Abstract
Mechanical thrombectomy is a ground breaking treatment for acute ischaemic stroke caused by occlusion of a large vessel. Its efficacy over intravenous thrombolysis has been proven in multiple trials with a lower number needed to treat than percutaneous coronary intervention for acute myocardial infarction. However, access to this key treatment modality remains limited with a considerable postcode lottery across the UK and many parts of the world. The evidence base for mechanical thrombectomy dates back to 2015. Since then, there have been important advances in establishing and widening the criteria for treatment. This narrative review aims to summarise the current evidence base and latest advances for physicians and academics with an interest in recanalisation treatments for acute ischaemic stroke.
Keywords: stroke, interventional radiology, neurology
Introduction
The treatment of acute ischaemic stroke was revolutionised in 2015 after the publication of several landmark randomised controlled trials.1–7 These trials compared best medical treatment with endovascular treatment and confirmed the benefit of mechanical thrombectomy in anterior circulation large vessel occlusion. The result was definitive, with a significant increase in the proportion of patients who were alive and independent at three months, and a number needed to treat of between three and seven. Figure 1 demonstrates the efficacy of mechanical thrombectomy (MT) when compared with other interventions.1 3–13 The HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke) trials7 led to the provision of rapid, arterial recanalisation in the form of endovascular treatment at comprehensive stroke centres for patients presenting with acute ischaemic stroke within 6 hours of symptom onset time. An estimated 10% of patients with stroke are eligible for mechanical thrombectomy, but in the UK approximately 3% of stroke patients are currently offered this treatment.14 15 Considering that the UK health service's Long Term Plan is to increase access to this vital stroke treatment, our aim is to describe the advances in mechanical thrombectomy and summarise the current evidence base to allow better patient selection and management of patients with acute ischaemic stroke.16
Figure 1.
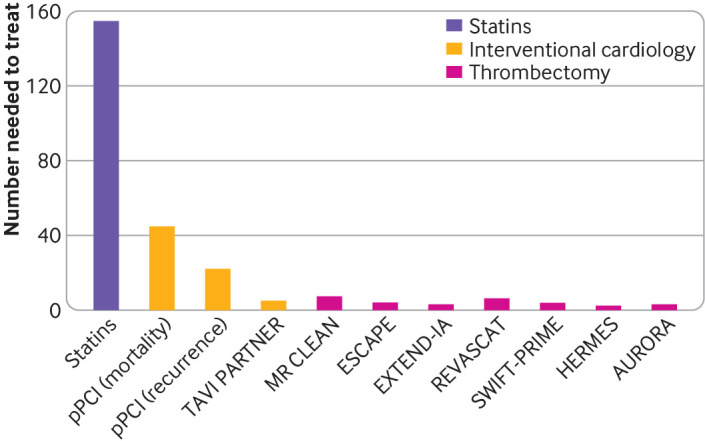
Comparison of the numbers needed to treat between landmark mechanical thrombectomy trials and other interventions. The figure shows the numbers needed to treat of the AURORA and HERMES meta-analyses and their respective randomised controlled trials (HERMES: MR CLEAN, ESCAPE, EXTEND-IA, REVASCAT, and SWIFT-PRIME; AURORA: DEFUSE-3 and DAWN1 3–10), which looked at achieving functional independence at 90 days. In the TAVI PARTNER trial, the number needed to treat looked at preventing death within five years.13 In the trials on primary percutaneous coronary intervention (pPCI), the number needed to treat was to prevent short term mortality and short term recurrence.12 For statins, the number needed to treat was to prevent one stroke11
Sources and selection criteria
Between September 2022 and November 2022, we conducted a search of all articles in English relating to mechanical thrombectomy for acute ischaemic stroke in the following databases: PubMed, Embase, OVID, and Google Scholar. There were no date restrictions. We searched alternative iterations of mechanical thrombectomy and stroke such as “endovascular therapy,” “intra-arterial clot retrieval,” “endovascular thrombectomy,” “acute ischaemic stroke,” and “acute stroke.” Priority was given to peer reviewed systematic reviews, meta-analyses, and randomised controlled trials as well as existing clinical guidelines, because they had the most impact on changing clinical practice.
Big five trials on mechanical thrombectomy
Thrombectomy has been conducted for over two decades, but the evidence base proving efficacy and cost effectiveness was initially lacking. In the early 2010s, the first three randomised controlled trials looking at interventional procedures failed to show benefit of MT but had their limitations, including one trial (IMS-III), which did not require direct vascular imaging evidence of large vessel occlusion for the selection of patients.17–19 This trial was swiftly followed in 2015 with the publication of five randomised controlled trials comparing the efficacy of MT (using second generation thrombectomy devices) in addition to intravenous thrombolysis where eligible versus intravenous thrombolysis alone.1 3–6 All five trials focused on the anterior circulation exclusively, and four of the trials recruited patients in windows of up to 8 hours from symptom onset. Outcomes were measured using the modified Rankin score, which is an ordinal score from 0 to 6, with 0 meaning free of disability and six meaning death.20 MR CLEAN was the first positive trial to be published.6 The results were compelling and prompted the data safety monitoring boards of the remaining trials to conduct interim analyses, leading to the early stoppage of these trials. The five trials (table 1) included over 1200 patients in total, and their data were combined in the HERMES meta-analysis.7 The researchers of this trial concluded that MT led to significantly better “good functional outcomes,” as defined by a modified Rankin score of 0-2. Patients treated with MT achieved the primary outcome in 46% of participants, compared with 26.5% receiving the best medical treatment (adjusted common odds ratio 2.49, 95% confidence interval 1.76 to 3.53, P<0.001). The number needed to treat to achieve a reduction in modified Rankin score of 1 point in one patient was 2.6. The pooled data allowed the meta-analysis to investigate the efficacy of thrombectomy across a variety of patient characteristics and showed that adult patients benefited from thrombectomy across all age groups and geographical locations. The benefit remained in patients who did not receive intravenous thrombolysis.
Table 1.
Key characteristics of particlpants who received mechanical thrombectomy in randomised controlled trials of acute ischaemic stroke, included in the HERMES collaboration meta-analysis7
| MR CLEAN | REVASCAT | ESCAPE | SWIFT-PRIME | EXTEND-IA | |
| Study and patient characteristics | |||||
| Location | Netherlands | Spain | International | International | Australia/New Zealand |
| No of centres | 16 | 4 | 22 | 60 | 10 |
| Duration | 4 years | 2 years | 2 years | 5 years | 3 years |
| Imaging | CT or MRI | CT or MRI | CT | CT or MRI | CT/CTP or MRI |
| Sample size (No of participants) | 500 | 206 | 316 | 196 | 70 |
| Median NIHSS | 17 | 17 | 16 | 17 | 17 |
| Median ASPECTS | 9 | 9 | 9 | 9 | 7 |
| Main inclusion criteria | |||||
| Age | ≥18 years | 18-80 years* | ≥18 years | 18-85 years | ≥18 years |
| Symptom onset | 0-6 hours | 0-8 hours | 0-12 hours | 0-6 hours | 0-6 hours |
| Large vessel occlusion type | ICA, M1, M2, A1, A2 | ICA, M1 | ICA, M1, M2† | ICA, M1 | ICA, M1, M2 |
| Modified Rankin score before stroke | Not used | ≤1 | NA‡ | ≤1 | ≤2 |
| NIHSS cut-off point | ≥2 | >5 | >5 | 8-30 | Nil |
| ASPECTS | Not used | >6§ | >6 | NA¶ | NA** |
| Findings for patients receiving mechanical thrombectomy | |||||
| Thrombolytic treatment | Yes (89%) | Yes (68%) | Yes (75%) | Mandated (100%) | Mandated (100%) |
| Successful recanalisation†† | 75.4% | 66.0% | 72.4% | 88.0% | 86.2% |
| 90 day modified Rankin score 0-2 rate (best medical treatment) | 19.1% | 28.2% | 29.3% | 35.0% | 40.0% |
| 90 day modified Rankin score 0-2 rate (mechanical thrombectomy) | 32.6% | 43.7% | 53.0% (P<0.001) |
60.0% (P<0.001) |
71.0% (P=0.001) |
| Number needed to treat | 7.4 | 6.5 | 4 | 4 | 3 |
| Rate of symptomatic intracerebral haemorrhage | 7.7% | 1.9% | 3.6% | 0.0% | 0.0% |
| Death (%) | 18.9% | 18.4% | 10.4% | 9.0% | 8.6% |
| Mortality difference between mechanical thrombectomy and best medical treatment | No difference | No difference | 50% reduction | No difference | No difference |
CT=computed tomography; MRI=magnetic resonance imaging; CTP=computed tomography perfusion; NIHSS=National Institute of Health Stroke Scale; ASPECTS=Alberta stroke programme early computed tomography score; NA=not available; ICA=internal carotid artery; DWI=diffusion weighted imaging.
*After 160 patients were enrolled, patients up to age 85 years were included with ASPECTS >8.
†M2 occlusions were included if involving more than one M2 vessel.
‡Barthel index was used.
§ASPECTS >6 on CT or ASPECTS >5 on MRI-DWI.
¶Advanced imaging, either CT or MRI, was used to establish core infarct size with varying criteria based on age and imaging modality.
**Advanced imaging, either CT or MRI, was used to establish mismatch ratio >1.2 and infarct core lesion <70 mL.
††As defined by thrombolysis in cerebral infarction 2b or 3.
Strict time criteria were applied to the patients recruited in the HERMES collaboration.7 ESCAPE had the longest inclusion window of 12 hours from symptom onset.4 SWIFT-PRIME, MR CLEAN, and EXTEND-IA recruited patients up to 6 hours from symptom onset and REVASCAT up to 8 hours.3 5 6 A subsequent meta-analysis demonstrated that most benefit was received from thrombectomy when conducted under 7 hours and 18 minutes.21 This reinforced the idea of the time clock; and similar to intravenous thrombolysis, an emphasis was placed on rapid recanalisation and reperfusion if good functional independence was to be achieved.
Following the results of the HERMES meta-analysis, best practice guidelines were updated in the USA, Canada, Europe, and the UK and mechanical thrombectomy became the preferred method for patients who have acute ischaemic stroke and presenting with an anterior circulation large vessel occlusion.
Procedural considerations of mechanical thrombectomy
MT is an endovascular procedure performed under fluoroscopic guidance and involves recanalisation of an intracranial occlusion by removing a thrombus using a retrievable stent (stent retriever), aspiration catheter, or a combination of both techniques. The procedure is typically performed via transfemoral arterial access but can be performed via the radial artery. A biaxial or triaxial catheter system is used, with progressively smaller calibre catheters sited within the distal vasculature. A large bore guide catheter is positioned proximally in the target great vessel, and digital subtraction angiography is acquired after injection of iodinated contrast confirming the large vessel occlusion.
Two main techniques for clot retrieval exist. A direct aspiration technique involves navigation of an aspiration catheter to the occlusion followed by syringe or pump driven aspiration.22 Alternatively, deployment of a stent retriever involves navigating a microwire and microcatheter beyond the occlusion. The stent retriever is unsheathed within the thrombus, allowing time for integration with the stent before being retrieved along with the thrombus. A combined stent retrieval aspiration technique can be used by additionally placing an aspiration catheter at the proximal margin of the thrombus.23 The COMPASS study showed no significant difference between the two approaches with respect to both good functional outcome at 90 days (modified Rankin score 0-2, 52% (95% confidence interval 43.8% to 60.3%) in aspiration arm v 50% (41.6% to 57.4%) in stent retrieval) and recanalisation rates. Aspiration approach had the added advantage of reduced costs and an 11 minute reduction in time to recanalisation (P=0.02).24
The second generation thrombectomy devices of the HERMES trials, including Solitaire (Medtronic) or Trevo (Stryker) stent retrievers, had improved successful reperfusion rates of 71% compared with first generation thrombectomy devices as defined by modified thrombolysis in cerebral infarction grades 2b or 3 (figure 2).7
Figure 2.
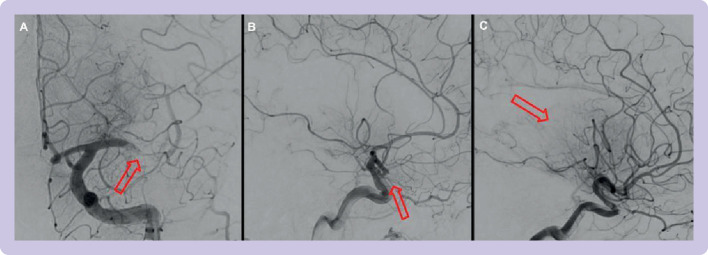
Angiographic runs during thrombectomy procedures performed at Imperial College Healthcare Trust. mTICI=modified thrombolysis in cerebral infarction; MCA=middle cerebral artery; arrows=area with a lack of contrast flow. (A) Left M1 occlusion with some degree of anterograde perfusion=mTICI 1. (B) Left M2 proximal occlusion with less than 50% of the affected MCA territory being perfused=mTICI 2a. (C) Left M2 distal occlusion with more than 50% of the affected MCA territory being perfused=mTICI 2b. Examples of mTICI 0 and mTICI 3 are shown in figure 3
Achieving complete recanalisation on the first pass of a thrombectomy device (as shown in figure 3) is referred to as the first pass effect, which is an independent predictor of good clinical outcome (modified Rankin score 0-2, 61.3% in first pass effect v 35.3%; odds ratio 1.7, 95% confidence interval 1.10 to 2.70).25
Figure 3.
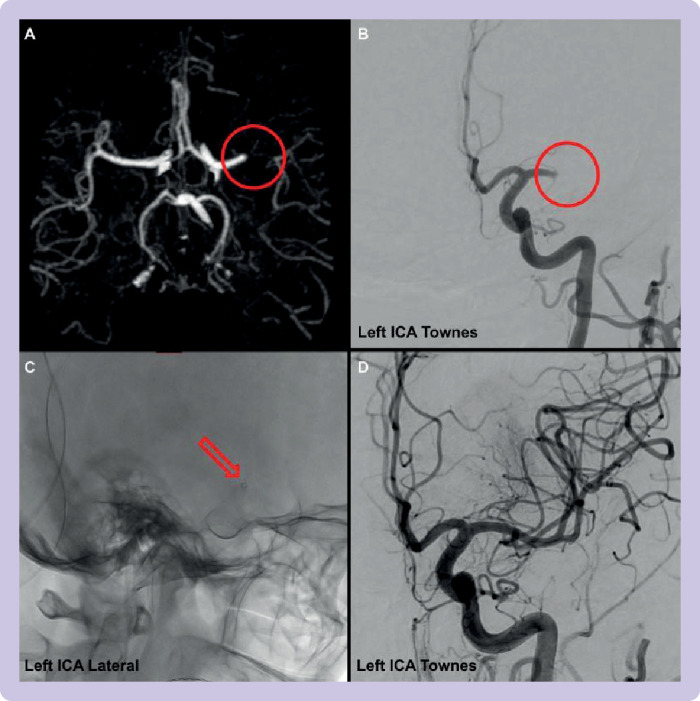
Patient with proximal large vessel occlusion in left M1 vessel. mTICI=thrombolysis in cerebral infarction; ICA=internal carotid artery; Townes=angled anteroposterior view. (A) Appearance of large vessel occlusion on computed tomography angiography. (B) Initial angiographic run with mTICI score 0 (ie, no flow distal to the occlusion). (C) Placement of aspiration catheter (small ring that is the distal end of the catheter) at the site of vessel occlusion. (D) Reperfusion after aspiration with mTICI score 3
When patients are selected for thrombectomy, practice varies between centres about performing the procedure under general anaesthesia or with conscious sedation. General anaesthesia confers the advantages of immobilisation to enable safe endovascular intervention with short delays in the induction of patients. By contrast, with conscious sedation, patients have faster times from door to groin puncture, but with the possibility of patient agitation complicating the procedure.26 Initial meta-analysis data from the HERMES trials and three single centre, randomised controlled trials raised concern that general anaesthesia led to worse outcomes (adjusted common odds ratio 1.53, 95% confidence interval 1.14 to 2.04, P=0.004).27 Recent meta-analysis from newer trials has suggested that, with a small effect size, patients treated under general anaesthesia achieve better functional outcomes (modified Rankin score 0-2, odds ratio 1.11, 95% confidence interval 1.03 to 1.2, P=0.007).28 More work is needed to determine superiority. Patient factors should be considered when determining suitability for general anaesthesia.
Optimal management of peri-procedural blood pressure is important because within infarcted and penumbral tissue, cerebral autoregulation is impaired. In persistent large vessel occlusion, a retrospective analysis of 80 patients showed that blood pressure fluctuations and drops in blood pressure contributed to penumbral tissue loss.29 In the case of hypertension, if patients receive intravenous thrombolysis, the National Institute for Health and Care Excellence recommend that blood pressure should be less than 185 mm Hg (systolic) and 110 mm Hg (diastolic) to reduce the risk of haemorrhagic transformation.30 The BP-TARGET randomised controlled trial including 324 patients failed to demonstrate a reduction in rates of radiological haemorrhagic transformation, with more intensive blood pressure lowering to 100-120 mm Hg systolic for the first 24-36 hours after thrombectomy.31 In ENCHANTED2-MT, a multicentre, international, randomised controlled trial of 821 patients, researchers showed that intensive management of systolic blood pressure below 120 mm Hg led to worsening functional outcomes on the shift analysis for modified Rankin score at 90 days (odds ratio 1.37, 95% confidence interval 1.07 to 1.76, P=0.01).32
Patient selection
After the adoption of MT globally, pathways have been developed to identify eligible patients with stroke who have large vessel occlusion. Patients either present to comprehensive stroke centres that are MT capable or to local primary stroke centres, which can refer to the nearest comprehensive stroke centre for definitive management. The key goal is rapid clinical and radiological assessment in tandem by stroke physicians and interventional neuroradiologists.
For physicians, the National Institutes of Health Stroke Scale (NIHSS) is an 11-category rating scale developed to act as a framework for the identification and monitoring of neurological deficits seen with stroke syndromes.33 Based on data from the HERMES trials, MT protocols select patients with at least moderate strokes with an NIHSS score ≥6.34
Once a patient has been determined to have a clinically significant clinical stroke syndrome, it is essential to clarify the pre-morbid level of independence. The HERMES trials excluded patients who were functionally dependent before their stroke. As such, in the UK, MT is often reserved for patients with a modified Rankin score between 0 and 2.
Subsequent brain and vascular imaging in the form of non-contrast computed tomography (CT) and CT angiography should be performed to identify a large vessel occlusion and the degree of established early ischaemic change. Class 1a evidence supports the use of MT in patients with a proximal large vessel occlusion involving the terminal internal carotid artery and/or the proximal M1 branch of the middle cerebral artery.7 MR CLEAN, EXTEND-IA, and ESCAPE did additionally include patients with M2 occlusions. The degree of early ischaemic change is defined by the ASPECTS (Alberta Stroke Protocol Early CT Score, figure 4). In the HERMES collaboration, patients with ASPECTS <6 were excluded because they had extensive early ischaemic change, suggesting less benefit to be gained from MT.
Figure 4.
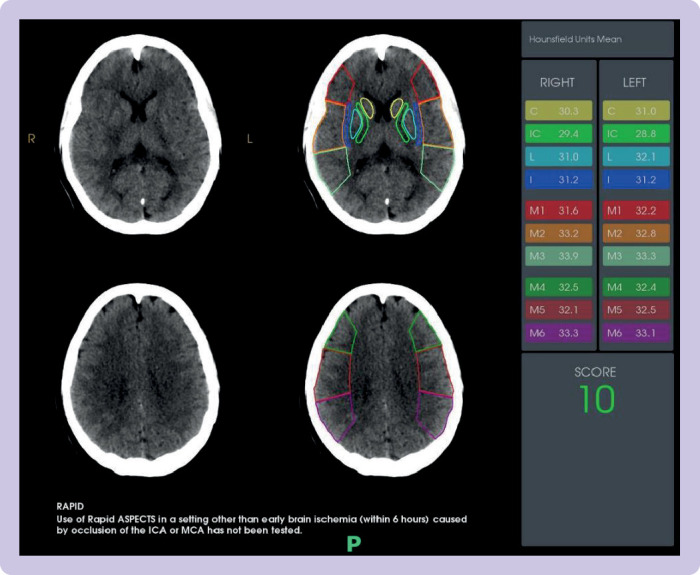
Demonstration of ASPECTS (Alberta Stroke Protocol Early Computed Tomography Score) on non-contrast computed tomography, in a patient presenting to Imperial College Healthcare Trust comprehensive stroke centre with images acquired from the Rapid.AI software package. The middle cerebral artery territory is divided into 10 regions, including six cortical and four deep grey matter structures. Hounsfield units are used to identify areas of early ischaemic change and one point is subtracted from the total score of 10 for each region affected
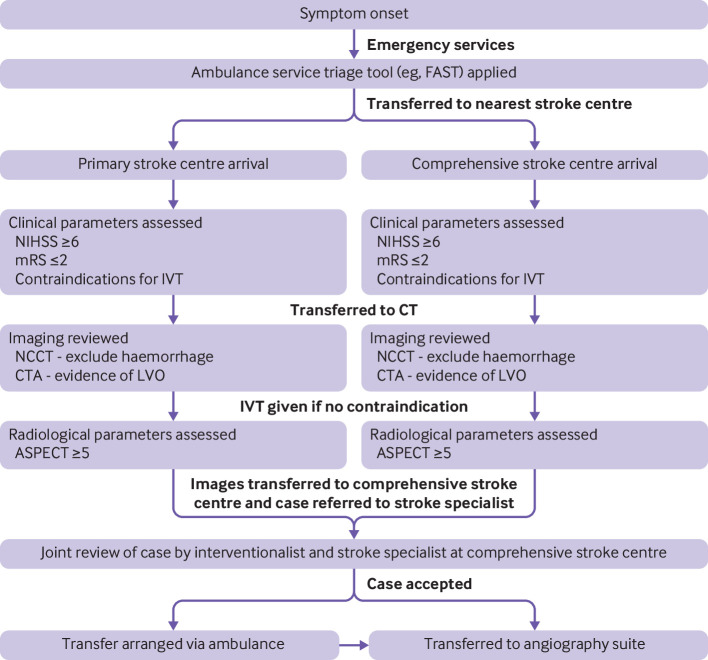
Patients with acute ischaemic stroke caused by an large vessel occlusion presenting within the early window of 6 hours from stroke symptom onset are eligible for MT (see example flowchart in figure 5) if they:
Figure 5.
Example workflow of a patient presenting with symptoms consistent with acute ischaemic stroke secondary to large vessel occlusion (LVO) within the standard time windows (0-6 hours from symptom onset). Intravenous thrombolysis (IVT) is considered in all cases if no contraindications. Eventual thrombectomy will be offered in the nearest comprehensive stroke centre that is mechanical thrombectomy capable. These eligibility criteria are an example of how patients are selected in the UK but might vary worldwide; some of the expansions of these criteria are discussed throughout the present review. NIHSS=National Institutes of Health Stroke Scale; mRS=modified Rankin score; NCCT=non-contrast computed tomography; CTA=computed tomography angiography; ASPECT=Alberta Stroke Protocol Early Computed Tomography Score; FAST=face arm speech time
Are functionally independent at baseline (modified Rankin score of 0-2)
Have at least a moderate stroke syndrome (NIHSS ≥6)
Do not show extensive areas of early ischaemia on non-contrast CT (for example, a cut-off of ASPECTS ≥5 can be used).
Mechanical thrombectomy for extended time windows (6-24 hours)
The HERMES collaboration clearly defined the importance of the time clock and showed that early intervention led to more favourable outcomes. Beyond 7 hours and 20 minutes after symptom onset, patients were less likely to achieve functional independence within three months.21 Only two of the five HERMES trials included any patients presenting in the late window (defined as 6-24 hours from symptom onset or last known being well (ie, when the patient was last known to be without the signs and symptoms of the current stroke or at their baseline)). Patients with unclear symptom onset or those with wake-up syndromes, representing up to 20% of presentations of acute ischaemic stroke, were excluded.35
When a large vessel occlusion occurs, deeper structures supplied by the vascular territory that lack collateral blood supply will infarct first. The areas of brain tissue with irreversible ischaemic damage are known as the ischaemic core. However, the part of the vascular territory surviving on collateral flow (ie, small vessels supplying ischaemic tissue through retrograde flow) is known as the ischaemic penumbra; this area of potentially salvageable brain tissue demonstrates reversible ischaemia if reperfusion is achieved. Patients can be phenotypically different and progress to complete infarction at different rates. In one study, 25% of patients termed as fast progressors lose 27 million neurons per minute, compared with 55% of patients termed as slow progressors who lose 35 000 neurons per minute. The quality of collateral flow was the predominant factor influencing infarct growth.36
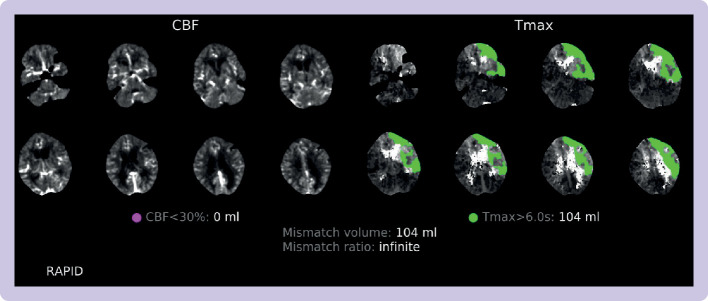
Advanced neuroimaging techniques such as magnetic resonance imaging (MRI) and CT perfusion can be used to evaluate the size of the ischaemic core and viable penumbra. The two landmark trials evaluating patients presenting in the late windows were the DAWN (n=206) and DEFUSE-3 (n=182) trials.9 10 The DEFUSE-3 trial incorporated radiological mismatch between core and penumbra to define its inclusion criteria. Patients presenting 6-16 hours from last known being well, inclusive of wake-up syndromes, were included if they had a core infarct volume <70 mL and a mismatch ratio between the core and penumbra of ≥1.8 (figure 6 shows an example). A different paradigm of a clinical radiological mismatch up to 24 hours was used in the DAWN trial. Patients had large clinical deficits but small ischaemic cores on advanced imaging. The clinical deficit, defined by NIHSS, was used as a surrogate marker for penumbra volume. Both trials showed that with the use of advanced imaging techniques, this carefully selected group of late presenters with a substantial core penumbra mismatch had improved functional neurological recovery. A limitation of these trials was inclusion of patients with unclear onset time or wake-up syndromes. Patients were included on the basis of their time last known well, but the true onset of stroke symptoms could have been shortly before waking. Fifty per cent of patients in DEFUSE-3 and 55% of patients in DAWN had a wake-up syndrome.
Figure 6.
Computed tomography perfusion and radiological mismatch, acquired from the Rapid.AI software package, for a patient presenting to Imperial College Healthcare Trust in the late window (ie, 6-24 hours from symptom onset or last known being well). The DAWN and DEFUSE-3 trials9 10 defined core infarct as volume of brain tissue with cerebral blood flow (CBF) <30% (highlighted on the sequences on the left) and penumbra as the mismatch in volume between the hypoperfusion lesion (ie, the volume of brain tissue where it takes >6 seconds for the contrast bolus to reach maximal density (Tmax), seen on the sequences on the right). Key values at the bottom of the figure are the core infarct volume (which must be <70 mL), mismatch ratio (which must be >1.8), and penumbra or mismatch volume (which must be >15 mL)
Most patients presenting in the late window who do not yet have large core infarction are slow progressors, and the impact of time is less relevant in this subgroup. The AURORA meta-analysis assimilated DAWN and DEFUSE-3 with individual patient data from the HERMES collaboration when patients presented in the late window.8 Level 1 evidence across a range of prespecified subgroups showed a clear benefit of MT. The main criticism of DAWN and DEFUSE-3 was countered as witnessed syndromes in the late window benefited from MT (odds ratio 2.78, 95% confidence interval 1.22 to 6.31, P=0.015). MT for highly selected patients in late windows had a number needed to treat of 3, a similar level of benefit to treatment in early windows.
Thrombectomy trials of the extended window have been conducted in resource rich, comprehensive stroke centres with access to neuroradiologists and advanced imaging techniques. However, in many parts of the world including the UK, there is limited access to advanced imaging. The “Getting It Right First Time” review in 2019 of stroke services in the UK showed that less than 10% of centres had access to CT perfusion and that up to an additional 2.7% of patients with acute ischaemic stroke could qualify for thrombectomy with the use of the DAWN/DEFUSE-3 criteria.37 38 In the UK, a review of Sentinel Stroke National Audit Programme (SSNAP) data showed that more patients were treated in the late window without CT perfusion than those who had CT perfusion (668 v 378).39 It is unclear at this stage whether patients fare worse without advanced imaging. The review of the SSNAP data showed that despite no significant difference in functional outcome between the two cohorts, the shift analysis did trend in favour of patients treated after CT perfusion. The odds ratio for futile recanalisation was lower in patients selected by CT perfusion. By contrast, a multinational cohort study by Nguyen et al showed no significant difference in modified Rankin score outcomes for 1604 patients selected in the late window for thrombectomy by non-contrast CT, CT perfusion, or MRI.40 Furthermore, data from a retrospective review looking at 142 patients ineligible for thrombectomy based on DAWN/DEFUSE-3 criteria who had so-called off-label thrombectomy still had a 30% rate of achieving functional independence (which was better than the outcomes for best medical treatment arms of the HERMES collaboration).41
CT perfusion is not without interpretation challenges, and the need for alternatives to advanced neuroimaging techniques has prompted two avenues of research.38 The ESCAPE trial used CT angiography collateral score to grade the degree of collateral flow in the territory of vessel occlusion in comparison to the contralateral hemisphere.4 MR CLEAN-LATE is a randomised controlled trial that rated so-called late presenters, including those who had wake-up strokes with proximal, anterior circulation, large vessel occlusion (including M2) according to collateral flow status and randomised to either MT or best medical treatment alone.42 Patients with absent collaterals and those eligible based on DAWN/DEFUSE-3 criteria were excluded. This multicentre randomised controlled trial recruited 500 patients and those randomised to endovascular treatment arm showed an improvement in modified Rankin score at 90 days (adjusted common odds ratio 1.67, 95% confidence interval 1.20 to 2.32).43 This treatment effect was similar to that seen in the initial HERMES collaboration trials and supports the selection of patients for MT without advanced imaging techniques.
The second approach being considered is demonstrated in the RESILIENT-EXTEND trial from Brazil, which is ongoing.44 The trial uses an age adjusted, modified clinical ASPECTS mismatch based on non-contrast CT and ASPECTS alone without the aid of advanced imaging techniques. In a recent meta-analysis of six observational registries covering simplified stroke imaging selection modality for MT in the extended time window, researchers found no significant difference in proportion of patients with modified Rankin score 0-2 at 180 days, based on selection by either non-contrast CT/CT angiography modality or advanced CT perfusion. However, mortality in the non-CT perfusion group was higher.45
At present, level 1a evidence still supports the use of advanced imaging, MRI, or CT perfusion for the selection of patients in the extended time window. However, recent publication of the MR CLEAN-LATE trial and upcoming results from the RESILIENT-EXTEND trial might lead to further widening of these imaging selection criteria.
Direct mechanical thrombectomy
Intravenous thrombolysis is the standard of care for patients with acute ischaemic stroke where there are no contraindications, and it can be delivered within 4.5 hours of symptom onset. Intravenous thrombolysis has its risks and benefits to consider especially when proceeding to thrombectomy. It has an associated risk of symptomatic intracranial haemorrhage.46 By contrast, there are proposed benefits of intravenous thrombolysis in terms of thrombus softening to aid recanalisation, and potential for recanalisation in cases of MT failure or residual distal emboli not amenable to MT.47
To date, six randomised controlled trials of similar design have been conducted which compare direct MT with bridging intravenous thrombolysis.48–53 Minor differences such as the alteplase dose or inclusion of M2 occlusions existed. In direct MT, the effect size as compared with bridging intravenous thrombolysis is thought to be small. Thus, aside from MR CLEAN NO-IV, the trials opted for a non-inferiority trial design with varying margins for significance. Most of these trials did not reach the threshold for non-inferiority, which is reflected in the ESO-ESMINT group’s recommendations after they conducted a study level meta-analysis of these six trials, comprising 2331 patients.54 Compared with patients randomised to bridging treatment, the odds ratio for good outcome in patients randomised to MT alone was 0.93 (95% confidence interval 0.79 to 1.10, P=0.38). Non-inferiority criteria was not met, based on the maximum clinically acceptable non-inferiority margin of 5.0%.
Models of pre-hospital pathways to thrombectomy
The trials of direct thrombectomy generally recruited patients who presented directly to MT capable comprehensive stroke centres.48–53 However, most centres offering stroke services in the world are not MT capable, and therefore require a secondary transfer. This requirement has led to the development of two main models of access to thrombectomy: namely, the drip and ship model and the mothership model.
In the drip and ship model, patients assessed to have a suspected stroke by pre-hospital healthcare workers are taken to their nearest primary stroke centre. After identification of a large vessel occlusion at the primary stroke centre, patients are referred to the nearest comprehensive stroke centre. Thrombolytic treatment is offered at the primary stroke centre when eligible. This paradigm offers quicker access to intravenous thrombolysis and more refined patient selection for those transferred to comprehensive stroke centres.
In the mothership model, patients assessed to be having a stroke in the pre-hospital setting are sent directly to comprehensive stroke centres that are MT capable. Although these patients might receive easier access to MT, the increased patient flow could overburden comprehensive stroke centres with patients who have non-strokes or who do not have large vessel occlusions. There might also be delays to the use of intravenous thrombolysis if patients are transferred to comprehensive stroke centres that are further away while bypassing primary stroke centres that are thrombolysis capable.
The two major meta-analyses conducted on these pathways included several observational studies and between one and two randomised controlled trials.55 56 Both studies showed that the mothership paradigm had an improved rate of achieving functional independence compared with the drip and ship model, and patients treated under the drip and ship paradigm had higher rates of symptomatic intracranial haemorrhage (odds ratio 1.49, 95% confidence interval 1.22 to 1.81).57 The effect of geography, variety of pre-hospital screening tools used, and heterogeneity of the studies limited the robustness of findings from these meta-analyses.
The RACECAT trial conducted in a non-urban area of Catalonia looked to answer some of these queries.58 A total of 1401 patients were enrolled in the pre-hospital setting using the RACE tool. A score ≥5 had a positive predictive value of 42% for large vessel occlusion and negative predictive value of 94% for large vessel occlusion. In this trial, the pre-hospital crew had to discuss with the neurologist at the comprehensive stroke centre before randomisation to either drip and ship or mothership paradigms.59 No significant difference was seen in the primary outcome (modified Rankin score 0-3 at 90 days) or in mortality at 90 days. Patients triaged to the mothership model were less likely to receive intravenous thrombolysis (47.5% v 60.4%; odds ratio 0.59, 95% confidence interval 0.45 to 0.76) and were more likely to receive thrombectomy (48.8% v 39.4%; 1.13 to 1.89) than those triaged to the drip and ship model. The workflow metrics for both thrombolysis (door to needle) and thrombectomy (door to groin puncture) were particularly impressive and questions might be raised about the comparability of these data to the real world setting.
Newer pathways of care are being trialled, including:
Drip and drive: the neuro-interventional team are taken to the primary stroke centre to conduct MT.60
Mobile stroke unit: ambulances equipped with CT angiography capability and staff trained to give thrombolytic treatments travel directly to patients.61
Direct transfer to angiography suite: patients with large stroke syndromes are transferred to the angiography suite where flat detector CT is performed. A single centre, randomised controlled trial of 174 patients in Barcelona showed reduced groin puncture times of direct to angiography suite compared with usual care (18 minutes v 42 minutes, P<0.001), with reduced disability on ordinal shift analysis of modified Rankin score (odds ratio 2.2, 95% confidence interval 1.2 to 4.1, P=0.009).62
More international and multicentre trials are needed to confirm the generalisability of these findings, especially in urban areas.
Basilar thrombectomy
The landmark five trials from the HERMES collaboration that led to changes in acute ischaemic stroke care in 2015 focused on patients with stroke in the proximal anterior circulation.4 However, an estimated 20% of strokes affect the posterior circulation, where the predominant supply is the basilar artery. Strokes secondary to basilar artery occlusions lead to an estimated 80% rate of dependency or mortality.
Posterior circulation strokes often present in a less specific manner with a fluctuating clinical course. In this patient cohort, patients can present with a myriad of symptoms and signs including dizziness, unilateral limb weakness, dysarthria, and gait ataxia that are under-represented in the NIHSS tool. The BATMAN collaboration has developed a POST-NIHSS score to help physicians identify the most disabling signs and symptoms of basilar artery occlusions.63
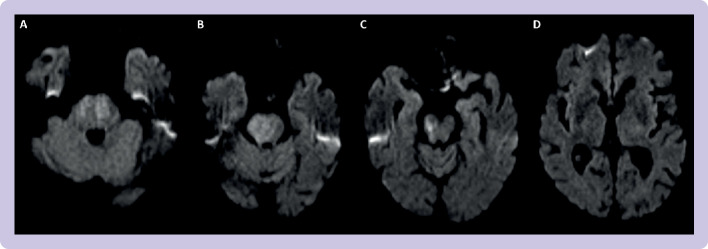
The subsequent radiological assessment also differs. A posterior circulation, acute stroke prognosis early CT score (pc-ASPECTS, figure 7)64 was created and subsequently validated to prognosticate for basilar artery occlusions. Favourable functional outcome was predominantly seen in patients with pc-ASPECTS ≥8.65 Owing to the clinical importance of brainstem ischaemia, an alternative scoring system known as the pons-midbrain index (figure 8) has been developed.66 A retrospective analysis of 158 patients in the BASICS dataset showed that for comatose patients, a pons-midbrain index score <3 conferred a lower mortality than those with greater degrees of brainstem infarction; however, the pons-midbrain index score did not correlate with functional outcome.67
Figure 7.
Use of pc-ASPECTS (posterior circulation acute stroke prognosis early computed tomography scoring) to assess posterior circulation. There are eight areas of assessment. A point is lost for early ischaemic change in each of thalamic, cerebellar, and occipital lobes; and 2 points lost for pontine and midbrain changes. This image, from Imperial College shows Healthcare Trust, shows a basilar artery stroke with a pc-ASPECTS score of 4 (2 for pontine and 2 for midbrain changes). (A) Ischaemia in bilateral pons. (B) Ischaemia of right hemipons. (C) Ischaemia of right midbrain. (D) No ischaemia in thalamus or occipital lobes. In this assessment, pc-ASPECTS scoring was based on diffusion weighted imaging
Figure 8.
Use of pons-midbrain index on computed tomography angiogram source imaging (CTASI) of a basilar artery stroke managed at Imperial College Healthcare Trust. Scores of 0 (no hypoattenuation), 1 (<50% hypoattenuation), or 2 (>50% hypoattenuation) are attributed to each half of the pons and midbrain with a total score of 8. Images A and B show a pons-midbrain index score of 5 (2 right pons, 1 left pons, 1 right midbrain, 1 left midbrain). Image C highlights how closely CTASI can correlate to MRI (magnetic resonance imaging)-DWI (diffusion weighted imaging)
Four randomised controlled trials have investigated the use of MT in basilar artery occlusions. The first two, BASICS and BEST, had slow recruitment and high crossover rates, respectively, and did not show significant improvements in achieving the primary outcome measure (modified Rankin score 0-3 at 90 days).68–71 The BASICS trial had very high rates of concurrent intravenous thrombolysis treatment in both arms, and subgroup analysis suggested a possible benefit of MT in those patients with NIHSS>10. Subsequently, ATTENTION and BAOCHE have been published with results in favour of MT. ATTENTION recruited patients with basilar artery occlusions within a symptom onset window of 0-12 hours. BAOCHE recruited late presenters with basilar artery occlusions (symptom onset window of 6-24 hours).
A meta-analysis (table 2) of the randomised controlled trials showed significant improvement in the rates of individuals with a good functional outcome (modified Rankin score 0-3, relative risk 1.54, 95% confidence interval 1.16 to 2.06, P=0.003) and functional independence (modified Rankin score 0-2, relative risk 1.69, 95% confidence intervals 1.05 to 2.71, P=0.03) in those who were treated with thrombectomy when compared with best medical treatment alone.72 MT was associated with a higher level of symptomatic intracranial haemorrhage (relative risk 7.12, 95% confidence intervals 2.16 to 23.54, P=0.001), although the mortality rate was significantly lower in the intervention group (relative risk 0.76, 95% confidence intervals 0.65 to 0.89, P<0.001). The outcome of these data has successfully answered the uncertainties surrounding MT for basilar artery occlusions and will lead to change in practice.
Table 2.
Key characteristics of participants who received mechanical thrombectomy for basilar artery occlusions from four randomised controlled trials of acute ischaemic stroke included in the meta-analysis by Malik et al72
| BASICS | BEST | ATTENTION | BAOCHE | |
| Study characteristics | ||||
| Location | International | China | China | China |
| No of centres | 23 | 28 | 48 | 36 |
| Duration | 8 years | 2 years | 2 years | 6 years |
| Imaging | CT or MRI | CT or MRI | CT or MRI | CT or MRI |
| Sample size (No of participants) | 300 | 131 | 507 | 217 |
| Main inclusion criteria | ||||
| Age (years) | 18-85* | ≥18 | ≥18 | 18-80 |
| Symptom onset | 0-6 hours | 0-8 hours | 0-12 hours | 6-24 hours |
| Pre-stroke modified Rankin score | ≤2 | ≤2 | ≤2 | ≤ 1 |
| NIHSS cut-off point | ≥10† | ≥6 | ≥10 | ≥10‡ |
| pc-ASPECTS | NA§ | ≥6¶ | ≥6** | ≥6 |
| Main outcomes (%) | ||||
| Successful recanalisation | 72 | 71 | 91 | 88 |
| 90 day modified Rankin score 0-2 rate | ||||
| Best medical treatment | 30 | 28 | 11 | 14 |
| Mechanical thrombectomy | 35 | 33 | 33 | 39 |
| Symptomatic intracranial haemorrhage rate (mechanical thrombectomy) | 4.5 | 8.0 | 5.0 | 8.8 |
| Death (mechanical thrombectomy) | 38.3 | 33 | 36.7 | 30.9 |
CT=computed tomography; MRI=magnetic resonance imaging; NIHSS=National Institutes of Health Stroke Scale; pc-ASPECTS=posterior circulation acute stroke prognosis early CT scoring; NA=not available.
*Owing to slow recruitment, patients older than 85 years were recruited after 91 enrolments.
†NIHSS<10 was allowed after interim analysis, owing to slow recruitment.
‡NIHSS cut-off point was later modified to ≥6, owing to slow recruitment.
§Although pc-ASPECTS was not used, patients were excluded if they had extensive bilateral brainstem ischaemia.
¶Patients with pons-midbrain index score ≥3 were also excluded.
**In patients older than 80 years, pc-ASPECTS ≥8 was used.
Tandem occlusions
Tandem occlusion refers to an acute ischaemic stroke secondary to an intracranial large vessel occlusion and concurrent extracranial internal carotid artery occlusion or severe stenosis. This occurs in about 10-20% of presentations of large vessel occlusion.73 Tandem lesions are typically caused by carotid artery disease and, less frequently, acute dissection. Tandem occlusions can be challenging cases, depending on the clinical, technical, and anatomical factors involved that affect decisions whether to:
Take an extracranial or intracranial approach first.
Stent the extracranial internal carotid artery and/or perform percutaneous angioplasty.
Not perform an acute carotid intervention.
Acute stenting of the extracranial internal carotid artery can increase intracranial clot lysis, treat the cause of the stroke, and reduce the incidence of recurrence.74 Acute stenting requires early initiation of potent antithrombotic treatment and therefore carries increased potential risk of haemorrhagic transformation, particularly in those individuals with large core infarctions and those receiving intravenous thrombolysis. Additionally, potential periprocedural risks are associated with additional interventional manoeuvres and a risk of in-stent thrombosis. But to reach and perform mechanical thrombectomy of the target intracranial large vessel occlusion, antegrade carotid angioplasty or stenting might be needed to allow passage to the intracranial circulation.
A recent international survey of stroke experts highlighted this treatment uncertainty with equipoise in the optimal management of tandem occlusions reported in 75% of respondents.75 A meta-analysis by Dufort et al of 1373 patients with tandem occlusions demonstrated a favourable modified Rankin score outcome at 90 days (odds ratio 1.43, 95% confidence interval 1.07 to 1.91) with intervention compared with no carotid intervention.76 In a German registry (GSR-ET), the intracranial approach was found to result in quicker flow restoration by about 20 minutes with a shift towards good outcomes.77
Furthermore, pooled analysis from the French, prospective, multicentre observational ETIS (Endovascular Treatment in Ischaemic Stroke) and the international TITAN (Thrombectomy in Tandem Lesions) registries included 603 patients with tandem occlusions.78 Three hundred and forty one of these patients were treated with acute stenting of the extracranial internal carotid artery and had higher odds of favourable outcome (odds ratio 1.09, 95% confidence interval 1.01 to 1.19, P=0.04) and successful reperfusion (1.19, 1.11 to 1.27, P<0.001). Despite higher rates of any intracranial haemorrhage in the stenting group, symptomatic intracranial haemorrhage, and parenchymal haematoma type 2 were not significantly different. The clinical benefit shown at 90 days from extracranial, internal carotid artery stenting was not found in tandem occlusion secondary to dissection.78
The current observational data suggest that acute stenting of the extracranial internal carotid artery in tandem occlusions results in favourable functional outcomes without substantial increase in symptomatic intracranial haemorrhage or periprocedural complications. But specific patient factors must be considered, particularly core infarct volume, anatomy and haemodynamics, patency of the circle of Willis, and underlying causes. Two randomised controlled trials evaluating the management of tandem occlusions are currently in progress (Tandem Occlusion Trial (EASI-TOC) and the TITAN trial), which will provide more definitive answers soon.79 80
Large core thrombectomy
The likelihood of achieving functional independence after mechanical thrombectomy has until recently been presumed to be lower in patients with a low ASPECTS at baseline, given the larger area of established tissue infarction. There are also associated higher risks of haemorrhagic transformation.81 These patients develop an increased frequency of post-stroke complications, have prolonged periods of hospital admission, and have higher mortality rates. RESCUE-Japan LIMIT was the first large core randomised controlled trial to publish and randomised 203 patients between mechanical thrombectomy and best medical treatment for patients who had an ASPECTS between 3 and 5.82 Despite higher rates of any intracranial haemorrhage in the thrombectomy arm (58% v 31.4%, P<0.001), 31% of patients who received thrombectomy still achieved the primary outcome (ambulatory independence, modified Rankin score 0-3 at 90 days) versus 12.7% who received best medical treatment (relative risk 2.43, 95% confidence interval 1.35 to 4.37).
The SELECT-2 and ANGEL-ASPECTS trials terminated early after the publication of RESCUE-Japan LIMIT, and showed that endovascular treatment in large core infarction (ASPECTS 3-5) had improved functional outcomes as defined by a primary outcome of modified Rankin score shift at 90 days (1 point improvement). Secondary outcomes for both randomised controlled trials also showed significantly improved rates of achieving functional independence (modified Rankin score 0-2).83 84 While the core criteria was based on non-contrast CT ASPECTS, there were high rates of advanced imaging to validate the findings, with 85% of patients in RESCUE-Japan LIMIT having MRI-ASPECTS and 98% of SELECT-2 patients having CT perfusion. As a result of these three trials, large core thrombectomy in selected patients has now been incorporated into the latest Stroke National Clinical Guidelines.85
Future directions
It is still unclear which is the optimal pre-hospital pathway to enable fast access to MT in both urban and non-urban areas. Several unanswered questions remain about the use of thrombectomy in a wider patient population. Some of the important areas of ongoing research are summarised below.
Mild stroke/low NIHSS
An NIHSS score of 6 has been shown to have a high positive predictive value for any vessel occlusion.34 Three of five HERMES trials excluded patients with mild strokes. However, patients can have minor stroke syndromes (defined by NIHSS 0-5) in the presence of an large vessel occlusion. These patients have good collateralisation but are at risk of early neurological deterioration if the collaterals fail.86 No level 1a data support the use of MT in minor stroke (defined by NIHSS 0-5). A systematic review and meta-analysis showed the safety and feasibility of MT in minor acute ischaemic stroke across a series of observational and retrospective studies.87 With respect to superiority, a multicentre cohort study of 301 patients indicated no difference in functional independence outcome (modified Rankin score 0-1 at 90 days) between those patients treated with MT and best medical treatment.88 A retrospective analysis of 312 patients in the Swiss Registry confirmed these findings and demonstrated a higher mortality in the MT group (9.3% v 2.8%; P=0.06).89 Currently, two randomised controlled trials are in progress (MOSTE and ENDOLOW), which aim to provide a definitive answer to MT in minor stroke.90 91
Medium vessel occlusion
Medium vessel occlusion refers to occlusions of the more distal branches of the intracranial circulation, namely, M2/3 of the middle cerebral artery, A2/3 of the anterior cerebral artery, and P2/3 of the posterior cerebral artery. These occlusions were largely excluded from the initial HERMES trials. They account for 25-40% of acute ischaemic strokes and could benefit from MT.92 Observational data from registries show promising results in medium vessel occlusions, especially in relation to the M2 segment.93–98 Ongoing randomised controlled trials investigating this area include ESCAPE-MeVO and DISTAL.
High pre-morbid dependency
Some centres are investigating the benefit of mechanical thrombectomy in patients with moderate to severe disability before stroke (with modified Rankin score scores of 3-5).99 100 These patients range from those who are able to independently ambulate but require assistance with some activities, to those who are bedbound and completely dependent. A systematic review and meta-analysis of six observational studies including 591 patients with pre-stroke dependency found a higher rate of unfavourable clinical outcome and a higher mortality rate when undergoing endovascular treatment than in those with no previous disability.101 However, a substantial proportion of these patients recovered well after treatment, indicating possible benefit in selected patients. More data from randomised controlled trials are needed, and investigators should reconsider what constitutes a so-called good outcome. The primary outcome for the original thrombectomy trial was a return to functional independence, which is not relevant in this cohort of patients with functional dependency.
Guidelines
Table 3 compares the three prominent guidelines for UK and Ireland (National Clinical Guideline for Stroke), North America (American Heart Association), and Europe (European Stroke Organisation/European Society of Minimally Invasive Neurological Therapy). The guidelines are concordant with their recommendations for the use of thrombectomy in the early and late windows.85 102 103 Bridging treatment with intravenous thrombolysis is recommended by all three. While the American Stroke Association recommends consideration of MT in patients with a pre-stroke modified Rankin score of 0-1, the National Clinical Guideline for Stroke extends this for a modified Rankin score of 0-2 and the European Stroke Organisation focuses on the radiological parameters. The American Stroke Association provides two separate recommendations for large vessel occlusions and medium vessel occlusions. All three sets of guideiness refer to the type of device and clot extraction technique used. While the National Clinical Guideline for Stroke moves away from advanced imaging except for in the very late windows (12-24 hours), the American Heart Association and European Stroke Organisation offer alternative strategies to advanced imaging and the optimal targets for peri-procedural hypertension management. The European Stroke Organisation considers pre-hospital pathways in thrombectomy but does not recommend one particular strategy.
Table 3.
Key recommendations for mechanical thrombectomy from three prominent guidelines in the UK and Ireland, North America, and Europe
| National Clinical Guideline for Stroke (UK and Ireland) | American Heart Association (North America) | European Stroke Organisation/European Society of Minimally Invasive Neurological Therapy (Europe) | |
| Treatment within 6 hours of symptom onset | Recommends | Recommends | Recommends |
| Treatment within 6-24 hours of symptom onset | Recommends with advanced imaging in the 12-24 hour window | Recommends with advanced imaging | Recommends with advanced imaging or CT angiography collateral scores up to 12 hours |
| Bridging treatment with intravenous thrombolysis in early window | Recommends | Recommends | Recommends |
| Clinical parameters for consideration of mechanical thrombectomy | NIHSS >5 and modified Rankin score 0-2; ASPECTS >2 in 0-12 hour window and with advanced imaging in 12-24 hours | NIHSS >5 and modified Rankin score 0-1; ASPECTS >5 | ASPECTS >5 |
| Vessel occlusions than mechanical thrombectomy is recommended for | Proximal anterior circulation, basilar, and vertebral | Proximal anterior circulation and M2/M3 within 6 hours | Not mentioned |
| Use of alternatives to advanced imaging | Not mentioned | Consider using collateral status up to 12 hours | Consider using collateral status up to 12 hours |
| Treatment of peri-procedural hypertension | Not mentioned | Maintain blood pressure <185/110 mm Hg even if intravenous thrombolysis not given | Maintain blood pressure <180/105 mm Hg |
| Pre-hospital models of patient identification for large vessel occlusion | References validated tools (eg, FAST/ROSIER) | Not mentioned | Does not recommend either drip and ship or mothership model over other |
| Use of thromboaspiration or stent retriever | Recommends both can be used | Thromboaspiration is recommended as non-inferior to stent retrievers | Does not recommend thromboaspiration alone |
NIHSS=National Institutes of Health Stroke Scale; ASPECTS=Alberta stroke programme early computed tomography score; FAST=face arms speech time; ROSIER=recognition of stroke in the emergency room; CT=computed tomography.
Conclusion
Stroke medicine has evolved rapidly in the past 30 years, epitomised by the considerable difference that mechanical thrombectomy has made to the lives of patients. In the early 2000s, the emphasis was on proving that thrombectomy was safe and possible. The HERMES collaboration justified its superiority and use in a tightly selected cohort of patients. In the past seven years, we have come a long way in expanding the initial selection criteria.7 As our technology, infrastructure, and understanding improves, the most pressing priority now is equal access for all appropriate patients, for this ground breaking treatment.
Questions for future research.
Is mechanical thrombectomy effective in comparison to best medical treatment in patients presenting with minor stroke syndromes (National Institutes of Health Stroke Scale <6)?
Is mechanical thrombectomy effective in comparison to best medical treatment in patients identified to have either primary or secondary medium vessel occlusion?
Is mechanical thrombectomy effective in patients who have moderate to severe disability before stroke?
What are the most effective interventions for patients presenting with tandem occlusions?
Patient involvement.
No patients were asked for input in the creation of this article.
Footnotes
Twitter: @SomaBanerjee73
Contributors: OR contributed to the design of the work, and drafted and critically revised the manuscript for critically important intellectual content. CH contributed to the drafting of sections relating to the technical aspects of thrombectomy and tandem occlusions. AM, JK, LD, and KL critically revised for critically important intellectual content. SB contributed to the conception, design, and drafting of the work, and critically revised for important intellectual content. OR, CH, AB, JK, LD, KL, and SB gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: SB declares KOL consulting for RapidAI.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 2.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–47. 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 3.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 8.Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 H from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet 2022;399:249–58. 10.1016/S0140-6736(21)01341-6 [DOI] [PubMed] [Google Scholar]
- 9.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 10.Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke 2017;12:896–905. 10.1177/1747493017701147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011:CD004816. 10.1002/14651858.CD004816.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huynh T, Perron S, O’Loughlin J, et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction. Circulation 2009;119:3101–9. 10.1161/CIRCULATIONAHA.108.793745 [DOI] [PubMed] [Google Scholar]
- 13.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 14.McMeekin P, Flynn D, James M, et al. Updating estimates of the number of UK stroke patients eligible for endovascular thrombectomy: incorporating recent evidence to facilitate service planning. Eur Stroke J 2021;6:349–56. 10.1177/23969873211059471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SSNAP - national. Available: https://www.strokeaudit.org/results/Clinical-audit/National-Results.aspx [Accessed 12 Dec 2022].
- 16.NHS Long Term Plan . Stroke care. Available: https://www.longtermplan.nhs.uk/online-version/chapter-3-further-progress-on-care-quality-and-outcomes/better-care-for-major-health-conditions/stroke-care/#ref [Accessed 12 Dec 2022].
- 17.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012;380:1241–9. 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 18.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914–23. 10.1056/NEJMoa1212793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012;380:1231–40. 10.1016/S0140-6736(12)61299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 21.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 22.Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J NeuroIntervent Surg 2014;6:260–4. 10.1136/neurintsurg-2014-011125 [DOI] [PubMed] [Google Scholar]
- 23.Deshaies EM. Tri-axial system using the Solitaire-FR and penumbra aspiration microcatheter for acute mechanical thrombectomy. J Clin Neurosci 2013;20:1303–5. 10.1016/j.jocn.2012.10.037 [DOI] [PubMed] [Google Scholar]
- 24.Turk AS, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a Multicentre, randomised, open label, blinded outcome, non-inferiority trial. The Lancet 2019;393:998–1008. 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 25.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018;49:660–6. 10.1161/STROKEAHA.117.020315 [DOI] [PubMed] [Google Scholar]
- 26.Feil K, Herzberg M, Dorn F, et al. General anesthesia versus conscious sedation in mechanical thrombectomy. J Stroke 2021;23:103–12. 10.5853/jos.2020.02404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell BCV, van Zwam WH, Goyal M, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol 2018;17:47–53. 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 28.Lee C-W, Chang Y-P, Huang Y-T, et al. General anesthesia but not conscious sedation improves functional outcome in patients receiving Endovascular Thrombectomy for acute ischemic stroke: a meta-analysis of randomized clinical trials and trial sequence analysis. Front Neurol 2022;13:1017098. 10.3389/fneur.2022.1017098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong H-G, Kim BJ, Kim H, et al. Blood pressure drop and penumbral tissue loss in nonrecanalized emergent large vessel occlusion. Stroke 2019;50:2677–84. 10.1161/STROKEAHA.119.025426 [DOI] [PubMed] [Google Scholar]
- 30.NICE . Recommendations | stroke and transient ischaemic attack in over 16S: diagnosis and initial management | guidance |. 2019.
- 31.Mazighi M, Richard S, Lapergue B, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. The Lancet Neurology 2021;20:265–74. 10.1016/S1474-4422(20)30483-X [DOI] [PubMed] [Google Scholar]
- 32.Yang P, Song L, Zhang Y, et al. Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (Enchanted2/MT): a Multicentre, open-label, blinded-endpoint, randomised controlled trial. Lancet 2022;400:1585–96. 10.1016/S0140-6736(22)01882-7 [DOI] [PubMed] [Google Scholar]
- 33.Lyden P. Using the National Institutes of health stroke scale. Stroke 2017;48:513–9. 10.1161/STROKEAHA.116.015434 [DOI] [PubMed] [Google Scholar]
- 34.Heldner MR, Hsieh K, Broeg-Morvay A, et al. Clinical prediction of large vessel occlusion in anterior circulation stroke: mission impossible? J Neurol 2016;263:1633–40. 10.1007/s00415-016-8180-6 [DOI] [PubMed] [Google Scholar]
- 35.Rubin MN, Barrett KM. What to do with wake-up stroke. Neurohospitalist 2015;5:161–72. 10.1177/1941874415576204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vagal A, Aviv R, Sucharew H, et al. Collateral clock is more important than time clock for tissue fate. Stroke 2018;49:2102–7. 10.1161/STROKEAHA.118.021484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reports - getting it right first time - GIRFT. Available: https://gettingitrightfirsttime.co.uk/girft-reports/ [Accessed 12 Dec 2022].
- 38.Vagal A, Wintermark M, Nael K, et al. Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology 2019;93:888–98. 10.1212/WNL.0000000000008481 [DOI] [PubMed] [Google Scholar]
- 39.Dhillon PS, Butt W, Podlasek A, et al. Perfusion imaging for endovascular thrombectomy in acute ischemic stroke is associated with improved functional outcomes in the early and late time windows. Stroke 2022;53:2770–8. 10.1161/STROKEAHA.121.038010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen TN, Abdalkader M, Nagel S, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol 2022;79:22–31. 10.1001/jamaneurol.2021.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai SM, Rocha M, Molyneaux BJ, et al. Thrombectomy 6-24 hours after stroke in trial ineligible patients. J NeuroIntervent Surg 2018;10:1033–7. 10.1136/neurintsurg-2018-013915 [DOI] [PubMed] [Google Scholar]
- 42.Pirson FAVA, Hinsenveld WH, Goldhoorn R-JB, et al. MR CLEAN-LATE, a multicenter randomized clinical trial of Endovascular treatment of acute ischemic stroke in the Netherlands for late arrivals: study protocol for a randomized controlled trial. Trials 2021;22:160. 10.1186/s13063-021-05092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olthuis SGH, Pirson FAV, Pinckaers FME, et al. Endovascular treatment versus no endovascular treatment after 6-24 H in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: a multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet 2023;401:1371–80. 10.1016/S0140-6736(23)00575-5 [DOI] [PubMed] [Google Scholar]
- 44.ClinicalTrials.gov . Randomization of Endovascular treatment in acute ischemic stroke in the extended time window - full text view. Available: https://clinicaltrials.gov/ct2/show/NCT04256096 [Accessed 12 Dec 2022].
- 45.Dong Z, Deng S, Zhang J, et al. Simplified stroke imaging selection modality for endovascular thrombectomy in the extended time window: systematic review and meta-analysis. J Neurointerv Surg 2022. 10.1136/jnis-2022-019556 [Epub ahead of print 7 Dec 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larrue V, von Kummer R, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with Recombinant tissue plasminogen activator. Stroke 2001;32:438–41. 10.1161/01.STR.32.2.438 [DOI] [PubMed] [Google Scholar]
- 47.Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke a topical review. Stroke 2017;48:2912–8. 10.1161/STROKEAHA.117.017208 [DOI] [PubMed] [Google Scholar]
- 48.Mitchell PJ, Yan B, Churilov L, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 H of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet 2022;400:116–25. 10.1016/S0140-6736(22)00564-5 [DOI] [PubMed] [Google Scholar]
- 49.Fischer U, Kaesmacher J, Strbian D, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. The Lancet 2022;400:104–15. 10.1016/S0140-6736(22)00537-2 [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA 2021;325:244. 10.1001/jama.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treurniet KM, LeCouffe NE, Kappelhof M, et al. MR CLEAN-NO IV: intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion-study protocol for a randomized clinical trial. Trials 2021;22:141. 10.1186/s13063-021-05063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med 2020;382:1981–93. 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 53.Zi W, Qiu Z, Li F, et al. Effect of Endovascular treatment alone vs intravenous Alteplase plus Endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA 2021;325:234–43. 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turc G, Tsivgoulis G, Audebert HJ, et al. European Stroke Organisation (ESO)-European society for minimally invasive neurological therapy (ESMINT) expedited recommendation on indication for intravenous Thrombolysis before mechanical Thrombectomy in patients with acute ischemic stroke and anterior circulation large vessel occlusion. J NeuroIntervent Surg 2022;14:209–27. 10.1136/neurintsurg-2021-018589 [DOI] [PubMed] [Google Scholar]
- 55.Romoli M, Paciaroni M, Tsivgoulis G, et al. Mothership versus drip-and-ship model for mechanical thrombectomy in acute stroke: a systematic review and meta-analysis for clinical and radiological outcomes. J Stroke 2020;22:317–23. 10.5853/jos.2020.01767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohamed A, Fatima N, Shuaib A, et al. Comparison of mothership versus drip-and-ship models in treating patients with acute ischemic stroke: a systematic review and meta-analysis. Int J Stroke 2022;17:141–54. 10.1177/17474930211013285 [DOI] [PubMed] [Google Scholar]
- 57.Ismail M, Armoiry X, Tau N, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg 2019;11:14–9. 10.1136/neurintsurg-2018-014249 [DOI] [PubMed] [Google Scholar]
- 58.Pérez de la Ossa N, Abilleira S, Jovin TG, et al. Effect of direct transportation to thrombectomy-capable center vs local stroke center on neurological outcomes in patients with suspected large-vessel occlusion stroke in Nonurban areas: the RACECAT randomized clinical trial. JAMA 2022;327:1782–94. 10.1001/jama.2022.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke 2014;45:87–91. 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 60.Brekenfeld C, Goebell E, Schmidt H, et al. 'Drip-and-drive’: shipping the neurointerventionalist to provide mechanical thrombectomy in primary stroke centers. J Neurointerv Surg 2018;10:932–6. 10.1136/neurintsurg-2017-013634 [DOI] [PubMed] [Google Scholar]
- 61.Turc G, Hadziahmetovic M, Walter S, et al. Comparison of mobile stroke unit with usual care for acute ischemic stroke management: a systematic review and meta-analysis. JAMA Neurol 2022;79:281. 10.1001/jamaneurol.2021.5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Requena M, Olivé-Gadea M, Muchada M, et al. Direct to angiography suite without stopping for computed tomography imaging for patients with acute stroke: a randomized clinical trial. JAMA Neurol 2021;78:1099. 10.1001/jamaneurol.2021.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alemseged F, Rocco A, Arba F, et al. Posterior National Institutes of health stroke scale improves prognostic accuracy in posterior circulation stroke. Stroke 2022;53:1247–55. 10.1161/STROKEAHA.120.034019 [DOI] [PubMed] [Google Scholar]
- 64.Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008;39:2485–90. 10.1161/STROKEAHA.107.511162 [DOI] [PubMed] [Google Scholar]
- 65.Puetz V, Khomenko A, Hill MD, et al. Extent of hypoattenuation on CT angiography source images in basilar artery occlusion: prognostic value in the basilar artery international cooperation study. Stroke 2011;42:3454–9. 10.1161/STROKEAHA.111.622175 [DOI] [PubMed] [Google Scholar]
- 66.Schaefer PW, Yoo AJ, Bell D, et al. CT angiography-source image hypoattenuation predicts clinical outcome in posterior circulation strokes treated with intra-arterial therapy. Stroke 2008;39:3107–9. 10.1161/STROKEAHA.108.517680 [DOI] [PubMed] [Google Scholar]
- 67.Pallesen LP, Khomenko A, Dzialowski I, et al. CT-angiography source images indicate less fatal outcome despite coma of patients in the basilar artery international cooperation study. Int J Stroke 2017;12:145–51. 10.1177/1747493016669886 [DOI] [PubMed] [Google Scholar]
- 68.Tao C, Qureshi AI, Yin Y, et al. Endovascular treatment versus best medical management in acute basilar artery occlusion strokes: results from the ATTENTION multicenter Registry. Circulation 2022;146:6–17. 10.1161/CIRCULATIONAHA.121.058544 [DOI] [PubMed] [Google Scholar]
- 69.Jovin TG, Li C, Wu L, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 2022;387:1373–84. 10.1056/NEJMoa2207576 [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020;19:115–22. 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 71.Nguyen TN, Strbian D. Endovascular therapy for stroke due to basilar artery occlusion: a BASIC challenge at BEST. Stroke 2021;52:3410–3. 10.1161/STROKEAHA.121.035948 [DOI] [PubMed] [Google Scholar]
- 72.Malik A, Drumm B, D’Anna L, et al. Mechanical thrombectomy in acute basilar artery stroke: a systematic review and meta-analysis of randomized controlled trials. BMC Neurol 2022;22:496. 10.1186/s12883-022-03015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubiera M, Ribo M, Delgado-Mederos R, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke 2006;37:2301–5. 10.1161/01.STR.0000237070.80133.1d [DOI] [PubMed] [Google Scholar]
- 74.Sivan-Hoffmann R, Gory B, Armoiry X, et al. Stent-retriever Thrombectomy for acute anterior ischemic stroke with tandem occlusion: a systematic review and meta-analysis. Eur Radiol 2017;27:247–54. 10.1007/s00330-016-4338-y [DOI] [PubMed] [Google Scholar]
- 75.Jacquin G, Poppe AY, Labrie M, et al. Lack of consensus among stroke experts on the optimal management of patients with acute tandem occlusion. Stroke 2019;50:1254–6. 10.1161/STROKEAHA.118.023758 [DOI] [PubMed] [Google Scholar]
- 76.Dufort G, Chen BY, Jacquin G, et al. Acute carotid Stenting in patients undergoing thrombectomy: a systematic review and meta-analysis. J NeuroIntervent Surg 2021;13:141–5. 10.1136/neurintsurg-2020-015817 [DOI] [PubMed] [Google Scholar]
- 77.Feil K, Herzberg M, Dorn F, et al. Tandem lesions in anterior circulation stroke: analysis of the German stroke registry-endovascular treatment. Stroke 2021;52:1265–75. 10.1161/STROKEAHA.120.031797 [DOI] [PubMed] [Google Scholar]
- 78.Anadani M, Marnat G, Consoli A, et al. Endovascular therapy of anterior circulation tandem occlusions: pooled analysis from the TITAN and ETIS registries. Stroke 2021;52:3097–105. 10.1161/STROKEAHA.120.033032 [DOI] [PubMed] [Google Scholar]
- 79.ClinicalTrials.gov . Endovascular acute stroke intervention - Tandem occlusion trial - full text view. Available: https://clinicaltrials.gov/ct2/show/NCT04261478 [Accessed 12 Dec 2022].
- 80.Zhu F, Hossu G, Soudant M, et al. Effect of emergent carotid Stenting during endovascular therapy for acute anterior circulation stroke patients with tandem occlusion: a multicenter, randomized, clinical trial (TITAN) protocol. Int J Stroke 2021;16:342–8. 10.1177/1747493020929948 [DOI] [PubMed] [Google Scholar]
- 81.Cucchiara B, Kasner SE, Tanne D, et al. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the stroke-acute ischemic NXY treatment (SAINT) I and SAINT II trials. Stroke 2009;40:3067–72. 10.1161/STROKEAHA.109.554386 [DOI] [PubMed] [Google Scholar]
- 82.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med 2022;386:1303–13. 10.1056/NEJMoa2118191 [DOI] [PubMed] [Google Scholar]
- 83.Sarraj A, Hassan AE, Abraham MG, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med 2023;388:1259–71. 10.1056/NEJMoa2214403 [DOI] [PubMed] [Google Scholar]
- 84.Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med 2023;388:1272–83. 10.1056/NEJMoa2213379 [DOI] [PubMed] [Google Scholar]
- 85.National clinical guideline for stroke. Available: https://www.strokeguideline.org/ [Accessed 30 Apr 2023].
- 86.Slawski D, Heit JJ. Treatment challenges in acute minor ischemic stroke. Front Neurol 2021;12:723637. 10.3389/fneur.2021.723637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCarthy DJ, Tonetti DA, Stone J, et al. More expansive horizons: a review of endovascular therapy for patients with low NIHSS scores. J Neurointerv Surg 2021;13:146–51. 10.1136/neurintsurg-2020-016583 [DOI] [PubMed] [Google Scholar]
- 88.Dargazanli C, Arquizan C, Gory B, et al. Mechanical thrombectomy for minor and mild stroke patients harboring large vessel occlusion in the anterior circulation: a multicenter cohort study. Stroke 2017;48:3274–81. 10.1161/STROKEAHA.117.018113 [DOI] [PubMed] [Google Scholar]
- 89.Manno C, Disanto G, Bianco G, et al. Outcome of endovascular therapy in stroke with large vessel occlusion and mild symptoms. Neurology 2019;93:e1618–26. 10.1212/WNL.0000000000008362 [DOI] [PubMed] [Google Scholar]
- 90.ClinicalTrials.gov . Endovascular therapy for low NIHSS ischemic strokes - full text view. Available: https://clinicaltrials.gov/ct2/show/NCT04167527 [Accessed 12 Dec 2022].
- 91.ClinicalTrials.gov . Minor stroke therapy evaluation - full text view. Available: https://www.clinicaltrials.gov/ct2/show/NCT03796468 [Accessed 9 Dec 2022].
- 92.Ospel JM, Goyal M. A review of endovascular treatment for medium vessel occlusion stroke. J Neurointerv Surg 2021;13:623–30. 10.1136/neurintsurg-2021-017321 [DOI] [PubMed] [Google Scholar]
- 93.Sweid A, Head J, Tjoumakaris S, et al. Mechanical thrombectomy in distal vessels: revascularization rates, complications, and functional outcome. World Neurosurg 2019;130:e1098–104. 10.1016/j.wneu.2019.07.098 [DOI] [PubMed] [Google Scholar]
- 94.Shek K, Alcock S, Ghrooda E, et al. Effectiveness and safety of endovascular thrombectomy for large versus medium vessel occlusions: a single-center experience. J Neurointerv Surg 2022;14:434–8. 10.1136/neurintsurg-2021-017502 [DOI] [PubMed] [Google Scholar]
- 95.Bhogal P, Bücke P, Aguilar Pérez M, et al. Mechanical thrombectomy for M2 occlusions: a single-centre experience. Intervent Neurol 2017;6:117–25. 10.1159/000458161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarraj A, Sangha N, Hussain MS, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol 2016;73:1291–6. 10.1001/jamaneurol.2016.2773 [DOI] [PubMed] [Google Scholar]
- 97.Flores A, Tomasello A, Cardona P, et al. Endovascular treatment for M2 occlusions in the era of Stentrievers: a descriptive multicenter experience. J Neurointerv Surg 2015;7:234–7. 10.1136/neurintsurg-2014-011100 [DOI] [PubMed] [Google Scholar]
- 98.Dorn F, Lockau H, Stetefeld H, et al. Mechanical thrombectomy of M2-occlusion. J Stroke Cerebrovasc Dis 2015;24:1465–70. 10.1016/j.jstrokecerebrovasdis.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 99.Goldhoorn R-JB, Verhagen M, Dippel DWJ, et al. Safety and outcome of endovascular treatment in prestroke-dependent patients: results from MR CLEAN registry. Stroke 2018;49:2406–14. 10.1161/STROKEAHA.118.022352 [DOI] [PubMed] [Google Scholar]
- 100.Tanaka K, Yamagami H, Yoshimoto T, et al. Endovascular therapy for acute ischemic stroke in patients with prestroke disability. JAHA 2021;10:20783. 10.1161/JAHA.121.020783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adamou A, Gkana A, Mavrovounis G, et al. Outcome of Endovascular Thrombectomy in pre-stroke dependent patients with acute ischemic stroke: A systematic review and meta-analysis. Frontiers in Neurology 2022;13:.:880046. 10.3389/fneur.2022.880046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for Healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:E344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 103.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical Thrombectomy in acute ischemic stroke. J NeuroIntervent Surg 2019. 10.1136/neurintsurg-2018-014569 [Epub ahead of print 26 Feb 2019]. [DOI] [PubMed] [Google Scholar]