Abstract
Polycythemia vera is a Philadelphia chromosome–negative myeloproliferative neoplasm that results in increased myeloproliferation. It is a debilitating disease characterized by the overproduction of red blood cells, but it also can result in increased white blood cells and platelets. Patients experience a shortened overall survival due to an increased risk of thrombotic events, including stroke, myocardial infarction, pulmonary embolism, and deep vein thrombosis. Current treatment strategies in clinical practice are driven by mitigating the risk of these thrombotic events by reducing patients’ hematocrit. In addition to thrombosis risk, polycythemia vera patients have constitutional symptoms such as fatigue, itching, bone pain, erythromelalgia, and splenomegaly. An increased risk of transformation of their disease to acute myeloid leukemia and/or myelofibrosis can also affect long-term survival in polycythemia vera. Additional research has identified other risk factors, such as increased white blood cells, increased platelet count, and cytokine levels, which can alter the prognosis of the disease. In this review, we will discuss the current treatment strategies in polycythemia vera and determine if incorporating additional biomarkers as endpoints is feasible in clinical practice.
Polycythemia vera (PV) is a chronic and debilitating myeloproliferative neoplasm (MPN) with life-threatening complications. It was first formally identified in 1892 as “maladie de Vaquez,” by French physician Louis Henri Vaquez. Polycythemia vera is the most common MPN, although rarely is it malignant, with an incidence ranging from 2.5 to 10 out of 100,000 (Spivak, 2018). The indolent nature of the disease is often misleading, and prior to 2008, PV was characterized as belonging to a group of myeloproliferative disorders (Tefferi et al., 2009). In 2005, the discovery of a gain-of-function mutation involving the Janus kinase, where valine is substituted for phenylalanine at 617 codon on exon 14, JAK2V617F (JAK2), was first published (Baxter et al., 2005; James et al., 2005; Kralovics et al., 2005, Zhao et al., 2005). This was the confirmation that PV indeed is a malignant process in which its pathogenesis leads to clonally driven overactivation of the JAK-STAT pathway resulting in the overproliferation of hematopoietic compartments such as erythrocytosis (PV), thrombocytosis (PV, essential thrombocytosis [ET], and myelofibrosis [MF]), and leukocytosis (PV, ET, and MF). In addition to overproduction of red blood cells (RBC), white blood cells (WBC), and platelets (PLT), overactivation of the JAK-STAT pathway leads to overproduction of cytokines, manifesting in patient symptoms (pruritus, fatigue, bone pain) and fibrotic formation that can result in disease transformation to myelofibrosis (MF) or acute myeloid leukemia (AML), as well as an increase in thrombotic risk (Tefferi et al., 2021; Tefferi et al., 2009).
DIAGNOSIS OF POLYCYTHEMIA VERA
The advances in science have provided improvements in the accuracy of PV diagnosis and separating that from secondary erythrocytosis. Patients with PV will harbor the JAK2 mutation in 97% of cases, and the remaining 3% will have mutations of exon 12 or MPL (Tefferi et al., 2021). Despite such a clear correlation with mutation and disease, there is still a discrepancy on how exactly to diagnose patients properly. In 2016, the World Health Organization (WHO) revised the criteria for PV diagnosis as seen in Table 1. This update represented specific changes to the hemoglobin (Hgb) requirement, with a 2 g/dL drop from 18.5 g/dL to 16.5 g/dL in men and decrease from 16.5 g/dL to 16.0 g/dL in women. This change was controversial among MPN experts, although it is important to point out that focusing on Hgb is not representative of the disease as a whole. A diagnosis of PV requires at least three major criteria or two major criteria with the decreased erythropoietin minor criterion to satisfy the diagnosis (Arber et al., 2016; Barbui et al., 2017). The diagnosis of PV does not outwardly require a bone marrow biopsy; however, the importance of obtaining a biopsy at the time of diagnosis cannot be understated, as this can provide valuable baseline information and provide context for presentations that are not straightforward.
Table 1. 2016 WHO Criteria for Polycythemia Vera Diagnosis.
Major criteria
|
|
Minor criterion Subnormal serum erythropoietin level |
| Diagnosis of PV requires meeting either all 3 major criteria, or the first 2 major criteria and the minor criterionb |
Note. Information from WHO (2016).
More than 25% above mean normal predicted value.
Criterion number 2 (bone marrow biopsy biopsy) may not be required in cases with sustained absolute erythrocytosis: hemoglobin levels > 18.5 g/dL in men (hematocrit, 55.5%) or > 16.5 g/dL in women (hematocrit, 49.5%) if major criterion 3 and the minor criterion are present. However, initial myelofibrosis (present in up to 20% of patients) can only be detected by performing a bone marrow biopsy; this finding may predict a more rapid progression to overt myelofibrosis (post–polycythemia vera myelofibrosis).
EPIDEMIOLOGY OF POLYCYTHEMIA VERA
Over 90% of patients with MPNs present with a driver mutation encoding JAK2, MPL, or CALR. (Kralovics et al., 2005; Pikman et al, 2006; Klampfl et al., 2013). These mutations lead to constitutive activation of the JAK-STAT pathway resulting in overproliferation of cells and inflammatory cytokines. Based on this, it is helpful to think that MPNs operate on a spectrum of disease that can be very heterogenous where there are overlapping symptoms and phenotypic presentations such as increased WBC and PLT, which can occur across the diagnoses (PV, ET, MF, etc.) as a result of this overactivation. Differentiating these conditions is often difficult, and a bone marrow biopsy can provide the morphologic and molecular information needed to assess and diagnose patients properly. Identification of cellularity, fibrotic formation, and pleomorphic changes can confirm the diagnosis of PV, while also excluding the possibility of an MPN overlap syndrome, which could manifest in the form of fibrosis with dysplasia in the setting of increased Hgb and JAK2 positivity (Ellis et al., 1986; Spivak, 2018; Kuendgen et al., 2021). Bone marrow is also essential in differentiating JAK2-positive ET from masked PV, which was highlighted in the 2016 WHO revision to MPN classification (Arber et al., 2016). Masked PV patients mimic ET in isolated thrombocytosis but lack the hemoglobin and hematocrit (HCT) criteria necessary for PV diagnosis, and are managed differently than true ET patients (Maslah et al., 2020).
Polycythemia vera is seen more commonly in patients over the age of 60 (63.1% vs. 36.9%), with a slight predilection to male sex (57.1% vs. 42.9%). The incidence of PV has been stable from 2002 to 2016, despite the discovery of the JAK2 mutation in 2005. Overall survival (OS) is shortened for patients with PV, which varies based on age, with median OS of 13.5 years from diagnosis across all age groups. Of the three major MPNs (PV, ET, and MF), patients with PV have a longer predicted OS when compared with patients with ET, and patients with MF have the shortest OS (Tefferi et al., 2014).
Overall survival is specifically impacted in patients with PV by an increased risk for thrombotic events (TE), including deep vein thrombosis (DVT), myocardial infarction (MI), pulmonary embolus (PE), and cerebrovascular accident (CVA). In a prospective analysis of 2,485 patients with PV (REVEAL study), 468 patients (18.8%) had a TE at the time of enrollment. Of those patients experiencing a TE, 218 patients (218/468, 46.5%) experienced arterial thrombosis with the majority experiencing CVA (57.7%; Grunwald et al., 2018). Age is a specific risk factor in PV patients for TEs. A recent Surveillance, Epidemiology, and End Results (SEER) data analysis of patients with PV showed an expected increase in TEs at 28.4%, while arterial events were similar to those in the REVEAL study at 46% (Pemmaraju et al., 2022). These data sets highlight that although there is significant risk with TEs, concern among clinicians should be paid to the risk of arterial events like MI and CVA, which can lead to long-term consequences affecting quality of life in a patient population where OS can span beyond a decade.
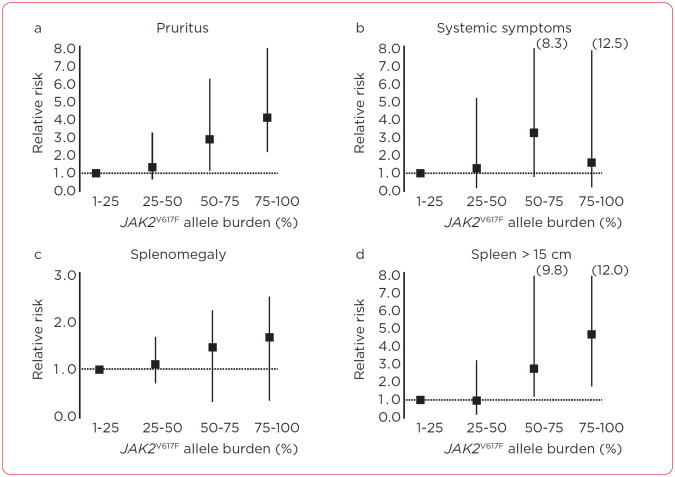
It is important to acknowledge that PV, although a malignant process, is chronic, with specific features of the disease contributing to risk assessment for thrombosis and transformation at diagnosis. Currently, there are numerous therapies for patients with PV, although the overall goal of treatment is not to eliminate the malignant clone as in other hematologic malignancies. The mainstay of treatment is to control the HCT, as this is the only factor that has been studied in a prospective manner that can affect thrombosis-free survival but not overall survival (NCCN, 2023; Barbui et al., 2018; Marchioli et al., 2013). White blood count ≥ 11 × 109/L in patients with PV has been reported as an independent risk of thrombosis (Gerds et al., 2021). Isolating the effect of lowering the WBC from the HCT is difficult, as most cytoreductive therapies that reduce WBC also affect the HCT, so no data can conclusively show that lower WBC alone reduces TE risk. JAK2 allele burden is a measurement of mutant allele vs. wild-type allele in hematopoietic cells (Passamonti & Rumi, 2009). This measurement has been shown in a prospective manner to correlate with an increased risk for thrombosis, cardiovascular (CV) events, and symptoms related to disease (Figure 1; Vannucchi et al., 2007). There is variability at which the quantified allelic burden ratio represents the highest risk for TE. Some data sets have seen 75% as representing increased risk for TE, whereas other data sets have seen increased risk at 50% or even as low as 20% (Passamonti et al., 2010; Guglielmelli et al., 2021; Vannucchi et al., 2007). Despite this evidence, the basic goals of therapy have not changed in PV; we remain with HCT of 45% as the guiding marker of treatment response.
Figure 1.
Effect of JAK2V617F allele burden on the risk of pruritus, systemic symptoms, and splenomegaly in various patient categories. Information from Vannuchi et al. (2007).
The objective of this review is to discuss in more detail the physiology behind why we treat patients who have PV with phlebotomy (PHL), either in conjunction with cytoreductive therapy or as a stand-alone treatment with low-dose aspirin (acetylsalicylic acid, ASA). We will discuss newer diagnostics as well as clinical trial endpoints to ask the question: is it time to look beyond just the HCT in treating patients with PV?
HEMATOCRIT IN POLYCYTHEMIA VERA
In 1967, the polycythemia vera study group (PVSG) was organized and functioned to provide diagnostic and therapeutic guidance for clinicians managing PV patients. They organized several multicenter prospective studies looking at the role of PHL, chemotherapy, and radiotherapy to manage erythrocytosis in PV patients. They also produced a trial evaluating the role of aspirin in thrombosis prevention in PV patients (Streiff et al., 2002). In 1978, a publication that identified the increased incidence of TEs related to HCT established the concept that patients with HCT from 46% to 52% had higher rates of TEs, thus recommending HCT < 45% for patients with PV (Pearson & Wetherley-Mein, 1978). PVSG-01 and ECLAP (European Collaboration on Low-dose Aspirin in PV) were prospective studies looking at PHL alone vs. the use of myelosuppressive agents and aspirin for HCT control, looking at 471 and 1,638 patients with PV, respectively. Despite the fact that these are still some of the largest prospective studies in PV, neither study was able to demonstrate that keeping HCT below 52% prevented TEs in PV (Marchioli et al., 2013).
The concept of keeping patients with PV with HCT < 45% came with the results of the CYTO-PV study. This trial demonstrated tight control of the HCT as defined by keeping patients < 45% led to a reduced risk of CV TEs in comparison with those patients managed between 45% to 50% HCT. Patients received either PHL, cytoreductive agents, or both in an effort to keep their HCT below 45% (low HCT group) or between 45% to 50% (high HCT group; Marchioli et al., 2013). An HCT of 45% creates difficulty when considering the sex of the patient. Females are different phenotypically and hormonally from men, resulting in different normal ranges. Males have 10 times the amount of testosterone than women. The resulting differences are that females have smaller red cell mass than men, and thus require a lower HCT target of 42% (Spivak, 2018).
It has been established then, that controlling HCT to a < 45% threshold can reduce TEs in PV, although the impact on disease drivers and OS has not been prospectively established in the PV population. Inherently, 20% of patients with PV will have TEs either at diagnosis or shortly after (Tefferi et al., 2013). These TEs can represent significant incidents in the form of MI, PE, and stroke, thus potentially impacting patients long term. In fact, augmentation of HCT with PHL alone may not be enough. In a subset analysis of the ECLAP study looking at 1,042 patients with PV and the same HCT goal, hydroxyurea demonstrated an advantage over PHL by reducing the incidence of fatal and nonfatal CV events (7.9% vs. 13.2%). That same analysis also showed an increase in the incidence of myelofibrosis transformation in those patients only receiving PHL, further suggesting that reducing HCT with PHL does not address underlying mechanisms of disease (Barbui et al., 2017).
CONCEPT OF COMPLETE HEMATOLOGIC RESPONSE
Current European LeukemiaNet (ELN) response criteria for PV were designed to provide criteria that are clinically relevant, practical, and reproducible for clinical trials of newer agents. Complete hematologic response (CHR) is currently defined as a response in PV where a patient's WBC is ≤ 10 × 109/L, HCT is < 45%, and PLT count is < 400 × 109/L. This definition of response from a collective of the three parameters is not based on the medical benefit of achieving these levels in combination, but rather the increased risk associated with the individual markers in PV (Barosi et al., 2009; Barbui et al., 2013).
Leukocytosis emerged as a risk feature in the ECLAP study where there was an increase in the incidence of arterial thrombosis among patients having WBC > 10 × 109/L. The CYTO-PV trial followed and also demonstrated that patients in the high HCT group (45%–50%) not only had a four times higher risk of death from CV death or major thrombosis, but these patients also had higher WBC than those patients in the low HCT group (Barbui et al., 2015). The REVEAL study demonstrated prospectively that elevated WBC > 10 × 109/L, HCT > 45%, and PLT > 400 × 109/L were associated with increased thrombotic risk. WBC > 11 × 109/L was associated with the highest risk of TE while PLT count > 600 × 109/L was associated with increased risk, although this was not statistically significant (Table 2; p > 0.05; Gerds et al., 2021). White blood count and its effect on transformation has also been evaluated in several retrospective study sets with varying results. These studies lacked longitudinal data, and thus a retrospective analysis looking at PV patients and their blood counts over time was performed in an effort to elucidate if there is an association with TE or transformation in PV patients. No increased risk of TE was identified, although patients with a WBC trajectory of ≥ 15 × 109/L and ≥ 35 × 109/L had a 5- and 24-fold increase in risk for transformation to MF or AML, respectively (Ronner et al., 2020).
Table 2. Association Between Blood Count Values and Thrombotic Events.
| Analysis | HR (95% CI) | p value |
|---|---|---|
| Association between elevated HCT and TEs | ||
| Age, y | 1.03 (1.01–1.046) | .0026 |
| Male sex (M vs. F) | 0.54 (0.362–0.799) | .0021 |
| Disease duration, y | 0.98 (0.952–1.017) | .3438 |
| History of TE (Y vs. N) | 2.49 (1.667–3.717) | < .0001 |
| Treatment (HU vs. none) | 0.95 (0.626–1.435) | .8004 |
| Treatment (any other vs. none) | 0.78 (0.39–1.577) | .4951 |
| HCT (> 45% vs. ≤ 45%) | 1.84 (1.234–2.749) | .0028 |
| Association between elevated WBC count and TEs | ||
| Age, y | 1.02 (1.007–1.042) | .0068 |
| Male sex (M vs. F) | 0.58 (0.393–0.858) | .0063 |
| Disease duration, y | 0.98 (0.947–1.014) | .2488 |
| History of TE (Y vs. N) | 2.42 (1.623–3.619) | < .0001 |
| Treatment (HU vs. none) | 0.98 (0.651–1.486) | .9386 |
| Treatment (any other vs. none) | 0.67 (0.335–1.325) | .2468 |
| WBC (> 11 vs. ≤ 11 × 109/L) | 2.35 (1.598–3.465) | <.0001 |
| Association between elevated WBC count and TEs (4 WBC levels: < 7, ≥ 7 to < 8.5, ≥ 8.5 to < 11, and ≥ 11 × 109/L) | ||
| Age, y | 1.02 (1.006–1.042) | .0076 |
| Male sex (M vs. F) | 0.59 (0.396–0.865) | .0071 |
| Disease duration, y | 0.98 (0.948–1.015) | .2646 |
| History of TE (Y vs. N) | 2.42 (1.618–3.608) | <.0001 |
| Treatment (HU vs. none) | 1.00 (0.66–1.509) | .9932 |
| Treatment (any other vs. none) | 0.67 (0.336–1.328) | .2496 |
| WBC (≥ 7 to < 8.5 vs. < 7 × 109/L) | 1.01 (0.504–2.022) | .9778 |
| WBC (≤ 8.5 to < 11 vs. < 7 × 109/L) | 1.40 (0.76–2.595) | .2790 |
| WBC (> 11 vs. < 7 × 109/L) | 2.61 (1.594–4.262) | .0001 |
| Association between elevated PLT count (> 400 × 109/L) and TEs | ||
| Age, y | 1.03 (1.01–1.046) | .0022 |
| Male sex (M vs. F) | 0.62 (0.416–0.914) | .0162 |
| Disease duration, y | 0.99 (0.953–1.019) | .3901 |
| History of TE (Y vs. N) | 2.45 (1.64–3.654) | <.0001 |
| Treatment (HU vs. none) | 0.87 (0.58–1.319) | .5223 |
| Treatment (any other vs. none) | 0.66 (0.334–1.324) | .2456 |
| PLT (> 400 vs. ≤ 400 × 109/L) | 1.60 (1.088–2.359) | .0170 |
| Association between elevated PLT count (> 600 × 109/L) and TEs | ||
| Age, y | 1.03 (1.01–1.046) | .0019 |
| Male sex (M vs. F) | 0.59 (0.398–0.872) | .0081 |
| Disease duration, y | 0.98 (0.951–1.017) | .3316 |
| History of TE (Y vs. N) | 2.43 (1.627–3.626) | <.0001 |
| Treatment (HU vs. none) | 0.89 (0.585–1.34) | .5657 |
| Treatment (any other vs. none) | 0.67 (0.335–1.331) | .2517 |
| PLT (> 600 vs. ≤ 600 × 109/L) | 1.37 (0.763–2.468) | .2913 |
Note. F = female; HCT = hematocrit; HR = hazard ratio; HU = hydroxyurea; M = male; PLT = platelet; TE = thrombotic event; WBC = white blood cell. Significant values are indicated in bold font. Treatment and categorical blood counts were included as time-dependent covariates in the models. Other treatments include interferon, busulfan, chlorambucil, anagrelide, and ruxolitinib.
Thrombocytosis has a less clear correlation with the increased risk for TEs. In the aforementioned REVEAL subset analysis, PLT count needed to be adjusted to > 600 × 109/L in order to individually show an increase in the risk of thrombosis. Additionally, this still was not statistically significant, but rather approached clinical significance at that adjustment (Gerds et al., 2021). As an additional analysis to the Ronner and colleagues study, Tremblay and colleagues (2021) posited that achieving PLT counts < 400 × 109/L was linked with an increase in transformation to MF or AML, a curious yet intriguing finding, which only conflates how to evaluate thrombocytosis in the PV patient. In contrast, extreme thrombocytosis (> 1,000 × 109/L) in patients with PV can induce an acquired Von Willebrand state where an increase in bleeding is possible (Tefferi & Barbui, 2019).
DRAWBACKS OF FOCUSING SOLELY ON HCT
Currently, HCT is the beacon guiding treatment in PV because this is the measurement that has been shown to decrease the incidence of TEs (Marchioli et al., 2013). Alhough HCT is the most clinically significant measurement from a lab value perspective, using this measure as a sole marker for disease monitoring may lead to inaccurate comprehensive assessment. (Spivak, 2018). Polycythemia vera is a disease that also carries the risk of transformation and a significant symptom burden. Focusing merely on the risk of thrombosis, one may lose sight of the other goals of therapy, including improvement in symptoms and addressing risk transformation, especially since these patients often live with their disease for long periods of time (Barosi et al., 2013).
Dr. William Dameshek warned of the pitfalls of focusing on treating numbers such as HCT for the pure sake of “normalizing” the number, “whether the patient needs it or not.” Understanding how increased HCT contributes to the overall presentation is critical to managing the disease. Patients can have increased risk yet still have normal HCT because it is the red cell mass that matters in the fluid dynamics of PV. As an example of this, as patients receive phlebotomy for treatment, depletion of iron can occur. In the face of decreased iron stores, the red cells are smaller and thus occupy less of the space in the HCT. This results in persistent increased red cell mass despite a potentially normal HCT. With this persistent increased red cell mass present, thrombotic risk may still be increased (Spivak, 2018). Relative red cell mass increases can be seen with inflammatory cytokines and expression of hypoxia-inducible factor 1 alpha (HIF1α) and HIF2α. Hypoxia-inducible factors are cytokines that respond to hypoxia driving reduction in hepcidin, the master regulator of iron metabolism, leading to increased erythropoiesis. In the face of decreased iron stores, this can lead to increased red cell mass and subsequent thrombosis (Gordeuk et al., 2011; Song et al., 2021)
Expanding on this research into the role of cytokines, a relationship of the hematopoietic stem cells and overexpression of JAK/STAT signaling results in increases in inflammatory cytokines (C-reactive protein, IL-6, and pentraxin-3) and contributes to the prothrombotic state of PV (Lussana & Rambaldi, 2017). In patients with PV, iron deficiency resulting from phlebotomy demonstrated an increase in prothrombotic gene transcript levels raising the potential for thrombosis in these patients (Song et al., 2021). In addition to the prothrombotic state contribution, inflammatory cytokines seen in PV play a critical role in the development of constitutional symptoms. Elevated levels of IL-1α, CCL4, TNFα, and IL-6 are linked to increases in spleen, bone pain, and fatigue, as well as fibrosis formation and transformation of disease from PV to MF or AML (Baldauf et al., 2022). Reducing circulating cytokines in patients with PV can help to ameliorate constitutional symptoms. This has been done with therapies that target the JAK2 mutant cell with improvement in symptoms. Targeting the HCT alone through phlebotomy has shown no such clinical benefit as this reduces the number but does not affect the underlying process of the disease.
SUMMARY
Patients with PV have decreased OS compared with that of historical controls. These reductions in OS are the result of disease progression, transformation to AML or MF, and complications related to TEs. Although the decrease in OS is notable, patients still often live with their disease for many years, all the while experiencing the constitutional symptoms that impact their quality of life. Thus far, treatment for PV has focused on mitigation of thrombotic risk through management of HCT (Tefferi & Barbui, 2019). Understanding the fluid dynamics involved with HCT is important to understanding the risks to the patient. Even if “controlled,” HCT may be misrepresented in the face of increased red cell mass. Reducing the HCT alone may neglect other concerning aspects of the disease in transformation and symptom control. In addition, focusing solely on HCT may not account for additional factors that can lead to increased thrombosis risk in PV (Spivak, 2018).
The ELN criteria have been created with the aim of providing clinically meaningful endpoints for new agents in PV to benefit patients. These criteria were not formed with the idea of supplanting the current treatment strategy, but rather endpoints for clinical trials, which are not always as reliable as real-world evidence. The prospective benefits of CHR in PV patients are still lacking at this time. Despite this, the evidence showing the risk associated with uncontrolled hematopoiesis in PV is considered valid enough to be included in such criteria. Incorporation of the Myeloproliferative Neoplasm Symptom Assessment Form total symptom score (MPN-SAF TSS) into clinical practice as a way to demonstrate symptom benefit is a priority to a patient population that lives with this disease for long periods (Barbui et al., 2013). With the additional subset analysis of prospective registry studies such as REVEAL, perhaps we will see recommendations change to pursue managing PV beyond a single number and take a more holistic approach.
Footnotes
Mr. Waggoner is an employee of PharmaEssentia US.
References
- Arber, D. A., Orazi, A., Hasserjian, R., Thiele, J., Borowitz, M. J., Le Beau, M. M.,…Vardiman, J. W. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127(20), 2391–2405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- Baldauf, C. K., Müller, P., Haage, T. R., Adam-Frey, S., Lokau, J., Garbers, C., & Fischer, T. (2022). Anti-IL-6 cytokine treatment has no impact on elevated hematocrit or splenomegaly in a polycythemia vera mouse model. Blood Advances, 6(2), 399–404. 10.1182/bloodadvances.2021004379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui, T., Tefferi, A., Vannucchi, A. M., Passamonti, F., Silver, R. T., Hoffman,…Barosi, G. (2018). Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia, 32(5), 1057–1069. 10.1038/s41375-018-0077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui, T., Masciulli, A., Marfisi, M. R., Tognoni, G., Finazzi, G., Rambaldi, A., & Vannucchi, A. (2015). White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood, 126(4), 560–561. 10.1182/blood-2015-04-638593 [DOI] [PubMed] [Google Scholar]
- Barbui, T., Thiele, J., Gisslinger, H., Carobbio, A., Vannucchi, A. M., & Tefferi, A. (2017). Diagnostic impact of the 2016 revised who criteria for polycythemia vera. American Journal of Hematology. 92(5), 417–419. 10.1002/ajh.24684 [DOI] [PubMed] [Google Scholar]
- Barbui, T., Vannucchi, A. M., Finazzi, G., Finazzi, M. C., Masciulli, A., Carobbio, A.,…Tognoni, G. (2017). A reappraisal of the benefit-risk profile of hydroxyurea in polycythemia vera: A propensity-matched study. American Journal of Hematology, 92(11), 1131–1136. 10.1002/ajh.24851 [DOI] [PubMed] [Google Scholar]
- Barosi, G., Mesa, R., Finazzi, G., Harrison, C., Kiladjian, J. J., Lengfelder, E.,…Barbui, T. (2013). Revised response criteria for polycythemia vera and essential thrombocythemia: An ELN and IWG-MRT consensus project. Blood, 121(23), 4778–4781. 10.1182/blood-2013-01-478891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barosi, G., Birgegard, G., Finazzi, G., Griesshammer, M., Harrison, C., Hasselbalch, H. C.,… Barbui, T. (2009). Response criteria for essential thrombocythemia and polycythemia vera: Result of a European LeukemiaNet consensus conference. Blood, 113(20), 4829–4833. 10.1182/blood-2008-09-176818 [DOI] [PubMed] [Google Scholar]
- Baxter, E. J., Scott, L. M., Campbell, P. J., East, C., Fourouclas, N., Swanton, S.,…Cancer Genome Project (2005). Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet, 365(9464), 1054–1061. 10.1016/S0140-6736(05)71142-9 [DOI] [PubMed] [Google Scholar]
- Ellis J. T., Peterson P., Geller S. A., & Rappaport H. (1986). Studies of the bone marrow in polycythemia vera and the evolution of myelofibrosis and second hematologic malignancies. Seminars in Hematology, 23(2), 144–155. [PubMed] [Google Scholar]
- Gerds, A. T., Mesa, R. A., Burke, J. M., Grunwald, M. R., Stein, B. L., Scherber, R.,…Oh, S. (2021). A real-world evaluation of the association between elevated blood counts and thrombotic events in polycythemia vera (analysis of data from the REVEAL Study). Blood, 138(Supplement 1), 239–239. 10.1182/blood-2021-148509 [DOI] [Google Scholar]
- Gordeuk, V. R., Miasnikova, G. Y., Sergueeva, A. I., Niu, X., Nouraie, M., Okhotin, D. J.,…Prchal, J. T. (2011). Chuvash polycythemia VHLR200W mutation is associated with down-regulation of hepcidin expression. Blood, 118(19), 5278–5282. 10.1182/blood-2011-03-345512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald, M. R., Stein, B. L., Boccia, R. V., Oh, S. T., Paranagama, D., Parasuraman, S.,…Mesa, R. (2018). Clinical and disease characteristics from REVEAL at time of enrollment (baseline): Prospective observational study of patients with polycythemia vera in the United States. Clinical Lymphoma, Myeloma & Leukemia, 18(12), 788–795.e2. 10.1016/j.clml.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmelli, P., Loscocco, G. G., Mannarelli, C., Rossi, E., Mannelli, F., Ramundo, F.,…Vannucchi, A. M. (2021). JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer Journal, 11(12), 199. 10.1038/s41408-021-00581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, C., Ugo, V., Le Couédic, J. P., Staerk, J., Delhommeau, F., Lacout, C.,…Vainchenker, W. (2005). A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature, 434(7037), 1144–1148. 10.1038/nature03546 [DOI] [PubMed] [Google Scholar]
- Kaplan, M. E., Mack, K., Goldberg, J. D., Donovan, P. B., Berk, P. D., & Wasserman, L. R. (1986). Long-term management of polycythemia vera with hydroxyurea: a progress report. Seminars in Hematology, 23(3), 167–171. [PubMed] [Google Scholar]
- Klampfl, T., Gisslinger, H., Harutyunyan, A. S., Nivarthi, H., Rumi, E., Milosevic, J. D.,…Kralovics, R. (2013). Somatic mutations of calreticulin in myeloproliferative neoplasms. The New England Journal of Medicine, 369(25), 2379–2390. 10.1056/NEJMoa1311347 [DOI] [PubMed] [Google Scholar]
- Kralovics, R., Passamonti, F., Buser, A. S., Teo, S. S., Tiedt, R., Passweg, J. R.,…Skoda, R. C. (2005). A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England Journal of Medicine, 352(17), 1779–1790. 10.1056/NEJMoa051113 [DOI] [PubMed] [Google Scholar]
- Kuendgen, A., Kasprzak, A., & Germing, U. (2021). Hybrid or mixed myelodysplastic/myeloproliferative disorders - Epidemiological features and overview. Frontiers in Oncology, 11, 778741. 10.3389/fonc.2021.778741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussana, F., & Rambaldi, A. (2017). Inflammation and myeloproliferative neoplasms. Journal of Autoimmunity, 85, 58–63. 10.1016/j.jaut.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Maslah, N., Soret, J., Dosquet, C., Vercellino, L., Belkhodja, C., Schlageter, M. H.,…Giraudier, S. (2020). Masked polycythemia vera: Analysis of a single center cohort of 2480 red cell masses. Haematologica, 105(3), e95–e97. 10.3324/haematol.2018.215582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioli, R., Finazzi, G., Specchia, G., Cacciola, R., Cavazzina, R., Cilloni, D.,…CYTO-PV Collaborative Group (2013). Cardiovascular events and intensity of treatment in polycythemia vera. The New England Journal of Medicine, 368(1), 22–33. 10.1056/NEJMoa1208500 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network . (2023). NCCN Clinical Practice Guidelines in Oncology: Myeloproliferative Neoplasms. V1.2023. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
- Passamonti, F., & Rumi, E. (2009). Clinical relevance of JAK2 (V617F) mutant allele burden. Haematologica, 94(1), 7–10. 10.3324/haematol.2008.001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti, F., Rumi, E., Pietra, D., Elena, C., Boveri, E., Arcaini, L.,…Cazzola, M. (2010). A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia, 24(9), 1574–1579. 10.1038/leu.2010.148 [DOI] [PubMed] [Google Scholar]
- Pearson, T. C., & Wetherley-Mein, G. (1978). Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet, 2(8102), 1219–1222. 10.1016/s0140-6736(78)92098-6 [DOI] [PubMed] [Google Scholar]
- Pemmaraju, N., Gerds, A. T., Yu, J., Parasuraman, S., Shah, A., Xi, A.,…Verstovsek, S. (2022). Thrombotic events and mortality risk in patients with newly diagnosed polycythemia vera or essential thrombocythemia. Leukemia Research, 115, 106809. 10.1016/j.leukres.2022.106809 [DOI] [PubMed] [Google Scholar]
- Pikman, Y., Lee, B. H., Mercher, T., McDowell, E., Ebert, B. L., Gozo, M.,…Levine, R. L. (2006). MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Medicine, 3(7), e270. 10.1371/journal.pmed.0030270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronner, L., Podoltsev, N., Gotlib, J., Heaney, M. L., Kuykendall, A. T., O’Connell, C., Shammo, J.,… Mascarenhas, J. (2020). Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood, 135(19), 1696–1703. 10.1182/blood.2019003347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J., Soo, J. K., Thiagarajan, P., & Prchal, J. (2021). Iron deficiency in polycythemia vera increases hif activity and transcription of prothrombotic genes. Blood, 138(supplement 1), 2549. 10.1182/blood-2021-153221 [DOI] [Google Scholar]
- Spivak, J. L. (2018). Polycythemia vera. Current Treatment Options in Oncology, 19(2), 12. 10.1007/s11864-018-0529-x [DOI] [PubMed] [Google Scholar]
- Streiff, M. B., Smith, B., & Spivak, J. L. (2002). The diagnosis and management of polycythemia vera in the era since the Polycythemia Vera Study Group: A survey of American Society of Hematology members’ practice patterns. Blood, 99(4), 1144–1149. 10.1182/blood.v99.4.1144 [DOI] [PubMed] [Google Scholar]
- Tefferi, A., Guglielmelli, P., Larson, D. R., Finke, C., Wassie, E. A., Pieri, L.,…Vannucchi, A. M. (2014). Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood, 124(16), 2507–2615. 10.1182/blood-2014-05-579136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi, A., Vannucchi, A. M., & Barbui, T. (2021). Polycythemia vera: Historical oversights, diagnostic details, and therapeutic views. Leukemia, 35(12), 3339–3351. 10.1038/s41375-021-01401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi, A., Rumi, E., Finazzi, G., Gisslinger, H., Vannucchi, A. M., Rodeghiero, F.,…Barbui, T. (2013). Survival and prognosis among 1545 patients with contemporary polycythemia vera: An international study. Leukemia, 27(9), 1874–1881. 10.1038/leu.2013.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi, A., & Barbui, T. (2019). Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. American Journal of Hematology, 94(1), 133–143. [DOI] [PubMed] [Google Scholar]
- Tefferi, A., Thiele, J., & Vardiman, J. W. (2009). The 2008 World Health Organization classification system for myeloproliferative neoplasms: order out of chaos. Cancer, 115(17), 3842–3847. 10.1002/ajh.25303 [DOI] [PubMed] [Google Scholar]
- Tremblay, D., Srisuwananukorn, A., Ronner, L., Podotslev, N., Gotlib, J., Heaney, M.,…Mascarhenas, J. (2021). European LeukemiaNet (ELN) response predicts disease progression but not thrombosis or death in polycythemia vera (PV): An analysis of a multicenter database. Blood, 138(supplement 1), 240. 10.1182/blood-2021-148020 [DOI] [Google Scholar]
- Vannucchi, A. M., Antonioli, E., Guglielmelli, P., Longo, G., Pancrazzi, A., Ponziani, V.,…MPD Research Consortium (2007). Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia, 21(9), 1952–1959. 10.1038/sj.leu.2404854 [DOI] [PubMed] [Google Scholar]
- Zhao, R., Xing, S., Li, Z., Fu, X., Li, Q., Krantz, S. B., & Zhao, Z. J. (2005). Identification of an acquired JAK2 mutation in polycythemia vera. The Journal of Biological Chemistry, 280(24), 22788–22792. 10.1074/jbc.C500138200 [DOI] [PMC free article] [PubMed] [Google Scholar]