Objective:
To evaluate the safety and efficacy of endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) using a lumen-apposing metal stent (LAMS).
Background:
For patients with acute cholecystitis who are poor surgical candidates, EUS-GBD using a LAMS is an important treatment alternative to percutaneous gallbladder drainage.
Methods:
We conducted a regulatory-compliant, prospective multicenter trial at 7 tertiary referral centers in the United States of America and Belgium. Thirty consecutive patients with mild or moderate acute cholecystitis who were not candidates for cholecystectomy were enrolled between September 2019 and August 2021. Eligible patients had a LAMS placed transmurally with 30 to 60-day indwell if removal was clinically indicated, and 30-day follow-up post-LAMS removal. Endpoints included days until acute cholecystitis resolution, reintervention rate, acute cholecystitis recurrence rate, and procedure-related adverse events (AEs).
Results:
Technical success was 93.3% (28/30) for LAMS placement and 100% for LAMS removal in 19 patients for whom removal was attempted. Five (16.7%) patients required reintervention. Mean time to acute cholecystitis resolution was 1.6±1.5 days. Acute cholecystitis symptoms recurred in 10.0% (3/30) after LAMS removal. Five (16.7%) patients died from unrelated causes. Procedure-related AEs were reported to the FDA in 30.0% (9/30) of patients, including one fatal event 21 days after LAMS removal; however, no AEs were causally related to the LAMS.
Conclusions:
For selected patients with acute cholecystitis who are at elevated surgical risk, EUS-GBD with LAMS is an alternative to percutaneous gallbladder drainage. It has high technical and clinical success, with low recurrence and an acceptable AE rate. Clinicaltrials.gov, Number: NCT03767881.
Keywords: acute cholecystitis, clinical study, drainage, gallbladder, metal stents
Approximately 600,000 outpatient1 and 300,000 inpatient2 cholecystectomies were performed in the United States in 2018. Laparoscopic cholecystectomy is the gold standard to treat acute cholecystitis in patients who are eligible for surgery.3 For patients who are not surgical candidates, percutaneous, endoscopic transpapillary, and endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) approaches are treatment options at centers with available expertise.4 Percutaneous gallbladder drainage (PT-GBD) is the most common nonoperative method for gallbladder decompression in patients unfit for surgery. However, drain-related complications (20%–75%), including dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use.5,6 Although EUS-GBD may have similar technical and clinical success compared with percutaneous drainage, it has been reported to use fewer hospital resources, result in fewer adverse events (AEs), improve pain scores, and decrease the need for repeat gallbladder drainage.7
Endoscopic approaches include transpapillary gallbladder drainage and EUS-GBD. Transpapillary gallbladder drainage is performed during endoscopic retrograde cholangiopancreatography (ERCP) but is associated with lower technical and clinical success rates than either PT-GBD or EUS-GBD.8 EUS-GBD creates an iatrogenic fistula between the gallbladder and the gastrointestinal tract.9 EUS-GBD was first performed using double-pigtail plastic stents (DPPS),10,11 followed by tubular self-expanding metal stents, which were not ideal due to their risk of leakage, longer length (contralateral wall injury and occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations due to their short length and large flanges,9 and since 2014 have been used for EUS-GBD.12 Two meta-analyses concluded that EUS-GBD using LAMS has greater safety8 and/or higher clinical success13 and/or technical success than PT-GBD or transpapillary drainage for patients with acute cholecystitis at high surgical risk. In a 2020 randomized superiority trial, EUS-GBD improved outcomes compared with PT-GBD in patients who were not surgical candidates.14 No LAMS has been approved for EUS-GBD in the United States. This study was conducted under a United States Food and Drug Administration (FDA)-approved protocol to evaluate LAMS for EUS-GBD in nonsurgical patients needing gallbladder decompression.
METHODS
Study Design
This was a prospective, multicenter, and consecutive single-arm trial to evaluate the safety and efficacy of EUS-GBD using a LAMS as an alternative to PT-GBD in patients with acute cholecystitis who were at high risk or unsuitable for surgery, conducted under an FDA Investigational Device Exemption (IDE#: G170190). All sites provided Ethics Committee/Institutional Review Board approval of the study protocol and informed consent. The study was overseen by an Independent Data Review board consisting of 3 physicians who did not participate in the study: 1 gastroenterologist (T. H. B.), 1 surgeon with experience performing cholecystectomy (L. S.), and 1 interventional radiologist (E. S.). Patients who met all eligibility criteria received the AXIOS LAMS with an electrocautery-enhanced delivery system (Boston Scientific Corporation) with 30 to 60-day stent indwell (unless medically recommended otherwise), and 3-day and 30-day follow-up after prosthesis removal. At the time of study initiation, the AXIOS, stent, and electrocautery-enhanced delivery system were cleared in the United States only for use to facilitate endoscopic drainage of symptomatic pancreatic pseudocysts or walled-off necrosis with ≥70% fluid content and was approved in Europe and other countries outside of the United States for the same use and for biliary tract drainage, including gallbladder drainage in patients with acute cholecystitis who are at high risk for surgery.
Patient Population
Eligible patients were 18 years or older, in need of intervention for symptomatic acute cholecystitis of Grade I/mild (healthy patient with mild local inflammatory changes and without organ dysfunction) or Grade II/moderate [defined by any of the following: leukocytosis (>18,000 cells per mm3), palpable, tender mass in the right upper quadrant, symptom duration >72 hours, marked local inflammation] per the Tokyo guidelines,15 were referred for percutaneous drainage of the gallbladder and were not surgical candidates because of advanced age, anesthetic risk, significant comorbidities, and/or overall health.
Exclusion criteria included Grade III/severe cholecystitis, imaging consistent with perforated, extensive gangrenous or ischemic gallbladder, hepatic abscess, ascites, coagulopathy requiring ongoing full anticoagulation, bleeding diathesis, prior surgical treatment of acute cholecystitis, current percutaneous drainage or history of PT-GBD without acute cholecystitis-free period after percutaneous drainage removal, distance between gallbladder wall and duodenal or gastric wall >1 cm on ultrasound, intervening gastric varices or vessels within a 1 cm radius of the device insertion location, allergies or contraindications to any of the device materials, pregnancy, prisoners, and other vulnerable populations.
Study Visits
A baseline visit included informed consent, eligibility assessment, preprocedure transabdominal ultrasound or computed tomography scan, medical history and examination, current medications, and laboratory tests for markers of inflammation and cholestasis. Within 5 days of this visit, EUS-GBD was completed, followed by daily symptom assessment until the resolution of acute cholecystitis. Thirty to 60 days after EUS-GBD, the stent was removed if acute cholecystitis resolved unless the LAMS removal procedure was medically contraindicated. A post-stent removal visit was conducted 72 hours later. An end-of-study visit was conducted at 30 days (±7 days) post-stent removal or 90 days (±14 days) post-stent placement, whichever occurred first. At any point during the study, additional visits, laboratory tests, imaging, or reintervention (eg, stent removal or patency assessment) could be performed if indicated.
Outcomes
The primary efficacy endpoint was the time to resolution of acute cholecystitis. Resolution was defined as achievement of at least 2 of 3 categories used to define cholecystitis without deterioration of the third: (1) fever resolution (body temperature <100.5°F), (2) at least a 4-point decrease in the pain score from the onset of acute cholecystitis, and (3) white blood cell count <12,000/cm3.
The secondary efficacy endpoint was the rate of reinterventions affecting the biliary tract for reasons other than the management of confirmed malignant biliary stenosis, including but not limited to stent migration, stent occlusion by gallbladder stones, and luminal debridement.
Additional endpoints included the technical success of stent placement and removal, stent patency, recurrence of acute cholecystitis, cumulative hospital days from initial stent placement to resolution of acute cholecystitis, and AEs related to the LAMS placement, indwell, or removal.
Statistical Analyses
Primary Efficacy Endpoint Performance Goal
Based on estimates of time to clinical resolution of cholecystitis from published studies of EUS-guided16 and PT-GBD,17 a performance goal of 3.5 days was used. The estimated mean and standard error from a Kaplan-Meier analysis was used to determine a one-sided 97.8% upper confidence interval of mean days to clinical resolution of cholecystitis. The performance goal for the primary efficacy endpoint would be met if the upper bound of the 97.8% confidence was <3.5 days.
Secondary Efficacy Endpoint Performance Goal
On the basis of estimates of proportions of patients with reinterventions from published studies of EUS-guided and PT-GBD,18,19 a performance goal of 46.2% was determined. The performance goal for the secondary efficacy endpoint would be met if the upper bound of the 99.7% one-sided Clopper-Pearson confidence interval of proportion of patients with reintervention was less than the performance goal of 46.2%.
Sample Size Calculation
Using a 1-sided test with a type I error (α) of 2.2%, power (1-β) of 80.4%, and allowing for 10% attrition, 30 patients needed to be enrolled.
Data Analyses
Descriptive statistics were tabulated for all intentions to treat subjects. The mean, standard deviation, minimum, and maximum were used to describe continuous variables; the median and interquartile range were calculated where appropriate. Frequency tables were used to summarize discrete variables. Proportions of subjects with AEs were determined. All analyses were performed using SAS version 9.4 (SAS Institute).
RESULTS
Baseline Patient Characteristics
Of 64 patients screened, 30 (46.9%) met eligibility criteria and were enrolled after providing written informed consent to participate in the study (Fig. 1). Among the 30 enrolled patients, the mean age was 75.2±14.1 (range, 34.0–94.0) years, mean body mass index was 26.9±6.3 (range, 18.2–44.1), and median days as onset of first acute cholecystitis symptoms were 4.0 (range, 1.0–48.0) (Supplemental Digital Content Table 1, http://links.lww.com/SLA/E384). Seven (23.3%) patients had Grade I and 23 (76.7%) had Grade II acute cholecystitis.
FIGURE 1.
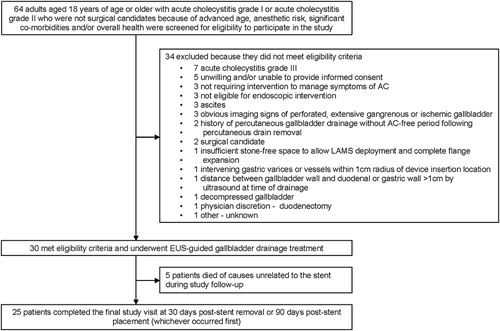
Patient flow through the study. AC indicates acute cholecystitis; EUS, endoscopic ultrasound; LAMS, lumen-apposing metal stent.
Technical Success and Index Procedure Characteristics
Twenty-eight (93.3%) patients had general anesthesia and 2 (6.7%) had conscious sedation for the index LAMS placement procedure (Table 1). A LAMS was placed in 28 (93.3%) patients with a mean stent placement time of 5.6±6.0 (range, 1.0–34.0) minutes and a mean drainage procedure time (initial puncture to scope out) of 6.8±5.6 (range, 1.0–30.0) minutes.
TABLE 1.
Index LAMS Placement Procedure Characteristics
| Characteristic | Mean±SD or % (n/N) |
|---|---|
| Anesthesia | |
| General | 93.3 (28/30) |
| Conscious sedation | 6.7 (2/30) |
| LAMS placement time (min) (delivery catheter entry to removal) | 5.6±6.0 |
| Drainage procedure time (min) (Initial puncture to scope out) | 6.8±5.6 |
| LAMS placed during: | |
| Index procedure | 100.0 (30/30) |
| Reintervention | 0.0 (0/30) |
| LAMS size (attempted) | |
| 10 mm×10 mm | 43.3 (13/30) |
| 15 mm×10 mm | 56.7 (17/30) |
| Additional LAMS placement attempted | 4.5 (1/22) |
| LAMS implanted | 93.3 (28/30) |
| Not implanted | 6.7 (2/30) |
| Stent retracted with scope removal | 3.3 (1/30) |
| Stent was deployed but did not penetrate the gallbladder wall | 3.3 (1/30) |
| LAMS size (implanted) | |
| 10 mm×10 mm | 39.3 (11/28) |
| 15 mm×10 mm | 60.7 (17/28) |
| LAMS patency assessment | |
| Drainage was visualized through the LAMS | 93.3 (28/30) |
| Drainage visualized fluoroscopically | 43.3 (13/30) |
| Endoscopic observation of the inner walls of the gallbladder through the study LAMS | 43.3 (13/30) |
| Additional endoscopic interventions through LAMS | |
| Dilation of LAMS performed | 10.0 (3/30) |
| Diameter of dilation (mm) | 9.7±3.5 |
| Interventions | |
| None | 63.3 (19/30) |
| Aspiration of bile for culture | 13.3 (4/30) |
| Aspiration of pus/sludge | 6.7 (2/30) |
| Stone removal | 6.7 (2/30) |
| Sludge removal | 10.0 (3/30) |
| Lavage | 10.0 (3/30) |
| A second nonstudy stent was placed (Solus stent) | 3.3 (1/30) |
| Stent removal | 3.3 (1/30) |
| Zosyn antibiotic given | 3.3 (1/30) |
LAMS indicates lumen-apposing metal stent.
Twenty (66.7%) patients underwent successful transduodenal LAMS placement, 8 (26.7%) patients had successful transgastric placement, and 2 (6.7%) patients did not receive a LAMS after planned transduodenal placement. Eleven of 13 attempted 10 mm×10 mm LAMS placements and all seventeen 15 mm×10 mm attempted LAMS placements were successful (Table 1). One LAMS placement was aborted due to intervening vessels, and 1 LAMS was dislodged during placement, managed by endoscopic clip closure of the puncture site. Drainage through the stent was visualized endoscopically in all the other 28 patients.
At the time of EUS-GBD, 17 other interventions were performed in 11 patients including aspiration of bile for culture (4), aspiration of pus or sludge (2), stone removal (2), sludge removal (3), lavage (3), placement of a nonstudy stent (1), or stent removal after misdeployment(1), or administration of piperacillin/tazobactam antibiotic (1).
At LAMS removal, stent patency was observed in 22 patients (100%). Of these, 19 patients had successful LAMS removal, and the remaining 3 patients did not have LAMS removal due to age and preexisting conditions (2) or permanent obstruction of cystic duct (1). Among the 8 patients who did not have patency assessed, 2 did not have a study stent implanted, 3 died before the stent removal visit, 1 patient had end-stage metastatic cancer, 1 patient was entering hospice (heart failure), and no reason was given for 1 patient.
Primary Efficacy Endpoint: Time to Resolution of Acute Cholecystitis
Symptom resolution was reported in all 30 patients, at a mean of 1.60±1.50 (range, 1.00–8.00) days (Table 2). This result met the primary efficacy endpoint performance goal (upper 97.8% confidence bound of the mean days to clinical resolution of 2.15 days being less than the performance goal of 3.5 days).
TABLE 2.
Primary Efficacy and Safety Results (N=30)
| Endpoint | % (n/N) |
|---|---|
| Primary efficacy endpoint | |
| Symptom resolution: temp<100.5°F, 4-point decrease in pain score or WBC <12,000 cm3 with improvement in at least 2 categories without deterioration in the third category (95% CI) | 100.0 (30/30) (88.4–100.0) |
| Days to resolution (97.8% one-sided CI) | 1.60±1.50 (range, 1.00–8.00) (0.0, 2.15) |
| Secondary efficacy endpoint | |
| Reintervention, migration or occlusion rate (99.7% one-sided CI) | 16.7 (5/30) (0.0–42.0) |
Secondary Efficacy Endpoint: Rate of Reintervention
Reintervention, migration, or occlusion occurred in 5 (16.7%; 99.7% one-sided CI, 0.0%–42.0%) patients (Table 2). There were no stent occlusions. This result met the secondary efficacy endpoint performance goal. One patient posttransgastric EUS-GBD had an inward stent migration found incidentally at the time of LAMS removal on day 35. Along with the removal of this stent and stones, DPPS were placed to maintain the still-patent fistula. Another patient underwent a balloon dilation of the cholecystoduodenostomy at the time of scheduled LAMS removal to aid in the removal of residual stones; the LAMS was replaced with 3 DPPS. Two patients underwent cholecystectomy: one patient 2 days after technical failure and misdeployment of the LAMS, another patient 45 days after removal of a transduodenal LAMS developed recurrent cholecystitis and was scheduled for a cholecystectomy (after the end of study). A fifth patient had a biliary stent placement in the left hepatic duct performed 23 days after the index transduodenal EUS-GBD for abdominal pain, nausea, and vomiting unrelated to the LAMS.
Acute Cholecystitis Recurrence
Recurrence of acute cholecystitis was reported in 3 of 30 patients (10%). All 3 cases were after the resolution of the baseline acute cholecystitis and after the removal of the LAMS. One patient who had transgastric LAMS placement was hospitalized with recurrent acute cholecystitis 21 days after LAMS removal. Although 2 DPPS had been placed, these had passed spontaneously at the time of recurrent cholecystitis. This patient developed septic shock and died 1 day later. A second patient had transduodenal LAMS placement and was reported to have recurrent acute cholecystitis 45 days after LAMS removal. At that time, this patient was scheduled to have a cholecystectomy at a future date (out of study purview). The third patient had transgastric LAMS placement, then later had 1 DPPS placed on the day of LAMS removal and gallbladder stone removal. Fifteen days later the patient was treated for suspected recurrent cholecystitis and for concurrent choledocholithiasis with a DPPS exchange.
Length of Hospitalization
The mean time of hospitalization after EUS-GBD was 5.5±6.6 (range, 1.0–35.0) days.
Adverse Events
Eleven AEs related to the stent, stent placement, or removal procedure, including 1 fatal, were reported in 9 (30.0%) patients during the study period (Table 3). At the time the AE was reported, the LAMS had not been removed in 8 of these patients and had been removed in 1 patient. Two reports of diarrhea, 2 of fatigue, 2 of bleeding that did not require a blood transfusion, 2 of sepsis (including 1 fatal), and 1 report each of pleural effusion, abdominal pain, and hypomagnesemia were documented. Mean days to onset of these AEs was 19.8±20.7 (range, 0.0–56.0), and mean days to resolution was 4.7±6.3 (range, 0.0–18.0).
TABLE 3.
AEs Related to Stent, Stent Placement or Removal Procedure (N=30 Patients)
| Adverse event | No. of events | % patients (n/N) |
|---|---|---|
| Any related AE | 11 | 30.0 (9/30) |
| Diarrhea | 2 | 6.7 (2/30) |
| Fatigue | 2 | 6.7 (2/30) |
| Fatal septic shock | 1 | 3.3 (1/30) |
| Sepsis | 1 | 3.3 (1/30) |
| Pleural effusion | 1 | 3.3 (1/30) |
| Upper abdominal pain | 1 | 3.3 (1/30) |
| Hypomagnesaemia | 1 | 3.3 (1/30) |
| Mucosal injury with moderate bleeding | 1 | 3.3 (1/30) |
| Moderate bleeding at stent site | 1 | 3.3 (1/30) |
Each patient had 1 or more of the listed AEs; rows are not mutually exclusive.
One patient with fatal septic shock had a 15 mm transgastric LAMS followed by a resolution of cholecystitis. At elective stent removal, food and stone found in the gallbladder were cleaned out endoscopically and a small gallbladder polyp was biopsied. Two DPPS were placed to maintain this fistula, however, they were not found (presumed spontaneous migration) migrated at the time of the recurrent cholecystitis with resultant septic shock and death.
DISCUSSION
In this prospective, multicenter study of patients with mild or moderate acute cholecystitis who were not candidates for cholecystectomy, EUS-GBD using LAMS showed high technical and clinical success, low recurrence of acute cholecystitis and a low rate of AEs attributable to the stent, or procedure despite a patient population with multiple comorbidities.
After its introduction in the 1980s, laparoscopic cholecystectomy quickly became the standard of care for patients with symptomatic gallstones, prompting a shift in surgery from the inpatient to outpatient setting between 1996 and 2006.20 PT-GBD has been performed since the 1970s and has historically been considered the gold standard for palliation in high-risk surgical patients.8,21–23 Use of ERCP for selective cannulation of the cystic duct was reported in 1984,24 and transpapillary gallbladder drainage was subsequently developed.23 EUS-GBD was first documented in a case report using a DPPS in 2007.10 An electrocautery-enhanced LAMS was developed and subsequently used for this procedure in 2014; this streamlined the drainage process with fewer instrument changes and has become the most widely used prosthesis for EUS-GBD.25–27
Three comparative retrospective observational studies suggested similar efficacy and similar or improved safety for EUS-GBD compared with PT-GBD in nonsurgical candidates with symptomatic gallbladder disease.5,18,19 In a 2020 superiority trial, 80 consecutive patients aged 18 years and older with grade II and III acute calculous cholecystitis15 and high-risk for cholecystectomy due to poor premorbid condition were randomized to EUS-GBD (using the same LAMS as in the current study) versus PT-GBD.14 EUS-GBD significantly reduced 1-year AEs compared with PT-GBD, [10/39 patients (25.6%) vs 31/40 patients (77.5%); P <0.001]. Thirty-day AEs [5 (12.8%) vs 19 (47.5%); P=0.001] and the reintervention rate [1/39 (2.6%) vs 8/40 (20%); P=0.001] were also significantly lower for EUS-GBD.14 Although these results are promising, some experts have cautioned that current data do not support that EUS-GBD can replace PT-GBD as the standard of care yet, citing limited long-term outcome data and lack of consensus regarding the need for LAMS removal after cholecystitis resolution.28 Although there are reports of long-term placement of LAMS resulting in significant tissue overgrowth precluding removal,29 placement of a DPPS through the LAMS, or replacing it with 2 DPPS at 4 to 6 weeks might mitigate this risk.30 The European Society of Gastrointestinal Endoscopy recommends performing transduodenal rather than transgastric EUS-GBD, as antral LAMS placement has been associated with more symptom recurrence owing to food impaction and a higher risk of a buried LAMS.31 Although no head-to-head comparisons have been performed between transduodenal versus transgastric approaches, the authors support the former whenever possible based on available evidence.31
Because of consistent findings that EUS-GBD has similar or greater efficacy and safety compared with PT-GBD, clearance for this expanded indication for use of the studied LAMS in the United States of America is desirable. The main barrier to wider use is the technical challenge, including the use of multiple endoscopic modalities such as therapeutic EUS, wire manipulation, and stent deployment.32 In a study including 48 patients undergoing EUS-GBD (37 with LAMS, 9 with FCSEMS, and 2 with plastic stents) by a single operator over 5 years, the median procedure time of 41 (range, 16–121) minutes was achieved at the 19th procedure.32 Clinical success was achieved in 35 (72.9%) patients, and 9 (18.8%) patients experienced AEs occurring evenly throughout the learning curve in this study.32 These findings suggest that, at centers with adequate personnel and resources, EUS-GBD can be introduced safely. Other barriers to increased use include uncertainty regarding the optimal duration of stenting for EUS-GBD, how to reduce stent migrations and how to minimize the number of endoscopic procedures over the long term.27
EUS-GBD in our study was limited to patients deemed to be at elevated risk for surgery. Although 1 patient did go on to successful interval cholecystectomy after EUS-GBD in this study (as have others in different studies), a multidisciplinary discussion with surgical colleagues is advisable before using EUS-GBD over transpapillary gallbladder drainage or PT-GBD in a potential future surgical candidate.33 The concern is that a cholecystoenteric fistula and peri-cholecystic adhesions could make a surgery more difficult or risky, and may require open cholecystectomy rather than laparoscopic. Feasibility and safety of surgery after EUS-GBD require further study before being established as a routine option in this setting.
EUS-GBD must also be recognized as one among the several treatment alternatives that have demonstrated efficacy and long-term safety for patients with acute cholecystitis who are not surgical candidates. For example, in a retrospective study of consecutive nonoperative patients with acute cholecystitis, transpapillary gallbladder drainage with 1 or 2 DPPS showed long-term success (absence of recurrence of cholecystitis and need for repeat unplanned GB therapy) in 95.9% (47/49) of patients with AEs in only 5.9% (3/49) over a mean follow-up of 453 days after the drainage procedure (range, 18–1879).34 Three of 18 patients with single stents required additional prescribed ERCPs for routine stent exchanges, whereas none with 2 stents placed at the index procedure required an additional ERCP procedure.34
The first prospective study of a LAMS for EUS-GBD (11 transgastric, 19 transduodenal; thirteen 10 mm×10 mm, and seventeen 10 mm×15 mm LAMS) reported clinical success in 26 (96%) patients, recurrent cholecystitis due to LAMS obstruction in 2 (7%), and possible stent-related or procedure-related serious adverse events in 4 (13%) over mean follow-up 298 days for all patients and 364 days for patients alive at the end of the study.29 These results suggest that longer LAMS indwell than was used in our study might be efficacious and safe for most patients, though significant tissue overgrowth was mentioned as a problem in 10% of patients in the cited study.29 PT-GBD is still the most common and most widely available method of gallbladder drainage and would be preferred for some patients who were ineligible for our study (eg, due to ascites or contraindications to endoscopy). In patients with acute cholecystitis who have prolonged or indefinite nonsurgical status, any of these treatments or more than one in tandem might be selected based on the patient’s disease characteristics, available expertise, and preferred devices and procedures at a given site.
Our study had strengths and limitations. Being a prospectively collected data set with strict guidelines, every AE, even when not related to the LAMS or procedure, was reported. This was a small, single-arm nonrandomized study conducted by expert endoscopists at large university medical centers. The favorable results therefore might not be reproducible at centers with lower interventional EUS procedural volumes, operators with less experience, or fewer specialty resources. Results may also vary among centers because a standardized technique of LAMS deployment (eg, wire-guided vs direct, fluoroscopy vs not, dilation, and DPPS placement in the same session in some cases) is lacking. Also, follow-up was limited to a maximum of 90 days post-stent placement, so long-term maintenance of the cholecystoenteric fistula or incidence of fistula closure after removal of the LAMS could not be estimated, but maintenance of the fistula with pigtail stents is strongly recommended, especially if the cystic duct is irreversibly obstructed.
CONCLUSIONS
Consistent with current published data, we found EUS-GBD with LAMS to have good efficacy and safety in the management of acute cholecystitis in patients with elevated risk for cholecystectomy. Additional studies regarding the optimal length of LAMS indwell, placement of a concomitant DPPS, longer-term efficacy and safety of EUS-GBD, and the impact of EUS-GBD on subsequent cholecystectomy are needed.
Acknowledgments
The authors acknowledge Andrew S. Ross, MD for his contributions to the study, and Boston Scientific Corporation employee Margaret Gourlay, MD, MPH for writing assistance.
Supplementary Material
Footnotes
The data, analytic methods, and study materials for this study may be made available to other researchers in accordance with the Boston Scientific Data Sharing Policy (http://www.bostonscientific.com/en-US/data-sharing-requests.html).
Grant supported by Boston Scientific Corporation.
S.S.I. and J.A.P.: concept and design. S.S.I., N.R.S., A.C.S., R.J.S., P.C., F.F.W., L.S., T.H.B., E.S., R.A.K., and S.W.v.d.M.: acquisition, analysis, or interpretation of data. S.S.I. and J.A.P.: drafting of the manuscript. S.S.I., N.R.S., A.C.S., R.J.S., P.C., F.F.W., L.S., T.H.B., E.S., R.A.K., J.A.P., E.M., E.H., and S.W.V.: critical revision of the manuscript for important intellectual content. E.M: statistical analysis. J.A.P.: obtained funding. S.S.I. and J.A.P.: supervision. S.S.I. and J.A.P.: administrative, technical, or material support.
S.S.I.: consultant for Boston Scientific and Gore. N.R.S.: consultant Boston Scientific, consultant Mauna Kea, advisory board and consultant STERIS, advisory board EndoscopyNow, consultant and advisory board Medtronic. A.C.S.: consultant/investigator relationship with Boston Scientific, Apollo Endosurgery, Enterasense and Endo-TAGSS; consultant fees from ERBE, GI Dynamics, Intuitive, and Olympus. R.J.S.: Advisory Board Member, Consultant, and recipient of unrestricted educational grant, and a research grant from Boston Scientific, Inc., and Advisory Board Member, Consultant, and recipient of unrestricted educational grant from Olympus, Inc. P.C.: consultant Boston Scientific, Medtronic; Advisory Council Medtronic. F.F.W. research funding to the institution—Steris/CSA Medical, Cancer Prevention Pharmaceuticals, PCI Biotech, Boston Scientific, and Cook Medical. L. Swanstrom: Advisory Board Member, Consultant, or recipient of research grants from Boston Scientific, Medtronic, Auris, Quelon, Taurus, USGI; Equity owner or patent holder, Wolf, virtual incision, Fractyl, Myka labs, Human Extensions. T.H.B.: consultant for Ambu, Boston Scientific, Cook Endoscopy, ConMed, Olympus, Medtronic, W.L. Gore. E. Shlomovitz: consultant and recipient of a research grant from Boston Scientific. R.A.K.: research support from Boston Scientific and the NIH. J.A.P., E. McMullen, and E. Ho: full-time employees of Boston Scientific Corporation. S.W.v.d.M.: consultancy fees from Boston Scientific and Cook Endoscopy (both 2012 to present); Boston Chair in Interventional Endoscopy (2018 to 2021), Cook Chair in Portal Hypertension (2021).
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Shayan S. Irani, Email: shayan.irani@virginiamason.org.
Neil R. Sharma, Email: nrsharma219@yahoo.com.
Andrew C. Storm, Email: Storm.Andrew@mayo.edu.
Raj J. Shah, Email: raj.shah@ucdenver.edu.
Prabhleen Chahal, Email: chahalp@ccf.org.
Field F. Willingham, Email: field.willingham@emory.edu.
Lee Swanstrom, Email: lswanstrom@gmail.com.
Todd H. Baron, Email: todd_baron@med.unc.edu.
Eran Shlomovitz, Email: eran.shlomovitz@uhn.ca.
Richard A. Kozarek, Email: richard.kozarek@virginiamason.org.
Joyce A. Peetermans, Email: Joyce.Peetermans@bsci.com.
Edmund McMullen, Email: Edmund.McMullen@bsci.com.
Evelyne Ho, Email: Evelyne.Ho@bsci.com.
Schalk W. van der Merwe, Email: schalk.vandermerwe@uzleuven.be.
REFERENCES
- 1.Sejpal DV, Trindade AJ, Lee C, et al. Digital cholangioscopy can detect residual biliary stones missed by occlusion cholangiogram in ERCP: a prospective tandem study. Endosc Int Open. 2019;7:E608–E614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litynski GS. Profiles in laparoscopy: Mouret, Dubois, and Perissat: the laparoscopic breakthrough in Europe (1987-1988). JSLS. 1999;3:163–167. [PMC free article] [PubMed] [Google Scholar]
- 3.Pucher PH, Brunt LM, Davies N, et al. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc. 2018;32:2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podboy A, Yuan J, Stave CD, et al. Comparison of EUS-guided endoscopic transpapillary and percutaneous gallbladder drainage for acute cholecystitis: a systematic review with network meta-analysis. Gastrointest Endosc. 2021;93:797–804 e791. [DOI] [PubMed] [Google Scholar]
- 5.Teoh AYB, Serna C, Penas I, et al. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy. 2017;49:130–138. [DOI] [PubMed] [Google Scholar]
- 6.Ostapenko A, Liechty S, Manuia E, et al. The Rise and fall of percutaneous cholecystostomy in a community hospital: is it possible to turn the tide of history? J Gastrointest Surg. 2022;26:602–607. [DOI] [PubMed] [Google Scholar]
- 7.Kedia P, Sharaiha RZ, Kumta NA, et al. Endoscopic gallbladder drainage compared with percutaneous drainage. Gastrointest Endosc. 2015;82:1031–1036. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui A, Kunda R, Tyberg A, et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an International, Multicenter Study. Surg Endosc. 2019;33:1260–1270. [DOI] [PubMed] [Google Scholar]
- 9.Jain D, Bhandari BS, Agrawal N, et al. Endoscopic ultrasound-guided gallbladder drainage using a lumen-apposing metal stent for acute cholecystitis: a systematic review. Clin Endosc. 2018;51:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007;65:735–737. [DOI] [PubMed] [Google Scholar]
- 11.Kwan V, Eisendrath P, Antaki F, et al. EUS-guided cholecystenterostomy: a new technique (with videos). Gastrointest Endosc. 2007;66:582–586. [DOI] [PubMed] [Google Scholar]
- 12.Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75:870–876. [DOI] [PubMed] [Google Scholar]
- 13.Mohan BP, Khan SR, Trakroo S, et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020;52:96–106. [DOI] [PubMed] [Google Scholar]
- 14.Teoh AYB, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomised multicentre controlled superiority trial (DRAC 1). Gut. 2020;69:1085–1091. [DOI] [PubMed] [Google Scholar]
- 15.Yokoe M, Takada T, Strasberg SM, et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:35–46. [DOI] [PubMed] [Google Scholar]
- 16.Mendez A, Mancera-Maldonado JL, Castañeda F. “Complications of percutaneous cholecystostomy.” Semin Intervent Radiol. 1994;11:283–286. [Google Scholar]
- 17.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399–404. [DOI] [PubMed] [Google Scholar]
- 18.Irani S, Ngamruengphong S, Teoh A, et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol. 2017;15:738–745. [DOI] [PubMed] [Google Scholar]
- 19.Tyberg A, Saumoy M, Sequeiros EV, et al. EUS-guided versus percutaneous gallbladder drainage: isn’t it time to convert? J Clin Gastroenterol. 2018;52:79–84. [DOI] [PubMed] [Google Scholar]
- 20.Barakat MT, Girotra M, Choudhary A, et al. A prospective evaluation of radiation-free direct solitary cholangioscopy for the management of choledocholithiasis. Gastrointest Endosc. 2018;87:584–589. e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn F. Cholecystostomy in the high risk patient with biliary tract disease. Ann Surg. 1977;185:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elyaderani M, Gabriele OF. Percutaneous cholecystostomy and cholangiography in patients with obstructive jaundice. Radiology. 1979;130:601–602. [DOI] [PubMed] [Google Scholar]
- 23.Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010;71:1038–1045. [DOI] [PubMed] [Google Scholar]
- 24.Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37–40. [PubMed] [Google Scholar]
- 25.Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc. 2014;80:1171. [DOI] [PubMed] [Google Scholar]
- 26.Sobani ZA, Ling C, Rustagi T. Endoscopic ultrasound-guided gallbladder drainage. Dig Dis Sci. 2021;66:2154–2161. [DOI] [PubMed] [Google Scholar]
- 27.Posner H, Widmer J. EUS guided gallbladder drainage. Transl Gastroenterol Hepatol. 2020;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JJ, Liu XC, Chen XQ, et al. Clinical value of DPOC for detecting and removing residual common bile duct stones (video). BMC Gastroenterol. 2019;19:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter D, Teoh AY, Itoi T, et al. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut. 2016;65:6–8. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Yoshida M, Suzuki Y, et al. Long-term outcomes of endoscopic gallbladder drainage for cholecystitis in poor surgical candidates: an updated comprehensive review. J Clin Med. 2021;10:4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Wanrooij RLJ, Bronswijk M, Kunda R, et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022;54:310–332. [DOI] [PubMed] [Google Scholar]
- 32.Tyberg A, Jha K, Shah S, et al. EUS-guided gallbladder drainage: a learning curve modified by technical progress. Endosc Int Open. 2020;8:E92–E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaura K, Bazerbachi F, Sawas T, et al. Surgical outcomes of ERCP-guided transpapillary gallbladder drainage versus percutaneous cholecystostomy as bridging therapies for acute cholecystitis followed by interval cholecystectomy. HPB (Oxford). 2020;22:996–1003. [DOI] [PubMed] [Google Scholar]
- 34.Storm AC, Vargas EJ, Chin JY, et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021;94:742–748. e741. [DOI] [PubMed] [Google Scholar]


