Abstract
Strains of the feline immunodeficiency virus (FIV) presently under investigation exhibit distinct patterns of in vitro tropism. In particular, the adaptation of FIV for propagation in Crandell feline kidney (CrFK) cells results in the selection of strains capable of forming syncytia with cell lines of diverse species origin. The infection of CrFK cells by CrFK-adapted strains appears to require the chemokine receptor CXCR4 and is inhibited by its natural ligand, stromal cell-derived factor 1α (SDF-1α). Here we found that inhibitors of CXCR4-mediated infection by human immunodeficiency virus type I (HIV-1), such as the bicyclam AMD3100 and short peptides derived from the amino-terminal region of SDF-1α, also blocked infection of CrFK by FIV. Nevertheless, we observed differences in the ranking order of the peptides as inhibitors of FIV and HIV-1 and showed that such differences are related to the species origin of CXCR4 and not that of the viral envelope. These results suggest that, although the envelope glycoproteins of FIV and HIV-1 are substantially divergent, FIV and HIV-1 interact with CXCR4 in a highly similar manner. We have also addressed the role of CXCR4 in the life cycle of primary isolates of FIV. Various CXCR4 ligands inhibited infection of feline peripheral blood mononuclear cells (PBMC) by primary FIV isolates in a concentration-dependent manner. These ligands also blocked the viral transduction of feline PBMC by pseudotyped viral particles when infection was mediated by the envelope glycoprotein of a primary FIV isolate but not by the G protein of vesicular stomatitis virus, indicating that they act at an envelope-mediated step and presumably at viral entry. These findings strongly suggest that primary and CrFK-adapted strains of FIV, despite disparate in vitro tropisms, share usage of CXCR4.
Strains of the feline immunodeficiency virus (FIV) presently under study are distinguished by dichotomous patterns of in vitro tropism. While primary isolates of FIV generally infect primary feline T lymphocytes, as well as long-term feline T-lymphoid cell lines and macrophages, a subset of such isolates may readily be adapted for propagation in a feline fibroblastic cell line, Crandell feline kidney (CrFK) cells (4, 5, 9, 32, 50). Such adaptation creates viral strains that induce syncytia not only in feline but also in human and simian cell lines (30, 34), thus broadening tropism, inasmuch as the formation of syncytia reflects tropism. Patterns of in vitro tropism have also been used to differentiate primary isolates of human immunodeficiency virus type 1 (HIV-1). Macrophage-tropic isolates, predominant early in infection, may be readily propagated in macrophages but not in established T-cell lines, while T-tropic isolates, whose presence is generally associated with disease progression, replicate poorly in macrophages but efficiently in established T-cell lines (40, 53).
Such selectivity for particular host cell types has recently been illuminated by the identification of chemokine receptors as cofactors for viral entry. Biological phenotype has been shown to be associated with the use of particular chemokine receptors for viral entry (reviewed in references 16 and 25); while macrophage-tropic viruses are highly selective for CCR5, T-tropic viruses, including laboratory-adapted viruses, are distinguished by their ability to use CXCR4, although primary T-tropic viruses generally retain the capacity to use CCR5. Accordingly, infection by different strains of HIV-1 is inhibited by the natural ligands of their corresponding chemokine receptor, that is, stromal cell-derived factor 1α (SDF-1α) for CXCR4 (1, 28) and macrophage inflammatory proteins 1α and 1β and regulated-upon-activation, normal T expressed and secreted protein for CCR5 (6).
Similar to T-tropic isolates of HIV-1, strains of FIV adapted for propagation in CrFK cells appear to use the chemokine receptor CXCR4 for infection. Indeed, the formation of syncytia between human cells and chronically infected CrFK cells was inhibited by a monoclonal antibody (MAb) directed against human CXCR4 (47). Furthermore, ectopic expression of feline or human CXCR4 in nonpermissive human cells allowed the formation of syncytia with chronically infected CrFK cells (48), and infection of CrFK cells was inhibited by human SDF-1α (17). While these findings do not provide an immediate explanation for host cell range differences between FIV strains, they raise the possibility that primary isolates of FIV fail to infect CrFK cells because, unlike CrFK-adapted strains, they are unable to use CXCR4.
In the present study, we have sought low-molecular-weight inhibitors of FIV among known ligands for human CXCR4. In particular, we have examined the effects of short peptides derived from the amino-terminal portion of SDF-1α and the bicyclam AMD3100—both previously shown to inhibit infection by CXCR4-dependent strains of HIV-1 (10, 15, 19, 39)—on infection of CrFK cells. Furthermore, we have examined the effects of CXCR4 ligands on infection of feline peripheral blood mononuclear cells (PBMC) by primary strains of FIV, in order to determine whether the use of CXCR4 by CrFK-tropic but not primary FIV governs tropism.
MATERIALS AND METHODS
Tissue culture.
U373MG (14), HeLa, and 293T cell lines, as well as the ID10 clone (29) of CrFK, were cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml (complete DMEM). The feline T-lymphoid cell line FL-4 (49), which is chronically infected with the Petaluma strain of FIV, was cultivated in RPMI 1640 with fetal calf serum and antibiotics as described for CrFK cells (complete RPMI). Feline PBMC were isolated from the blood of specific-pathogen-free cats by density gradient centrifugation and activated for 3 days in complete RPMI containing 5 μg of concanavalin A (ConA) per ml, 50 μM 2-mercaptoethanol (2-ME), and 10 mM HEPES.
Virus.
Stocks of the laboratory-adapted Petaluma isolate (32) of FIV were derived from the supernatant of the FL-4 cell line. Stocks of the primary FIV isolates Be, Le, and Wi (26), as well as Wo (27), were derived from the supernatants of acutely infected PBMC. We define as primary those viral isolates that have been cultivated only in feline PBMC and for a limited number of passages (two to four). An HIV-1 stock was prepared by transient transfection of HeLa cells with the LAI molecular clone (31).
CXCR4 ligands.
Synthetic human SDF-1α was the generous gift of F. Baleux (Institut Pasteur, Paris, France). Synthetic peptides representing the amino-terminal portion of human SDF-1α and derivatives thereof (15) are shown in Fig. 1. The bicyclam AMD3100 was kindly provided by D. Schols (Rega Institute for Medical Research, Leuven, Belgium).
FIG. 1.
Amino acid sequences of the amino terminus of human SDF-1α and related synthetic peptides. Peptide residues representing substitutions to the wild-type SDF-1α sequence are underscored.
Antibodies.
The purified 6H8 murine MAb, raised against a peptide representing the amino-terminal 28 amino acids of human CXCR4 (23), was generously provided by A. Amara (Institut Pasteur). Purified monoclonal murine immunoglobulin G1 (MOPC-21; Sigma Chemical Company) was used as the isotype-matched control.
Expression vectors.
The env gene of a primary FIV isolate was cloned by PCR from DNA extracted from the lymph nodes of a cat with feline AIDS that had been experimentally infected by the Wo isolate 5.5 years previously. PCR fragments containing the entire env sequence were initially cloned into the plasmid pCR-Script Amp SK(+) (Stratagene). The env gene was then subcloned in the NotI and SacI sites of the plasmid VR1012 (kindly provided by VICAL Inc., San Diego, Calif.), placing env under the transcriptional control of the cytomegalovirus immediate early gene enhancer-promoter. The VRGWo2 clone, whose sequence will be reported elsewhere, was selected for use in this study. The env gene of the CrFK-adapted molecular clone 34TF10 (42) was cloned by PCR from the pTΔ20 expression vector (30). PCR fragments containing the entire env sequence were cloned in the PstI and BamHI sites of the plasmid VR1012, yielding VR34TF10. The pNL-Luc-E−R+ vector, containing an NL4-3 HIV-1 provirus defective in envelope glycoprotein and in which the nef gene has been replaced by a gene (luc) encoding luciferase (7), was kindly provided by T. Dragic and E. Landau (both at the Aaron Diamond AIDS Research Center, New York, N.Y.) and was used in complementation assays. The pVSVg vector, expressing the G protein of vesicular stomatitis virus (VSV) under the transcriptional control of the cytomegalovirus immediate early gene promoter (51), was the kind gift of A. Miyanohara (University of California, San Diego, La Jolla). Vectors expressing human (33) and feline (48) CXCR4, the latter kindly provided by B. Willett (University of Glasgow Veterinary School, Glasgow, United Kingdom), were also used.
Inhibition of FIV infection. (i) CrFK cells.
CrFK cells were resuspended at a concentration of 105 cells/ml in complete DMEM, and 0.4 ml was dispensed in the wells of 48-well tissue culture plates (4 × 104 cells per well) and cultivated overnight. The medium was removed from adherent CrFK cells in quadruplicate wells and replaced with 0.2 ml of serial dilutions of CXCR4 ligands prepared in complete DMEM. Following an incubation period of 15 min, 0.2 ml of a Petaluma stock, diluted so as to contain approximately 100 50% tissue culture infectious doses, calculated according to the method of Reed and Muench (35), was added to the wells. Infection was allowed to proceed for 2 h, at which time the ligands and virus were removed. The wells were washed once with 0.5 ml of complete DMEM, and cells were then cultivated in a medium containing the original dilution of CXCR4 ligands. Three days following infection, cells were fixed with acetone-methanol (50:50 [vol/vol]) and infected cells were enumerated following immunocytochemical staining for the FIV capsid protein, p25, as described elsewhere (38).
(ii) PBMC.
Serial dilutions of CXCR4 ligands were prepared in complete RPMI with HEPES and 2-ME, and 50 μl was dispensed in each well of transfer tubes (Costar). Activated feline PBMC were resuspended at a concentration of 4 × 106 cells/ml in complete RPMI with HEPES, 2-ME, and 200 U of recombinant human interleukin 2 (IL-2) per ml, and 100 μl was added to triplicate or quadruplicate wells (4 × 105 cells per well). Following an incubation period of 15 min, 50 μl of viral stock, diluted so as to contain approximately 100 50% tissue culture infectious doses (35), was added to the wells. Infection was allowed to proceed for 24 h, at which time the PBMC were washed twice with 0.5 ml of complete RPMI, resuspended in 200 μl of complete RPMI containing HEPES, 2-ME, and 100 U of IL-2 per ml, and transferred to 96-well tissue culture plates. Half the medium was replaced 4 days later, and aliquots of 10 μl were removed after 4 and 7 days for analysis of reverse transcriptase activity. Quantitative densitometry of autoradiography film was performed with the software program NIH Image V1.54 (27a).
Inhibition of HIV-1 infection.
U373MG cells (human astroglioma cells expressing CD4 and bearing the lacZ gene of Escherichia coli under the transcriptional control of the HIV-1 long terminal repeat) were seeded in six-well plates and transfected with plasmids coding for either human or feline CXCR4 by using the calcium phosphate method. Eighteen hours after transfection the cells were trypsinized and transferred to 24-well plates. Forty-eight hours after transfection peptides were added at indicated final concentrations and the cells were infected with HIV-1LAI. The cells were fixed with 0.5% glutaraldehyde 24 h after infection and stained with the X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) substrate (11). Blue cells were scored under ×20 magnification.
FIV pseudotypes.
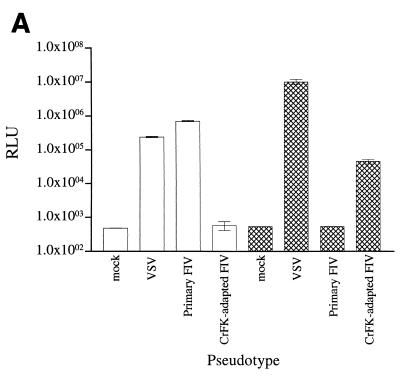
The VRGWo2 and VR34TF10 vectors, expressing the envelope glycoprotein of primary and CrFK-adapted strains of FIV, respectively, and the pVSVg vector, expressing the G protein of VSV, were used to pseudotype HIV-1 pNL-Luc-E−R+. 293T cells (1.5 × 106 cells) were seeded in 10-cm-diameter petri dishes and cotransfected by using the calcium phosphate technique with 10 μg of VRGWo2 and 5 μg of pNL-Luc-E−R+ or with 7.5 μg of VR34TF10 and 7.5 μg of pNL-Luc-E−R+ to generate FIV-pseudotyped virions or with 7.5 μg of both pNL-Luc-E−R+ and pVSVg to generate VSV-pseudotyped virions. The medium was changed 8 h after transfection, and supernatants were collected 24 (VRGWo2 and pVSVg) or 40 (VR34TF10) h later. The content of the HIV-1 Gag protein p24 in the supernatants was measured by using a commercial enzyme-linked immunosorbent assay kit (Innogenetics, Zwijndrecht, Belgium). Pseudotyped virus was used to infect mitogen-activated feline PBMC (essentially as described below) or CrFK cells (6 × 105 cells per well of 48-well plates), with the quantities of p24 indicated (see the legend to Fig. 6A). Cells were washed twice with phosphate-buffered saline 48 h after infection, lysed, and analyzed as described below.
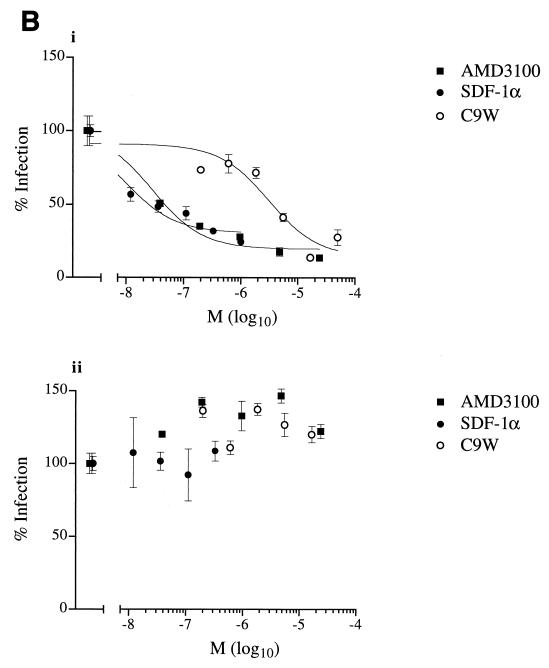
FIG. 6.
FIV entry. (A) Infection of primary feline PBMC and CrFK cells by virions pseudotyped with the envelope glycoprotein from primary and adapted FIV or the G protein of VSV. The transduction of PBMC was performed with approximately 20, 400, and 15 ng of p24 for primary FIV envelope glycoprotein, adapted FIV glycoprotein envelope, and G protein, respectively. The transduction of CrFK cells was performed with approximately 200, 800, and 15 ng of p24 for primary FIV envelope glycoprotein, adapted FIV envelope glycoprotein, and G protein, respectively. Results for feline PBMC (white bars) and CrFK cells (crosshatched bars) are means ± standard errors of the mean (error bars) for triplicate wells. RLU, relative light units. (B) Effects of CXCR4 ligands on entry. The transduction of primary feline PBMC by virions pseudotyped with the envelope glycoprotein from a primary FIV isolate (panel i) or the G protein of VSV (panel ii) was examined in the presence of SDF-1α, the bicyclam AMD3100, or peptide C9W. Luciferase activity (% infection) is expressed as a percentage of luciferase activity without an inhibitor. Results are means (M) ± standard errors of the mean (error bars) for triplicate wells.
Inhibition of FIV entry.
Mitogen-activated PBMC were dispensed in transfer tubes (Corning Costar Corporation, Cambridge, Mass.) at 4 × 105 cells per well in 200 μl of complete RPMI containing HEPES, 2-ME, and 100 U of IL-2 per ml. A medium (100 μl) containing indicated concentrations of SDF-1α, the bicyclam AMD3100, or SDF-1α-derived peptide was added to triplicate wells prior to the addition of 100 μl of pseudotyped virus (containing approximately 20 ng of p24 for VRGWo2 supernatants and 15 ng of p24 for VSV supernatants). The supernatant of cells transfected only with pNL-Luc-E−R+ was used as a control. After overnight incubation, cells were washed twice with phosphate-buffered saline. Cells were cultured for a further 48 h and then washed and lysed in 100 μl of luciferase lysis buffer (Promega France, Charbonnières, France). The amount of luciferase activity in 10 μl of lysate was measured by using Promega luciferase kit reagents (Promega) in a luminometer.
Toxicity.
The toxicity of CXCR4 ligands for feline PBMC was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Briefly, PBMC were cultivated under the conditions used for the inhibition of infection but in the absence of virus. Twenty-four hours after infection, PBMC were washed once with 0.5 ml of complete RPMI, resuspended in 100 μl of a solution containing 300 μg of MTT per ml in complete RPMI containing HEPES, 2-ME, and 100 U of IL-2 per ml, and transferred to 96-well plates. PBMC were cultivated for 3 h in the presence of MTT. The converted dye was solubilized by the addition of 150 μl of acidified isopropanol and measured spectrophotometrically at 540 nm.
Flow cytometry.
The expression of CXCR4 on feline cells was examined by flow cytometry with an EPICS Elite flow cytometer (Coulter, Margency, France) after immunostaining of CrFK cells or feline PBMC. Suspensions of CrFK cells were prepared by treating monolayers with 0.02% EDTA. PBMC were examined prior to activation and 24, 48, and 72 h after stimulation with ConA. Cells were labelled by conventional methods with, for approximately 5 × 105 cells, 1 μg of purified anti-CXCR4 (6H8) or isotype-matched MAb as the primary antibody and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Southern Biotechnology, Birmingham, Ala.) as the secondary antibody. Cells were fixed with 1% paraformaldehyde following labelling.
RESULTS
Various CXCR4 ligands inhibit infection of CrFK cells by CrFK-adapted virus.
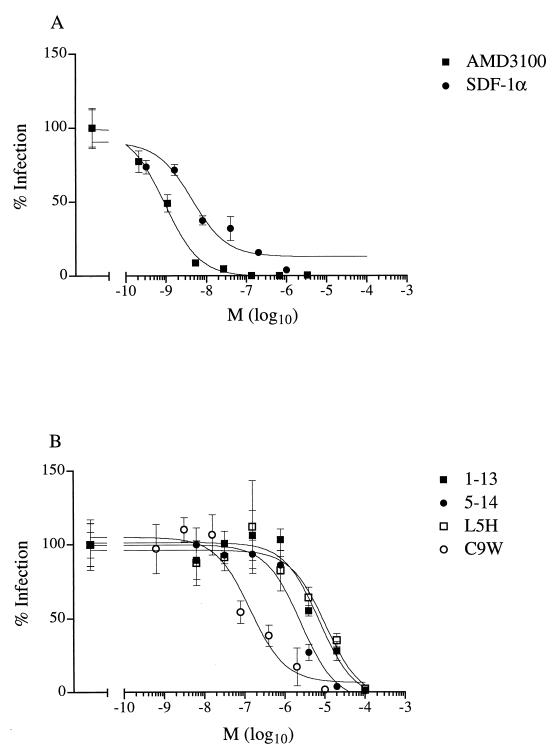
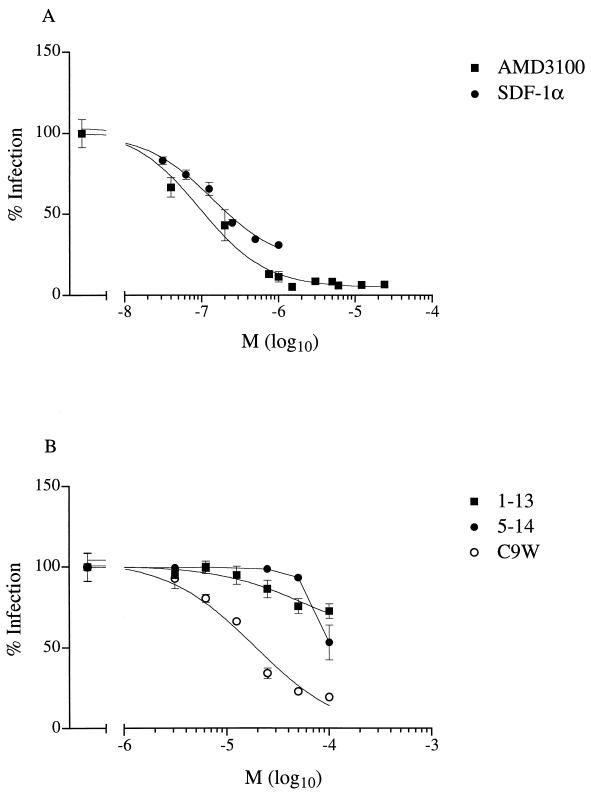
In order to identify low-molecular-weight inhibitors of CrFK-adapted FIV, we examined the effects of various CXCR4 ligands after the infection of the CrFK cell line. We observed that not only human SDF-1α, as previously shown by Hosie et al. (17), but also the bicyclam AMD3100 inhibited infection by a CrFK-adapted strain (Petaluma) of FIV (50% inhibitory concentration [IC50] of 4 and 0.9 nM, respectively) (Fig. 2A). We have previously shown that short peptides derived from the amino-terminal portion of human SDF-1α inhibit infection by CXCR4-dependent strains of HIV-1 (15). For HIV-1, all inhibitory peptides were located at the amino terminus, as exemplified by wild-type peptides comprising residues 1 to 13 (peptide 1-13) or residues 5 to 14 (peptide 5-14). The substitution of histidine for leucine at residue 5 and tryptophan for cysteine at position 9 created peptides—L5H and C9W, respectively—with superior inhibitory activities against HIV-1. We examined the effects of the wild-type peptide and substitution analogues on infection of the CrFK cell line (Fig. 2B). Similar to previous observations regarding HIV-1, peptides representing the amino-terminal portion of SDF-1α inhibited the infection of CrFK cells by a CrFK-adapted strain (Petaluma). However, while the C9W peptide proved to be a more efficient inhibitor than wild-type peptides 1-13 and 5-14 (IC50 of 0.2, 7, and 3 μM, respectively), the substitution of histidine for leucine (peptide L5H) did not improve the inhibitory activity (IC50 of 10 μM) against FIV (Fig. 2B).
FIG. 2.
Effects of CXCR4 ligands on infection of CrFK cells by CrFK-adapted virus. Ligands were SDF-1α or the bicyclam AMD3100 (A) or synthetic peptides based on the amino terminus of SDF-1α (B). The numbers of infected cells are expressed as percentages of the number of infected cells without an inhibitor. Results are the means (M) ± standard errors of the mean (error bars) of triplicate (SDF-1α) or quadruplicate (AMD3100 and peptides) wells.
Relative inhibitory activities of SDF-1α-derived peptides depend upon receptor origin.
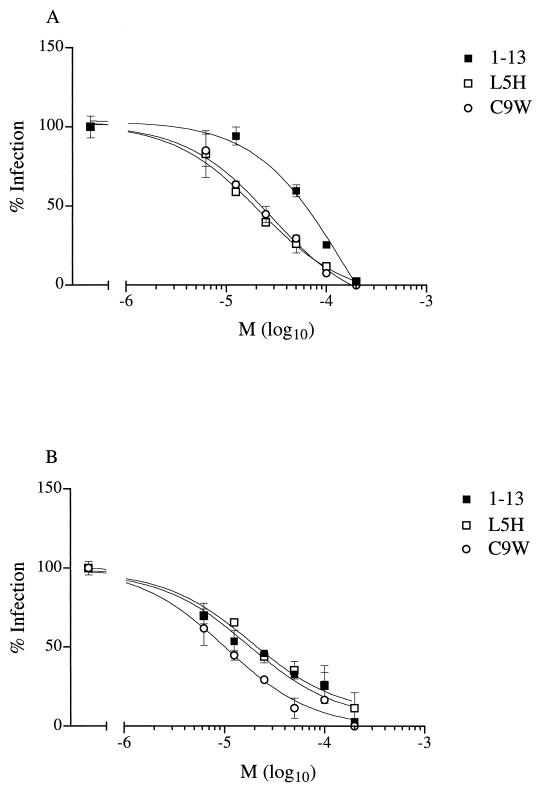
Having observed differences in the ranking order of inhibitory activities of peptides derived from SDF-1α on HIV-1 and FIV, we addressed the question of whether these differences stemmed from the virus or receptor. We compared the inhibitory activities of wild-type, L5H, and C9W peptides on the entry of a CXCR4-dependent strain of HIV-1 when entry was mediated by feline or human CXCR4. When entry was mediated by human CXCR4, both substitution analogues exhibited inhibitory activities superior to that of the wild-type peptide (Fig. 3A). By contrast, when entry was mediated by feline CXCR4, the wild-type and L5H peptides exhibited activities inferior to that of the C9W peptide (Fig. 3B). These results suggest that, despite the strong homology between feline and human CXCR4 and the substantial divergence of the envelope glycoproteins of FIV and HIV-1, the extent of inhibition mediated by a given CXCR4 ligand depends upon the species origin of CXCR4 and not that of the viral envelope.
FIG. 3.
Inhibitory activities of peptides derived from the SDF-1α sequence on HIV-1 entry mediated by human CXCR4 and feline CXCR4. Infection of cells transfected by human (A) or feline (B) CXCR4 was examined in the presence of wild-type peptide 1-13 and substitution analogues L5H and C9W. The numbers of infected cells are expressed as percentages of the number of infected cells without an inhibitor, after correcting for the background level. Results are means (M) ± standard errors of the mean (error bars) for wells. In the absence of an inhibitor, wells contained 440 ± 37 and 117 ± 10 positive cells for human and feline CXCR4, respectively.
Various CXCR4 ligands inhibit infection of primary feline blasts by primary virus.
We wished to determine whether infection by primary isolates of FIV, unlike infection of CrFK cells by CrFK-adapted FIV strains, was independent of CXCR4. To this end, we have examined the effect of CXCR4 ligands on infection of feline blasts by the primary FIV isolate Wo. The Wo isolate does not infect CrFK cells and repeated attempts to adapt Wo for propagation in this cell line have failed (26). Somewhat unexpectedly, both SDF-1α and the bicyclam AMD3100 inhibited infection in a dose-dependent manner (IC50 of approximately 140 and 90 nM, respectively) (Fig. 4A). Peptides representing the amino-terminal portion of SDF-1α also inhibited infection of feline PBMC by primary FIV (Fig. 4B). Moreover, the relative efficiencies of the peptides resembled that observed for CrFK-adapted virus in CrFK cells; that is, C9W was a considerably better inhibitor (IC50 of 20 μM) than wild-type peptides 1-13 and 5-14 (IC50 > 100 μM). Inhibition by the C9W peptide did not appear to be secondary to toxicity for PBMC, since no reduction in cell viability was observed at a concentration of 50 μM by MTT test (data not shown).
FIG. 4.
Effects of CXCR4 ligands on infection of primary feline PBMC by the primary FIV isolate Wo. Ligands were SDF-1α or the bicyclam AMD3100 (A) or synthetic peptides based on the amino terminus of SDF-1α (B). Reverse transcriptase activity (% infection) is expressed as a percentage of reverse transcriptase activity without an inhibitor. Results are means (M) ± standard errors of the mean (error bars) for quadruplicate wells.
Usage of CXCR4 is a common feature of infection by primary FIV isolates.
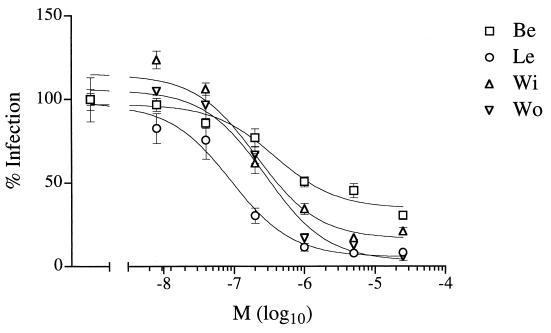
In order to determine whether usage of CXCR4 is frequent among primary isolates of FIV, we examined whether the bicyclam AMD3100 would inhibit infection by diverse primary strains of FIV representing independent clinical isolates. We observed a marked concentration-dependent reduction in infection of feline blasts by all four primary isolates examined (Fig. 5), although sensitivity to AMD3100 varied substantially, with approximate IC50 ranging from 90 nM to 1 μM for the Le and Be isolates, respectively.
FIG. 5.
Effect of the CXCR4 ligand AMD3100 on infection of primary feline PBMC by the primary FIV isolates Be, Le, Wi, and Wo. Reverse transcriptase activity (% infection) is expressed as a percentage of reverse transcriptase activity without an inhibitor. Results are means (M) ± standard errors of the mean (error bars) for quadruplicate wells.
Various CXCR4 ligands inhibit transduction of primary feline blasts.
We wished to determine whether CXCR4 ligands exerted their effect on infection by primary FIV isolates at an envelope-mediated step, which might reflect the inhibition of viral entry. To this end, we devised a heterologous complementation assay that permitted the use of HIV-1-based reporter gene constructs and in which the effects of CXCR4 ligands on a single round of infection could be evaluated. The envelope glycoprotein (VRGWo2) of the primary FIV isolate, like the G protein of VSV, permitted the efficient production of pseudotyped virus and transduction of primary feline PBMC but not CrFK cells with luc (Fig. 6A). By contrast, the envelope glycoprotein (VR34TF10) of the CrFK-adapted FIV isolate did not allow transduction of primary feline blasts, although it did allow transduction—albeit at high doses—of CrFK cells (Fig. 6A). These results show that the Wo isolate and the 34TF10 clone fail to infect CrFK cells and PBMC, respectively, owing to a restriction imposed at an envelope-mediated step, in accordance with conclusions drawn from other studies (30). We then examined whether the CXCR4 ligands would inhibit transduction and whether such inhibition would be restricted to infection mediated by the FIV envelope. Diverse CXCR4 ligands—SDF-1α, AMD3100, and the C9W peptide—reduced luciferase activity in a dose-dependent manner, but only when viral particles were pseudotyped with the FIV envelope glycoprotein (Fig. 6A). No such inhibition was observed when viral particles were pseudotyped with the G protein of VSV (Fig. 6B); rather, the ligands appeared to improve transduction to a slight extent, perhaps by enhancing the activation of primary feline blasts.
Surface expression of CXCR4 on feline lymphocytes increases upon mitogenic activation.
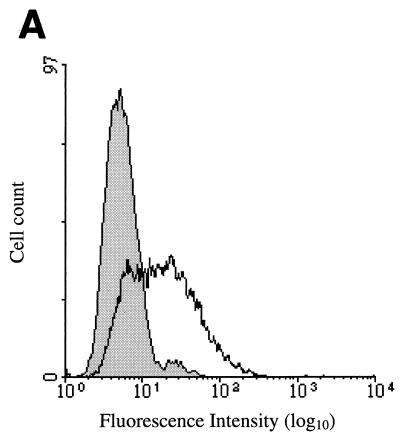
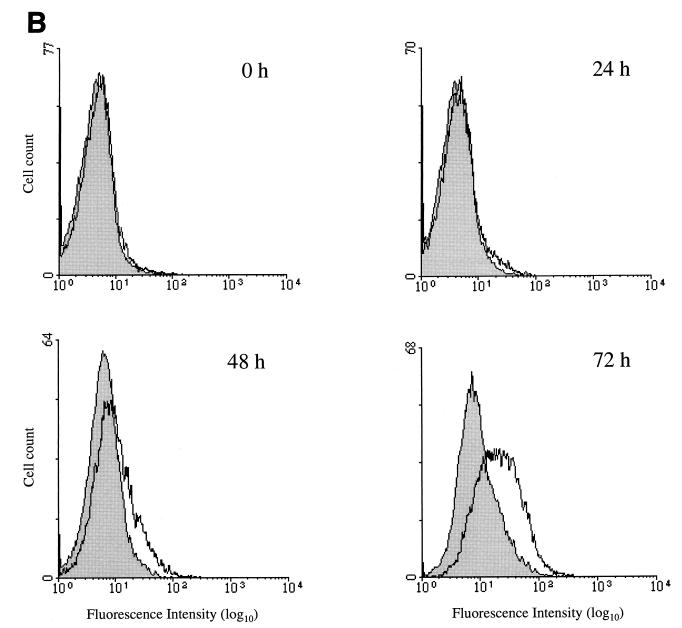
We have examined the surface expression of CXCR4 on cells susceptible to infection by CrFK-tropic and primary strains of FIV by flow cytometry, by using a monoclonal antibody (6H8) raised against a peptide corresponding to the amino terminus of human CXCR4. In agreement with the results of Hosie et al. (17), CXCR4 was detected at the surface of CrFK cells (mean logarithms of fluorescence intensity of 17.0 and 5.5 for anti-CXCR4 and isotype-matched control antibodies, respectively) (Fig. 7A). We also examined the surface expression of CXCR4 on feline PBMC as a function of the time of mitogenic activation. Immediately after isolation and prior to activation, little or no expression of CXCR4 was detected (Fig. 7B). Upon culture in the presence of ConA, the surface expression of CXCR4 increased over the time period examined (3 days) (mean logarithms of fluorescence intensity of 19.7 and 8.7 for anti-CXCR4 and isotype-matched control antibodies, respectively).
FIG. 7.
Surface expression of feline CXCR4 on cellular targets of FIV. Surface expression of feline CXCR4 on CrFK cells (A) and during mitogenic activation of feline PBMC (B) was examined by flow cytometry following immunostaining. Histograms show analysis with anti-CXCR4 (white) and isotype-matched control (shaded) antibodies. CXCR4 expression of PBMC was determined after 0, 24, 48, and 72 h of mitogenic stimulation. Cell count is plotted against fluorescence intensity.
DISCUSSION
Here we have shown that various CXCR4 ligands, previously shown to inhibit infection by CXCR4-dependent strains of HIV-1, inhibited the infection of the CrFK cell line by CrFK-adapted FIV. Furthermore, diverse CXCR4 ligands inhibited the infection of feline PBMC by primary isolates of FIV. Upon examination of a single round of infection, such ligands inhibited the transduction of primary PBMC when viral envelope proteins were furnished by a primary isolate of FIV, but not VSV. We interpret these results as meaning that feline CXCR4 is used by at least some primary strains of FIV for viral entry. The usage of CXCR4, initially described for CrFK-adapted strains (47, 48), may thus be extended to primary isolates of FIV. These findings have profound implications for the pathogenesis of FIV infection.
Diverse ligands for human CXCR4, including human SDF-1α, the bicyclam AMD3100, and SDF-1α-derived peptides, inhibited the infection of the CrFK cell line by CrFK-adapted FIV and feline lymphocytes by primary strains of FIV. This finding is in agreement with the high degree of homology—94.9% amino acid identity—between CXCR4 of feline and human origins (48). Furthermore, the predicted primary structure of mature human and feline SDF-1α is perfectly conserved (22). The use of ligands to evaluate the usage of particular chemokine receptors in viral infection requires that the ligand be highly selective for the receptor in question. The bicyclam AMD3100, in that it inhibits the infection of CXCR4- but not CCR5-dependent strains of HIV-1, has been considered highly selective, among human chemokine receptors, for CXCR4 (10, 19, 39). While selectivity for human CXCR4 does not in itself ensure selectivity for feline CXCR4, preferential binding to feline CXCR4 may be inferred when results obtained in feline cells with structurally diverse CXCR4 ligands converge. Thus, the inhibition of FIV infection by diverse CXCR4 ligands, as observed in the present study, may be interpreted as implicating CXCR4 in FIV infection.
The apparent efficacy of CXCR4 ligands as inhibitors of FIV infection depends upon the cellular model examined. In particular, inhibition is apparently less efficient for the infection of primary feline PBMC by primary FIV strains than for infection of CrFK cells by CrFK-adapted virus. This discrepancy would appear to be largely attributable to differences in the cellular substrate, since it is observed even when the infectivity of a single viral preparation (Petaluma) is compared in the two cellular contexts (data not shown). Although analysis of surface expression of CXCR4 suggests that the steady-state level of CXCR4 is no higher on feline blasts than on CrFK cells, a requirement for higher concentrations of ligands to saturate CXCR4 sites may potentially be related to the kinetics of CXCR4 recycling to the cell surface or the structural heterogeneity in different cell types (21). Alternatively, the reduced efficacy of CXCR4 ligands may be attributed to the use of additional chemokine receptors present on primary PBMC. Multiple receptors could be present on the same target cells or, since primary PBMC represent a highly heterogeneous population, could define subpopulations of lymphocytes. The usage of alternate receptors, however, would appear to account for a small portion of entry events, in that infection is markedly reduced in primary PBMC by CXCR4 ligands, albeit at concentrations superior to those required in CrFK cells to obtain similar levels of inhibition. It should also be noted that the effect of CXCR4 ligands on primary blasts is unlikely to be null: we observed enhanced infection by virions pseudotyped with the VSV G protein in the presence of CXCR4 ligands. This suggests that the inhibitory effect of CXCR4 ligands on entry mediated by CXCR4 may be offset by a stimulatory effect on viral transcription, or other postentry steps of the viral life cycle, once entry is achieved.
Diverse cellular targets of FIV—CrFK cells, and PBMC—express CXCR4 at the plasma membrane. Moreover, surface expression of CXCR4 increased markedly upon mitogenic stimulation of feline PBMC. Similar observations have been made for human CXCR4 (2). The increase in surface expression of CXCR4 on feline blasts is contemporaneous with an increase in susceptibility to FIV infection (37). It is thus likely that the activation of lymphocytes impinges on the viral replicative cycle not only at postentry steps, such as transcription, but also at entry.
Upon comparison of the inhibitory activities of various peptide ligands of CXCR4 on infection by FIV and HIV-1, we observed that the relative efficacies of the peptides depended on whether infection was mediated by feline or human CXCR4. Since the inhibitory activity of the SDF-1α-derived peptides is presumed to be related principally to receptor occupancy (15), it is likely that the substitutions made in the wild-type sequence, giving rise to the L5H and C9W peptides, improved the inhibitory activity against HIV-1 by increasing the affinity for human CXCR4. Presumably the C9W, but not L5H, substitution improved the affinity for feline CXCR4, resulting in an enhanced inhibitory activity against FIV, as well as HIV-1, when infection was mediated by feline CXCR4. These differences in inhibitory activity are likely to reflect structural discrepancies between feline and human CXCR4, despite their high degree of homology (48). The structural diversity in these highly related receptors is suggested by differences not only in ligand binding but also in coreceptor activity (46). Thus, while minor differences in chemokine receptors gave rise to readily detectable differences in the relative efficacies of SDF-1α-derived peptides, the substantial structural divergence in the envelope glycoproteins of FIV and HIV-1 did not. This would appear to suggest that FIV and HIV-1 use CXCR4 for entry in a similar fashion and, furthermore, that interactions with CXCR4 permitting fusion may be highly restricted in nature.
Despite the distinct host ranges exhibited by CrFK-adapted and primary viruses, both types of virus appear to use CXCR4 preferentially. The molecular basis for FIV tropism remains enigmatic, although several plausible explanations may be advanced. Should we assume that the entry of FIV requires an interaction between the viral envelope and CXCR4 exclusively, CXCR4 expression on CrFK cells must permit a productive interaction with the envelope glycoprotein from CrFK-adapted virus but not from primary virus. While the comparison of surface expression of CXCR4 on CrFK cells and PBMC suggests that the quantity of CXCR4 on CrFK should not be limiting for primary virus, CXCR4 expression may be qualitatively different in CrFK, perhaps owing to cell-specific processing events. Should, however, we suppose that CXCR4 generally behaves as a coreceptor for FIV, that is, in conjunction with a cofactor assuming a role similar to that of CD4 in infection by primate lentiviruses, other hypotheses may be formulated. First, CrFK-adapted virus, while using the same chemokine receptor as primary virus, may have gained the use of a CD4-like cofactor expressed on CrFK cells. This hypothesis would require that the cofactor be expressed on cell lines originating from highly divergent species. An alternate hypothesis, appealing in its simplicity and analogy with HIV, may be put forward. Strains of HIV-1 and -2 selected for growth in transformed T-cell lines use CXCR4 and, infrequently, gain independence from CD4 (12, 13, 36). It is possible that laboratory-adapted FIV strains, like CD4-independent strains of HIV, may have gained independence from a CD4-like cofactor, required by most primary isolates for entry. Such an eventuality, already suggested by others (48), is in keeping with the promiscuity of the envelope glycoprotein of CrFK-adapted virus. Finally, other as yet undefined cell-type-associated factors may account for the failure of primary strains of FIV to infect CXCR4-expressing CrFK cells; in this regard it is noteworthy that human macrophages, despite expression of CXCR4, are under certain circumstances refractory to infection by HIV-1 CXCR4-dependent strains (44, 52).
The tropism of HIV-1 in vitro, principally related to the preferential usage of either CXCR4 or CCR5, is thought to be determined, at least in part, by the third variable region (V3) of the extracellular envelope glycoprotein (reviewed in references 20 and 25). In particular, the usage of CXCR4 by HIV-1 is associated with substitutions that increase the net charge of the V3 region (18). These findings underpin an electrostatic model of interaction between the viral envelope and CXCR4 by which the positively charged V3 region of the X4 envelope binds to negatively charged residues of the extracellular domains of CXCR4 (3, 24). Curiously, the adaptation of FIV for propagation in CrFK cells results in the selection of virus with substitutions that increase the net charge of the FIV V3 region (41, 45) in a manner highly analogous to that observed for HIV-1. However, since both host range variants of FIV use CXCR4 despite V3 polymorphism, substitutions in the V3 region appear to affect FIV tropism in a manner unrelated to coreceptor selection. Moreover, an apparently unrelated substitution in the transmembrane glycoprotein has also been found sufficient to confer tropism for CrFK cells (43). It therefore seems likely that the host range variation of FIV implies a global modification in the envelope structure. Should CrFK-adapted strains be analogous to CD4-independent strains of HIV, substitutions conferring CrFK cell tropism may perhaps prime the viral envelope for fusion, obviating the need for an interaction with a principal receptor to trigger fusion (36).
The discovery that at least some primary FIV isolates use CXCR4 has implications for the pathogenesis of FIV infection. Strains of HIV-1 that predominate during primary and asymptomatic infection selectively use CCR5, while the progression to disease is associated with the emergence of virus with broader chemokine receptor usage and, in particular, that are capable of using CXCR4 (8). Regarding FIV, the primary strains that we have examined were isolated from cats presenting clinical symptoms of feline AIDS and thus may well correspond to late-stage isolates. It will be of considerable interest to know whether FIV isolates that predominate during primary and asymptomatic infection use CXCR4 and whether the emergence of viral variants that use CXCR4 predicts disease progression and clinical decline. The definition of chemokine receptor usage during the course of FIV infection and, in particular, its relation to pathogenesis could ultimately contribute to understanding the role of CXCR4-dependent viruses in pathogenic processes leading to human immunodeficiency.
While at least some primary isolates of FIV use CXCR4 for entry into physiologically relevant target cells, the simian lentiviruses, with the exception of a recently described isolate from a mandrill (39a), have not been reported to use CXCR4. FIV infection thus emerges as the sole animal model for human AIDS in which the role of CXCR4 usage in pathogenesis may be studied and in which the utility of CXCR4 as a target for therapeutic intervention may be examined. Since, however, the efficacy of CXCR4 ligands as viral inhibitors depends on the species origin of CXCR4, in vitro studies to define optimal ligands for feline CXCR4 will necessarily precede the in vivo assessment of inhibitors of FIV infection.
ACKNOWLEDGMENTS
We thank Ali Amara, Françoise Baleux, Dominique Schols, and Brian Willett for donation of reagents. We are also grateful to Isabelle Bouchaert for assistance with flow cytometry experiments.
J.R. and N.H. were supported by grants from Sidaction and the Agence Nationale pour la Recherche contre le SIDA. This work was supported by the European concerted action FAVEUR.
REFERENCES
- 1.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 2.Bleul C C, Wu L, Hoxie J A, Springer T A, MacKay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brelot A, Heveker N, Adema K, Hosie M J, Willett B, Alizon M. Effect of mutations in the second extracellular loop of CXCR4 on its utilization by human and feline immunodeficiency viruses. J Virol. 1999;73:2576–2586. doi: 10.1128/jvi.73.4.2576-2586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown W C, Bissey L D, Logan K S, Pedersen N C, Elder J H, Collisson E W. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991;65:3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner D, Pedersen N C. Infection of peritoneal macrophages in vitro and in vivo with feline immunodeficiency virus. J Virol. 1989;63:5483–5488. doi: 10.1128/jvi.63.12.5483-5488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocchi F, De Vico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 7.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency type I in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 8.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandell R A, Fabricant C G, Nelson-Rees W A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK) In Vitro. 1973;9:176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- 10.Donzella G A, Schols D, Lin S W, Esté J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clerq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 11.Dragic T, Hazan U, Alizon M. Detection of cell fusion mediated by the envelopes of human retroviruses by transactivation of a reporter gene. Viral gene techniques. Methods Mol Genet. 1995;7:218–236. [Google Scholar]
- 12.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briand P, Hazan U. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis Turner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 14.Harrington R, Geballe A P. Cofactor requirements for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heveker N, Montes M, Germeroth L, Amara A, Trautmann A, Alizon M, Schneider-Mergener J. Dissociation of the signalling and antiviral properties of SDF-1-derived peptides. Curr Biol. 1998;8:369–376. doi: 10.1016/s0960-9822(98)70155-1. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman T L, Doms R W. Chemokines and coreceptors in HIV/SIV-host interactions. AIDS. 1998;12:S17–S26. [PubMed] [Google Scholar]
- 17.Hosie M J, Broere N, Hesselgesser J, Turner J D, Hoxie J A, Neil J C, Willett B J. Modulation of feline immunodeficiency infection by stromal cell-derived factor. J Virol. 1998;72:2097–2104. doi: 10.1128/jvi.72.3.2097-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiken C L, de Jong J J, Baan E, Keulen W, Tersmette M, Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992;66:4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labrosse B, Brelot A, Heveker N, Sol N, Schols D, De Clercq E, Alizon M. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 1998;72:6381–6388. doi: 10.1128/jvi.72.8.6381-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazawa, T. Personal communication.
- 23.Mondor I, Moulard M, Ugolini S, Klasse P-J, Hoxie J, Amara A, Delaunay T, Wyatt R, Sodroski J, Sattentau Q J. Interactions among HIV gp120, CD4, and CXCR4: dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology. 1998;248:394–405. doi: 10.1006/viro.1998.9282. [DOI] [PubMed] [Google Scholar]
- 24.Moore J P, Binley J. HIV envelope’s letters boxed into shape. Nature. 1998;393:630–631. doi: 10.1038/31359. [DOI] [PubMed] [Google Scholar]
- 25.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 26.Moraillon, A. Unpublished observations.
- 27.Moraillon A, Barre-Sinoussi F, Parodi A, Moraillon R, Dauguet C. In vitro properties and experimental pathogenic effect of three feline immunodeficiency viruses (FIV) isolated from cats with terminal disease. Vet Microbiol. 1992;31:41–54. doi: 10.1016/0378-1135(92)90140-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.National Institutes of Health. NIH Image V1.54. National Institutes of Health, Bethesda, Md.
- 28.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzano-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 29.Osborne R, Rigby M, Siebelink K, Neil J C, Jarrett O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J Gen Virol. 1994;75:3641–3645. doi: 10.1099/0022-1317-75-12-3641. [DOI] [PubMed] [Google Scholar]
- 30.Pancino G, Castelot S, Sonigo P. Differences in feline immunodeficiency virus (FIV) host cell range correlate with envelope fusogenic properties. Virology. 1995;206:796–806. doi: 10.1006/viro.1995.1002. [DOI] [PubMed] [Google Scholar]
- 31.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 33.Pleskoff O, Sol N, Labrosse B, Alizon M. Human immunodeficiency virus strains differ in their ability to infect CD4+ cells expressing the rat homolog of CXCR-4 (fusin) J Virol. 1997;71:3259–3262. doi: 10.1128/jvi.71.4.3259-3262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poeschla E M, Looney D J. CXCR4 is required by a nonprimate lentivirus: heterologous expression of feline immunodeficiency virus in human, rodent, and feline cells. J Virol. 1998;72:6858–6866. doi: 10.1128/jvi.72.8.6858-6866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 36.Reeves J D, Schulz T F. The CD4-independent tropism of human immunodeficiency virus type 2 involves several regions of the envelope protein and correlates with a reduced activation threshold for envelope-mediated fusion. J Virol. 1997;71:1453–1465. doi: 10.1128/jvi.71.2.1453-1465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson, J. Unpublished observations.
- 38.Richardson J, Fossati I, Moraillon A, Castelot S, Sonigo P, Pancino G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J Gen Virol. 1996;77:759–771. doi: 10.1099/0022-1317-77-4-759. [DOI] [PubMed] [Google Scholar]
- 39.Schols D, Struyf S, Damme J V, Esté J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.Schols D, De Clercq E. The simian immunodeficiency virus mnd(GB-1) strain uses CXCR4, not CCR5, as coreceptor for entry in human cells. J Gen Virol. 1998;79:2203–2205. doi: 10.1099/0022-1317-79-9-2203. [DOI] [PubMed] [Google Scholar]
- 40.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E Y, van Steenwijk R P, Lange J M A, Eeftink Schattenkerk J K M, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siebelink K H J, Karlas J A, Rimmelzwaan G F, Osterhaus A D M E. A determinant of feline immunodeficiency virus involved in CrFK tropism. Vet Immunol Immunopathol. 1995;46:61–69. doi: 10.1016/0165-2427(94)07006-s. [DOI] [PubMed] [Google Scholar]
- 42.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vahlenkamp T W, Vershoor E J, Schuurman N N M P, van Vliet A L W, Horzinek M C, Egberink H F, de Ronde A. A single amino acid substitution in the transmembrane envelope glycoprotein of feline immunodeficiency virus alters cellular tropism. J Virol. 1997;71:7132–7135. doi: 10.1128/jvi.71.9.7132-7135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi A G, Vercelli D. Cutting edge: CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J Immunol. 1998;161:2084–2088. [PubMed] [Google Scholar]
- 45.Verschoor E J, Boven L A, Blaak H, van Vliet A L W. A single mutation within the V3 envelope neutralization domain of feline immunodeficiency virus determines its tropism for CRFK cells. J Virol. 1995;69:4752–4757. doi: 10.1128/jvi.69.8.4752-4757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willett B J, Adema K, Heveker N, Brelot A, Picard L, Alizon M, Turner J D, Hoxie J A, Peiper S, Neil J C, Hosie M. The second extracellular loop of CXCR4 determines its function as a receptor for feline immunodeficiency virus. J Virol. 1998;72:6475–6481. doi: 10.1128/jvi.72.8.6475-6481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willett B J, Hosie M J, Shaw A R E, Neil J C. Inhibition of feline immunodeficiency infection by CD9 antibody operates after viral entry and is independent of virus tropism. J Gen Virol. 1997;78:611–618. doi: 10.1099/0022-1317-78-3-611. [DOI] [PubMed] [Google Scholar]
- 48.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto J K, Ackley C D, Zochlinski H, Louie H, Pembroke E, Torten M, Hansen H, Munn R, Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto J K, Sparger E, Ho E W, Andersen P R, O’Connor T P, Mandell C P, Lowenstine L, Munn R, Pedersen N C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1258. [PubMed] [Google Scholar]
- 51.Yee J-K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D H. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]