmem119-EGFP mice. CD47 blocking antibody was found previously to enhance microglia/macrophage activation in and around the hematoma hence reducing ICH-induced brain injury.10,11 Three days after ICH, there was a significant increase in the number of perihematomal MDMs in the anti-CD47 group (ICH+anti-CD47 group) compared to the IgG group (ICH + IgG group) (164 ± 29 vs. 113 ± 32 cells/mm2 in ICH + IgG group, n = 6 per group, p = 0.0149, Figure 2(a) and (c)). There was no difference in the number of MDMs present within the hematoma core (18 ± 22 vs. 8 ± 10 cells/mm2 in ICH + IgG group, n = 6 per group, p > 0.05, Figure 2(a) and (b)) or ipsilateral BG (19 ± 21 vs. 11 ± 7 cells/mm2 in ICH + IgG group, n = 6 per group, p > 0.05, Figure 2(a) and (d)). Additionally there was no statistically significant difference in the percentage of MDMs among all F4/80 positive phagocytes (hematoma core, 34.1 (6.8, 49.6) vs. 10.8 (0.0, 53.1) % in ICH + IgG group; perihematomal, 21.6 ± 5.0 vs. 21.5 ± 4.8% in ICH + IgG group; Ipsilateral BG, 3.6 ± 4.0 vs. 3.0 ± 2.3% in ICH + IgG group, n = 6 per group, p > 0.05 in all three areas, Figure 2(a) to (d)).
Figure 2.
CD47 blocking antibody increases the number of monocyte-derived macrophages (MDMs) 3 days after intracerebral hemorrhage (ICH); (a) Representative image of GFP (green fluorescent protein; green) and F4/80 (red) double staining in Tmem119-EGFP mice in ICH+anti-CD47 group and ICH + IgG group 3 days post hemorrhage. Regions of interest were the hematoma core, peri-hematoma, and ipsilateral basal ganglia (BG) areas. (b) Quantification data on the number and percentage of hematoma core F4/80(+)GFP(−) cells. (c) Quantification data on the number and percentage of perihematomal F4/80(+)GFP(−) cells and (d) Quantification data on the number and percentage of ipsilateral BG F4/80(+)GFP(−) cells. *P < 0.05 vs the ICH + IgG group. Scale bars are 50 µm. Values are means ± SD or median (quartile 1, quartile 3); n = 6 per group.
Interestingly, at post-hemorrhage day 7, there was a 3 fold increase in the number of MDMs in the hematoma core in the anti-CD47 group (90 (73, 152) vs. 28 (9, 83) cells/mm2 in ICH + IgG group, n = 6 per group, p = 0.0368, Figure 3(a) and (b)), and a significant increase in the number of perihematomal MDMs compared to controls (194 ± 87 vs. 71 ± 25 cells/mm2 in ICH + IgG group, p = 0.0074, Figure 3(a) and (c)). Notably, the number of perihematomal MDMs in the anti-CD47 treatment group did not follow the natural history of descending trend from day 3 to day 7 like the number changes in the ICH + IgG group. In contrast, there was a slight non-significant increase of the perihematomal MDMs from day 3 to day 7 with CD47 treatment (164 ± 29 vs. 194 ± 87 cells/mm2, ICH+CD47 day 3 vs. day7, n = 6 per group, p > 0.05). No significant difference in the number of MDMs was found in the ipsilateral BG (25 ± 26 vs. 8 ± 5 cells/mm2 in ICH + IgG group, n = 6 per group, p > 0.05, Figure 3(a) and (d)). The percentage of peri-hematoma MDM among all phagocytes was significantly higher post hemorrhage day 7 anti-CD47 group compared to IgG control (12.7 ± 5.6 vs. 6.1 ± 2.0% in ICH + IgG group, p = 0.0210, Figure 3(a) and (c)). The percentage of the MDMs was similar in the hematoma core (32.9 ± 15.5 vs. 39.4 ± 26.0% in ICH + IgG group, p > 0.05, Figure 3(a) and (b)) and the ipsilateral BG (3.0 ± 2.6 vs. 1.3 ± 0.9% in ICH + IgG group, p > 0.05, Figure 3(a) and (d)).
Figure 3.
CD47 blocking antibody increases the number and extends the duration of phagocytic activity of monocyte-derived macrophages (MDMs) 7 days after intracerebral hemorrhage (ICH); (a) Representative image of GFP (green fluorescent protein; green) and F4/80 (red) double staining in Tmem119-EGFP mice in ICH+anti-CD47 group and ICH + IgG group 7 days post hemorrhage. Regions of interest were the hematoma core, peri-hematoma, and ipsilateral basal ganglia (BG) areas. (b) Quantification data on the number and percentage of hematoma core F4/80(+)GFP(−) cells. (c) Quantification data on the number and percentage of perihematomal F4/80(+)GFP(−) cells and (d) Quantification data on the number and percentage of ipsilateral BG F4/80(+)GFP(−) cells. *P < 0.05 vs the ICH + IgG group. **P < 0.001 vs the ICH + IgG group. Scale bars are 50 µm. Values are means ± SD or median (quartile 1, quartile 3); n = 6 per group.
CD47 blocking antibody increased the number of microglia
Green fluorescence positive microglia were used to observe microglia response to ICH in different regions of the BG according to the hematoma border. Examining the brains 3 days after ICH, we found a significant increase in microglia in the perihematomal area (571 ± 40 vs. 439 ± 62 cells/mm2 in ICH + IgG group, n = 6 per group, p = 0.0015, Figure 4(a) and (b), as well as in ipsilateral BG area (389 ± 31 vs. 336 ± 46 cells/mm2 in ICH + IgG group, p = 0.0434, Figure 4(a) and (b)) with CD47 blocking antibody. However, there was no difference in microglia number in the hematoma core (34 ± 17 vs. 46 ± 23 cells/mm2 in ICH + IgG group, p > 0.05, Figure 4(a) and (b)) or the contralateral BG (198 (193, 225) vs. 193(188, 205) cells/mm2, in ICH + IgG group, p > 0.05, Figure 4(a) and (b)) between two groups.
Figure 4.
CD47 blocking antibody increases the number of microglia in ipsilateral basal ganglia. (a) Representative native EGFP (enhanced green fluorescent protein; green) fluorescence images of different brain regions from a coronal section of Tmem119-EGFP in ICH+anti-CD47 group and ICH + IgG group 3 days and 7 days post hemorrhage. Regions of interest were hematoma core, peri-hematoma, ipsilateral basal ganglia (BG) areas, and contralateral BG. (b) Quantification data on the number of GFP(+) cells in different brain regions 3 days after hemorrhage and (c) Quantification data on the number of GFP(+) cells in different brain regions 7 days after hemorrhage. *P < 0.05 vs the ICH + IgG group. **P < 0.001 vs the ICH + IgG group. Scale bars are 50 µm. Values are means ± SD or median (quartile 1, quartile 3); n = 6 per group.
The impact of CD47 blocking antibody on microglia was more prominent by day 7 after ICH. A significant increase of microglia was observed in the hematoma core (309 ± 193 vs. 122 ± 46 cells/mm2 in ICH + IgG group, p = 0.0432, Figure 4(a) and (c)), and the perihematomal area (1349 ± 162 vs. 1009 ± 153 cells/mm2 in ICH + IgG group, p = 0.0039, Figure 4(a) and (c)), but not the ipsilateral BG (664 ± 121 vs. 579 ± 115 cells/mm2 in ICH + IgG group, p > 0.05, Figure 4(a) and (c)) or contralateral BG(193 ± 10 vs. 188 ± 12 cells/mm2 in ICH + IgG group, p > 0.05, Figure 4(a) and (c)).
CD47 blocking antibody increased the number of giant cells and soma size of microglia
We investigated the effect of CD47 blocking antibody on F4/80 positive giant cells, which we believe have stronger phagocytotic function. Our results showed that co-injection of CD47 blocking antibody significantly increased the number of perihematomal giant cells (122 ± 29 vs. 90 ± 19 cells/mm2 in the ICH + IgG group, p = 0.0483, Figure 5(a) to (c)). Additionally, the percentage of microglia in giant cells was doubled in the CD47 blocking antibody group compared to the IgG group (60 ± 19 vs. 33 ± 15% in the ICH + IgG group, p = 0.0196, Figure 5(a), (b) and (d)). We also compared the soma size of the cells in the two subgroups and found that the soma size of MDMs in the ICH+CD47 group was similar to the ICH + IgG group (290 ± 70 vs. 244 ± 68 µm2 for ICH + IgG, n = 6 per group, p > 0.05, Figure 5(a), (b) and (e)). However, the average soma size of microglia was significantly increased in the CD47 antibody group compared to the microglia in the IgG group (162 ± 19 vs. 103 ± 19 µm2 for ICH + IgG, n = 6 per group, p < 0.001, Figure 5(a), (b) and (e)), while still smaller than the MDMs in the ICH+CD47 group (p = 0.0015).
Figure 5.
CD 47 blocking antibody increased the number of giant cells and phagocytosis activity of microglia 3 days after intracerebral hemorrhage (ICH); (a) Representative hematoxylin and eosin stained image of giant cells engulfed red blood cells around the hematoma in ICH + IgG group and ICH+anti-CD47 group. Scale bar is 20 µm. (b) Representative image of GFP (green fluorescent protein; green) and F4/80 (red) double staining. * indicates F4/80(+)GFP(−) giant cells. # indicates F4/80(+)GFP(+) giant cells. Scale bar is 20 µm. (c) Quantification data on number of giant cells in perihematomal area. (d) Quantification data on percentage of F4/80(+)GFP(−) cell in giant cells in perihematomal area and (e) Quantification data on soma size of perihematomal F4/80(+)GFP(+) and F4/80(+)GFP(−) cells. *P < 0.05 vs the ICH + IgG group. Values are means ± SD; n = 6 per group.
Clodronate liposomes depleted both infiltrating MDMs and microglia and aggravated neuronal death after ICH
To examine whether infiltrating MDMs and microglia have phagocytic ability, clodronate liposomes co-injection with blood was used to induce the death of phagocytes that ingested the compound. 26 Three days after ICH, the number of MDMs in the clodronate liposomes group was reduced in all 3 ipsilateral areas compared to the control liposomes group. There was almost full MDM depletion in the hematoma core (0 (0, 8) vs. 18 (10, 22) cells/mm2 in the control group, n = 6 per group, p = 0.0346, Figure 6(a) and (b)), and a 77% decrease of perihematomal MDMs (33 (18, 62) vs. 142 (111, 170) cells/mm2 in control liposomes group, p = 0.0108, Figure 6(a) and (c)), as well as a near full depletion of MDMs in the ipsilateral BG area(0 (0, 13) vs. 8 (6, 24) cells/mm2 in control group, n = 6 per group, p > 0.05, Figure 6(a) and (d)).
Figure 6.
Clodronate liposomes deplete infiltrating monocyte-derived macrophages (MDMs) and microglia after intracerebral hemorrhage (ICH); (a) Representative image of GFP (green fluorescent protein; green) and F4/80 (red) double staining in Tmem119-EGFP mice in ICH+Clodonate group and ICH+Control group 3 days post hemorrhage. Regions of interest were the hematoma core, peri-hematoma, and ipsilateral basal ganglia (BG) areas. (b) Quantification data on the number of hematoma core F4/80(+)GFP(−) cells and F4/80(+)GFP(+) cells. (c) Quantification data on the number of perihematomal F4/80(+)GFP(−) cells and F4/80(+)GFP(+) cells. (d) Quantification data on the number of ipsilateral BG F4/80(+)GFP(−) cells and F4/80(+)GFP(+) cells. (e) Representative image of DARPP-32 (red) immunoreacitivity in Tmem119-EGFP mice of ICH+Clodonate group and ICH+Control group 3 days post hemorrhage and (f) Quantification data of neuronal loss of ipsilateral BG. *P < 0.05 vs the ICH + IgG group. **P < 0.001 vs the ICH+Control group. Scale bars are 50 μm. Values are means ± SD or median (quartile 1, quartile 3); n = 6 per group.
Clodronate liposomes treatment also depleted microglia compared to the control liposomes treatment group. Thus, the clodronate liposomes depleted almost all the microglia in the hematoma core (0 (0, 8) vs. 45 (34, 75) cells/mm2, in ICH+Control group, n = 6 per group, p = 0.0022, Figure 6(a) and (b)), 80% of microglia in the perihematomal area (90 ± 47 vs. 459 ± 98 cells/mm2 in ICH+Control group, p < 0.001, Figure 6(a) and (c)), as well as 68% of the microglia in the ipsilateral BG area (114 ± 47 vs. 352 ± 51 cells/mm2 in ICH+Control group, p < 0.001, Figure 6(a) and (d)). DARPP-32 is a specific neuronal marker in the basal ganglia. Clodronate liposomes aggravated neuronal loss (24.4 ± 3.3 vs. 16.6 ± 2.4% in control liposome group, p = 0.001, Figure 6(e) and (f)) at day 3 post ICH.
Prx2 but not thrombin contributed to MDM infiltration after ICH
To investigate the mechanisms underlying MDM infiltration after ICH, we injected Prx2 (a protein release on red blood cell lysis) into the BG of the Tmem119-EGFP mice. One day after Prx2 injection, there was a significant increase in both microglia (507 ± 109 vs. 211 ± 15 cells/mm2 in contralateral side, p < 0.001, Figure 7(a) and (c)) and MDMs (356 ± 101 vs. 11 ± 4 cells/mm2 in contralateral side, p < 0.001, Figure 7(a) and (c)) around the injection site. While the thrombin injection caused a significant increase in the number of microglia (314 ± 43 vs. 205 ± 10 cells/mm2 in contralateral BG, p < 0.001, Figure 7(b) and (d)), there was no increase in MDMs (15 ± 8 vs. 8 ± 2 cells/mm2 in contralateral BG, p > 0.05, Figure 7(b) and (d)) around the injection site. The number of microglia and MDMs in contralateral BG in both groups were similar to those in the sham group
Figure 7.
Peroxiredoxin 2 (Prx2) but not thrombin induces the infiltration of monocyte-derived macrophages (MDMs). (a) Representative images of GFP (green fluorescent protein; green) and F4/80 (red) double staining in Tmem119-EGFP mice 1 day after intracerebral Prx2 injection. * indicates F4/80(+)GFP(−) cells. (b) Representative images of GFP (green) and F4/80 (red) double staining in Tmem119-EGFP mice 1 day after fluorescent thrombin injection. (c) Quantification data on the number of F4/80(+)GFP(+) cells and F4/80(+)GFP(−) cells 1 day after Prx2 injection and (d) Quantification data on the number of F4/80(+)GFP(+) cells and F4/80(+)GFP(−) cells 1 day after thrombin injection. **P < 0.001 vs. contralateral side. Dash line indicates the mean value of the sham animal. Values are means ± SD; n = 6 per group. Scale bars are 50 µm in the 2 upper panels, 20 μm in the lower panel.
Discussion
This study described the distinct characteristic of MDMs and microglia after ICH in a mouse model: 1) In a mouse ICH model, MDMs infiltrated the brain and formed a layer of cells adjacent to the hematoma by day 3, many of which presented as giant cells engorged with erythrocytes, while the number of MDMs decreased from day 3 to day 7; 2) CD47 blocking antibody increased the number of MDMs around and inside the hematoma and extended the phagocytosis activity of MDMs up until day 7; CD 47 blocking antibody also increased the number and soma size of microglia around the hematoma at day 3 and day 7; 3) Clodronate liposomes substantially depleted MDMs and microglia in and around the hematoma and aggravate neuronal loss by day 3 after hemorrhage; 4) Intracerebral injection of Prx2 but not thrombin, attracted MDMs into the brain parenchyma.
Brain phagocytes mediate the internalization and defense against pathogens in brain pathological conditions. Apart from the innate immune response, phagocytes play important roles in tissue remodeling and brain recovery.14,27,28 Monocytes, representing 4% of mice and 10% of human blood nucleated cells, have distinct roles in tissue homeostasis, and contribute to different pathologies. Recent evidence indicates that monocytes do not substantially contribute to microglia populations in the steady state.29,30 The blood-brain barrier normally limits peripheral leukocytes, including monocytes, entering the central nervous system. However, in the setting of brain injury and blood-brain barrier disruption, monocytes can infiltrate into the parenchyma and become MDMs. Researchers have also found that the acceleration of hematoma clearance is beneficial to brain recovery after both ICH and IVH in rodent models.10,31 Hence, a better understanding of the phagocytosis and phagocytic cells after ICH could facilitate the development of novel therapeutic approaches to ICH as well as other forms of brain hemorrhage.
In our previous study, 19 we were able to detect an enhanced green fluorescent signal in perihematomal microglia at day 3 and day 7 after experimental ICH in Tmem119-EGFP mice, along with an increased number of microglia. Similarly, microglia can be found with enhanced fluorescence signals after intracerebral thrombin injection. The current study also found a strong green fluorescent signal 3 days after ICH in Tmem119-EGFP mice (Figure 1). Another study found that Tmem119-tdTomato reporter mice exposed to laser injury showed robust activation of Tmem119-tdTomato positive microglia. 32 Additionally, Mercurio et al. 33 reported an upregulation of Tmem119 gene expression in frozen brain sections after traumatic brain injury (Day 7), along with increased microglial activation. However, Tmem119 downregulation has also been reported after isolation by fluorescence-activated cell sorting; reduced Tmem119 mRNA levels (50%) in CD11b+CD45Int microglia were found on day 1 post-ICH, though this was still significantly higher than the expression of Tmem119 in sham-operated mice and after 3 days of culture, there is a self-recovery of Tmem119 mRNA expression in microglia. 34 Masuda et al., using single-cell sequencing, also found the Tmem119 gene is downregulated in several models of neurodegeneration and demyelination. 22 Taken together, although Tmem119 gene regulation in microglia in activation and disease needs further clarification, it may differ between diseases and the time course of disease.
To further rule out misidentified MDMs due to false negative microglia, we co-stained microglia-specific markers 22 Hexb and Sall1 in the Tmem119-EGFP mice after ICH. Masuda et al. 22 reported that Hexb expression was unaffected by brain pathology. We found these two other microglia markers colocalized with GFP in showing activated microglia in the perihematomal area (supplement figure 1). We rarely identified Hexb(+) or Sall1(+) cells that were GFP(−) indicating that the EGFP-Tmem119 mouse is a useful tool for identifying microglia after ICH (at 3 days) and that GFP(−) cells likely are MDMs.
According to the data presented in the current study, both microglia and MDMs have the phagocytosis capacity in ICH, with evidence being the increased number and morphology change seen on day 3 and day 7, along with the depletion for both cell groups on day 3 by clodronate liposomes induced death of cells that ingested the compound. 26 However, it remains unclear how the depletion of MDMs and microglia with clodronate liposomes affects brain hemorrhage. Our study revealed a significant reduction in perihematomal MDMs and microglia in the ipsilateral basal ganglia three days after hemorrhage. Moreover, clodronate drastically reduced perihematomal giant cells. The aggravation of neuronal loss at day 3 post ICH by clodronate liposomes suggests that the phagocytosis activity of MDMs and microglia plays a critical role in brain injury after ICH and may allow the design of appropriate therapeutic strategies.
The difference between MDMs and microglia has been discussed in several brain pathologies including multiple sclerosis, 35 Alzheimer's disease, 36 ischemic stroke, 37 and brain hemorrhage. 38 Most of those studies used cytometry-based assays to sort out the two groups and subsequently assess the distinct characteristics of MDMs and microglia. Tmem119 is a cell-surface protein with unknown functions and it is a highly expressed microglia-specific marker in both mice and human. 18 Here, we utilized the Tmem119-EFGP mice line which has a robust green fluorescent gene labeling of microglia with an intact endogenous expression of the Tmem119 gene. 17 Previously, we have used the Tmem119-EFGP mice to demonstrate the microglia death after ICH by day 1 and proliferation by days 3 and 7 as a natural history of microglia response to ICH. 19 In this study, we focused on the changes of MDMs versus microglia and, in particular, their phagocytic activity, as well as their response to phagocyte depletion with clodronate liposomes and phagocytic enhancement by CD47 blocking antibody. With the microglia identified with gene editing, the MDMs population can subsequently be established with a generalized macrophage marker (such as F4/80). In this study, MDMs in the brain sections were defined as those F4/80 positive cells without green fluorescence (F4/80(+)GFP(−) cells). Therefore, our finding is a good add-on to the existing understanding of MDMs and microglia, and provides unique insights into MDM activation and modulation after ICH.
Using this technique to separate MDMs and microglia, we were able to identify a layer of phagocytes in the perihematomal region which were predominantly giant cells. The formation of giant cells has been reported in ICH 24 and other CNS diseases such as viral encephalitis and amyotrophic lateral sclerosis. 39 We previously found that giant cells might enhance hematoma clearance and have greater phagocytic capacity. 24 In this study, we found that the MDMs have a larger soma size and unique tissue distribution, and that the number of MDMs peaked around day 3 with the formation of giant cells and then decreased dramatically on day 7. Moreover, we observed a high phagocytosis activity in these giant cells, as indicated by their soma size and the amount of red blood cell inclusion. Chang and colleagues utilized flow cytometry to identify phagocytes that exhibited PKH-26-erythrocyte signal as cells with erythrophagocytosis ability. 38 Therefore, we recognize giant phagocytes that have engulfed red blood cells as having even greater phagocytic ability. We also confirmed the presence of similar giant cells through F4/80 and GFP co-staining. Our data suggest that the MDMs might have a greater phagocytosis ability than microglia, as the number of giant cells that originated from MDMs (F4/80(+)GFP(−)) was almost three times higher than the number that originated from microglia (F4/80(+)GFP(+)).
With the CD47 blocking antibody co-injection, we observed an increase in the number of MDMs around the hematoma on day 3, which persisted up until day 7. The results showed that co-injection of CD47 blocking antibody increased the number of perihematomal giant cells and the percentage of microglia that were giant cells. Additionally, the overall soma size of microglia in the CD47 antibody group was significantly increased compared to microglia in the IgG group, but they were still smaller than the MDMs in the ICH+CD47 group. These findings suggest that CD47 blocking antibody enhances the phagocytotic function of MDMs and microglia, potentially leading to better clearance of debris and promoting tissue repair following ICH. According to previous studies, accelerating hematoma clearance by microglia/MDMs phagocytosis in ICH benefits functional outcome.11,40,41 In a mouse ICH model, our previous study demonstrated that CD47 blocking antibody could accelerate hematoma clearance and reduce ICH-induced brain swelling, neuronal loss, and functional deficits. 10 Similarly, benefits with a CD47 blocking antibody treatment were found in a rat ICH model. 11 Taken together, these findings suggest MDMs (as well as microglia) may be a key participant in phagocytic removal of the blood clot and its natural history may be manipulated by phagocytic enhancement, suggesting a future cell-specific therapeutic strategy for ICH.
However, the role of MDMs in brain injury and brain recovery after ICH is controversial. Some previous studies demonstrate that enhancement of the erythrophagocytosis promptly can promote brain recovery after ICH,11,40,41 and MDMs rather than microglia are the main phagocyte in the erythrophagocytosis. 38 Therefore, pharmacologic targets to enhance phagocytosis after ICH have been sought after. One such example is bexarotene, which increases macrophage expression of phagocytosis receptors Axl and CD36, thus boosting erythrophagocytosis both in vitro and in vivo models of ICH, 40 indicating the enhancement of MDMs recruitment might be beneficial. However, the influx of myelomonocytic cells caused severe neuroinflammation leading to brain edema, and prolonged blockage of MDMs with anti-adhesion molecule therapy attenuated brain edema, but also promoted neuronal damage and fibrosis by interfering with vascular repair. 42 A human study also found an independent association between higher absolute monocyte count at admission and 30-day fatality in non-traumatic ICH. 43 Thus, further investigation is needed to better understand the role of MDMs after ICH.
The underlying regulation of monocyte recruitment warrants further investigation. It has been demonstrated that ICH can modulate bone marrow cells that restrain distal brain inflammation, which could be a self-protective interaction. 44 One possible mechanism involves the CC-chemokine ligand 2 and its receptor. 45 Depletion of monocytes using a CCR2-specific antibody is neuro-protective in an experimental autoimmune encephalomyelitis model. 45 In addition, the interleukin (IL)-4)/signal transducer and activator of transcription 6 (STAT6) axis was found to play an important role in modulation of microglia/macrophage function after ICH. 46
After ICH, lysis of red blood cells is associated with neuronal death, brain edema, and neurological deficits. 47 Peroxiredoxin-2 (PRX-2) is one of the six members of the peroxiredoxin family, and the third most abundant protein in red blood cells. 48 It has been reported that PRX-2 release into parenchyma is involved in the progression of brain injury after ICH by initiating neuroinflammation in both rat 49 and mouse 50 ICH models. Since PRX-2 levels in erythrocytes are very high (5.6 mg/ml), 51 30 µl of blood with a hematocrit of 42% could potentially release 71 µg PRX-2. Neurons could also release PRX-2 after injury. To better mimic the gradual development of hemolysis after ICH, 10 µg PRX-2 was injected into the basal ganglia in this study, which was also used in a previous mouse study. 50 That study showed significant neutrophil infiltration and microglia/macrophage activation, as well as brain injury.
Thrombin, a key component in the clotting cascade, is increased in brain parenchyma after ICH. 52 This increase is primarily due to prothrombin entry from blood, either with a hemorrhage or following blood-brain barrier disruption. Thrombin is believed to play a major role in brain pathology following hemorrhagic stroke, leading to brain edema, neuronal death, and deterioration of functional outcome. 52 Gong et al. 53 measured thrombin activity in the ipsilateral basal ganglia after a striatal 100 µl blood injection in rats and found thrombin activities of 3.3 and 2.4 U/g at 1 and 24 hours after ICH, compared to 0.1 U/g in saline-injected rats. Since the mouse brain is approximately 1/4–1/5 of the weight of the rat brain, 0.5 U of thrombin was used in this study. A similar dose (0.4 U) caused significant neutrophil infiltration and microglia/macrophage activation, as well as brain injury in previous studies. 54
Here we found that intracerebral Prx2 can attracted MDMs into the mouse brain while thrombin did not, indicating that the hemolysis and subsequent release of neurotoxic components play a role in the initiation of MDM infiltration. Our findings provide a novel perspective and evidence of how brain hemorrhage initiates clot removal and promotes brain recovery.
Some limitations of the study include: (1) The CD47 blocking antibody was co-injected with the autologous blood. Future studies are needed to test the effect of different routes and optimal doses of CD47 blocking antibody in monocyte recruitment and microglia activation after ICH. (2) Only young animals were used in this study; future experiments need to examine changes in MDMs and microglia in aged animals. (3) We only observe the changes of MDMs and microglia up to day 7 and ICH-induced brain recovery was not examined here. (4) Sex differences were not tested. (5) Rat-derived Prx2 and thrombin were used in this study.
In conclusion, this study utilized genetic modification to differentiate between microglia and MDM populations. We found monocytes gathered around the hematoma and forming a layer of giant erythrophagocytic cells after ICH in a mouse model. The infiltration of MDMs can be depleted by clodronate liposomes and enhanced by CD47 blocking antibody. CD47 blocking antibody can also enhance the phagocytotic activity of microglia. Erythrocyte degradation product Prx2 can attract MDMs to the brain parenchyma whereas plasma components thrombin, did not. Additional work is warranted to explore the interaction of MDMs and microglia in the ICH brain and how specific pharmacological strategies targeting monocytes or microglia will reduce brain damage after ICH.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Y.H., R.F.K., and G.X. were supported by grants supported by the National Institutes of Health (NIH) [NS-096917, NS-106746, NS-112394 and NS-116786].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
F.Y., Y. H., R. F. K., H. J. L. G., and G. X. participated in the experimental design. F.Y., J. Y., K. G. H., and S. K. conducted animal surgery, performed experiments, and performed data analysis. F.Y. drafted the manuscript. K. G. H., and S. K. revised the manuscript. Y. H., R. F. K., H. J. L. G., and G. X. supervised this project and revised the manuscript. F.Y., and G. X. approved the final version of the manuscript on behalf of all the authors.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009; 8: 355–369. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 2022; 53: e282–e361. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson DA, Pandey AS, Thompson BG, et al. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 2018; 134: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397. [DOI] [PubMed] [Google Scholar]
- 5.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013; 382: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanley DF, Thompson RE, Rosenblum M, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019; 393: 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldmann T, Wieghofer P, Jordao MJ, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 2016; 17: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson DA, Keep RF, Hua Y, et al. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: from macro to micro. J Cereb Blood Flow Metab 2018; 38: 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren H, Han R, Chen X, et al. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: an update. J Cereb Blood Flow Metab 2020; 40: 1752–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing C, Bian L, Wang M, et al. Enhancement of hematoma clearance with CD47 blocking antibody in experimental intracerebral hemorrhage. Stroke 2019; 50: 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao C, Keep RF, Xi G, et al. CD47 blocking antibody accelerates hematoma clearance after intracerebral hemorrhage in aged rats. Transl Stroke Res 2020; 11: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Grotta J, Gonzales N, et al. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 2009; 40: S92–94. [DOI] [PubMed] [Google Scholar]
- 13.Ajami B, Bennett JL, Krieger C, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 2007; 10: 1538–1543. [DOI] [PubMed] [Google Scholar]
- 14.Reu P, Khosravi A, Bernard S, et al. The lifespan and turnover of microglia in the human brain. Cell Rep 2017; 20: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kierdorf K, Masuda T, Jordao MJC, et al. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci 2019; 20: 547–562. [DOI] [PubMed] [Google Scholar]
- 16.Miron VE, Priller J. Investigating microglia in health and disease: challenges and opportunities. Trends Immunol 2020; 41: 785–793. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser T, Feng G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 2019; 6: ENEURO.0448-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 2016; 113: E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye F, Yang J, Hua Y, et al. Novel approach to visualize microglia death and proliferation after intracerebral hemorrhage in mice. Stroke 2022; 53: e472–e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao S, Zheng M, Hua Y, et al. Hematoma changes during clot resolution after experimental intracerebral hemorrhage. Stroke 2016; 47: 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda T, Amann L, Sankowski R, et al. Novel hexb-based tools for studying microglia in the CNS. Nat Immunol 2020; 21: 802–815. [DOI] [PubMed] [Google Scholar]
- 23.Heindl S, Gesierich B, Benakis C, et al. Automated morphological analysis of microglia after stroke. Front Cell Neurosci 2018; 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei J, Wang M, Jing C, et al. Multinucleated giant cells in experimental intracerebral hemorrhage. Transl Stroke Res 2020; 11: 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shtaya A, Bridges LR, Esiri MM, et al. Rapid neuroinflammatory changes in human acute intracerebral hemorrhage. Ann Clin Transl Neurol 2019; 6: 1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 2010; 605: 189–203. [DOI] [PubMed] [Google Scholar]
- 27.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017; 23: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 28.Tay TL, Mai D, Dautzenberg J, et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 2017; 20: 793–803. [DOI] [PubMed] [Google Scholar]
- 29.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14: 392–404. [DOI] [PubMed] [Google Scholar]
- 30.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye F, Hua Y, Keep RF, et al. CD47 blocking antibody accelerates hematoma clearance and alleviates hydrocephalus after experimental intraventricular hemorrhage. Neurobiol Dis 2021; 155: 105384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan C, Elyaman W. A new understanding of TMEM119 as a marker of microglia. Front Cell Neurosci 2022; 16: 902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercurio D, Fumagalli S, Schafer MK, et al. Protein expression of the microglial marker Tmem119 decreases in association with morphological changes and location in a mouse model of traumatic brain injury. Front Cell Neurosci 2022; 16: 820127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Lan X, Han X, et al. Expression of Tmem119/Sall1 and Ccr2/CD69 in FACS-Sorted microglia- and monocyte/Macrophage-Enriched cell populations after intracerebral hemorrhage. Front Cell Neurosci 2018; 12: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prineas JW, Parratt JDE. Multiple sclerosis: microglia, monocytes, and macrophage-mediated demyelination. J Neuropathol Exp Neurol 2021; 80: 975–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theriault P, ElAli A, Rivest S. The dynamics of monocytes and microglia in Alzheimer's disease. Alzheimers Res Ther 2015; 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritzel RM, Patel AR, Grenier JM, et al. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation 2015; 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CF, Goods BA, Askenase MH, et al. Divergent functions of tissue-resident and blood-derived macrophages in the hemorrhagic brain. Stroke 2021; 52: 1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fendrick SE, Xue QS, Streit WJ. Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene. J Neuroinflammation 2007; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CF, Massey J, Osherov A, et al. Bexarotene enhances macrophage erythrophagocytosis and hematoma clearance in experimental intracerebral hemorrhage. Stroke 2020; 51: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Lan X, Han X, et al. Microglia-derived interleukin-10 accelerates post-intracerebral hemorrhage hematoma clearance by regulating CD36. Brain Behav Immun 2021; 94: 437–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastorakos P, Mihelson N, Luby M, et al. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci 2021; 24: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh KB, Sekar P, Langefeld CD, et al. Monocyte count and 30-Day case fatality in intracerebral hemorrhage. Stroke 2015; 46: 2302–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi SX, Shi K, Liu Q. Brain injury instructs bone marrow cellular lineage destination to reduce neuroinflammation. Sci Transl Med 2021; 13 [DOI] [PubMed] [Google Scholar]
- 45.Mildner A, Mack M, Schmidt H, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 2009; 132: 2487–2500. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Chen Z, Yu F, et al. IL-4/STAT6 signaling facilitates innate hematoma resolution and neurological recovery after hemorrhagic stroke in mice. Proc Natl Acad Sci U S A 2020; 117: 32679–32690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006; 5: 53–63. [DOI] [PubMed] [Google Scholar]
- 48.Low FM, Hampton MB, Peskin AV, et al. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 2007; 109: 2611–2617. [DOI] [PubMed] [Google Scholar]
- 49.Bian L, Zhang J, Wang M, et al. Intracerebral hemorrhage-induced brain injury in rats: the role of extracellular peroxiredoxin 2. Transl Stroke Res 2020; 11: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Novakovic N, Hua Y, et al. Role of lipocalin-2 in extracellular peroxiredoxin 2-induced brain swelling, inflammation and neuronal death. Exp Neurol 2021; 335: 113521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal 2008; 10: 1621–1630. [DOI] [PubMed] [Google Scholar]
- 52.Ye F, Garton HJL, Hua Y, et al. The role of thrombin in brain injury after hemorrhagic and ischemic stroke. Transl Stroke Res 2021; 12: 496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong Y, Xi G, Hu H, et al. Increase in brain thrombin activity after experimental intracerebral hemorrhage. Acta Neurochir Suppl 2008; 105: 47–50. [DOI] [PubMed] [Google Scholar]
- 54.Mao S, Xi G, Keep RF, et al. Role of lipocalin-2 in thrombin-induced brain injury. Stroke 2016; 47: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]