Abstract
During the late phase of adult T-cell leukemia/lymphoma, a severe lymphoproliferative disorder caused by human T-cell leukemia virus type 1 (HTLV-1), leukemic cells no longer produce interleukin-2. Several studies have reported the lack of the Src-like protein tyrosine kinase Lck and overexpression of Lyn and Fyn in these cells. In this report we demonstrate that, in addition to the downregulation of TCR, CD45, and Lck (which are key components of T-cell activation), HTLV-1-infected cell lines demonstrate a large increase of FynB, a Fyn isoform usually poorly expressed in T cells. Furthermore, similar to anergic T cells, Fyn is hyperactive in one of these HTLV-1-infected T-cell lines, probably as a consequence of Csk downregulation. A second family of two proteins, Zap-70 and Syk, relay the signal of T-cell activation. We demonstrate that in contrast to uninfected T cells, Zap-70 is absent in HTLV-1-infected T cells, whereas Syk is overexpressed. In searching for the mechanism responsible for FynB overexpression and Zap-70 downregulation, we have investigated the ability of the Tax and Rex proteins to modulate Zap-70 expression and the alternative splicing mechanism which gives rise to either FynB or FynT. By using Jurkat T cells stably transfected with the tax and rex genes or inducibly expressing the tax gene, we found that the expression of Rex was necessary to increase fynB expression, suggesting that Rex controls fyn gene splicing. Conversely, with the same Jurkat clones, we found that the expression of Tax but not Rex could downregulate Zap-70 expression. These results suggest that the effect of Tax and Rex must cooperate to deregulate the pathway of T-cell activation in HTLV-1-infected T cells.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATL), an aggressive lymphoproliferative disorder, and is also responsible for tropical spastic paraparesis, a chronic neurological disease. In vitro, HTLV-1 can infect several types of cells, but it transforms only human T lymphocytes. This observation suggests that T-cell-specific events induced by HTLV-1 infection may trigger the lymphoproliferative process. T lymphocytes can be activated by the stimulation of the T-cell receptor (TCR)-CD3 complex with processed antigen in association with self-major histocompatibility complex (MHC) gene products. One of the earliest detectable consequences of receptor ligation is the tyrosine phosphorylation of multiple cellular substrates. The tyrosine phosphorylation events are regulated sequentially by two classes of protein tyrosine kinases (PTKs), the Src family and the Syk/Zap-70 family. First, the Src family kinase members Lck or Fyn phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) contained within the CD3 and ζ subunits of the TCR complex (10, 23). Second, the Syk/Zap-70 family of PTKs are recruited to the receptor complex. Once bound to the ITAMs, Zap-70 becomes phosphorylated on several tyrosine residues (45), possibly as a result of both autophosphorylation and phosphorylation by Lck (12).
Previous studies have shown that HTLV-1-infected T cells exhibit altered expression of PTKs. For example, Lck is not expressed in HTLV-1-infected T cells, whereas two other Src family proteins, Lyn and Fyn, are overexpressed (18, 26, 42). In addition, all the HTLV-1-infected T cells used in this report demonstrate a downregulation of TCR and CD45. In the present study, we demonstrate that, in addition to Lck deficiency and to the reduction of TCR and CD45, disregulation of Fyn and Zap-70 may contribute to the unresponsive state of these cells as characterized by the inability to produce interleukin-2 (IL-2). We first demonstrated that FynB, a Fyn isoform principally expressed in brain and poorly expressed in T cells (15), is strongly upregulated in HTLV-1-infected T-cell lines and that the viral protein Rex is likely to be involved in the control of the splicing event that gives rise to this isoform. We also demonstrated that one of the HTLV-1-infected T-cell lines, C91, exhibits a hyperactive Fyn enzyme which does not result from mutations. Rather, we found that Csk, a PTK involved in the negative control of Src protein activities, was poorly expressed in this cell line compared to other T cells. We then observed that Zap-70, which is expressed at high levels in T cells, is absent in several HTLV-1-infected T cells, whereas Syk, which is mostly expressed in B cells, mast cells, platelets, and immature T cells, is expressed in HTLV-1-infected T cells. We demonstrated that Tax expression is sufficient to induce this downregulation of Zap-70.
Because recent evidence suggests that Syk can function independently of Lck and CD45 (13), we evaluated the effect of TCR stimulation on MT-2, an HTLV-1-infected cell line characterized by the absence of Lck, Zap-70, and CD45 and by a relatively high expression of Syk. Whereas this stimulation increased the tyrosine phosphorylation of two proteins, Syk phosphorylation and activity remained unchanged. Our findings imply that several PTK abnormalities induced by Tax and Rex are responsible for HTLV-1-infected cell line hyporesponsiveness to TCR stimulation and that Syk does not functionally compensate for the decrease of Zap-70 in these cells.
MATERIALS AND METHODS
Cells.
All cell lines were maintained in RPMI with Glutamax supplemented with 10% fetal calf serum, penicillin, and streptomycin (Life Technologies, Inc.).
CEM is a cell line derived from peripheral blood from a patient with an acute lymphoblastic leukemia. Jcl20 is a derivative mutant of the human leukemia Jurkat T-cell line which expresses both Zap-70 and Syk. HUT-78 is a human cutaneous T-cell lymphoma derived from the peripheral blood of a patient with Sezary syndrome. H9 is a single cell clone derived from HUT-78. MT-2 and MT-4 have been established by cocultivation of adult T-cell leukemia (ATL) cells and human cord blood lymphocytes (26). MT-2, but not MT-4, is an HTLV-1 producing T-cell line. C91 cells are HTLV-1-transformed human cord blood T cells (27). ATL2 cells have been established by cocultivation of ATL cells and human cord blood lymphocytes. C8166 is a human umbilical cord blood lymphocyte cell line that carries, but does not express, the HTLV-1 genome, except for the tax gene. Several clones of Jurkat T cells stably producing Tax and/or Rex proteins have been used in this study (22, 43): C9 is a clone of Jurkat cells stably transfected with a simian virus 40 (SV40)-Neo expression vector; C50, was stably cotransfected with a MT-Tax expression vector and an SV40-Neo expression vector; and E5 and E12 were stably cotransfected with pMTenvXLTR, together with an SV40-Neo expression vector. The JPX-9 cell line (kindly provided by K. Sugamura, Tohoku University, Sendai, Japan) was generated by the stable introduction of a Tax expression plasmid under the control of the human metallothionein promoter; the induction of Tax was performed in the presence of CdCl2 (30 μM), ZnCl2 (100 μM), or ZnSO4 (100 μM) for 24 h.
Antisera.
The following antisera were used. Anti-Lck 7229 (kindly provided by A. Veillette) is a polyclonal rabbit antiserum raised against a fusion protein encompassing amino acids 2 to 148 of mouse Lck (1). Fyn-specific polyclonal antiserum (kindly provided by A. Veillette) is directed against residues 25 to 141 of the murine Fyn sequence (17). This antiserum recognizes sequences that are common to FynT and FynB. Rabbit anti-Syk serum was kindly provided by N. Taylor. Anti-Zap-70 918 (46) is a polyclonal rabbit antiserum generated against a TrpE fusion protein encompassing amino acids 253 to 329 of human Zap-70. Anti-Csk is a polyclonal antibody (a gift from G. Dumesnil). For antiphosphotyrosine immunoblotting experiments, we used the mouse monoclonal antibody (MAb) 4G10 (Upstate Biotechnology, Inc., Lake Placid, N.Y.). T cells were activated by stimulation with anti-TCR mouse MAb (anti-immunoglobulin M [IgM] MAb) Vit-3 (kindly provided by W. Knapp). Anti-Tax (kindly provided by L. Coscoy) is a monoclonal murine antiserum.
Total extracts of treated and nontreated cells.
For the treatment with anti-CD3, T cells were activated with anti-TCR mouse MAb Vit-3 at 37°C (1:100 dilution of ascites in RPMI).
Cells were lysed by adding an equal volume of 2× TNE (100 mM Tris, pH 8.0; 2% Nonidet P-40; 40 mM EDTA), supplemented with 20 μg of each of the protease inhibitors leupeptin, aprotinin, N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), N-α-p-tosyl-l-lysine chloromethyl ketone (TLCK), and phenylmethylsulfonyl fluoride per ml, as well as the phosphatase inhibitors sodium fluoride (100 mM) and sodium orthovanadate (2 mM).
Immunoprecipitations.
Cells were lysed as described above. Specific polypeptides were then recovered by immunoprecipitation from equivalent amounts of cellular proteins by using anti-Lck, anti-Fyn, anti-Zap, or anti-Syk serum. Immune complexes were collected with Staphylococcus aureus protein A (Pansorbin; Calbiochem). Then, immunoprecipitates were washed three times in lysis buffer and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequent immunoblots were obtained by using the protocol outlined below.
Immunoblots.
Immunoblots were performed according to a previously described protocol (44). Antiphosphotyrosine immunoblots were performed with mouse MAb 4G10 at 0.3 μg/ml. For anti-Lck, anti-Fyn, anti-Zap, and anti-Syk immunoblots, we used antiserum at a 1/1,000 dilution (for enhanced chemiluminescence [ECL]) or at a 1/200 dilution (for 125I-labeled protein A), as indicated in the figure legends, and proteins transferred to Immobilon membranes (Millipore) were visualized with the Amersham ECL system (for direct immunoblotting of total cell extracts) or by incubation with 125I-labeled protein A (Amersham) (for immunoblotting of the immunoprecipitates). Immunoreactive products were detected by autoradiography, and 125I-labeled proteins were quantitated with a PhosphorImager (Molecular Dynamics).
Immune-complex kinase assay.
Cells were lysed with TNE as described above. Then, 500 μg of protein from the lysates was incubated with 3 μl of the Fyn antibody, and immunoprecipitation performed as described above except that the immunoprecipitates were washed three times with 1× TNE supplemented with sodium orthovanadate (1 mM), once with 1× TNE supplemented with sodium orthovanadate (1 mM) and NaCl (1 M), and once again in 1× TNE supplemented with sodium orthovanadate (1 mM). The kinase reactions were then performed in a buffer containing 20 mM HEPES (pH 7.5) and 10 mM MnCl2. The reactions were conducted for 4 min at room temperature in the presence of acid-denatured rabbit muscle enolase (Sigma) as a substrate, in addition to 100 μM nonradioactive ATP and 12.5 μCi of [γ-32P]ATP. Data were quantitated with a PhosphorImager (Molecular Dynamics).
RNA preparation and Northern blot analysis.
RNA was extracted in Trizol (Gibco-BRL). First, 10 μg of RNA was separated on a formaldehyde-agarose gel and transferred to a Hybond membrane (Amersham). The RNA on the filter was then hybridized with the 32P-labeled DNA fragment (zap MscI fragment). Hybridization was carried out at 42°C in buffer containing 50% (vol/vol) formamide and 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The filter was washed in 0.1× SSC for 20 min at 68°C.
Semiquantitative reverse transcription-PCR (RT-PCR).
RNA was extracted in Trizol (Gibco-BRL) according to recommendations of the manufacturer. PCR and runoff procedures were similar to those described for TCR repertoire analysis (14). The primers used in this case were as follows: for PCR, hfyn9 (5′-GGATACTACATTACCACCCG-3′) (sense primer, ∼50 bp upstream of the exon 7 region of fyn) and hfyn2 (5′-CTGGCTACGGAATTGAAAGC-3′) (antisense primer, ∼800 bp downstream of the exon 7 of fyn); for run-off, hfyn10 (5′-FAM-GTGTTTCCATACCAGGTACC-3′) (antisense primer, directly downstream of the exon 7 of fyn), labeled at the 5′ end with a fluorescent FAM fluorophore.
The fluorescent runoff products (which differ in size by 9 nucleotides depending on whether the initial template was the fynT or the fynB isoform, since exon 7 is 9 nucleotides longer in the latter case) were then loaded onto a denaturing acrylamide gel in an automated sequencer (ABI 373A; Applied Biosystems, Foster City, Calif.), along with size standards. The pattern of fluorescent bands was recorded and then converted into profiles by using the Immunoscope software (developed by C. Pannetier and coworkers [14]), which provides the length of the fluorescent DNA fragments (in nucleotides) and the fluorescence intensities.
RESULTS
HTLV-1 chronically infected T cells show a reduced expression of TCR and CD45.
The transmembrane tyrosine phosphatase CD45 is believed to act as a positive regulator of Src family kinases by dephosphorylating their negative C-terminal regulatory tyrosine and, consequently, CD45 is a critical component of early T-cell signalling.
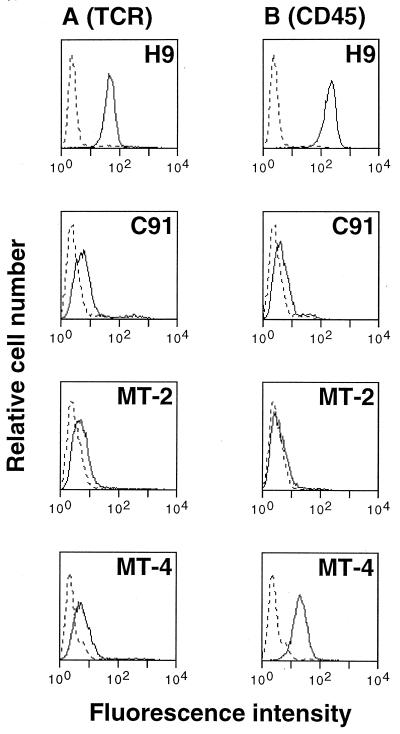
To determine the level of TCR and CD45 expressed at the surface of chronically HTLV-1-infected T cells, we used flow cytometry (Fig. 1). H9 noninfected T cells were used as a positive control for the expression of these surface molecules. Three HTLV-1-infected T-cell lines, C91, MT-2, and MT-4, were assayed. Anti-TCR staining results with the TCR MAb Vit-3 were similar for each clone and showed a weak surface expression when compared to the negative (fluorescein isothiocyanate-labeled goat anti-mouse [GAM-FITC] alone) and the positive control H9. Compared to H9, anti-CD45 staining indicated a decreased expression on MT-4 cells, a markedly reduced expression on C91 cells, and no expression on MT-2 cells.
FIG. 1.
TCR and CD45 expression are downregulated in HTLV-1-infected T cells. Cells from H9 (an uninfected T-cell line) and from C91, MT-2, and MT-4 (three HTLV-1-infected cell lines) were stained with GAM-FITC alone (dotted line) or with antibodies to TCR (Vit-3) (A) or CD45 (B) followed by GAM-FITC. The cell number is shown on the y axis, and the fluorescence intensity in log units is shown on the x axis.
Lck is absent in IL-2-independent HTLV-1-infected T cells.
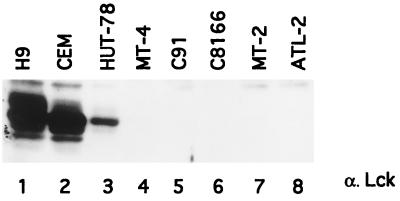
By immunoblotting of total cell lysates with an antiserum directed against a fusion protein encompassing amino acids 2 to 148 of Lck, we confirmed that Lck was expressed at high levels in H9 and CEM T cells (Fig. 2, lanes 1 and 2). Its expression was reduced in HUT-78 (lane 3) and absent in all of the HTLV-1-infected T-cell lines tested (MT-4, C91, C8166, MT-2, and ATL-2) (lanes 4 to 8), as previously reported (17).
FIG. 2.
Expression of Lck tyrosine kinase in HTLV-1-infected or uninfected T-cell lines. Lysates (50 μg of protein) from H9 (lane 1), CEM (lane 2), HUT-78 (lane 3), or HTLV-1-infected cell lines and from MT-4, C91, C8166, MT-2, and ATL-2 (lanes 4 to 8) were subjected to SDS-PAGE and Western blotting with anti-Lck antibody.
Fyn is hyperactive in C91, an HTLV-1-infected T-cell line.
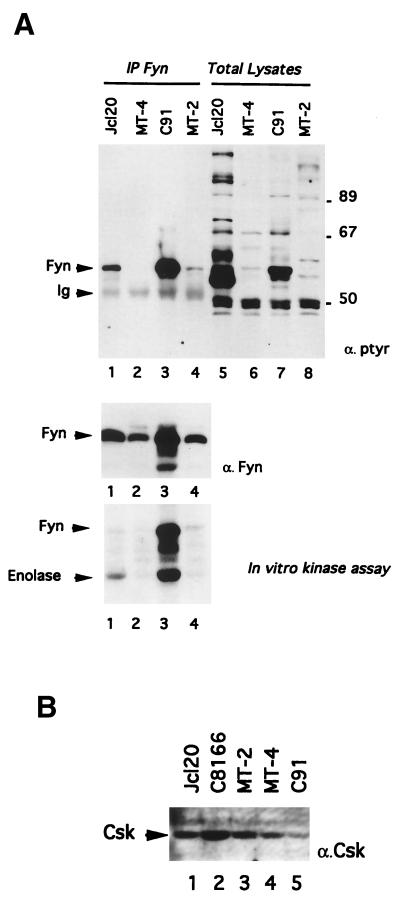
In anergic CD4+ peripheral T cells, a state characterized by their hyporesponsiveness to TCR stimulation, Fyn activity is elevated (2, 13, 28). To evaluate whether similar events occur in hyporesponsive HTLV-1-infected cell lines, we decided to determine the level of phosphorylation and the activity of Fyn. The experiment depicted in Fig. 3 indicates that a 59-kDa protein (p59) is highly phosphorylated in total lysates from C91 cells (Fig. 3A, top panel, lane 7) compared to Jurkat (lane 5) and other HTLV-1-infected cells (lanes 6 and 8). In an attempt to demonstrate that this protein is Fyn, lysates from C91 were immunoprecipitated with antibodies against Fyn. The phosphotyrosine content of Fyn was then measured by antiphosphotyrosine immunoblotting (Fig. 3, top panel, lanes 1 to 4). A strong signal (compare lanes 3 and 1) was detected in Fyn immunoprecipitates from C91 cells. A weak Fyn phosphorylation could also be detected in Fyn immunoprecipitates from MT-2 (lane 4). However, no basal tyrosine phosphorylation could be detected from MT-4 Fyn immunoprecipitates (lane 2). To evaluate the possibility that Fyn hyperphosphorylation in C91 cells could result from its overexpression, the immunoblot shown in Fig. 3 (top panel) was stripped and reprobed with anti-Fyn antibodies (middle panel). Comparison of the antiphosphotyrosine with the anti-Fyn immunoblots indicated that the twofold overexpression of Fyn in C91 cells (lane 3) compared to its expression in Jcl20 (lane 1) could not fully explain its hyperphosphorylation. In order to analyze the basal activity of Fyn in C91 cells in the other HTLV-1-infected cell lines and in Jurkat cells, Fyn was immunoprecipitated and the immune complexes were assayed for phosphorylation of the Fyn substrate enolase. As shown in Fig. 3 (bottom panel, lane 3) the intrinsic activity of Fyn in MT-2 and MT-4 cell lines is weak (lanes 2 and 4), probably as a consequence of the absence of CD45 in MT-2 and its low expression in MT-4 (see Fig. 1), but Fyn activity was elevated in the C91 cell line (lane 3) despite the weak expression of CD45 (see Fig. 1). However, compared to its level of expression in Jcl20, C8166, MT-2, and MT-4 (Fig. 3B, lanes 1 to 4), we found that Csk expression was downregulated in C91 cells (lane 5), thus providing a potential explanation for Fyn hyperactivity.
FIG. 3.
Enhanced tyrosine phosphorylation and kinase activity of Fyn parallels the decreased expression of Csk in an HTLV-1-infected T-cell line, C91. (A) After cell lysis, Fyn was immunoprecipitated and probed by immunoblotting with either antiphosphotyrosine (top panel, lanes 1 to 4) or anti-Fyn (middle panel) antibodies. Lanes 5 to 8 represent total cell lysates. The bottom panel represents the enzymatic kinase activity of Fyn as assessed by immune-complex kinase reactions in the presence of enolase. In this figure, the positions of Fyn, immunoglobulins (Ig), and enolase are indicated by arrows on the left. (B) Direct Western blot of Csk. Lysates (50 μg of protein) from Jcl20 (lane 1), C8166 (lane 2), MT-2 (lane 3), MT-4 (lane 4), or C91 (lane 5) were subjected to SDS-PAGE and Western blotting with anti-Csk antibody.
Rex is required to increase fynB expression in most HTLV-1-infected cell lines.
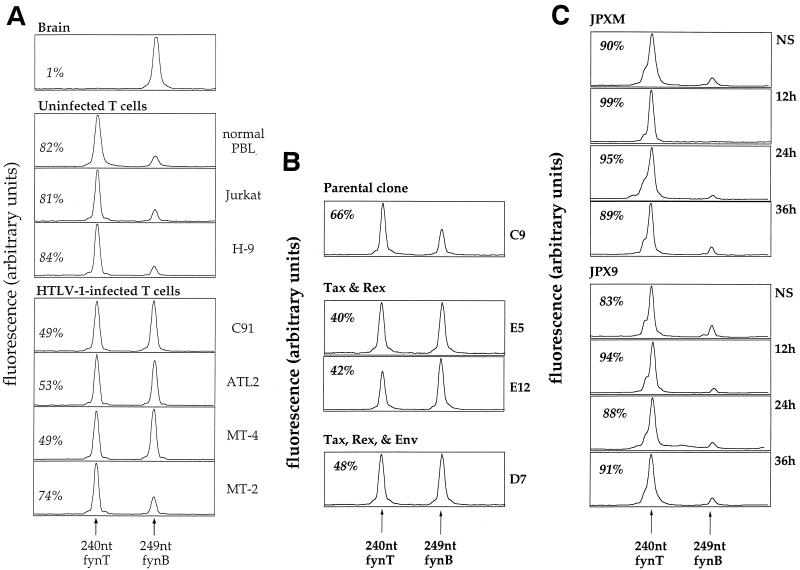
In addition to Csk downregulation, a mutation of the fyn gene in C91 cells could explain the high intrinsic activity of Fyn in this cell line. To address this question, we amplified the cDNA by PCR and sequenced the coding sequence of fyn. This sequence did not reveal any mutation that might account for its hyperactivity. However, the sequence started to be blurred at the beginning of exon 7 (data not shown). As these overlapping sequences were compatible with a mixture of the two fyn isoforms, fynT and fynB, we measured the ratio of fynT/fynB expression in C91 by semiquantitative RT-PCR. We took advantage of the fact that exon 7 in fynB contains three more codons than exon 7 in fynT. Thus, a PCR product encompassing fynB exon 7 will be 9 nucleotides longer. As shown in Fig. 4A, fynB is the only detectable isoform in the brain, whereas fynT is the major isoform in peripheral blood lymphocytes (82%) and in T-cell lines (81% in Jurkat cells and 84% in H9). In contrast, we found that fynB expression increased significantly in most HTLV-1-infected cell lines and that the two isoforms were equally expressed in C91, ATL2, and MT-4. Finally, sequencing of fynB and fynT cDNAs in C91, after cloning of the PCR products, did not reveal any mutation.
FIG. 4.
(A) Relative mRNA levels of the T and B isoforms of fyn in various cell types. mRNA from different cell types was amplified by semiquantitative RT-PCR by using fyn-specific primers bordering exon 7 (which differs in length by three codons between the T and B isoforms). This PCR product was then subjected to a runoff reaction with a fluorescent primer so that the runoff product encompasses the exon 7 region. The length in nucleotides of this runoff product is expected to be 240 nucleotides with a fynT template and 249 nucleotides with a fynB template. This runoff product was then electrophoresed in an automated sequencer, along with size standards. The band pattern for each sample was converted into a profile as shown. The fluorescence and length in nucleotides of the various bands was determined with the Immunoscope software. The percentage of the fynT isoform is indicated in italics in each panel. (B) Relative mRNA levels of the T and B isoforms of fyn in Jurkat clones stably expressing Tax, Rex, and Env. mRNA from different Jurkat clones expressing neomycin resistance alone or together with viral proteins (Tax, Rex, and Env) were amplified by semiquantitative RT-PCR by using fyn-specific primers as described for panel A. The percentage of the fynT isoform is indicated in italics in each panel. (C) Quantification of fynT and fynB mRNAs during a study of the kinetics of induction of Tax by CdCl2 in JPXM and JPX9 cells. JPXM and JPX9 were cultured in medium alone (NS) or in medium containing 30 μM CdCl2 for 12, 24, or 36 h. mRNA was amplified by semiquantitative RT-PCR by using fyn-specific primers as described for panel A. The percentage of the fynT isoform is indicated in italics in each panel.
In order to provide direct evidence for the involvement of an HTLV-1 gene product in fynB upregulation, we used Jurkat cells stably transfected with an expression vector containing the neomycin resistance gene alone (C9) or Jurkat clones expressing either tax or both tax and rex genes (we have been unable to obtain Jurkat clones stably expressing detectable levels of Rex), as indicated in Fig. 4B. The comparison of fynB mRNA expression in these Jurkat clones indicate that in the presence of Tax and Rex, fynB mRNA is upregulated. To elucidate the role of Tax, we examined fynB expression in the JPX9 clone of Jurkat cells containing the wild-type tax gene under the control of an inducible promoter. In the presence of 30 μM CdCl2, no upregulation of fynB could be observed in the JPX9 clone after 12, 24, or 36 h, as in the JPXM clone containing a variant of Tax mutated in the activation domain (Fig. 4C), whereas NF-κB nuclear translocation (dependent on Tax expression) was visible at 12 h (data not shown). We obtained the same results after treatment with estradiol of Jurkat clone cells carrying a fusion protein between Tax and the estradiol receptor (data not shown).
These results suggest that Rex controls the splicing event that gives rise to FynT or FynB.
Expression of Zap-70 and Syk in HTLV-1-infected T-cell lines.
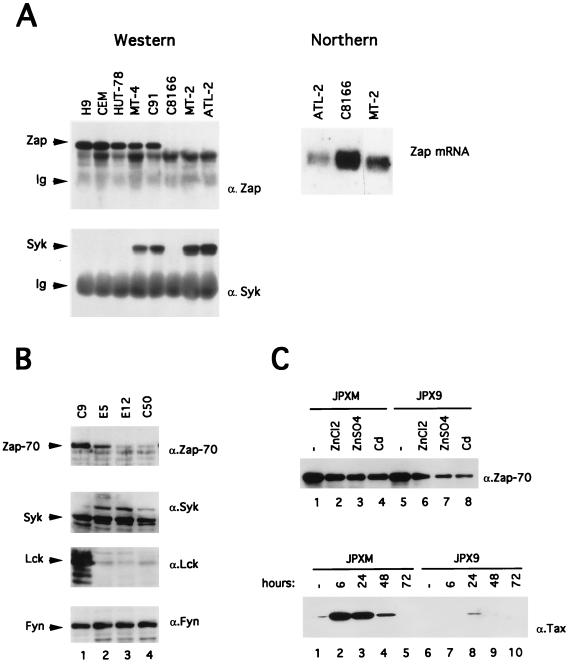
In addition to the Src family kinases, Zap-70 and Syk constitute a second family of PTKs involved in TCR signalling. We have tested whether a dysregulation of those two proteins may play a role in the hyporesponsiveness of HTLV-1-infected T cells. We thus compared the expression of Zap-70 and Syk in HTLV-1-infected and uninfected T-cell lines. Zap-70 protein was absent in three HTLV-1-infected cell lines, C8166, MT-2, and ATL-2 (Fig. 5A, top panel), whereas it was expressed slightly less in MT-4, C91, and HUT-78 (a human cutaneous T-cell lymphoma) than in H9 and CEM cells. We then asked whether the decreased expression of Zap-70 in HTLV-1-infected cell lines could be compensated for by an increase in Syk expression. Indeed, when compared with H9, CEM, and HUT-78, Syk expression was upregulated in the HTLV-1-infected cell lines MT-4, C91, MT-2, and ATL-2, but not in C8166, a human umbilical cord blood lymphocyte cell line that does not express the full HTLV-1 genome.
FIG. 5.
(A) Expression of Zap-70 and Syk in HTLV-1-infected T cells. For the Western blotting experiment (left part), cells (5 × 106) were lysed as described in Materials and Methods, and Zap-70 and Syk proteins were immunoprecipitated from 1 mg of cellular proteins with rabbit anti-Zap-70 or anti-Syk antisera. Zap-70 and Syk polypeptides were subsequently detected by using anti-Zap-70 or anti-Syk immunoblotting. The positions of the Zap, Syk, and immunoglobulins (Ig) are shown on the left. For the Northern blotting analysis (right part), 10 μg of total RNA from ATL-2, C8166, or MT-2 were electrophoresed, blotted, and hybridized with a 32P-labeled zap probe. (B) Western blot analysis of Zap-70 (α.Zap-70), Syk (α.Syk), Lck (α.Lck), and Fyn (α.Fyn) in Jurkat C9 cells, Tax- and Rex-positive cells (E5 and E12), and Tax-positive cells (C50). A total of 50 μg of protein was loaded into each lane. (C) In the top panel, a Western blot analysis of Zap-70 in JPXM and JPX9 cells treated 24 h with medium alone (lanes 1 and 5) or with heavy metals (lanes 2 to 4 and lanes 6 to 8) is shown. In the bottom panel, JPXM and JPX9 were cultured in medium alone (lanes 1 and 6) or in medium containing 30 μM CdCl2 (lanes 2 to 5 and lanes 7 to 10) for 6, 24, 48, or 72 h as indicated. Then, 50 μg of the extract was assayed to determine wild-type or mutated Tax expression by Western blotting.
In order to determine at which level Zap-70 expression is controlled, the three HTLV-1-infected cell lines (ATL-2, C8166, and MT-2) which do not express Zap-70 were assayed for the expression of zap by Northern blot analysis (Fig. 5A). These cells demonstrated detectable amounts of zap transcript, suggesting that zap gene expression is blocked at a posttranscriptional level.
Tax downregulates the expression of Zap-70 in HTLV-1-infected T-cell lines.
Since Tax is the only viral protein expressed by C8166 cells and since Zap-70 is not expressed in this cell line (Fig. 5A), we investigated the role played by this protein in the downregulation of Zap-70 by using Jurkat T cells that stably expressed Tax (C50) or Tax and Rex (E5 and E12) (Fig. 5B). Compared to control Jurkat cells (lane 1), two clones, E5 and E12 (lanes 2 and 3), which express both Tax and Rex, and one clone, C50 (lane 4), which expresses only Tax, were found to exhibit a decreased expression of Zap-70. We monitored in parallel the expression of Syk, Lck, and Fyn by immunoblotting and found that Tax-expressing clones exhibit a strong downregulation of Lck expression but no modification of the level of expression of Syk and Fyn (lanes 2 to 4).
To further evaluate the effect of Tax on Zap-70 expression, we then used JPXM and JPX9 cells, which are subclones of Jurkat cells stably transformed with a plasmid carrying, respectively, mutant or wild-type tax sequences under the control of the human metallothionein promoter (Fig. 5C). Expression of Zap-70 was assayed by Western Blot after a 24-h culture of cells with either medium alone (Fig. 5C, top panel, lanes 1 and 5) or medium containing 100 μM ZnCl2 (lanes 2 and 6), 100 μM ZnSO4 (lanes 3 and 7), or 30 μM CdCl2 (lanes 4 and 8). Treatment of JPXM cells with these inducers led to a slight reduction of Zap-70 expression (compare lanes 2 to 4 to lane 1). However, treatment of JPX9 cells led to an important downregulation of Zap-70 (compare lanes 6 to 8 to lane 5). Thus, these data clearly demonstrate that Tax induces a downregulation of Zap-70 expression. Western blot analysis of lysates prepared from JPXM (Fig. 5C, bottom panel, lanes 1 to 5) and JPX9 (lanes 6 to 10) at 6, 24, 48, and 72 h after induction with CdCl2 revealed that Tax was more expressed in JPXM than in JPX9.
Altered tyrosine phosphorylations and Syk activity in TCR-stimulated HTLV-1-infected cell lines.
Previous reports suggested that the prerequisites for signal transduction by Syk or Zap-70 differed strikingly (16, 27, 29, 30, 49). While both associate with ITAMs, Syk has a higher intrinsic activity than Zap-70 (29, 30), and this should allow Syk to function even when TCR expression is low. These studies also demonstrated that Syk is not as dependent on Src family kinases as Zap-70 and that Syk-mediated signals can even occur in the absence of activation of Src family members by CD45 (16, 27, 30, 49). Because HTLV-1-infected cells exhibit a decreased surface expression of CD45, an absence of Lck, and for some of them of Zap-70, we decided to evaluate the biochemical activity of Syk.
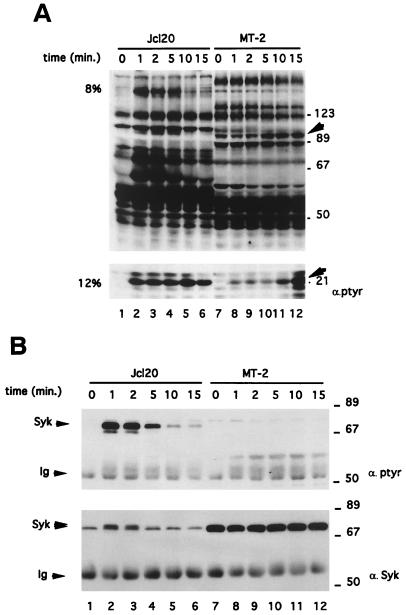
We examined one of the earliest steps in the TCR-mediated response of MT-2 cells, the induction of the tyrosine phosphorylation of cellular proteins. To precisely define the kinetics of phosphorylation of these targets, we performed a parallel time course study of tyrosine phosphorylation induction in Jcl20 and MT-2 cells (Fig. 6). Cells were activated for variable lengths of time by the addition of the anti-TCR Vit-3 antibody. Whole-cell lysates were directly subjected to SDS-PAGE (Fig. 6A) or first to Syk immunoprecipitation (Fig. 6B). The antiphosphotyrosine immunoblot of whole-cell lysates revealed that the pattern of basal phosphoprotein induction was different in Jcl20 and MT-2 (Fig. 6A, compare lanes 1 and 7). Upon engagement of TCR, tyrosine protein phosphorylation was enhanced in Jcl20 (lanes 1 to 6). In marked contrast, the MT-2 cell line did not show any significant induction of tyrosine phosphoproteins after TCR stimulation (lanes 7 to 12). In these cells, only two substrates, migrating at approximately 21 and 90 kDa, exhibited increased tyrosine phosphorylation after TCR stimulation, with delayed kinetics compared to Jcl20. We next tested the impact of TCR stimulation on the state of tyrosine phosphorylation of Syk in Jcl20 and MT-2 (Fig. 6B). This experiment shows that Jcl20 cells exhibit a marked increase in TCR-induced tyrosine phosphorylation of Syk (Fig. 6B, lanes 1 to 6 [top panel]), which was concomitant with the appearance of a higher-molecular-weight form (lanes 1 to 6 [bottom panel]). In MT-2 cells, Syk was not found to be inducibly tyrosine phosphorylated (top panel, lanes 7 to 12) despite its strong expression (bottom panel, compare lanes 7 to 12 to lanes 1 to 6). These results were extended to other HTLV-1-infected cells with the same results as found for MT-2 cell line (data not shown).
FIG. 6.
Phosphorylation of 21- and 90-kDa substrates but not of Syk in TCR-induced MT-2 cells. Jcl20 or MT-2 cells were stimulated for the indicated periods of time with anti-TCR MAb Vit-3 and subsequently lysed in Nonidet P-40 containing buffer. (A) Proteins (50 μg) from whole-cell lysates were separated on 8% (top panel) or 12% (bottom panel) SDS-PAGE gels and probed with antiphosphotyrosine antibody. The 90- and 21-kDa tyrosine phosphorylated proteins are indicated by arrowheads. The positions of prestained molecular-mass markers are shown on the right. (B) With the same extracts, anti-Syk immunoprecipitation was followed by antiphosphotyrosine immunoblotting (top panel) or anti-Syk immunoblotting (bottom panel). The positions of Syk and the heavy chain of IgG (Ig) are indicated on the left, while the molecular-mass markers are shown on the right.
DISCUSSION
We report here several characteristics of HTLV-1-infected T-cell lines that have important implications for the mechanism of HTLV-1-induced T-cell hyporesponsiveness. During the late phase of ATL, leukemic cells no longer produce IL-2 (for a review, see reference 25), and all of the HTLV-1-infected cell lines we used showed only a minimal production of IL-2 mRNA, as assessed by quantitative RT-PCR (data not shown). In agreement with previous studies analyzing TCR responses in HTLV-1-infected T-cell lines (18, 26, 34, 48), we demonstrated that these cells exhibit altered expression and function of several key components of the T-cell signalling pathway. All of the cell lines used in the present study demonstrated a downregulation of TCR and CD45 expression and a modification of expression and kinase activity of Fyn isoforms and of Syk/Zap-70 kinases.
We first confirmed the absence of Lck in several HTLV-1-infected T-cell lines, and then focused our attention on Fyn. As a result of an alternative usage of exon 7, Fyn is expressed as two isoforms, FynB and FynT, differing only in a 52-amino-acid region localized on the end of the SH2 domain and the beginning of the kinase domain. While FynB accumulates in the brain, FynT is expressed predominantly in T lymphocytes. Fusaki and coworkers have shown that an HTLV-1-infected T-cell line, Hayai, overexpressed Fyn and exhibited a constitutive phosphorylation of Sam68, an RNA-binding protein that has been identified as being associated with Src during mitosis (18). We found that Fyn phosphorylation and activity is dramatically increased in another HTLV-1-infected T-cell line, C91 (Fig. 3), despite the low expression of CD45 (Fig. 1). Other cell lines producing HTLV-1 viruses (and thus expressing all of the viral proteins) do not demonstrate an increase in Fyn activity; we can therefore rule out the possibility that the regulation of Fyn activity is due to the expression of a viral protein, as is the case for Lck and the Saimiri herpesvirus Tip-484 membrane protein or for the Lyn kinase and the Epstein-Barr virus LMP2a protein (9, 32). Rather, we decided to evaluate two other hypotheses which could explain Fyn hyperactivity in C91; first, a defect of Csk expression (a protein tyrosine kinase which regulates Src family protein activities by phosphorylating their C-terminal tyrosine) and, second, an activating mutation of Fyn. We indeed found that Csk was poorly expressed in C91 cells. Thus, this result provides a potential explanation for the increased Fyn activity in this cell line. In searching for mutations of fyn which could account for its abnormal phosphorylation and hyperactivity in C91 cells, we performed direct sequencing of PCR-amplified fyn mRNA. We did not observe such mutations (unpublished results), but instead we found that the fynB/fynT ratio was increased in most HTLV-1-infected cell lines (Fig. 4). Among several possible hypotheses to explain this finding, we favor the idea that these Fyn defects correlate with the anergic state of HTLV-1-infected T cells: first, Fyn from anergic T cells exhibits an increased constitutive tyrosine kinase activity as in the C91 cell line (8, 19, 37, 39); second, Davidson and colleagues have demonstrated in an antigen-specific mouse T-cell hybridoma that, in contrast to an active form of FynT, an active form of FynB is unable to mediate IL-2 production in response to antigen stimulation (17). It is possible, therefore, that in HTLV-1-infected cell lines FynB overexpression could compete with FynT expression and repress its ability to mediate IL-2 production.
To get further insight into the mechanism leading to HTLV-1-infected-T-cell unresponsiveness, we compared the levels of expression of Zap-70 and Syk proteins (Fig. 5A). The results of our analysis showed that Syk was expressed in all of the HTLV-1-infected cell lines tested, except for C8166 which no longer expresses most of the HTLV-1 genome. In addition, Zap-70 was absent from three HTLV-1 cell lines. However, Northern blot analysis (Fig. 5A) demonstrated that the expression of zap-70 mRNA was conserved in these cells, suggesting that the expression of the zap-70 gene product was blocked at a posttranscriptional level.
Among the proteins encoded by HTLV-1, Tax and Rex have been shown to modulate the expression of cellular genes or gene products by different mechanisms. Tax is a nuclear protein which activates viral and cellular genes by interacting directly or indirectly with cellular transcription factors, including CREB/ATF and the Rel/NF-κB families; Rex works through cis-acting elements, referred to as Rex response elements (RXRE), by promoting the nucleocytoplasmic export of unspliced and singly spliced mRNAs that encode the gag-pol and env gene products of HTLV-1. Functional analyses have revealed that Rex contains an amino-terminal region which mediates binding to the RXRE (3, 6, 20), a leucine-rich carboxy-terminal activation domain which was shown to function as a nuclear export signal (7, 21), and a domain responsible for the multimerization of Rex (5, 33). It was thus interesting to investigate whether Tax or Rex is implicated in the regulation of fyn, zap-70, and syk gene expression. In order to test this hypothesis, we used Jurkat cells stably transfected with tax and rex or with tax only. Expression of the fynB transcript and the Zap-70 protein was compared in these cells. We found that tax gene expression was sufficient to inhibit Zap-70 expression but was unable to modulate the expression of Syk and Fyn (Fig. 5B). Furthermore, the induction of Tax in JPX9 cells, a subclone of Jurkat cells stably transformed with a plasmid carrying the tax gene under the control of an inducible promoter, led to the downregulation of Zap-70 (Fig. 5C). As previously reported (31), we have also confirmed that the expression of HTLV-1 Tax protein was sufficient to repress the lck gene (Fig. 5B). Besides Tax, it is also likely that Rex could influence the expression of cellular gene products. In order to identify the precise mechanism by which HTLV-1 infection modulates fynT/fynB (exon 7 alternative splicing), we measured the respective amounts of fynB and fynT in the Jurkat clones expressing Tax and Rex. We observed that in the presence of Rex, fynB expression was clearly increased (Fig. 4B), but we could not demonstrate that this effect of Rex was independent of the presence of Tax. In fact, it has been shown in vitro that Rex, in addition to its role in the nucleus-to-cytoplasm transport of viral mRNA, was a potent inhibitor of splicing (2, 4). Moreover, a recent report demonstrates that for another Rex-like protein, the Rev protein of equine infectious anemia virus, the requirements for alternative splicing and nuclear export functions are different (22). Concerning our observations, it is unclear whether Rex directly affects fyn gene alternative splicing or only the nucleus-to-cytoplasm transport of fynB mRNA. However, because our experiments have been done with total cellular RNA, we favor the first hypothesis. In addition, the fact that fynB expression was only slightly elevated in MT-2 cells, despite the presence of Rex, suggests that cellular component(s) essential for this process might be deficient in these cells (Fig. 4A).
Finally, our results indicate that Tax and Rex cooperate in HTLV-1-infected T cells to deregulate the expression of PTKs, and we therefore decided to analyze in these cells the variation of cellular tyrosine phosphorylation after TCR activation.
According to a sequential model of tyrosine kinase activation, it has been proposed that TCR-CD4 engagement by antigen and MHC receptors leads in a first step to Lck-mediated tyrosine phosphorylation of ITAMs and, in a second step, to the recruitment of Zap-70 to the receptor and its subsequent phosphorylation by Lck (for a review, see reference 47). However, current biochemical and genetic evidence is compatible with the idea that in T cells, Syk is not as dependent on Lck kinase activity as Zap-70. First, transient expression in Cos cells of Zap-70 or Syk (11) or stimulation of chimeric receptors containing Zap-70 or Syk (27) has demonstrated that, in contrast to Zap-70, Syk phosphorylation is not dependent on an Src family kinase expression and also that Syk can directly activate the T-cell signalling pathway. Secondly, Syk endogenous expression in a CD45-deficient Jurkat line and Syk transfection in the Lck-deficient JCAM1.6 Jurkat T cells can overcome the requirement for either CD45 or Lck (13). Indeed, it has been recently suggested that Syk can, like Lck, initiate the cascade of activation in T cells by directly phosphorylating the ITAMs (30, 49) and that Syk is activated upon interaction with doubly phosphorylated ITAMs (24, 38, 41, 49). However, it has been demonstrated in B cells deficient in Lyn (28) and by cotransfection experiments that Syk phosphorylation and kinase activity can be regulated by Lyn expression (40, 49), suggesting that Syk is differentially regulated in B and T cells. In the context of HTLV-1 infection in T cells, we asked whether the strong expression of Syk (reference 42 and Fig. 5) might bypass the absence of Lck and the altered expression of CD45 (reference 26 and Fig. 1 and 2). We actually observed an increase in the phosphorylation of only two substrates (one of 90 kDa and the other of 21 kDa) in the MT-2 cell line upon TCR cross-linking (Fig. 6), and we could not detect an inducible phosphorylation of Syk in all of the HTLV-1-infected T cells we analyzed (Fig. 6 and data not shown). This suggests that Syk, although abundant in these cells, is not activated correctly and therefore is unable to compensate for the absence of Lck and Zap-70.
In conclusion, several observations have suggested that HTLV-1-infected T cells exhibit altered expression or activity of key molecules implicated in T-cell activation: the expression of the TCR-CD3 complex is modulated (34; this study); the expression of CD45 is downmodulated (this study); and the expression of Lck is absent in IL-2-independent cells, whereas the expression of Lyn is upregulated (26, 48). Moreover, in two cell lines, Hayai and C91 (18; this study), the expression of Fyn is high and a more-detailed study has revealed that its activity is also elevated in C91 cells, probably as a consequence of Csk downregulation (this study). Finally, we have also demonstrated that the ratio between fynB and fynT, two isoforms of fyn, is elevated compared to uninfected T cells and that the expression of Zap-70 is decreased or absent, whereas that of Syk is conversely upregulated. Concerning the mechanism(s) responsible for these defects, it has been shown that Tax is sufficient to repress the expression of Lck (31), and we demonstrate here that Tax is also responsible for the decreased expression of Zap-70, probably through a posttranscriptional mechanism. We further show that Rex is necessary for increased fynB expression. Further study is required to define the precise mechanism by which Rex controls fyn splicing and also to identify the regions of this molecule involved in this process.
ACKNOWLEDGMENTS
We thank G. Langsley for critical reading of the manuscript. We also thank A. Veillette and O. Acuto for critical reading of the manuscript and for providing various reagents. We thank L. Tuosto and F. Dautry for helpful discussion. We thank N. Taylor for the gift of anti-Syk antibody, W. Knapp for providing Vit-3 anti-TCR antibody, L. Coscoy for anti-Tax antibody, and G. Dumesnil for anti-Csk antibody. We also thank J.-L. Moreau for help with the flow cytometry.
This research was sponsored in part by grants from l’Association pour la Recherche sur le Cancer, l’Agence Nationale de Recherche contre le SIDA, INSERM, and the Ligue Nationale Française contre le Cancer to A.I. R.W. is the recipient of long-term fellowship from SIDACTION and Pasteur-Weizmann. J.-P.L. is the recipient of a Pasteur Institute fellowship.
REFERENCES
- 1.Abraham N, Miceli M C, Parnes J C, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature (London) 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 2.Bakker A, Li X, Ruland C T, Stephens D M, Black A C, Rosenblatt J D. Human T-cell leukemia virus type 2 Rex inhibits pre-mRNA splicing in vitro at an early stage of spliceosome formation. J Virol. 1996;70:5511–5518. doi: 10.1128/jvi.70.8.5511-5518.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballaun C, Farrington G K, Dobrovnik M, Rusche J, Hauber J, Böhnlein E. Functional analysis of human T-cell leukemia virus type 1 Rex response element: direct RNA binding of Rex protein correlates with in vivo activity. J Virol. 1991;65:4408–4413. doi: 10.1128/jvi.65.8.4408-4413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black A C, Ruland C T, Yip M T, Luo J, Tran B, Kalsi A, Quan E, Aboud M, Chen I S Y, Rosenblatt J D. Human T-cell leukemia virus type II Rex binding and activity require an intact splice donor site and a specific RNA secondary structure. J Virol. 1991;65:6645–6653. doi: 10.1128/jvi.65.12.6645-6653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogerd H, Greene W C. Dominant negative mutants of human T-cell leukemia virus type 1 Rex and human immunodeficiency virus type 1 Rev fail to multimerize in vivo. J Virol. 1993;67:2496–2502. doi: 10.1128/jvi.67.5.2496-2502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogerd H P, Huckaby G L, Ahmed Y F, Hanly S M, Greene W C. The type I human T-cell leukemia virus (HTLV-I) Rex trans-activator binds directly to the HTLV-I Rex and the type 1 human immunodeficiency virus Rev RNA response elements. Proc Natl Acad Sci USA. 1991;88:5704–5708. doi: 10.1073/pnas.88.13.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogerd H P, Fridell R A, Benson R E, Jian H, Cullen B R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boussiotis V A, Freeman G J, Berezovskaya A, Barber D L, Nadler L M. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt A L, Bolen J B, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with Src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhardt A L, Stealey B, Rowley R B, Mahajan S, Prendergast M, Fargnoli J, Bolen J B. Temporal regulation of non-transmembrane protein tyrosine kinase enzyme activity following T cell antigen receptor engagement. J Biol Chem. 1994;269:23642–23647. [PubMed] [Google Scholar]
- 11.Chan A C, Iwashima M, Turck C W, Weiss A. ZAP-70: a 70 kD protein tyrosine kinase that associates with the TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 12.Chan A C, Dalton M, Johson R, Kong G-H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu D H, Spits H, Peyron J-F, Rowley R B, Bolen J B, Weiss A. The Syk protein tyrosine kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- 14.Cochet M, Pannetier C, Regnault A, Darche S, Leclerc C, Kourilsky P. Molecular detection and in vivo analysis of the specific T cell response to a protein antigen. Eur J Immunol. 1992;22:2639–2647. doi: 10.1002/eji.1830221025. [DOI] [PubMed] [Google Scholar]
- 15.Cooke M P, Perlmutter R M. Expression of a novel form of fyn proto-oncogene in hematopoietic cells. New Biol. 1989;1:66–74. [PubMed] [Google Scholar]
- 16.Couture C, Baier G, Altman A, Mustelin T. p56lck-independent activation and tyrosine phosphorylation of p72syk by T-cell antigen receptor/CD3 stimulation. Proc Natl Acad Sci USA. 1994;91:5301–5305. doi: 10.1073/pnas.91.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson D, Chow L M L, Fournel M, Veillette A. Differential regulation of T cell antigen responsiveness by isoforms of the Src-related tyrosine protein kinase p59fyn. J Exp Med. 1992;175:1483–1492. doi: 10.1084/jem.175.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusaki N, Iwamatsu A, Iwashima M, Fujisawa J-I. Interaction between Sam68 and Src family tyrosine kinases, Fyn and Lck, in T cell receptor signaling. J Biol Chem. 1997;272:6214–6219. doi: 10.1074/jbc.272.10.6214. [DOI] [PubMed] [Google Scholar]
- 19.Gajewski T F, Qian D, Fields P, Fitch F W. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci USA. 1994;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassman R, Berchtold S, Aepinus C, Ballaun C, Boehnlein E, Fleckenstein B. In vitro binding of human T-cell leukemia virus Rex proteins to the Rex response element of viral transcripts. J Virol. 1991;65:3721–3727. doi: 10.1128/jvi.65.7.3721-3727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakata Y, Umemoto T, Matsushita S, Shida H. Involvement of human CRM1 (exportin 1) in the export and multimerization of the Rex protein of human T-cell leukemia virus type 1. J Virol. 1998;72:6602–6607. doi: 10.1128/jvi.72.8.6602-6607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris M E, Gontarek R R, Derse D, Hope T J. Differential requirements for alternative splicing and nuclear export functions of equine infectious anemia virus Rev protein. Mol Cell Biol. 1998;18:3889–3899. doi: 10.1128/mcb.18.7.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwashima M, Irving B A, Van Oers N S C, Chan A C, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Sakamoto H, Appella E, Siraganian R P. Conformational changes induced in the protein tyrosine kinase p72syk by tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. J Biol Chem. 1996;271:27962–27968. doi: 10.1128/mcb.16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 26.Koga Y, Oh-Hori N, Sato H, Yamamoto N, Kimura G, Nomoto K. Absence of transcription of lck (lymphocyte specific protein tyrosine kinase) message in IL-2 independent, HTLV-1-transformed T cell lines. J Immunol. 1989;142:4493–4499. [PubMed] [Google Scholar]
- 27.Kolanus W, Romeo C, Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- 28.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Tanigushi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1735. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latour S, Chow L M L, Veillette A. Differential intrinsic enzymatic activity of Syk and Zap-70 protein-tyrosine kinases. J Biol Chem. 1996;271:22782–22790. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- 30.Latour S, Fournel M, Veillette A. Regulation of T-cell antigen receptor signalling by Syk tyrosine protein kinase. Mol Cell Biol. 1997;17:4434–4441. doi: 10.1128/mcb.17.8.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemasson I, Robert-Hebmann V, Hamaia S, Duc Dodon M, Gazzolo L, Devaux C. Transrepression of lck gene expression by human T-cell leukemia virus type 1-encoded p40tax. J Virol. 1997;71:1975–1983. doi: 10.1128/jvi.71.3.1975-1983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund T, Medveczky M M, Medveczky P G. Herpesvirus Saimiri Tip-484 membrane protein markedly increases p56lck activity in T cells. J Virol. 1997;71:378–382. doi: 10.1128/jvi.71.1.378-382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malim M H, Cullen B R. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991;65:241–248. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka M, Hattori T, Chosa T, Tsuda H, Kuwata S, Yoshida M, Uchiyama T, Takatsuki K. T3 surface molecule on adult T cell leukemia are modulated in vivo. Blood. 1986;4:1070–1076. [PubMed] [Google Scholar]
- 35.Miyoshi I, Taguchi H, Kubonishi I, Yoshimoto S, Ohtsuki Y, Shiraishi Y, Akagi T. Type C virus-producing cell lines derived from adult T cell leukemia. GANN. 1982;28:219–228. [Google Scholar]
- 36.Popovic M, Lange-Wantzin G, Sarin P S, Mann D, Gallo R. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci USA. 1983;80:5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quill H, Riley M P, Cho E A, Casnellie J E, Reed J E. Anergic Th1 cells express altered levels of the protein tyrosine kinases Lck and p59fyn. J Immunol. 1992;149:2887–2893. [PubMed] [Google Scholar]
- 38.Rowley R B, Burkhardt A L, Chao H-G, Matsueda G R, Bolen J B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 39.Salojin K, Zhang J, Cameron M, Gill B, Arreaza G, Ochi A, Delovitch T L. Impaired plasma membrane targeting of Grb2-murine Son of Sevenless (mSOS) complex and differential activation of the Fyn-T cell receptor (TCR)-ζ-Cb1 pathway mediate T cell hyporesponsiveness in autoimmune nonobese diabetic mice. J Exp Med. 1997;186:887–897. doi: 10.1084/jem.186.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharenberg A M, Lin S, Cuenod B, Yamamura H, Kinet J P. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO J. 1995;14:3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiue L, Zoller M J, Brugge J S. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- 42.Uchiumi F, Semba K, Yamanashi Y, Fujisawa J I, Yoshida M, Inoue K, Toyoshima K, Yamamoto T. Characterization of the promoter region of the src family gene lyn and its transactivation by human T-cell leukemia virus type I-encoded p40tax. Mol Cell Biol. 1992;12:3784–3795. doi: 10.1128/mcb.12.9.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valentin H, Lemasson I, Hamaia S, Cassé H, König S, Devaux C, Gazzolo L. Transcriptional activation of the vascular cell adhesion molecule-1 gene in T lymphocytes expressing human T-cell leukemia virus type 1 Tax protein. J Virol. 1997;71:8522–8530. doi: 10.1128/jvi.71.11.8522-8530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veillette A, Horak I D, Bolen J B. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 1988;2:385–401. [PubMed] [Google Scholar]
- 45.Watts J D, Affolter M, Krebs D L, Wange R L, Samelson L E, Aebersold R. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase Zap-70. J Biol Chem. 1994;269:29520–29529. [PubMed] [Google Scholar]
- 46.Weil R, Veillette A. Intramolecular and extramolecular mechanisms repress the catalytic function of p56lck in resting T-lymphocytes. J Biol Chem. 1994;269:22830–22838. [PubMed] [Google Scholar]
- 47.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 48.Yamanashi Y, Mori S, Yoshida M, Kishimoto T, Inoue K, Yamamoto T, Toyoshima K. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc Natl Acad Sci USA. 1989;86:6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoller K E, MacNeil I A, Brugge J S. Protein tyrosine kinase Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]