Abstract
Background:
The National Cancer Registry of Panama (NCRP) was established in 1974. In 1984, histological confirmation became mandatory. The now pathology-based registry has evolved and has been a population-based cancer registry (PBCR) since 2012 with cancer-specific Web-based reporting software. Herein, we characterize the main features in its development that may help readers understand its evolution and improvements that are needed to be in line with international standards.
Methods:
We describe the major components of the NCRP using its structure, processes, and a results framework for 3 major periods since its inception: 1974–1999, 2000–2011, and 2012 to present.
Results:
The NCRP has always been linked to the Ministry of Health of Panama. Until the end of its second period, it operated as a pathology-based registry and all staff worked part time. Currently, the NCRP is based on passive reporting through a Web-based system set up for both public and private health institutions, covering 77% of the existing health-care institutions in the nation. The number of cases with unknown age were less than 10 per year and primary tumors with unknown origin were at most 3%. The proportion of death certificate only (DCO) cases decreased 5% in 18 years. Men are more likely to have DCO than women (odds ratio, 1.53; 95% CI, 1.48–1.58).
Discussion:
The NCRP has evolved, achieving significant improvements and progress over the years. Yet, much remains to be done. To provide internationally comparable, valid, and timely cancer incidence data, the NCRP should continue to improve its quality and coverage and provide continuous staff training on cancer registry procedures.
Keywords: cancer control, neoplasms, Panama, population-based cancer registries
Introduction
Cancer incidence calculated using registration data plays a key role in guiding cancer control plans and in the evaluation of interventions. To achieve these goals, reliable, high-quality data compiled through population-based cancer registries (PBCRs) is crucial.1,2 These goals constitute important challenges, particularly for low- and middle-income countries in Africa, Latin America, and Asia where less than 10% of the population is currently covered by a high-quality PBCR.3
Despite these figures, many Latin American countries are trying to improve their cancer registries. As of 2014, almost 98 registries covered approximately 20% of the population in the region.4 In Central America, only Costa Rica has a PBCR with national coverage that has met the quality standards for contributing to Cancer Incidence in Five Continents global statistics.5 Very recently, Guatemala, Honduras, El Salvador, and Nicaragua have documented advances towards reliable data for cancer control.4,6
Panama is a country of over 4.2 million inhabitants divided into 10 provinces and 5 comarcas (a Spanish name for indigenous territories)7, the latter of which are areas mostly populated by several indigenous groups, representing an estimated 12.3% of the total population.8 For 2014, cancer ranked second as a cause of death after cardiovascular diseases, accounting for approximately 16.8% of the total national mortality.9
Since 1974, the National Cancer Registry of Panama (NCRP) has been functioning as a pathology-based registry, until recently with incomplete coverage. As of 2000, the NCRP allocated annual budgets, and data began to be captured in databases.10 In 2012, the NCRP initiated a new phase, transitioning to a PBCR instead of a pathology-based registry. In 2015, Panama received an expert consult in the framework of the Global Initiative for Cancer Registry Development, a program led by the International Agency for Research on Cancer (IARC) that is geared to improve coverage and quality of cancer registration at a global and regional level.4
This article describes the major characteristics and changes introduced in the NCRP since its inception in 1974, with emphasis on its latter evolution. We also discuss practical implications of attaining a better coverage and quality of data.
Methods
In this review, we list the critical features of a PBCR that are recommended at an international level,11 organizing them in terms of structure, process, and results. Using this scheme, we summarize the main organizational and process characteristics of the NPCR during 3 periods: from its inception in 1974 until 1999, from 2000 until 2011, and from 2012 onward. The information was compiled using the documentation of the NPCR as well as a personal interview with one of the coauthors who has been working closely with the registry since 1974.
We compare the number of cancer cases and the crude rates reported annually in each of the periods, and we also compare the quality indicators in the available years of the last period.
Results
The main characteristics referring to structure and process through each of the periods are summarized in Table 1.
Table 1.
Main Characteristics of the Panama Cancer Registry by Main Periods
| PBCR aspects | Periods of the registry | ||
|---|---|---|---|
| 1974-1999 | 2000-2011 | From 2012 onward | |
| Structure | |||
| Regulation and plans | Inception of the National Cancer Registry of Panama (NCRP) (1974) Mandatory reporting of cancer (MoH Resolution) (1984) |
National Commission of the NCRP (2000) Regulation of the NCRP Commissionand its functions (2011) |
Executive order for a new NCRP (2012) National NCDs control strategy(2014) |
| Organization and structure | MoH-Planning and Statistics Directorate Advisory Committee | MoH-Planning and Statistics Directorate Advisory Committee | MoH-Planning and Statistics Directorate Advisory Committee |
| Physical location | Oncological Institute and then MoH Planning Directorate | MoH Planning Directorate | MoH Planning Directorate |
| Director | Part time dedication (10%) | All directors part time (10%) 2000-2009 MD oncologist 2009-2011 MD public health |
All directors part time (10%) 2012-2013 MD public health 2014-2018 MD public health |
| Staff at central office | 1974-1984: 1 coordinator and1 technician 1984-1992: 1 coordinator (part time) 1993-1999: 1 coordinator and 3 technicians (part time) |
2000-2011: 1 coordinator (part time) and 2 technicians (part time) | 1 Coordinator (part time) 3 Statisticians (part time) 1 Register specialist (part time) 1 Epidemiologist (part time) 1 IT specialist (part time) 1 pathologist (occasional) |
| Financing | No funds for many years MoH, NGOs (1993-1999) | MoH, PAHO (2003-2004) | MoH; NGO (supporting mainly training) |
| Process | |||
| Type of registry | Histopathological | Histopathological | PBCR |
| Case definition | Cancer cases diagnosed in Panama, confirmed by histopathology | Cancer cases diagnosed in Panama, confirmed by histopathology | Cancer cases diagnosed in Panama, including benign CNS tumors; in situ breast and cervix |
| Coverage | National | National | National; estimated 77% |
| Sources and data collection | Pathology labs from public and private hospitals (3) Hospital discharges; death certificates; clinical records Passive data collection |
Pathology labs from public and private hospitals (3) Hospital discharges; death certificates; clinical records Fox-Prox software data collection.Semi-decentralized |
Passive to central level through web |
| Coding | 1974-1979: ICD-8th revision 1980-1984: ICD-9th revision 1998: ICD-10 |
ICD-10 Since 2009 ICD-O-3 |
ICD-10 ICD-O-3 |
| Manual of procedures | Procedures Manual 198427 | 2003 Induction manual27 2011 Manual of Procedures27 |
2012 Procedures manual (2nd Ed)27 2016 Procedures manual (3rd Ed)27 |
| Dissemination | Periodic results disseminated in printed form and uploaded to website (5 reports available)27 | Periodic results disseminated in printed form and uploaded to website27 | 2012-201827 2019-2020 Preliminary findings27 |
| Training of staff | 1 staff member went to IARC Summer Course (1998) International Registry Course in Lima(1998) |
2 staff members had a mentorship stay in Cuba (2011; PAHO financed) | 5 staff in GICR training course (2015 Panama; 2016 Ecuador) 2 staff members had a mentorshipstay (2016; GICR financed) |
| Evaluation | None | 2010 external evaluation | IARC external evaluation and recommendations |
CNS, central nervous system; IARC, International Agency for Cancer Research; GICR, Global Initiative for Cancer Registration; MD, medical doctor; MoH, Ministry of Health; NCDs, noncommunicable diseases; NGO, nongovernmental organizations; PAHO, Pan American Health Organization; PBCR, population-based cancer registry.
1974–1999
As a pathology-based cancer registry, NCRP was founded in 1974 when the Ministry of Health (MoH) established a national commission for cancer registry. The NCRP inception was based at the National Oncological Institute, but soon after, the registry was transferred within the MoH to the Department of Registry and Health Statistics.
NCRP data sources included public hospitals in the country with pathology or cytology departments as well as the National Mortality Registry, handled by the Instituto Nacional de Estadística y Censo (National Institute of Statistics and Census; INEC). Only cases with histological confirmation of a cancer were accepted and reported. Until 1984, data collection was done manually. After 1984, the staff at the statistics departments working in public hospitals, together with pathologists, sent information to the NCRP. Data from private hospitals were collected manually through active casefinding by NCRP personnel. Topography was coded according to the International Classification of Diseases (ICD) using ICD-8 (1974–1984) and ICD-9 (1984–1999). This was based mainly on descriptions regarding the site of origin in the pathology report.
The aim of the registry, sources of information, and case reporting criteria were defined in the first procedures manual published by a multisectoral committee at the MoH.12 By 1984, a resolution by the MoH made notification of cancer cases to NCRP mandatory for both public and private health institutions at the national level.12 In the same year, the International Classification of Diseases for Oncology (ICD-O) was introduced.
As of 1993, records were stored in electronic format (FoxPro databases), allowing the detection and elimination of duplicates, as well as the recording of multiple primary cancer cases. With the use of Microsoft Excel, it was also possible to merge NCPR data with National Mortality Registry data. The NCPR director during this period was an oncologist who worked for 10 years pro bono on a part-time basis. The remainder of the staff was also part-time, supported with funds provided through Fundacancer, a nongovernmental organization.
The first official printed report, published in 1990, described the incidence and mortality of cancer from data collected during 1974–1984.13 Unstable political circumstances in the country and limited funding severely hindered more frequent publication of reports. Nevertheless, in 1994, several reports were published for the 1985–1992 period.14 In 1998, one staff member took an IARC summer course in cancer epidemiology.
2000–2011
The Executive Order 384/2000 installed the National Commission of the NCRP, improved the financial status of the registry, and allowed training registrars at the regional hospitals.15 The same year, the Executive Order 384/2000 was issued, ICD-10 was introduced (with a Spanish version available in 1998) and, by the year 2009, the ICD-O-3 began to be utilized. From 2004–2011, the registry gradually began to decentralize, and the need for consultancies and ongoing mentoring with international partners was recognized.
For the first time, in 2009, the NCRP director had a public health background, though he only dedicated an estimated 10% of his time to the registry. In 2010, NCRP had an external evaluation by the Ibero-American Network of Cancer Epidemiology and Information Systems (REDEPICAN)16, which resulted in the identification of its strengths and weaknesses. The main results of the evaluation were that the registry should become a PBCR rather than pathology-based, and it must improve the quality of the data to comply with international standards. Shortly after, a new registry was designed, aiming to establish it as a PBCR with national coverage.
From 2012 Onward
In 2012, based on the decision to convert the registry into a PBCR, its inclusion criteria changed. The number of registry staff at the central level increased and efforts to include new data sources (public and private) were put into effect, including changes in the procedure manual.17
Cases were defined as any invasive neoplasm, as well as in situ breast and cervical cancers, and benign neoplasms of the central nervous system. Cases include all those diagnosed in Panama, regardless of nationality (including nonresidents, although nonresidents were excluded from the analysis).
Currently, the NCRP collects data from 77% of the health institutions with cancer-related services at a national level (Table 2). Cases are abstracted by 44 staff-trained members affiliated in the institutions (26 facilities in 8 provinces). Cases are abstracted using structured case notification forms and a Web-based registry information system. The forms and copies of the pathology reports (if available) are sent to the NCRP central office, where the registry staff compares and validates the information. LINK PLUS software is used to identify duplicates.18
Table 2.
Private and Public Health Institutions Reporting to the National Cancer Registry of Panama in 2017
| Province | Hospital | Public/Private | Reporting | Estimated population coverage |
|---|---|---|---|---|
| Coclé | Rafael Estevez | Public | X | 282,560 |
| Aquilino Tejeira | Public | X | 227,786 | |
| Colon | Manuel Amador Guerrero | Public | X | 268,002 |
| Centro Médico del Caribe | Private | X | ND | |
| 4 Altos | Private | X | ND | |
| Chiriquí | Dr. Rafael Hernández | Public | 448,329 | |
| José Domingo de Obaldía | Public | 279,588 | ||
| Chiriquí | Private | ND | ||
| Clínica Hospital Mae Lewis | Private | ND | ||
| Herrera | Gustavo Nelson Collado | Public | X | 117,826 |
| Cecilio Castillero | Public | X | 117,826 | |
| San Juan Bautista | Private | ND | ||
| Venancio Villarreal | Private | ND | ||
| Los Santos | Joaquín P Franco | Public | X | 91,550 |
| Panama | Dr. Arnulfo Arias Madrid | Public | X | NC |
| Regional de Chepo | Public | X | 97,825 | |
| Especialidades Pediátricas Omar Torrijos Herrera | Public | X | NC | |
| Regional Docente 24 de Diciembre | Public | X | 236,733 | |
| Dra. Susana Jones Cano (San Judas Tadeo) | Public | X | NC | |
| San Miguel Arcángel | Public | X | 566,736 | |
| Santo Tomás | Public | X | NC | |
| Hospital del Niño | Public | X | NC | |
| Instituto Oncológico | Public | X | NC | |
| Centro Médico Paitilla | Private | X | ND | |
| Clínica Hospital Nacional | Private | X | ND | |
| Clínica Hospital Río Abajo | Private | ND | ||
| Punta Pacífica | Private | X | ND | |
| Clínica Hospital San Fernando | Private | X | ND | |
| Clínica Hospital Santa Fe | Private | X | ND | |
| Clínica Hospital Panamericano | Private | X | ND | |
| Policlínica Dr. Santiago Barraza | Public | X | 51,111 | |
| Veraguas | Santiago Luis Chicho Fábrega | Public | X | 243,491 |
| Panama Oeste | Nicolás Solano | Public | X | 518,013 |
| Total | 33 | 26 | ND |
NC, national coverage (tertiary referral hospitals); ND, not determined as patients from different regions may attend private hospitals. *Based on 2014 estimates.
A cancer specific Web-based information system performs automatized checks based on IARC software tools to check data validity and consistency.19 Multiple neoplasms are defined according to the IARC rules. When inconsistencies are observed, the NCRP contacts the regional departments of registries and statistics to ask for clarification. There is a close collaboration between the NCRP and staff of the National Mortality Registry at the INEC. Thus, the performance of the vital statistics system of Panama has been described as high quality for recent years.20,21
The NCRP reports the standard quality control indicators for population-based cancer registries: numbers and proportion of cases with death-certificate only (DCO), unknown age, and primary tumor. Morphologically verified cases include clinical investigation, image studies, and microscopic findings while patients are alive.
The NCRP continues to be located at the MoH, based at the Department of Registries and Health Statistics within the Planning Directorate. All registry staff have part-time dedication to the registry. The NCRP director dedicates 10% of their time to the registry. A statistician, an information specialist, and an epidemiologist have half-time contracts. In addition, 2 statisticians and a registry specialist dedicate about 70% of their time to the NCRP. Since 2016, a pathologist helps pro bono with resolving doubts on coding the correct diagnosis of difficult cases.
Until 2016, new employees recruited by the NCRP were trained on the job, which included a 3-day compulsory internship. Recently, the NCRP has designed a formal training program for cancer registration at a basic and middle level, which has been offered annually to the team members working at the central level as well as the registrars working at the reporting institutions. Through the Global Initiative for Cancer Registry Development, 2 staff members had the opportunity to undergo a 2-week mentorship in 2015 at the Cancer Registry of Uruguay, and 2 staff members participated in a cancer analysis course in Quito, Ecuador.
In 2015, an IARC expert performed a second external evaluation and found that, although the registry had made important improvements in becoming population-based, the proportion of verified cases without morphology was still relatively low, and some important information sources were not yet reporting to the registry.
From its inception, the NCRP has operated with continuous financial support from the MoH. Technical support has been provided by several organizations, such as the Pan American Health Organization, Fundacancer, and the National Commission for Tobacco Control. Financial resources are assigned by the MoH through the Department of Planning, under which the registry functions. Panama has a selective tax on tobacco consumption, which partly finances the NCRP.22 Funding is limited, which hinders possibilities for hiring full-time staff and the sustainability of registry operations. Currently, the staff members working at the NCRP are shared with other MoH services, adversely influencing the timely reporting of data.
The NCRP adheres to the confidentiality guidelines described in the manual of procedures, including personal data protection and confidentiality agreements signed by the cancer registrars.
Number of Cases and Crude Cumulative Incidence by Periods
The number of cases and the crude cumulative incidence rate (CCIR) are presented in Table 3. At the beginning of the first period in 1974, there were 1,216 cancer cases recorded with a CCIR of 75.1 per 100,000 inhabitants.14 At the beginning of the second period in 2000, the number of cases increased to 4,227, with a CCIR of 143.4 per 100,000 inhabitants (134.7 in males and 152.2 in females). At the beginning of the third period in 2012, the number of cases increased to 5,929 with a CCIR of 156.3 per 100,000 inhabitants (149.1 in males and 164.1 in females).23
Table 3.
Number of Cases and Crude Cumulative Incidence in the National Cancer Registry of Panama by Main Periods, Sex, and Year (1974–2018)
| Males | Females | Total | |||||
|---|---|---|---|---|---|---|---|
| Period | Year | Cases (N) | Crude incidence rate (100,000) | Cases (N) | Crude incidence rate (100,000) | Cases (N) | Crude incidence rate (100,000) |
| 1974-1999 | 1974 | NA | NA | NA | NA | 1,216 | 75.1 |
| 1984 | NA | NA | NA | NA | 2,930 | 137.3 | |
| 1985 | 1,177 | 105.9 | 1,720 | 160.9 | 2,897 | 132.9 | |
| 2000-2011 | 2000 | 2,005 | 134.7 | 2,222 | 152.2 | 4,227 | 143.4 |
| 2001 | 2,069 | 136.4 | 2,298 | 154.5 | 4,367 | 145.4 | |
| 2002 | 2,133 | 138.1 | 2,238 | 147.7 | 4,371 | 142.8 | |
| 2003 | 2,388 | 151.8 | 2,378 | 154.1 | 4,766 | 152.9 | |
| 2004 | 2,305 | 144.0 | 2,449 | 155.8 | 4,754 | 149.9 | |
| 2005 | 2,421 | 148.6 | 2,433 | 152.1 | 4,854 | 151.7 | |
| 2006 | 2,378 | 143.6 | 2,603 | 159.9 | 4,981 | 150.8 | |
| 2007 | 2,433 | 144.5 | 2,602 | 157.2 | 5,035 | 150.8 | |
| 2008 | 2,441 | 142.6 | 2,560 | 152.1 | 5,001 | 147.3 | |
| 2009 | 2,728 | 156.9 | 2,704 | 158.0 | 5,432 | 157.4 | |
| 2010 | 2,544 | 144.1 | 2,616 | 150.5 | 5,160 | 147.2 | |
| 2011 | 2,642 | 141.2 | 2,864 | 154.6 | 5,506 | 147.9 | |
| From 2012 onward | 2012 | 2,837 | 149.1 | 3,092 | 164.1 | 5,929 | 156.5 |
| 2013 | 2,932 | 151.6 | 3,175 | 165.7 | 6,107 | 158.6 | |
| 2014 | 3,015 | 153.4 | 3,354 | 172.2 | 6,369 | 162.7 | |
| 2015 | 2,651 | 132.8 | 3,071 | 155.1 | 5,722 | 143.9 | |
| 2016 | 3,118 | 153.9 | 3,657 | 181.8 | 6,775 | 167.8 | |
| 2017 | 3,614 | 175.8 | 3,983 | 195.0 | 7,597 | 181.3 | |
| 2018 | 3,389 | 162.5 | 4,150 | 200.2 | 7,539 | 181.3 | |
NA, not available.
Quality Indicators of the Register of the Third Period
Since 2012, the number of recorded cases with unknown ages was less than 10 per year, and the proportion of cases with unknown primary reached a maximum of 3.0% in 2014.
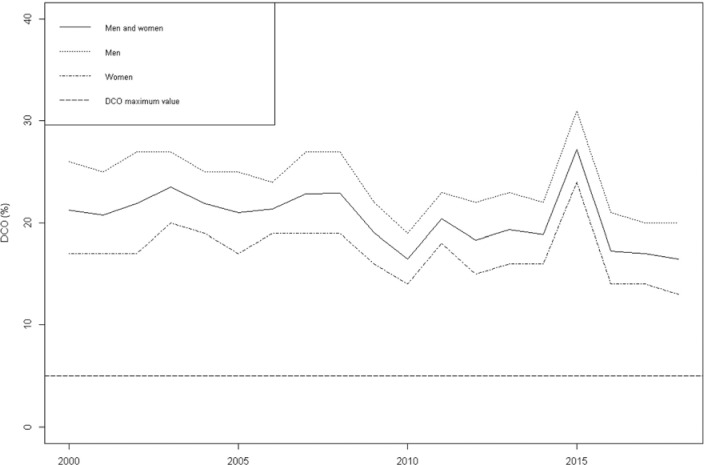
Figure 1 shows the proportion of cases diagnosed with DCO during third period, from 21% in 2012 to 16% in 2018. Using a reconstruction of data using published proportions of DCO by sex and year, the association of these later 2 variables with DCO was assessed using unconditional logistic regression (Google Collaboratory Notebook DCO Cancer Register Model Panama 2000-2018.ipynb; https://shorturl.at/duyV1). There is no published information of DCO proportions by sex since 2016. After adjustments by sex, the DCO decreased to 0.015% per year (OR, 0.985; 95% CI, 0.982–0.988). Men with cancer were more likely to have a DCO diagnosis than women (OR, 1.53; 95% CI, 1.48–1.58).
Figure 1.
Death-Certificate Only (DCO) Diagnosis in the National Register of Cancer of Panama, 2000–2018
Source: Published data from the National Register of Cancer of Panama. https://www.minsa.gob.pa/contenido/registro-nacional-del-cancer
Discussion
This paper provides an overview of the transition of the NCRP from being pathology-based to a population-based cancer registry. The decision at a high political level and the legal framework has helped to make PBCR a priority and to obtain financing, allowing the restructuring of the registry and training of the staff through the years.
Cancer registration needs sufficient resources to produce high-quality statistics, and the benefit gained through data-driven policies can be significant.24 Direct financing of the NCRP and increasing its resources may allow the hiring of staff exclusively for the registry, which will improve its sustainability and the quality and timeliness of data reporting. Despite the limitation of having human resources dedicating part time to the NCRP, important work has been done on the integration of new employees, focusing on the growth and sustainably of these new human resources over time. Adequate staffing of the registry must be ensured for the development of population-based cancer registration in low and middle-income areas and countries.11
The current coverage of 77% of the existing institutions needs improvement, and there is a constant necessity for quality and procedure controls in the local reporting facilities. The Web-based system has allowed a daily registration and continuous feedback on the inconsistencies among variables. Nevertheless, this does not guarantee correct processes of case-finding and reporting. For the near future, there are plans to increase the coverage of reporting sources to 100% and to work intensely on the report of quality indicators of the data through collaborations with the IARC. In many low- and middle-income countries, facilities related to cancer care appear and disappear frequently. The monitoring and identification of new facilities in the country requires constant attention and a close relationship with the Department of Registries and Health Statistics, which is the entity issuing the codes to the facilities. In addition, the inclusion of laboratories and radiography centers with computer tomography scans may be an important source of information.
The proportion of cases with unknown age and primary tumors are, all together, below 3%. Although there is a decrease of proportion of DCO, it is still above 10%, with men having a higher rate than women. A high DCO rate increases the uncertainty of the calculation of cancer survival in a PBCR,25 and it needs to be decreased further.
External evaluations are a crucial step to undertake, mainly in low- and middle-income countries, as it fosters resources and support toward a common objective.26 IARC and REDEPICAN have provided support and shared knowledge in designing the new NCRP. Efforts to standardize data collection tools and increase awareness of the cancer registry have been advocated through audits and the promotion of staff to visit other PBCR and training courses.
In conclusion, the NCRP has evolved, achieving significant improvements and progress over the years. However, much remains to be done. To provide internationally comparable, valid, and timely cancer incidence data, the NCRP should constantly improve quality and coverage, thereby allowing for a greater confidence in the areas of prevention, and planning efficiently the cancer control programs through the country. It is expected that, in the future, the NCRP will serve different purposes, from exploring socioeconomic inequalities and geographic variation within the country to the prediction of trends in long-term survival. Continuous staff training on cancer registry procedures, database record management, and statistics are essential for the improvement and growth of the NCRP.
Acknowledgements
The authors thank the staff working at the National Department of Registries and Health Statistics for their cooperation. A special thanks to Dr. Arturo Rebollón, Leticia Fernandez, Julio Santamaría, Guillermina McLeary, for their previous support to the NCRP and to Fundacancer for the financial support.
References
- 1.Parkin DM. The role of cancer registries in cancer control. Int J Clin Oncol. 2008;13(2):102-111. [DOI] [PubMed] [Google Scholar]
- 2.Piñeros M, Znaor A, Mery L, Bray F. A global cancer surveillance framework within noncommunicable disease surveillance: making the case for population-based cancer registries. Epidemiol Rev. 2017;39(1):161-169. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents Vol. XI. International Agency for Research on Cancer; 2021. [Google Scholar]
- 4.Piñeros M, Abriata MG, Mery L, Bray F. Cancer registration for cancer control in Latin America: a status and progress report. Rev Panam Salud Publica. 2017;41:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman D, Sierra MS. Cancer in Central and South America: Introduction. Cancer Epidemiol. 2016;44:S3-S10. [DOI] [PubMed] [Google Scholar]
- 6.Frech S, Piñeros M, Frazier L, et al. Advancing reliable data for cancer control in the Central America Four region. J Glob Oncol. 2018;4(4):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adames Y, Rivera J. Diagnóstico de la Población Indígena de Panamá con base en los Censos de Población y Vivienda de 2010. 2015. https://www.inec.gob.pa/archivos/P6571INDIGENA_FINAL_FINAL.pdf
- 8.Instituto Nacional de Estadística y Censo . Boletín 15. Estimaciones de la población de la república, provincia, comarca indígena por distrito, según sexo y edad; 2010–20. 2013. https://www.inec.gob.pa/publicaciones/Default3.aspx?ID_PUBLICACION=499&ID_CATEGORIA=3&ID_SUBCATEGORIA=10
- 9.Roa R, Santamaría I, Ruiloba A. Boletín Estadístico: Anuario 2015. Ministerio de Salud de Panamá (Panama Ministry of Health), Dirección de Planificación; 2016. https://www.minsa.gob.pa/sites/default/files/publicacion-general/boletin_nacional_2015.pdf [Google Scholar]
- 10.Politis M, Higuera G, Chang LR, Gomez B, Bares J, Motta J. Trend analysis of cancer mortality and incidence in Panama, using Joinpoint regression analysis. Medicine (Baltimore). 2015;94(24):e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray F, Znaor A, Cueva P, et al. Planning and Developing Population-Based Cancer Registration in Low- and Middle-Income Settings. International Agency for Research on Cancer; 2014. http://www.iarc.fr/en/publications/pdfs-online/treport-pub/treport-pub43/index.php [PubMed] [Google Scholar]
- 12.Manual de Procedimiento del Registro Nacional del Cáncer. Ministerio de Salud de Panamá (Panama Ministry of Health); 1984. https://www.minsa.gob.pa/sites/default/files/general/1984-manual_de_procedimientos_-registro_nacional_del_cancer.pdf [Google Scholar]
- 13.Trinidad Castillero J, Abadía C, Morales EA, et al. Registro Nacional de Cáncer 1974–1984. Ministerio de Salud; 1990. https://www.minsa.gob.pa/sites/default/files/general/1._boletin_estadistico_del_registro_nacional_de_cancer_1974-1984.pdf [Google Scholar]
- 14.Ruiloba A, Rodríguez de Causadias ML, Mojica L, McLearly G, Patiño de I. Principales Causas de Tumores Malignos en la República De Panamá Años : 1985–2009. 2018. https://www.minsa.gob.pa/sites/default/files/general/1985_-2009_estadisticas_finales_tumores_malignos_.pdf
- 15.Ministerio de Salud de Panamá (Panama Ministry of Health). Panamanian Executive Decree 384/2000. 2000. [Google Scholar]
- 16.Navarro C, Antonio Molina J, Barrios E, et al. Evaluación externa de registros de cáncer de base poblacional: la Guía REDEPICAN para América Latina. Rev Panam Salud Publica. 2013;34(5). [PubMed] [Google Scholar]
- 17.Fernández Garrote ML, Rodríguez Causadias de ML. Manual de Procedimientos Registro Nacional de Cancer De Panamá Versión 3. 2018. [Google Scholar]
- 18.Link Plus. National Program of Cancer Registries website. Accessed December 6, 2022. https://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm [Google Scholar]
- 19.Ferlay J, Burkhard C, Whelan S, Parkin DM. Check and conversion programs for cancer registries (IARC/IACR Tools for Cancer Registries). 2005. [Google Scholar]
- 20.Wang H, Abajobir AA, Abate KH, et al. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1084-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikkelsen L, Phillips DE, Abouzahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386(10001):1395-1406. [DOI] [PubMed] [Google Scholar]
- 22.Asamblea Nacional. Panamanian Law 69/2009. 2009. [Google Scholar]
- 23.Roa R, Santamaría I, Rebollón Guardado A, et al. Registro Nacional Del Cancer De Panamá, Boletín Estadístico, Año 2012. 2017. https://www.minsa.gob.pa/sites/default/files/general/boletin_2012_rncp.pdf
- 24.Saraiya M, Tangka FKL, Asma S, Richardson LC. Importance of economic evaluation of cancer registration in the resource limited setting: laying the groundwork for surveillance systems. Cancer Epidemiol. 2016;45(suppl 1):S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner H, Holleczek B. Deriving valid population-based cancer survival estimates in the presence of nonnegligible proportions of cancers notified by death certificates only. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2480-2486. [DOI] [PubMed] [Google Scholar]
- 26.Arrossi S. Cancer registration and information systems in Latin America. Lancet Oncol. 2015;16(14):1400-1401. [DOI] [PubMed] [Google Scholar]
- 27.Ministerio de Salud de Panamá. Website of Panama's National Cancer Registry (Registro Nacional de Cáncer de Panamá). https://www.minsa.gob.pa/contenido/registro-nacional-del-cancer