Précis:
After the open bleb revision following PreserFlo, the mean postoperative intraocular pressure (IOP) was lowered from 26.4 ± 9.9 mm Hg to 12.9 ± 5.6 mm Hg at 1 month and 15.9 ± 4.1 mm Hg at 12 months.
Purpose:
The purpose of this study was to evaluate the effectiveness and safety of an open bleb revision with mitomycin-C (MMC) for bleb fibrosis after PreserFlo MicroShunt implantation.
Patients and Methods:
We performed a retrospective analysis of 27 consecutive patients with bleb fibrosis after PreserFlo MicroShunt Implantation that underwent an open revision with MMC 0.2 mg/mL applied for 3 minutes at the Department of Ophthalmology of the Mainz, University Medical Center, Mainz, Germany. Demographic data, such as age, sex, glaucoma type, number of glaucoma medications, IOP before and after PreserFlo implantation and revision, complications, and reoperations within 12 months, were analyzed.
Results:
Twenty-seven patients (27 eyes) received an open revision after previous PreserFlo Microshunt implantation and consecutive bleb fibrosis. The mean preoperative IOP was 26.4 ± 9.9 mm Hg before revision, 7.0 ± 2.7 mm Hg (P < 0.001) in the first week after the revision, and 15.9 ± 4.1 mm Hg at 12 months (P = 0.02). Four patients needed IOP-lowering medication after 12 months. One patient had a positive Seidel test and needed a conjunctival suture. Four patients required a second procedure due to recurring bleb fibrosis.
Conclusions:
At 12 months, open revision with MMC for bleb fibrosis after failed PreserFlo implantation effectively and safely reduced IOP with a similar medication burden.
Key Words: PreserFlo, revision, bleb failure, glaucoma
PreserFlo MicroShunt (Santen Pharmaceutical Co. Ltd.) is a trans-scleral, bleb-forming implantation, which lowers intraocular pressure (IOP) and has a good safety profile.1–8 It is becoming more widely used as the surgical learning curve is steeper than for the trabeculectomy and the intraoperative and postoperative follow-up routine is not as challenging. Thus, this device is an alternative for trabeculectomy in patients with open angle glaucoma with high IOP.3,8–10
Despite optimistic postoperative results in terms of the IOP reduction or the number of eye drops needed, postoperative complications, such as bleb fibrosis, are commonly seen after the PreserFlo implantation. According to recent studies, the frequency of bleb fibrosis varies between 11.8% and 19%7,11,12 6–12 months after implantation. The most commonly used surgical technique to restore filtration is bleb needling. This method involves a needle carefully used to separate adhesions within the bleb. Bleb needling carries the risk of serious complications, such as conjunctival erosiones,13 endophthalmitis,14 as well as damage to the implant or hypotony with maculopathy and choroidal detachment.15–17
To avoid complications and to be more effective when the PreserFlo implantation is embedded in fibrotic tissue, we used an alternative technique instead bleb needling and performed a revision by opening the conjunctiva and tenon in case of bleb fibrosis after PreserFlo implantation.
We present, to the best of our knowledge, the first data on the efficacy and safety of an open revision after PreserFlo implantation in bleb fibrosis.
PATIENTS AND METHODS
Study Design
This is a retrospective, monocentric analysis of consecutive cases of patients with bleb fibrosis after PreserFlo implantation at the Department of Ophthalmology of the Mainz, University Medical Center, Mainz, Germany. All revisions were conducted by 2 surgeons between September 2020 and January 2022. Demographic data on age, sex, type of glaucoma, lens status, previous surgeries, number of antiglaucoma medications, IOP, number of 5-fluorouracil (5-FU) injections postoperatively, complications, and reoperations were collected.
Primary and Secondary Outcomes
The primary outcome was IOP reduction after open revision. The secondary outcomes were the number of glaucoma medications, postoperative complications, and reoperations after the open revision procedure.
Surgical Technique
Our revision technique is as follows: a radial incision of the conjunctiva and tenon was performed ~4 mm away from the implantation. The underlying sclera was revealed, as shown in Figure 1A. Cauterization was performed when needed. The stent was exposed, and its flow was checked (Fig. 1B). The fibrotic tissue was removed when needed. Then, the tenon’s tissue was opened to the posterior and lateral sides using blunt scissors. Mitomycin-C (MMC) (0.2 mg/mL) was applied for 3 minutes using a Merocel sponge (Figure 1C). Then, Tenon’s capsule was reattached with 8-0 vicryl suture, followed by a continuous suture (vicryl 8-0) of the conjunctiva (Figure 1D). Every patient received a 5-FU subconjunctival injection in the temporal inferior quadrant after the revision, starting on the first postoperative day. Only in the case of Seidel positivity, signs of hypotony, such as flattening of the anterior chamber, choroidal detachment, or corneal erosion, no subconjunctival 5-FU injections were performed.
FIGURE 1.

Surgical technique of open revision in case of bleb fibrosis after PreserFlo Microshunt implantation. A, Radial incision of the conjunctiva and tenon. B, Stent exposure and aqueous humor outflow through the implantation are checked. C, Application of the MMC (0.02 mg/mL) for 3 minutes. D, Reattachment of tenon’s capsule with 8-0 vicryl suture, followed by a continuous suture (vicryl 8-0) of the conjunctiva. MMC indicates mitomycin-C.
Statistics
Statistical analysis was performed using GraphPad Prism9, Version 9.5.1 (GraphPad Software) for Mac. For statistical analysis, best corrected visual acuity was converted into a logarithm of the minimum angle of resolution scale. Categorical variables were presented as absolute and relative frequencies, whereas mean and SD were computed for approximately normal-distributed continuous variables. Continuous variables were compared with the Wilcoxon test. We performed an uncorrected Welch t test for age between men and women. All statistics were 2-sided and P value < 0.05 was considered statistically significant.
RESULTS
Twenty-seven eyes of 27 patients were included in this retrospective analysis having elevated IOP associated with bleb fibrosis after PreserFlo implantation and subsequent open revision.
Demographic Data
Of the included patients, 56% (n = 15) were females. Primary open angle glaucoma (POAG) was the most common diagnosis, (n = 14, 52%). Four patients had pseudoexfoliation glaucoma, 2 normal tension glaucomas, 2 neovascular glaucomas, 1 pigment dispersion glaucoma, 1 irido-corneo-endothlial-syndrome, 1 Axenfeld-Rieger syndrome, and 1 ocular hypertension.
The highest reported IOP ever in these patients was 33.2 ± 9.3 mm Hg. 41% (n = 11) of patients were pseudophakic. Of the patients, 33% (n = 9) had ocular surgery before PreserFlo implantation, apart from cataract surgery (Table 1).
TABLE 1.
Demographic Data of Study Cohort
| n (%) | |
|---|---|
| Sex | |
| Female | 15 (55.6) |
| Male | 12 (44.4) |
| Age | |
| Mean±SD | 66.9±12.9 |
| Range (y) | 29–86 |
| Diagnosis | |
| POAG | 14 (52) |
| Pseudoexfoliation glaucoma | 4 (15) |
| Other | 9 (33) |
| Prior surgeries (before Preserflo implantation) | |
| Cataract surgery | 11 (40.7) |
| Trabeculectomy | 2 (7.4) |
| SLT | 3 (11.1) |
| Cyclophotocoagulation | 3 (11.1) |
| Cyclocryocoagulation | 3 (11.1) |
| Vitrectomy | 3 (11.1) |
| Others | 4 (14.8) |
POAG indicates primary open angle glaucoma; SLT, selective laser trabeculoplasty.
Patient’s Characteristics at the Time of PreserFlo Implantation
At the time of PreserFlo implantation, patients’ mean age was 66.9 ± 12.9 years (mean ± SD). They used on average 2.9 ± 1.6 glaucoma medications, and visual acuity was 0.4 ± 0.4 (logarithm of the minimum angle of resolution). Of PreserFlo implantation, 96.3% (n = 26) were done in our clinic and 1.7 ± 1.5 subconjunctival 5-FU injections were performed after PreserFlo implantation. Before PreserFlo implantation, the mean preoperative IOP was 26.6 ± 9.8 mm Hg and decreased to 8.7 ± 4.6 mm Hg (−67.3%) within the first week (P < 0.0001). The patient’s demographic data are presented in Table 1.
Open Revision of PreserFlo in All Patients
The time between the initial PreserFlo implantation and the first occurrence of an elevated IOP was 5.2 ± 6.2 months. The mean preoperative IOP before the open revision of the PreserFlo implantation was 26.4 ± 9.9 mm Hg. On the first postoperative day, IOP was 8.9 ± 5.7 mm Hg (−66%) P < 0.0001). After one week, IOP was reduced to 7.0 ± 2.7 mm Hg (−73% compared with preoperatively) (P < 0.0001).
After 1 month, the mean IOP was 12.9 ± 5.9 mm Hg (−51%) (P < 0.0001), 12.1 ± 4.5 mm Hg (−54%) at 3 months (P = 0.0002), 16.3 ± 7.4 mm Hg (−38%) at 6 months (P = 0.01), and 15.9 ± 4.1 mm Hg (−40%) at 12 months (P =0.02) (Fig. 2).
FIGURE 2.
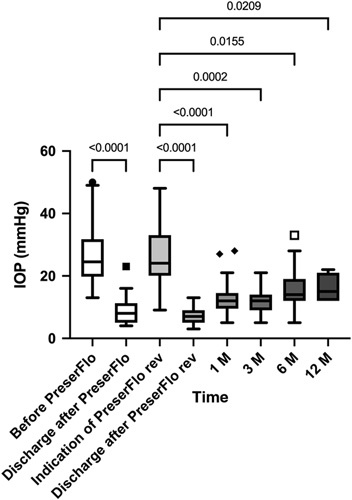
IOP before and after initial PreserFlo Microshunt implantation and open revision in the total study group (n = 27 eyes). IOP indicates intraocular pressure; rev, revision.
2.9±2.2 subconjunctival 5-FU injections were performed within 8.9 ± 9.5 days after open revision of the PreserFlo implantation.
Glaucoma Medication
At the indication of the PreserFlo revision, the number of medications was 0.9 ± 1.4. After 1 month, no glaucoma medication was needed to control the IOP (P = 0.009). After 3 months, the number of medications was 0.2 ± 0.6 (P = 0.04), after 6 months 0.6 ± 1.1 (P=0.79), and 0.7 ± 1.1 in 4 patients (14.8%) after 12 months to reduce IOP (P = 0.99). The glaucoma medication data are presented in Figure 3. There was a statistically significant reduction in the glaucoma medication up to 3 months after the revision.
FIGURE 3.
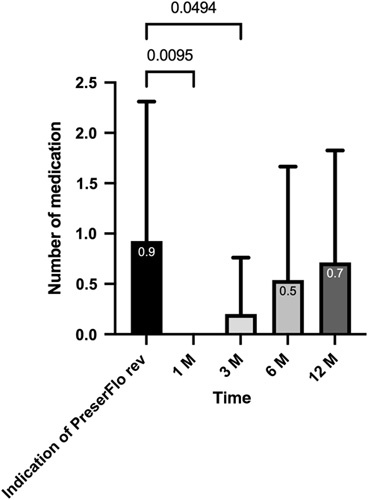
Glaucoma medication after open revision of PreserFlo Microshunt implantation. rev indicates revision.
Complications
One eye (4%) presented with a positive Seidel test, and needed a conjunctival suture in local anesthesia within the first week after the revision.
Reoperations
A total of 4 patients (15%) received another procedure later on as bleb fibrosis after open revision of PreserFlo occurred again. One patient received a trabeculectomy having had a vitrectomy and trabeculectomy before the initial PreserFlo implantation. Another patient having iridocorneal endothelial syndrome received a Paul glaucoma drainage implantation to control IOP. A 29-year-old patient with Axenfeld-Rieger received a second open revision of the PreserFlo and later on a Paul glaucoma drainage implantation. The fourth patient developed a cilio-lenticular block after initial PreserFlo implantation. Subsequently, cataract surgery, iridectomy, and vitrectomy were performed. This patient received a second open revision of PreserFlo to control IOP and has been stable since then.
Sensitivity Analysis on Patients With Primary Open Angle Glaucoma Without Previous Glaucoma Surgery
In patients with POAG who did not receive other previous surgeries than cataract surgery (n = 14), the mean preoperative IOP before the open revision of the PreserFlo was 24.0 ± 6.8 mm Hg and was reduced to 7.0 ± 2.6 mm Hg (−70.8%) in the first week/at discharge, (P < 0.0001). After 1 month, the mean postoperative IOP was 11.7 ± 6.0 mm Hg (−51.3%) (P = 0.0016), 10.3 ± 3.2 (−57.1%) mm Hg at 3 months (P = 0.0019), 13.3±4.8 mm Hg (−44.6%) at 6 months (P = 0.11) and 16.0 ± 5.3 mm Hg (−33.3%) at 12 months (P = 99). The Data on IOP fluctuation in patients with POAG after the initial PreserFlo implantation and revision are presented in Figure 4.
FIGURE 4.

IOP before and after PreserFlo Microshunt implantation and open revision in patients with POAG without previous glaucoma surgery. IOP indicates intraocular pressure; POAG, primary open angle glaucoma; rev, revision.
DISCUSSION
To the best of our knowledge, this is the first study describing the results of an open bleb revision for bleb fibrosis after PreserFlo Microshunt implantation. We present 1-year results of an open bleb revision with MMC, showing promising outcomes in terms of IOP reduction and reduction of IOP lowering medications. The incidence of complications and the need for subsequent revisions were found to be low in our study.
The efficacy and safety of primary PreserFlo Microshunt implantation have been well described. Mean IOP reduction varies between 29% and 60% one year after initial implantation, depending on the study, MMC concentration, and placement of MMC.2,4,5,7,10 Our IOP reduction 12 months after the revision was 39% for the total study group and 33% for patients with POAG without previous glaucoma surgery. This shows that an open revision for bleb fibrosis after the PreserFlo Microshunt implantation can be successful in restoring the function of the implant.
Bleb fibrosis following glaucoma surgeries, such as trabeculectomy or XEN implantation, is a major cause of surgical failure and is thus widely discussed among glaucoma surgeons due to its common occurrence and clinical significance. Similar, newer surgical procedures, such as PreserFlo Microshunt implantation, have also demonstrated a notable percentage of bleb fibrosis, often necessitating additional surgical interventions. Bleb fibrosis occurs among 3.4% after 3 months, 15.6% after 9 months, 19.0% after 12 months, and up to 53% after 4 years after PreserFlo Microshunt implantation.1,7,18–21 Up to now, there is no recommended surgical procedure in case of bleb fibrosis for PreserFlo Microshunt implantation due to the lack of data.
Some surgeons perform needling procedures,2 others lean towards revision with opening the conjunctiva and tenon,22 whereas others do not attempt any revision, but do further incisional glaucoma surgery.4 Given that the most commonly observed bleb after PreserFlo Microshunt implantation is localized beneath Tenon’s capsule posterior to the limbus, as reported by Ibarz Barberá et al23 in 2022, and considering the deep positioning of the device, an open revision seems to be a suitable approach.22 It allows the removal of scar tissue surrounding the tube, which is usually responsible for stopping the flow.1
Bleb needling after trabeculectomy24,25 or XEN implantation can result in challenges.26 Insufficient IOP reduction, the postoperative need for glaucoma medication, further revisions, and serious complications have been described in the literature.16,17,24–26 Learned from these experiences, we propose open bleb revision in case of bleb fibrosis after PreserFlo Microshunt implantation, including the usage of MMC. Previous research has demonstrated that the usage of antimetabolites reduces the risk of bleb failure after trabeculectomy,27–30 MMC is also used during the initial PreserFlo implantation. However, Ibarz Barbera et al1 discussed that there is maybe a need for additional investigation on whether MMC is needed in the mid-posterior region of the eye where the PreserFlo Microshunt drains and the Tenon capsule is thicker. In this area, a larger amount of fibroblasts is residing.
In the proposed open revision technique, we used MMC, 0.2 mg/mL as antimetabolites, followed by subconjunctival 5-FU injections once a day starting on the first postoperative day.
However, the 5-FU and MMC usage can lead to bleb leak, blebitis, or endophthalmitis.31 In our study, there was no severe side effect in any of the patients who underwent the open revision, but one patient needed a further conjunctival suture due to bleb leakage.
When comparing our results with a study of open revision after XEN implantation, the average reduction in IOP was similar at 37.5%, and there was a 65% reduction in the number of required IOP-lowering medications at 1 year. However, 4 out of the 16 patients in the XEN study required bleb needling with 5-FU injection in the first 12 months after revision surgery, and one eye experienced bleb-related endophthalmitis.32
Similar results are reported for XEN-needling: Ali et al33 showed a mean IOP reduction of 33% at 6 months and 38% at 12 months. There are advantages and disadvantages of bleb needling and open bleb revision. Needling is a less invasive procedure compared with open bleb revision. It involves using a thin needle to create small punctures within the bleb.24 In contrast, open revision typically requires a larger incision and more extensive manipulation of the bleb, which can increase the risk of complications. The needling involves minimal use of additional surgical materials and requires less operating room time, which can result in cost savings for patients and health care systems.
Our study has several limitations. First, it is a retrospective single-center study with no control group. Second, almost half of the cases with bleb fibrosis were cases with “off-label” use of PreserFlo Microshunt implantation (secondary open angle glaucoma) and thus might have had a different fibrosis risk. To account for this fact, we conducted a sensitivity analysis including only patients with POAG and no previous glaucoma surgery. Bleb revision was performed by 2 surgeons in a real-world clinical setting, with both surgeons using the same surgical technique with the same intra and postoperative management. The case number of this study was relatively low when analyzing severe but rare complications, which should be incorporated into future prospective studies.
CONCLUSION
The proposed open revision technique with MMC in case of bleb fibrosis after PreserFlo Microshunt implantation showed sufficient IOP reduction and eye drop freedom with only a few side effects. A revision of the Preserflo Microshunt implantation in case of bleb fibrosis should be considered before discussing other surgical options.
Footnotes
Disclosure: The authors declare no conflict of interest.
Contributor Information
Alicja Strzalkowska, Email: alicja.m.bula@gmail.com.
Piotr Strzalkowski, Email: peterstrzalkowski@gmail.com.
Esther M. Hoffmann, Email: ehoffman@uni-mainz.de.
Norbert Pfeiffer, Email: norbert.pfeiffer@unimedizin-mainz.de.
Alexander K. Schuster, Email: alexander.schuster@uni-mainz.de.
REFERENCES
- 1.Ibarz Barberá M, Martínez-Galdón F, Caballero-Magro E, et al. Efficacy and safety of the PreserFlo microshunt with mitomycin C for the treatment of open angle glaucoma. J Glaucoma. 2022;31:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batlle JF, Corona A, Albuquerque R. Long-term results of the PRESERFLO MicroShunt in Patients with primary open-angle glaucoma from a single-center nonrandomized study. J Glaucoma. 2021;30:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhayani R, Martínez de la Casa JM, Figus M, et al. Short-term safety and efficacy of PreserfloTM Microshunt in glaucoma patients: a multicentre retrospective cohort study. Eye. 2022;37:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheres LMJ, Kujovic-Aleksov S, Ramdas WD, et al. XEN® gel stent compared to PRESERFLOTM MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 2021;99:e433–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fea AM, Laffi GL, Martini E, et al. Effectiveness of microshunt in patients with primary open-angle and pseudoexfoliative glaucoma: a retrospective European Multicenter Study. Ophthalmol Glaucoma. 2022;5:210–218. [DOI] [PubMed] [Google Scholar]
- 6.Pillunat KR, Herber R, Haase MA, et al. PRESERFLOTM MicroShunt versus trabeculectomy: first results on efficacy and safety. Acta Ophthalmol. 2022;100:e779–e790. [DOI] [PubMed] [Google Scholar]
- 7.Baker ND, Barnebey HS, Moster MR, et al. Ab-externo microshunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021;128:1710–1721. [DOI] [PubMed] [Google Scholar]
- 8.Schlenker MB, Durr GM, Michaelov E, et al. Intermediate outcomes of a novel standalone Ab externo SIBS microshunt with mitomycin C. Am J Ophthalmol. 2020;215:141–153. [DOI] [PubMed] [Google Scholar]
- 9.Pinchuk L, Riss I, Batlle JF, et al. The development of a micro‐shunt made from poly (styrene‐ block ‐isobutylene‐ block ‐styrene) to treat glaucoma. J Biomed Mater Res B Appl Biomater. 2017;105:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riss I, Batlle J, Pinchuk L, et al. One-year results on the safety and efficacy of the InnFocus MicroShunt™ depending on placement and concentration of mitomycin C. J Fr Ophtalmol. 2015;38:855–860. [DOI] [PubMed] [Google Scholar]
- 11.Vastardis I, Fili S, Perdikakis G, et al. Preliminary results of Preserflo Microshunt versus Preserflo Microshunt and Ologen implantation. Eye Vis (Lond). 2021;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barberá MI, Martínez-Galdón F, Caballero-Magro E, et al. Efficacy and safety of the Preserflo MicroShunt with mitomycin C for the treatment of open angle glaucoma. J Glaucoma. 2022;31:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaels L, Holland L, Mercieca K. Trans-conjunctival erosion of a novel SIBS microshunt after revision surgery using mitomycin C. J Glaucoma. 2021;30:e349–e351. [DOI] [PubMed] [Google Scholar]
- 14.Brambati M, Bettin P, Ramoni A, et al. A case of endophthalmitis following needling procedure after PRESERFLO® Micro Shunt implantation. Eur J Ophthalmol. 2022;32:NP83–NP86. [DOI] [PubMed] [Google Scholar]
- 15.Olivari S, Cutolo CA, Negri L, et al. XEN implant fracture during needling procedure. J Glaucoma. 2019;28:1086–1089. [DOI] [PubMed] [Google Scholar]
- 16.Broadway DC, Bloom PA, Bunce C, et al. Needle revision of failing and failed trabeculectomy blebs with adjunctive 5-fluorouracil: survival analysis. Ophthalmology. 2004;111:665–673. [DOI] [PubMed] [Google Scholar]
- 17.Feldman RM, Tabet RR. Needle revision of filtering blebs. J Glaucoma. 2008;17:594–600. [DOI] [PubMed] [Google Scholar]
- 18.Durr GM, Schlenker MB, Samet S, et al. One-year outcomes of stand-alone ab externo SIBS microshunt implantation in refractory glaucoma. Br J Ophthalmol. 2022;106:71–79. [DOI] [PubMed] [Google Scholar]
- 19.Lenzhofer M, Kersten-Gomez I, Sheybani A, et al. Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Experiment Ophthalmol. 2019;47:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quaranta L, Micheletti E, Carassa R, et al. Efficacy and safety of PreserFlo® MicroShunt after a failed trabeculectomy in eyes with primary open-angle glaucoma: a retrospective study. Adv Ther. 2021;38:4403–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawiroredjo SSM, Bramer WM, Pawiroredjo ND, et al. Efficacy of the PRESERFLO MicroShunt and a meta-analysis of the literature. J Clin Med Res. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seuthe AM, Erokhina M, Szurman P, et al. One year results of PRESERFLO® MicroShunt implantation for refractory glaucoma. J Glaucoma. 2023;32:414–419. [DOI] [PubMed] [Google Scholar]
- 23.Ibarz Barberá M, Morales Fernández L, Tañá Rivero P, et al. Anterior-segment optical coherence tomography of filtering blebs in the early postoperative period of ab externo SIBS microshunt implantation with mitomycin C: morphological analysis and correlation with intraocular pressure reduction. Acta Ophthalmol. 2022;100:e192–e203. [DOI] [PubMed] [Google Scholar]
- 24.Mercieca K, Drury B, Bhargava A, et al. Trabeculectomy bleb needling and antimetabolite administration practices in the UK: a glaucoma specialist national survey. Br J Ophthalmol. 2018;102:1244–1247. [DOI] [PubMed] [Google Scholar]
- 25.Rotchford AP, King AJW. Needling revision of trabeculectomies bleb morphology and long-term survival. Ophthalmology. 2008;115:1148–1153.e4. [DOI] [PubMed] [Google Scholar]
- 26.Midha N, Gillmann K, Chaudhary A, et al. Efficacy of needling revision after XEN gel stent implantation: a prospective study. J Glaucoma. 2020;29:11–14. [DOI] [PubMed] [Google Scholar]
- 27.Costa VP, Arcieri ES, Freitas TG. Long-term intraocular pressure control of eyes that developed encapsulated blebs following trabeculectomy. Eye. 2006;20:304–308. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Simon GJ, Glovinsky Y. Needle revision of failed filtering blebs augmented with subconjunctival injection of mitomycin C. Ophthalmic Surg Lasers Imaging. 2003;34:94–99. [PubMed] [Google Scholar]
- 29.Mardelli PG, Lederer CM, Jr, Murray PL, et al. Slit-lamp needle revision of failed filtering blebs using mitomycin C. Ophthalmology. 1996;103:1946–1955. [DOI] [PubMed] [Google Scholar]
- 30.Ewing RH, Stamper RL. Needle revision with and without 5-fluorouracil for the treatment of failed filtering blebs. Am J Ophthalmol. 1990;110:254–259. [DOI] [PubMed] [Google Scholar]
- 31.Narayanaswamy AK, Lee K, Zhen M, et al. Randomized, controlled trial of a sustained delivery formulation of 5-fluorouracil for the treatment of failing blebs. Ophthalmology. 2012;119:314–320. [DOI] [PubMed] [Google Scholar]
- 32.Linton E, Au L. Technique of Xen implant revision surgery and the surgical outcomes: a retrospective interventional case series. Ophthalmol Ther. 2020;9:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali ZC, Moshin N, Hakim MT, et al. Two-year outcomes of XEN implantation with minimal bleb needling. J Curr Glaucoma Pract. 2022;16:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


