Supplemental Digital Content is Available in the Text.
Background:
Repository corticotrophin injection (RCI, Acthar Gel) and intravenous methylprednisolone (IVMP) improve the rate but not the extent of visual recovery following acute optic neuritis. RCI has adrenal-stimulating and melanocortin receptor-stimulating properties that may endow it with unique anti-inflammatory properties relative to IVMP.
Methods:
Individuals with acute optic neuritis of less than 2 weeks duration were prospectively enrolled and randomized 1:1 to receive either RCI or IVMP. Peripapillary retinal nerve fiber layer (pRNFL) and ganglion cell plus inner plexiform layer thickness (GC + IPL) were serially evaluated by OCT. In addition, patient-reported outcomes (PROs) for changes in fatigue, mood, visual function, depression, and quality of life (QOL) were measured, and high and low contrast visual acuity were recorded.
Results:
Thirty-seven subjects were enrolled (19 RCI; 18 IVMP); the average time from symptom to treatment was 8.8 days. At 6 months, there was no difference in the primary outcome: loss of average pRNFL thickness in the affected eye (RCI vs IVMP: −13.1 vs −11.7 µm, P = 0.88) 6 months after randomization. Additional outcomes also showed no difference between treatment groups: 6-month attenuation of GC + IPL thickness (RCI vs IVMP: −13.8 vs −12.0 µm, P = 0.58) and frequency of pRNFL swelling at 1 month (RCI vs IVMP: 63% vs 72%, P = 0.73) and 3 months (RCI vs IVMP: 26% vs 31%, P = 0.99). Both treatments resulted in improvement in visual function and PROs.
Conclusions:
Treatment of acute optic neuritis with RCI or IVMP produced no clinically meaningful differences in optic nerve structure or visual function.
Administration of high-dose corticosteroids is the recommended treatment for acute optic neuritis,1 Corticotropin or high-dose intravenous solumedrol accelerate visual recovery but do not improve long-term visual outcomes.2,3 Although the anti-inflammatory and immunosuppressive effects of corticosteroids are broad,4 corticotropin possesses immunomodulatory effects that extend beyond its ability to promote the synthesis and release of glucocorticoids through the hypothalamic–pituitary–adrenal axis. Through its binding to multiple melanocortin receptor (MCR) subtypes, corticotropin may modulate adaptive and innate immune cell activity5 and promote axonal sprouting and outgrowth.6
Optical coherence tomography (OCT) is a sensitive and reproducible method for measuring optic nerve and retinal injury. Both peripapillary retinal nerve fiber layer (pRNFL) and ganglion cell plus inner plexiform layer (GC + IPL) thinning can be detected weeks after optic neuritis and become fully manifest by 6 months.7,8 In addition, in the setting of acute optic neuritis, pRNFL thickening, presumably from post-inflammatory axoplasmic stasis, is evident in recently affected eyes and may persist for up to 3 months.9 The aim of this study was to use serial OCT to determine whether there are measurable anti-inflammatory and/or neuroprotective effects that distinguish repository corticotrophin injection (RCI) from high-dose IV methylprednisolone for the treatment of acute optic neuritis and whether such differences are subsequently manifest clinically in validated patient-reported outcomes.
METHODS
Study Design
We performed a multicenter, randomized active control phase 4 trial involving 3 academic US hospital centers (University of Colorado, University of Texas Southwestern, and University of Pennsylvania). Local institutional review board approval was provided at each site, and the study was conducted in accordance with the provisions of the International Conference on Harmonization Guidelines for Good Clinical Practice. The study is registered with ClinicalTrials.gov (NCT01838174).
Participants
We enrolled adult patients (18–55 years old, inclusive) with a clinical diagnosis of unilateral acute demyelinating optic neuritis within 14 days of symptom onset (vision loss, eye pain, pain on movement, or color vision impairment). The diagnosis of optic neuritis was made by the referring physician and did not require additional MRI and clinical confirmation. The qualifying episode of optic neuritis needed to be the first clinical episode of optic neuritis in the affected eye. Exclusion criteria related to the diagnosis of optic neuritis included (1) bilateral clinical optic neuritis or (2) severe disc edema, no light perception vision, lack of pain, hemorrhage, retinal exudates, or optic atrophy in the affected eye. A study protocol is available at ClinicalTrials.gov (NCT01838174). All participants submitted written informed consent.
Randomization and Masking
Participants were randomized (1:1) in an unmasked fashion to receive either repository corticotropin injection (RCI, Acthar Gel, Mallinckrodt Pharmaceuticals, Bridgewater, NJ) or intravenous methylprednisolone (IVMP). The randomization sequence was generated by computer at the University of Colorado and assigned to subjects without stratification or minimization factors. All sites locally enrolled patients.
Procedures
RCI was administered subcutaneously: 80 units (U) daily for 5 days followed by 40 U daily for 10 days. IVMP (1,000 mg) was administered intravenously daily for 3 days followed by 11 days of oral prednisone (60 mg daily). Instructions for SQ RCI administration were provided by the investigator, and the first injection was observed on site. IVMP was provided either at the patient's home by home health agency nurse or through a local infusion center. No other immunosuppressants were permitted during the 6-month study period; however, those participants with multiple sclerosis (MS) or subsequently diagnosed with MS were allowed to continue, alter, or initiate disease modifying therapy beginning 12 weeks after enrollment at the discretion of their treating physician (Table 1).
TABLE 1.
Summary of demographic and baseline data
| Overall, n (%) | RCI, n (%) | IVSM, n (%) | P | |
| 37 | 19 (51.35%) | 18 (48.65%) | ||
| Female, n (%) | 27 (73.0%) | 15 (78.9%) | 12 (66.7%) | 0.48* |
| Age (y) | ||||
| Mean (SD) | 34.9 (7.2) | 36.0 (5.5) | 33.8 (8.7) | 0.23† |
| Median | 34.2 | 35.8 | 33.0 | |
| Time from symptom onset to treatment (d) | ||||
| Mean (SD) | 8.8 (3.2) | 8.5 (2.8) | 9.1 (3.7) | 0.68† |
| Median | 8 | 8 | 9 | |
| Baseline affected eye HCVA score | ||||
| Mean (SD) | 23.5 (22.7) | 22.9 (24.3) | 24.0 (21.7) | 0.76† |
| Median | 22.0 | 17.0 | 22.0 | |
| Previous MS diagnosis, n (%) | ||||
| Yes | 7 (18.9%) | 3 (15.8%) | 4 (22.2%) | 0.69* |
| No | 24 (64.9) | 13 (68.4) | 11 (61.1%) | |
| Prior optic neuritis fellow eye, N (%) | ||||
| Yes | 4 (10.8) | 2 (10.5) | 2 (11.1) | 0.99* |
| No | 33 (89.2) | 17 (89.5) | 16 (88.9) |
Fisher exact test.
Wilcoxon rank-sum test.
HCVA, high-contrast visual acuity; IVMP, intravenous methylprednisolone; MS, multiple sclerosis; RCI, repository corticotrophin injection.
Study visits were conducted at months 0 (within 2 weeks of optic neuritis onset), 1, 3, and 6. Each visit included testing of high-contrast (HCVA, Early Treatment Diabetic Retinopathy Study charts) and low-contrast (LCVA, Sloan 2.5% and 1.25% letter acuity charts) visual acuity, color vision testing (Farnsworth D-15), and spectral domain (SD) OCT of the optic disc and macula. Furthermore, Modified Fatigue Impact Scale (MFIS), Beck Depression Inventory (BDI), 25-Item National Eye Institute Visual Function Questionnaire and 10-item supplement Neuro-ophthalmic Supplement (NEI-VFQ-25 + NOS-10), and the Multiple Sclerosis Quality of Life 54-item questionnaire (MSQOL-54) were administered. Automated perimetry (30-2 Threshold Test, SITA-Standard) was performed at months 0 and 6. SD OCT images of the optic disc (Optic Disc Cube 200 × 200) and macula (Macular Cube 512 × 128) were obtained using a Cirrus device (Carl Zeiss Meditec, Dublin, CA; Software version 7.0.1.290), and pRNFL and GC + IPL thicknesses were measured using the ONH and RNFL Analysis and Ganglion Cell Analysis packages. Swelling of the pRNFL was defined as any quadrant or sector with an affected/contralateral thickness eye ratio ≥1.1.9
Outcomes
Outcomes and adverse events were evaluated in an unblinded manner. The primary outcome was the difference in the mean pRNFL thickness in the affected eye at 6 months minus the mean pRNFL thickness in the contralateral eye at baseline adjusted for the baseline mean pRNFL thickness of the contralateral eye. The secondary outcome was the frequency of optic nerves with RNFL swelling at 1 and 3 months. Tertiary exploratory outcomes included (1) the difference in the mean GC + IPL thickness in the affected eye at 6 months minus the mean GC + IPL thickness in the contralateral eye at baseline adjusted for the baseline mean GC + IPL thickness of the contralateral eye and (2) fatigue, mood, visual acuity, and quality of life in patients as measured by MFIS, BDI, HCVA, 2.5% and 1.25% LCVA, NEI-VFQ-25 + NOS-10, and the MSQOL-54.
Adverse events were assessed by the investigator for severity, relationship to study treatment, and potential cause. All adverse events were evaluated by the local investigator for criteria of a severe adverse event. Since both active arms used approved therapies for acute optic neuritis, there was no data safety monitoring board. If and when it was necessary, appropriate medical care was provided for all adverse events.
Statistical Analysis
Initial sample size calculations were based on the primary endpoint, considering a 50% treatment difference (estimated mean pRNFL thicknesses of 77.9 and 85.8 μm; standard deviations 13 and 8.3 μm), a 2-sided alpha of 5%, and a power of 80%. To assess the average difference in pRNFL and GC + IPL thickness losses between treatment groups at 6 months after treatment, linear regression models were fitted with the outcome as the difference in baseline fellow eye thickness minus 6-month affected eye thickness. Baseline fellow eye thickness and study center were included as covariates in these models. The presence of pRNFL swelling for each subject at each timepoint was defined as the ratio of affected eye pRNFL thickness to fellow eye pRNFL thickness ratios greater than 1.1 in any quadrant or sector. Differences in frequency of pRNFL swelling between treatment groups at each timepoint were assessed using the Fisher exact test. VF-25 + NOS-10 composite scores,10 MFIS scores,11 MSQOL-54 scores,12 and Beck Depression Inventory scores13 were calculated and assessed between treatment groups at all timepoints separately using the Wilcoxon rank-sum test. Differences in high-contrast visual acuity (HCVA) and low-contrast visual acuity (LCVA) among treatment groups between baseline and 6 months were assessed using the Wilcoxon signed-rank test, and the differences in improvement in HCVA and LCVA between baseline and 6 months between treatment groups were assessed using the Wilcoxon rank-sum test. All analysis was performed using R version 4.0.2.
RESULTS
Subject Characteristics
From October 2013 to October 2020, 38 subjects were screened at 3 participating sites in the United States. Thirty-seven subjects were enrolled; 19 were randomized to receive RCI, and 18 were randomized to IVMP (see consort diagram in Supplemental Digital Content, http://links.lww.com/WNO/A693). Recruitment was halted in August 2021, before reaching the planned enrolment of 60 subjects, due to challenges posed by the COVID pandemic.
Of the 37 subjects assigned to either RCI or IVMP, 19 (100%) and 17 (94·4%), respectively, were included in the study analyses. Thirty-six (97·2%) subjects completed the 6-month study protocol; 1 patient was lost to follow-up before month 3, whereas a second patient missed the month 3 visit but returned for the final month 6 visit. Most subjects were women (n = 27, 73·0%) with no history of MS (n = 24, 64·9%); 4 subjects had a history of optic neuritis in the fellow eye. Subject age, baseline acuity in the affected eye, and the average time from symptom onset to treatment did not differ between treatment groups (Table 1).
Baseline clinical and laboratory data were available for 31 subjects. 30/31 subjects had a relative afferent pupillary defect (RAPD) in the affected eye; the subject without a RAPD had suffered prior optic neuritis in the fellow eye. 29/31 subjects had a clinical MRI of the orbits, and 28/29 subjects had MRI confirmation of their optic neuritis. 26/31 subjects were tested for serum AQP4-IgG, and 10/31 subjects were tested for serum MOG-IgG. No subjects were seropositive for AQP4-IgG; however, 2 subjects were seropositive for MOG-IgG. One subject had a high titer (1:100), was diagnosed with myelin–oligodendrocyte glycoprotein–associated disease (MOGAD), and randomized to corticotrophin. The second subject had a low, nondiagnostic, titer of 1:20 and was subsequently diagnosed with MS.
Efficacy
The study did not meet the primary endpoint. There was no clinically relevant difference between the treatment groups in the mean pRNFL thickness at 6 months between the affected eye and the baseline contralateral eye (mean [SD]: −13·1 [15·5] vs −11·7 [12·0] µm, P = 0·88). Similarly, there was no difference between treatment groups in the GC + IPL thickness (µm) when comparing the affected and baseline contralateral eye (mean [SD]: −13·8 [10·7] vs −12·0 [12·4] µm, P = 0·58) (Table 2). The frequency of eyes with RNFL edema was not different between the treatment groups at any time point, either at the inception of our ascertainment or during the recovery period (Table 2).
TABLE 2.
Optical coherence tomography measures of pRNFL thickness, GC + IPL thickness, and pRNFL swelling
| Mean (SD) or n (%) | RCI | IVSM | P | |||||||||
| Baseline (n = 19) | 1 mo (n = 19) | 3 mo (n = 19) | 6 mo (n = 19) | Baseline (n = 18) | 1 mo (n = 18) | 3 mo (n = 16) | 6 mo (n = 17) | Baseline | 1 mo | 3 mo | 6 mo | |
| Affected eye pRNFL thickness (µm) | 112.0 (31.2) | 98.2 (17.3) | 80.3 (17.0) | 78.8 (15.9) | 125 (63.2) | 93.4 (12.2) | 80.3 (12.7) | 78.6 (12.5) | 0.33* | 0.31* | 0.75* | 0.98* |
| Difference in affected and baseline contralateral pRNFL thickness (µm) | 20.7 (29.3) | 7.16 (13.2) | −10.7 (14.6) | −13.1 (15.5) | 35.0 (61.4) | 3.33 (8.82) | −9.69 (11.6) | −11.7 (12.0) | 0.26† | 0.29† | 0.90† | 0.88† |
| Affected eye GC + IPL thickness (µm) | 76.5 (9.42) | 66.7 (8.64) | 63.8 (9.56) | 65.9 (11.2) | 76.9 (15.5) | 70.3 (9.92) | 68.6 (11.6) | 67.4 (11.5) | 0.97* | 0.36* | 0.28* | 0.65* |
| Difference in affected and baseline contralateral GC + IPL thickness (µm) | −1.8 (8.7) | −11.5 (9.5) | −14.5 (11.0) | −13.8 (10.7) | −2.1 (13.7) | −8.7 (10.4) | −10.4 (12.1) | −12.0 (12.4) | 0.99‡ | 0.31‡ | 0.25‡ | 0.58‡ |
| Frequency of pRNFL swelling§ | 17 (89.4%) | 12 (63.2%) | 5 (26.3%) | 4 (21.1%) | 12 (66.7%) | 13 (72.2%) | 5 (31.3%) | 4 (28.6%) | 0.12‖ | 0.73‖ | 0.99‖ | 0.99‖ |
Significance assessed using linear regression controlling for study center.
Significance assessed using linear regression controlling for baseline contralateral eye RNFL thickness and study center.
Significance assessed using linear regression controlling for baseline contralateral eye GC + IPL thickness and study center.
Number of subjects with pRNFL thickness in any quadrant or sector with an affected/contralateral eye ratio ≥1.1.
Fisher exact test for count data.
GC + IPL, ganglion cell plus inner plexiform layer; IVMP, intravenous methylprednisolone; mM, microns; pRNFL, peripapillary retinal nerve fiber layer; RCI, repository corticotrophin injection.
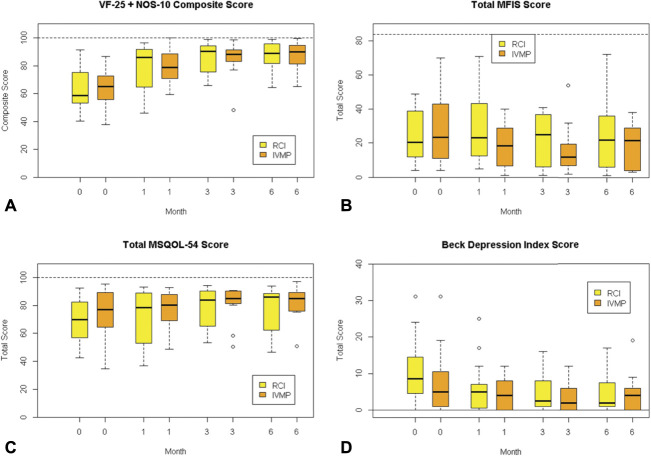
We evaluated HCVA, LCVA, and multiple patient-reported outcomes: VF-25 + NOS-10, MFIS, BDI, and MSQOL-54. HCVA, 2.5% LCVA and 1.25% LCVA significantly improved from baseline in the affected eye of the RCI and IVMP treatment groups; however, there was no difference in the level of improvement in the visual acuity between treatment groups (Table 3). There were no differences between RCI-treated and IVMP-treated subjects in any patient-reported outcomes: VFQ-25 + NOS-10, MFIS, MSQOL-54, and BDI (Fig. 1). VFQ-25 + NOS-10 improved to a similar extent with both treatments.
TABLE 3.
High-contrast (HCVA) and low-contrast (LCVA) visual acuity
| Mean (SD) | RCI | IVSM | Between-Group Comparison of Improvement | ||||||
| Baseline | 6 mo | Improvement* | P † | Baseline | 6 mo | Improvement* | P † | P ‡ | |
| Affected eye | |||||||||
| HCVA | 22.9 (24.3) | 51.4 (12.3) | 28.5 (18.3) | <0.01 | 24.0 (21.7) | 49.7 (21.8) | 27.9 (23.4) | <0.01 | 0.90 |
| 1.25% LCVA | 0.0 (0.0) | 8.4 (11.3) | 8.4 (11.3) | 0.02 | 1.5 (5.7) | 7.6 (11.5) | 7.6 (11.5) | 0.02 | 0.89 |
| 2.5% LCVA | 2.1 (4.9) | 18.8 (15.0) | 16.7 (13.6) | <0.01 | 2.7 (9.3) | 17.6 (14.2) | 17.1 (14.7) | <0.01 | 0.98 |
| Fellow eye | |||||||||
| HCVA | 51.5 (5.8) | 53.9 (7.7) | 2.4 (5.9) | 0.16 | 56.6 (9.4) | 62.4 (5.1) | 6.1 (8.6) | 0.01 | 0.21 |
| 1.25% LCVA | 14.4 (9.2) | 15.4 (8.3) | 0.9 (7.5) | 0.84 | 19.1 (11.3) | 19.9 (10.7) | 1.2 (9.7) | 0.22 | 0.44 |
| 2.5% LCVA | 28.2 (6.8) | 29.3 (10.0) | 1.1 (9.9) | 0.66 | 31.5 (13.1) | 33.9 (10.3) | 3.0 (14.4) | 0.09 | 0.44 |
Improvement calculated as the 6-month score minus baseline score.
P-value for differences among groups calculated using the Wilcoxon signed-rank test.
P-value for difference between groups calculated using the Wilcoxon rank-sum test.
IVMP, intravenous methylprednisolone; RCI, repository corticotrophin injection.
FIG. 1.
Box plots of the median and interquartile range for the scores of the (A) 25-item National Eye Institute Visual Function Questionnaire and 10-item supplement Neuro-ophthalmic Supplement (NEI-VFQ-25 + NOS-10), (B) Modified Fatigue Impact Scale (Total MFIS), (C) Multiple Sclerosis Quality of Life 54-item questionnaire (total MSQOL-54 score), and (D) Beck Depression Index score for each treatment arm at 0, 1, 3, and 6 months. Dashed lines represent the upper bound of possible scores. The highest possible score for the Beck Depression Index is 63 (not shown).
Safety
Adverse events were reported by 71% of subjects receiving RCI and 61% of subjects receiving IVMP. The majority were rated as mild (RCI: 95% and IVMP: 79%). The most frequently reported adverse events were injection site reactions (RCI: 42%), insomnia (RCI: 26%; IVMP: 17%), and dyspepsia (RCI: 21%; IVMP: 22%). There were no serious adverse events.
DISCUSSION
Corticotrophin binding to MCRs may affect neuroinflammation by modulating steroidogenesis, peripheral immune cell functioning, and CNS neuroprotection and repair.5 Corticotrophin and analogs of alpha-melanocyte stimulating hormone have been shown to inhibit experimental autoimmune encephalomyelitis and experimental autoimmune uveitis in rodents.14,15 In addition, corticotrophin and MCR agonists have protected oligodendrocytes in vitro from cell death after oxidative, apoptotic, and excitotoxic stress.16
We sought to determine whether corticotrophin signaling through MCRs can provide additional therapeutic benefits when compared with IVMP. In the current study, we compared 2 approved agents, RCI and IVMP, for the treatment of acute optic neuritis. Both treatment arms demonstrated significant recovery of HCVA and LCVA, and no differences were noted in the extent of pRNFL and GC + IPL thinning at 1, 3, and 6 months after vision loss. Similarly, no difference in the fraction of eyes with pRNFL thickening (i.e., edema) was observed at any timepoint. Patient-reported outcomes of visual function, quality of life, and depression during the 6-month recovery period were also indistinguishable across the 2 treatment arms. The frequency of adverse events was not different between treatment groups, although reporting may have been affected by the open label and unblinded design.
The trial was discontinued before reaching planned enrollment. Although underpowered for detecting small differences in OCT outcomes, pRNFL and GC + IPL measures were markedly similar between the treatment arms (Table 2), while using high speed, high-definition SD-OCT, a retinal imaging paradigm characterized by a low test-retest variability (i.e., low coefficient of variation). Given the similar OCT outcomes noted in the current trial, we calculated sample group sizes for future 1-sided noninferiority studies for the primary outcome of pRNFL thickness at 6 months. For a margin of equivalence of 8 μM (10% of the mean value of the current study), group sample sizes of 51 achieve 80% power to detect noninferiority using a 1-sided, 2-sample t test using a 0.025 alpha level. The margin of equivalence in the current trial was limited to 15 μM.
Recent clinical trials have evaluated whether the addition of phenytoin or erythropoietin to IVMP lessens pRNFL loss after acute optic neuritis.17,18 The reduction in the mean pRNFL thickness observed in the RCI and IVMP treatment arms of the current trial are quite similar to those observed in the placebo group of the erythropoietin trial (14·65 μM)17 and the combination therapy group (phenytoin plus IVMP) of the phenytoin trial (16·69 μM).18 The mean pRNFL thickness loss observed in the placebo arm of the phenytoin trial (23·79 μM) was more extensive than in other studies and may have exaggerated the neuroprotective effect of the sodium channel blocker.18 Indeed, in a follow-up study, there was no difference at 6 months between the mean serum concentration of neurofilament heavy chain and neurofilament light chain proteins between treatment groups, and there was no correlation between the concentration of the serum neurofilament markers and OCT metrics.19
Treatment with RCI or IVMP resulted in no differences in clinical or patient-reported outcomes in the current study. Given the excellent visual recovery observed in the placebo group of the Optic Neuritis Treatment Trial,2 it is not surprising that differences in HCVA and LCVA were not evident. Furthermore, it was encouraging to observe that patient-reported measures of visual function, fatigue, quality of life, and depression were also indistinguishable among 2 approved and conventional treatment protocols for acute ON. The results indicate that both IVMP-treated and RCI-treated patients should expect similar rates and extent of visual recovery.
The rates of adverse events were comparable; dyspepsia was frequently reported in both treatment groups, and injection site reactions and insomnia were the most frequently reported adverse event associated with RCI treatment.
This is the first clinical trial that we know of to compare 2 registered and standard-of-care treatments for acute optic neuritis. Although the power of the study was limited by curtailed enrollment, the overlapping structural and clinical outcomes indicate that potential additional anti-inflammatory and neuroprotective features of corticotrophin signaling in acute optic neuritis are likely to be modest when compared with high-dose intravenous methylprednisolone. Another potential study limitation was the inclusion of subjects with prior ON in the contralateral eye. In these subjects, the measured pRNFL and GC + IPL loss in the affected eye will be limited by the extent of prior thinning in the fellow eye. Since only 2 subjects in each treatment group had prior contralateral ON, the effect is likely to be small.
Differences between RCI and IVMP may be evident for rarer causes of acute optic neuritis (e.g., aquaporin-4-IgG seropositive neuromyelitis optica spectrum disease or MOGAD)20 or with more rapid treatment.21 Although only a single MOGAD optic neuritis patient was enrolled in this study, the low number may have been the result of exclusion criteria (bilateral optic neuritis and severe disc edema or hemorrhage) and the limited availability of commercial MOG-IgG testing. Nevertheless, the data from our trial may be helpful in powering future studies aimed at the identification of therapeutic and neuroprotective agents while using a common signature syndrome for acute ON as a template from which to detect and monitor treatment effects. The outcomes derived from 2 different, albeit effective interventions for acute ON serves to support the construct validity of using a commonly occurring and eloquent inflammatory demyelinating syndrome. Discovery innovation, such as our employment of acute ON, provides further evidence to support the contention that the anterior visual network can serve as a “high-throughput” and vertically integrated neuroscience system. We hypothesize that such a system can be instrumental for interrogating the pathobiology of a variety of neuroimmunologic and neuroinflammatory disorders with prospects whereby the translation of newly elucidated mechanisms of disease will lead to the rational design of clinical trials that focus on “precision medicine” whereby we can ultimately treat each individual patient, in conjunction with their specific working diagnosis, individually.
STATEMENT OF AUTHORSHIP
Conception and design: J. L. Bennett, E. M. Frohman; Acquisition of data: J. L. Bennett, E. M. Frohman, R. K. Johnson, C. Mizenko, J. C. DuPont, T. C. Frohman, K. S. Shindler; Analysis and interpretation of data: J. L. Bennett, E. M. Frohman, N. C. Grove, B. D. Wagner, A. M. Lynch, T. C. Frohman, K. S. Shindler. Drafting the manuscript: J. L. Bennett; Revising the manuscript for intellectual content: J. L. Bennett, E. M. Frohman, N. C. Grove, B. D. Wagner, A. M. Lynch, T. C. Frohman, K. S. Shindler. Final approval of the completed manuscript: All authors.
Footnotes
This trial was supported by an independent investigator grants from Mallinckrodt Pharmaceuticals (St. Louis, MO) to J. L. Bennett, E. M. Frohman and K. S. Shindler. Mallinckrodt Pharmaceuticals provided funding for the study and repository corticotropin injection (Acthar Gel). Mallinckrodt Pharmaceuticals was not involved in the design of the study, the collection of data, the analysis of data, or the drafting of the manuscript. J. L. Bennett receives additional support from National Eye Institute grants R01-EY022936, R01-, and R21-032399.
J. L. Bennett reports payment for consultation from MedImmune/Viela Bio/Horizon Therapeutics, Alexion, Chugai, Clene Nanomedicine, Genentech, Genzyme, Mitsubishi Tanabe Pharma, Reistone Biopharma, TG Therapeutics, Antigenomycs, and Roche; personal fees from AbbVie; grants from Novartis, Mallinckrodt, and Alexion; and has a patent for Aquaporumab issued. E. M. Frohman reports payment or honoraria from Biogen, Alexion, Genzyme, Novartis, and Janssen, and leadership/fiduciary role in National MS Society. K. S. Shindler reports payment for consultation from Noveome Biotherapeutics; grants from Noveome, Gyroscope Therapeutics, NIH, and Mallinckrodt; speakers honorarium from Santen Pharmaceuticals; author royalties from UpToDate; membership on a Scientific Advisory Board with stock options from Noveome; fees from various law firms and insurance companies for medical expert witness testimony; and has a patent for RGC-selective neuroprotective gene therapies. N. C. Grove, R. K. Johnson, C. Mizenko, J. C. Dupont, B. D. Wagner, A. M. Lynch and T. Frohman report no relevant disclosures.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jneuro-ophthalmology.com).
Contributor Information
Nathan C. Grove, Email: NATHAN.GROVE@CUANSCHUTZ.EDU.
Ruth K. Johnson, Email: Rukojo313@gmail.com.
Christopher Mizenko, Email: christopher.mizenko@cuanschutz.edu.
Joan C. DuPont, Email: DupontJ@pennmedicine.upenn.edu.
Brandie D. Wagner, Email: brandie.wagner@cuanschutz.edu.
Anne M. Lynch, Email: ANNE.LYNCH@CUANSCHUTZ.EDU.
Teresa C. Frohman, Email: teresafrohman@gmail.com.
Kenneth S. Shindler, Email: kenneth.shindler@pennmedicine.upenn.edu.
Elliot M. Frohman, Email: Elliot.frohman@austin.utexas.edu.
REFERENCES
- 1.Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2015;2015:CD001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck RW, Cleary PA, Anderson MM, Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326:581–588. [DOI] [PubMed] [Google Scholar]
- 3.Bowden AN, Bowden PM, Friedmann AI, Perkin GD, Rose FC. A trial of corticotrophin gelatin injection in acute optic neuritis. J Neurol Neurosurg Psychiatry. 1974;37:869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberman AC, Budzinski ML, Sokn C, Gobbini RP, Steininger A, Arzt E. Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front Endocrinol (Lausanne). 2018;9:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisak RP, Benjamins JA. Melanocortins, melanocortin receptors and multiple sclerosis. Brain Sci. 2017;7:104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindqvist N, Napankangas U, Lindblom J, Hallbook F. Proopiomelanocortin and melanocortin receptors in the adult rat retino-tectal system and their regulation after optic nerve transection. Eur J Pharmacol. 2003;482:85–94. [DOI] [PubMed] [Google Scholar]
- 7.Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77:517–528. [DOI] [PubMed] [Google Scholar]
- 8.Costello F, Pan YI, Yeh EA, Hodge W, Burton JM, Kardon R. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry. 2015;86:1369–1373. [DOI] [PubMed] [Google Scholar]
- 9.Kupersmith MJ, Mandel G, Anderson S, Meltzer DE, Kardon R. Baseline, one and three month changes in the peripapillary retinal nerve fiber layer in acute optic neuritis: relation to baseline vision and MRI. J Neurol Sci. 2011;308:117–123. [DOI] [PubMed] [Google Scholar]
- 10.Petrillo J, Balcer L, Galetta S, Chai Y, Xu L, Cadavid D. Initial impairment and recovery of vision-related functioning in participants with acute optic neuritis from the RENEW trial of opicinumab. J Neuroophthalmol. 2019;39:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritvo P, Fischer J, Miller D, Andrews H, Paty D, LaRocca N. Multiple Sclerosis Quality of Live Inventory: A User's Manual. New York, NY: National Multiple Sclerosis Society, 1997. [Google Scholar]
- 12.Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 14.Mykicki N, Herrmann AM, Schwab N, et al. Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease. Sci Transl Med. 2016;8:362ra146. [DOI] [PubMed] [Google Scholar]
- 15.Ng TF, Dawit K, Taylor AW. Melanocortin receptor agonists suppress experimental autoimmune uveitis. Exp Eye Res. 2022;218:108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamins JA, Nedelkoska L, Lisak RP. Melanocortin receptor subtypes are expressed on cells in the oligodendroglial lineage and signal ACTH protection. J Neurosci Res. 2018;96:427–435. [DOI] [PubMed] [Google Scholar]
- 17.Lagreze WA, Kuchlin S, Ihorst G, et al. Safety and efficacy of erythropoietin for the treatment of patients with optic neuritis (TONE): a randomised, double-blind, multicentre, placebo -controlled study. Lancet Neurol. 2021;20:991–1000. [DOI] [PubMed] [Google Scholar]
- 18.Raftopoulos R, Hickman SJ, Toosy A, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:259–269. [DOI] [PubMed] [Google Scholar]
- 19.Raftopoulos R, Kuhle J, Grant D, et al. Neurofilament results for the phase II neuroprotection study of phenytoin in optic neuritis. Eur J Neurol. 2021;28:587–594. [DOI] [PubMed] [Google Scholar]
- 20.Akaishi T, Takeshita T, Himori N, et al. Rapid administration of high-dose intravenous methylprednisolone improves visual outcomes after optic neuritis in patients with AQP4-IgG-positive NMOSD. Front Neurol. 2020;11:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale GH, Petersen T, Bacher Svendsen K, Christensen T, Houen G, Bek T. Time to steroid treatment in severe acute optic neuritis. Brain Behav. 2018;8:e01032. [DOI] [PMC free article] [PubMed] [Google Scholar]